A Cluster-Based Approach for Identifying Prognostic microRNA Signatures in Digestive System Cancers
Abstract
:1. Introduction
2. Results
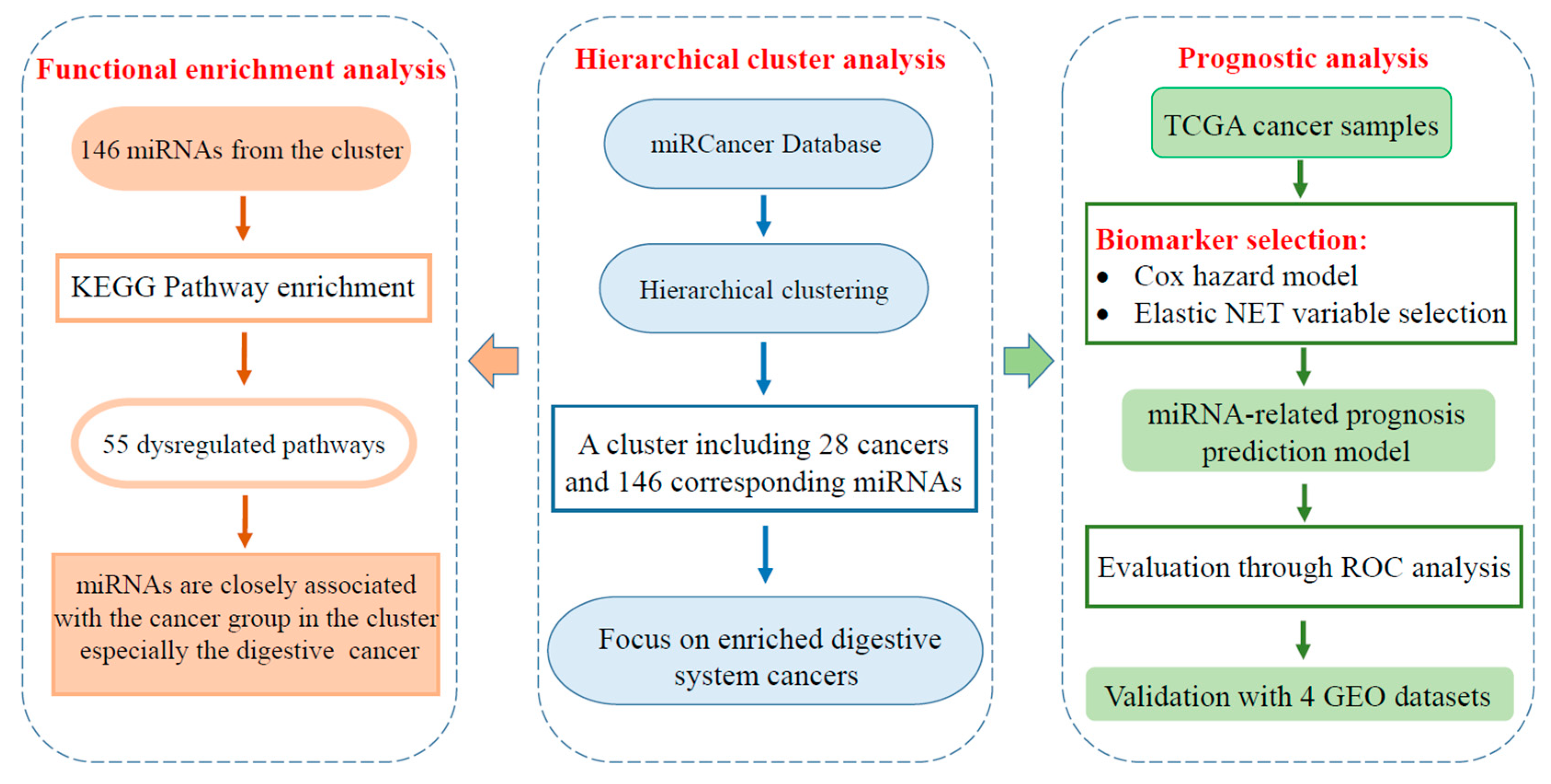
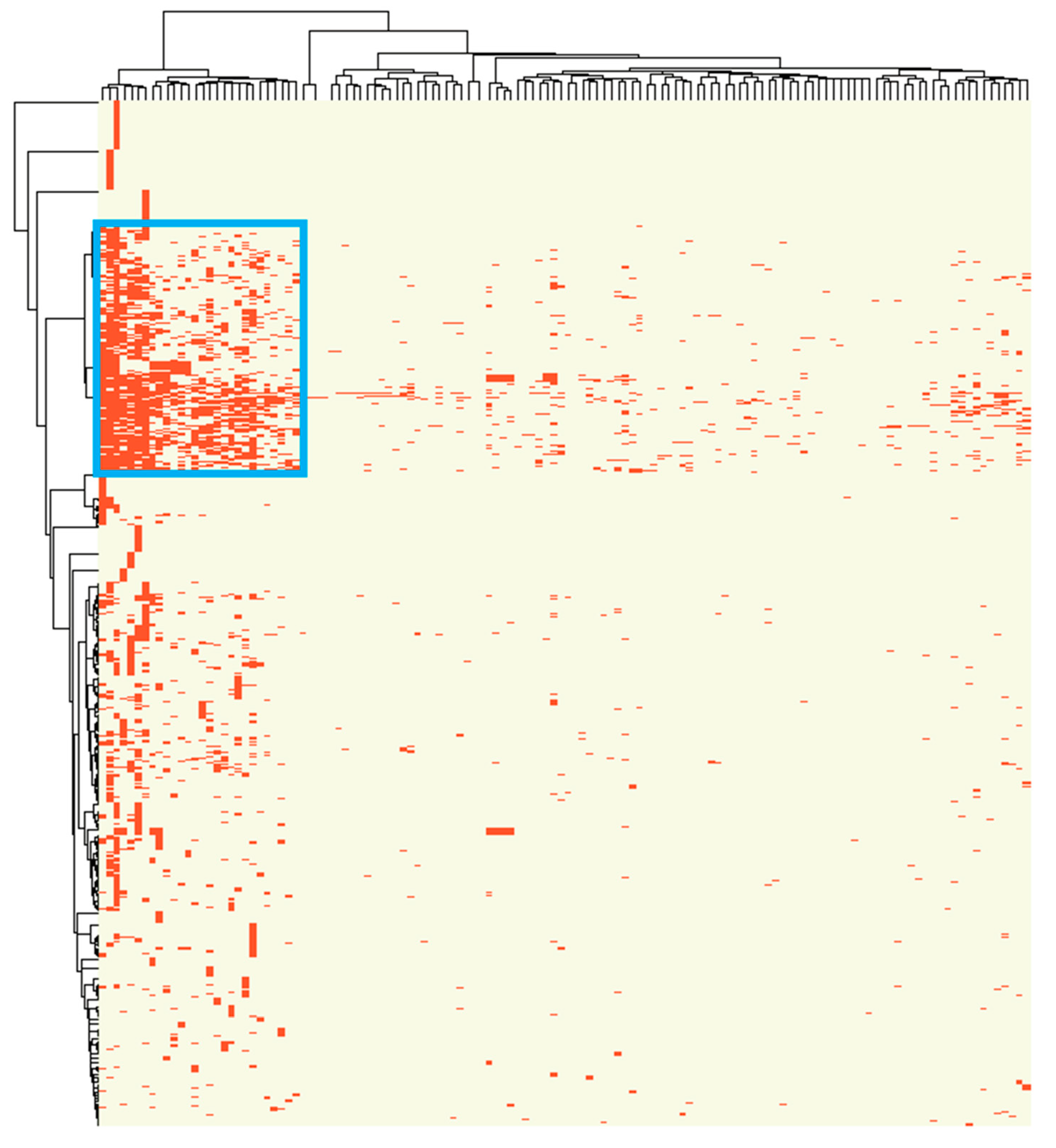
2.1. A Distinct Cluster of Association between miRNA Dysregulation and Cancer
2.2. Cluster Associated miRNAs Were Involved in Digestive System Related Pathways
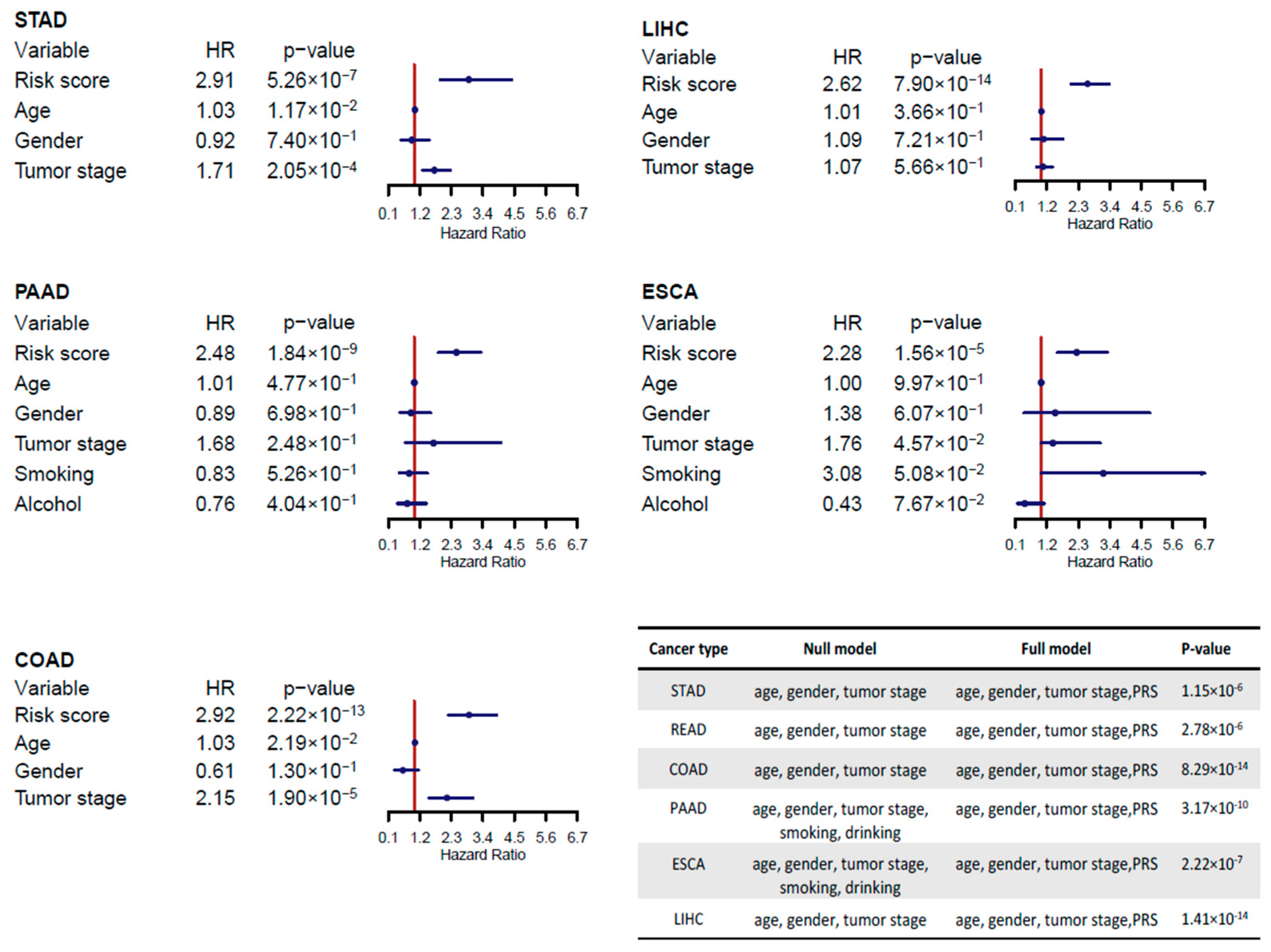
2.3. miRNA Signatures for Digestive System Cancer Prognosis
2.4. Cluster-Based miRNA Prognostic Signatures Are Superior to Cancer-Specific Signatures
2.5. Validation of the Prognostic miRNA Signatures Using Independent Datasets
3. Discussion
4. Materials and Methods
4.1. miRNA-Cancer Association
4.2. miRNA and Clinical Data of Digestive System Cancers
4.3. Identification and Evaluation of Prognostic miRNAs
4.4. Comparison between the Cluster-Based Approach and the Cancer-Specific Approach
4.5. Validation of Prognostic miRNAs
4.6. Pathway Enrichment Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wild, C.P.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wu, Y.; Yang, J.; Yang, D.; Fang, X. Progress in the treatment of advanced gastric cancer. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Xu, S.; Wu, Y.; Wu, B.; Liao, D.J.; Xu, N.; Wang, G. Management and survival analysis of elderly patients with a cancer in the digestive system who refused to receive anticancer treatments. Support Care Cancer 2018, 26, 2333–2339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappell, M.S. Pathophysiology, clinical presentation, and management of colon cancer. Gastroenterol. Clin. N. Am. 2008, 37, 1–24. [Google Scholar] [CrossRef]
