Dissecting the Roles of Cuticular Wax in Plant Resistance to Shoot Dehydration and Low-Temperature Stress in Arabidopsis
Abstract
1. Introduction
2. Results
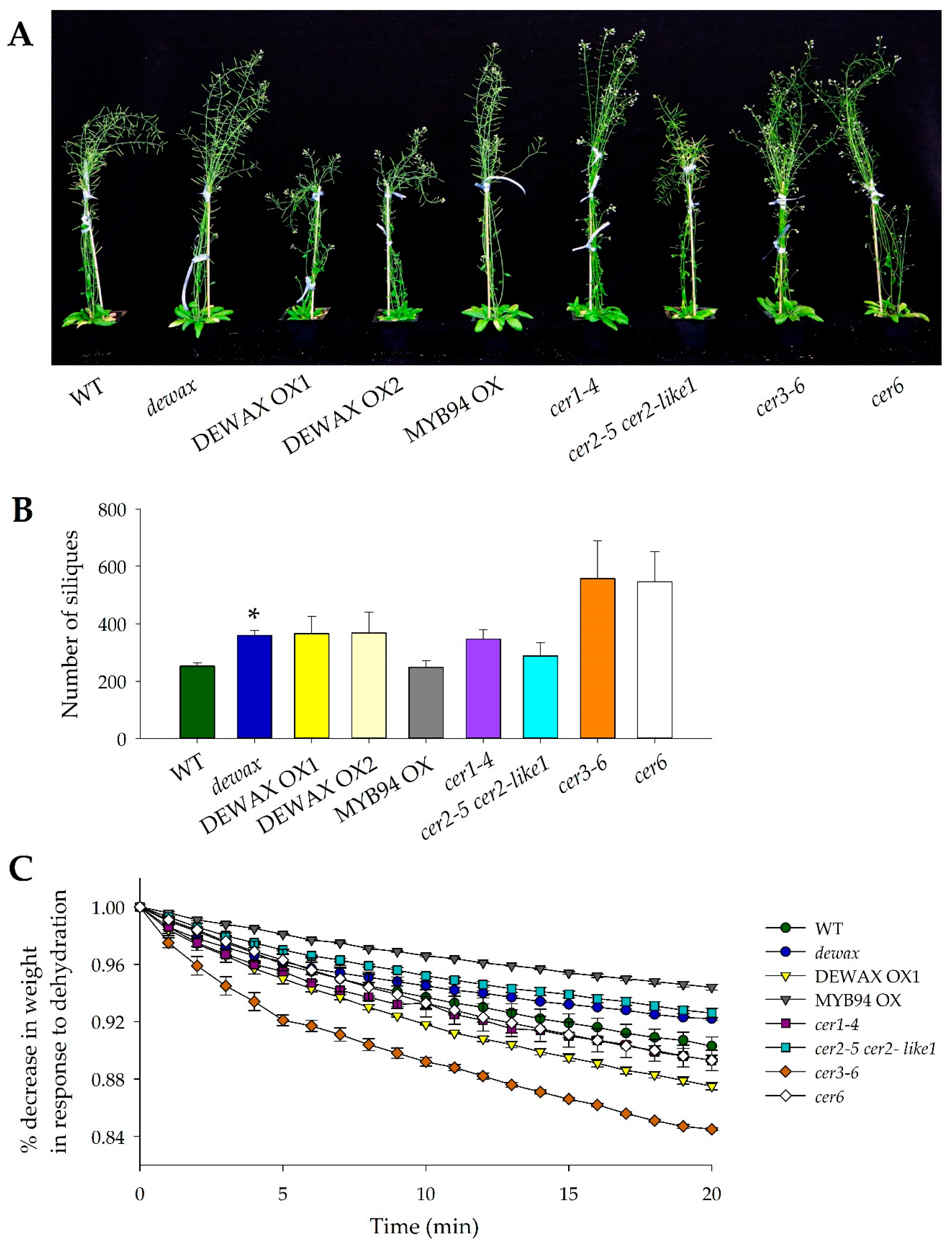
2.1. Higher Accumulation of Cuticular Wax Does Not Inhibit the Reproductive Yield in Arabidopsis
2.2. The Mutant Allele cer3-6 Is Highly Sensitive to Shoot Dehydration
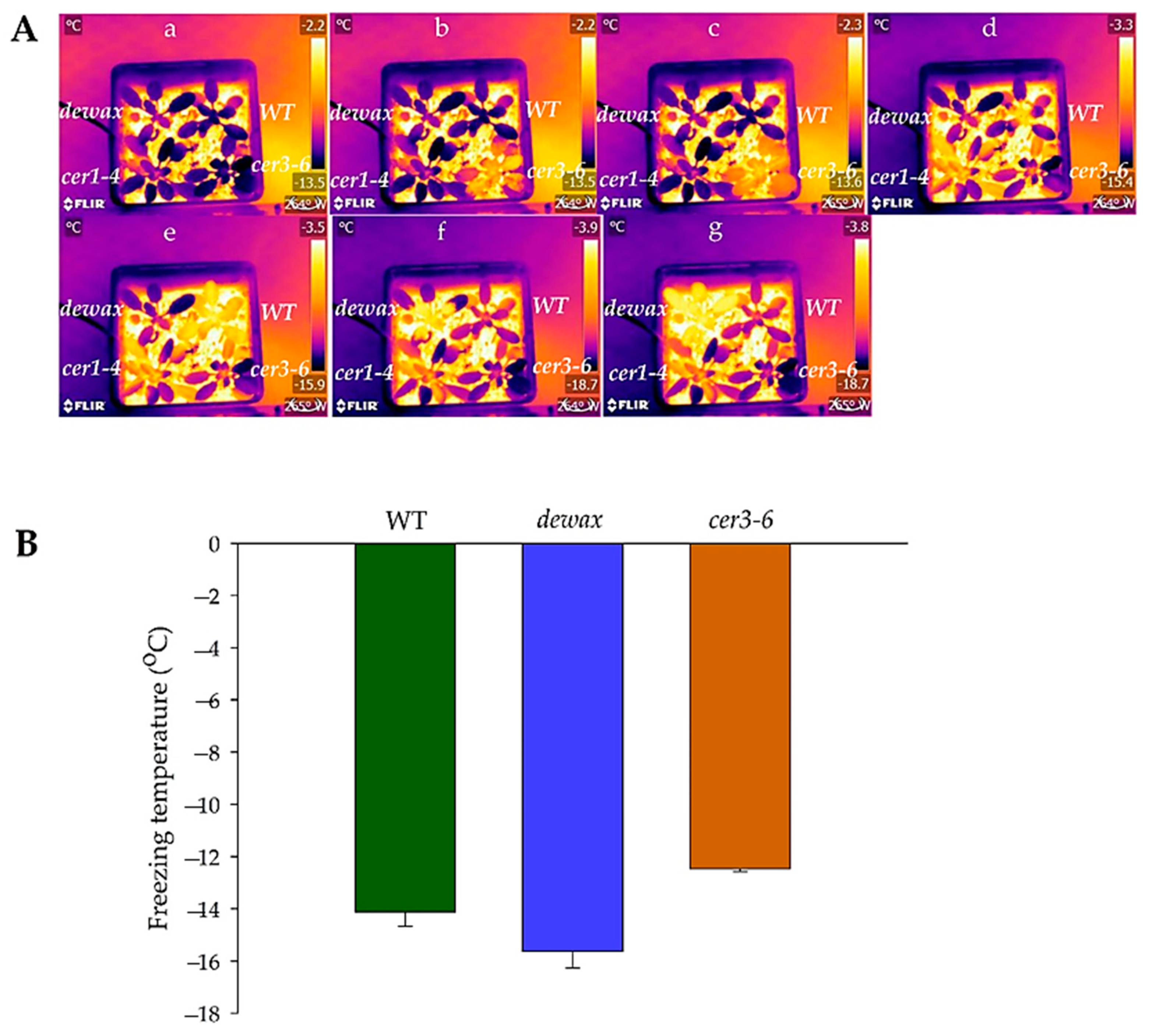
2.3. Cold Acclimated cer3-6 and Dewax Display Contrasting Ice Nucleation at Warmer Subzero Temperatures
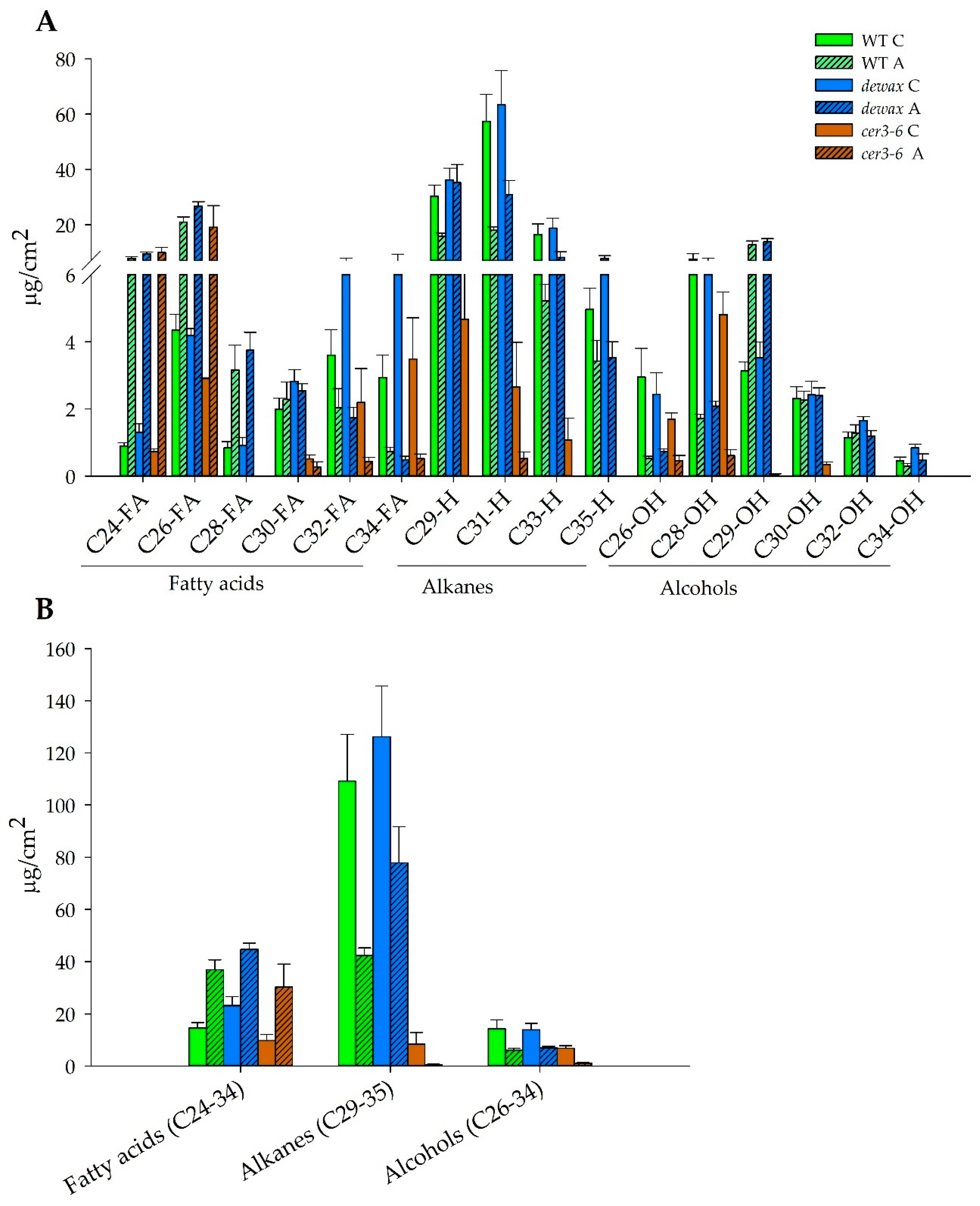
2.4. Accumulation of Hydrophobic Wax Deposition in the Cuticle Decreases under Cold Acclimation
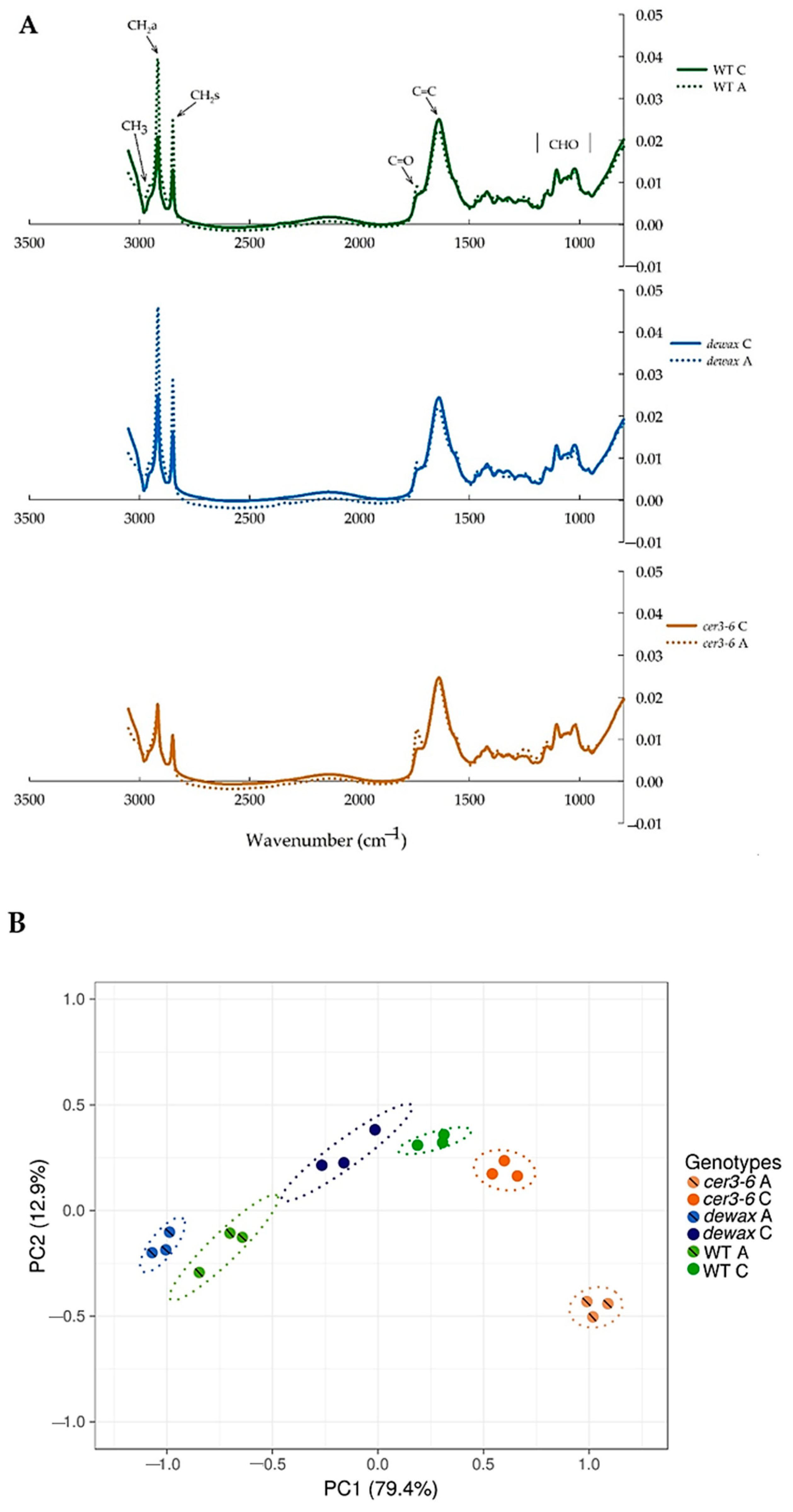
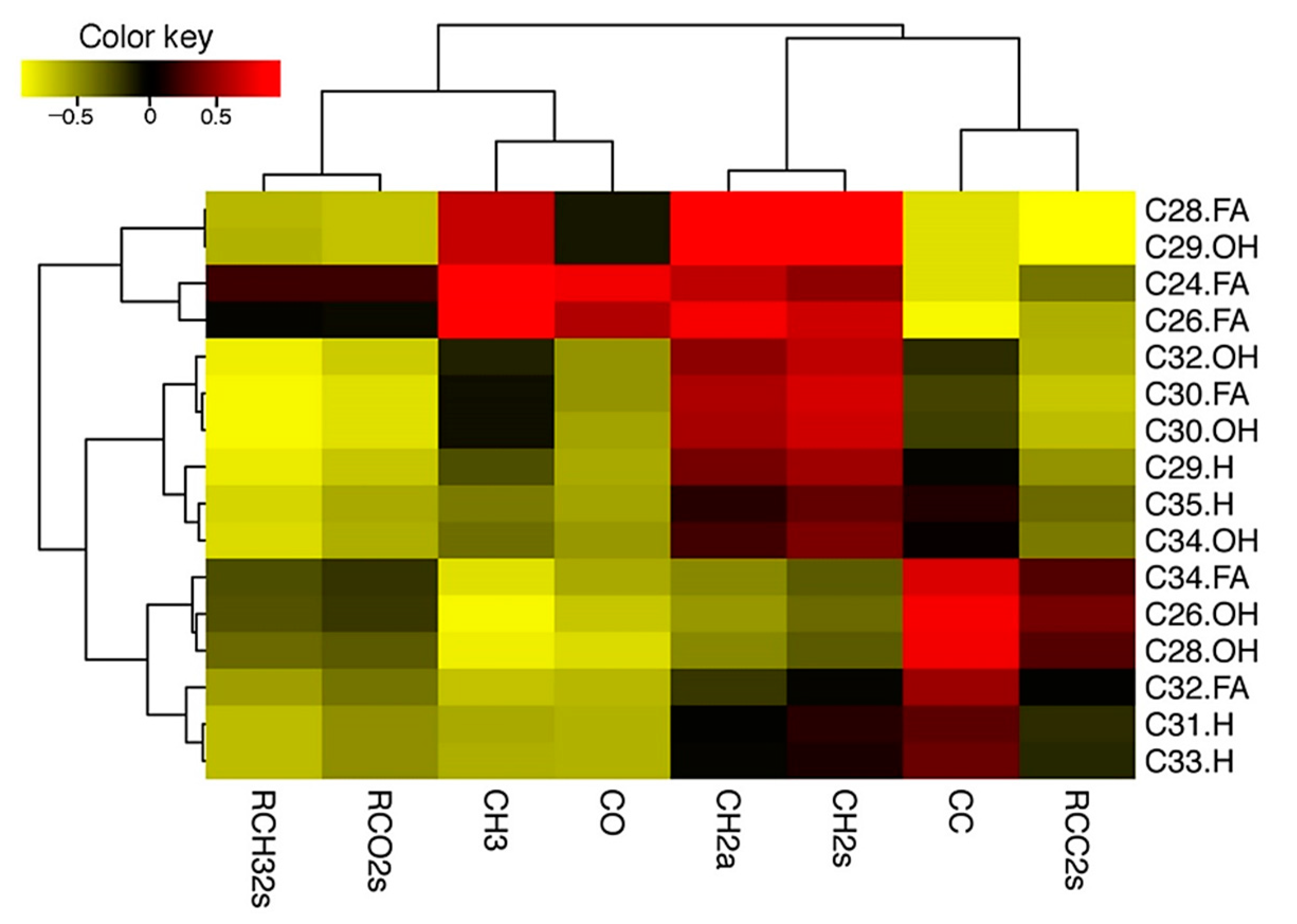
2.5. ATR-FTIR Analysis of Leaf Epidermal Surfaces Identify Changes in Lipid Accumulation in Response to Cold Acclimation
3. Discussion
3.1. Arabidopsis Requires an Intact Alkane Biosynthetic Pathway, Mediated by CER3 and CER1, to Resist against Dehydration and Frost
3.2. Higher Accumulation of Wax in the Cuticle Does Not Inhibit the Reproductive Yield in Arabidopsis
3.3. Cold Acclimation-Induced Compositional Changes of Wax Constituents in the Cuticle
3.4. ATR-FTIR Based Analysis Further Revealed Cold-Acclimation Driven Leaf Cuticular Changes in Arabidopsis
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Arabidopsis Shoot Dehydration Assay
4.3. Cold Acclimation
4.4. Freezing Treatment and Thermal Imaging
4.5. ATR-FTIR Analysis of Rosette Leaf Cuticular Waxes
4.6. Correlation and Principal Component Analysis
4.7. Wax Extraction and GC-MS Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, S.B.; Suh, M.C. Advances in the understanding of cuticular waxes in Arabidopsis thaliana and crop species. Plant Cell Rep. 2015, 34, 557–572. [Google Scholar] [CrossRef]
- Xue, D.; Zhang, X.; Lu, X.; Chen, G.; Chen, Z.H. Molecular and evolutionary mechanisms of cuticular wax for plant drought tolerance. Front. Plant Sci. 2017, 8, 621. [Google Scholar] [CrossRef] [PubMed]
- Yeats, T.H.; Rose, J.K.C. The formation and function of plant cuticles. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, T.; Griffiths, D.W. The effects of stress on plant cuticular waxes. New Phytol. 2006, 171, 469–499. [Google Scholar] [CrossRef] [PubMed]
- Dhanyalakshmi, K.H.; Soolanayakanahally, R.Y.; Rahman, T.; Tanino, K.T.; Nataraja, K.N. Leaf Cuticular Wax, a Trait for Multiple Stress Resistance in Crop Plants. In Abiotic and Biotic Stress in Plants; De Oliveira, B.A., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Samuels, L.; Kunst, L.; Jetter, R. Sealing plant surfaces: Cuticular wax formation by epidermal cells. Annu. Rev. Plant. Biol. 2008, 59, 683–707. [Google Scholar] [CrossRef]
