Intricacies of GABAA Receptor Function: The Critical Role of the β3 Subunit in Norm and Pathology
Abstract
:1. Introduction
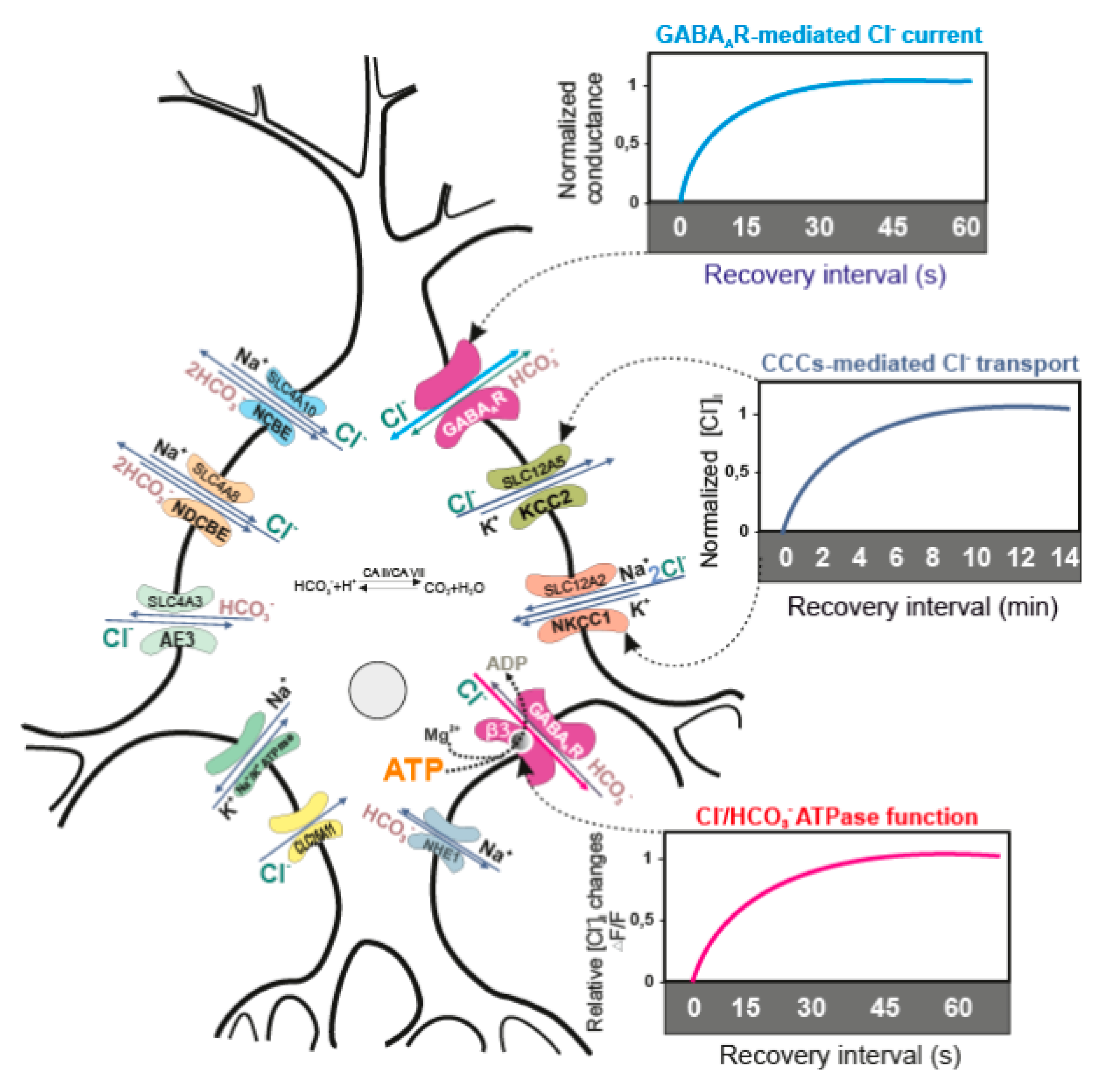
2. CCCs and GABAAR Activity
2.1. Role of KCC2
2.2. Role of NKCC1
2.3. CCCs and Neurological Disorders
3. Role of GABAAR/Cl−, HCO3− ATPase
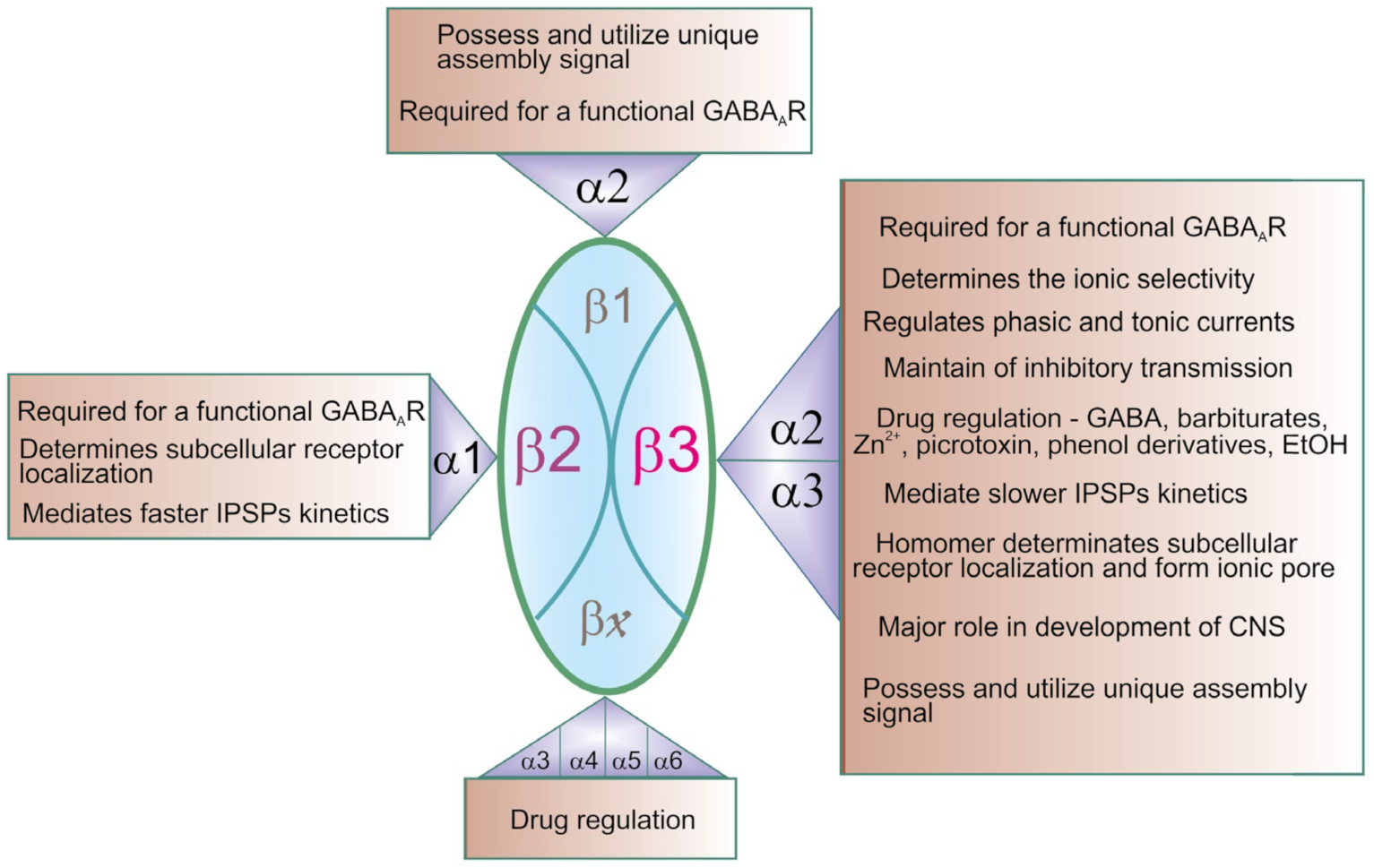
4. Role of the β3 Subunit in GABAAR Function
5. The β3 Subunit and Human Diseases
5.1. Epilepsy
5.2. Autism
5.3. Alzheimer’s Disease (AD)
5.4. Parkinson’s Disease (PD)
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Rahmati:, N.; Hoebeek, F.E.; Peter, S.; De Zeeuw, C.I. Chloride Homeostasis in Neurons with Special Emphasis on the Olivocerebellar System: Differential Roles for Transporters and Channels. Front. Cell. Neurosci. 2018, 12, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruffin, V.A.; Salameh, A.I.; Boron, W.F.; Parker, M.D. Intracellular pH regulation by acid-base transporters in mammalian neurons. Front. Physiol. 2014, 5, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raimondo, J.V.; Richards, B.A.; Woodin, M.A. Neuronal chloride and excitability—The big impact of small changes. Curr. Opin. Neurobiol. 2017, 43, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.S.; Artoni, P.; Landi, S.; Cozzolino, O.; Parra, R.; Pracucci, E.; Trovato, F.; Szczurkowska, J.; Luin, S.; Arosio, D.; et al. Simultaneous two-photon imaging of intracellular chloride concentration and pH in mouse pyramidal neurons in vivo. Proc. Natl. Acad. Sci. USA 2017, 114, 8770–8779. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, A.; Jedlicka, P.; Luhmann, H.J.; Kilb, W. Interactions between Membrane Resistance, GABA-A Receptor Properties, Bicarbonate Dynamics and Cl--Transport Shape Activity-Dependent Changes of Intracellular Cl- Concentration. Int. J. Mol. Sci. 2019, 20, 1416. [Google Scholar] [CrossRef] [Green Version]
- Hübner, C.A.; Holthoff, K. Anion transport and GABA signaling. Front. Cell. Neurosci. 2013, 7, 177. [Google Scholar] [CrossRef] [Green Version]
- Staley, K.J.; Proctor, W.R. Modulation of mammalian dendritic GABAA receptor function by the kinetics of Cl− and HCO3− transport. J. Physiol. Lond. 1999, 519, 693–712. [Google Scholar] [CrossRef]
- Mueller, A.L.; Taube, J.S.; Schwartzkroin, P.A. Development of hyperpolarizing inhibitory postsynaptic potentials and hyperpolarizing response to gamma-aminobutyric acid in rabbit hippocampus studied in vitro. J. Neurosci. 1984, 4, 860–867. [Google Scholar] [CrossRef] [Green Version]
- Ben-Ari, Y. The GABA excitatory/inhibitory developmental sequence: A personal journey. Neuroscience 2014, 279, 187–219. [Google Scholar] [CrossRef] [Green Version]
- Müller, W.; Misgeld, U.; Lux, H.D. gamma-Aminobutyric acid-induced ion movements in the guinea pig hippocampal slice. Brain Res. 1989, 484, 184–191. [Google Scholar] [CrossRef]
- Ferrini, F.; Perez-Sanchez, J.; Ferland, S.; Lorenzo, L.E.; Godin, AG.; Plasencia-Fernandez, I.; Cottet, M.; Castonguay, A.; Wang, F.; Salio, C.; et al. Differential chloride homeostasis in the spinal dorsal horn locally shapes synaptic metaplasticity and modality-specific sensitization. Nat. Commun. 2020, 11, 3935. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, A.T.; Ledri, M.; Melis, M.; Ledri, L.N.; Andersson, M.; Kokaia, M. Altered Chloride Homeostasis Decreases the Action Potential Threshold and Increases Hyperexcitability in Hippocampal Neurons. eNeuro 2018, 4, ENEURO.0172-17.2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staley, K.J.; Soldo, B.L.; Proctor, W.R. Ionic mechanisms of neuronal excitation by inhibitory GABAA receptors. Science 1995, 269, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Perkins, K.L.; Wong, R.K. Ionic basis of the postsynaptic depolarizing GABA response in hippocampal pyramidal cells. J. Neurophysiol. 1996, 76, 3886–3894. [Google Scholar] [CrossRef]
- Kaila, K. Ionic basis of GABAA receptor channel function in the nervous system. Prog. Neurobiol. 1994, 42, 489–537. [Google Scholar] [CrossRef]
- Isomura, Y.; Sugimoto, M.; Fujiwara-Tsukamoto, Y.; Yamamoto-Muraki, S.; Yamada, J.; Fukuda, A. Synaptically activated Cl--accumulation responsible for depolarizing GABAergic responses in mature hippocampal neurons. J. Neurophysiol. 2003, 90, 2752–2756. [Google Scholar] [CrossRef] [Green Version]
