Role of the HSP70 Co-Chaperone SIL1 in Health and Disease
Abstract
1. Introduction
2. The ER-Resident HSP70 Chaperone, BiP
2.1. BiP’s ATPase Cycle
2.2. ERdj Co-Factors for BiP
2.3. BiP’s Nucleotide Exchange Factors
GRP170
3. SIL1
3.1. Discovery and Expression
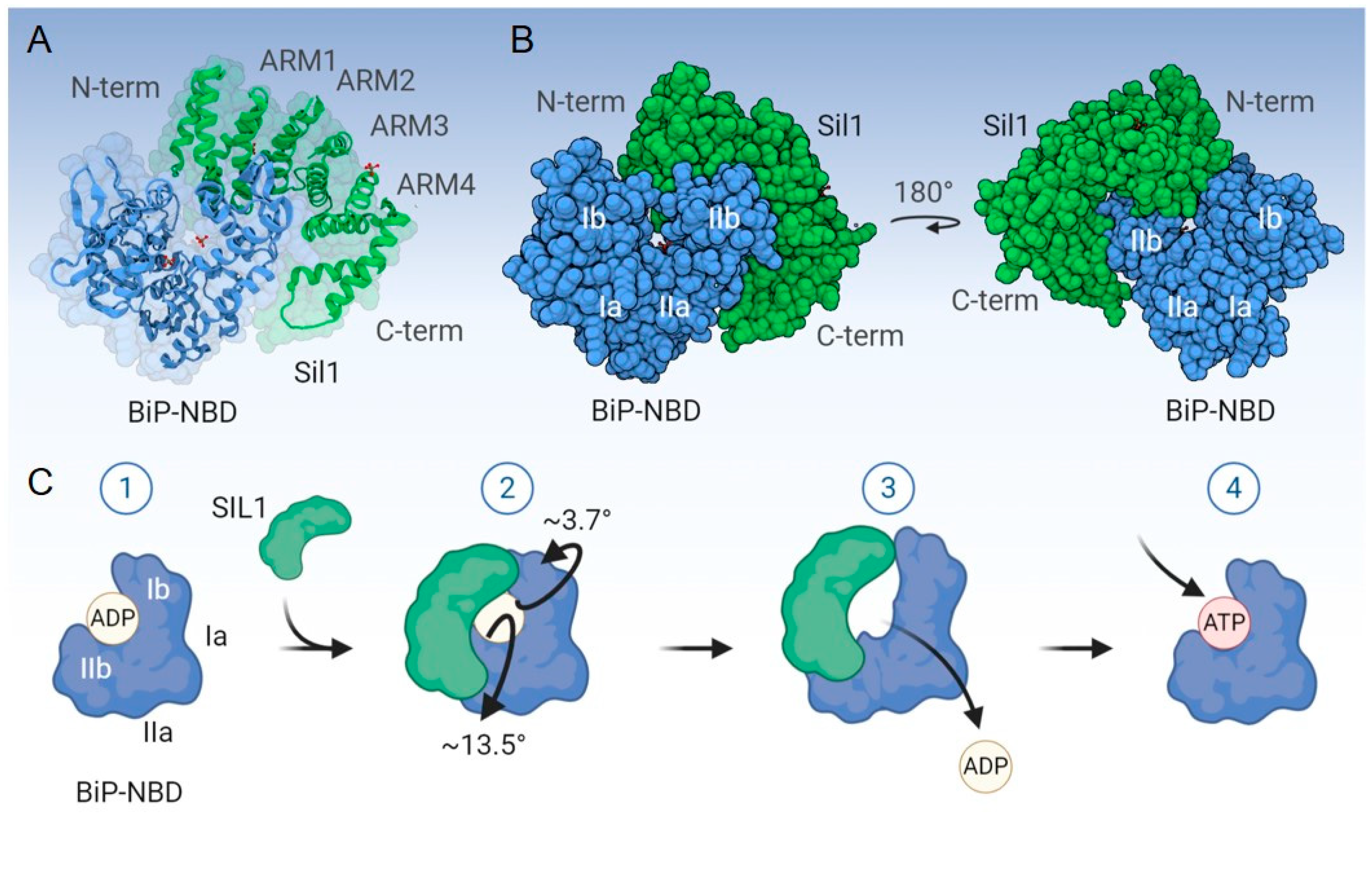
3.2. Structure, Mechanism of Nucleotide Exchange, and Expression
4. Marinesco-Sjögren Syndrome
4.1. Mechanistic Insights into MSS-Associated Pathologies
4.1.1. The Neurological Features of MSS and the Role of SIL1
4.1.2. The Myopathy in MSS
4.1.3. Metabolic Features Associated with SIL1 Depletion
4.1.4. Bilateral Cataracts
4.1.5. Role of SIL1 in B-lymphocytes
4.2. Why Does the Loss of SIL1 in Humans and Mice Selectively Affect Some Tissues while Sparing Others?
4.3. Linkage of SIL1 with Non-MSS Pathologies
5. Potential Strategies to Treat MSS
5.1. Chemical Chaperones
5.2. Gene Therapy for MSS
5.3. Modulation of UPR Signaling
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ER | Endoplasmic Reticulum |
| ERdjs | ER-resident DnaJ-like Proteins |
| NEF | Nucleotide Exchange Factors |
| MSS | Marinesco-Sjögren Syndrome |
| ERQC | ER Protein Quality Control |
| HSP | Heat Shock Protein |
| UPS | Ubiquitin Proteasomal System |
| ERAD | ER-associated Degradation |
| CNX | Calnexin |
| CRT | Calreticulin |
| Ig | Immunoglobulin |
| UPR | Unfolded Protein Response |
| ATP | Adenosine Triphosphate |
| NBD | Nucleotide-binding Domain |
| SBD | Substrate-binding Domain |
| SRP | Signal Recognition Particle |
| Sil1 | Suppressor of Ire1 and Lhs1 deletion 1 |
| BAP | BiP-associated Protein |
| ARM | Armadillo-like Repeats |
| PBMCs | Peripheral Blood Mononuclear Cells |
| AARS | Alanyl tRNA Synthetase |
| IR | Insulin Receptor |
| IGF1R | Insulin-like Growth Factor Receptor |
| MIGIRKO | Muscle-Specific Dual Knockout of IR And IGF1R |
| NMJ | Neuromuscular Junctions |
| LBLs | Lymphoblastoid Lines |
| i.p.GTT | Intraperitoneal Glucose Tolerance Test |
| i.p.ITT | Intraperitoneal Insulin Tolerance Test |
| ALS | Amyotrophic Lateral Sclerosis |
| PHGDH | Phosphoglycerate dehydrogenase |
| ATXN10 | Ataxin-10 |
| PBA | 4-Phenylbutyrate |
| TUDCA | Tauroursodeoxycholic Acid |
| SCA10 | Spinocerebellar Ataxia Type 10 |
| RIPK1 | Receptor-interacting Serine/Threonine-Protein Kinase 1 |
References
- Dancourt, J.; Barlowe, C. Protein sorting receptors in the early secretory pathway. Annu. Rev. Biochem. 2010, 79, 777–802. [Google Scholar] [CrossRef]
- Braakman, I.; Bulleid, N.J. Protein Folding and Modification in the Mammalian Endoplasmic Reticulum. Annu. Rev. Biochem. 2011, 80, 71–99. [Google Scholar] [CrossRef]
- Pobre, K.F.R.; Poet, G.J.; Hendershot, L.M. The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends. J. Biol. Chem. 2019, 294, 2098–2108. [Google Scholar] [CrossRef]
- Lamriben, L.; Graham, J.B.; Adams, B.M.; Hebert, D.N. N-Glycan-based ER Molecular Chaperone and Protein Quality Control System: The Calnexin Binding Cycle. Traffic 2016, 17, 308–326. [Google Scholar] [CrossRef]
- Schiene-Fischer, C. Multidomain Peptidyl Prolyl cis/trans Isomerases. Biochim. Biophys. Acta 2015, 1850, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Jansen, G.; Määttänen, P.; Denisov, A.Y.; Scarffe, L.; Schade, B.; Balghi, H.; Dejgaard, K.; Chen, L.Y.; Muller, W.J.; Gehring, K.; et al. An interaction map of endoplasmic reticulum chaperones and foldases. Mol. Cell. Proteom. MCP 2012, 11, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Bulleid, N.J. Disulfide bond formation in the mammalian endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Braakman, I.; Hebert, D.N. Protein folding in the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 2013, 5, a013201. [Google Scholar] [CrossRef]
- Guerriero, C.J.; Brodsky, J.L. The delicate balance between secreted protein folding and endoplasmic reticulum-associated degradation in human physiology. Physiol. Rev. 2012, 92, 537–576. [Google Scholar] [CrossRef] [PubMed]
- Fregno, I.; Molinari, M. Proteasomal and lysosomal clearance of faulty secretory proteins: ER-associated degradation (ERAD) and ER-to-lysosome-associated degradation (ERLAD) pathways. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 153–163. [Google Scholar] [CrossRef]
- Nakatsukasa, K.; Kamura, T.; Brodsky, J.L. Recent technical developments in the study of ER-associated degradation. Curr. Opin. Cell Biol. 2014, 29, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Rapoport, T.A. Mechanistic insights into ER-associated protein degradation. Curr. Opin. Cell Biol. 2018, 53, 22–28. [Google Scholar] [CrossRef]