- Hartgrink, H.H.; Jansen, E.P.; van Grieken, N.C.; van de Velde, C.J. Gastric cancer. Lancet 2009, 374, 477–490. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Wang, H. Precision diagnosis and treatment of liver cancer in China. Cancer Lett. 2018, 412, 283–288. [Google Scholar] [CrossRef]
- Huang, F.L.; Yu, S.J. Esophageal cancer: Risk factors, genetic association, and treatment. Asian J. Surg. 2018, 41, 210–215. [Google Scholar] [CrossRef]
- Halbrook, C.J.; Lyssiotis, C.A. Employing Metabolism to Improve the Diagnosis and Treatment of Pancreatic Cancer. Cancer Cell 2017, 31, 5–19. [Google Scholar] [CrossRef] [Green Version]
- Carleton, M.; Cleary, M.A.; Linsley, P.S. MicroRNAs and cell cycle regulation. Cell Cycle 2007, 6, 2127–2132. [Google Scholar] [CrossRef]
- Boehm, M.; Slack, F.J. MicroRNA control of lifespan and metabolism. Cell Cycle 2006, 5, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.; Song, G.; Roll, G.R.; Frandsen, N.M.; Willenbring, H. A microRNA-21 surge facilitates rapid cyclin D1 translation and cell cycle progression in mouse liver regeneration. J. Clin. Investig. 2012, 122, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Goodall, G.J.; Wickramasinghe, V.O. RNA in cancer. Nat. Rev. Cancer 2021, 21, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Sohel, M.M.H. Circulating microRNAs as biomarkers in cancer diagnosis. Life Sci. 2020, 248, 117473. [Google Scholar] [CrossRef] [PubMed]