- Ben-Ari, Y.; Khalilov, I.; Kahle, K.T.; Cherubini, E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist 2012, 18, 467–486. [Google Scholar] [CrossRef]
- Rivera, C.; Voipio, J.; Kaila, K.; Voipio, J.; Kaila, K. Two developmental switches in GABAergic signalling: The K+–Cl− cotransporter KCC2 and carbonic anhydrase CAVII. J. Physiol. 2005, 562, 27–36. [Google Scholar] [CrossRef]
- Yamada, J.; Okabe, A.; Toyoda, H.; Kilb, W.; Luhmann, H.J.; Fukuda, A. Cl− uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. J. Physiol. 2004, 557, 829–841. [Google Scholar] [CrossRef]
- Balena, T.; Woodin, M.A. Coincident pre- and postsynaptic activity downregulates NKCC1 to hyperpolarize E(Cl) during development. Eur. J. Neurosci. 2008, 27, 2402–2412. [Google Scholar] [CrossRef]
- Ratté, S.; Prescott, S.A. ClC-2 channels regulate neuronal excitability, not intracellular chloride levels. J. Neurosci. 2011, 31, 15838–15843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaila, K.; Price, T.J.; Payne, J.A.; Puskarjov, M.; Voipio, J. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat. Rev. Neurosci. 2014, 15, 637–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyon, N.; Vinay, L.; Prescott, S.A.; De Koninck, Y. Chloride Regulation: A Dynamic Equilibrium Crucial for Synaptic Inhibition. Neuron 2016, 89, 1157–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, M.; Fukuda, A. Development and regulation of chloride homeostasis in the central nervous system. Front. Cell. Neurosci. 2015, 9, 371. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, T.; Ghaffarian, N.; Philippot, C.; Seifert, G.; Steinhäuser, C.; Pape, H.C.; Blaesse, P. Differential regulation of chloride homeostasis and GABAergic transmission in the thalamus. Sci. Rep. 2018, 8, 13929. [Google Scholar] [CrossRef] [Green Version]
- Sieghart, W.; Sperk, G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr. Top. Med. Chem. 2002, 2, 795–816. [Google Scholar] [CrossRef]
- Sigel, E.; Steinmann, M.E. Structure, function, and modulation of GABAA receptors. J. Biol. Chem. 2012, 287, 40224–40231. [Google Scholar] [CrossRef] [Green Version]
- Michels, G.; Moss, S.J. GABAA receptors: Properties and trafficking. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 3–14. [Google Scholar] [CrossRef]
- Nutt, D. GABAA Receptors: Subtypes, Regional Distribution, and Function. J. Clin. Sleep Med. 2006, 2, S7–S11. [Google Scholar] [CrossRef] [Green Version]
- Connolly, C.N.; Wooltorton, J.R.; Smart, T.G.; Moss, S.J. Subcellular localization of gamma-aminobutyric acid type A receptors is determined by receptor beta subunits. Proc. Natl. Acad. Sci. USA 1996, 93, 9899–9904. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, Q.A.; Nicoll, R.A. The GABAA Receptor β Subunit Is Required for Inhibitory Transmission. Neuron 2018, 98, 718–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, M.L.; Timmermann, D.B.; Johansen, T.H.; Schousboe, A.; Varming, T.; Ahring, P.K. The beta subunit determines the ion selectivity of the GABAA receptor. J. Biol. Chem. 2002, 277, 41438–41447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Currin, C.B.; Trevelyan, A.J.; Akerman, C.J.; Raimondo, J.V. Chloride dynamics alter the input-output properties of neurons. PLoS Comput. Biol. 2020, 16, e1007932. [Google Scholar] [CrossRef]
- Janssen, M.J.; Yasuda, R.P.; Vicini, S. GABAA Receptor β3 Subunit Expression Regulates Tonic Current in Developing Striatopallidal Medium Spiny Neurons. Front. Cell. Neurosci. 2011, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Menzikov, S.A.; Zaichenko, D.M.; Moskovtsev, A.A.; Morozov, S.G.; Kubatiev, A.A. Ectopic GABAA receptor β3 subunit determines Cl−/HCO3−-ATPase and chloride transport in HEK 293FT cells. FEBS J. 2020. [Google Scholar] [CrossRef]
- Gerencser, G.A.; Zhang, J. Existence and nature of the chloride pump. Biochim. Biophys. Acta 2003, 1618, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Clausen, J.D.; Bublitz, M.; Arnou, B.; Olesen, C.; Andersen, J.P.; Mølle, J.V.; Nissen, P. Crystal Structure of the Vanadate-Inhibited Ca2+-ATPase. Structure. 2016, 24, 617–623. [Google Scholar] [CrossRef] [Green Version]
- Gong, P.; Hong, H.; Perkins, E.J. Ionotropic GABA receptor antagonism-induced adverse outcome pathways for potential neurotoxicity biomarkers. Biomark. Med. 2015, 11, 1225–1239. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Yoon, B.E. Altered GABAergic Signaling in Brain Disease at Various Stages of Life. Exp. Neurobiol. 2017, 26, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Braat, S.; Kooy, R.F. The GABAA Receptor as a Therapeutic Target for Neurodevelopmental Disorders. Neuron 2015, 86, 1119–1130. [Google Scholar] [CrossRef] [Green Version]
