Neuroprotective Effects of Testosterone in the Hypothalamus of an Animal Model of Metabolic Syndrome
Abstract
:1. Introduction
2. Results
2.1. MetS Phenotype Induced by HFD and Effects of TTh
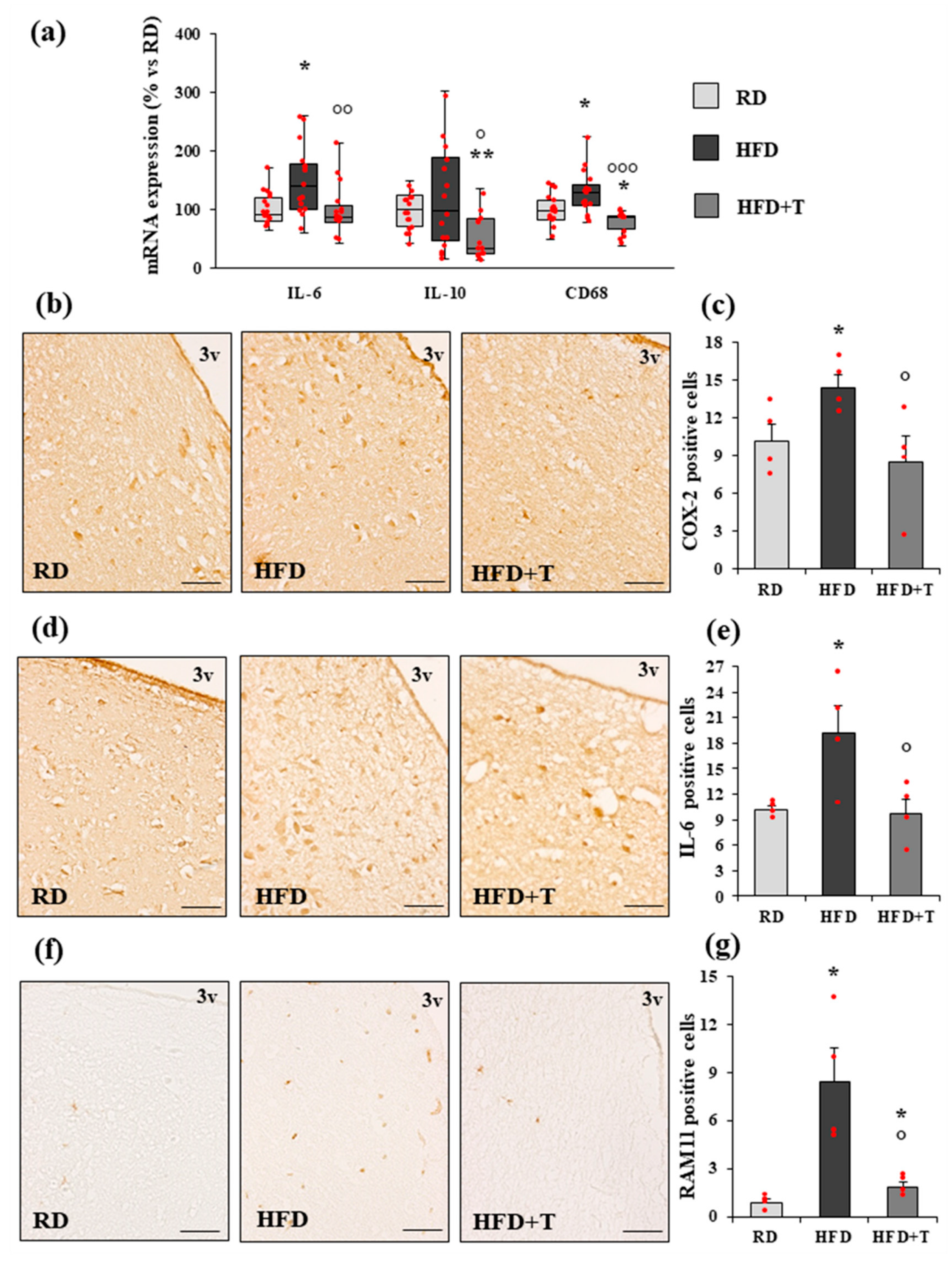
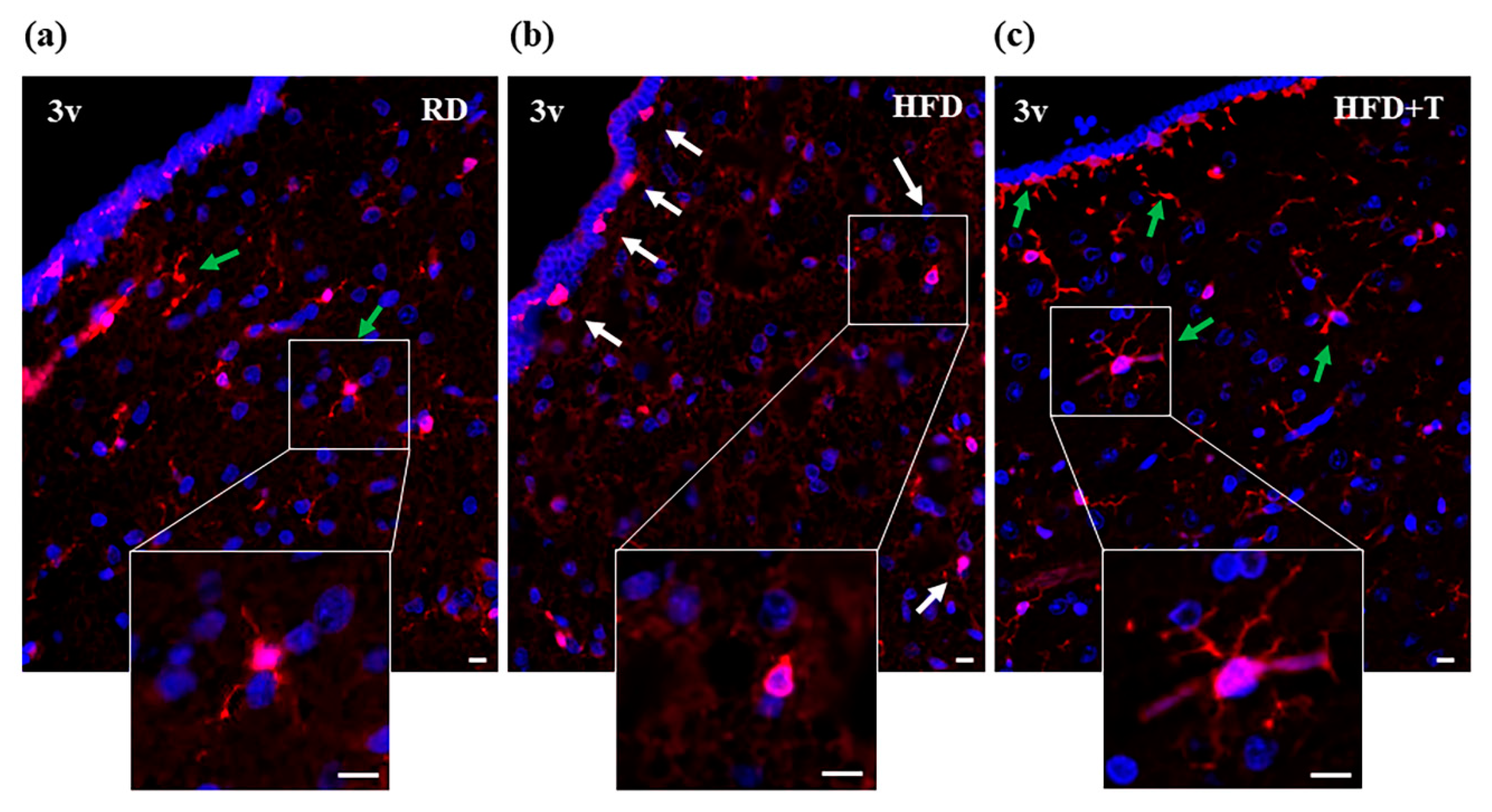
2.2. HFD-Induced Hypothalamic Inflammation and Effects of TTh
2.3. Gene Expression Analysis of Estrogen Receptors, Glucose Metabolism Regulators, and GnRH-Related Markers
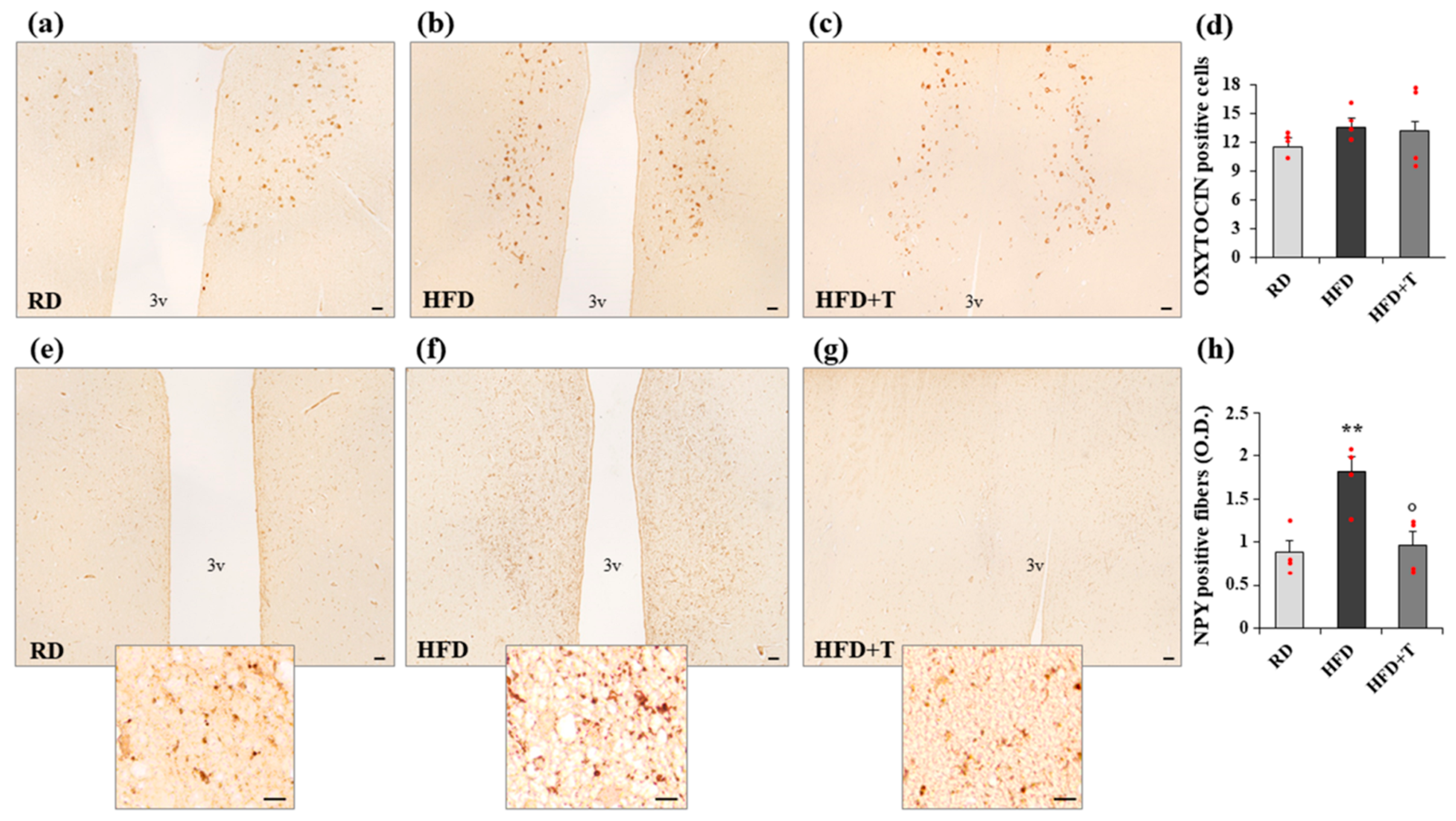
2.4. Modulation of Markers of Neurogenic/Neurodifferentiation and of the Melanocortin System Induced by HFD and TTh
3. Discussion
4. Materials and Methods
4.1. Animal Treatments
4.2. Measurement of Cholesterol, Triglycerides, Glycemia, T, and LH in Rabbit Serum
4.3. Oral Glucose Tolerance Test
4.4. RNA Extraction and Quantitative RT-PCR Analysis
4.5. Immunohistochemistry and Immunofluorescence
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AR | Androgen receptor |
| BBB | Blood–brain barrier |
| CD68 | Cluster of Differentiation 68 |
| ER | Estrogen receptor |
| GLUT | Glucose transporter |
| GnRH | Gonadotropin-releasing hormone |
| GPR30 | G protein-coupled Receptor 30 |
| HFD | High-fat diet |
| HPG | Hypothalamic–pituitary–gonadal |
| IBA1 | Ionized calcium binding adaptor molecule 1 |
| IL | Interleukin |
| IRS-1 | Insulin receptor substrate 1 |
| MC3R | Melanocortin receptor 3 |
| MC4R | Melanocortin receptor 4 |
| MetS | Metabolic syndrome |
| PVN | Paraventricular nucleus |
| T | Testosterone |
| TNF-α | Tumor necrosis factor α |
| TTh | Testosterone treatment |
References
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nat. Cell Biol. 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Jais, A.; Brüning, J.C. Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Investig. 2017, 127, 24–32. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.T.; Araujo, E.P.; Bordin, S.; Ashimine, R.; Zollner, R.L.; Boschero, A.C.; Saad, M.J.A.; Velloso, L.A. Consumption of a Fat-Rich Diet Activates a Proinflammatory Response and Induces Insulin Resistance in the Hypothalamus. Endocrinology 2005, 146, 4192–4199. [Google Scholar] [CrossRef] [Green Version]
