Peripheral Blood miRome Identified miR-155 as Potential Biomarker of MetS and Cardiometabolic Risk in Obese Patients
Abstract
:1. Introduction
2. Subjects and Methods
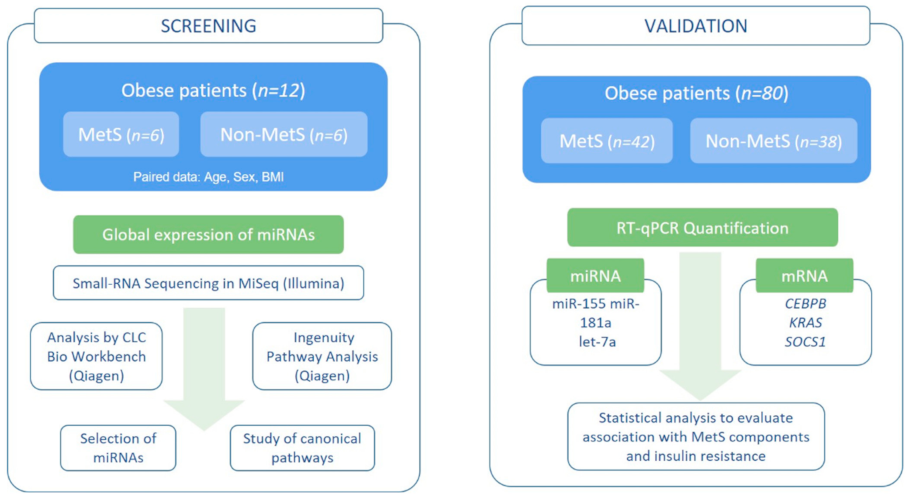
2.1. Patients and Study Design
2.2. Clinical Laboratory Analyses
2.3. RNA Extraction and Analysis
2.4. miRome Analysis by RNA-Seq
2.5. In Silico Analysis for Selection of miRNAs Target Genes
2.6. Expression of Selected miRNAs by Stem Loop RT-qPCR
2.7. Expression of Target mRNAs by qPCR
2.8. Statistical Analysis
3. Results
3.1. Characteristics of the Study Groups
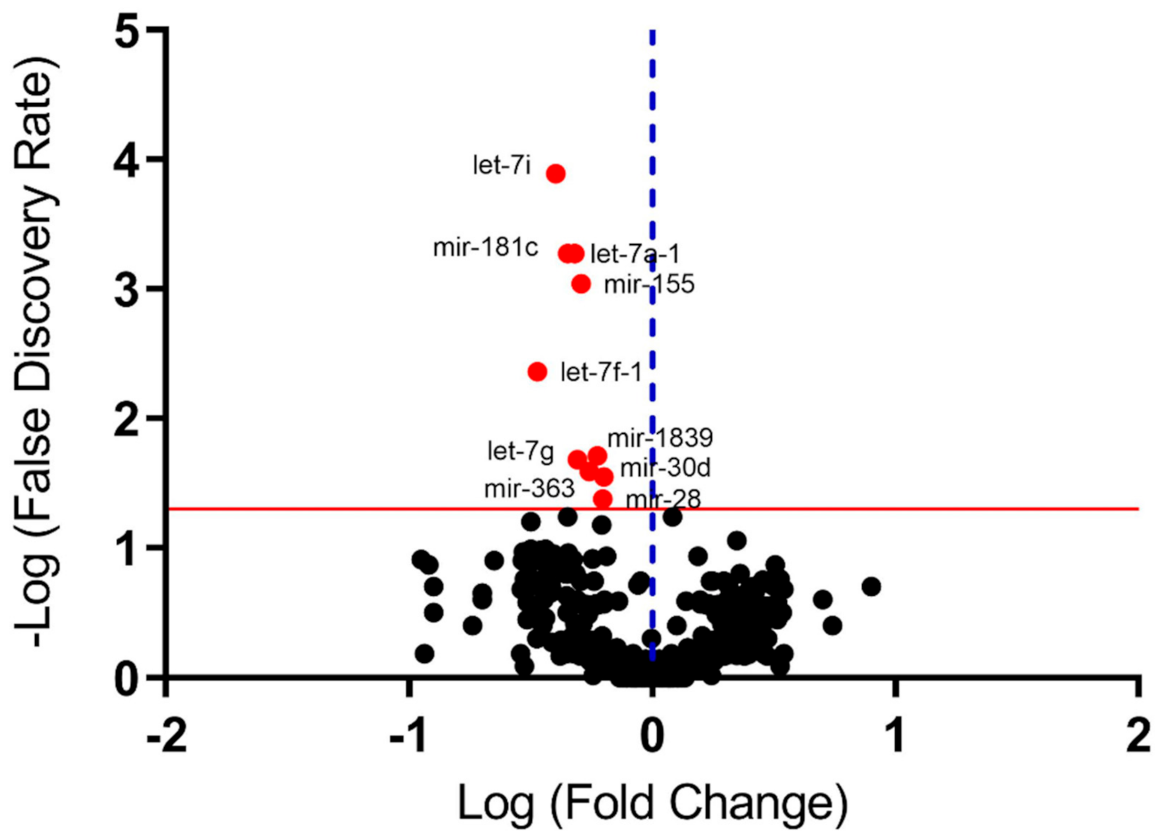
3.2. miRNome in Peripheral Blood (Screening Study)
3.3. Selected miR:mRNA Interactions Study
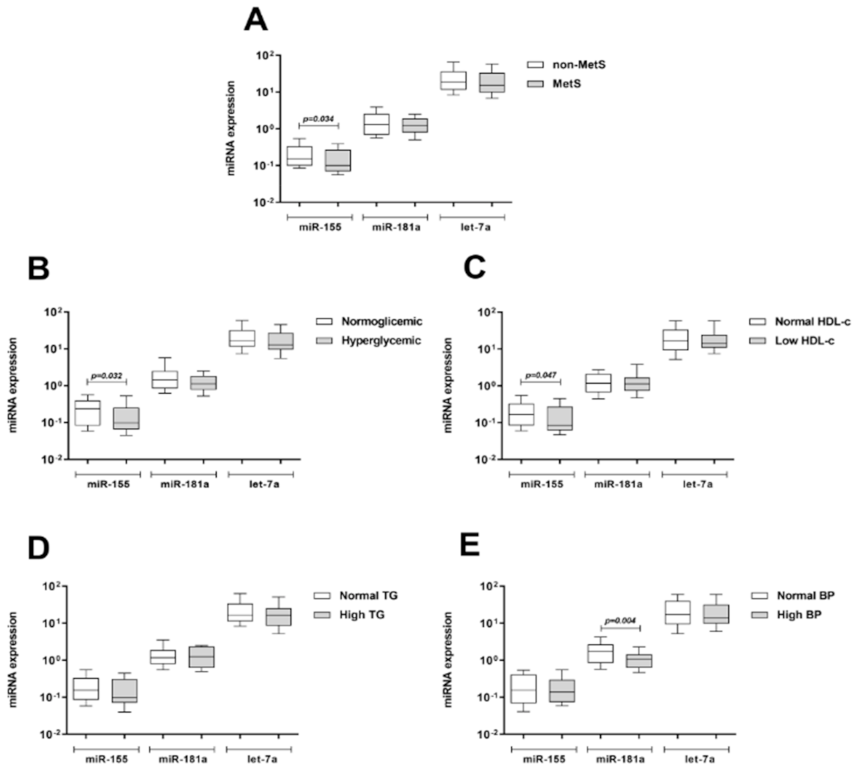
3.4. Expression of Selected miRNAs by qPCR (Validation Study)
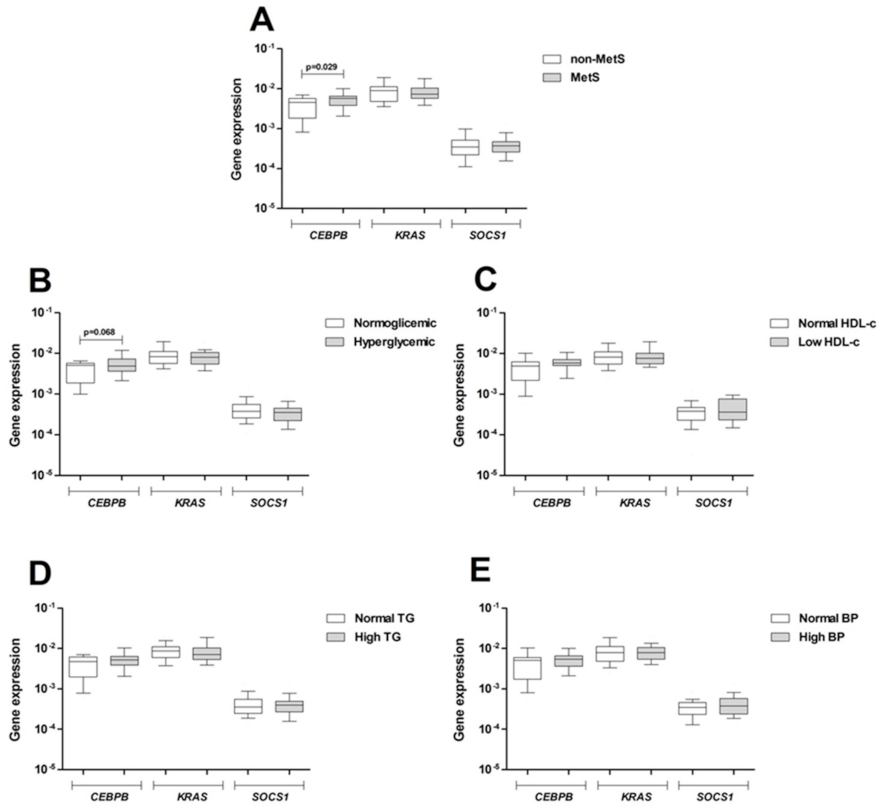
3.5. Expression of Target Genes in Peripheral Blood
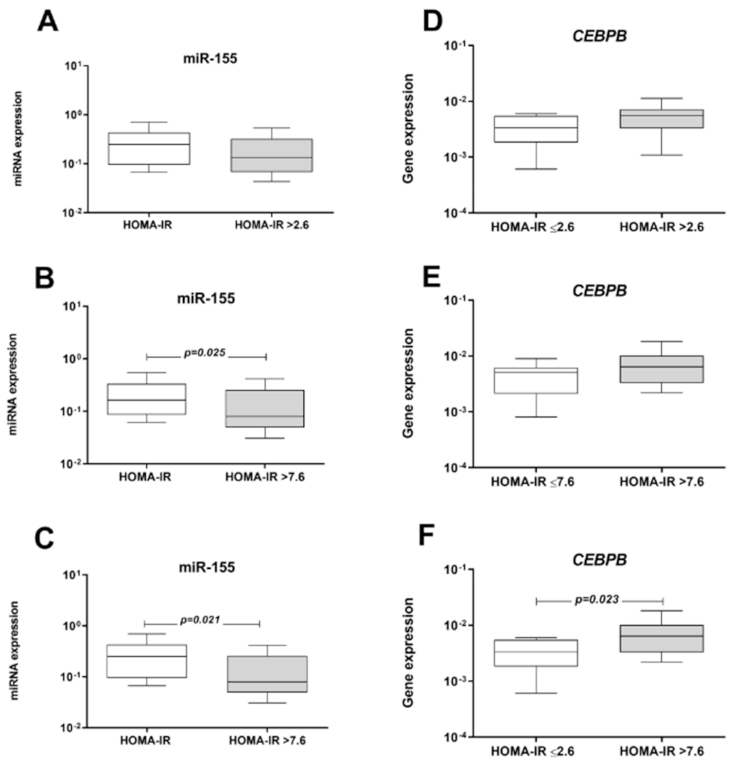
3.6. Influence of MetS Criteria and Insulin Resistance on miRNA and mRNA Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| CEBP | CCAAT Enhancer binding protein beta gene |
| FDR | False discovery rate |
| GLP-1 | Glucagon like peptide 1 |
| HbA1c | Glycated hemoglobin |
| HDL | High-density lipoprotein |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| hsCRP | High-sensitivity C-reactive protein |
| IL-1β | Interleukin 1β |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| IPA | Ingenuity Pathway Analysis |
| KRAS | KRAS proto-oncogene, GTPase |
| LDL | Low-density lipoprotein |
| MetS | Metabolic Syndrome |
| miRNAs | microRNAs |
| NF-kB | Nuclear factor Kappa B |
| PBMC | Peripheral blood mononuclear cells |
| RIN | RNA integrity number |
| RT-qPCR | Reverse transcription quantitative PCR |
| SOCS1 | Suppressor of cytokine signaling 1 |
| TNF-α | Tumor necrosis factor alpha |
| T2D | Type 2 diabetes |
| VLDL | Very low-density lipoprotein |
References
- Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 2014, 943162. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Fall, T.; Ingelsson, E. Genome-wide association studies of obesity and metabolic syndrome. Mol. Cell. Endocrinol. 2014, 382, 740–757. [Google Scholar] [CrossRef] [PubMed]