- Payne, J.A. Functional characterization of the neuronal-specific K-Cl cotransporter: Implications for [K+]o regulation. Am. J. Physiol. 1997, 273, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.R.; Sharp, J.W.; Kumari, V.G.; Wilson, M.; Payne, J.A. The neuron-specific K-Cl cotransporter, KCC2. Antibody development and initial characterization of the protein. J. Biol. Chem. 1999, 274, 12656–12664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillen, C.M.; Brill, S.; Payne, J.A.; Forbush, B. Molecular cloning and functional expression of the K-Cl cotransporter from rabbit, rat, and human. A new member of the cation-chloride cotransporter family. J. Biol. Chem. 1996, 271, 16237–16244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uvarov, P.; Ludwig, A.; Markkanen, M.; Soni, S.; Hübner, CA.; Rivera, C.; Airaksinen, M.S. Coexpression and heteromerization of two neuronal K-Cl cotransporter isoforms in neonatal brain. J. Biol. Chem. 2009, 284, 13696–13704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaesse, P.; Airaksinen, M.S.; Rivera, C.; Kaila, K. Cation-chloride cotransporters and neuronal function. Neuron 2009, 61, 820–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, J.A.; Stevenson, T.J.; Donaldson, L.F. Molecular Characterization of a Putative K-Cl Cotransporter in Rat Brain. J. Biol. Chem. 1996, 271, 16245–16252. [Google Scholar] [CrossRef] [Green Version]
- Chamma, I.; Chevy, Q.; Poncer, J.C.; Lévi, S. Role of the neuronal K-Cl co-transporter KCC2 in inhibitory and excitatory neurotransmission. Front. Cell. Neurosci. 2012, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Kakazu, Y.; Uchida, S.; Nakagawa, T.; Akaike, N.; Nabekura, J. Reversibility and cation selectivity of the K+-Cl- cotransport in rat central neurons. J. Neurophysiol. 2000, 84, 281–288. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.R.; Payne, J.A. Cation transport by the neuronal K+-Cl- cotransporter KCC2: Thermodynamics and kinetics of alternate transport modes. Am. J. Physiol. Cell Physiol. 2004, 287, 919–931. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Lovinger, D.; Delpire, E. Cortical neurons lacking KCC2 expression show impaired regulation of intracellular chloride. J. Neurophysiol. 2005, 93, 1557–1568. [Google Scholar] [CrossRef] [Green Version]
- Cordero-Erausquin, M.; Coull, J.A.; Boudreau, D.; Rolland, M.; De Koninck, Y. Differential maturation of GABA action and anion reversal potential in spinal lamina I neurons: Impact of chloride extrusion capacity. J. Neurosci. 2005, 25, 9613–9623. [Google Scholar] [CrossRef] [PubMed]
- Mercado, A.; Broumand, V.; Zandi-Nejad, K.; Enck, A.H.; Mount, D.B. A C-terminal domain in KCC2 confers constitutive K+-Cl- cotransport. J. Biol. Chem. 2006, 281, 1016–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viitanen, T.; Ruusuvuori, E.; Kaila, K.; Voipio, J. The K+-Cl− cotransporter KCC2 promotes GABAergic excitation in the mature rat hippocampus. J. Physiol. 2010, 588, 1527–1540. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.; Voipio, J.; Payne, J.A.; Ruusuvuori, E.; Lahtinen, H.; Lamsa, K.; Pirvola, U.; Saarma, M.; Kaila, K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 1999, 397, 251–255. [Google Scholar] [CrossRef] [PubMed]
- DeFazio, R.A.; Hablitz, J.J. Chloride accumulation and depletion during GABAA receptor activation in neocortex. Neuroreport 2001, 12, 2537–2541. [Google Scholar] [CrossRef]
- DeFazio, R.A.; Keros, S.; Quick, M.W.; Hablitz, J.J. Potassium-coupled chloride cotransport controls intracellular chloride in rat neocortical pyramidal neurons. J. Neurosci. 2000, 20, 8069–8076. [Google Scholar] [CrossRef] [Green Version]
- Rivera, C.; Voipio, J.; Thomas-Crusells, J.; Li, H.; Emri, Z.; Sipilä, S.; Payne, J.A.; Minichiello, L.; Saarma, M.; Kaila, K. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J. Neurosci. 2004, 24, 4683–4691. [Google Scholar] [CrossRef] [Green Version]
- Gulyás, A.I.; Sík, A.; Payne, J.A.; Kaila, K.; Freund, T.F. The KCl cotransporter, KCC2, is highly expressed in the vicinity of excitatory synapses in the rat hippocampus. Eur. J. Neurosci. 2001, 13, 2205–2217. [Google Scholar] [CrossRef]
