Reciprocal Changes in miRNA Expression with Pigmentation and Decreased Proliferation Induced in Mouse B16F1 Melanoma Cells by l-Tyrosine and 5-Bromo-2′-Deoxyuridine
Abstract
1. Introduction
2. Results
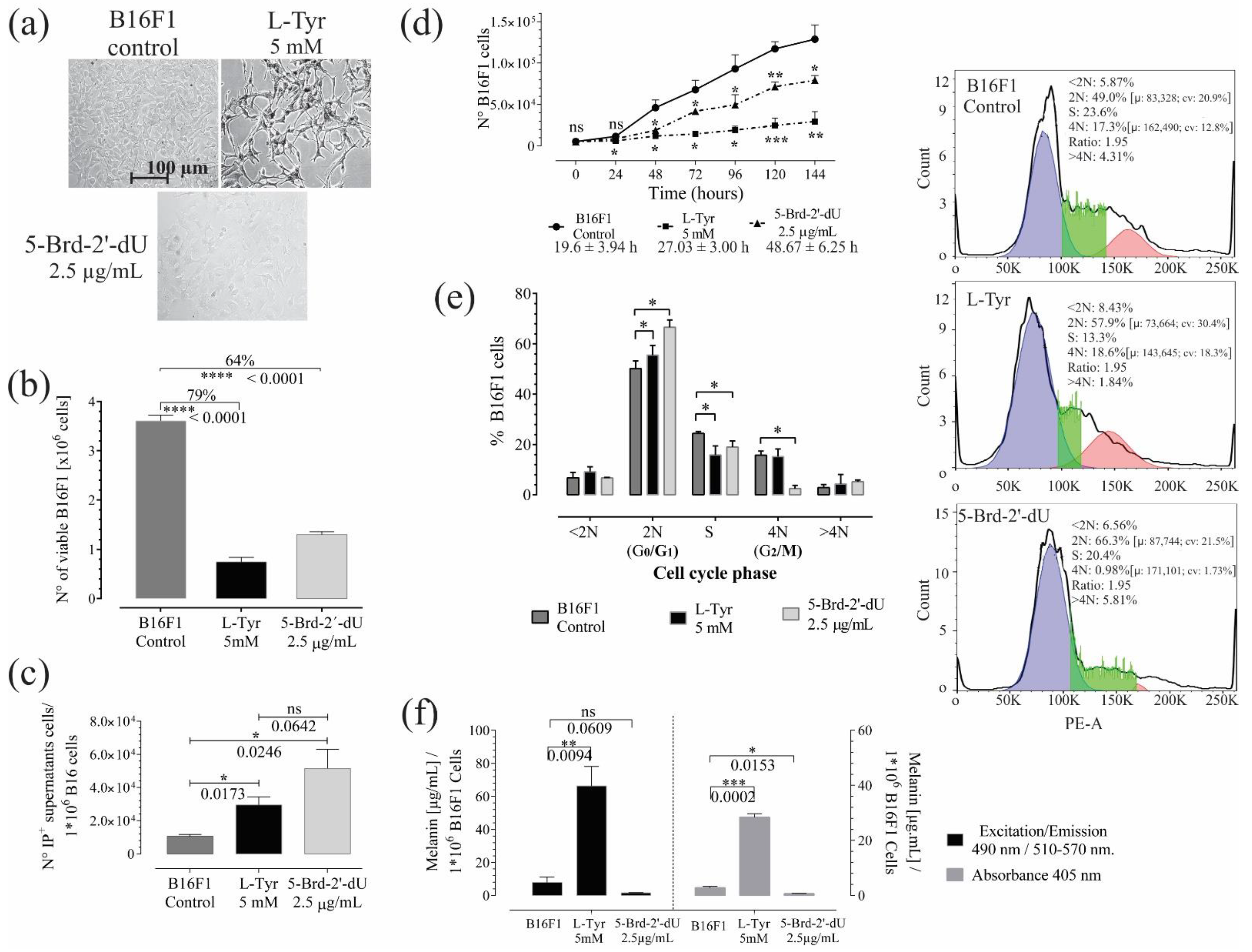
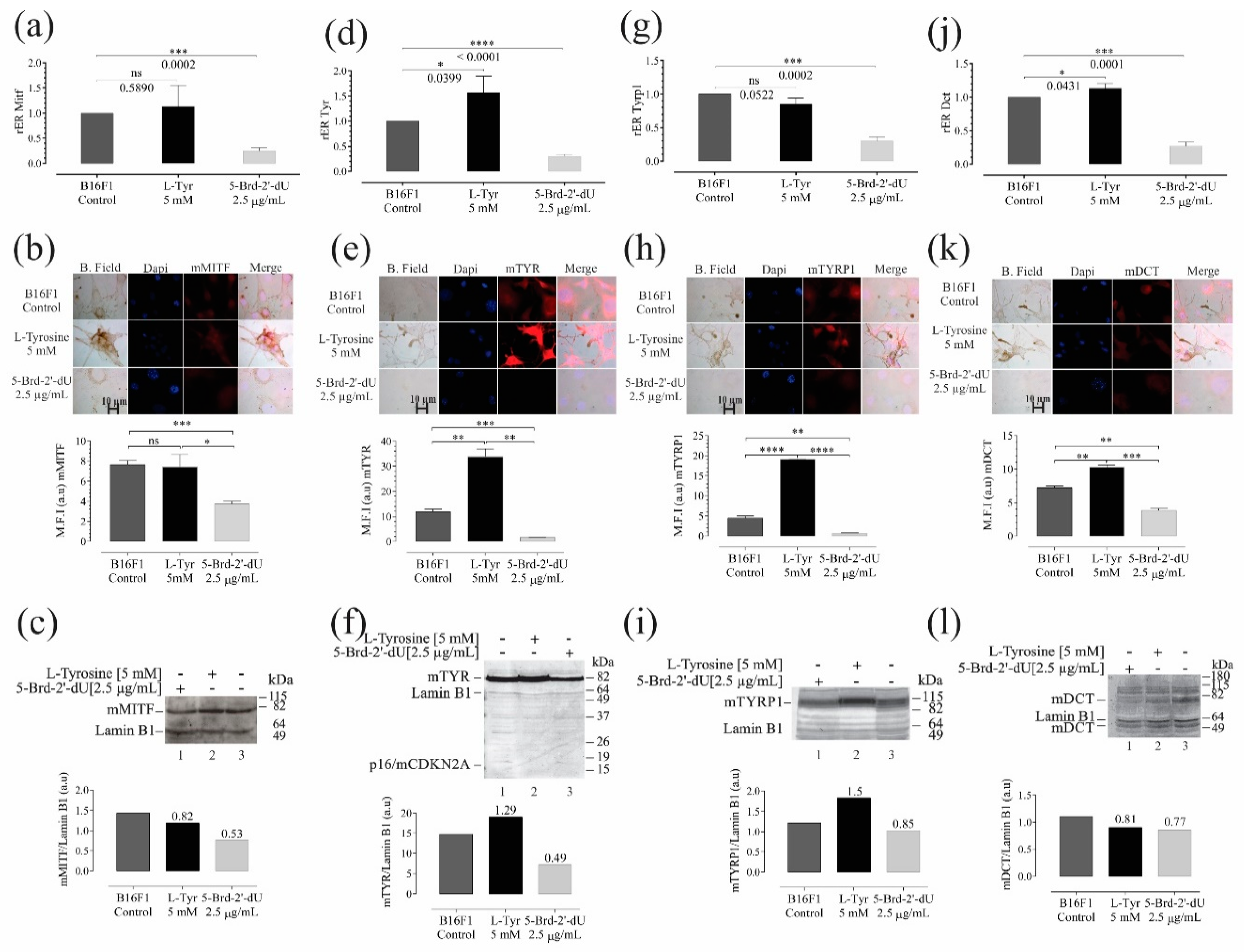
2.1. Decreased Proliferation and Pigmentation Changes in Melanoma B16F1 Cells
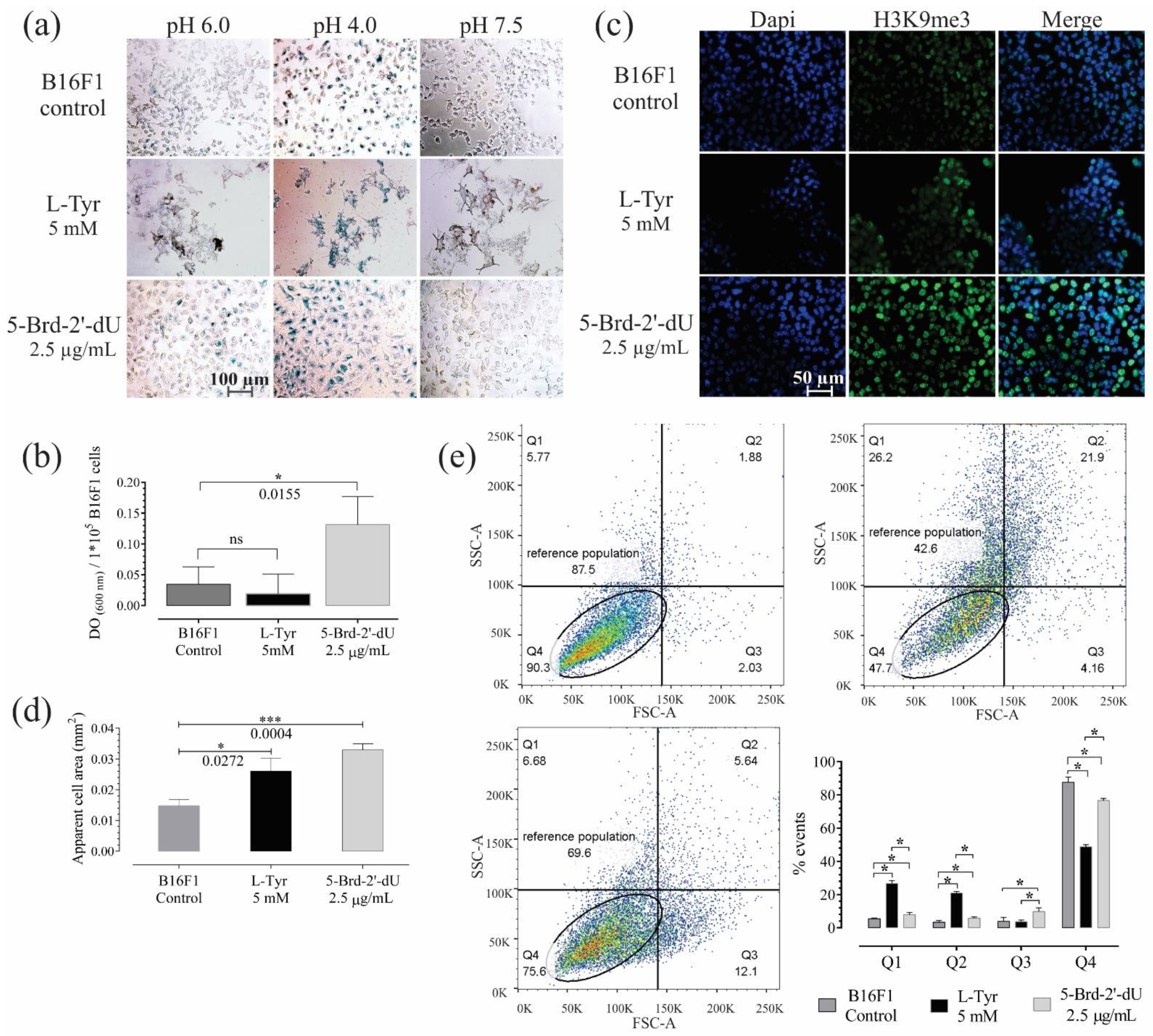
2.2. Induction of Replicative Senescence in B16F1 Cells Exposed to 5-Brd-2′-dU
2.3. Small RNAseq and Differential Expression Analysis of miRNAs
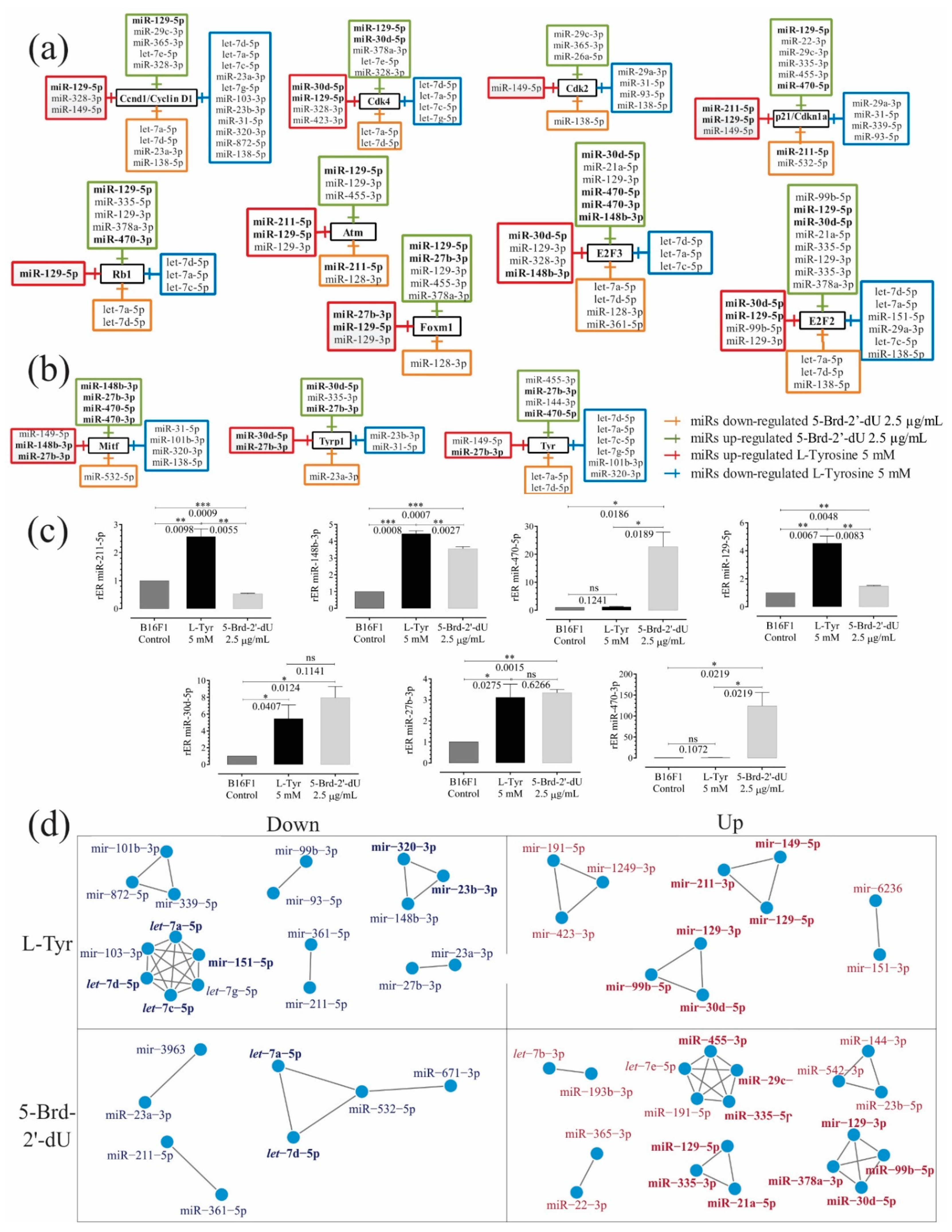
2.4. Confirmation of miRNAs Differential Expression by RT-qPCR Stem-Loop and Prediction of miRNA Targets Associated with Induced Phenotypes
2.5. Regulatory Networks and Co-Expression Networks (RC-miR)
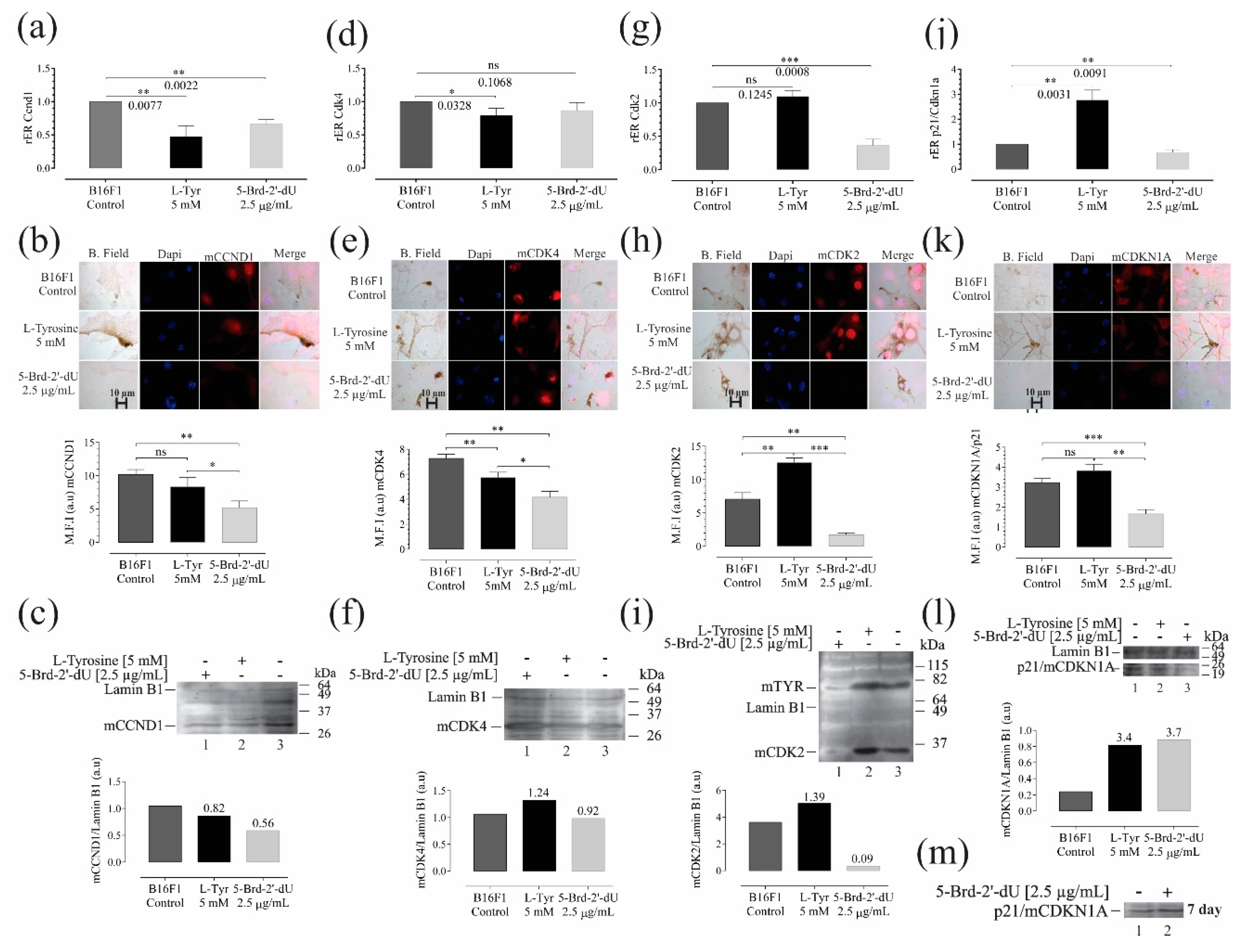
2.6. Analysis of Differential Expression mRNAs and Protein Level
3. Discussion
4. Materials and Methods
4.1. Cell Line Cultures
4.2. Induction of the Phenotype of Decreased Proliferation and Changes in Pigmentation by Expo-Sure to L-Tyrosine (L-Tyr) or 5-Bromo-2′-Deoxyuridine (5-Brd-2′-dU)
4.3. MTT Assay
4.4. Univariate Analysis of Cell Cycle by Incorporation of Propidium Iodide
4.5. Melanin Quantification
4.6. Β-Galactosidase Activity Associated with Senescence (S.A. β-Gal)
4.7. Protein Detection by Immunofluorescence
4.8. Protein Expression by Western Blot
4.9. Extraction of Total RNA and Enrichment of Small RNAs
4.10. Small RNAseq
4.11. Principal Components Analysis-PCA
4.12. Differential Expression Analysis of miRNAs from Sequencing Reads