- Thaler, J.P.; Yi, C.-X.; Schur, E.A.; Guyenet, S.J.; Hwang, B.H.; Dietrich, M.O.; Zhao, X.; Sarruf, D.A.; Izgur, V.; Maravilla, K.R.; et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Investig. 2012, 122, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Holland, W.L.; Bikman, B.T.; Wang, L.-P.; Yuguang, G.; Sargent, K.M.; Bulchand, S.; Knotts, T.A.; Shui, G.; Clegg, D.J.; Wenk, M.R.; et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid–induced ceramide biosynthesis in mice. J. Clin. Investig. 2011, 121, 1858–1870. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, G.; Zhang, H.; Karin, M.; Bai, H.; Cai, D. Hypothalamic IKKβ/NF-κB and ER Stress Link Overnutrition to Energy Imbalance and Obesity. Cell 2008, 135, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Morelli, A.; Sarchielli, E.; Comeglio, P.; Filippi, S.; Vignozzi, L.; Marini, M.; Rastrelli, G.; Maneschi, E.; Cellai, I.; Persani, L.; et al. Metabolic syndrome induces inflammation and impairs gonadotropin-releasing hormone neurons in the preoptic area of the hypothalamus in rabbits. Mol. Cell. Endocrinol. 2014, 382, 107–119. [Google Scholar] [CrossRef]
- Baufeld, C.; Osterloh, A.; Prokop, S.; Miller, K.R.; Heppner, F.L. High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol. 2016, 132, 361–375. [Google Scholar] [CrossRef] [Green Version]
- Puig, J.; Blasco, G.; Daunis-I-Estadella, J.; Molina, X.; Xifra, G.; Ricart, W.; Pedraza, S.; Fernández-Aranda, F.; Fernández-Real, J.M. Hypothalamic Damage Is Associated With Inflammatory Markers and Worse Cognitive Performance in Obese Subjects. J. Clin. Endocrinol. Metab. 2015, 100, E276–E281. [Google Scholar] [CrossRef] [Green Version]
- Cai, D.; Khor, S. “Hypothalamic Microinflammation” Paradigm in Aging and Metabolic Diseases. Cell. Metab. 2019, 30, 19–35. [Google Scholar] [CrossRef]
- Leisegang, K.; Henkel, R.; Agarwal, A.K. Obesity and metabolic syndrome associated with systemic inflammation and the impact on the male reproductive system. Am. J. Reprod. Immunol. 2019, 82, e13178. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Cai, D.; Yuan, M.; Frantz, D.F.; Melendez, P.A.; Hansen, L.; Lee, J.; Shoelson, S.E. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 2005, 11, 183–190. [Google Scholar] [CrossRef]
- Vignozzi, L.; Filippi, S.; Comeglio, P.; Cellai, I.; Sarchielli, E.; Morelli, A.; Rastrelli, G.; Maneschi, E.; Galli, A.; Vannelli, G.B.; et al. Nonalcoholic steatohepatitis as a novel player in metabolic syndrome-induced erectile dysfunction: An experimental study in the rabbit. Mol. Cell. Endocrinol. 2014, 384, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Itani, S.I.; Ruderman, N.B.; Schmieder, F.; Boden, G. Lipid-Induced Insulin Resistance in Human Muscle Is Associated With Changes in Diacylglycerol, Protein Kinase, C., and IκB-α. Diabetes 2002, 51, 2005–2011. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, G.K.; Yu, J.G.; Ofrecio, J.; Olefsky, J.M. Increased p85/55/50 Expression and Decreased Phosphotidylinositol 3-Kinase Activity in Insulin-Resistant Human Skeletal Muscle. Diabetes 2005, 54, 2351–2359. [Google Scholar] [CrossRef] [Green Version]
- Ehses, J.A.; Böni-Schnetzler, M.; Faulenbach, M.; Donath, M.Y. Macrophages, cytokines and β-cell death in Type 2 diabetes. Biochem. Soc. Trans. 2008, 36, 340–342. [Google Scholar] [CrossRef] [Green Version]
- Donath, M.Y.; Boni-Schnetzler, M.; Ellingsgaard, H.; Halban, P.A.; Ehses, J.A. Cytokine production by islets in health and diabetes: Cellular origin, regulation and function. Trends Endocrinol. Metab. 2010, 21, 261–267. [Google Scholar] [CrossRef]
- Prada, P.O.; Zecchin, H.G.; Gasparetti, A.L.; Torsoni, M.A.; Ueno, M.; Hirata, A.E.; Amaral, M.E.C.D.; Höer, N.F.; Boschero, A.C.; Saad, M.J.A. Western Diet Modulates Insulin Signaling, c-Jun N-Terminal Kinase Activity, and Insulin Receptor Substrate-1ser307 Phosphorylation in a Tissue-Specific Fashion. Endocrinology 2005, 146, 1576–1587. [Google Scholar] [CrossRef] [Green Version]
- Waise, T.M.Z.; Toshinai, K.; Naznin, F.; Namkoong, C.; Moin, A.S.M.; Sakoda, H.; Nakazato, M. One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice. Biochem. Biophys. Res. Commun. 2015, 464, 1157–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enriori, P.J.; Evans, A.E.; Sinnayah, P.; Jobst, E.E.; Tonelli-Lemos, L.; Billes, S.K.; Glavas, M.M.; Grayson, B.E.; Perello, M.; Nillni, E.A.; et al. Diet-Induced Obesity Causes Severe but Reversible Leptin Resistance in Arcuate Melanocortin Neurons. Cell Metab. 2007, 5, 181–194. [Google Scholar] [CrossRef]
- Moraes, J.C.; Coope, A.; Morari, J.; Cintra, D.E.; Roman, E.A.; Pauli, J.R.; Romanatto, T.; Carvalheira, J.B.; Oliveira, A.L.R.; Saad, M.J.; et al. High-Fat Diet Induces Apoptosis of Hypothalamic Neurons. PLoS ONE 2009, 4, e5045. [Google Scholar] [CrossRef]
- Pace, S.; Werz, O. Impact of Androgens on Inflammation-Related Lipid Mediator Biosynthesis in Innate Immune Cells. Front. Immunol. 2020, 11, 1356. [Google Scholar] [CrossRef]
- Winter, A.G.; Zhao, F.; Lee, R.K. Androgen deficiency and metabolic syndrome in men. Transl. Androl. Urol. 2014, 3, 50–58. [Google Scholar]
