Synthesis, Spectroscopy, Single-Crystal Structure Analysis and Antibacterial Activity of Two Novel Complexes of Silver(I) with Miconazole Drug
Abstract
:1. Introduction
2. Results and Discussion
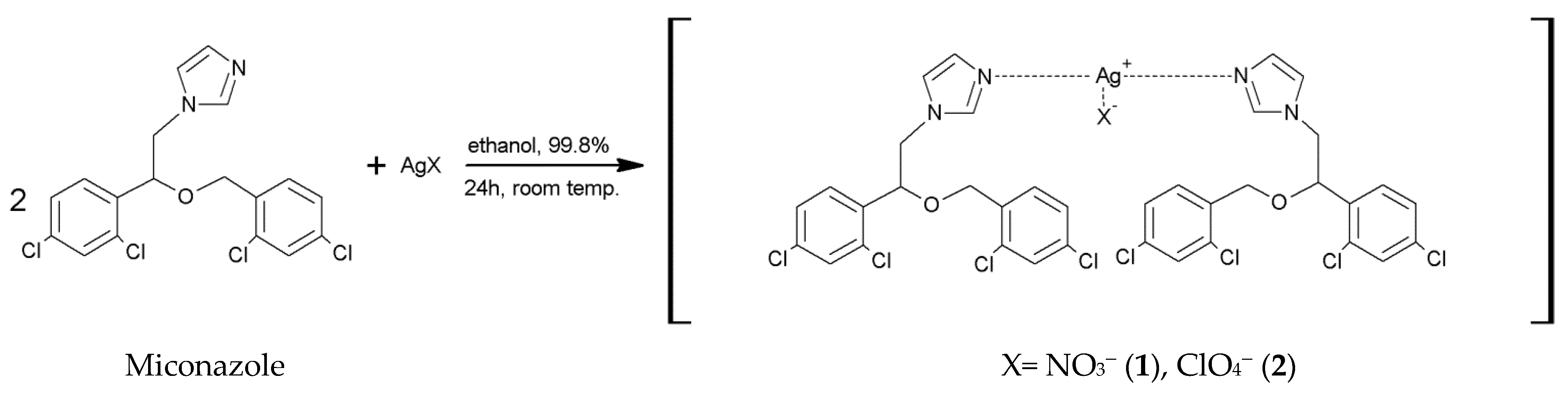
2.1. Synthesis of [Ag(MCZ)2NO3] (1) and [Ag(MCZ)2ClO4] (2)
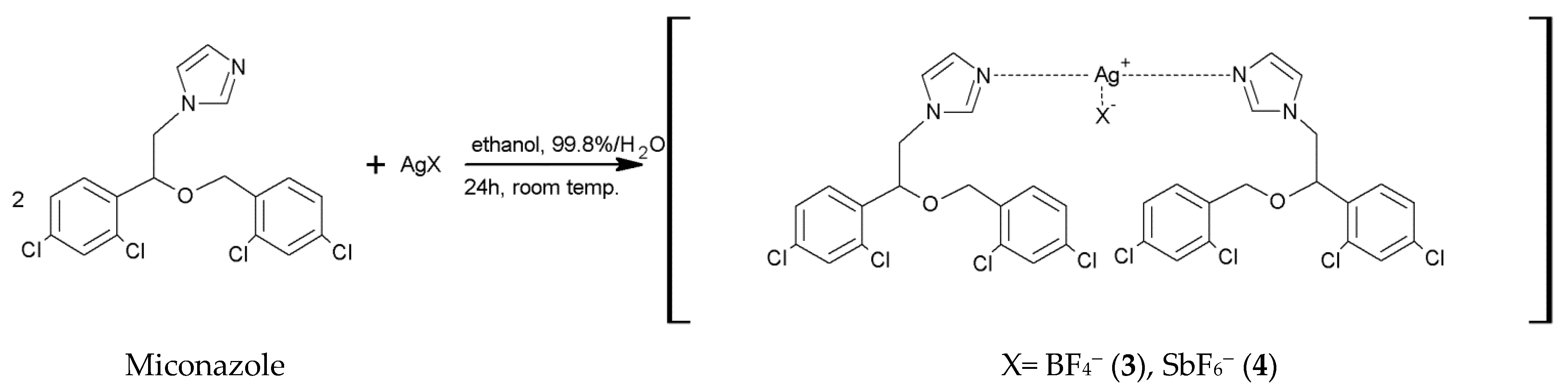
2.2. Synthesis of [Ag(MCZ)2BF4] (3) and [Ag(MCZ)2SbF6] (4)
2.3. NMR Spectroscopy
2.4. IR Spectroscopy
2.5. Light Stability
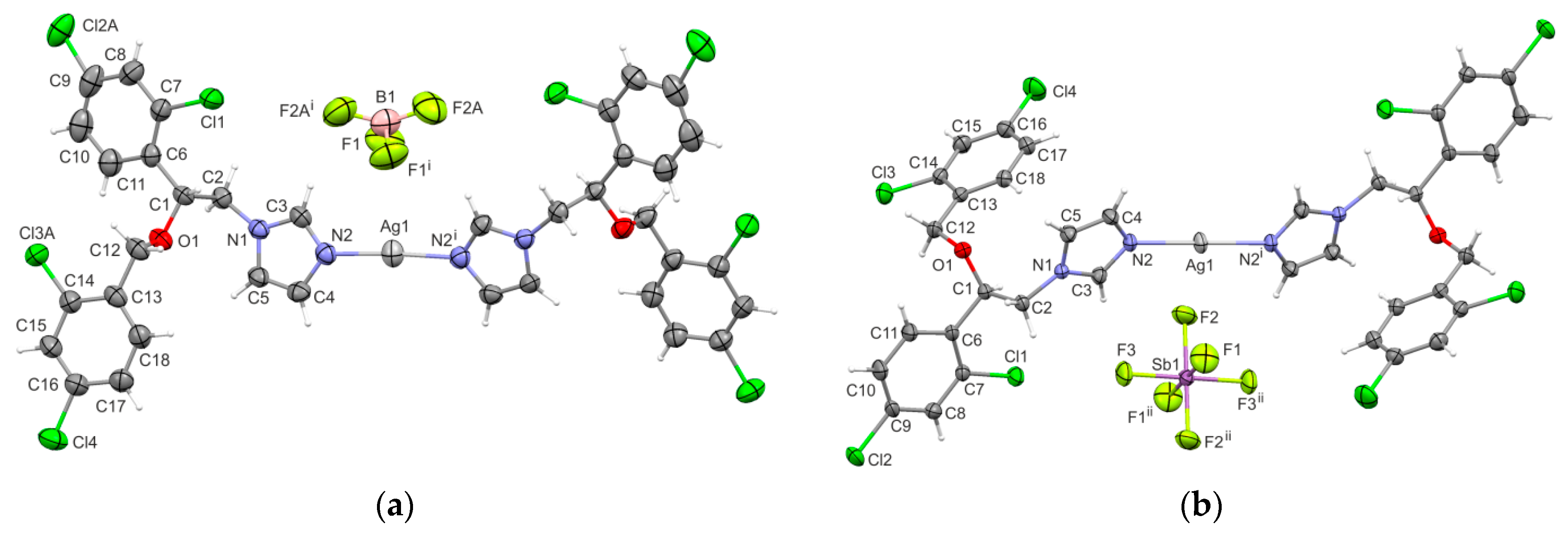
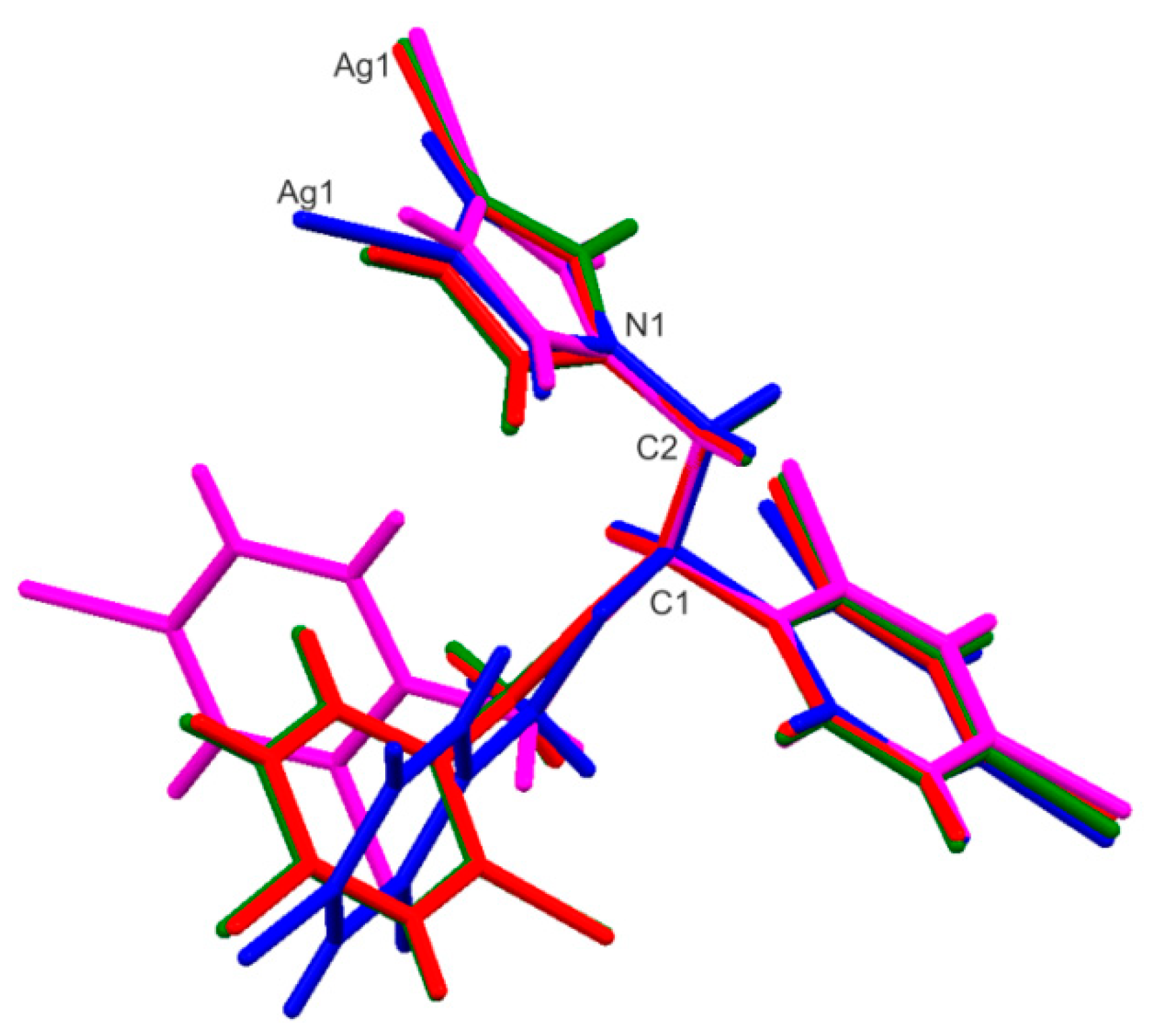
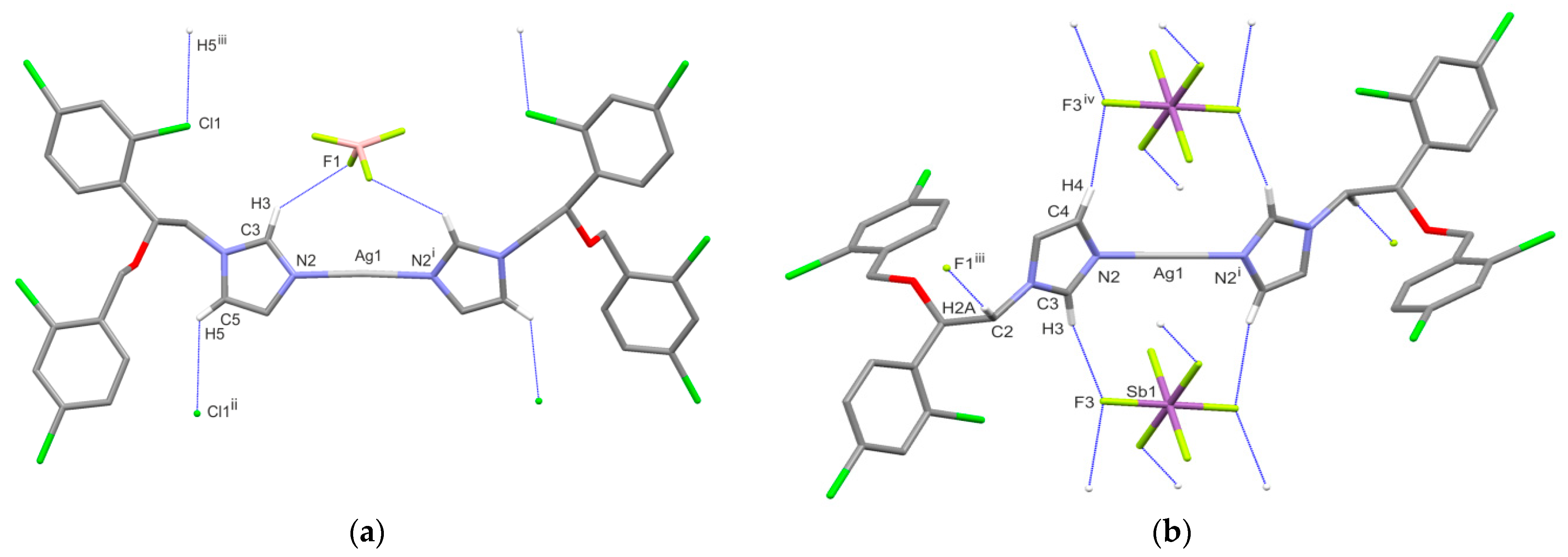
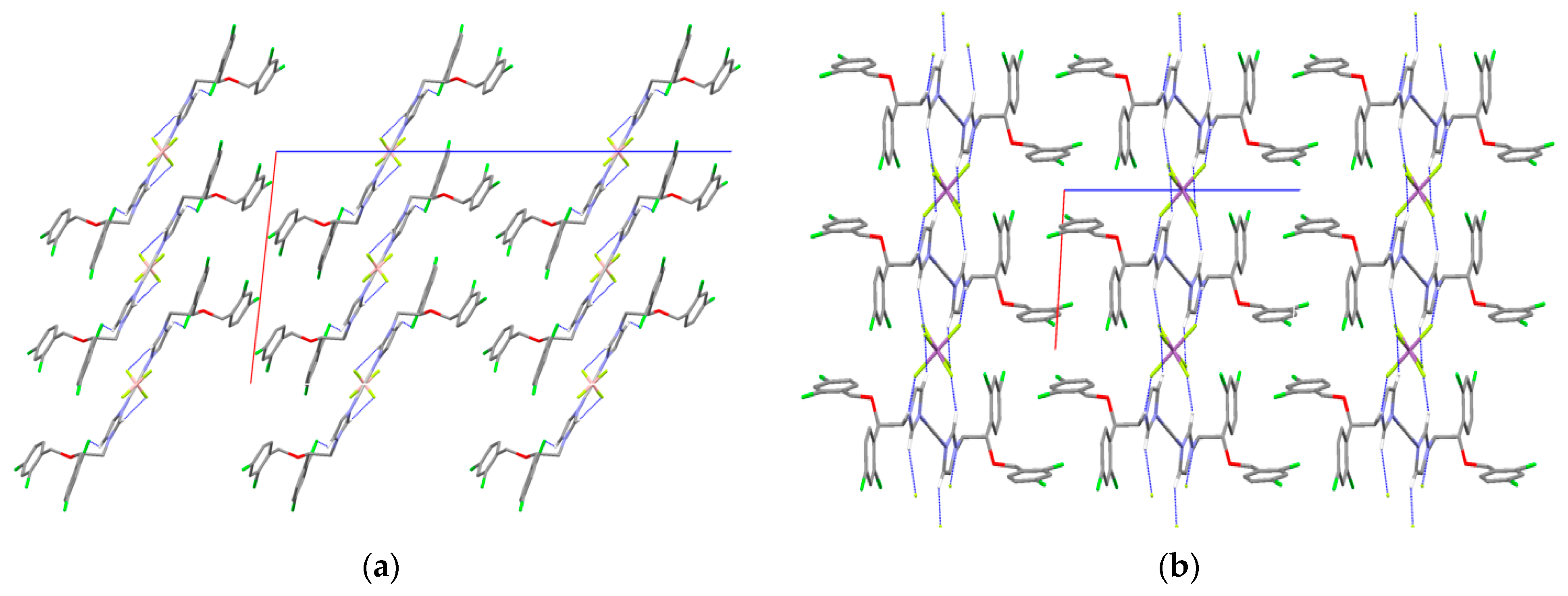
2.6. X-ray Single-Crystal Structure Determination
2.7. Antimicrobial Activity
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Synthesis
3.2.1. Synthesis of [Ag(Miconazole)2BF4] (3)
3.2.2. Synthesis of [Ag(Miconazole)2SbF6] (4)
3.3. Light Stability
3.4. X-ray Single-Crystal Diffraction
3.5. Chemistry
3.6. Antibacterial Activity Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charkhian, H.; Bodaqlouie, A.; Soleimannezhadbari, E.; Lotfollahi, L.; Shaykh-Baygloo, N.; Hosseinzadeh, R.; Yousefi, N.; Khodayar, M. Comparing the bacteriostatic effects of different metal nanoparticles against proteus vulgaris. Curr. Microbiol. 2020, 77, 2674–2684. [Google Scholar] [CrossRef]