- Lee, S.B.; Suh, M.C. Recent advances in cuticular wax biosynthesis and its regulation in Arabidopsis. Mol. Plant. 2013, 6, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Buschhaus, C.; Jetter, R. Composition differences between epicuticular and intracuticular wax substructures: How do plants seal their epidermal surfaces? J. Exp. Bot. 2011, 62, 841–853. [Google Scholar] [CrossRef]
- Bernard, A.; Joubès, J. Arabidopsis cuticular waxes: Advances in synthesis, export and regulation. Prog. Lipid. Res. 2013, 52, 110–129. [Google Scholar] [CrossRef]
- Bourdenx, B.; Bernard, A.; Domergue, F.; Pascal, S.; Léger, A.; Roby, D.; Pervent, M.; Vile, D.; Haslam, R.P.; Napier, J.A.; et al. Overexpression of Arabidopsis ECERIFERUM1 promotes wax very-long-chain alkane biosynthesis and influences plant response to biotic and abiotic stresses. Plant Physiol. 2011, 156, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Aarts, M.G.M.; Keijzer, C.J.; Stiekema, W.J.; Pereira, A. Molecular Characterization of the CER1 Gene of Arabidopsis Involved in Epicuticular Wax Biosynthesis and Pollen Fertility. Plant Cell 1995, 7, 2115. [Google Scholar] [CrossRef][Green Version]
- Bernard, A.; Domergue, F.; Pascal, S.; Jetter, R.; Renne, C.; Faure, J.D.; Haslam, R.P.; Napier, J.A.; Lessire, R.; Joubès, J. Reconstitution of plant alkane biosynthesis in yeast demonstrates that Arabidopsis ECERIFERUM1 and ECERIFERUM3 are core components of a very-long-chain alkane synthesis complex. Plant Cell 2012, 24, 3106–3118. [Google Scholar] [CrossRef] [PubMed]
- Pascal, S.; Bernard, A.; Deslous, P.; Gronnier, J.; Fournier-Goss, A.; Domergue, F.; Rowland, O.; Joubès, J. Arabidopsis CER1-LIKE1 Functions in a Cuticular Very-Long-Chain Alkane-Forming Complex. Plant Physiol. 2019, 179, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Goodwin, S.M.; Boroff, V.L.; Liu, X.; Jenks, M.A. Cloning and characterization of the WAX2 gene of Arabidopsis involved in cuticle membrane and wax production. Plant Cell 2003, 15, 1170–1185. [Google Scholar] [CrossRef]
- Rowland, O.; Lee, R.; Franke, R.; Schreiber, L.; Kunst, L. The CER3 wax biosynthetic gene from Arabidopsis thaliana is allelic to WAX2/YRE/FLP1. FEBS Lett. 2007, 581, 3538–3544. [Google Scholar] [CrossRef]