- Wang, C.; Shimizu-Okabe, C.; Watanabe, K.; Okabe, A.; Matsuzaki, H.; Ogawa, T.; Mori, N.; Fukuda, A.; Sato, K. Developmental changes in KCC1, KCC2, and NKCC1 mRNA expressions in the rat brain. Brain Res. Dev. Brain Res. 2002, 139, 59–66. [Google Scholar] [CrossRef]
- Tillman, L.; Zhang, J. Crossing the Chloride Channel: The Current and Potential Therapeutic Value of the Neuronal K+-Cl- Cotransporter KCC2. Biomed. Res. Int. 2019, 8941046. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, U.; Druzin, M.; Johansson, S. Cl− concentration changes and desensitization of GABAA and glycine receptors. J. Gen. Physiol. 2011, 138, 609–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Wang, J.; Jiang, J.; Zheng, X.; Justice, N.J.; Wang, K.; Ran, X.; Li, Y.; Huo, Q.; Zhang, J.; et al. APP modulates KCC2 expression and function in hippocampal GABAergic inhibition. eLife 2017, 6, e20142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, X.; Huguenard, J.R.; Prince, D.A. Impaired Cl- extrusion in layer V pyramidal neurons of chronically injured epileptogenic neocortex. J. Neurophysiol. 2005, 93, 2117–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyde, T.M.; Lipska, B.K.; Ali, T.; Mathew, S.V.; Law, A.J.; Metitiri, O.E.; Straub, R.E.; Ye, T.; Colantuoni, C.; Herman, M.M.; et al. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J. Neurosci. 2011, 31, 11088–11095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, I.; Chudotvorova, I. GABA neurotransmission and neural cation-chloride co-transporters: Actions beyond ion transport. Crit. Rev. Neurobiol. 2006, 18, 105–112. [Google Scholar] [CrossRef]
- Achilles, K.; Okabe, A.; Ikeda, M.; Shimizu-Okabe, C.; Yamada, J.; Fukuda, A.; Luhmann, H.J.; Kilb, W. Kinetic properties of Cl- uptake mediated by Na+-dependent K+-2Cl- cotransport in immature rat neocortical neurons. J. Neurosci. 2007, 27, 8616–8627. [Google Scholar] [CrossRef] [Green Version]
- Isenring, P.; Jacoby, S.C.; Payne, J.A.; Forbush, B. Comparison of Na-K-Cl cotransporters. NKCC1, NKCC2, and the HEK cell Na-K-Cl cotransporter. J. Biol. Chem. 1998, 273, 11295–11301. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Murali, S.G. Stimulation of Na+-K+-2Cl- cotransporter in neuronal cells by excitatory neurotransmitter glutamate. Am. J. Physiol. 1998, 275, 772–779. [Google Scholar] [CrossRef]
- Pond, B.B.; Berglund, K.; Kuner, T.; Feng, G.; Augustine, G.J.; Schwartz-Bloom, R.D. The chloride transporter Na+-K+-Cl- cotransporter isoform-1 contributes to intracellular chloride increases after in vitro ischemia. J. Neurosci. 2006, 26, 1396–1406. [Google Scholar] [CrossRef] [Green Version]
- Hannaert, P.; Alvarez-Guerra, M.; Pirot, D.; Nazaret, C.; Garay, R. Rat NKCC2/NKCC1 cotransporter selectivity for loop diuretic drugs. Naunyn-Schmiedebergs Arch. Pharmacol. 2002, 365, 193–199. [Google Scholar]
- Brumback, A.C.; Staley, K.J. Thermodynamic regulation of NKCC1-mediated Cl- cotransport underlies plasticity of GABAA signaling in neonatal neurons. J. Neurosci. 2008, 28, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Islas, C.; Chub, N.; Wenner, P. NKCC1 and AE3 appear to accumulate chloride in embryonic motoneurons. J. Neurophysiol. 2009, 101, 507–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, H.; Putzier, I.; Frings, S.; Kaupp, U.B.; Gensch, T. Chloride Accumulation in Mammalian Olfactory Sensory Neurons. J. Neurosci. 2004, 24, 7931–7938. [Google Scholar] [CrossRef] [PubMed]
- Nickell, W.T.; Kleene, N.K.; Kleene, S.J. Mechanisms of neuronal chloride accumulation in intact mouse olfactory epithelium. J. Physiol. 2007, 583, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, M.D.; Snyder, E.Y.; Hebert, S.C.; Delpire, E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: A possible mechanism underlying GABA’s excitatory role in immature brain. J. Neurobiol. 1997, 33, 781–795. [Google Scholar] [CrossRef]
- Nardou, R.; Yamamoto, S.; Chazal, G.; Bhar, A.; Ferrand, N.; Dulac, O.; Ben-Ari, Y.; Khalilov, I. Neuronal chloride accumulation and excitatory GABA underlie aggravation of neonatal epileptiform activities by phenobarbital. Brain 2011, 134, 987–1002. [Google Scholar] [CrossRef] [Green Version]