- da Silva Martins, L.H.; Rai, M.; Neto, J.M.; Gomes, P.W.P.; da Silva Martins, J.H. Silver: Biomedical applications and adverse effects. In Biomedical Applications of Metals; Rai, M., Ingle, A., Medici, S., Eds.; Springer: Cham, Switzerland, 2018; pp. 113–128. [Google Scholar]
- Frei, A. Metal Complexes, an Untapped Source of Antibiotic Potential? Antibiotics 2020, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Balazs, D.J.; Triandafillu, K.; Wood, P.; Chevolot, Y.; van Delden, C.; Harms, H.; Hollenstein, C.; Mathieu, H.J. Inhibition of bacterial adhesion on PVC endotracheal tubes by RF-oxygen glow discharge, sodium hydroxide and silver nitrate treatments. Biomaterials 2004, 25, 2139–2151. [Google Scholar] [CrossRef]
- Politano, A.D.; Campbell, K.T.; Rosenberger, L.H.; Sawyer, R.G. Use of silver in the prevention and treatment of infections: Silver review. Surg. Infect. 2013, 14, 8–20. [Google Scholar] [CrossRef]
- Alexander, J.W. History of the medical use of silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [Green Version]
- Klasen, H.J. Historical review of the use of silver in the treatment of burns I: Early uses. Burns 2000, 26, 117–130. [Google Scholar] [CrossRef]