- Kurata, T.; Kawabata-Awai, C.; Sakuradani, E.; Shimizu, S.; Okada, K.; Wada, T. The YORE-YORE gene regulates multiple aspects of epidermal cell differentiation in Arabidopsis. Plant J. 2003, 36, 55–66. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Gai, X.; Ren, J.; Liu, X.; Cai, Y.; Wang, Q.; Ren, H. Cucumis sativus L. WAX2 Plays a Pivotal Role in Wax Biosynthesis, Influencing Pollen Fertility and Plant Biotic and Abiotic Stress Responses. Plant Cell Physiol. 2015, 56, 1339–1354. [Google Scholar] [CrossRef] [PubMed]
- Rowland, O.; Zheng, H.; Hepworth, S.R.; Lam, P.; Jetter, R.; Kunst, L. CER4 encodes an alcohol-forming fatty acyl-coenzyme A reductase involved in cuticular wax production in Arabidopsis. Plant Physiol. 2006, 142, 866–877. [Google Scholar] [CrossRef]
- Millar, A.A.; Clemens, S.; Zachgo, S.; Giblin, E.M.; Taylor, D.C.; Kunst, L. CUT1, an Arabidopsis Gene Required for Cuticular Wax Biosynthesis and Pollen Fertility, Encodes a Very-Long-Chain Fatty Acid Condensing Enzyme. Plant Cell 1999, 11, 825. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haslam, T.M.; Mañas-Fernández, A.; Zhao, L.; Kunst, L. Arabidopsis ECERIFERUM2 Is a component of the fatty acid elongation machinery required for fatty acid extension to exceptional lengths. Plant Physiol. 2012, 160, 1164–1174. [Google Scholar] [CrossRef]
- Panikashvili, D.; Savaldi-Goldstein, S.; Mandel, T.; Yifhar, T.; Franke, R.B.; Höfer, R.; Schreiber, L.; Chory, J.; Aharoni, A. The Arabidopsis DESPERADO/AtWBC11 transporter is required for cutin and wax secretion. Plant Physiol. 2007, 145, 1345–1360. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Broeckling, C.D.; Blancaflor, E.B.; Sledge, M.K.; Sumner, L.W.; Wang, Z.Y. Overexpression of WXP1, a putative Medicago truncatula AP2 domain-containing transcription factor gene, increases cuticular wax accumulation and enhances drought tolerance in transgenic alfalfa (Medicago sativa). Plant J. 2005, 42, 689–707. [Google Scholar] [CrossRef]
- Seo, P.J.; Lee, S.B.; Suh, M.C.; Park, M.J.; Park, C.M. The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis. Plant Cell. 2011, 23, 1138–1152. [Google Scholar] [CrossRef]
- Aharoni, A.; Dixit, S.; Jetter, R.; Thoenes, E.; Van Arkel, G.; Pereira, A. The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell. 2004, 16, 2463–2480. [Google Scholar] [CrossRef]
- Broun, P.; Poindexter, P.; Osborne, E.; Jiang, C.Z.; Riechmann, J.L. WIN1, a transcriptional activator of epidermal wax accumulation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 4706–4711. [Google Scholar] [CrossRef]