- Filippi, S.; Vignozzi, L.; Morelli, A.; Chavalmane, A.K.; Sarchielli, E.; Fibbi, B.; Saad, F.; Sandner, P.; Ruggiano, P.; Vannelli, G.B.; et al. Testosterone Partially Ameliorates Metabolic Profile and Erectile Responsiveness to PDE5 Inhibitors in an Animal Model of Male Metabolic Syndrome. J. Sex. Med. 2009, 6, 3274–3288. [Google Scholar] [CrossRef]
- Morelli, A.; Vignozzi, L.; Maggi, M. Hypogonadotropic hypogonadism and metabolic syndrome: Insights from the high-fat diet experimental rabbit animal model. Minerva Endocrinol. 2016, 41, 240–249. [Google Scholar]
- Maneschi, E.; Morelli, A.; Filippi, S.; Cellai, I.; Comeglio, P.; Mazzanti, B.; Mello, T.; Calcagno, A.; Sarchielli, E.; Vignozzi, L.; et al. Testosterone treatment improves metabolic syndrome-induced adipose tissue derangements. J. Endocrinol. 2012, 215, 347–362. [Google Scholar] [CrossRef] [Green Version]
- Sarchielli, E.; Comeglio, P.; Filippi, S.; Cellai, I.; Guarnieri, G.; Guasti, D.; Rapizzi, E.; Rastrelli, G.; Bani, D.; Vannelli, G.B.; et al. Testosterone improves muscle fiber asset and exercise performance in a metabolic syndrome model. J. Endocrinol. 2020, 245, 259–279. [Google Scholar] [CrossRef]
- Vignozzi, L.; Morelli, A.; Sarchielli, E.; Comeglio, P.; Filippi, S.; Cellai, I.; Maneschi, E.; Serni, S.; Gacci, M.; Carini, M.; et al. Testosterone protects from metabolic syndrome-associated prostate inflammation: An experimental study in rabbit. J. Endocrinol. 2012, 212, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Morelli, A.; Comeglio, P.; Filippi, S.; Sarchielli, E.; Cellai, I.; Vignozzi, L.; Yehiely-Cohen, R.; Maneschi, E.; Gacci, M.; Carini, M.; et al. Testosterone and farnesoid X receptor agonist INT-747 counteract high fat diet-induced bladder alterations in a rabbit model of metabolic syndrome. J. Steroid Biochem. Mol. Biol. 2012, 132, 80–92. [Google Scholar] [CrossRef]
- Mignone, J.L.; Kukekov, V.; Chiang, A.-S.; Steindler, D.; Enikolopov, G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J. Comp. Neurol. 2004, 469, 311–324. [Google Scholar] [CrossRef]
- Michaud, J.L.; Rosenquist, T.; May, N.R.; Fan, C.-M. Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev. 1998, 12, 3264–3275. [Google Scholar] [CrossRef] [Green Version]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morelli, A.; Filippi, S.; Comeglio, P.; Sarchielli, E.; Cellai, I.; Pallecchi, M.; Bartolucci, G.; Danza, G.; Rastrelli, G.; Corno, C.; et al. Physical activity counteracts metabolic syndrome-induced hypogonadotropic hypogonadism and erectile dysfunction in the rabbit. Am. J. Physiol. Metab. 2019, 316, E519–E535. [Google Scholar] [CrossRef]