- Jo, Y.-K.; Kim, B.H.; Jung, G. Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Dis. 2009, 93, 1037–1043. [Google Scholar] [CrossRef] [Green Version]
- Mirsattari, S.M.; Hammond, R.R.; Sharpe, M.D.; Leung, F.; Young, G.B. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology 2004, 62, 1408–1410. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.M.; Peana, M.G.; Crisponi, G.; Nurchi, V.M.; Lachowicz, J.I.; Remelli, M.; Zoroddu, M.A. Silver coordination compounds: A new horizon in medicine. Coord. Chem. Rev. 2016, 349, 327–328. [Google Scholar] [CrossRef]
- Klasen, H.J. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 2000, 26, 131–138. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of silver on burn wound infection control and healing: Review of the literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Schaller, U.C.; Klauss, V. Is Crede’s prophylaxis for ophthalmia neonatorum still valid? Bull. World Health Organ. 2001, 79, 262–263. [Google Scholar]
- Oriel, J.D. Eminent venereologists 5: Carl Credé. Genitourin. Med. 1991, 67, 67–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, C. Barnes, MD: the physician who spun silver into gold. Available online: https://hekint.org/2020/08/18/albert-c-barnes-md-the-physician-who-spun-silver-into-gold/ (accessed on 29 January 2021).