- Sajeevan, R.S.; Nataraja, K.N.; Shivashankara, K.S.; Pallavi, N.; Gurumurthy, D.S.; Shivanna, M.B. Expression of Arabidopsis SHN1 in Indian Mulberry (Morus indica L.) Increases Leaf Surface Wax Content and Reduces Post-harvest Water Loss. Front. Plant Sci. 2017, 8, 418. [Google Scholar] [CrossRef]
- Go, Y.S.; Kim, H.; Kim, H.J.; Suh, M.C. Arabidopsis cuticular wax biosynthesis is negatively regulated by the DEWAX gene encoding an AP2/ERF-type transcription factor. Plant Cell. 2014, 26, 1666–1680. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, H.U.; Suh, M.C. MYB94 and MYB96 additively activate cuticular wax biosynthesis in Arabidopsis. Plant Cell Physiol. 2016, 57, 2300–2311. [Google Scholar] [CrossRef]
- Lee, S.B.; Suh, M.C. Cuticular wax biosynthesis is up-regulated by the MYB94 transcription factor in Arabidopsis. Plant Cell Physiol. 2015, 56, 48–60. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, W.; Li, W. Genetic interactions underlying the biosynthesis and inhibition of β-diketones in wheat and their impact on glaucousness and cuticle permeability. PLoS ONE 2013, 8, e54129. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Chai, G.; Li, C.; Hu, Y.; Chen, X.; Wang, Z. Developmental Changes in Composition and Morphology of Cuticular Waxes on Leaves and Spikes of Glossy and Glaucous Wheat (Triticum aestivum L.). PLoS ONE 2015, 10, e0141239. [Google Scholar] [CrossRef]
- Sánchez, F.J.; Manzanares, M.; De Andrés, E.F.; Tenorio, J.L.; Ayerbe, L. Residual transpiration rate, epicuticular wax load and leaf colour of pea plants in drought conditions. Influence on harvest index and canopy temperature. Eur. J. Agron. 2001, 15, 57–70. [Google Scholar] [CrossRef]
- Jenks, M.A.; Andersen, L.; Teusink, R.S.; Williams, M.H. Leaf cuticular waxes of potted rose cultivars as affected by plant development, drought and paclobutrazol treatments. Physiol. Plant. 2001, 112, 62–70. [Google Scholar] [CrossRef]
- Cameron, K.D.; Teece, M.A.; Smart, L.B. Increased accumulation of cuticular wax and expression of lipid transfer protein in response to periodic drying events in leaves of tree tobacco. Plant Physiol. 2006, 140, 176–183. [Google Scholar] [CrossRef]
- Kosma, D.K.; Bourdenx, B.; Bernard, A.; Parsons, E.P.; Lü, S.; Joubès, J.; Jenks, M.A. The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol. 2009, 151, 1918–1929. [Google Scholar] [CrossRef]
- Wisniewski, M.; Fuller, M. Ice nucleation and deep supercooling in plants: New insights using infrared thermography. Cold Adapt. Org. 1999, 105–118. [Google Scholar] [CrossRef]