- Ishida, Y.; Yamamoto, A.; Kitamura, A.; Lamandé, S.R.; Yoshimori, T.; Bateman, J.F.; Kubota, H.; Nagata, K. Autophagic Elimination of Misfolded Procollagen Aggregates in the Endoplasmic Reticulum as a Means of Cell Protection. Mol. Biol. Cell 2009, 20, 2744–2754. [Google Scholar] [CrossRef]
- Kruse, K.B.; Brodsky, J.L.; McCracken, A.A. Characterization of an ERAD Gene as VPS30/ATG6 Reveals Two Alternative and Functionally Distinct Protein Quality Control Pathways: One for Soluble Z Variant of Human α-1 Proteinase Inhibitor (A1PiZ) and Another for Aggregates of A1PiZ. Mol. Biol. Cell 2006, 17, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S. Emerging Principles of Selective ER Autophagy. J. Mol. Biol. 2020, 432, 185–205. [Google Scholar] [CrossRef]
- Grumati, P.; Dikic, I.; Stolz, A. ER-phagy at a glance. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef]
- Munro, S.; Pelham, H.R. An Hsp70-like protein in the ER: Identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell 1986, 46, 291–300. [Google Scholar] [CrossRef]
- Haas, I.G.; Wabl, M. Immunoglobulin heavy chain binding protein. Nature 1983, 306, 387–389. [Google Scholar] [CrossRef]
- Morris, J.A.; Dorner, A.J.; Edwards, C.A.; Hendershot, L.M.; Kaufman, R.J. Immunoglobulin binding protein (BiP) function is required to protect cells from endoplasmic reticulum stress but is not required for the secretion of selective proteins. J. Biol. Chem. 1997, 272, 4327–4334. [Google Scholar] [CrossRef]
- Hendershot, L.M.; Ting, J.; Lee, A.S. Identity of the immunoglobulin heavy-chain-binding protein with the 78,000-dalton glucose-regulated protein and the role of posttranslational modifications in its binding function. Mol. Cell. Biol. 1988, 8, 4250–4256. [Google Scholar] [CrossRef]
- Ng, D.T.; Hiebert, S.W.; Lamb, R.A. Different roles of individual N-linked oligosaccharide chains in folding, assembly, and transport of the simian virus 5 hemagglutinin-neuraminidase. Mol. Cell. Biol. 1990, 10, 1989–2001. [Google Scholar] [CrossRef] [PubMed]
- Flynn, G.C.; Pohl, J.; Flocco, M.T.; Rothman, J.E. Peptide-binding specificity of the molecular chaperone BiP. Nature 1991, 353, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Blond-Elguindi, S.; Cwirla, S.E.; Dower, W.J.; Lipshutz, R.J.; Sprang, S.R.; Sambrook, J.F.; Gething, M.J. Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell 1993, 75, 717–728. [Google Scholar] [CrossRef]
- Bole, D.G.; Hendershot, L.M.; Kearney, J.F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J. Cell Biol. 1986, 102, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, L.M.; Kearney, J.F. A role for human heavy chain binding protein in the developmental regulation of immunoglobin transport. Mol. Immunol. 1988, 25, 585–595. [Google Scholar] [CrossRef]
- Skowronek, M.H.; Hendershot, L.M.; Haas, I.G. The variable domain of nonassembled Ig light chains determines both their half-life and binding to the chaperone BiP. Proc. Natl. Acad. Sci. USA 1998, 95, 1574–1578. [Google Scholar] [CrossRef]
- Lièvremont, J.-P.; Rizzuto, R.; Hendershot, L.; Meldolesi, J. BiP, a Major Chaperone Protein of the Endoplasmic Reticulum Lumen, Plays a Direct and Important Role in the Storage of the Rapidly Exchanging Pool of Ca2+. J. Biol. Chem. 1997, 272, 30873–30879. [Google Scholar] [CrossRef]
- Mayer, M.P.; Gierasch, L.M. Recent advances in the structural and mechanistic aspects of Hsp70 molecular chaperones. J. Biol. Chem. 2019, 294, 2085–2097. [Google Scholar] [CrossRef]
- Hendershot, L.; Wei, J.; Gaut, J.; Melnick, J.; Aviel, S.; Argon, Y. Inhibition of immunoglobulin folding and secretion by dominant negative BiP ATPase mutants. Proc. Natl. Acad. Sci. USA 1996, 93, 5269–5274. [Google Scholar] [CrossRef]
- Paton, A.W.; Beddoe, T.; Thorpe, C.M.; Whisstock, J.C.; Wilce, M.C.; Rossjohn, J.; Talbot, U.M.; Paton, J.C. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature 2006, 443, 548–552. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Andreasson, C.; Barducci, A.; Cheetham, M.E.; Cyr, D.; Emanuelsson, C.; Genevaux, P.; Gestwicki, J.E.; Goloubinoff, P.; Huerta-Cepas, J.; et al. Function, evolution, and structure of J-domain proteins. Cell Stress Chaperones 2019, 24, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Kityk, R.; Kopp, J.; Mayer, M.P. Molecular Mechanism of J-Domain-Triggered ATP Hydrolysis by Hsp70 Chaperones. Mol. Cell 2018, 69, 227–237.e224. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zong, Y.; Su, J.; Li, H.; Zhu, H.; Columbus, L.; Zhou, L.; Liu, Q. Conformation transitions of the polypeptide-binding pocket support an active substrate release from Hsp70s. Nat. Commun. 2017, 8, 1201. [Google Scholar] [CrossRef]