- Vaupel, E. Arthur Eichengrn—Tribute to a Forgotten Chemist, Entrepreneur, and German Jew. Angew. Chem. Int. Ed. 2005, 44, 3344–3355. [Google Scholar] [CrossRef]
- Wescott, D.C.; Pusey, B. The Management of Blenorrhœa Neonatorum, with Especial Reference to the Duties of the Nurse. The Am. J. Nurs. 1901, 1, 635–639. [Google Scholar] [CrossRef]
- Fromm, K.M. Silver coordination compounds with antimicrobial properties. Appl. Organometal. Chem. 2013, 27, 683–687. [Google Scholar] [CrossRef]
- De Gracia, C.G. An open study comparing topical silver sulfadiazine and topical silver sulfadiazine-cerium nitrate in the treatment of moderate and severe burns. Burns 2001, 27, 67–74. [Google Scholar] [CrossRef]
- Fuller, F.W. The side effects of silver sulfadiazine. J. Burn Care Res. 2009, 30, 464–470. [Google Scholar] [CrossRef]
- Aziz, Z.; Hassan, B.A.R. The effects of honey compared to silver sulfadiazine for the treatment of burns: A systematic review of randomized controlled trials. Burns 2017, 43, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Nímia, H.H.; Carvalho, V.F.; Isaac, C.; Souza, F.Á.; Gemperli, R.; Paggiaro, A.O. Comparative study of Silver Sulfadiazine with other materials for healing and infection prevention in burns: A systematic review and meta-analysis. Burns 2019, 45, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska-Lis, U.; Felczak, A.; Chęcińska, L.; Zawadzka, K.; Patyna, E.; Lisowska, K.; Ochocki, J. Synthesis, characterization and antimicrobial activity of water-soluble silver(i) complexes of metronidazole drug and selected counter-ions. Dalton Trans. 2015, 44, 8178–8189. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska-Lis, U.; Felczak, A.; Chęcińska, L.; Szabłowska-Gadomska, I.; Patyna, E.; Małecki, M.; Lisowska, K.; Ochocki, J. Antibacterial Activity and Cytotoxicity of Silver(I) Complexes of Pyridine and (Benz)Imidazole Derivatives. X-ray Crystal Structure of [Ag(2,6-di(CH2OH)py)2]NO3. Molecules 2016, 21, 87. [Google Scholar] [CrossRef] [Green Version]
- Glišic´, B.Ð.; Senerovic, L.; Comba, P.; Wadepohl, H.; Veselinovic, A.; Milivojevic, D.R.; Djuran, M.I.; Nikodinovic-Runic, J. Silver(I) complexes with phthalazine and quinazoline as effective agents against pathogenic Pseudomonas aeruginosa strains. J. Inorg. Biochem. 2016, 155, 115–128. [Google Scholar] [CrossRef]
- Savić, N.D.; Petković, B.B.; Vojnovic, S.; Mojicevic, M.; Wadepohl, H.; Olaifa, K.; Marsili, E.; Nikodinovic-Runic, J.; Djuran, M.I.; Glišić, B.Đ. Dinuclear silver(I) complexes with a pyridine-based macrocyclic type of ligand as antimicrobial agents against clinically relevant species: The influence of the counteranion on the structure diversification of the complexes. Dalton Trans. 2020, 49, 10880–10894. [Google Scholar] [CrossRef]
- Smoleński, P.; Jaros, S.W.; Pettinari, C.; Lupidi, G.; Quassinti, L.; Bramucci, M.; Vitali, L.A.; Petrelli, D.; Kochel, A.; Kirillov, A.M. New water-soluble polypyridine silver(I) derivatives of 1,3,5-triaza-7-phosphaadamantane (PTA) with significant antimicrobial and antiproliferative activities. Dalton Trans. 2013, 42, 6572–6581. [Google Scholar] [CrossRef]
- Santos, A.F.; Ferreira, I.P.; Takahashi, J.A.; Rodrigues, G.L.S.; Pinheiro, C.B.; Teixeira, L.R.; Rochaa, W.R.; Beraldo, H. Silver(I) complexes with 2-acetylpyridinebenzoylhydrazones exhibit antimicrobial effects against yeast and filamentous fungi. New J. Chem. 2018, 42, 2125–2132. [Google Scholar] [CrossRef]
- Andrejevic´, T.P.; Nikolic´, A.M.; Glišic´, B.Ð.; Wadepohl, H.; Vojnovic, S.; Zlatovic´, M.; Petkovic´, M.; Nikodinovic-Runic, J.; Opsenica, I.M.; Djuran, M.I. Synthesis, structural characterization and antimicrobial activity of silver(I) complexes with 1-benzyl-1H-tetrazoles. Polyhedron 2018, 154, 325–333. [Google Scholar] [CrossRef]
- Gandra, R.M.; Carron, P.M.; Fernandes, M.F.; Ramos, L.S.; Mello, T.P.; Aor, A.C.; Branquinha, M.H.; McCann, M.; Devereux, M.; Santos, A.L.S. Antifungal Potential of Copper(II), Manganese(II) and Silver(I) 1,10-Phenanthroline Chelates Against Multidrug-Resistant Fungal Species Forming the Candida haemulonii Complex: Impact on the Planktonic and Biofilm Lifestyles. Front. Microbiol. 2017, 8, 1257. [Google Scholar] [CrossRef]