4.13. Prediction of Molecular Targets (mRNAs) of microRNAs by TargetScan Mouse
4.14. RT-qPCR-Stem-Loop for miRNAs and RT-PCR for mRNAs
4.15. Construction of Co-Expression Networks for miRNAs (RC-miR)
4.16. Regulation Networks and Functional Enrichment
4.17. Statistic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aftab, M.N.; Dinger, M.E.; Perera, R.J. The role of microRNAs and long non-coding RNAs in the pathology, diagnosis, and management of melanoma. Arch. Biochem. Biophys. 2014, 563, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Damsky, W.E.; Theodosakis, N.; Bosenberg, M. Melanoma metastasis: New concepts and evolving paradigms. Oncogene 2014, 33, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Haass, N.K.; Smalley, K.S.; Li, L.; Herlyn, M. Adhesion, migration and communication in melanocytes and melanoma. Pigment Cell Res. 2005, 18, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, D.; Leung, E.Y.; Baguley, B.C.; Finlay, G.J.; Askarian-Amiri, M.E. Epigenetic regulation in human melanoma: Past and future. Epigenetics 2015, 10, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T. Epigenetic regulation by long noncoding RNAs. Science 2012, 338, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Glud, M.; Gniadecki, R. MicroRNAs in the pathogenesis of malignant melanoma. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M. MicroRNAs in melanoma biology. Adv. Exp. Med. Biol. 2013, 774, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Mione, M.; Bosserhoff, A. MicroRNAs in melanocyte and melanoma biology. Pigment Cell Melanoma Res. 2015, 28, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Ameres, S.L.; Zamore, P.D. Diversifying