- Wisniewski, M.; Glenn, D.M.; Fuller, M.P. Use of a hydrophobic particle film as a barrier to extrinsic ice nucleation in tomato plants. J. Am. Soc. Hortic. Sci. 2002, 127, 358–364. [Google Scholar] [CrossRef]
- Reyes-Díaz, M.; Ulloa, N.; Zúñiga-Feest, A.; Gutiérrez, A.; Gidekel, M.; Alberdi, M.; Corcuera, L.J.; Bravo, L.A. Arabidopsis thaliana avoids freezing by supercooling. J. Exp. Bot. 2006, 57, 3687–3696. [Google Scholar] [CrossRef] [PubMed]
- Hoermiller, I.I.; Ruschhaupt, M.; Heyer, A.G. Mechanisms of frost resistance in Arabidopsis thaliana. Planta 2018, 248, 827–835. [Google Scholar] [CrossRef]
- Jefferson, P.G. Genetic variation for epicuticular wax production in Altai wildrye populations that differ in glaucousness. Crop Sci. 1994, 34, 367–371. [Google Scholar] [CrossRef]
- Kim, K.S.; Park, S.H.; Jenks, M.A. Changes in leaf cuticular waxes of sesame (Sesamum indicum L.) plants exposed to water deficit. J. Plant Physiol. 2007, 164, 1134–1143. [Google Scholar] [CrossRef]
- Guo, J.; Xu, W.; Yu, X.; Shen, H.; Li, H.; Cheng, D.; Liu, A.; Liu, J.; Liu, C.; Zhao, S.; et al. Cuticular Wax Accumulation Is Associated with Drought Tolerance in Wheat Near-Isogenic Lines. Front. Plant Sci. 2016, 7, 1809. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ni, E.; Yang, J.; Zhou, H.; Liang, H.; Li, J.; Jiang, D.; Wang, Z.; Liu, Z.; Zhuang, C. Rice OsGL1-6 is involved in leaf cuticular wax accumulation and drought resistance. PLoS ONE 2013, 8, e65139. [Google Scholar] [CrossRef]
- Johnson, D.A.; Richards, R.A.; Turner, N.C. Yield, Water Relations, Gas Exchange, and Surface Reflectances of Near-Isogenic Wheat Lines Differing in Glaucousness. Crop. Sci. 1983, 23, 318–325. [Google Scholar] [CrossRef]
- González, A.; Ayerbe, L. Effect of terminal water stress on leaf epicuticular wax load, residual transpiration and grain yield in barley. Euphytica 2010, 172, 341–349. [Google Scholar] [CrossRef]
- Burke, M.J.; Gusta, L.V.; Quamme, H.A.; Weiser, C.J.; Li, P.H. Freezing and injury in plants. Ann. Rev. Plant Physiol. 1976, 27, 507–528. [Google Scholar] [CrossRef]
- Pearce, R.S.; Ashworth, E.N. Cell shape and localization of ice in leaves of overwintering wheat during frost stress in the field. Planta 1992, 188, 324–331. [Google Scholar] [CrossRef]
- Wisniewski, M.; Lindow, S.E.; Ashworth, E.N. Observations of Ice Nucleation and Propagation in Plants Using Infrared Video Thermography. Plant Physiol. 1997, 113, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.R.B.; Wisniewski, M. Low-temperature tolerance of blackcurrant flowers. Hortic. Sci. 1999, 34, 855–859. [Google Scholar] [CrossRef]
- Wordmaster, B.A.A.; Palta, J.P.; Wisniewski, M. Ice nucleation and propagation in cranberry uprights and fruit using infrared video thermography. J. Am. Soc. Hortic. Sci. 1999, 124, 619–625. [Google Scholar] [CrossRef]
- Sakai, A. Freezing avoidance mechanism of primordial shoots of conifer buds. Plant Cell Physiol. 1979, 20, 1381–1390. [Google Scholar] [CrossRef]
- Beck, E.; Schulze, E.D.; Senser, M.; Scheibe, R. Equilibrium freezing of leaf water and extracellular ice formation in Afroalpine ‘giant rosette’ plants. Planta 1984, 162, 276–282. [Google Scholar] [CrossRef]
- Guy, C.L. Cold acclimation and freezing stress tolerance: Role of protein metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1990, 41, 187–223. [Google Scholar] [CrossRef]
- Fuller, M.; Hamed, F.; Wisniewski, M.; Glenn, D.M. Protection of plants from frost using hydrophobic particle film and acrylic polymer. Ann. App. Biol. 2003, 143, 93–97. [Google Scholar] [CrossRef]
- Wisniewski, M.; Gusta, L.; Neuner, G. Adaptive mechanisms of freeze avoidance in plants: A brief update. Environ. Exp. Bot. 2014, 99, 133–149. [Google Scholar] [CrossRef]
- Willick, I.R.; Lahlali, R.; Vijayan, P.; Muir, D.; Karunakaran, C.; Tanino, K.K. Wheat flag leaf epicuticular wax morphology and composition in response to moderate drought stress are revealed by SEM, FTIR-ATR and synchrotron X-ray spectroscopy. Physiol. Plant. 2018, 162, 316–332. [Google Scholar] [CrossRef]
- Liu, X.; Bourgault, R.; Galli, M.; Strable, J.; Chen, Z.; Feng, F.; Dong, J.; Molina, I.; Gallavotti, A. The FUSED LEAVES1-ADHERENT1 regulatory module is required for maize cuticle development and organ separation. New Phytol. 2020. [Google Scholar] [CrossRef]
- Türker-Kaya, S.; Huck, C.W. A review of mid-infrared and near-infrared imaging: Principles, concepts and applications in plant tissue analysis. Molecules 2017, 22, 168. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Haslam, T.; Kunst, L. Wax Analysis of Stem and Rosette Leaves in Arabidopsis thaliana. Bio-Protocol 2013, 3. [Google Scholar] [CrossRef]