- Sahua, S.C.; Zhengb, J.; Grahamb, L.; Chenc, L.; Ihried, J.; Youricka, J.J.; Sprandoa, R.L. Comparative cytotoxicity of nanosilver in human liver HepG2 and colon Caco2 cells in culture. J. Appl. Toxicol. 2014, 34, 1155–1166. [Google Scholar] [CrossRef]
- Thati, B.; Noble, A.; Creaven, B.S.; Walsh, M.; McCann, M.; Kavanagh, K.; Devereux, M.; Egan, D.A. RETRACTED: In vitro anti-tumour and cyto-selective effects of coumarin-3-carboxylic acid and three of its hydroxylated derivatives, along with their silver-based complexes, using human epithelial carcinoma cell lines. Cancer Lett. 2007, 248, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.A.; Southerland, M.R.; Youngs, W.J. Recent Developments in the Medicinal Applications of Silver-NHC Complexes and Imidazolium Salts. Molecules 2017, 22, 1263. [Google Scholar] [CrossRef] [PubMed]
- Mfouo-Tynga, I.; El-Hussein, A.; Abdel-Harith, M.; Abrahamse, H. Photodynamic ability of silver nanoparticles in inducing cytotoxic effects in breast and lung cancer cell lines. Int. J. Nanomed. 2014, 9, 3771–3780. [Google Scholar]
- Dziedzic, A.; Kubina, R.; Bułdak, R.J.; Skonieczna, M.; Cholewa, K. Silver Nanoparticles Exhibit the Dose-Dependent Anti-Proliferative Effect against Human Squamous Carcinoma Cells Attenuated in the Presence of Berberine. Molecules 2016, 21, 365. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.-J.; Liu, H.-Z.; Ye, C.-Z.; Lou, J.-F.; Liu, Y.; Xing, D.-X.; Li, S.-P.; Liu, S.-L.; Song, L.-Z. Synthesis, characterization and in vitro cytotoxic properties of new silver(I) complexes of two novel Schiff bases derived from thiazole and pyrazine. Polyhedron 2014, 71, 119–132. [Google Scholar] [CrossRef]
- Żyro, D.; Śliwińska, A.; Szymczak-Pajor, I.; Stręk, M.; Ochocki, J. Light Stability, Pro-Apoptotic and Genotoxic Properties of Silver (I) Complexes of Metronidazole and 4-Hydroxymethylpyridine against Pancreatic Cancer Cells In Vitro. Cancers 2020, 12, 3848. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Ganellin, C.R. Analogue-Based Drug Discovery; John Wiley & Sons: Hoboken, NJ, USA, 2006; p. 502. [Google Scholar]
- World Health Organization. Model List of Essential Medicines; 21st List, 2019; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Sriram, B.; Agarwal, P.K.; Tee, N.W.S.; Rajadurai, V.S. Systemic Candidiasis in Extremely Low Birthweight (ELBW) Neonates Despite the Routine Use of Topical Miconazole Prophylaxis: Trends, Risk Factors and Outcomes over an 11-Year Period. Ann. Acad. Med. Singap. 2014, 43, 255–262. [Google Scholar]
- Boyen, F.; Verstappen, K.M.; De Bock, M.; Duim, B.; Weese, J.S.; Schwarz, S.; Haesebrouck, F.; Wagenaar, J.A. In vitro antimicrobial activity of miconazole and polymyxin B against canine meticillin-resistant Staphylococcus aureus and meticillin-resistant Staphylococcus pseudintermedius isolates. Vet. Dermatol. 2012, 23, 381-e70. [Google Scholar] [CrossRef]
- Hensel, P.; Austel, M.; Wooley, R.E.; Keys, D.; Ritchie, B.W. In vitro and in vivo evaluation of a potentiated miconazole aural solution in chronic Malassezia otitis externa indogs. Vet. Dermatol. 2009, 20, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kar, H.K. Solid state compatibility studies of miconazole using thermal and spectroscopic methods. Adv. Anal. Chem. 2015, 5, 51–55. [Google Scholar]
- Nenoff, P.; Koch, D.; Krüger, C.; Drechsel, C.; Mayser, P. New insights on the antibacterial efficacy of miconazole in vitro. Mycoses 2017, 60, 552–557. [Google Scholar] [CrossRef] [Green Version]
- Piérard, G.E.; Hermanns-Lê, T.; Delvenne, P.; Piérard-Franchimont, C. Miconazole, a pharmacological barrier to skin fungal infections. Expert Opin. Pharmacother. 2012, 13, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, S.; Brunetto, P.S.; Gagnon, J.; Priebe, M.; Giese, B.; Fromm, K.M. Nanobio silver: Its interactions with peptides and bacteria, and its uses in medicine. Chem. Rev. 2013, 113, 4708–4754. [Google Scholar] [CrossRef] [Green Version]
- Russell, A.D.; Hugo, W.B. 7 antimicrobial activity and action of silver. Prog. Med. Chem. 1994, 31, 351–370. [Google Scholar] [PubMed]