- Loh, H.-Y.; Norman, B.P.; Lai, K.-S.; Rahman, N.M.A.N.A.; Alitheen, N.B.M.; Osman, M.A. The Regulatory Role of MicroRNAs in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 4940. [Google Scholar] [CrossRef] [Green Version]
- Daoud, A.Z.; Mulholland, E.J.; Cole, G.; McCarthy, H.O. MicroRNAs in Pancreatic Cancer: Biomarkers, prognostic, and therapeutic modulators. BMC Cancer 2019, 19, 1130. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Tao, Y.; Shan, L.; Chen, R.; Jiang, H.; Qian, Z.; Cai, F.; Ma, L.; Yu, Y. The Role of MicroRNAs in Hepatocellular Carcinoma. J. Cancer 2018, 9, 3557–3569. [Google Scholar] [CrossRef]
- Bidarra, D.; Constâncio, V.; Barros-Silva, D.; Ramalho-Carvalho, J.; Moreira-Barbosa, C.; Antunes, L.; Maurício, J.; Oliveira, J.; Henrique, R.; Jerónimo, C. Circulating MicroRNAs as Biomarkers for Prostate Cancer Detection and Metastasis Development Prediction. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.-L.; Tsai, Y.-M.; Lien, C.-T.; Kuo, P.-L.; Hung, J.-Y. The Roles of MicroRNA in Lung Cancer. Int. J. Mol. Sci. 2019, 20, 1611. [Google Scholar] [CrossRef] [Green Version]
- Di, Z.; Di, M.; Fu, W.; Tang, Q.; Liu, Y.; Lei, P.; Gu, X.; Liu, T.; Sun, M. Integrated Analysis Identifies a Nine-microRNA Signature Biomarker for Diagnosis and Prognosis in Colorectal Cancer. Front. Genet. 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Angius, A.; Uva, P.; Pira, G.; Muroni, M.R.; Sotgiu, G.; Saderi, L.; Uleri, E.; Caocci, M.; Ibba, G.; Cesaraccio, M.R.; et al. Integrated Analysis of miRNA and mRNA Endorses a Twenty miRNAs Signature for Colorectal Carcinoma. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Cai, Q.; Jiang, Z.; Liu, B.; Zhu, Z.; Li, C. Prognostic role of microRNA-21 in gastric cancer: A meta-analysis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2014, 20, 1668–1674. [Google Scholar] [CrossRef] [Green Version]
- Gramantieri, L.; Fornari, F.; Callegari, E.; Sabbioni, S.; Lanza, G.; Croce, C.M.; Bolondi, L.; Negrini, M. MicroRNA involvement in hepatocellular carcinoma. J. Cell. Mol. Med. 2008, 12, 2189–2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, P.J.; Getz, G.; Korbel, J.O.; Stuart, J.M.; Jennings, J.L.; Stein, L.D.; Perry, M.D.; Nahal-Bose, H.K.; Ouellette, B.F.F.; Li, C.H.; et al. Pan-cancer analysis of whole genomes. Nature 2020, 578, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Bergstrom, K.S.; Xia, L. Mucin-type O-glycans and their roles in intestinal homeostasis. Glycobiology 2013, 23, 1026–1037. [Google Scholar] [CrossRef]
- Velcich, A.; Yang, W.; Heyer, J.; Fragale, A.; Nicholas, C.; Viani, S.; Kucherlapati, R.; Lipkin, M.; Yang, K.; Augenlicht, L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 2002, 295, 1726–1729. [Google Scholar] [CrossRef]
- Ueda, T.; Volinia, S.; Okumura, H.; Shimizu, M.; Taccioli, C.; Rossi, S.; Alder, H.; Liu, C.-G.; Oue, N.; Yasui, W.; et al. Relation between microRNA expression and progression and prognosis of gastric cancer: A microRNA expression analysis. Lancet Oncol. 