- Thaler, J.P.; Guyenet, S.J.; Dorfman, M.D.; Wisse, B.E.; Schwartz, M.W. Hypothalamic Inflammation: Marker or Mechanism of Obesity Pathogenesis? Diabetes 2013, 62, 2629–2634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillemot-Legris, O.; Muccioli, G.G. Obesity-Induced Neuroinflammation: Beyond the Hypothalamus. Trends Neurosci. 2017, 40, 237–253. [Google Scholar] [CrossRef]
- Vegeto, E.; Villa, A.; Della Torre, S.; Crippa, V.; Rusmini, P.; Cristofani, R.; Galbiati, M.; Maggi, A.; Poletti, A. The Role of Sex and Sex Hormones in Neurodegenerative Diseases. Endocr. Rev. 2020, 41, 273–319. [Google Scholar] [CrossRef]
- Frye, C.; McCormick, C. Androgens Are Neuroprotective in the Dentate Gyrus of Adrenalectomized Female Rats. Stress 2000, 3, 185–194. [Google Scholar] [CrossRef]
- Galea, L.A. Gonadal hormone modulation of neurogenesis in the dentate gyrus of adult male and female rodents. Brain Res. Rev. 2008, 57, 332–341. [Google Scholar] [CrossRef]
- Rosario, E.R.; Pike, C.J. Androgen regulation of β-amyloid protein and the risk of Alzheimer’s disease. Brain Res. Rev. 2008, 57, 444–453. [Google Scholar] [CrossRef] [Green Version]
- Moser, V.A.; Christensen, A.; Liu, J.; Zhou, A.G.; Yagi, S.; Beam, C.R.; Galea, L.A.; Pike, C.J. Effects of aging, high-fat diet, and testosterone treatment on neural and metabolic outcomes in male brown Norway rats. Neurobiol. Aging 2019, 73, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, R.; Tong, Y.; Chen, P.; Shen, Y.; Miao, S.; Liu, X. Neuroprotection by dihydrotestosterone in LPS-induced neuroinflammation. Neurobiol. Dis. 2020, 140, 104814. [Google Scholar] [CrossRef]
- Atallah, A.; Mhaouty-Kodja, S.; Grange-Messent, V. Chronic depletion of gonadal testosterone leads to blood–brain barrier dysfunction and inflammation in male mice. Br. J. Pharmacol. 2016, 37, 3161–3175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, V.M. New Insights into the Control of Pulsatile GnRH Release: The Role of Kiss1/Neurokinin B Neurons. Front. Endocrinol. 2012, 3, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyamugenda, E.; Griffin, H.; Russell, S.; Cooney, K.A.; Kowalczyk, N.S.; Islam, I.; Phelan, K.D.; Baldini, G. Selective Survival of Sim1/MC4R Neurons in Diet-Induced Obesity. IScience 2020, 23, 101114. [Google Scholar] [CrossRef]