- Yan, M.; Li, J.; Sha, B. Structural analysis of the Sil1-Bip complex reveals the mechanism for Sil1 to function as a nucleotide-exchange factor. Biochem. J. 2011, 438, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Masso-Welch, P.; Di, Y.P.; Cai, J.W.; Shen, J.W.; Subjeck, J.R. The 170-kDa glucose-regulated stress protein is an endoplasmic reticulum protein that binds immunoglobulin. Mol. Biol. Cell 1993, 4, 1109–1119. [Google Scholar] [CrossRef]
- Park, J.; Easton, D.P.; Chen, X.; MacDonald, I.J.; Wang, X.Y.; Subjeck, J.R. The chaperoning properties of mouse grp170, a member of the third family of hsp70 related proteins. Biochemistry 2003, 42, 14893–14902. [Google Scholar] [CrossRef]
- Dierks, T.; Volkmer, J.; Schlenstedt, G.; Jung, C.; Sandholzer, U.; Zachmann, K.; Schlotterhose, P.; Neifer, K.; Schmidt, B.; Zimmermann, R. A microsomal ATP-binding protein involved in efficient protein transport into the mammalian endoplasmic reticulum. EMBO J. 1996, 15, 6931–6942. [Google Scholar] [CrossRef]
- Craven, R.A.; Egerton, M.; Stirling, C.J. A novel Hsp70 of the yeast ER lumen is required for the efficient translocation of a number of protein precursors. EMBO J. 1996, 15, 2640–2650. [Google Scholar] [CrossRef]
- Saris, N.; Holkeri, H.; Craven, R.A.; Stirling, C.J.; Makarow, M. The Hsp70 homologue Lhs1p is involved in a novel function of the yeast endoplasmic reticulum, refolding and stabilization of heat-denatured protein aggregates. J. Cell Biol. 1997, 137, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Hanninen, A.L.; Simola, M.; Saris, N.; Makarow, M. The cytoplasmic chaperone hsp104 is required for conformational repair of heat-denatured proteins in the yeast endoplasmic reticulum. Mol. Biol. Cell 1999, 10, 3623–3632. [Google Scholar] [CrossRef]
- Boisramé, A.; Beckerich, J.-M.; Gaillardin, C. Sls1p, an Endoplasmic Reticulum Component, Is Involved in the Protein Translocation Process in the Yeast Yarrowia lipolytica. J. Biol. Chem. 1996, 271, 11668–11675. [Google Scholar] [CrossRef]
- Kabani, M.; Beckerich, J.M.; Gaillardin, C. Sls1p stimulates Sec63p-mediated activation of Kar2p in a conformation-dependent manner in the yeast endoplasmic reticulum. Mol. Cell. Biol. 2000, 20, 6923–6934. [Google Scholar] [CrossRef] [PubMed]
- Boisramé, A.; Kabani, M.; Beckerich, J.-M.; Hartmann, E.; Gaillardin, C. Interaction of Kar2p and Sls1p Is Required for Efficient Co-translational Translocation of Secreted Proteins in the YeastYarrowia lipolytica. J. Biol. Chem. 1998, 273, 30903–30908. [Google Scholar] [CrossRef] [PubMed]
- Tyson, J.R.; Stirling, C.J. LHS1 and SIL1 provide a lumenal function that is essential for protein translocation into the endoplasmic reticulum. EMBO J. 2000, 19, 6440–6452. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.T.; Shen, Y.; Hendershot, L.M. BAP, a Mammalian BiP-associated Protein, Is a Nucleotide Exchange Factor That Regulates the ATPase Activity of BiP. J. Biol. Chem. 2002, 277, 47557–47563. [Google Scholar] [CrossRef]
- Hale, S.J.; Lovell, S.C.; de Keyzer, J.; Stirling, C.J. Interactions between Kar2p and its nucleotide exchange factors Sil1p and Lhs1p are mechanistically distinct. J. Biol. Chem. 2010, 285, 21600–21606. [Google Scholar] [CrossRef]
- Senderek, J.; Krieger, M.; Stendel, C.; Bergmann, C.; Moser, M.; Breitbach-Faller, N.; Rudnik-Schoneborn, S.; Blaschek, A.; Wolf, N.I.; Harting, I.; et al. Mutations in SIL1 cause Marinesco-Sjogren syndrome, a cerebellar ataxia with cataract and myopathy. Nat. Genet. 2005, 37, 1312–1314. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Uhlen, M.; Oksvold, P.; Fagerberg, L.; Lundberg, E.; Jonasson, K.; Forsberg, M.; Zwahlen, M.; Kampf, C.; Wester, K.; Hober, S.; et al. Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol. 2010, 28, 1248–1250. [Google Scholar] [CrossRef]
- Berglund, L.; Björling, E.; Oksvold, P.; Fagerberg, L.; Asplund, A.; Szigyarto, C.A.; Persson, A.; Ottosson, J.; Wernérus, H.; Nilsson, P.; et al. A genecentric Human Protein Atlas for expression profiles based on antibodies. Mol. Cell. Proteom. MCP 2008, 7, 2019–2027. [Google Scholar] [CrossRef]