microRNA sequence and function. Nat. Rev. Mol. Cell Biol. 2013, 14, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.C. Genetics of melanoma progression: The rise and fall of cell senescence. Pigment Cell Melanoma Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.E.; Bemis, L.; Norris, D.A.; Shellman, Y.G. miR in melanoma development: miRNAs and acquired hallmarks of cancer in melanoma. Physiol. Genom. 2013, 45, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, C.C.; Cheng, H.H.; Tewari, M. MicroRNA profiling: Approaches and considerations. Nat. Rev. Genet. 2012, 13, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Shain, A.H.; Yeh, I.; Kovalyshyn, I.; Sriharan, A.; Talevich, E.; Gagnon, A.; Dummer, R.; North, J.; Pincus, L.; Ruben, B.; et al. The Genetic Evolution of Melanoma from Precursor Lesions. N. Engl. J. Med. 2015, 373, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.A.; Strasberg Rieber, M.; Rieber, M. Decrease in actin gene expression in melanoma cells compared to melanocytes is partly counteracted by BrdU-induced cell adhesion and antagonized by L-tyrosine induction of terminal differentiation. Biochem. Biophys. Res. Commun. 1995, 216, 84–89. [Google Scholar] [CrossRef]
- Rieber, M.; Rieber, M.S. Cyclin-dependent kinase 2 and cyclin A interaction with E2F are targets for tyrosine induction of B16 melanoma terminal differentiation. Cell Growth Differ. 1994, 5, 1339–1346. [Google Scholar]
- Strasberg Rieber, M.; Rieber, M. Suppression of cyclin D1 but not cdk4 or cyclin A with induction of melanoma terminal differentiation. Biochem. Biophys. Res. Commun. 1995, 216, 422–427. [Google Scholar] [CrossRef]