2010, 11, 136–146. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Yin, S.; Sun, R.; Zhang, S.; Fu, M.; Wu, Y.; Zhang, T.; Khaliq, J.; Li, Y. miR-140-5p suppresses the proliferation, migration and invasion of gastric cancer by regulating YES1. Mol. Cancer 2017, 16, 139. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Z.; Yang, Y.; Luo, M.; Zhang, M.; Wang, X.; Liu, L.; Hou, N.; Guo, Q.; Song, T.; et al. MiR-99b-5p and miR-203a-3p Function as Tumor Suppressors by Targeting IGF-1R in Gastric Cancer. Sci. Rep. 2018, 8, 10119. [Google Scholar] [CrossRef] [Green Version]
- Grossi, I.; Salvi, A.; Baiocchi, G.; Portolani, N.; De Petro, G. Functional Role of microRNA-23b-3p in Cancer Biology. Microrna 2018, 7, 156–166. [Google Scholar] [CrossRef]
- Chen, L.; Xiao, H.; Wang, Z.-H.; Huang, Y.; Liu, Z.-P.; Ren, H.; Song, H. miR-29a suppresses growth and invasion of gastric cancer cells in vitro by targeting VEGF-A. BMB Rep. 2014, 47, 39–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wald, P.; Liu, X.S.; Pettit, C.; Dillhoff, M.; Manilchuk, A.; Schmidt, C.; Wuthrick, E.; Chen, W.; Williams, T.M. Prognostic value of microRNA expression levels in pancreatic adenocarcinoma: A review of the literature. Oncotarget 2017, 8, 73345–73361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botla, S.K.; Savant, S.; Jandaghi, P.; Bauer, A.S.; Mücke, O.; Moskalev, E.A.; Neoptolemos, J.P.; Costello, E.; Greenhalf, W.; Scarpa, A.; et al. Early Epigenetic Downregulation of microRNA-192 Expression Promotes