- Atlas, H.P. Available online: https://www.proteinatlas.org/ENSG00000120725-SIL1 (accessed on 29 November 2020).
- Monaco, G.; Lee, B.; Xu, W.; Mustafah, S.; Hwang, Y.Y.; Carré, C.; Burdin, N.; Visan, L.; Ceccarelli, M.; Poidinger, M.; et al. RNA-Seq Signatures Normalized by mRNA Abundance Allow Absolute Deconvolution of Human Immune Cell Types. Cell Rep. 2019, 26, 1627–1640.e1627. [Google Scholar] [CrossRef]
- Adachi, Y.; Yamamoto, K.; Okada, T.; Yoshida, H.; Harada, A.; Mori, K. ATF6 Is a Transcription Factor Specializing in the Regulation of Quality Control Proteins in the Endoplasmic Reticulum. Cell Struct. Funct. 2008, 33, 75–89. [Google Scholar] [CrossRef]
- Lee, A.S. The glucose-regulated proteins: Stress induction and clinical applications. Trends Biochem. Sci. 2001, 26, 504–510. [Google Scholar] [CrossRef]
- Anttonen, A.K.; Mahjneh, I.; Hamalainen, R.H.; Lagier-Tourenne, C.; Kopra, O.; Waris, L.; Anttonen, M.; Joensuu, T.; Kalimo, H.; Paetau, A.; et al. The gene disrupted in Marinesco-Sjogren syndrome encodes SIL1, an HSPA5 cochaperone. Nat. Genet. 2005, 37, 1309–1311. [Google Scholar] [CrossRef]
- Siegenthaler, K.D.; Pareja, K.A.; Wang, J.; Sevier, C.S. An unexpected role for the yeast nucleotide exchange factor Sil1 as a reductant acting on the molecular chaperone BiP. Elife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Marinesco, G. Novelle maladie familiale caracterisee pare une cataracte congenitale et un arret du development somato-neurophysique. L’Encephale 1931, 26, 97–109. [Google Scholar]
- Sjogren, T. Hereditary congenital spinocerebellar ataxia accompanied by congenital cataract and oligophrenia; a genetic and clinical investigation. Confin. Neurol. 1950, 10, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Alter, M.; Talbert, O.R.; Croffead, G. Cerebellar ataxia, congenital cataracts, and retarded somatic and mental maturation. Report of cases of Marinesco-Sjogren syndrome. Neurology 1962, 12, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Krieger, M.; Roos, A.; Stendel, C.; Claeys, K.G.; Sonmez, F.M.; Baudis, M.; Bauer, P.; Bornemann, A.; de Goede, C.; Dufke, A.; et al. SIL1 mutations and clinical spectrum in patients with Marinesco-Sjogren syndrome. Brain 2013, 136, 3634–3644. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Imai, K.; Yoshida, K.; Okuno, Y.; Muramatsu, H.; Shiraishi, Y.; Chiba, K.; Tanaka, H.; Miyano, S.; Kojima, S.; et al. Whole-exome sequence analysis of ataxia telangiectasia-like phenotype. J. Neurol. Sci. 2014, 340, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Superneau, D.W.; Wertelecki, W.; Zellweger, H.; Bastian, F. Myopathy in Marinesco-Sjogren syndrome. Eur. Neurol. 1987, 26, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Suga, K.; Tsugawa, S.; Sakuma, K.; Tachi, N.; Chiba, S.; Imamura, S. Muscle pathology in Marinesco-Sjogren syndrome: A unique ultrastructural feature. Brain Dev. 1996, 18, 64–67. [Google Scholar] [CrossRef]
- Sewry, C.A.; Voit, T.; Dubowitz, V. Myopathy with unique ultrastructural feature in Marinesco-Sjogren syndrome. Ann. Neurol. 1988, 24, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Komiyama, A.; Tanabe, Y.; Katafuchi, Y.; Ohtaki, E.; Nonaka, I. Myopathy in Marinesco-Sjogren syndrome: An ultrastructural study. Acta Neuropathol. 1990, 80, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Mahjneh, I.; Anttonen, A.K.; Somer, M.; Paetau, A.; Lehesjoki, A.E.; Somer, H.; Udd, B. Myopathy is a prominent feature in Marinesco-Sjogren syndrome: A muscle computed tomography study. J. Neurol. 2006, 253, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Torbergsen, T.; Stalberg, E.; Aasly, J.; Lindal, S. Myopathy in Marinesco-Sjogren syndrome: An electrophysiological study. Acta Neurol. Scand. 1991, 84, 132–138. [Google Scholar] [CrossRef]
- Phan, V.; Cox, D.; Cipriani, S.; Spendiff, S.; Buchkremer, S.; O’Connor, E.; Horvath, R.; Goebel, H.H.; Hathazi, D.; Lochmüller, H.; et al. SIL1 deficiency causes degenerative changes of peripheral nerves and neuromuscular junctions in fish, mice and human. Neurobiol. Dis. 2019, 124, 218–229. [Google Scholar] [CrossRef]
- Lagier-Tourenne, C.; Tranebaerg, L.; Chaigne, D.; Gribaa, M.; Dollfus, H.; Silvestri, G.; Betard, C.; Warter, J.M.; Koenig, M. Homozygosity mapping of Marinesco-Sjogren syndrome to 5q31. Eur. J. Hum. Genet. Ejhg 2003, 11, 770–778. [Google Scholar] [CrossRef]