- Nardou, R.; Ben-Ari, Y.; Khalilov, I. Bumetanide, an NKCC1 antagonist, does not prevent formation of epileptogenic focus but blocks epileptic focus seizures in immature rat hippocampus. J. Neurophysiol. 2009, 101, 2878–2888. [Google Scholar] [CrossRef] [Green Version]
- Dzhala, V.I.; Kuchibhotla, K.V.; Glykys, J.C.; Kahle, K.T.; Swiercz, W.B.; Feng, G.; Kuner, T.; Augustine, G.J.; Bacskai, B.J.; Staley, K.J. Progressive NKCC1-dependent neuronal chloride accumulation during neonatal seizures. J. Neurosci. 2010, 30, 11745–11761. [Google Scholar] [CrossRef]
- Mazarati, A.; Shin, D.; Sankar, R. Bumetanide inhibits rapid kindling in neonatal rats. Epilepsia 2009, 50, 2117–2122. [Google Scholar] [CrossRef] [Green Version]
- Rheims, S.; Ryvlin, P.; Scherer, C.; Minotti, L.; Hoffmann, D.; Guenot, M.; Mauguière, F.; Benabid, AL.; Kahane, P. Analysis of clinical patterns and underlying epileptogenic zones of hypermotor seizures. Epilepsia 2008, 49, 2030–2040. [Google Scholar] [CrossRef]
- Kilb, W.; Sinning, A.; Luhmann, H.J. Model-specific effects of bumetanide on epileptiform activity in the in-vitro intact hippocampus of the newborn mouse. Neuropharmacology 2007, 53, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Kühlbrandt, W. Biology, structure and mechanism of P-type ATPases. Nat. Rev. Mol. Cell Biol. 2004, 5, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Fatima-Shad, K.; Barry, P.H. Anion permeation in GABA- and glycine-gated channels of mammalian cultured hippocampal neurons. Proc. Biol. Sci. 1993, 253, 69–75. [Google Scholar] [PubMed]
- Bormann, J.; Hamill, O.P.; Sakmann, B. Mechanism of anion permeation through channels gated by glycine and -aminobutyric acid in mouse cultured spinal neurons. J. Physiol. Lond. 1987, 385, 243–286. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, K.; Zheng, C.; Liu, Q.; Chang, Y.; Kerrigan, J.F.; Wu, J. Functional rundown of gamma-aminobutyric acid (A) receptors in human hypothalamic hamartomas. Ann. Neurol. 2011, 69, 664–672. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.T. Allosteric modulation of GABAA receptors by extracellular ATP. Mol. Brain 2014, 24, 6. [Google Scholar] [CrossRef] [Green Version]
- Stelzer, A.; Kay, A.R.; Wong, R.K. GABAA-receptor function in hippocampal cells is maintained by phosphorylation factors. Science 1988, 241, 339–341. [Google Scholar] [CrossRef]
- Rapallino, M.V.; Cupello, A.; Hydén, H. An electrogenic ionic pump derived from an ionotropic receptor: Assessment of a candidate. Cell Mol. Neurobiol. 1999, 19, 681–690. [Google Scholar] [CrossRef]
- Cupello, A. Neuronal transmembrane chloride electrochemical gradient: A key player in GABAA receptor activation physiological effect. Amino Acids. 2003, 24, 335–346. [Google Scholar] [CrossRef]
- Menzikov, S.A.; Morozov, S.G. Involvement of brain GABAAR-coupled Cl-/HCO3--ATPase in phenol-induced the head-twitching and tremor responses in rats. Neurotoxicology 2019, 71, 122–131. [Google Scholar] [CrossRef]
- Menzikov, S. Dual character of GABA action on Cl--transport by the reconstituted Cl-/HCO3--ATPase from rat brain. J. Recept. Signal Transduct. Res. 2018, 38, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Menzikov, S.A. Neuronal multifunctional ATPase. Biophys. Rev. Lett. 2013, 8, 213–227. [Google Scholar] [CrossRef]
- Menzikov, S. Biochemical properties of the sensitivity to GABAAergic ligands, Cl-/HCO3--ATPase isolated from fish (Cyprinus carpio) olfactory mucosa and brain. Fish Physiol. Biochem. 2018, 44, 583–597. [Google Scholar] [CrossRef]
- Menzikov, S.; Menzikova, O. Interaction of pentobarbital with GABAergic drugs acting on the Cl--ATPase activity of the plasma membranes from bream brain (Abramis brama L.). Neurosci. Lett. 2002, 334, 161–164. [Google Scholar] [CrossRef]
- Zezula, J.; Slany, A.; Sieghart, W. Interaction of allosteric ligands with GABAA receptors containing one, two, or three different subunits. Eur. J. Pharm. 1996, 301, 207–214. [Google Scholar] [CrossRef]
- Davies, P.A.; Kirkness, E.F.; Hales, T.G. Modulation by general anaesthetics of rat GABAA receptors comprised of α1β3 and β3 subunits expressed in human embryonic kidney 293 cells. Br. J. Pharmacol. 1997, 120, 899–909. [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.L.; Macdonald, R.L. Single channel properties of recombinant GABAA receptors containing gamma 2 or delta subtypes expressed with alpha 1 and beta 3 subtypes in mouse L929 cells. J. Physiol. 1997, 505, 283–297. [Google Scholar] [CrossRef]