Pancreatic Cancer Progression. Cancer Res. 2016, 76, 4149–4159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhayat, S.A.; Abdeen, B.; Köhler, G.; Senninger, N.; Haier, J.; Mardin, W.A. MicroRNA-100 and microRNA-21 as markers of survival and chemotherapy response in pancreatic ductal adenocarcinoma UICC stage II. Clin. Epigenet. 2015, 7, 132. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Zubair, H.; Srivastava, S.K.; Singh, S.; Singh, A.P. Insights into the Role of microRNAs in Pancreatic Cancer Pathogenesis: Potential for Diagnosis, Prognosis, and Therapy. Adv. Exp. Med. Biol. 2015, 889, 71–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, B.; Ding, Q.; Han, H.; Wu, D. miRCancer: A microRNA–cancer association database constructed by text mining on literature. Bioinformatics 2013, 29, 638–644. [Google Scholar] [CrossRef]
- Chen, D.T.; Hernandez, J.M.; Shibata, D.; McCarthy, S.M.; Humphries, L.A.; Clark, W.; Elahi, A.; Gruidl, M.; Coppola, D.; Yeatman, T. Complementary strand microRNAs mediate acquisition of metastatic potential in colonic adenocarcinoma. J. Gastrointest. Surg. 2012, 16, 905–912. [Google Scholar] [CrossRef]
- Wei, R.; Huang, G.L.; Zhang, M.Y.; Li, B.K.; Zhang, H.Z.; Shi, M.; Chen, X.Q.; Huang, L.; Zhou, Q.M.; Jia, W.H.; et al. Clinical significance and prognostic value of microRNA expression signatures in hepatocellular carcinoma. Clin. Cancer Res. 2013, 19, 4780–4791. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; He, P.; Wang, J.; Schetter, A.; Tang, W.; Funamizu, N.; Yanaga, K.; Uwagawa, T.; Satoskar, A.R.; Gaedcke, J.; et al. A Novel MIF Signaling Pathway Drives the Malignant Character of Pancreatic Cancer by Targeting NR3C2. Cancer Res. 2016, 76, 3838–3850. [Google Scholar] [CrossRef] [Green Version]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-miRPath v3.0: Deciphering microRNA function with experimental support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef]

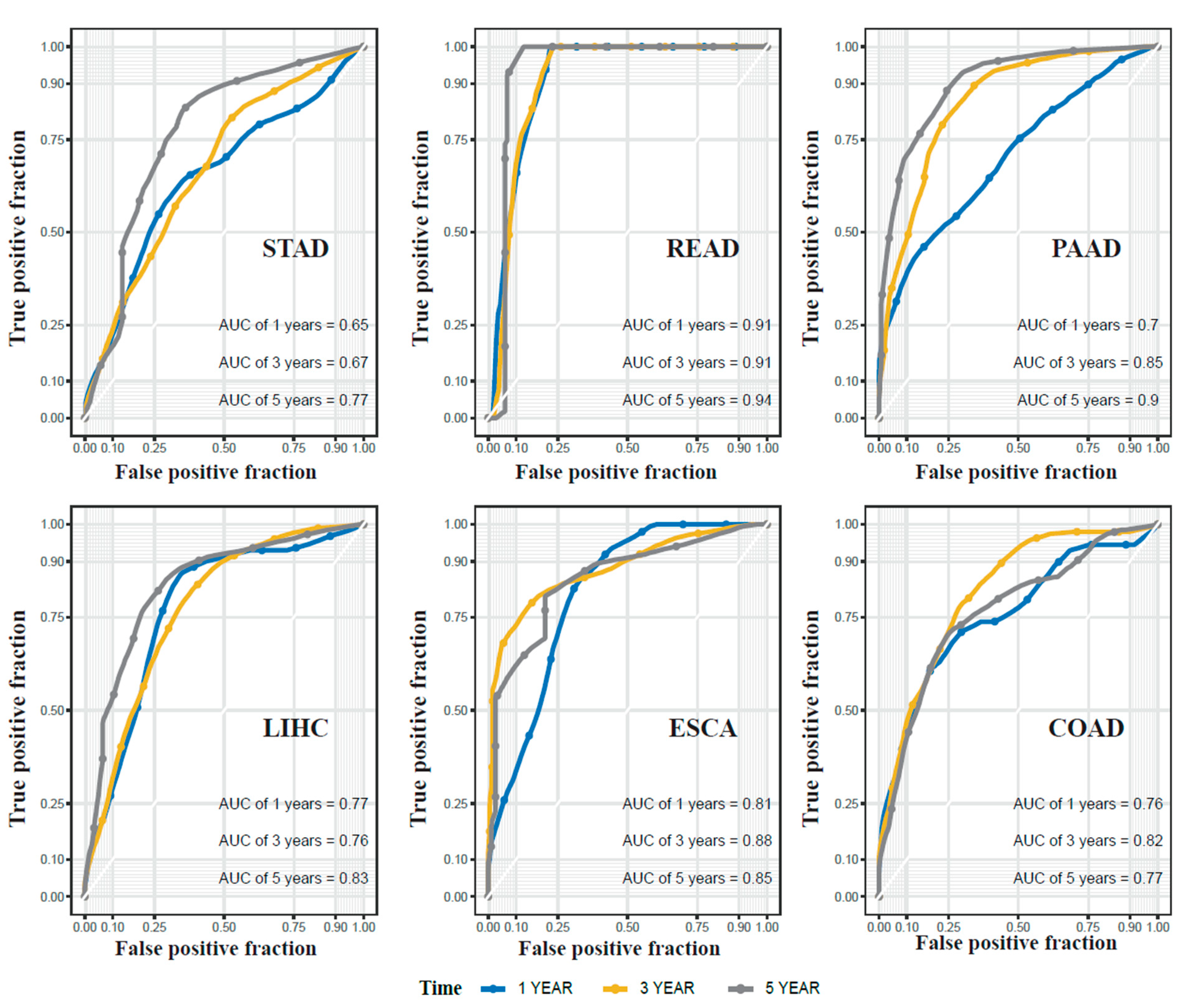
| Cancer | Method | Full Dataset | Cross-Validation | ||
|---|---|---|---|---|---|
| AUC-3 | AUC-5 | AUC-3 | AUC-5 | ||
| STAD | cluster-based | 0.67 | 0.77 | 0.62 | 0.67 |
| cancer-specific | 0.61 | 0.67 | 0.57 | 0.6 | |
| combined | 0.73 | 0.72 | 0.67 | 0.66 | |
| PAAD | cluster-based | 0.85 | 0.9 | 0.72 | 0.74 |
| cancer-specific | 0.82 | 0.83 | 0.67 | 0.64 | |
| combined | 0.86 | 0.93 | 0.69 | 0.72 | |
| ESCA | cluster-based | 0.88 | 0.85 | 0.74 | 0.7 |
| cancer-specific | 0.78 | 0.72 | 0.68 | 0.61 | |
| combined | 0.86 | 0.81 | 0.73 | 0.68 | |
| COAD | cluster-based | 0.82 | 0.77 | 0.69 | 0.61 |
| cancer-specific | 0.77 | 0.74 | 0.6 | 0.55 | |
| combined | 0.82 | 0.79 | 0.67 | 0.61 | |
| LIHC | cluster-based | 0.76 | 0.83 | 0.65 | 0.68 |
| cancer-specific | 0.78 | 0.84 | 0.7 | 0.76 | |
| combined | 0.78 | 0.84 | 0.7 | 0.76 | |
| READ | cluster-based | 0.91 | 0.94 | - | - |
| cancer-specific | 0.9 | 0.92 | - | - | |
| combined | 0.92 | 0.91 | - | - | |
| Cancer | Method | HR (95% CI) | p-Value | AUC-3 | AUC-5 |
|---|---|---|---|---|---|