- Anttonen, A.K.; Siintola, E.; Tranebjaerg, L.; Iwata, N.K.; Bijlsma, E.K.; Meguro, H.; Ichikawa, Y.; Goto, J.; Kopra, O.; Lehesjoki, A.E. Novel SIL1 mutations and exclusion of functional candidate genes in Marinesco-Sjogren syndrome. Eur. J. Hum. Genet. Ejhg 2008, 16, 961–969. [Google Scholar] [CrossRef][Green Version]
- Howes, J.; Shimizu, Y.; Feige, M.J.; Hendershot, L.M. C-terminal mutations destabilize SIL1/BAP and can cause Marinesco-Sjogren syndrome. J. Biol. Chem. 2012, 287, 8552–8560. [Google Scholar] [CrossRef]
- Ichhaporia, V.P. The Role of BiP Co-chaperone SIL1 in Marinesco-Sjögren Syndrome Pathogenesis. Theses Dissertations (ETD) 2018, 469. [Google Scholar] [CrossRef]
- Inaguma, Y.; Hamada, N.; Tabata, H.; Iwamoto, I.; Mizuno, M.; Nishimura, Y.V.; Ito, H.; Morishita, R.; Suzuki, M.; Ohno, K.; et al. SIL1, a causative cochaperone gene of Marinesco-Sojgren syndrome, plays an essential role in establishing the architecture of the developing cerebral cortex. EMBO Mol. Med. 2014, 6, 414–429. [Google Scholar] [CrossRef] [PubMed]
- Mimura, N.; Yuasa, S.; Soma, M.; Jin, H.; Kimura, K.; Goto, S.; Koseki, H.; Aoe, T. Altered Quality Control in the Endoplasmic Reticulum Causes Cortical Dysplasia in Knock-In Mice Expressing a Mutant BiP. Mol. Cell. Biol. 2008, 28, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Ichhaporia, V.P.; Sanford, T.; Howes, J.; Marion, T.N.; Hendershot, L.M.; Gilmore, R. Sil1, a nucleotide exchange factor for BiP, is not required for antibody assembly or secretion. Mol. Biol. Cell 2015, 26, 420–429. [Google Scholar] [CrossRef]
- Zhao, L.; Longo-Guess, C.; Harris, B.S.; Lee, J.W.; Ackerman, S.L. Protein accumulation and neurodegeneration in the woozy mutant mouse is caused by disruption of SIL1, a cochaperone of BiP. Nat. Genet. 2005, 37, 974–979. [Google Scholar] [CrossRef]
- Reinhold, A.; Scheer, I.; Lehmann, R.; Neumann, L.M.; Michael, T.; Varon, R.; Von Moers, A. MR imaging features in Marinesco-Sjogren syndrome: Severe cerebellar atrophy is not an obligatory finding. Ajnr. Am. J. Neuroradiol. 2003, 24, 825–828. [Google Scholar]
- Kitao, Y.; Hashimoto, K.; Matsuyama, T.; Iso, H.; Tamatani, T.; Hori, O.; Stern, D.M.; Kano, M.; Ozawa, K.; Ogawa, S. ORP150/HSP12A Regulates Purkinje Cell Survival: A Role for Endoplasmic Reticulum Stress in Cerebellar Development. J. Neurosci. 2004, 24, 1486–1496. [Google Scholar] [CrossRef]
- Weitzmann, A.; Volkmer, J.; Zimmermann, R. The nucleotide exchange factor activity of Grp170 may explain the non-lethal phenotype of loss of Sil1 function in man and mouse. FEBS Lett. 2006, 580, 5237–5240. [Google Scholar] [CrossRef]
- Andreasson, C.; Rampelt, H.; Fiaux, J.; Druffel-Augustin, S.; Bukau, B. The endoplasmic reticulum Grp170 acts as a nucleotide exchange factor of Hsp70 via a mechanism similar to that of the cytosolic Hsp110. J. Biol. Chem. 2010, 285, 12445–12453. [Google Scholar] [CrossRef]
- Behnke, J.; Hendershot, L.M. The large Hsp70 Grp170 binds to unfolded protein substrates in vivo with a regulation distinct from conventional Hsp70s. J. Biol. Chem. 2014, 289, 2899–2907. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Rosales, C.; Seburn, K.; Ron, D.; Ackerman, S.L. Alteration of the unfolded protein response modifies neurodegeneration in a mouse model of Marinesco-Sjogren syndrome. Hum. Mol. Genet. 2010, 19, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Ron, D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 2011, 13, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, A.; Nonaka, I.; Hirayama, K. Muscle pathology in Marinesco-Sjogren syndrome. J. Neurol. Sci. 1989, 89, 103–113. [Google Scholar] [CrossRef]
- Zimmer, C.; Gosztonyi, G.; Cervos-Navarro, J.; von Moers, A.; Schroder, J.M. Neuropathy with lysosomal changes in Marinesco-Sjogren syndrome: Fine structural findings in skeletal muscle and conjunctiva. Neuropediatrics 1992, 23, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Ichhaporia, V.P.; Kim, J.; Kavdia, K.; Vogel, P.; Horner, L.; Frase, S.; Hendershot, L.M. SIL1, the endoplasmic-reticulum-localized BiP co-chaperone, plays a crucial role in maintaining skeletal muscle proteostasis and physiology. Dis. Models Mech. 2018, 11. [Google Scholar] [CrossRef]