- Pigeyre, M.; Yazdi, F.T.; Kaur, Y.; Meyre, D. Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin. Sci. 2016, 130, 943–986. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Kumar, P.; Mahalingam, K. Molecular genetics of human obesity: A comprehensive review. Comptes Rendus Biol. 2017, 340, 87–108. [Google Scholar] [CrossRef]
- Brown, A.E.; Walker, M. Genetics of Insulin Resistance and the Metabolic Syndrome. Curr. Cardiol. Rep. 2016, 18, 75. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, R.; Cerda, A.; Genvigir, F.D.; Sampaio, M.F.; Armaganijan, D.; Bernik, M.M.; Dorea, E.L.; Hirata, M.H.; Hinuy, H.M.; Hirata, R.D. Leptin receptor gene polymorphisms are associated with adiposity and metabolic alterations in Brazilian individuals. Arq. Bras. Endocrinol. Metabol. 2013, 57, 677–684. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, R.; Moraes, T.I.; Cerda, A.; Hirata, M.H.; Fajardo, C.M.; Sousa, M.C.; Dorea, E.L.; Bernik, M.M.; Hirata, R.D. ADIPOQ and IL6 variants are associated with a pro-inflammatory status in obeses with cardiometabolic dysfunction. Diabetol. Metab. Syndr. 2015, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Manriquez, V.; Aviles, J.; Salazar, L.; Saavedra, N.; Seron, P.; Lanas, F.; Fajardo, C.M.; Hirata, M.H.; Hirata, R.D.C.; Cerda, A. Polymorphisms in Genes Involved in the Leptin-Melanocortin Pathway are Associated with Obesity-Related Cardiometabolic Alterations in a Southern Chilean Population. Mol. Diagn. Ther. 2018, 22, 101–113. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, S.; Li, H.; Xiang, H.; Peng, J.; Jiang, S. MicroRNAs: Emerging roles in adipogenesis and obesity. Cell. Signal. 2014, 26, 1888–1896. [Google Scholar] [CrossRef]
- Abente, E.J.; Subramanian, M.; Ramachandran, V.; Najafi-Shoushtari, S.H. MicroRNAs in obesity-associated disorders. Arch. Biochem. Biophys. 2016, 589, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.; Lawson, H.A. The Epigenetics of Metabolic Syndrome. Physiol. Genom. 2018, 50, 947–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deiuliis, J.A. MicroRNAs as regulators of metabolic disease: Pathophysiologic significance and emerging role as biomarkers and therapeutics. Int. J. Obes. 2016, 40, 88–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Párrizas, M.; Novials, A. Circulating microRNAs as biomarkers for metabolic disease. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 591–601. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; Bohl, M.; Gregersen, S.; Hermansen, K.; O’Driscoll, L. Blood-Based Biomarkers for Metabolic Syndrome. Trends Endocrinol. Metab. 2016, 27, 363–374. [Google Scholar] [CrossRef]
- de Fátima Costa, A.; Franco, O.L. Insights into RNA transcriptome profiling of cardiac tissue in obesity and hypertension conditions. J. Cell. Physiol. 2015, 230, 959–968. [Google Scholar] [CrossRef]
- Lu, D.; Thum, T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat. Rev. Cardiol. 2019, 16, 661–674. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034-1. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhang, B.H.; Shen, C.A.; Zhu, B.W.; An, H.Y.; Zheng, B.; Xu, S.B.; Sun, J.C.; Sun, P.C.; Zhang, W.; Wang, J.; et al. Insight into miRNAs related with glucometabolic disorder. Biomed. Pharmacother. 2019, 111, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Elton, T.S.; Selemon, H.; Elton, S.M.; Parinandi, N.L. Regulation of the MIR155 host gene in physiological and pathological processes. Gene 2013, 532, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.; Meese, E.; Keller, A. Specific miRNA Disease Biomarkers in Blood, Serum and Plasma: Challenges and Prospects. Mol. Diagn. Ther. 2016, 20, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, Y.; Wu, J. TNFα-induced up-regulation of miR-155 inhibits adipogenesis by down-regulating early adipogenic transcription factors. Biochem. Biophys. Res. Commun. 2011, 414, 618–624. [Google Scholar] [CrossRef]
- Engin, A.B. MicroRNA and Adipogenesis. Adv. Exp. Med. Biol. 2017, 960, 489–509. [Google Scholar]
- Hulsmans, M.; Sinnaeve, P.; Van der Schueren, B.; Mathieu, C.; Janssens, S.; Holvoet, P. Decreased miR-181a expression in monocytes of obese patients is associated with the occurrence of metabolic syndrome and coronary artery disease. J. Clin. Endocrinol. Metab. 2012, 97, E1213–E1218. [Google Scholar] [CrossRef] [Green Version]