- Baldini, G.; Phelan, K.D. The melanocortin pathway and control of appetite-progress and therapeutic implications. J. Endocrinol. 2019, 241, R1–R33. [Google Scholar] [CrossRef]
- Xi, D.; Gandhi, N.; Lai, M.; Kublaoui, B.M. Ablation of Sim1 Neurons Causes Obesity through Hyperphagia and Reduced Energy Expenditure. PLoS ONE 2012, 7, e36453. [Google Scholar] [CrossRef]
- Tolson, K.P.; Gemelli, T.; Meyer, D.; Yazdani, U.; Kozlitina, J.; Zinn, A.R. Inducible Neuronal Inactivation of Sim1 in Adult Mice Causes Hyperphagic Obesity. Endocrinology 2014, 155, 2436–2444. [Google Scholar] [CrossRef] [Green Version]
- Kublaoui, B.M.; Holder, J.J.L.; Gemelli, T.; Zinn, A.R. Sim1 Haploinsufficiency Impairs Melanocortin-Mediated Anorexia and Activation of Paraventricular Nucleus Neurons. Mol. Endocrinol. 2006, 20, 2483–2492. [Google Scholar] [CrossRef]
- Ghoussaini, M.; Stutzmann, F.; Couturier, C.; Vatin, V.; Durand, E.; Lecoeur, C.; Degraeve, F.; Heude, B.; Tauber, M.; Hercberg, S.; et al. Analysis of the SIM1 Contribution to Polygenic Obesity in the French Population. Obesity 2010, 18, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- El Khattabi, L.; Guimiot, F.; Pipiras, E.; Andrieux, J.; Baumann, C.; Bouquillon, S.; Delezoide, A.-L.; Delobel, B.; Demurger, F.; Dessuant, H.; et al. Incomplete penetrance and phenotypic variability of 6q16 deletions including SIM1. Eur. J. Hum. Genet. 2014, 23, 1010–1018. [Google Scholar] [CrossRef] [Green Version]
- Nyamugenda, E.; Trentzsch, M.; Russell, S.; Miles, T.; Boysen, G.; Phelan, K.D.; Baldini, G. Injury to hypothalamic Sim1 neurons is a common feature of obesity by exposure to high-fat diet in male and female mice. J. Neurochem. 2019, 149, 73–97. [Google Scholar] [CrossRef] [PubMed]
- Wahab, F.; Khan, I.U.; Polo, I.R.; Zubair, H.; Drummer, C.; Shahab, M.; Behr, R. Irisin in the primate hypothalamus and its effect on GnRH in vitro. J. Endocrinol. 2019, 241, 175–187. [Google Scholar] [CrossRef]
- Hashemi, M.-S.; Ghaedi, K.; Salamian, A.; Karbalaie, K.; Emadi-Baygi, M.; Tanhaei, S.; Nasr-Esfahani, M.; Baharvand, H. Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience 2013, 231, 296–304. [Google Scholar] [CrossRef]
- Piya, M.K.; Harte, A.L.; Sivakumar, K.; Tripathi, G.; Voyias, P.D.; James, S.; Sabico, S.; Al-Daghri, N.M.; Saravanan, P.; Barber, T.M.; et al. The identification of irisin in human cerebrospinal fluid: Influence of adiposity, metabolic markers, and gestational diabetes. Am. J. Physiol. Metab. 2014, 306, E512–E518. [Google Scholar] [CrossRef]