- Rieber, M.; Strasberg-Rieber, M. Induction of p53 and melanoma cell death is reciprocal with down-regulation of E2F, cyclin D1 and pRB. Int. J. Cancer 1998, 76, 757–760. [Google Scholar] [CrossRef]
- Flórez Vargas, Ó.R.; Gomez, L.A. Expresión Diferencial de dos microRNAs Asociados con el Silenciamiento de la Ciclina D1 en Células de Melanoma B16 en Senescencia Inducida por la 5-Bromo-2-desoxiuridina.Tesis de Maestría; Universidad Nacional de Colombia: Bogotá, Colombia, 2008. [Google Scholar]
- Silagi, S.; Bruce, S.A. Suppression of malignancy and differentiation in melanotic melanoma cells. Proc. Natl. Acad. Sci. USA 1970, 66, 72–78. [Google Scholar] [CrossRef]
- Guerra, L.; Bover, L.; Mordoh, J. Differentiating effect of L-tyrosine on the human melanoma cell line IIB-MEL-J. Exp. Cell Res. 1990, 188, 61–65. [Google Scholar] [CrossRef]
- Garcia, R.I.; Werner, I.; Szabo, G. Effect of 5-bromo-2′-deoxyuridine on growth and differentiation of cultured embryonic retinal pigment cells. In Vitro 1979, 15, 779–788. [Google Scholar] [CrossRef]
- Levkoff, L.H.; Marshall, G.P., II; Ross, H.H.; Caldeira, M.; Reynolds, B.A.; Cakiroglu, M.; Mariani, C.L.; Streit, W.J.; Laywell, E.D. Bromodeoxyuridine inhibits cancer cell proliferation in vitro and in vivo. Neoplasia 2008, 10, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, C.A.; Gomez, L.A. Extracción y Solubilidad de la Melanina Total Producida por Células de Melanoma Murino B16 Expuestas al Aminoácido L-Tirosina; Instituto Nacional de Salud: Bogotá, Colombia, 2005.