- D’Amore, S.; Vacca, M.; Cariello, M.; Graziano, G.; D’Orazio, A.; Salvia, R.; Sasso, R.C.; Sabbà, C.; Palasciano, G.; Moschetta, A. Genes and miRNA expression signatures in peripheral blood mononuclear cells in healthy subjects and patients with metabolic syndrome after acute intake of extra virgin olive oil. Biochim. Biophys. Acta 2016, 1861, 1671–1680. [Google Scholar] [CrossRef]
- Karolina, D.S.; Tavintharan, S.; Armugam, A.; Sepramaniam, S.; Pek, S.L.; Wong, M.T.; Lim, S.C.; Sum, C.F.; Jeyaseelan, K. Circulating miRNA profiles in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 2012, 97, E2271–E2276. [Google Scholar] [CrossRef] [Green Version]
- Xihua, L.; Shengjie, T.; Weiwei, G.; Matro, E.; Tingting, T.; Lin, L.; Fang, W.; Jiaqiang, Z.; Fenping, Z.; Hong, L. Circulating miR-143-3p inhibition protects against insulin resistance in Metabolic Syndrome via targeting of the insulin-like growth factor 2 receptor. Transl. Res. 2018, 205, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Al-Rawaf, H.A. Circulating microRNAs and adipokines as markers of metabolic syndrome in adolescents with obesity. Clin. Nutr. 2018, 38, 2231–2238. [Google Scholar] [CrossRef]
- Wang, Y.T.; Tsai, P.C.; Liao, Y.C.; Hsu, C.Y.; Juo, S.H. Circulating microRNAs have a sex-specific association with metabolic syndrome. J. Biomed. Sci. 2013, 20, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Hernando, C.; Ramírez, C.M.; Goedeke, L.; Suárez, Y. MicroRNAs in metabolic disease. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 178–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahdavi, R.; Ghorbani, S.; Alipoor, B.; Panahi, G.; Khodabandehloo, H.; Esfahani, E.N.; Razi, F.; Meshkani, R. Decreased Serum Level of miR-155 is Associated with Obesity and its Related Metabolic Traits. Clin. Lab. 2018, 64, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Tryggestad, J.B.; Teague, A.M.; Sparling, D.P.; Jiang, S.; Chernausek, S.D. Macrophage-Derived microRNA-155 Increases in Obesity and Influences Adipocyte Metabolism by Targeting Peroxisome Proliferator-Activated Receptor Gamma. Obesity 2019, 27, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ding, Y.; Liang, B.; Lin, J.; Kim, T.K.; Yu, H.; Hang, H.; Wang, K. A Systematic Study of Dysregulated MicroRNA in Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2017, 18, 456. [Google Scholar] [CrossRef]