- Ferrante, C.; Orlando, G.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; Vacca, M.; et al. Central inhibitory effects on feeding induced by the adipo-myokine irisin. Eur. J. Pharmacol. 2016, 791, 389–394. [Google Scholar] [CrossRef]
- Wahab, F.; Shahab, M.; Behr, R. Hypothesis: Irisin is a metabolic trigger for the activation of the neurohormonal axis governing puberty onset. Med. Hypotheses 2016, 95, 1–4. [Google Scholar] [CrossRef]
- Manfredi-Lozano, M.; Roa, J.; Tena-Sempere, M. Connecting metabolism and gonadal function: Novel central neuropeptide pathways involved in the metabolic control of puberty and fertility. Front. Neuroendocr. 2018, 48, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Monami, M.; Rastrelli, G.; Aversa, A.; Tishova, Y.; Saad, F.; Lenzi, A.; Forti, G.; Mannucci, E.; Maggi, M. Testosterone and Metabolic Syndrome: A Meta-Analysis Study. J. Sex. Med. 2011, 8, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.; Hoermann, R.; Wittert, G.; Yeap, B.B. Effects of testosterone treatment on glucose metabolism and symptoms in men with type 2 diabetes and the metabolic syndrome: A systematic review and meta-analysis of randomized controlled clinical trials. Clin. Endocrinol. 2014, 83, 344–351. [Google Scholar] [CrossRef]
- Bhasin, S.; Brito, J.P.; Cunningham, G.R.; Hayes, F.J.; Hodis, H.N.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Wu, F.C.; Yialamas, M.A. Testosterone Therapy in Men With Hypogonadism: An Endocrine Society* Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1715–1744. [Google Scholar] [CrossRef] [Green Version]
- Corona, G.; Rastrelli, G.; Maggi, M. Diagnosis and treatment of late-onset hypogonadism: Systematic review and meta-analysis of TRT outcomes. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Poli, G.; Sarchielli, E.; Guasti, D.; Benvenuti, S.; Ballerini, L.; Mazzanti, B.; Armignacco, R.; Cantini, G.; Lulli, M.; Chortis, V.; et al. Human fetal adrenal cells retain age-related stem- and endocrine-differentiation potential in culture. FASEB J. 2018, 33, 2263–2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| RD (n = 20) | HFD (n = 19) | P HFD vs. RD | HFD + T (n = 18) | P HFD + T vs. RD | P HFD + T vs. HFD | |
|---|---|---|---|---|---|---|
| Total body weight (g) | 3890.5 (3753.5–3988.2) | 3733.0 (3479.7–3861.2) | 0.058 | 3788.0 (3506.5–4174.2) | 0.558 | 0.548 |
| Blood glucose (g/L) | 0.95 (0.82–1.13) | 1.82 (1.59–2.24) | <0.0001 | 1.49 (1.06–1.74) | 0.001 | 0.002 |