- Taylor, P.M.; Thomas, P.; Gorrie, G.H.; Connolly, C.N.; Smart, T.G.; Moss, S.J. Identification of amino acid residues within GABAA receptor beta subunits that mediate both homomeric and heteromeric receptor expression. J. Neurosci. 1999, 19, 6360–6371. [Google Scholar] [CrossRef] [Green Version]
- Hannan, S.; Smart, T.G. Cell surface expression of homomeric GABAA receptors depends on single residues in subunit transmembrane domains. J. Biol. Chem. 2018, 293, 13427–13439. [Google Scholar] [CrossRef] [Green Version]
- Miller, P.S.; Aricescu, A.R. Crystal structure of a human GABAA receptor. Nature 2014, 512, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Ratra, G.S.; Kamita, S.G.; Casida, J.E. Role of human GABAA receptor beta3 subunit in insecticide toxicity. Toxicol. Appl. Pharmacol. 2001, 172, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Darnieder, L.M.; Deeb, T.Z.; Moss, S.J. Regulation of GABAARs by phosphorylation. Adv. Pharmacol. 2015, 72, 97–146. [Google Scholar] [PubMed] [Green Version]
- Houston, C.M.; Hosie, A.M.; Smart, T.G. Distinct regulation of beta2 and beta3 subunit-containing cerebellar synaptic GABAA receptors by calcium/calmodulin-dependent protein kinase II. J. Neurosci. 2008, 28, 7574–7584. [Google Scholar] [CrossRef] [PubMed]
- Bollan, K.; King, D.; Robertson, L.A.; Brown, K.; Taylor, P.M.; Moss, S.J.; Connolly, C.N. GABAA receptor composition is determined by distinct assembly signals within alpha and beta subunits. J. Biol. Chem. 2003, 278, 4747–4755. [Google Scholar] [CrossRef] [Green Version]
- Gingrich, K.J.; Roberts, W.A.; Kass, R.S. Dependence of the GABAA receptor gating kinetics on the alpha-subunit isoform: Implications for structure-function relations and synaptic transmission. J. Physiol. 1995, 489, 529–543. [Google Scholar] [CrossRef]
- Ramadan, E.; Fu, Z.; Losi, G.; Homanics, G.E.; Neale, J.H.; Vicini, S. GABAA receptor beta3 subunit deletion decreases alpha2/3 subunits and IPSC duration. J. Neurophysiol. 2003, 89, 128–134. [Google Scholar] [CrossRef] [Green Version]
- Chiodi, C.G.; Baptista-Hon, D.T.; Hunter, W.N.; Hales, T.G. Amino acid substitutions in the human homomeric β3 GABAA receptor that enable activation by GABA. J. Biol. Chem. 2019, 294, 2375–2385. [Google Scholar] [CrossRef] [Green Version]
- Kaila, K.; Ruusuvuori, E.; Seja, P.; Voipio, J.; Puskarjov, M. GABA actions and ionic plasticity in epilepsy. Curr. Opin. Neurobiol. 2014, 26, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Gurba, K.N.; Hernandez, C.C.; Hu, N.; Macdonald, R.L. GABRB3 mutation, G32R, associated with childhood absence epilepsy alters α1β3γ2L γ-aminobutyric acid type A (GABAA) receptor expression and channel gating. J. Biol. Chem. 2012, 287, 12083–12097. [Google Scholar] [CrossRef] [Green Version]
- Absalom, N.L.; Ahring, P.K.; Liao, V.W.; Balle, T.; Jiang, T.; Anderson, L.L.; Arnold, J.C.; McGregor, I.S.; Bowen, M.T.; Chebib, M. Functional genomics of epilepsy-associated mutations in the GABAA receptor subunits reveal that one mutation impairs function and two are catastrophic. J. Biol. Chem. 2019, 294, 6157–6171. [Google Scholar] [CrossRef]
- Janve, V.S.; Hernandez, C.C.; Verdier, K.M.; Hu, N.; Macdonald, R.L. Epileptic encephalopathy de novo GABRB mutations impair γ-aminobutyric acid type A receptor function. Ann. Neurol. 2016, 79, 806–825. [Google Scholar] [CrossRef] [PubMed]
- Møller, R.S.; Wuttke, T.V.; Helbig, I.; Marini, C.; Johannesen, K.M.; Brilstra, E.H.; Vaher, U.; Borggraefe, I.; Talvik, I.; Talvik, T.; et al. Mutations in GABRB3: From febrile seizures to epileptic encephalopathies. Neurology 2017, 88, 483–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epi4K Consortium; Epilepsy Phenome/Genome Project; Allen, A.S.; Berkovic, S.F.; Cossette, P.; Delanty, N.; Dlugos, D.; Eichler, E.E.; Epstein, M.P.; Glauser, T.; et al. De novo mutations in epileptic encephalopathies. Nature 2013, 501, 217–221. [Google Scholar] [PubMed] [Green Version]