- Klöting, N.; Berthold, S.; Kovacs, P.; Schön, M.R.; Fasshauer, M.; Ruschke, K.; Stumvoll, M.; Blüher, M. MicroRNA expression in human omental and subcutaneous adipose tissue. PLoS ONE 2009, 4, e4699. [Google Scholar] [CrossRef] [Green Version]
- Khamaneh, A.M.; Alipour, M.R.; Sheikhzadeh Hesari, F.; Ghadiri Soufi, F. A signature of microRNA-155 in the pathogenesis of diabetic complications. J. Physiol. Biochem. 2015, 71, 301–309. [Google Scholar] [CrossRef]
- Corral-Fernández, N.E.; Salgado-Bustamante, M.; Martínez-Leija, M.E.; Cortez-Espinosa, N.; García-Hernández, M.H.; Reynaga-Hernández, E.; Quezada-Calvillo, R.; Portales-Pérez, D.P. Dysregulated miR-155 expression in peripheral blood mononuclear cells from patients with type 2 diabetes. Exp. Clin. Endocrinol. Diabetes 2013, 121, 347–353. [Google Scholar] [CrossRef]
- Lin, X.; Qin, Y.; Jia, J.; Lin, T.; Chen, L.; Zeng, H.; Han, Y.; Wu, L.; Huang, S.; Wang, M.; et al. MiR-155 Enhances Insulin Sensitivity by Coordinated Regulation of Multiple Genes in Mice. PLoS Genet. 2016, 12, e1006308. [Google Scholar] [CrossRef] [Green Version]
- Mazloom, H.; Alizadeh, S.; Pasalar, P.; Esfahani, E.N.; Meshkani, R. Downregulated microRNA-155 expression in peripheral blood mononuclear cells of type 2 diabetic patients is not correlated with increased inflammatory cytokine production. Cytokine 2015, 76, 403–408. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, N.; Wang, Z.; Ai, D.M.; Cao, Z.Y.; Pan, H.P. Decreased MiR-155 Level in the Peripheral Blood of Non-Alcoholic Fatty Liver Disease Patients may Serve as a Biomarker and may Influence LXR Activity. Cell. Physiol. Biochem. 2016, 39, 2239–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faccini, J.; Ruidavets, J.B.; Cordelier, P.; Martins, F.; Maoret, J.J.; Bongard, V.; Ferrières, J.; Roncalli, J.; Elbaz, M.; Vindis, C. Circulating miR-155, miR-145 and let-7c as diagnostic biomarkers of the coronary artery disease. Sci. Rep. 2017, 7, 42916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Wei, Y.; Geißler, C.; Abschlag, K.; Corbalán Campos, J.; Hristov, M.; Möllmann, J.; Lehrke, M.; Karshovska, E.; Schober, A. Hyperlipidemia-Induced MicroRNA-155-5p Improves β-Cell Function by Targeting. Diabetes 2017, 66, 3072–3084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, M.; Xu, Z.; Ding, T.; Kuang, D.M.; Zheng, L. MicroRNA-155 regulates inflammatory cytokine production in tumor-associated macrophages via targeting C/EBPbeta. Cell. Mol. Immunol. 2009, 6, 343–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, K.; Fuster, J.J.; Walsh, K. Adipokines: A link between obesity and cardiovascular disease. J. Cardiol. 2014, 63, 250–259. [Google Scholar] [CrossRef] [Green Version]
| Variables | Total Group (n = 80) | MetS (n = 48) | non-MetS (n = 32) | p-Value | |
|---|---|---|---|---|---|
| Demographic and Clinical Data | Age in years | 48.8 ± 8.2 | 49.7 ± 8.0 | 47.4 ± 8.2 | 0.261 |
| Gender [woman], % | 67 (54) | 61 (29) | 78 (25) | 0.095 | |
| Hypertension, % | 45 (36) | 54 (26) | 31 (10) | 0.043 | |
| Dyslipidemia, % | 71 (57) | 85 (41) | 50 (16) | <0.001 | |
| Insulin resistance, % | 75 (60) | 83 (40) | 63 (20) | 0.035 | |
| Type 2 diabetes, % | 28 (22) | 33 (16) | 19 (6) | 0.146 | |
| Additional MetS criteria #, % | |||||
| 0 | 19 (15) | 0 (0) | 47 (15) | <0.001 | |
| 1 | 21 (17) | 0 (0) | 53 (17) | ||
| 2 | 33 (26) | 54 (26) | 0 (0) | ||
| 3 | 20 (16) | 33 (16) | 0 (0) | ||
| 4 | 7 (6) | 13 (6) | 0 (0) | ||
| Alcohol consumption, % | 7.5 (6) | 6.2 (3) | 9.4 (3) | 0.603 | |
| Tobacco smoking, % | 19 (15) | 21 (10) | 15 (5) | 0.559 | |
| Anthropometric Measures | BMI, Kg/m2 | 33.8 ± 4.2 | 35.1 ± 3.2 | 32.1 ± 3.8 | 0.002 |
| Waist circumference, cm | 105.1 ± 13.0 | 111.3 ± 10.2 | 95.9 ± 11.2 | <0.001 | |
| Waist-hip ratio | 0.90 ± 0.09 | 0.94 ± 0.09 | 0.84 ± 0.07 | <0.001 | |
| Fat mass, % | 36.8 ± 6.1 | 37.6 ± 5.6 | 35.7 ± 6.5 | 0.185 | |
| Metabolic Profile | Glucose, mg/dL | 105 ± 27 | 112 ± 33 | 95 ± 9 | <0.001 |
| Insulin, mU/L | 23.2 ± 22.9 | 27.8 ± 17.7 | 16.6 ± 10.7 | 0.018 | |
| HOMA-IR | 6.62 ± 9.22 | 8.5 ± 11.5 | 3.97 ± 2.78 | 0.016 | |
| HbA1c, % | 6.22 ± 1.64 | 6.65 ± 1.97 | 5.57 ± 0.47 | 0.001 | |
| Total cholesterol, mg/dL | 210 ± 39 | 214 ± 43 | 206 ± 33 | 0.356 | |
| LDL cholesterol, mg/dL | 128 ± 32 | 129 ± 35 | 126 ± 27 | 0.710 | |
| HDL cholesterol, mg/dL | 51 ± 14 | 49 ± 16 | 56 ± 13 | 0.028 | |
| VLDL cholesterol, mg/dL | 30 ± 15 | 34 ± 16 | 23 ± 12 | 0.001 | |
| Triglycerides, mg/dL | 149 ± 77 | 172 ± 80 | 116 ± 59 | 0.001 |
| miRNA | Fold Change | p-Value | FDR |
|---|---|---|---|
| let-7a-1 | −2.22433 | 9.00 × 10−6 | 0.000531 |
| let-7f-1 | −2.9723 | 0.000123 | 0.004344 |
| let-7g | −2.03151 | 0.000827 | 0.020901 |
| let-7i | −2.48811 | 7.25 × 10−7 | 0.000128 |
| mir-28 | −1.59773 | 0.002377 | 0.04208 |
| mir-30d | −1.57958 | 0.001446 | 0.02843 |
| mir-155 | −1.96078 | 2.07 × 10−5 | 0.000916 |
| mir-181a | −2.08434 | 8.60 × 10−6 | 0.000531 |
| mir-363 | −1.6846 | 0.000663 | 0.019573 |
| mir-1839 | −1.81056 | 0.001164 | 0.025752 |
| Variables | r | p-Value |
|---|---|---|
| CEBPB mRNA | −0.431 | <0.001 |
| HOMA-IR | −0.256 | 0.036 |
| Adiponectin | 0.292 | 0.017 |
| Fibrinogen | −0.252 | 0.029 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerda, A.; Amaral, A.A.; de Oliveira, R.; Moraes, T.I.; Braga, A.A.; Graciano-Saldarriaga, M.E.; Fajardo, C.M.; Hirata, T.D.C.; Bonezi, V.; Campos-Salazar, A.B.; et al. Peripheral Blood miRome Identified miR-155 as Potential Biomarker of MetS and Cardiometabolic Risk in Obese Patients. Int. J. Mol. Sci. 2021, 22, 1468. https://doi.org/10.3390/ijms22031468
Cerda A, Amaral AA, de Oliveira R, Moraes TI, Braga AA, Graciano-Saldarriaga ME, Fajardo CM, Hirata TDC, Bonezi V, Campos-Salazar AB, et al. Peripheral Blood miRome Identified miR-155 as Potential Biomarker of MetS and Cardiometabolic Risk in Obese Patients. International Journal of Molecular Sciences. 2021; 22(3):1468. https://doi.org/10.3390/ijms22031468
Chicago/Turabian StyleCerda, Alvaro, Adonai Aralim Amaral, Raquel de Oliveira, Tamiris Invencioni Moraes, Aécio Assunção Braga, Magda Elizabeth Graciano-Saldarriaga, Cristina Moreno Fajardo, Thiago Dominguez Crespo Hirata, Vivian Bonezi, Antony Brayan Campos-Salazar, and et al. 2021. "Peripheral Blood miRome Identified miR-155 as Potential Biomarker of MetS and Cardiometabolic Risk in Obese Patients" International Journal of Molecular Sciences 22, no. 3: 1468. https://doi.org/10.3390/ijms22031468