- Fernandes, B.; Matama, T.; Guimaraes, D.; Gomes, A.; Cavaco-Paulo, A. Fluorescent quantification of melanin. Pigment Cell Melanoma Res. 2016, 29, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, K.; Tritsch, G.L.; Moore, G.E. Tyrosine utilization by pigmented hamster melanoma cells cultured in vitro. Int. J. Cancer 1968, 3, 446–453. [Google Scholar] [CrossRef]
- Wrathall, J.R.; Oliver, C.; Silagi, S.; Essner, E. Suppression of pigmentation in mouse melanoma cells by 5-bromodeoxyuridine: Effects on tyrosinase activity and melanosome formation. J. Cell Biol. 1973, 57, 406–423. [Google Scholar] [CrossRef]
- Masterson, J.C.; O’Dea, S. 5-Bromo-2-deoxyuridine activates DNA damage signalling responses and induces a senescence-like phenotype in p16-null lung cancer cells. Anticancer Drugs 2007, 18, 1053–1068. [Google Scholar] [CrossRef]
- Suzuki, T.; Michishita, E.; Ogino, H.; Fujii, M.; Ayusawa, D. Synergistic induction of the senescence-associated genes by 5-bromodeoxyuridine and AT-binding ligands in HeLa cells. Exp. Cell Res. 2002, 276, 174–184. [Google Scholar] [CrossRef]
- Suzuki, T.; Minagawa, S.; Michishita, E.; Ogino, H.; Fujii, M.; Mitsui, Y.; Ayusawa, D. Induction of senescence-associated genes by 5-bromodeoxyuridine in HeLa cells. Exp. Gerontol. 2001, 36, 465–474. [Google Scholar] [CrossRef]
- Pasztor, L.M.; Hu, F. An amelanotic variant of B16 malignant melanoma. Cancer Res. 1972, 32, 1769–1774. [Google Scholar]
- Gomez, L.A.; Strasberg Rieber, M.; Rieber, M. PCR-mediated differential display and cloning of a melanocyte gene decreased in malignant melanoma and up-regulated with sensitization to DNA damage. DNA Cell Biol. 1996, 15, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Nunez, S.; Heard, E.; Narita, M.; Lin, A.W.; Hearn, S.A.; Spector, D.L.; Hannon, G.J.; Lowe, S.W. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 2003, 113, 703–716. [Google Scholar] [CrossRef]
- Epstein, W.L.; Fukuyama, K.; Drake, T.E. Ultrastructural effects of thymidine analogs in melanosomes and virus activation in cloned hamster melanoma cells in culture. Yale J. Biol. Med. 1973, 46, 471–481. [Google Scholar]
- Price, P.M. The effect of 5-bromodeoxyuridine on messenger RNA production in cultured cells. Biochim. Biophys. Acta 1976, 447, 304–311. [Google Scholar] [CrossRef]
- Rieber, M.; Rieber, M.S.; Urbina, C.; Lira, R. Differential response of adherent and unanchored melanoma cells to bromodeoxyuridine evidenced by specific lectin-binding protein changes. Int. J. Cancer 1989, 43, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.W.; Rehli, M.; Bosserhoff, A.K. miRNA expression profiling in melanocytes and melanoma cell lines reveals miRNAs associated with formation and progression of malignant melanoma. J. Investig. Dermatol. 2009, 129, 1740–1751. [Google Scholar] [CrossRef] [PubMed]
- Gomez, L.A. Aplicación de microarreglos de cADN para estudiar algunos determinantes moleculares de la supresión del crecimiento celular en cáncer. Biomedica 2009, 29. [Google Scholar]
- Kato, J.; Matsushime, H.; Hiebert, S.W.; Ewen, M.E.; Sherr, C.J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993, 7, 331–342. [Google Scholar] [CrossRef]
- Bates, S.; Bonetta, L.; MacAllan, D.; Parry, D.; Holder, A.; Dickson, C.; Peters, G. CDK6 (PLSTIRE) and CDK4 (PSK-J3) are a distinct subset of the cyclin-dependent kinases that associate with cyclin D1. Oncogene 1994, 9, 71–79. [Google Scholar]
- Escobar, L.M. Expresión Diferencial del gen Rock alfa e Inhibición del Crecimiento de Células de Melanoma Humano y Murino Inducido por la Genisteina y la L-Tirosina In Vitro.Tesis de Maestría; Pontificia Universidad Javeriana: Bogotá, Colombia, 2000. [Google Scholar]
- Melnikova, V.O.; Bolshakov, S.V.; Walker, C.; Ananthaswamy, H.N. Genomic alterations in spontaneous and carcinogen-induced murine melanoma cell lines. Oncogene 2004, 23, 2347–2356. [Google Scholar] [CrossRef]
- Shin, S.Y.; Kim, C.G.; Lim, Y.; Lee, Y.H. The ETS family transcription factor ELK-1 regulates induction of the cell cycle-regulatory gene p21(Waf1/Cip1) and the BAX gene in sodium arsenite-exposed human keratinocyte HaCaT cells. J. Biol. Chem. 2011, 286, 26860–26872. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.; Lin, A.W.; McCurrach, M.E.; Beach, D.; Lowe, S.W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997, 88, 593–602. [Google Scholar] [CrossRef]
- Charrier-Savournin, F.B.; Chateau, M.T.; Gire, V.; Sedivy, J.; Piette, J.; Dulic, V. p21-Mediated nuclear retention of cyclin B1-Cdk1 in response to genotoxic stress. Mol. Biol. Cell 2004, 15, 3965–3976. [Google Scholar] [CrossRef] [PubMed]
- Bunz, F.; Dutriaux, A.; Lengauer, C.; Waldman, T.; Zhou, S.; Brown, J.P.; Sedivy, J.M.; Kinzler, K.W.; Vogelstein, B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 1998, 282, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, I.; Draetta, G.; Karsenti, E. Activation of the phosphatase activity of human cdc25A by a cdk2-cyclin E dependent phosphorylation at the G1/S transition. EMBO J. 1994, 13, 4302–4310. [Google Scholar] [CrossRef] [PubMed]