- Macdonald, R.L.; Kang, J.Q.; Gallagher, M.J. Mutations in GABAA receptor subunits associated with genetic epilepsies. J. Physiol. 2010, 588, 1861–1869. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.C.; Zhang, Y.; Hu, N.; Shen, D.; Shen, W.; Liu, X.; Kong, W.; Jiang, Y.; Macdonald, R.L. GABAA Receptor Coupling Junction and Pore GABRB3 Mutations are Linked to Early-Onset Epileptic Encephalopathy. Sci. Rep. 2017, 7, 15903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papandreou, A.; McTague, A.; Trump, N.; Ambegaonkar, G.; Ngoh, A.; Meyer, E.; Scott, R.H.; Kurian, M.A. GABRB3 mutations: A new and emerging cause of early infantile epileptic encephalopathy. Dev. Med. Child. Neurol. 2016, 58, 416–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirker, S.; Schwarzer, C.; Czech, T.; Baumgartner, C.; Pockberger, H.; Maier, H.; Hauer, B.; Sieghart, W.; Furtinger, S.; Sperk, G. Increased Expression of GABAA Receptor β-Subunits in the Hippocampus of Patients with Temporal Lobe Epilepsy. J. Neuropathol. Exp. Neurol. 2003, 62, 820–834. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Olsen, R.W.; Medina, M.T.; Schwartz, E.; Alonso, M.E.; Duron, R.M.; Castro-Ortega, R.; Martinez-Juarez, I.E.; Pascual-Castroviejo, I.; Machado-Salas, J.; et al. Hyperglycosylation and reduced GABA currents of mutated GABRB3 polypeptide in remitting childhood absence epilepsy. Am. J. Hum. Genet. 2008, 82, 1249–1261. [Google Scholar] [CrossRef] [Green Version]
- Hörtnagl, H.; Tasan, R.O.; Wieselthaler, A.; Kirchmair, E.; Sieghart, W.; Sperk, G. Patterns of mRNA and protein expression for 12 GABAA receptor subunits in the mouse brain. Neuroscience 2013, 236, 345–372. [Google Scholar] [CrossRef] [Green Version]
- Homanics, G.E.; DeLorey, T.M.; Firestone, L.L.; Quinlan, J.J.; Handforth, A.; Harrison, N.L.; Krasowski, M.D.; Rick, C.E.; Korpi, E.R.; Mäkelä, R.; et al. Mice devoid of gamma-aminobutyrate type A receptor beta3 subunit have epilepsy, cleft palate, and hypersensitive behavior. Proc. Natl. Acad. Sci. USA 1997, 94, 4143–4148. [Google Scholar] [CrossRef] [Green Version]
- Moghbelinejad, S.; Rashvand, Z.; Khodabandehloo, F.; Mohammadi, G.; Nassiri-Asl, M. Modulation of the Expression of the GABAA Receptor β1 and β3 Subunits by Pretreatment with Quercetin in the KA Model of Epilepsy in Mice: -The Effect of Quercetin on GABAA Receptor Beta Subunits. J. Pharmacopunct. 2016, 19, 163–166. [Google Scholar]
- Khom, S.; Hintersteiner, J.; Luger, D.; Haider, M.; Pototschnig, G.; Mihovilovic, M.D.; Schwarzer, C.; Hering, S. Analysis of β-Subunit-dependent GABAA Receptor Modulation and Behavioral Effects of Valerenic Acid Derivatives. J. Pharmacol. Exp Ther. 2016, 357, 580–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khazipov, R.; Valeeva, G.; Khalilov, I. Depolarizing GABA and developmental epilepsies. CNS Neurosci. Ther. 2015, 21, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Sperk, G. Changes in GABAA receptors in status epilepticus. Epilepsia 2007, 48, 2382. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Mannal of Mental Disorders: DSM-V; American Psychiatric Association: Arlington, TX, USA, 2013. [Google Scholar]
- Sesarini, C.V. GABAergic neurotransmission alterations in autism spectrum disorders. Neurotransmitter 2015, 2, e1052. [Google Scholar]
- Rubenstein, J.L.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef]
- Cellot, G.; Cherubini, E. GABAergic signaling as therapeutic target for autism spectrum disorders. Front. Pediatr. 2014, 2, 70. [Google Scholar] [CrossRef] [Green Version]
- Pizzarelli, R.; Cherubini, E. Alterations of GABAergic signaling in autism spectrum disorders. Neural Plast. 2011, 297153. [Google Scholar] [CrossRef] [Green Version]
- DeLorey, T.M. GABRB3 gene deficient mice: A potential model of autism spectrum disorder. Int. Rev. Neurobiol. 2005, 71, 359–382. [Google Scholar]
- Delahanty, R.; Kang, J.; Brune, C.; Kistner, E.O.; Courchesne, E.; Cox, N.J.; Cook, E.H., Jr.; Macdonald, R.L.; Sutcliffe, J.S. Maternal transmission of a rare GABRB3 signal peptide variant is associated with autism. Mol. Psychiatry 2011, 16, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Guo, X.; Dong, X.; Han, Y.; Gao, L.; Su, Y.; Dai, W.; Zhang, X. GABAA receptor subunit gene polymorphisms predict symptom-based and developmental deficits in Chinese Han children and adolescents with autistic spectrum disorders. Sci. Rep. 2017, 7, 3290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, A.L.; Ma, D.; Whitehead, P.L.; Martin, E.R.; Wright, H.H.; Abramson, R.K.; Hussman, J.P.; Haines, J.L.; Cuccaro, M.L.; Gilbert, J.R.; et al. Investigation of autism and GABA receptor subunit genes in multiple ethnic groups. Neurogenetics 2006, 7, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provenzano, G.; Gilardoni, A.; Maggia, M.; Pernigo, M.; Sgadò, P.; Casarosa, S.; Bozzi, Y. Altered Expression of GABAergic Markers in the Forebrain of Young and Adult Engrailed-2 Knockout Mice. Genes (Basel) 2020, 11, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rissman, R.A.; Mobley, W.C. Implications for treatment: GABAA receptors in aging, Down syndrome and Alzheimer’s disease. J. Neurochem. 