- Liau, S.Y.; Read, D.C.; Pugh, W.J.; Furr, J.R.; Russell, A.D. Interaction of silver nitrate with readily identifiable groups: Relationship to the antibacterialaction of silver ions. Lett. Appl. Microbiol. 1997, 25, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.M.; Albering, J.H.; Barakat, A. Unexpected formation of polymeric silver (I) complexes of azine-type ligand via self-assembly of Ag-salts with isatin oxamohydrazide. R. Soc. Open Sci. 2018, 5, 180434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stryjska, K.K.; Radko, L.; Chęcińska, L.; Kusz, J.; Posyniak, A.; Ochocki, J. Synthesis, spectroscopy, light stability, single crystal analysis and in vitro cytotoxic activity on HepG2 liver cancer of two novel silver(I) complexes of miconazole. Int. J. Mol. Sci. 2020, 21, 3629. [Google Scholar] [CrossRef]
- Piel, G.; Evrard, B.; Fillet, M.; Llabres, G.; Delattre, L. Development of a non-surfactant parenteral formulation of miconazole by the use of cyclodextrins. Int. J. Pharm. 1998, 169, 15–22. [Google Scholar] [CrossRef]
- Paiva, I.L.; de Carvalho, G.S.G.; da Silva, A.D.; Corbi, P.P.; Bergamini, F.R.G.; Formiga, A.L.B.; Diniz, R.; do Carmo, W.R.; Leite, C.Q.F.; Pavan, F.R.; et al. Silver(I) complexes with symmetrical Schiff bases: Synthesis, structural characterization, DFT studies and antimycobacterial assays. Polyhedron 2013, 62, 104–109. [Google Scholar] [CrossRef]
- da Silva, S.A.; Leite, C.Q.; Pavan, F.R.; Masciocchi, N.; Cuin, A. Coordinative versatility of a Schiff base containing thiophene: Synthesis, characterization and biological activity of zinc(II) and silver(I) complexes. Polyhedron 2014, 79, 170–177. [Google Scholar] [CrossRef]
- Roca, S.; Vikić-Topić, D.; Plavec, J.; Šket, P.; Mihalić, Z.; Matković-Čalogović, D.; Popović, Z. Structural diversity of the Ag coordination sphere in complexes of silver(I) nitrate with 3-halopyridine. Characterization of the complexes in solution and in the solid state. Polyhedron 2016, 109, 166–175. [Google Scholar] [CrossRef]
- Santos, A.F.; Ferreira, I.P.; Pinheiro, C.B.; Santos, V.G.; Lopes, M.T.P.; Teixeira, L.R.; Rocha, W.R.; Rodrigues, G.L.S.; Beraldo, H. [Ag(L)NO3] Complexes with 2-Benzoylpyridine-Derived Hydrazones: Cytotoxic Activity and Interaction with Biomolecules. ACS Omega 2018, 3, 7027–7035. [Google Scholar] [CrossRef]
- Thomas, A.; Beena, P.; Abraham, E. Formulation Development and Evaluation of Niosomal Gel of Combined Anti-Fungal Agents. Int. J. Pharm. 2018, 8, 3–20. [Google Scholar]
- Ahmed, T.A.; Mahmoud, M.F.; Samy, A.M.; Badawi, A.A.; Gabr, K.E. Formulation, evaluation and optimization of miconazole nitrate tablet prepared by foam granulation technique. Int. J. Drug Deliver. 2011, 3, 712–733. [Google Scholar]
- Buchner, R.; Field, J.S.; Haines, R.J.; Ledwaba, L.P.; McGuire, R., Jr.; McMillin, D.R.; Munro, O.Q. Synthesis, crystal structure and solid state photoluminescence of [Pt(trpy)(C≡CPh)]SbF6 (trpy≡2,2’:6’,2’’-terpyridine). Inorg. Chim. Acta 2007, 360, 1633–1638. [Google Scholar] [CrossRef]
- Simpson, P.V.; Nagel, C.; Bruhn, H.; Schatzschneider, U. Antibacterial and Antiparasitic Activity of Manganese(I) Tricarbonyl Complexes with Ketoconazole, Miconazole, and Clotrimazole Ligands. Organometallics 2015, 34, 3809–3815. [Google Scholar] [CrossRef]
- Hasan, M.S.; Das, N. A detailed in vitro study of naproxen metal complexes in quest of new therapeutic possibilities. Alex. J. Med. 2017, 53, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Halim, H.F.; Nour El-Dien, F.A.; Mohamed, G.G.; Mohamed, N.A. Synthesis, spectroscopic, thermal characterization, and antimicrobial activity of miconazole drug and its metal complexes. J. Therm. Anal. Calorim. 2012, 109, 883–892. [Google Scholar] [CrossRef]
- Rigaku, O.D. CrysAlisPro Software System; Version 1.171. 38.41 k; Rigaku Coorporation: Oxford, UK, 2015. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Żesławska, E.; Korona-Głowniak, I.; Szczesio, M.; Olczak, A.; Żylewska, A.; Tejchman, W.; Malm, A. Structural analysis and antimicrobial activity of 2 [1H]-pyrimidinethione/selenone derivatives. J. Mol. Struct. 2017, 1142, 261–266. [Google Scholar] [CrossRef]