| OGTT (iAUC) | 121.1 (98.8–157.8) | 207.0 (187.2–221.9) | <0.0001 | 182.0 (170.2–229.0) | 0.001 | 0.064 |
| Cholesterol (mg/dL) | 48.0 (29.0–49.0) | 1464.0 (872.5–2,370.0) | <0.0001 | 1167.0 (769.5–1317.2) | <0.0001 | 0.095 |
| Triglycerides (mg/dL) | 65.0 (43.5–75.0) | 168.0 (114.0–256.0) | <0.0001 | 137.0 (83.0–344.5) | 0.001 | 0.504 |
| MAP (mmHg) | 85.0 (70.0–98.7) | 150.0 (131.2–165.0) | <0.0001 | 113.1 (106.2–122.7) | <0.0001 | <0.0001 |
| VAT(% of total weight) | 0.95 (0.83–1.10) | 1.11 (0.93–1.22) | 0.011 | 0.13 (0.07–0.25) | <0.0001 | <0.0001 |
| T (nmoles/L) | 6.72 (4.42–11.10) | 0.70 (0.69–1.19) | <0.0001 | 19.75 (9.68–39.40) | 0.001 | <0.0001 |
| LH (ng/mL) | 0.33 (0.12–0.65) | 0.06 (0.05–0.14) | 0.004 | 0.06 (0.04–0.10) | <0.0001 | 0.389 |
| Prostate weight(% of total weight) | 0.016 (0.012–0.021) | 0.009 (0.007–0.013) | 0.001 | 0.027 (0.021–0.029) | <0.0001 | <0.0001 |
| Seminal vesicle weight (% of total weight) | 0.014 (0.011–0.021) | 0.013 (0.011–0.016) | 0.595 | 0.055 (0.043–0.064) | <0.0001 | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarchielli, E.; Comeglio, P.; Filippi, S.; Cellai, I.; Guarnieri, G.; Marzoppi, A.; Cipriani, S.; Vignozzi, L.; Morelli, A.; Maggi, M. Neuroprotective Effects of Testosterone in the Hypothalamus of an Animal Model of Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 1589. https://doi.org/10.3390/ijms22041589
Sarchielli E, Comeglio P, Filippi S, Cellai I, Guarnieri G, Marzoppi A, Cipriani S, Vignozzi L, Morelli A, Maggi M. Neuroprotective Effects of Testosterone in the Hypothalamus of an Animal Model of Metabolic Syndrome. International Journal of Molecular Sciences. 2021; 22(4):1589. https://doi.org/10.3390/ijms22041589
Chicago/Turabian StyleSarchielli, Erica, Paolo Comeglio, Sandra Filippi, Ilaria Cellai, Giulia Guarnieri, Alessandra Marzoppi, Sarah Cipriani, Linda Vignozzi, Annamaria Morelli, and Mario Maggi. 2021. "Neuroprotective Effects of Testosterone in the Hypothalamus of an Animal Model of Metabolic Syndrome" International Journal of Molecular Sciences 22, no. 4: 1589. https://doi.org/10.3390/ijms22041589
APA StyleSarchielli, E., Comeglio, P., Filippi, S., Cellai, I., Guarnieri, G., Marzoppi, A., Cipriani, S., Vignozzi, L., Morelli, A., & Maggi, M. (2021). Neuroprotective Effects of Testosterone in the Hypothalamus of an Animal Model of Metabolic Syndrome. International Journal of Molecular Sciences, 22(4), 1589. https://doi.org/10.3390/ijms22041589