- Stead, E.; White, J.; Faast, R.; Conn, S.; Goldstone, S.; Rathjen, J.; Dhingra, U.; Rathjen, P.; Walker, D.; Dalton, S. Pluripotent cell division cycles are driven by ectopic Cdk2, cyclin A/E and E2F activities. Oncogene 2002, 21, 8320–8333. [Google Scholar] [CrossRef]
- Hirobe, T.; Ishikawa, A. l-tyrosine induces melanocyte differentiation in novel pink-eyed dilution castaneus mouse mutant showing age-related pigmentation. J. Dermatol. Sci. 2015, 80, 203–211. [Google Scholar] [CrossRef]
- Hirobe, T.; Wakamatsu, K.; Ito, S.; Abe, H.; Kawa, Y.; Mizoguchi, M. Stimulation of the proliferation and differentiation of mouse pink-eyed dilution epidermal melanocytes by excess tyrosine in serum-free primary culture. J. Cell Physiol. 2002, 191, 162–172. [Google Scholar] [CrossRef]
- Strasberg Rieber, M.; Welch, D.R.; Miele, M.E.; Rieber, M. p53-independent increase in p21WAF1 and reciprocal down-regulation of cyclin A and proliferating cell nuclear antigen in bromodeoxyuridine-mediated growth arrest of human melanoma cells. Cell Growth Differ. 1996, 7, 197–202. [Google Scholar]
- Rieber, M.; Rieber, M.S. Sensitization to radiation-induced DNA damage accelerates loss of bcl-2 and increases apoptosis and autophagy. Cancer Biol. Ther. 2008, 7, 1561–1566. [Google Scholar] [CrossRef]
- Nicetto, D.; Zaret, K.S. Role of H3K9me3 heterochromatin in cell identity establishment and maintenance. Curr. Opin. Genet. Dev. 2019, 55, 1–10. [Google Scholar] [CrossRef]
- Yu, Y.; Schleich, K.; Yue, B.; Ji, S.; Lohneis, P.; Kemper, K.; Silvis, M.R.; Qutob, N.; van Rooijen, E.; Werner-Klein, M.; et al. Targeting the Senescence-Overriding Cooperative Activity of Structurally Unrelated H3K9 Demethylases in Melanoma. Cancer Cell 2018, 33, 322–336. [Google Scholar] [CrossRef]
- Morasso, M.I.; Rieber, M.S.; Gil, F.; Rieber, M. Cell adhesion regulates melanoma specific differentiation and interactions with the 3′ region of the tyrosinase gene. Biochem. Biophys. Res. Commun. 1990, 172, 638–645. [Google Scholar] [CrossRef]
- Slominski, A. L-tyrosine induces synthesis of melanogenesis related proteins. Life Sci. 1989, 45, 1799–1803. [Google Scholar] [CrossRef]
- Glud, M.; Rossing, M.; Hother, C.; Holst, L.; Hastrup, N.; Nielsen, F.C.; Gniadecki, R.; Drzewiecki, K.T. Downregulation of miR-125b in metastatic cutaneous malignant melanoma. Melanoma Res. 2010, 20, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Kozubek, J.; Ma, Z.; Fleming, E.; Duggan, T.; Wu, R.; Shin, D.G.; Dadras, S.S. In-depth characterization of microRNA transcriptome in melanoma. PLoS ONE 2013, 8, e72699. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tetzlaff, M.T.; Liu, A.; Liegl-Atzwanger, B.; Guo, J.; Xu, X. Loss of microRNA-205 expression is associated with melanoma progression. Lab. Investig. 2012, 92, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.; Lorenz, P.; Gross, G.; Ibrahim, S.; Kunz, M. MicroRNA let-7b targets important cell cycle molecules in malignant melanoma cells and interferes with anchorage-independent growth. Cell Res. 2008, 18, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Sand, M.; Skrygan, M.; Sand, D.; Georgas, D.; Gambichler, T.; Hahn, S.A.; Altmeyer, P.; Bechara, F.G. Comparative microarray analysis of microRNA expression profiles in primary cutaneous malignant melanoma, cutaneous malignant melanoma metastases, and benign melanocytic nevi. Cell Tissue Res. 2013, 351, 85–98. [Google Scholar] [CrossRef]
- Diermeier, S.; Schmidt-Bruecken, E.; Kubbies, M.; Kunz-Schughart, L.A.; Brockhoff, G. Exposure to continuous bromodeoxyuridine (BrdU) differentially affects cell cycle progression of human breast and bladder cancer cell lines. Cell Prolif. 2004, 37, 195–206. [Google Scholar] [CrossRef]
- Michishita, E.; Nakabayashi, K.; Suzuki, T.; Kaul, S.C.; Ogino, H.; Fujii, M.; Mitsui, Y.; Ayusawa, D. 5-Bromodeoxyuridine induces senescence-like phenomena in mammalian cells regardless of cell type or species. J. Biochem. 1999, 126, 1052–1059. [Google Scholar]
- Silagi, S. Effects of 5-bromodeoxyuridine on tumorigenicity, immunogenicity, virus production, plasminogen activator, and melanogenesis of mouse melanoma cells. Int. Rev. Cytol. 1976, 45, 65–111. [Google Scholar]
- Thomas, L.; Chan, P.W.; Chang, S.; Damsky, C. 5-Bromo-2-deoxyuridine regulates invasiveness and expression of integrins and matrix-degrading proteinases in a differentiated hamster melanoma cell. J. Cell. Sci. 1993, 105, 191–201. [Google Scholar] [PubMed]
- Rauth, S.; Davidson, R.L. Suppression of tyrosinase gene expression by bromodeoxyuridine in Syrian hamster melanoma cells is not due to its incorporation into upstream or coding sequences of the tyrosinase gene. Somat. Cell Mol. Genet 1993, 19, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Paus, R. Are L-tyrosine and L-dopa hormone-like bioregulators? J. Theor. Biol. 1990, 143, 123–138. [Google Scholar] [CrossRef]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- Gunturiz, M.L.; Gómez, L.A. Expresión de Los Genes Que Regulan La Síntesis de Melanina: MITF-M, TRP1 y TRP2 En Células de Melanoma Maligno B16 y A375; Instituto Nacional de Salud: Bogotá, Colombia, 2013; p. 51.