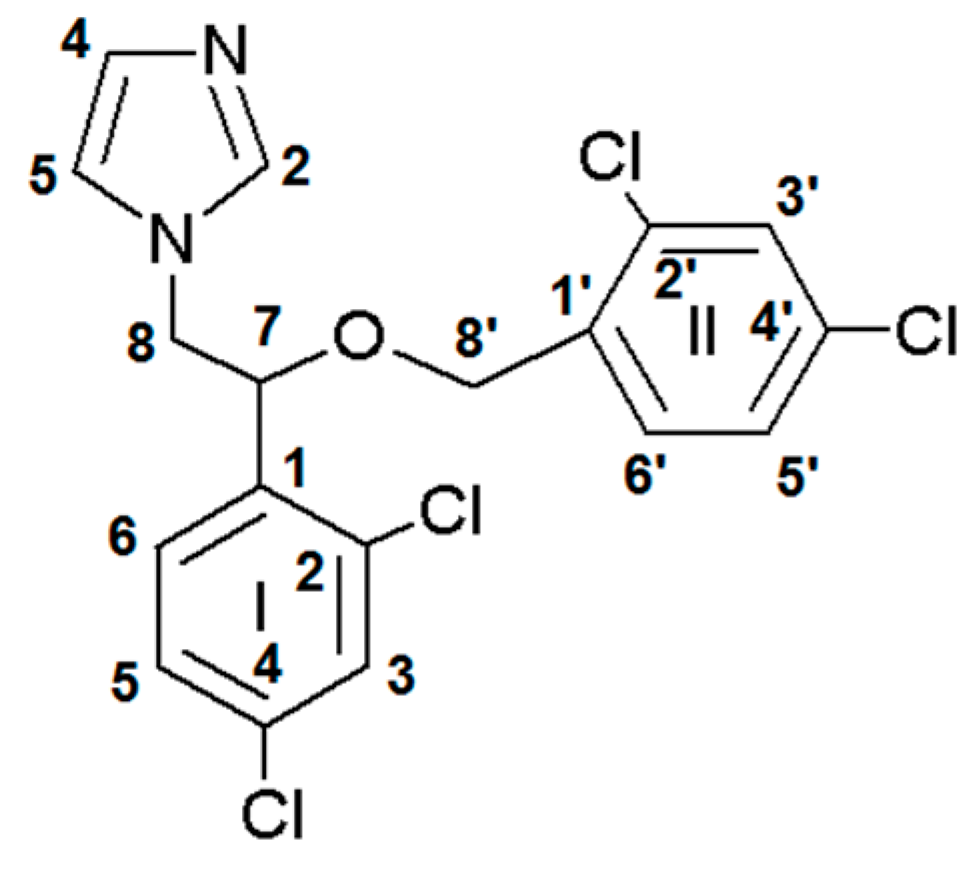
| Miconazole | Ag(MCZ)2NO3 [50] | Ag(MCZ)2ClO4 [50] | Ag(MCZ)2BF4 | Ag(MCZ)2SbF6 | |
|---|---|---|---|---|---|
| Proton | Chemical Shift (ppm) | Chemical Shift (ppm) | Chemical Shift (ppm) | Chemical Shift (ppm) | Chemical Shift (ppm) |
| Imidazole | |||||
| H2 | - | 8.08 | 8.20 | 8.17 | 8.13 |
| H4 | 7.26 | 7.32 | 7.33 | 7.33 | 7.33 |
| H5 | 7.27 | 7.34 | 7.34 | 7.34 | 7.34 |
| 2,4-Dichlorophenyl | |||||
| (Ring I) H3 | 7.37 | 7.40 | 7.40 | 7.41 | 7.42 |
| (Ring I) H5 | 7.20 | 7.29 | 7.29 | 7.29 | 7.29 |
| (Ring I) H6 | 7.16 | 7.08 | 7.09 | 7.07 | 7.05 |
| (Ring II) H3′ | 7.49 | 7.49 | 7.49 | 7.49 | 7.49 |
| (Ring II) H5′ | 7.33 | 7.37 | 7.39 | 7.39 | 7.41 |
| (Ring II) H6′ | 7.32 | 7.36 | 7.35 | 7.35 | 7.36 |
| Aliphatic-CH | 4.10 | 4.16 | 4.19 | 4.20 | 4.20 |
| 2-Aliphatic-CH2 | 4.27–4.52 | 4.29–4.55 | 4.32–4.56 | 4.33–4.56 | 4.34–4.56 |
| Miconazole | Ag(MCZ)2BF4 | Ag(MCZ)2SbF6 | |
|---|---|---|---|
| Carbon | Chemical Shift (ppm) | Chemical Shift (ppm) | Chemical Shift (ppm) |
| Imidazole | |||
| C2 | 138.31 | 140.40 | 140.41 |
| C4 | 120.43 | 121.50 | 121.49 |
| C5 | 125.76 | 127.89 | 127.89 |
| (Ring I) C1 | 135.01 | 134.61 | 134.61 |
| (Ring I) C2 | 134.55 | 134.40 | 134.39 |
| (Ring I) C3 | 129.74 | 129.82 | 129.81 |
| (Ring I) C4 | 134.09 | 134.31 | 134.31 |
| (Ring I) C5 | 129.07 | 129.56 | 129.57 |
| (Ring I) C6 | 128.31 | 129.14 | 129.15 |
| (Ring II) C1′ | 133.58 | 133.72 | 133.72 |
| (Ring II) C2′ | 133.53 | 133.67 | 133.67 |
| (Ring II) C3′ | 129.35 | 129.56 | 129.57 |
| (Ring II) C4′ | 134.13 | 131.38 | 131.38 |
| (Ring II) C5′ | 128.65 | 129.30 | 129.30 |
| (Ring II) C6′ | 127.86 | 128.42 | 128.42 |
| C7 | 77.49 | 76.95 | 76.95 |
| C8 | 50.42 | 50.97 | 50.97 |
| C8′ | 67.79 | 67.85 | 67.85 |
| H-Bond | D–H | H⋅⋅⋅A | D⋅⋅⋅A | D–H⋅⋅⋅A |
|---|---|---|---|---|
| 3 | ||||
| C3–H3⋅⋅⋅F1 | 0.93 | 2.53 | 3.292(7) | 140 |
| C5–H5⋅⋅⋅Cl1ii | 0.93 | 2.80 | 3.434(5) | 126 |
| 4 | ||||
| C2–H2A⋅⋅⋅F1iii | 0.97 | 2.37 | 3.313(4) | 163 |
| C3–H3⋅⋅⋅F1 | 0.93 | 2.35 | 3.221(4) | 156 |
| C4–H4⋅⋅⋅F3iv | 0.93 | 2.61 | 3.478(4) | 156 |
| Microorganism | Ag(MCZ)2NO3 | AgNO3 | Ag(MCZ)2ClO4 | AgClO4 | Ag(MCZ)2BF4 | AgBF4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (μmol/L) | (μmol/L) | (μmol/L) | (μmol/L) | (μmol/L) | (μmol/L) | |||||||
| Gram-positive bacteria | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| S. aureus ATCC 25923 | 1.95 | 15.57 | 22.81 | 45.61 | 3.76 | 30.15 | 18.84 | 37.68 | 1.90 | 30.51 | 20.10 | 80.41 |
| S. epidermidis ATCC 12228 | 1.95 | 15.57 | 5.73 | 11.40 | 3.76 | 30.15 | 9.42 | 75.36 | 1.90 | 30.51 | 5.05 | 40.21 |
| M. luteus ATCC 10240 | 0.49 | 15.57 | 11.40 | 22.81 | 1.88 | 7.51 | 18.84 | 75.36 | 0.96 | 7.60 | 10.05 | 80.41 |
| B. subtilis ATCC 6633 | 3.89 | 7.78 | 22.81 | 45.61 | 7.51 | 15.03 | 18.84 | 75.36 | 7.60 | 30.51 | 20.10 | 40.21 |
| B. cereus ATCC 10876 | 7.78 | 15.57 | 91.23 | 91.23 | 15.03 | 60.21 | 37.68 | 151.21 | 15.20 | 30.51 | 80.41 | 80.41 |
| E. faecalis ATCC 29212 | 15.57 | 31.24 | 45.61 | 91.23 | 30.15 | 120.42 | 37.68 | 151.21 | 30.51 | 60.92 | 40.21 | 80.41 |
| Gram-negative bacteria | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| S. Typhimurium ATCC 14028 | 62.38 | 62.38 | 22.81 | 91.23 | 120.42 | 120.42 | 18.84 | 37.68 | 121.83 | 243.66 | 20.10 | 40.21 |
| E. coli ATCC 25922 | 31.24 | 31.24 | 45.61 | 45.61 | 60.21 | 60.21 | 75.36 | 75.36 | 121.83 | 121.83 | 80.41 | 80.41 |
| P. mirabilis ATCC 12453 | 62.38 | 62.38 | 91.23 | 91.23 | 60.21 | 60.21 | 151.21 | 151.21 | 121.83 | 121.83 | 80.41 | 80.41 |
| K. pneumoniae ATCC 13883 | 62.38 | 62.38 | 45.61 | 45.61 | 120.42 | 120.42 | 37.68 | 75.36 | 121.83 | 121.83 | 40.21 | 40.21 |
| P. aeruginosa ATCC 9027 | 31.24 | 62.38 | 45.61 | 45.61 | 120.42 | 120.42 | 37.68 | 151.21 | 60.92 | 121.83 | 40.21 | 40.21 |
| Yeasts | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC |
| C. glabrata ATCC 90030 | 1.95 | 7.78 | 11.40 | 22.81 | 1.88 | 30.15 | 9.42 | 37.68 | 1.90 | 15.20 | 10.05 | 20.10 |
| C. albicans ATCC 102231 | 0.98 | 1.95 | 11.40 | 91.23 | 1.88 | 3.76 | 9.42 | 75.36 | 1.90 | 3.80 | 20.10 | 80.41 |