- Wang, H.; Ni, X.; Harris-Shultz, K. Molecular evolution of the plant ECERIFERUM1 and ECERIFERUM3 genes involved in aliphatic hydrocarbon production. Comput. Biol. Chem. 2019, 80, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Sun, Y.; Liu, T.; Wu, H.; An, P.; Shui, Z.; Wang, J.; Zhu, Y.; Li, C.; Wang, Y.; et al. TaCER1-1A is involved in cuticular wax alkane biosynthesis in hexaploid wheat and responds to plant abiotic stresses. Plant Cell Environ. 2019, 42, 3077–3091. [Google Scholar] [CrossRef] [PubMed]
| Genotype | Relative Nucleation Temperature |
|---|---|
| WT | 1 |
| dewax | 1.14 ± 0.06 * |
| cer3-6 | 0.87 ± 0.04 * |
| Fatty Acids (FA) | ||||||||
| C24-FA | C26-FA | C28-FA | C30-FA | C32-FA | C34-FA | Total FA | ||
| Least square means (LSM) | ||||||||
| Treatment (T) | ||||||||
| Non-acclimated (C) | 0.98 | 3.81 | 0.59 | 1.78 | 4.26 | 4.44 | 15.86 | |
| Cold-acclimated (A) | 9.01 | 22.26 | 2.30 | 1.70 | 1.41 | 0.59 | 37.26 | |
| Genotype (G) | ||||||||
| WT | 4.33 | 12.65 | 2.00 | 2.13 | 2.82 | 1.84 | 28.76 | |
| dewax | 5.34 | 15.49 | 2.33 | 2.68 | 4.37 | 3.70 | 33.91 | |
| cer3-6 | 5.32 | 10.97 | 0.00 | 0.39 | 1.32 | 2.01 | 20.01 | |
| Analysis of variance | ||||||||
| Source | DF | Pr ≥ F | ||||||
| T | 1 | <0.001 | <0.001 | <0.001 | n.s. 1 | <0.001 | <0.001 | <0.001 |
| G | 2 | n.s. | n.s. | <0.001 | <0.001 | 0.01 | n.s. | 0.02 |
| T × G | 2 | n.s. | n.s. | 0.03 | n.s. | n.s. | n.s. | n.s. |
| Alkanes (H) | ||||||||
| C29-H | C31-H | C33-H | C35-H | Total H | ||||
| LSM | ||||||||
| Treatment (T) | ||||||||
| C | 23.72 | 41.19 | 12.09 | 4.23 | 81.23 | |||
| A | 16.98 | 16.47 | 4.47 | 2.32 | 40.23 | |||
| Genotype (G) | ||||||||
| WT | 23.00 | 37.66 | 10.84 | 4.20 | 75.71 | |||
| dewax | 35.71 | 47.24 | 13.44 | 5.62 | 102.01 | |||
| cer3-6 | 2.33 | 1.60 | 0.54 | 0.00 | 4.47 | |||
| Analysis of variance | ||||||||
| Source | DF | Pr ≥ F | ||||||
| T | 1 | 0.04 | <0.001 | 0.004 | 0.002 | 0.002 | ||
| G | 2 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| T × G | 2 | n.s. | 0.05 | n.s. | 0.03 | n.s. | ||
| Alcohols (OH) | ||||||||
| C26-OH | C28-OH | C29-OH | C30-OH | C32-OH | C34-OH | Total OH | ||
| LSM | ||||||||
| Treatment (T) | ||||||||
| C | 2.36 | 6.28 | 2.23 | 1.70 | 0.93 | 0.43 | 13.94 | |
| A | 0.58 | 1.47 | 8.83 | 1.56 | 0.83 | 0.26 | 13.53 | |
| Genotype (G) | ||||||||
| WT | 1.74 | 4.59 | 7.92 | 2.30 | 1.22 | 0.38 | 18.13 | |
| dewax | 1.59 | 4.33 | 8.67 | 2.42 | 1.42 | 0.67 | 19.08 | |
| cer3-6 | 1.08 | 2.71 | 0.02 | 0.18 | 0.00 | 0.00 | 3.98 | |
| Analysis of variance | ||||||||
| Source | DF | Pr ≥ F | ||||||
| T | 1 | 0.002 | <0.001 | <0.001 | n.s. | n.s. | n.s. | n.s. |
| G | 2 | n.s. | n.s. | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| T × G | 2 | n.s. | n.s. | <0.001 | n.s. | n.s. | n.s. | n.s. |
| CH3 Stretching (2966–2950) | CH2 Asymmetric (2936–2894) | CH2 Symmetric (2871–2826) | C=O Stretching (1758–1726) | C=C Stretching (1706–1584) | Ratio (CH3:CH2s) | Ratio (C=O:CH2s) | Ratio (C=C:CH2s) | |
|---|---|---|---|---|---|---|---|---|
| CH3 | CH2a | CH2s | CO | CC | RCH32s | RCO2s | RCC2s | |
| WT C | 0.052 ± 0.027 | 0.27 ± 0.05 | 0.138 ± 0.025 | 0.109 ± 0.002 | 1.143 ± 0.015 | 0.378 | 0.791 | 8.310 |
| WT A | 0.077 ± 0.009 | 0.462 ± 0.039 | 0.216 ± 0.047 | 0.132 ± 0.007 | 1.023 ± 0.02 | 0.358 | 0.612 | 4.729 |
| dewax C | 0.051 ± 0.032 | 0.303 ± 0.07 | 0.162 ± 0.037 | 0.111 ± 0.005 | 1.127 ± 0.032 | 0.316 | 0.688 | 6.966 |
| dewax A | 0.07 ± 0.008 | 0.517 ± 0.019 | 0.24 ± 0.009 | 0.128 ± 0.003 | 1.014 ± 0.027 | 0.311 | 0.536 | 4.234 |
| cer3-6 C | 0.06 ± 0.014 | 0.255 ± 0.014 | 0.128 ± 0.01 | 0.116 ± 0.008 | 1.134 ± 0.02 | 0.472 | 0.912 | 8.889 |
| cer3-6 A | 0.069 ± 0.011 | 0.277 ± 0.014 * (p = 0.024); WT A vs. cer3-6 A) | 0.118 ± 0.019 * (p = 0.021); WT A vs. cer3-6 A) | 0.174 ± 005 ** (p = 0.008); WT A vs. cer3-6 A) | 1.081 ± 0.023 | 0.588 | 1.477 | 9.183 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, T.; Shao, M.; Pahari, S.; Venglat, P.; Soolanayakanahally, R.; Qiu, X.; Rahman, A.; Tanino, K. Dissecting the Roles of Cuticular Wax in Plant Resistance to Shoot Dehydration and Low-Temperature Stress in Arabidopsis. Int. J. Mol. Sci. 2021, 22, 1554. https://doi.org/10.3390/ijms22041554
Rahman T, Shao M, Pahari S, Venglat P, Soolanayakanahally R, Qiu X, Rahman A, Tanino K. Dissecting the Roles of Cuticular Wax in Plant Resistance to Shoot Dehydration and Low-Temperature Stress in Arabidopsis. International Journal of Molecular Sciences. 2021; 22(4):1554. https://doi.org/10.3390/ijms22041554
Chicago/Turabian StyleRahman, Tawhidur, Mingxuan Shao, Shankar Pahari, Prakash Venglat, Raju Soolanayakanahally, Xiao Qiu, Abidur Rahman, and Karen Tanino. 2021. "Dissecting the Roles of Cuticular Wax in Plant Resistance to Shoot Dehydration and Low-Temperature Stress in Arabidopsis" International Journal of Molecular Sciences 22, no. 4: 1554. https://doi.org/10.3390/ijms22041554
APA StyleRahman, T., Shao, M., Pahari, S., Venglat, P., Soolanayakanahally, R., Qiu, X., Rahman, A., & Tanino, K. (2021). Dissecting the Roles of Cuticular Wax in Plant Resistance to Shoot Dehydration and Low-Temperature Stress in Arabidopsis. International Journal of Molecular Sciences, 22(4), 1554. https://doi.org/10.3390/ijms22041554