2011, 117, 613–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Sun, H.; Chen, Z.; Xu, H.; Bu, G.; Zheng, H. Implications of GABAergic Neurotransmission in Alzheimer’s Disease. Front. Aging Neurosci. 2016, 8, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwakowsky, A.; Calvo-Flores, G.B.; Govindpani, K.; Waldvogel, H.J.; Faull, R.L. Gamma-aminobutyric acid A receptors in Alzheimer’s disease: Highly localized remodeling of a complex and diverse signaling pathway. Neural Regen. Res. 2018, 13, 1362–1363. [Google Scholar] [CrossRef]
- Kwakowsky, A.; Calvo-Flores Guzmán, B.; Pandya, M.; Turner, C.; Waldvogel, H.J.; Faull, R.L. GABAA receptor subunit expression changes in the human Alzheimer’s disease hippocampus, subiculum, entorhinal cortex and superior temporal gyrus. J. Neurochem. 2018, 145, 374–392. [Google Scholar] [CrossRef]
- Limon, A.; Reyes-Ruiz, J.M.; Miledi, R. Loss of functional GABAA receptors in the Alzheimer diseased brain. Proc. Natl. Acad. Sci. USA 2012, 109, 10071–10076. [Google Scholar] [CrossRef] [Green Version]
- Błaszczyk, J.W. Parkinson’s Disease and Neurodegeneration: GABA-Collapse Hypothesis. Front. Neurosci. 2016, 10, 269. [Google Scholar] [CrossRef] [Green Version]
- Gironell, A. The GABA Hypothesis in Essential Tremor: Lights and Shadows. Tremor. Other. Hyperkinet. Mov. (NY) 2014, 4, 254. [Google Scholar] [CrossRef]
- Calon, F.; Morissette, M.; Rajput, A.H.; Hornykiewicz, O.; Bédard, P.J.; Di Paolo, T. Changes of GABA receptors and dopamine turnover in the postmortem brains of parkinsonians with levodopa-induced motor complications. Mov. Disord. 2003, 18, 241–253. [Google Scholar] [CrossRef] [PubMed]
- van Nuland, A.J.M.; den Ouden, H.E.M.; Zach, H.; Dirkx, M.F.M.; van Asten, J.J.A.; Scheenen, T.W.J.; Toni, I.; Cools, R.; Helmich, R.C. GABAergic changes in the thalamocortical circuit in Parkinson’s disease. Hum. Brain Mapp. 2020, 41, 1017–1029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniele, A.; Panza, F.; Greco, A.; Logroscino, G.; Seripa, D. Can a Positive Allosteric Modulation of GABAergic Receptors Improve Motor Symptoms in Patients with Parkinson’s Disease? The Potential Role of Zolpidem in the Treatment of Parkinson’s Disease. Parkinsons Dis. 2016, 2531812. [Google Scholar] [CrossRef] [PubMed]
- Paris-Robidas, S.; Brochu, E.; Sintes, M.; Emond, V.; Bousquet, M.; Vandal, M.; Pilote, M.; Tremblay, C.; Di Paolo, T.; Rajput, A.H.; et al. Defective dentate nucleus GABA receptors in essential tremor. Brain 2012, 135, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Mograbi, K.M.; de Castro, A.C.; de Oliveira, J.A.; Sales, P.J.; Covolan, L.; Del Bel, E.A.; de Souza, A.S. Effects of GABAa receptor antagonists on motor behavior in pharmacological Parkinson’s disease model in mice. Physiol. Rep. 2017, 5, e13081. [Google Scholar] [CrossRef] [Green Version]
- Hoerbelt, P.; Lindsley, T.A.; Fleck, M.W. Dopamine directly modulates GABAA receptors. J. Neurosci. 2015, 35, 3525–3536. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menzikov, S.A.; Morozov, S.G.; Kubatiev, A.A. Intricacies of GABAA Receptor Function: The Critical Role of the β3 Subunit in Norm and Pathology. Int. J. Mol. Sci. 2021, 22, 1457. https://doi.org/10.3390/ijms22031457
Menzikov SA, Morozov SG, Kubatiev AA. Intricacies of GABAA Receptor Function: The Critical Role of the β3 Subunit in Norm and Pathology. International Journal of Molecular Sciences. 2021; 22(3):1457. https://doi.org/10.3390/ijms22031457
Chicago/Turabian StyleMenzikov, Sergey A., Sergey G. Morozov, and Aslan A. Kubatiev. 2021. "Intricacies of GABAA Receptor Function: The Critical Role of the β3 Subunit in Norm and Pathology" International Journal of Molecular Sciences 22, no. 3: 1457. https://doi.org/10.3390/ijms22031457
APA StyleMenzikov, S. A., Morozov, S. G., & Kubatiev, A. A. (2021). Intricacies of GABAA Receptor Function: The Critical Role of the β3 Subunit in Norm and Pathology. International Journal of Molecular Sciences, 22(3), 1457. https://doi.org/10.3390/ijms22031457