- Rauth, S.; Hoganson, G.E.; Davidson, R.L. Bromodeoxyuridine- and cyclic AMP-mediated regulation of tyrosinase in Syrian hamster melanoma cells. Somat. Cell Mol. Genet. 1990, 16, 583–592. [Google Scholar] [CrossRef]
- Haflidadottir, B.S.; Bergsteinsdottir, K.; Praetorius, C.; Steingrimsson, E. miR-148 regulates Mitf in melanoma cells. PLoS ONE 2010, 5, e11574. [Google Scholar] [CrossRef]
- Git, A.; Dvinge, H.; Salmon-Divon, M.; Osborne, M.; Kutter, C.; Hadfield, J.; Bertone, P.; Caldas, C. Systematic comparison of microarray profiling, real-time PCR, and next-generation sequencing technologies for measuring differential microRNA expression. RNA 2010, 16, 991–1006. [Google Scholar] [CrossRef]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]
- Tay, Y.; Zhang, J.; Thomson, A.M.; Lim, B.; Rigoutsos, I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature 2008, 455, 1124–1128. [Google Scholar] [CrossRef]
- Li, R.; Qian, N.; Tao, K.; You, N.; Wang, X.; Dou, K. MicroRNAs involved in neoplastic transformation of liver cancer stem cells. J. Exp. Clin. Cancer Res. 2010, 29, 169. [Google Scholar] [CrossRef]
- Tam, S.W.; Theodoras, A.M.; Shay, J.W.; Draetta, G.F.; Pagano, M. Differential expression and regulation of Cyclin D1 protein in normal and tumor human cells: Association with Cdk4 is required for Cyclin D1 function in G1 progression. Oncogene 1994, 9, 2663–2674. [Google Scholar]
- Couts, K.L.; Anderson, E.M.; Gross, M.M.; Sullivan, K.; Ahn, N.G. Oncogenic B-Raf signaling in melanoma cells controls a network of microRNAs with combinatorial functions. Oncogene 2013, 32, 1959–1970. [Google Scholar] [CrossRef]
- Chen, J.; Feilotter, H.E.; Pare, G.C.; Zhang, X.; Pemberton, J.G.; Garady, C.; Lai, D.; Yang, X.; Tron, V.A. MicroRNA-193b represses cell proliferation and regulates cyclin D1 in melanoma. Am. J. Pathol. 2010, 176, 2520–2529. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.W.; Bosserhoff, A.K. Integrin beta 3 expression is regulated by let-7a miRNA in malignant melanoma. Oncogene 2008, 27, 6698–6706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, S.; Ma, P.; Jing, Y.; Peng, H.; Gao, W.-Q.; Zhuang, G. Lin28B promotes melanoma growth by mediating a microRNA regulatory circuit. Carcinogenesis 2015, 36, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.Y.; Chang, C.C.; Lin, C.T.; Lai, C.H.; Peng, S.Y.; Ko, Y.J.; Tang, P.C. Let-7b-mediated suppression of basigin expression and metastasis in mouse melanoma cells. Exp. Cell Res. 2011, 317, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Serguienko, A.; Grad, I.; Wennerstrom, A.B.; Meza-Zepeda, L.A.; Thiede, B.; Stratford, E.W.; Myklebost, O.; Munthe, E. Metabolic reprogramming of metastatic breast cancer and melanoma by let-7a microRNA. Oncotarget 2015, 6, 2451–2465. [Google Scholar] [CrossRef]
- Long, J.; Menggen, Q.; Wuren, Q.; Shi, Q.; Pi, X. Long Noncoding RNA Taurine-Upregulated Gene1 (TUG1) Promotes Tumor Growth and Metastasis through TUG1/Mir-129-5p/Astrocyte-Elevated Gene-1 (AEG-1) Axis in Malignant Melanoma. Med. Sci. Monit. 2018, 24, 1547–1559. [Google Scholar] [CrossRef]
- Shen, X.; Kong, S.; Yang, Q.; Yin, Q.; Cong, H.; Wang, X.; Ju, S. PCAT-1 promotes cell growth by sponging miR-129 via MAP3K7/NF-kappaB pathway in multiple myeloma. J. Cell Mol. Med. 2020. [Google Scholar] [CrossRef]
- Diao, Y.; Jin, B.; Huang, L.; Zhou, W. MiR-129-5p inhibits glioma cell progression in vitro and in vivo by targeting TGIF2. J. Cell Mol. Med. 2018, 22, 2357–2367. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.; Yin, J.; Li, Y.; Li, R.; Wang, Z.; Zhou, X.; Jin, X.; Shen, F.; Yan, W.; You, Y. miR-129-5p targets Wnt5a to block PKC/ERK/NF-kappaB and JNK pathways in glioblastoma. Cell Death Dis. 2018, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ge, J.; Zhang, Z.; Zhou, W. MiR-129 inhibits cell proliferation and metastasis by targeting ETS1 via PI3K/AKT/mTOR pathway in prostate cancer. Biomed. Pharmacother. 2017, 96, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, Y.; Chen, X.; Wu, P.; Chen, D. Inactivation of the Wnt/beta-catenin signaling pathway underlies inhibitory role of microRNA-129-5p in epithelial-mesenchymal transition and angiogenesis of prostate cancer by targeting ZIC2. Cancer Cell Int. 2019, 19, 271. [Google Scholar] [CrossRef]