- Roos, A.; Buchkremer, S.; Kollipara, L.; Labisch, T.; Gatz, C.; Zitzelsberger, M.; Brauers, E.; Nolte, K.; Schroder, J.M.; Kirschner, J.; et al. Myopathy in Marinesco-Sjogren syndrome links endoplasmic reticulum chaperone dysfunction to nuclear envelope pathology. Acta Neuropathol. 2014, 127, 761–777. [Google Scholar] [CrossRef]
- Masiero, E.; Agatea, L.; Mammucari, C.; Blaauw, B.; Loro, E.; Komatsu, M.; Metzger, D.; Reggiani, C.; Schiaffino, S.; Sandri, M. Autophagy Is Required to Maintain Muscle Mass. Cell Metab. 2009, 10, 507–515. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.T.; Lauritzen, H.P.M.M.; Hirshman, M.F.; Smyth, G.; Goodyear, L.J.; Kahn, C.R. Differential Role of Insulin/IGF-1 Receptor Signaling in Muscle Growth and Glucose Homeostasis. Cell Rep. 2015, 11, 1220–1235. [Google Scholar] [CrossRef]
- O’Neill, B.T.; Lee, K.Y.; Klaus, K.; Softic, S.; Krumpoch, M.T.; Fentz, J.; Stanford, K.I.; Robinson, M.M.; Cai, W.; Kleinridders, A.; et al. Insulin and IGF-1 receptors regulate FoxO-mediated signaling in muscle proteostasis. J. Clin. Investig. 2016, 126, 3433–3446. [Google Scholar] [CrossRef] [PubMed]
- Lecker, S.H.; Jagoe, R.T.; Gilbert, A.; Gomes, M.; Baracos, V.; Bailey, J.; Price, S.R.; Mitch, W.E.; Goldberg, A.L. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004, 18, 39–51. [Google Scholar] [CrossRef]
- Demontis, F.; Piccirillo, R.; Goldberg, A.L.; Perrimon, N. Mechanisms of skeletal muscle aging: Insights from Drosophila and mammalian models. Dis. Models Mech. 2013, 6, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Edström, E.; Altun, M.; Hägglund, M.; Ulfhake, B. Atrogin-1/MAFbx and MuRF1 Are Downregulated in Aging-Related Loss of Skeletal Muscle. J. Gerontol. Ser. A 2006, 61, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Ittner, A.A.; Bertz, J.; Chan, T.Y.; van Eersel, J.; Polly, P.; Ittner, L.M. The nucleotide exchange factor SIL1 is required for glucose-stimulated insulin secretion from mouse pancreatic beta cells in vivo. Diabetologia 2014, 57, 1410–1419. [Google Scholar] [CrossRef]
- Bromberg, M.B.; Junck, L.; Gebarski, S.S.; McLean, M.J.; Gilman, S. The marinesco-sjögren syndrome examined by computed tomography, magnetic resonance, and 18f-2-fluoro-2-deoxy-d-glucose and positron emission tomography. Arch. Neurol. 1990, 47, 1239–1242. [Google Scholar] [CrossRef]
- Sanford, T. The Role of BiP Nucleotide Exchange Factor Sil1 in Immunoglobulin Biosynthesis. Theses Dissertations (ETD) 2012, 255. [Google Scholar] [CrossRef]
- Wang, J.; Takeuchi, T.; Tanaka, S.; Kubo, S.K.; Kayo, T.; Lu, D.; Takata, K.; Koizumi, A.; Izumi, T. A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J. Clin. Investig. 1999, 103, 27–37. [Google Scholar] [CrossRef]
- Vanhove, M.; Usherwood, Y.K.; Hendershot, L.M. Unassembled Ig heavy chains do not cycle from BiP in vivo but require light chains to trigger their release. Immunity 2001, 15, 105–114. [Google Scholar] [CrossRef]
- Kollipara, L.; Buchkremer, S.; Coraspe, J.A.G.; Hathazi, D.; Senderek, J.; Weis, J.; Zahedi, R.P.; Roos, A. In-depth phenotyping of lymphoblastoid cells suggests selective cellular vulnerability in Marinesco-Sjögren syndrome. Oncotarget 2017, 8, 68493–68516. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ezgu, F.; Krejci, P.; Li, S.; de Sousa, C.; Graham, J.M.; Hansmann, I.; He, W.; Porpora, K.; Wand, D.; Wertelecki, W.; et al. Phenotype-genotype correlations in patients with Marinesco-Sjögren syndrome. Clin. Genet. 2014, 86, 74–84. [Google Scholar] [CrossRef]
- de L’Etang, A.F.; Maharjan, N.; Cordeiro Braña, M.; Ruegsegger, C.; Rehmann, R.; Goswami, A.; Roos, A.; Troost, D.; Schneider, B.L.; Weis, J.; et al. Marinesco-Sjögren syndrome protein SIL1 regulates motor neuron subtype-selective ER stress in ALS. Nat. Neurosci. 2015, 18, 227. [Google Scholar] [CrossRef]
- Yang, J.H.; Wada, A.; Yoshida, K.; Miyoshi, Y.; Sayano, T.; Esaki, K.; Kinoshita, M.O.; Tomonaga, S.; Azuma, N.; Watanabe, M.; et al. Brain-specific Phgdh deletion reveals a pivotal role for L-serine biosynthesis in controlling the level of D-serine, an N-methyl-D-aspartate receptor co-agonist, in adult brain. J. Biol. Chem. 2010, 285, 41380–41390. [Google Scholar] [CrossRef]
- März, P.; Probst, A.; Lang, S.; Schwager, M.; Rose-John, S.; Otten, U.; Ozbek, S. Ataxin-10, the spinocerebellar ataxia type 10 neurodegenerative disorder protein, is essential for survival of cerebellar neurons. J. Biol. Chem. 2004, 279, 35542–35550. [Google Scholar] [CrossRef]