| COAD | cluster-based | 0.14 (0.04–0.47) | 0.0015 * | 0.76 | 0.81 |
| cancer-specific | 0.18 (0.06–0.52) | 0.0019 * | 0.77 | 0.73 | |
| combined | 0.11 (0.04–0.32) | 5.87 × 10−5 * | 0.81 | 0.83 | |
| LIHC | cluster-based | 0.22 (0.11–0.43) | 9.58 × 10−6 * | 0.82 | 0.82 |
| cancer-specific | 0.39 (0.23–0.65) | 0.0003 * | 0.66 | 0.69 | |
| combined | 0.39 (0.24–0.63) | 0.00014 * | 0.73 | 0.75 | |
| PAAD | cluster-based | 0.31 (0.17–0.58) | 0.0002 * | 0.74 | 0.79 |
| cancer-specific | 0.65 (0.32–1.34) | 0.2465 | 0.59 | 0.59 | |
| combined | 0.32 (0.18–0.59) | 0.00003 * | 0.81 | 0.89 | |
| ESCA | cluster-based | 0.67 (0.42–1.06) | 0.0871 | 0.57 | 0.58 |
| cancer-specific | 0.49 (0.31–0.79) | 0.0034 * | 0.65 | 0.65 | |
| combined | 0.64 (0.40–1.01) | 0.0583 | 0.61 | 0.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Cui, X.; Xiao, F.; Cai, G. A Cluster-Based Approach for Identifying Prognostic microRNA Signatures in Digestive System Cancers. Int. J. Mol. Sci. 2021, 22, 1529. https://doi.org/10.3390/ijms22041529
Zhou J, Cui X, Xiao F, Cai G. A Cluster-Based Approach for Identifying Prognostic microRNA Signatures in Digestive System Cancers. International Journal of Molecular Sciences. 2021; 22(4):1529. https://doi.org/10.3390/ijms22041529
Chicago/Turabian StyleZhou, Jun, Xiang Cui, Feifei Xiao, and Guoshuai Cai. 2021. "A Cluster-Based Approach for Identifying Prognostic microRNA Signatures in Digestive System Cancers" International Journal of Molecular Sciences 22, no. 4: 1529. https://doi.org/10.3390/ijms22041529