- Ya, G.; Wang, H.; Ma, Y.; Hu, A.; Ma, Y.; Hu, J.; Yu, Y. Serum miR-129 functions as a biomarker for colorectal cancer by targeting estrogen receptor (ER) beta. Pharmazie 2017, 72, 107–112. [Google Scholar] [CrossRef]
- Setijono, S.R.; Park, M.; Kim, G.; Kim, Y.; Cho, K.W.; Song, S.J. miR-218 and miR-129 regulate breast cancer progression by targeting Lamins. Biochem. Biophys. Res. Commun. 2018, 496, 826–833. [Google Scholar] [CrossRef]
- Zhao, J.J.; Lin, J.; Zhu, D.; Wang, X.; Brooks, D.; Chen, M.; Chu, Z.B.; Takada, K.; Ciccarelli, B.; Admin, S.; et al. miR-30-5p functions as a tumor suppressor and novel therapeutic tool by targeting the oncogenic Wnt/beta-catenin/BCL9 pathway. Cancer Res. 2014, 74, 1801–1813. [Google Scholar] [CrossRef]
- Ouzounova, M.; Vuong, T.; Ancey, P.B.; Ferrand, M.; Durand, G.; Le-Calvez Kelm, F.; Croce, C.; Matar, C.; Herceg, Z.; Hernandez-Vargas, H. MicroRNA miR-30 family regulates non-attachment growth of breast cancer cells. BMC Genom. 2013, 14, 139. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, J.; Zhao, J.; Bai, J. Mir-30d suppresses cell proliferation of colon cancer cells by inhibiting cell autophagy and promoting cell apoptosis. Tumour. Biol. 2017, 39. [Google Scholar] [CrossRef]
- Li, J.; Donath, S.; Li, Y.; Qin, D.; Prabhakar, B.S.; Li, P. miR-30 regulates mitochondrial fission through targeting p53 and the dynamin-related protein-1 pathway. PLoS Genet. 2010, 6, e1000795. [Google Scholar] [CrossRef]
- Wu, C.; Jin, B.; Chen, L.; Zhuo, D.; Zhang, Z.; Gong, K.; Mao, Z. MiR-30d induces apoptosis and is regulated by the Akt/FOXO pathway in renal cell carcinoma. Cell Signal 2013, 25, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Martinez, I.; Cazalla, D.; Almstead, L.L.; Steitz, J.A.; DiMaio, D. miR-29 and miR-30 regulate B-Myb expression during cellular senescence. Proc. Natl. Acad. Sci. USA 2011, 108, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Hong, L.; Xu, X.; Huang, S.; Herpai, D.; Li, L.; Xu, Y.; Truong, L.; Hu, W.Y.; Wu, X.; et al. miR-30 disrupts senescence and promotes cancer by targeting both p16(INK4A) and DNA damage pathways. Oncogene 2018, 37, 5618–5632. [Google Scholar] [CrossRef] [PubMed]
- Gaziel-Sovran, A.; Segura, M.F.; Di Micco, R.; Collins, M.K.; Hanniford, D.; Vega-Saenz de Miera, E.; Rakus, J.F.; Dankert, J.F.; Shang, S.; Kerbel, R.S.; et al. miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell 2011, 20, 104–118. [Google Scholar] [CrossRef] [PubMed]
- Rieber, M.S.; Rieber, M. Specific tyrosinases associated with melanoma replicative senescence and melanogenesis. Cancer Res. 1993, 53, 2469–2471. [Google Scholar] [PubMed]
- Sestakova, B.; Ondrusova, L.; Vachtenheim, J. Cell cycle inhibitor p21/ WAF1/ CIP1 as a cofactor of MITF expression in melanoma cells. Pigment Cell Melanoma Res. 2010, 23, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Widlund, H.R.; Horstmann, M.A.; Ramaswamy, S.; Ross, K.; Huber, W.E.; Nishimura, E.K.; Golub, T.R.; Fisher, D.E. Critical role of CDK2 for melanoma growth linked to its melanocyte-specific transcriptional regulation by MITF. Cancer Cell 2004, 6, 565–576. [Google Scholar] [CrossRef]
- Haddad, M.M.; Xu, W.; Schwahn, D.J.; Liao, F.; Medrano, E.E. Activation of a cAMP pathway and induction of melanogenesis correlate with association of p16(INK4) and p27(KIP1) to CDKs, loss of E2F-binding activity, and premature senescence of human melanocytes. Exp. Cell Res. 1999, 253, 561–572. [Google Scholar] [CrossRef]
- Dai, X.; Rao, C.; Li, H.; Chen, Y.; Fan, L.; Geng, H.; Li, S.; Qu, J.; Hou, L. Regulation of pigmentation by microRNAs: MITF-dependent microRNA-211 targets TGF-beta receptor 2. Pigment Cell Melanoma Res. 2015, 28, 217–222. [Google Scholar] [CrossRef]
- Vitiello, M.; Tuccoli, A.; D’Aurizio, R.; Sarti, S.; Giannecchini, L.; Lubrano, S.; Marranci, A.; Evangelista, M.; Peppicelli, S.; Ippolito, C.; et al. Context-dependent miR-204 and miR-211 affect the biological properties of amelanotic and melanotic melanoma cells. Oncotarget 2017, 8, 25395–25417. [Google Scholar] [CrossRef]
- