N-palmitoyl-D-glucosamine, A Natural Monosaccharide-Based Glycolipid, Inhibits TLR4 and Prevents LPS-Induced Inflammation and Neuropathic Pain in Mice
Abstract
:1. Introduction
2. Methods
2.1. Computational Methods
2.2. Cell Culture and Luciferase Assay
2.3. RNA Extraction and Quantitative PCR (qPCR)
2.4. 3-(4,5-Dimethylthiazol-2-yl)-2,5- Diphenyltetrazolium Bromide (MTT) Assay
2.5. TRPA1-HEK293 Cells and Calcium Assay
2.6. Animals
2.7. Induction of Keratitis
2.7.1. Treatments
- mice receiving an intrastromal injection of PBS and oral vehicle administration (2 μL) (Saline/Veh group);
- mice receiving an intrastromal injection of LPS and oral vehicle administration (2 μg/2 μL PBS) (LPS/Veh group);
- mice administered per os with PGA (5 mg/kg) 30 min before receiving an intrastromal injection of LPS (2 μg/2 μL PBS) (PGA + LPS group);
- mice receiving an intrastromal injection of LPS (2 μg/2 μL PBS) and after 30 min administered per os with PGA 5 mg/kg for three days (LPS + PGA group);
- mice receiving an intrastromal injection of PBS (2 μL) and after 30 min administered per os with PGA 5 mg/kg for three days (Saline/PGA group).
2.7.2. Clinical Score Evaluation
2.8. Cornea Immunohistochemistry
2.9. miRNA qRT-PCR
2.10. ELISA Assay
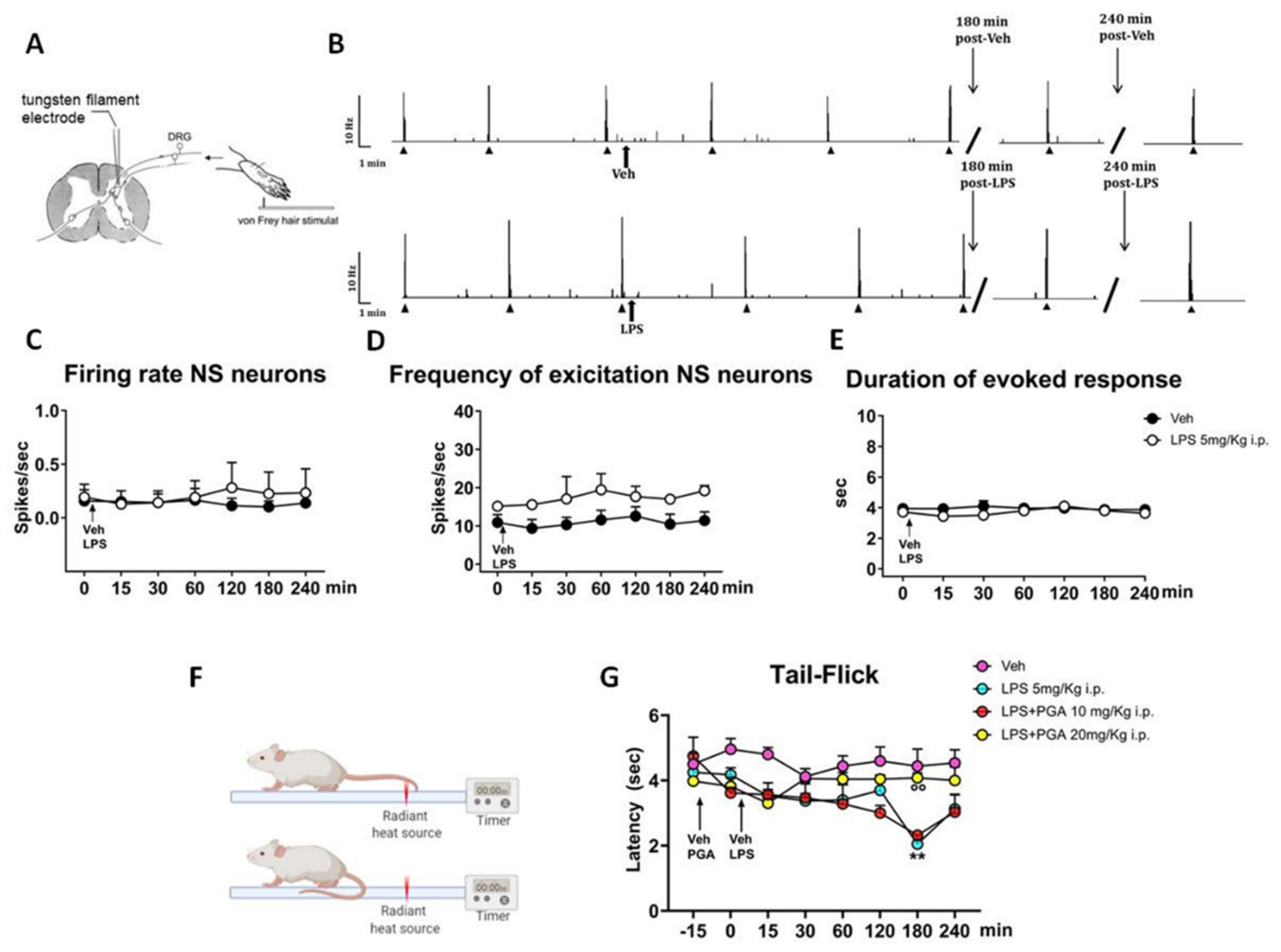
2.11. In Vivo Electrophysiological Recordings of Nociceptive Spinal Neurons (NS) in Combination with the Tail-Flick Test
2.12. Pain Models
2.12.1. Pain Measurement
2.12.2. Treatments
2.13. Electron Microscopy
3. Statistical Analysis
4. Results
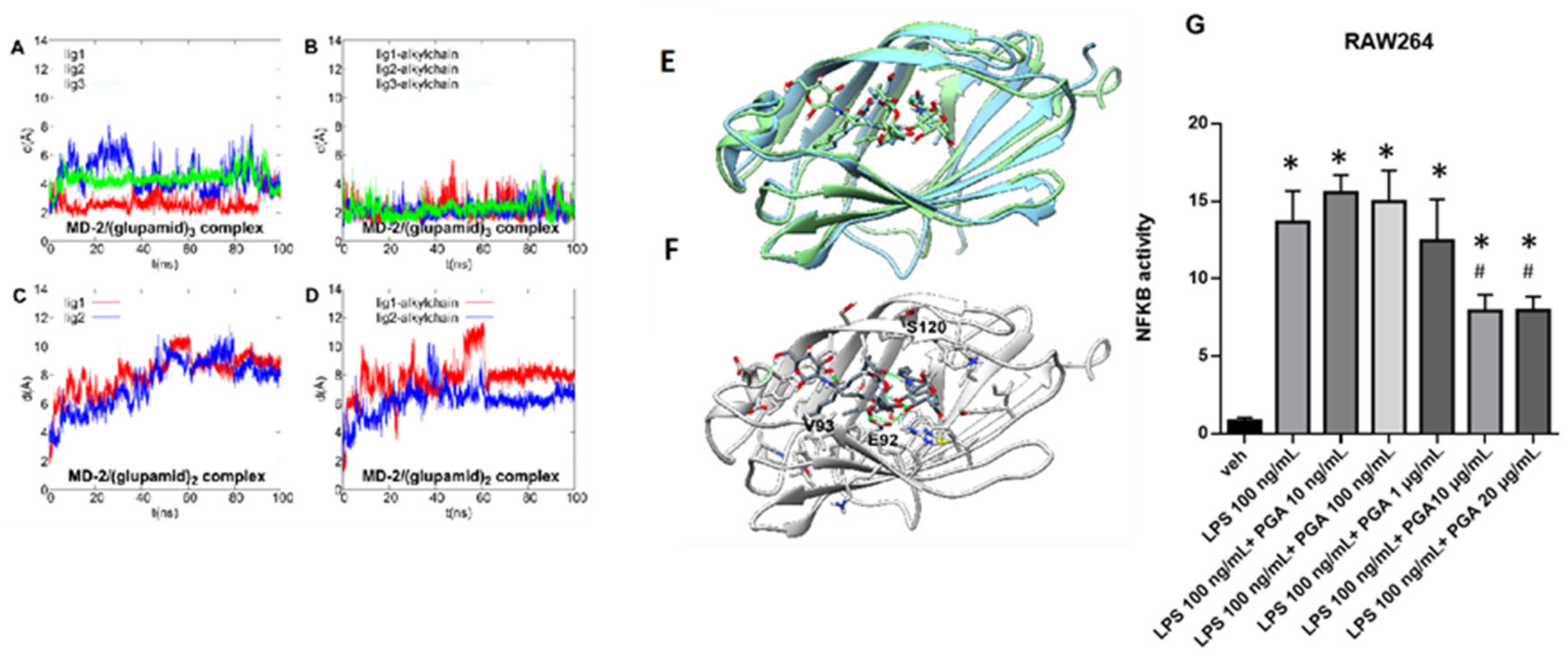
4.1. Theoretical Complex of MD-2 Protein with PGA
4.2. PGA Prevents NF-kB Activation in LPS Stimulated RAW264.7 Cells
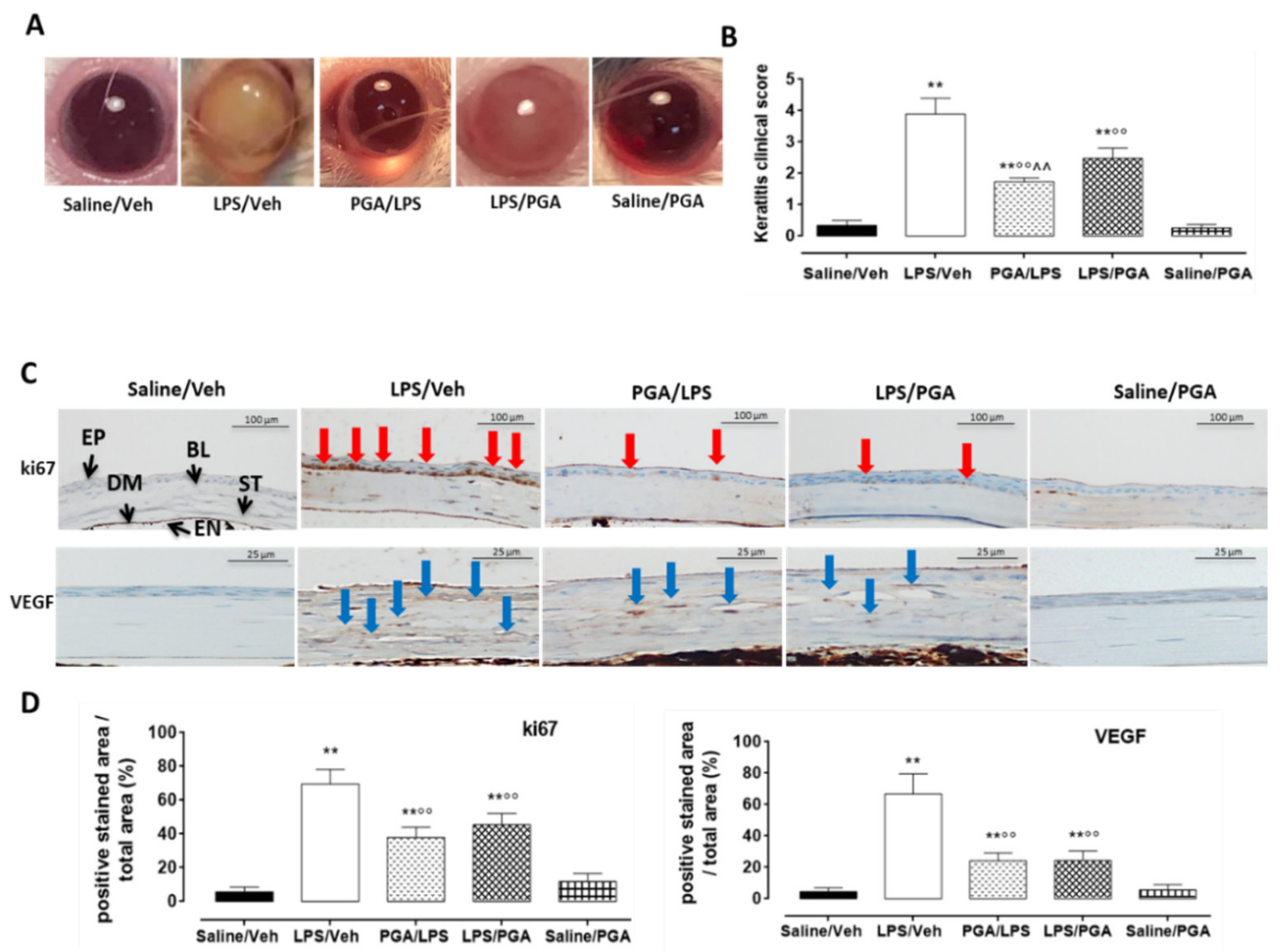
4.3. LPS-Induced Corneal Inflammation
4.3.1. Clinical Score
4.3.2. PGA Reduces ki67 and VEGF Labelling in LPS-Injected Mice
4.3.3. PGA Affects TLR4-Dependent Cytokine Release through miRNA Regulation
4.4. LPS Does Not Alter the Spinal Nociceptive Specific Neuron (NS) Activity, but Increases Responsiveness to a Thermal Stimulus in Naïve Mice
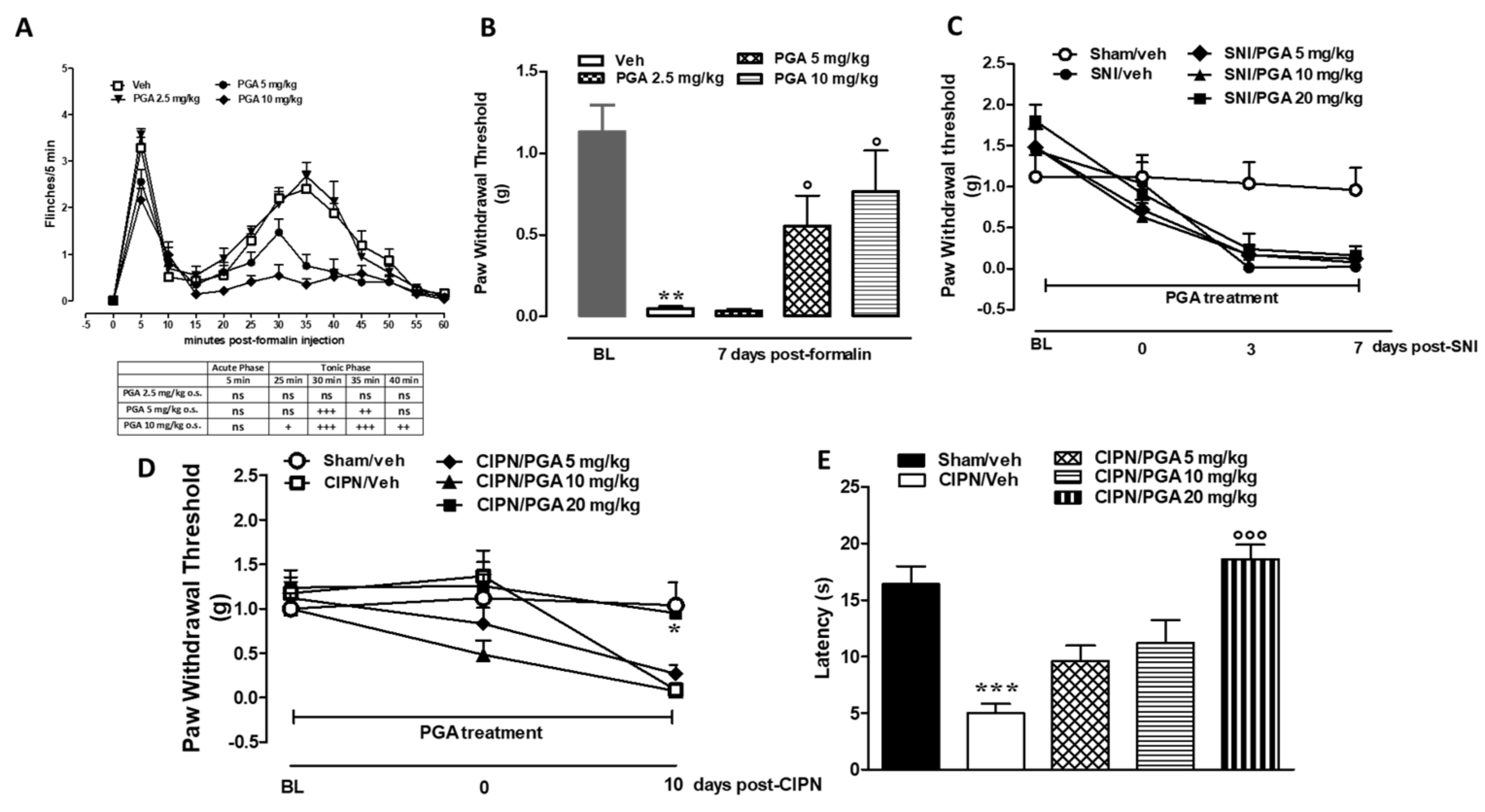
4.5. PGA Prevented the Formalin- or Oxaliplatin-, but not SNI-Induced Allodynia
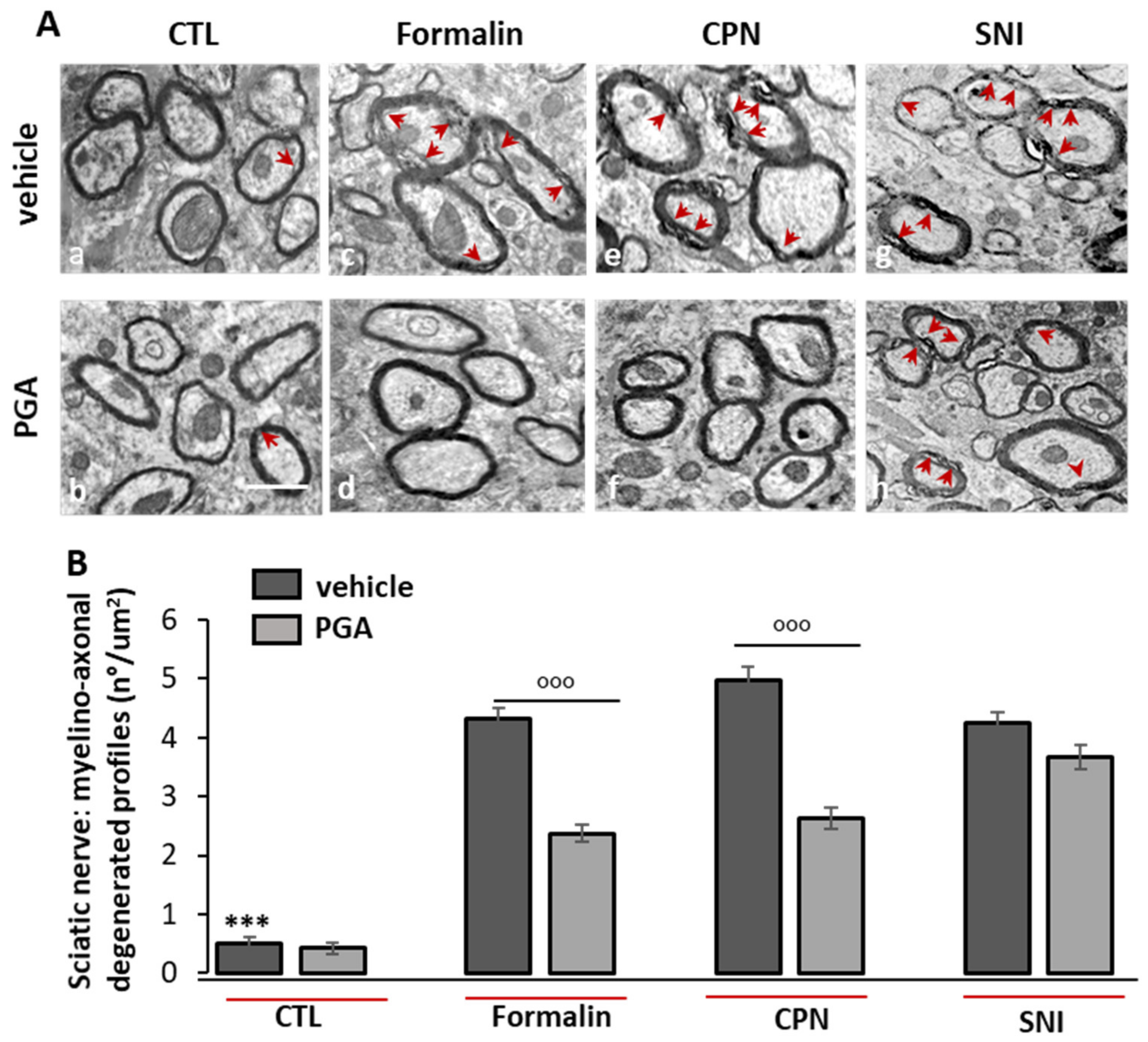
4.6. PGA Prevented the Formalin- or Oxaliplatin- but not SNI-Induced Myelino-Axonal Degeneration of Sciatic Nerve
PGA Does Not Induce TRPA1-Mediated Elevation of Intracellular Ca2+
5. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Marchand, F.; Perretti, M.; McMahon, S.B. Role of the immune system in chronic pain. Nat. Rev. Neurosci. 2005, 6, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Woolf, C.J. The neuropathic pain triad: Neurons, immune cells and glia. Nat. Neurosci. 2007, 10, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.K.; Old, E.A.; Malcangio, M. Neuropathic pain and cytokines: Current perspectives. J. Pain Res. 2013, 6, 803. [Google Scholar] [PubMed] [Green Version]
- Coraggio, V.; Guida, F.; Boccella, S.; Scafuro, M.; Paino, S.; Romano, D.; Maione, S.; Luongo, L. Neuroimmune-driven neuropathic pain establishment: A focus on gender differences. Int. J. Mol. Sci. 2018, 19, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, E. TLR4 signalling. Nat. Rev. Immunol. 2008, 8, 241. [Google Scholar] [CrossRef]
- Ospelt, C.; Gay, S. TLRs and chronic inflammation. Int. J. Biochem. Cell Biol. 2010, 42, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Halder, A.; Yadav, K.; Aggarwal, A.; Singhal, N.; Sandhir, R. Activation of TNFR1 and TLR4 following oxygen glucose deprivation promotes mitochondrial fission in C6 astroglial cells. Cell. Signal. 2020, 75, 109714. [Google Scholar] [CrossRef]
- Zhao, L.; Li, M.; Sun, K.; Su, S.; Geng, T.; Sun, H. Hippophae rhamnoides polysaccharides protect IPEC-J2 cells from LPS-induced inflammation, apoptosis and barrier dysfunction in vitro via inhibiting TLR4/NF-κB signaling pathway. Int. J. Biol. Macromol. 2020, 155, 1202–1215. [Google Scholar] [CrossRef]
- Christianson, C.A.; Dumlao, D.S.; Stokes, J.A.; Dennis, E.A.; Svensson, C.I.; Corr, M.; Yaksh, T.L. Spinal TLR4 mediates the transition to a persistent mechanical hypersensitivity after the resolution of inflammation in serum-transferred arthritis. Pain 2011, 152, 2881–2891. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Gao, Y.-J.; Ji, R.-R. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci. Bull. 2012, 28, 131–144. [Google Scholar] [CrossRef]
- Bettoni, I.; Comelli, F.; Rossini, C.; Granucci, F.; Giagnoni, G.; Peri, F.; Costa, B. Glial TLR4 receptor as new target to treat neuropathic pain: Efficacy of a new receptor antagonist in a model of peripheral nerve injury in mice. Glia 2008, 56, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Soto, R.; Yandamuri, S.; Stone, C.; Dickey, L.; Gomes-Neto, J.C.; Pastuzyn, E.D.; Bell, R.; Petersen, C.; Buhrke, K. The microbiota protects from viral-induced neurologic damage through microglia-intrinsic TLR signaling. Elife 2019, 8, e47117. [Google Scholar] [CrossRef] [PubMed]
- Philip-Hollingsworth, S.; Dazzo, F.; Hollingsworth, R. Structural requirements of Rhizobium chitolipooligosaccharides for uptake and bioactivity in legume roots as revealed by synthetic analogs and fluorescent probes. J. Lipid Res. 1997, 38, 1229–1241. [Google Scholar] [CrossRef]
- Das, A.; Srinivasan, M.; Ghosh, T.S.; Mande, S.S. Xenobiotic metabolism and gut microbiomes. PLoS ONE 2016, 11, e0163099. [Google Scholar] [CrossRef] [Green Version]
- Cordaro, M.; Siracusa, R.; Impellizzeri, D.; D’Amico, R.; Peritore, A.F.; Crupi, R.; Gugliandolo, E.; Fusco, R.; Di Paola, R.; Schievano, C. Safety and efficacy of a new micronized formulation of the ALIAmide palmitoylglucosamine in preclinical models of inflammation and osteoarthritis pain. Arthritis Res. Ther. 2019, 21, 254. [Google Scholar] [CrossRef] [Green Version]
- Costa, B.; Comelli, F.; Miolo, A.; Della Valle, M. Effect of Glupamid (N-palmitoyl-D-glucosamine) on knee osteoarthritis pain. In Proceedings of the 3rd World Veterinary Orthopaedic Congress, Bologna, Italy, 15–18 September 2010; pp. 15–18. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Case, D.; Cerutti, D.; Cheatham, T.; Darden, T.; Duke, R.; Giese, T.; Gohlke, H.; Goetz, A.; Greene, D.; Homeyer, N. AMBER 2016; University of California: San Francisco, CA, USA, 2016. [Google Scholar]
- Gotz, A.W.; Williamson, M.J.; Xu, D.; Poole, D.; Le Grand, S.; Walker, R.C. Routine microsecond molecular dynamics simulations with AMBER on GPUs. 1. Generalized born. J. Chem. Theory Comput. 2012, 8, 1542–1555. [Google Scholar] [CrossRef]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef] [Green Version]
- D’Aniello, E.; Fellous, T.; Iannotti, F.A.; Gentile, A.; Allarà, M.; Balestrieri, F.; Gray, R.; Amodeo, P.; Vitale, R.M.; Di Marzo, V. Identification and characterization of phytocannabinoids as novel dual PPARα/γ agonists by a computational and in vitro experimental approach. Biochim. Biophys. Acta BBA Gen. Subj. 2019, 1863, 586–597. [Google Scholar] [CrossRef]
- Borgonovo, G.; Zimbaldi, N.; Guarise, M.; De Nisi, P.; De Petrocellis, L.; Schiano Moriello, A.; Bassoli, A. Isothiocyanates and Glucosinolates from Sisymbrium officinale (L.) Scop.(“the Singers’ Plant”): Isolation and in Vitro Assays on the Somatosensory and Pain Receptor TRPA1 Channel. Molecules 2019, 24, 949. [Google Scholar] [CrossRef] [Green Version]
- Carlson, E.C.; Lin, M.; Liu, C.-Y.; Kao, W.W.; Perez, V.L.; Pearlman, E. Keratocan and lumican regulate neutrophil infiltration and corneal clarity in lipopolysaccharide-induced keratitis by direct interaction with CXCL1. J. Biol. Chem. 2007, 282, 35502–35509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrillo, F.; Trotta, M.C.; Bucolo, C.; Hermenean, A.; Petrillo, A.; Maisto, R.; Pieretti, G.; Pietropaolo, M.; Ferraraccio, F.; Gagliano, C. Resolvin D1 attenuates the inflammatory process in mouse model of LPS-induced keratitis. J. Cell. Mol. Med. 2020, 24, 12298–12307. [Google Scholar] [CrossRef] [PubMed]
- Stechschulte, S.U.; Joussen, A.M.; von Recum, H.A.; Poulaki, V.; Moromizato, Y.; Yuan, J.; D’Amato, R.J.; Kuo, C.; Adamis, A.P. Rapid ocular angiogenic control via naked DNA delivery to cornea. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1975–1979. [Google Scholar]
- Claybon, A.; Bishop, A.J. Dissection of a mouse eye for a whole mount of the retinal pigment epithelium. J. Vis. Exp. 2011, e2563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregory-Ksander, M.; Perez, V.L.; Marshak-Rothstein, A.; Ksander, B.R. Soluble Fas ligand blocks destructive corneal inflammation in mouse models of corneal epithelial debridement and LPS induced keratitis. Exp. Eye Res. 2019, 179, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Saika, S.; Ikeda, K.; Yamanaka, O.; Flanders, K.C.; Nakajima, Y.; Miyamoto, T.; Ohnishi, Y.; Kao, W.W.; Muragaki, Y.; Ooshima, A. Therapeutic effects of adenoviral gene transfer of bone morphogenic protein-7 on a corneal alkali injury model in mice. Lab. Investig. 2005, 85, 474–486. [Google Scholar] [CrossRef] [Green Version]
- Platania, C.B.M.; Maisto, R.; Trotta, M.C.; D’Amico, M.; Rossi, S.; Gesualdo, C.; D’Amico, G.; Balta, C.; Herman, H.; Hermenean, A. Retinal and circulating mi RNA expression patterns in diabetic retinopathy: An in silico and in vivo approach. Br. J. Pharmacol. 2019, 176, 2179–2194. [Google Scholar] [CrossRef]
- Alves, H.; Valentim, A.; Olsson, I.; Antunes, L. Intraperitoneal anaesthesia with propofol, medetomidine and fentanyl in mice. Lab. Anim. 2009, 43, 27–33. [Google Scholar] [CrossRef] [Green Version]
- McGaraughty, S.; Chu, K.L.; Perner, R.J.; DiDomenico, S.; Kort, M.E.; Kym, P.R. TRPA1 modulation of spontaneous and mechanically evoked firing of spinal neurons in uninjured, osteoarthritic, and inflamed rats. Mol. Pain 2010, 6, 1744–8069. [Google Scholar] [CrossRef] [Green Version]
- Telleria-Diaz, A.; Schmidt, M.; Kreusch, S.; Neubert, A.-K.; Schache, F.; Vazquez, E.; Vanegas, H.; Schaible, H.-G.; Ebersberger, A. Spinal antinociceptive effects of cyclooxygenase inhibition during inflammation: Involvement of prostaglandins and endocannabinoids. Pain 2010, 148, 26–35. [Google Scholar] [CrossRef]
- Boccella, S.; Guida, F.; Palazzo, E.; Marabese, I.; de Novellis, V.; Maione, S.; Luongo, L. Spared Nerve Injury as a Long-Lasting Model of Neuropathic Pain. In Neurotrophic Factors; Springer: Berlin/Heidelberg, Germany, 2018; pp. 373–378. [Google Scholar]
- Simone, D.A.; Khasabov, S.G.; Hamamoto, D.T. Changes in response properties of nociceptive dorsal horn neurons in a murine model of cancer pain. Sheng Li Xue Bao 2008, 60, 635–644. [Google Scholar] [PubMed]
- Bannon, A.W.; Malmberg, A.B. Models of nociception: Hot-plate, tail-flick, and formalin tests in rodents. Curr. Protoc. Neurosci. 2007, 41, 8.9.1–8.9.16. [Google Scholar] [CrossRef] [PubMed]
- Abbott, F.V.; Guy, E.R. Effects of morphine, pentobarbital and amphetamine on formalin-induced behaviours in infant rats: Sedation versus specific suppression of pain. Pain 1995, 62, 303–312. [Google Scholar] [CrossRef]
- Guida, F.; Luongo, L.; Marmo, F.; Romano, R.; Iannotta, M.; Napolitano, F.; Belardo, C.; Marabese, I.; D’Aniello, A.; De Gregorio, D. Palmitoylethanolamide reduces pain-related behaviors and restores glutamatergic synapses homeostasis in the medial prefrontal cortex of neuropathic mice. Mol. Brain 2015, 8, 1–15. [Google Scholar] [CrossRef]
- Cavaletti, G.; Tredici, G.; Petruccioli, M.; Donde, E.; Tredici, P.; Marmiroli, P.; Minoia, C.; Ronchi, A.; Bayssas, M.; Etienne, G.G. Effects of different schedules of oxaliplatin treatment on the peripheral nervous system of the rat. Eur. J. Cancer 2001, 37, 2457–2463. [Google Scholar] [CrossRef]
- Freireich, E.J.; Gehan, E.; Rall, D.; Schmidt, L.; Skipper, H. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother. Rep. 1966, 50, 219. [Google Scholar]
- Mannelli, L.D.C.; Lucarini, E.; Micheli, L.; Mosca, I.; Ambrosino, P.; Soldovieri, M.V.; Martelli, A.; Testai, L.; Taglialatela, M.; Calderone, V. Effects of natural and synthetic isothiocyanate-based H2S-releasers against chemotherapy-induced neuropathic pain: Role of Kv7 potassium channels. Neuropharmacology 2017, 121, 49–59. [Google Scholar] [CrossRef] [Green Version]
- Decosterd, I.; Woolf, C.J. Spared nerve injury: An animal model of persistent peripheral neuropathic pain. Pain 2000, 87, 149–158. [Google Scholar] [CrossRef]
- Guida, F.; De Gregorio, D.; Palazzo, E.; Ricciardi, F.; Boccella, S.; Belardo, C.; Iannotta, M.; Infantino, R.; Formato, F.; Marabese, I. Behavioral, Biochemical and Electrophysiological Changes in Spared Nerve Injury Model of Neuropathic Pain. Int. J. Mol. Sci. 2020, 21, 3396. [Google Scholar] [CrossRef]
- Belardo, C.; Iannotta, M.; Boccella, S.; Rubino, R.C.; Ricciardi, F.; Infantino, R.; Pieretti, G.; Stella, L.; Paino, S.; Marabese, I. Oral cannabidiol prevents allodynia and neurological dysfunctions in a mouse model of mild traumatic brain injury. Front. Pharmacol. 2019, 10, 352. [Google Scholar] [CrossRef]
- Mannelli, L.D.C.; Maresca, M.; Farina, C.; Scherz, M.W.; Ghelardini, C. A model of neuropathic pain induced by sorafenib in the rat: Effect of dimiracetam. Neurotoxicology 2015, 50, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, K.M.; Vornov, J.J.; Wu, Y.; Liu, Y.; Carozzi, V.A.; Rodriguez-Menendez, V.; Ballarini, E.; Alberti, P.; Pozzi, E.; Semperboni, S. Peripheral neuropathy induced by microtubule-targeted chemotherapies: Insights into acute injury and long-term recovery. Cancer Res. 2018, 78, 817–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visintin, A.; Halmen, K.A.; Latz, E.; Monks, B.G.; Golenbock, D.T. Pharmacological inhibition of endotoxin responses is achieved by targeting the TLR4 coreceptor, MD-2. J. Immunol. 2005, 175, 6465–6472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.M.; Park, B.S.; Kim, J.-I.; Kim, S.E.; Lee, J.; Oh, S.C.; Enkhbayar, P.; Matsushima, N.; Lee, H.; Yoo, O.J. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 2007, 130, 906–917. [Google Scholar] [CrossRef] [Green Version]
- Okun, E.; Barak, B.; Saada-Madar, R.; Rothman, S.M.; Griffioen, K.J.; Roberts, N.; Castro, K.; Mughal, M.R.; Pita, M.A.; Stranahan, A.M. Evidence for a developmental role for TLR4 in learning and memory. PLoS ONE 2012, 7, e47522. [Google Scholar] [CrossRef] [Green Version]
- Okun, E.; Griffioen, K.J.; Mattson, M.P. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011, 34, 269–281. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Qian, Y.; Fang, Q.; Zhong, P.; Li, W.; Wang, L.; Fu, W.; Zhang, Y.; Xu, Z.; Li, X.; et al. Author Correction: Saturated palmitic acid induces myocardial inflammatory injuries through direct binding to TLR4 accessory protein MD2. Nat. Commun. 2018, 9, 16185. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Young, D.W.; Gusovsky, F.; Chow, J.C. Cellular events mediated by lipopolysaccharide-stimulated Toll-like receptor 4 MD-2 is required for activation of mitogen-activated protein kinases and Elk-1. J. Biol. Chem. 2000, 275, 20861–20866. [Google Scholar] [CrossRef] [Green Version]
- Wan, P.; Xie, M.; Chen, G.; Dai, Z.; Hu, B.; Zeng, X.; Sun, Y. Anti-inflammatory effects of dicaffeoylquinic acids from Ilex kudingcha on lipopolysaccharide-treated RAW264. 7 macrophages and potential mechanisms. Food Chem. Toxicol. 2019, 126, 332–342. [Google Scholar]
- Yang, J.; Chen, Y.; Jiang, K.; Yang, Y.; Zhao, G.; Guo, S.; Deng, G. MicroRNA-106a provides negative feedback regulation in lipopolysaccharide-induced inflammation by targeting TLR4. Int. J. Biol. Sci. 2019, 15, 2308. [Google Scholar] [CrossRef]
- Pasquini, S.; Mugnaini, C.; Ligresti, A.; Tafi, A.; Brogi, S.; Falciani, C.; Pedani, V.; Pesco, N.; Guida, F.; Luongo, L. Design, synthesis, and pharmacological characterization of indol-3-ylacetamides, indol-3-yloxoacetamides, and indol-3-ylcarboxamides: Potent and selective CB2 cannabinoid receptor inverse agonists. J. Med. Chem. 2012, 55, 5391–5402. [Google Scholar] [CrossRef] [PubMed]
- Mugnaini, C.; Nocerino, S.; Pedani, V.; Pasquini, S.; Tafi, A.; De Chiaro, M.; Bellucci, L.; Valoti, M.; Guida, F.; Luongo, L. Investigations on the 4-Quinolone-3-Carboxylic Acid Motif Part 5: Modulation of the Physicochemical Profile of a Set of Potent and Selective Cannabinoid-2 Receptor Ligands through a Bioisosteric Approach. ChemMedChem 2012, 7, 920–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cighetti, R.; Ciaramelli, C.; Sestito, S.E.; Zanoni, I.; Kubik, Ł.; Ardá-Freire, A.; Calabrese, V.; Granucci, F.; Jerala, R.; Martín-Santamaría, S. Modulation of CD14 and TLR4. MD-2 activities by a synthetic lipid A mimetic. Chembiochem Eur. J. Chem. Biol. 2014, 15, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neal, M.D.; Jia, H.; Eyer, B.; Good, M.; Guerriero, C.J.; Sodhi, C.P.; Afrazi, A.; Prindle, T., Jr.; Ma, C.; Branca, M. Discovery and validation of a new class of small molecule Toll-like receptor 4 (TLR4) inhibitors. PLoS ONE 2013, 8, e65779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eslani, M.; Baradaran-Rafii, A.; Movahedan, A.; Djalilian, A.R. The ocular surface chemical burns. J. Ophthalmol. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Baradaran-Rafii, A.; Rahmati-Kamel, M.; Eslani, M.; Kiavash, V.; Karimian, F. Effect of hydrodynamic parameters on corneal endothelial cell loss after phacoemulsification. J. Cataract Refract. Surg. 2009, 35, 732–737. [Google Scholar] [CrossRef]
- Fabiani, C.; Barabino, S.; Rashid, S.; Dana, M.R. Corneal epithelial proliferation and thickness in a mouse model of dry eye. Exp. Eye Res. 2009, 89, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Ellenberg, D.; Azar, D.T.; Hallak, J.A.; Tobaigy, F.; Han, K.Y.; Jain, S.; Zhou, Z.; Chang, J.-H. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog. Retin. Eye Res. 2010, 29, 208–248. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.X.; Ma, J.-X. Ocular neovascularization: Implication of endogenous angiogenic inhibitors and potential therapy. Prog. Retin. Eye Res. 2007, 26, 1–37. [Google Scholar] [CrossRef]
- Chang, L.K.; Garcia-Cardeña, G.; Farnebo, F.; Fannon, M.; Chen, E.J.; Butterfield, C.; Moses, M.A.; Mulligan, R.C.; Folkman, J.; Kaipainen, A. Dose-dependent response of FGF-2 for lymphangiogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 11658–11663. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Sun, J.; Du, L.; Du, H.; Wang, L.; Mai, J.; Zhang, F.; Liu, P. Neuropilin-2 contributes to LPS-induced corneal inflammatory lymphangiogenesis. Exp. Eye Res. 2016, 143, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zohaib, A.; Li, Y.; Zhu, B.; Ye, J.; Wan, S.; Xu, Q.; Song, Y.; Chen, H.; Cao, S. miR-206 modulates lipopolysaccharide-mediated inflammatory cytokine production in human astrocytes. Cell. Signal. 2015, 27, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yu, J.; Jing, Y.; Zhang, J. MiR-106a aggravates sepsis-induced acute kidney injury by targeting THBS2 in mice model. Acta Cir. Bras. 2019, 34, e201900602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, N.; Cui, H.; Banerjee, S.; Tan, Z.; Salomao, R.; Fu, M.; Abraham, E.; Thannickal, V.J.; Liu, G. miR-27a regulates inflammatory response of macrophages by targeting IL-10. J. Immunol. 2014, 193, 327–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philippe, L.; Alsaleh, G.; Pichot, A.; Ostermann, E.; Zuber, G.; Frisch, B.; Sibilia, J.; Pfeffer, S.; Bahram, S.; Wachsmann, D. MiR-20a regulates ASK1 expression and TLR4-dependent cytokine release in rheumatoid fibroblast-like synoviocytes. Ann. Rheum. Dis. 2013, 72, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Jurga, A.M.; Rojewska, E.; Makuch, W.; Mika, J. Lipopolysaccharide from Rhodobacter sphaeroides (TLR4 antagonist) attenuates hypersensitivity and modulates nociceptive factors. Pharm. Biol. 2018, 56, 275–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchinson, M.R.; Zhang, Y.; Brown, K.; Coats, B.D.; Shridhar, M.; Sholar, P.W.; Patel, S.J.; Crysdale, N.Y.; Harrison, J.A.; Maier, S.F. Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: Involvement of toll-like receptor 4 (TLR4). Eur. J. Neurosci. 2008, 28, 20–29. [Google Scholar] [CrossRef] [Green Version]
- Putnam, S.; Criddle, C.; Lopez, A.; Wilkinson, K.A. Lipopolysaccharide-Induced Inflammation does not Alter Spinal Cord Excitability in Female or Male mice. FASEB J. 2016, 30, 1283.3. [Google Scholar]
- Rolls, A.; Shechter, R.; London, A.; Ziv, Y.; Ronen, A.; Levy, R.; Schwartz, M. Toll-like receptors modulate adult hippocampal neurogenesis. Nat. Cell Biol. 2007, 9, 1081–1088. [Google Scholar] [CrossRef]
- Guida, F.; Luongo, L.; Aviello, G.; Palazzo, E.; De Chiaro, M.; Gatta, L.; Boccella, S.; Marabese, I.; Zjawiony, J.K.; Capasso, R. Salvinorin A reduces mechanical allodynia and spinal neuronal hyperexcitability induced by peripheral formalin injection. Mol. Pain 2012, 8, 1744–8069. [Google Scholar] [CrossRef] [Green Version]
- Luongo, L.; Guida, F.; Boccella, S.; Bellini, G.; Gatta, L.; Rossi, F.; de Novellis, V.; Maione, S. Palmitoylethanolamide reduces formalin-induced neuropathic-like behaviour through spinal glial/microglial phenotypical changes in mice. CNS Neurol. Disord. Drug Targets Former. Curr. Drug Targets CNS Neurol. Disord. 2013, 12, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Abarca, A.B.; Avila-Rojas, S.H.; Barragán-Iglesias, P.; Pineda-Farias, J.B.; Granados-Soto, V. Formalin injection produces long-lasting hypersensitivity with characteristics of neuropathic pain. Eur. J. Pharmacol. 