- Wang, J.; Pareja, K.A.; Kaiser, C.A.; Sevier, C.S. Redox signaling via the molecular chaperone BiP protects cells against endoplasmic reticulum-derived oxidative stress. Elife 2014, 3, e03496. [Google Scholar] [CrossRef]
- Wang, J.; Sevier, C.S. Formation and Reversibility of BiP Protein Cysteine Oxidation Facilitate Cell Survival during and post Oxidative Stress. J. Biol. Chem. 2016, 291, 7541–7557. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-C.; Chu, J.; Lin, L.; Song, J.; Ning, L.-N.; Luo, H.-B.; Yang, S.-S.; Shi, Y.; Wang, Q.; Qu, N.; et al. SIL1 Rescued Bip Elevation-Related Tau Hyperphosphorylation in ER Stress. Mol. Neurobiol. 2016, 53, 983–994. [Google Scholar] [CrossRef]
- Labisch, T.; Buchkremer, S.; Phan, V.; Kollipara, L.; Gatz, C.; Lentz, C.; Nolte, K.; Vervoorts, J.; Coraspe, J.A.G.; Sickmann, A.; et al. Tracking Effects of SIL1 Increase: Taking a Closer Look Beyond the Consequences of Elevated Expression Level. Mol. Neurobiol. 2018, 55, 2524–2546. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, S.; Zhang, R.; Xin, T.; Pang, Q. SIL1 functions as an oncogene in glioma by AKT/mTOR signaling pathway. Oncotargets Ther. 2018, 11, 3775–3783. [Google Scholar] [CrossRef]
- Atlas, H.P. SIL1 is Prognostic, High Expression is Unfavourable in Glioma. Available online: https://www.proteinatlas.org/ENSG00000120725-SIL1/pathology/glioma (accessed on 17 September 2020).
- Fujitake, J.; Komatsu, Y.; Hataya, Y.; Nishikawa, A.; Eriguchi, M.; Mizuta, H.; Hayashi, M. A case of Marinesco-Sjogren syndrome: MRI observations of skeletal muscles, bone metabolism, and treatment with testosterone and risedronate. Intern. Med. (Tokyo Jpn.) 2011, 50, 145–149. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kawahara, G.; Hayashi, Y.K. Characterization of Zebrafish Models of Marinesco-Sjögren Syndrome. PloS ONE 2016, 11, e0165563. [Google Scholar] [CrossRef]
- Elia, A.E.; Lalli, S.; Monsurrò, M.R.; Sagnelli, A.; Taiello, A.C.; Reggiori, B.; La Bella, V.; Tedeschi, G.; Albanese, A. Tauroursodeoxycholic acid in the treatment of patients with amyotrophic lateral sclerosis. Eur. J. Neurol. 2016, 23, 45–52. [Google Scholar] [CrossRef]
- Cudkowicz, M.E.; Andres, P.L.; Macdonald, S.A.; Bedlack, R.S.; Choudry, R.; Brown, R.H., Jr.; Zhang, H.; Schoenfeld, D.A.; Shefner, J.; Matson, S.; et al. Phase 2 study of sodium phenylbutyrate in ALS. Amyotroph. Lateral Scler. 2009, 10, 99–106. [Google Scholar] [CrossRef]
- Wiley, J.C.; Pettan-Brewer, C.; Ladiges, W.C. Phenylbutyric acid reduces amyloid plaques and rescues cognitive behavior in AD transgenic mice. Aging Cell 2011, 10, 418–428. [Google Scholar] [CrossRef]
- Nunes, A.F.; Amaral, J.D.; Lo, A.C.; Fonseca, M.B.; Viana, R.J.; Callaerts-Vegh, Z.; D’Hooge, R.; Rodrigues, C.M. TUDCA, a bile acid, attenuates amyloid precursor protein processing and amyloid-β deposition in APP/PS1 mice. Mol. Neurobiol. 2012, 45, 440–454. [Google Scholar] [CrossRef]
- Paganoni, S.; Macklin, E.A.; Hendrix, S.; Berry, J.D.; Elliott, M.A.; Maiser, S.; Karam, C.; Caress, J.B.; Owegi, M.A.; Quick, A.; et al. Trial of Sodium Phenylbutyrate-Taurursodiol for Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2020, 383, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Paganoni, S.; Hendrix, S.; Dickson, S.P.; Knowlton, N.; Macklin, E.A.; Berry, J.D.; Elliott, M.A.; Maiser, S.; Karam, C.; Caress, J.B.; et al. Long-term survival of participants in the CENTAUR trial of sodium phenylbutyrate-taurursodiol in amyotrophic lateral sclerosis. Muscle Nerve 2020, 63, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kashimada, A.; Hasegawa, S.; Isagai, T.; Uchiyama, T.; Matsuo, M.; Kawai, M.; Goto, M.; Morio, T.; Hayashi, Y.K.; Takagi, M. Targeting the enhanced ER stress response in Marinesco-Sjögren syndrome. J. Neurol. Sci. 2018, 385, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.J.; Shi, Y.; Austin, R.C.; Werstuck, G.H. Valproate protects cells from ER stress-induced lipid accumulation and apoptosis by inhibiting glycogen synthase kinase-3. J. Cell Sci. 2005, 118, 89–99. [Google Scholar] [CrossRef]
- Das, I.; Png, C.W.; Oancea, I.; Hasnain, S.Z.; Lourie, R.; Proctor, M.; Eri, R.D.; Sheng, Y.; Crane, D.I.; Florin, T.H.; et al. Glucocorticoids alleviate intestinal ER stress by enhancing protein folding and degradation of misfolded proteins. J. Exp. Med. 2013, 210, 1201–1216. [Google Scholar] [CrossRef]
- Rosa, A.I.; Fonseca, I.; Nunes, M.J.; Moreira, S.; Rodrigues, E.; Carvalho, A.N.; Rodrigues, C.M.P.; Gama, M.J.; Castro-Caldas, M. Novel insights into the antioxidant role of tauroursodeoxycholic acid in experimental models of Parkinson’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2171–2181. [Google Scholar] [CrossRef]
- Tu, B.P.; Weissman, J.S. Oxidative protein folding in eukaryotes: Mechanisms and consequences. J. Cell Biol. 2004, 164, 341–346. [Google Scholar] [CrossRef]
- Gene Therapy: The View from NCATS. Hum. Gene Ther. 2016, 27, 7–13. [CrossRef]
- Malecaze, F.; Lubsen, N.H.; Serre, B.; Decha, A.; Duboue, M.; Penary, M.; Berg, D.; Arnaud, J.D.; Titeux, M.; Kremer, E.J.; et al. Lens cell targetting for gene therapy of prevention of posterior capsule opacification. Gene. Ther. 2006, 13, 1422–1429. [Google Scholar] [CrossRef]
- Nance, M.E.; Hakim, C.H.; Yang, N.N.; Duan, D. Nanotherapy for Duchenne muscular dystrophy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10. [Google Scholar] [CrossRef]
- Kodippili, K.; Hakim, C.H.; Pan, X.; Yang, H.T.; Yue, Y.; Zhang, Y.; Shin, J.H.; Yang, N.N.; Duan, D. Dual AAV Gene Therapy for Duchenne Muscular Dystrophy with a 7-kb Mini-Dystrophin Gene in the Canine Model. Hum. Gene. 2018, 29, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Han, S.O.; Ronzitti, G.; Arnson, B.; Leborgne, C.; Li, S.; Mingozzi, F.; Koeberl, D. Low-Dose Liver-Targeted Gene Therapy for Pompe Disease Enhances Therapeutic Efficacy of ERT via Immune Tolerance Induction. Mol. Ther. Methods Clin. Dev. 2017, 4, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Hwu, W.L.; Muramatsu, S.; Tseng, S.H.; Tzen, K.Y.; Lee, N.C.; Chien, Y.H.; Snyder, R.O.; Byrne, B.J.; Tai, C.H.; Wu, R.M. Gene therapy for aromatic L-amino acid decarboxylase deficiency. Sci. Transl. Med. 2012, 4, 134ra161. [Google Scholar] [CrossRef] [PubMed]
- Shahryari, A.; Saghaeian Jazi, M.; Mohammadi, S.; Razavi Nikoo, H.; Nazari, Z.; Hosseini, E.S.; Burtscher, I.; Mowla, S.J.; Lickert, H. Development and Clinical Translation of Approved Gene Therapy Products for Genetic Disorders. Front. Genet. 2019, 10, 868. [Google Scholar] [CrossRef]
- Kumar, S.R.; Markusic, D.M.; Biswas, M.; High, K.A.; Herzog, R.W. Clinical development of gene therapy: Results and lessons from recent successes. Mol. Ther. Methods Clin. Dev. 2016, 3, 16034. [Google Scholar] [CrossRef]
- Grande, V.; Ornaghi, F.; Comerio, L.; Restelli, E.; Masone, A.; Corbelli, A.; Tolomeo, D.; Capone, V.; Axten, J.M.; Laping, N.J.; et al. PERK inhibition delays neurodegeneration and improves motor function in a mouse model of Marinesco-Sjögren syndrome. Hum. Mol. Genet. 2018, 27, 2477–2489. [Google Scholar] [CrossRef]
- Das, I.; Krzyzosiak, A.; Schneider, K.; Wrabetz, L.; D’Antonio, M.; Barry, N.; Sigurdardottir, A.; Bertolotti, A. Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science 2015, 348, 239–242. [Google Scholar] [CrossRef]
- Krzyzosiak, A.; Sigurdardottir, A.; Luh, L.; Carrara, M.; Das, I.; Schneider, K.; Bertolotti, A. Target-Based Discovery of an Inhibitor of the Regulatory Phosphatase PPP1R15B. Cell 2018. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, H.; Li, L.; Liu, H.; Shi, W.; Yuan, X.; Wu, L. Structural Insights into IRE1 Functions in the Unfolded Protein Response. Curr. Med. Chem. 2016, 23, 4706–4716. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, C.M.; Garri, C.; Cain, E.L.; Ang, K.K.; Wilson, C.G.; Chen, S.; Hearn, B.R.; Jaishankar, P.; Aranda-Diaz, A.; Arkin, M.R.; et al. Ceapins are a new class of unfolded protein response inhibitors, selectively targeting the ATF6α branch. Elife 2016, 5. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichhaporia, V.P.; Hendershot, L.M. Role of the HSP70 Co-Chaperone SIL1 in Health and Disease. Int. J. Mol. Sci. 2021, 22, 1564. https://doi.org/10.3390/ijms22041564
Ichhaporia VP, Hendershot LM. Role of the HSP70 Co-Chaperone SIL1 in Health and Disease. International Journal of Molecular Sciences. 2021; 22(4):1564. https://doi.org/10.3390/ijms22041564
Chicago/Turabian StyleIchhaporia, Viraj P., and Linda M. Hendershot. 2021. "Role of the HSP70 Co-Chaperone SIL1 in Health and Disease" International Journal of Molecular Sciences 22, no. 4: 1564. https://doi.org/10.3390/ijms22041564
APA StyleIchhaporia, V. P., & Hendershot, L. M. (2021). Role of the HSP70 Co-Chaperone SIL1 in Health and Disease. International Journal of Molecular Sciences, 22(4), 1564. https://doi.org/10.3390/ijms22041564