| C. parapsilosis ATCC 22019 | 0.12 | 0.49 | 11.40 | 91.23 | 0.12 | 1.88 | 4.73 | 75.36 | 0.12 | 1.90 | 10.05 | 80.41 |
| Microorganism | Ag(MCZ)2SbF6 | AgSbF6 | MCZ | AgSD | ||||||||
| (μmol/L) | (μmol/L) | (μmol/L) | (μmol/L) | |||||||||
| Gram-positive bacteria | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||||
| S. aureus ATCC 25923 | 3.32 | 26.64 | 22.74 | 45.48 | 1.18 | 9.38 | 21.85 | 43.70 | ||||
| S. epidermidis ATCC 12228 | 3.32 | 26.64 | 5.69 | 22.74 | 1.18 | 9.38 | 43.70 | 43.70 | ||||
| M. luteus ATCC 10240 | 1.66 | 6.64 | 11.37 | 45.48 | 1.18 | 1.18 | 10.92 | 87.68 | ||||
| B. subtilis ATCC 6633 | 13.28 | 26.64 | 22.74 | 22.74 | 4.69 | 4.69 | 10.92 | 21.85 | ||||
| B. cereus ATCC 10876 | 13.28 | 53.19 | 91.25 | 182.22 | 4.69 | 4.69 | 43.70 | 350.14 | ||||
| E. faecalis ATCC 29212 | 26.64 | 106.38 | 45.48 | 91.25 | 9.38 | 18.75 | 87.68 | 87.68 | ||||
| Gram-negative bacteria | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||||
| S. Typhimurium ATCC 14028 | 212.77 | 212.77 | 22.74 | 45.48 | >1000 | Nd | 43.70 | 87.68 | ||||
| E. coli ATCC 25922 | 212.77 | 212.77 | 91.25 | 91.25 | >1000 | Nd | 43.70 | 87.68 | ||||
| P. mirabilis ATCC 12453 | 212.77 | 212.77 | 91.25 | 91.25 | >1000 | Nd | 21.85 | 43.70 | ||||
| K. pneumoniae ATCC 13883 | 212.77 | 212.77 | 45.48 | 45.48 | >1000 | Nd | 43.70 | 87.68 | ||||
| P. aeruginosa ATCC 9027 | 106.38 | 106.38 | 45.48 | 45.48 | >1000 | Nd | 21.85 | 43.70 | ||||
| Yeasts | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | ||||
| C. glabrata ATCC 90030 | 0.83 | 13.28 | 11.37 | 22.74 | 4.69 | 18.75 | 10.92 | 43.70 | ||||
| C. albicans ATCC 102231 | 1.66 | 3.32 | 11.37 | 91.25 | 4.69 | 9.38 | 10.92 | 43.70 | ||||
| C. parapsilosis ATCC 22019 | 0.10 | 0.83 | 11.37 | 91.25 | 0.07 | 1.18 | 5.46 | 43.70 | ||||
| 3 | 4 | |
|---|---|---|
| Empirical formula | C36H28AgCl8N4O2·BF4 | C36H28AgCl8N4O2·SbF6 |
| Formula weight | 1026.90 | 1175.84 |
| Crystal system | Monoclinic | Triclinic |
| Space group | C2/c | P |
| a (Å) | 15.6809(10) | 8.3845(6) |
| b (Å) | 8.5678(5) | 8.8813(5) |
| c (Å) | 30.601(2) | 14.9005(11) |
| α (°) | 90.000 | 91.261(5) |
| β (°) | 96.353(5) | 93.497(5) |
| γ (°) | 90.000 | 110.570(5) |
| V (Å3) | 4086.0(5) | 1035.77(12) |
| Z | 4 | 1 |
| T (K) | 293(1) | 293(1) |
| F (000) | 2048 | 576 |
| Dx (g∙cm−3) | 1.669 | 1.885 |
| µ (mm−1) | 1.074 | 1.71 |
| Wavelength (Å) | 0.71073 | 0.71073 |
| θ range (°) | 3.1–27.0 | 3.2–30.0 |
| Measured reflections | 17115 | 11524 |
| Unique reflections | 4455 | 6013 |
| Observed reflections [I > 2σ (I)] | 2961 | 4135 |
| Completeness to θmax (%) | 99.8 | 99.4 |
| Parameters/restraints | 284/47 | 265/0 |
| R [F2 > 2σ (F2)] | 0.065 | 0.040 |
| wR (all data) | 0.196 | 0.101 |
| S | 1.06 | 1.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stryjska, K.; Korona-Glowniak, I.; Chęcińska, L.; Kusz, J.; Ochocki, J. Synthesis, Spectroscopy, Single-Crystal Structure Analysis and Antibacterial Activity of Two Novel Complexes of Silver(I) with Miconazole Drug. Int. J. Mol. Sci. 2021, 22, 1510. https://doi.org/10.3390/ijms22041510
Stryjska K, Korona-Glowniak I, Chęcińska L, Kusz J, Ochocki J. Synthesis, Spectroscopy, Single-Crystal Structure Analysis and Antibacterial Activity of Two Novel Complexes of Silver(I) with Miconazole Drug. International Journal of Molecular Sciences. 2021; 22(4):1510. https://doi.org/10.3390/ijms22041510
Chicago/Turabian StyleStryjska, Karolina, Izabela Korona-Glowniak, Lilianna Chęcińska, Joachim Kusz, and Justyn Ochocki. 2021. "Synthesis, Spectroscopy, Single-Crystal Structure Analysis and Antibacterial Activity of Two Novel Complexes of Silver(I) with Miconazole Drug" International Journal of Molecular Sciences 22, no. 4: 1510. https://doi.org/10.3390/ijms22041510
APA StyleStryjska, K., Korona-Glowniak, I., Chęcińska, L., Kusz, J., & Ochocki, J. (2021). Synthesis, Spectroscopy, Single-Crystal Structure Analysis and Antibacterial Activity of Two Novel Complexes of Silver(I) with Miconazole Drug. International Journal of Molecular Sciences, 22(4), 1510. https://doi.org/10.3390/ijms22041510