2017, 797, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Boonen, B.; Alpizar, Y.A.; Meseguer, V.M.; Talavera, K. TRP channels as sensors of bacterial endotoxins. Toxins 2018, 10, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maione, S.; De Petrocellis, L.; de Novellis, V.; Moriello, A.S.; Petrosino, S.; Palazzo, E.; Rossi, F.S.; Woodward, D.; Di Marzo, V. Analgesic actions of N-arachidonoyl-serotonin, a fatty acid amide hydrolase inhibitor with antagonistic activity at vanilloid TRPV1 receptors. Br. J. Pharmacol. 2007, 150, 766–781. [Google Scholar] [CrossRef] [Green Version]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef] [Green Version]
- Boiko, N.; Medrano, G.; Montano, E.; Jiang, N.; Williams, C.R.; Madungwe, N.B.; Bopassa, J.C.; Kim, C.C.; Parrish, J.Z.; Hargreaves, K.M. TrpA1 activation in peripheral sensory neurons underlies the ionic basis of pain hypersensitivity in response to vinca alkaloids. PLoS ONE 2017, 12, e0186888. [Google Scholar] [CrossRef]
- Diogenes, A.; Ferraz, C.; Akopian, A.A.; Henry, M.; Hargreaves, K. LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J. Dental Res. 2011, 90, 759–764. [Google Scholar] [CrossRef]
- Meseguer, V.; Alpizar, Y.A.; Luis, E.; Tajada, S.; Denlinger, B.; Fajardo, O.; Manenschijn, J.-A.; Fernández-Pena, C.; Talavera, A.; Kichko, T. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun. 2014, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iannotta, M.; Belardo, C.; Trotta, M.C.; Iannotti, F.A.; Vitale, R.M.; Maisto, R.; Boccella, S.; Infantino, R.; Ricciardi, F.; Mirto, B.F.; et al. N-palmitoyl-D-glucosamine, A Natural Monosaccharide-Based Glycolipid, Inhibits TLR4 and Prevents LPS-Induced Inflammation and Neuropathic Pain in Mice. Int. J. Mol. Sci. 2021, 22, 1491. https://doi.org/10.3390/ijms22031491
Iannotta M, Belardo C, Trotta MC, Iannotti FA, Vitale RM, Maisto R, Boccella S, Infantino R, Ricciardi F, Mirto BF, et al. N-palmitoyl-D-glucosamine, A Natural Monosaccharide-Based Glycolipid, Inhibits TLR4 and Prevents LPS-Induced Inflammation and Neuropathic Pain in Mice. International Journal of Molecular Sciences. 2021; 22(3):1491. https://doi.org/10.3390/ijms22031491
Chicago/Turabian StyleIannotta, Monica, Carmela Belardo, Maria Consiglia Trotta, Fabio Arturo Iannotti, Rosa Maria Vitale, Rosa Maisto, Serena Boccella, Rosmara Infantino, Flavia Ricciardi, Benito Fabio Mirto, and et al. 2021. "N-palmitoyl-D-glucosamine, A Natural Monosaccharide-Based Glycolipid, Inhibits TLR4 and Prevents LPS-Induced Inflammation and Neuropathic Pain in Mice" International Journal of Molecular Sciences 22, no. 3: 1491. https://doi.org/10.3390/ijms22031491
APA StyleIannotta, M., Belardo, C., Trotta, M. C., Iannotti, F. A., Vitale, R. M., Maisto, R., Boccella, S., Infantino, R., Ricciardi, F., Mirto, B. F., Ferraraccio, F., Panarese, I., Amodeo, P., Tunisi, L., Cristino, L., D’Amico, M., di Marzo, V., Luongo, L., Maione, S., & Guida, F. (2021). N-palmitoyl-D-glucosamine, A Natural Monosaccharide-Based Glycolipid, Inhibits TLR4 and Prevents LPS-Induced Inflammation and Neuropathic Pain in Mice. International Journal of Molecular Sciences, 22(3), 1491. https://doi.org/10.3390/ijms22031491









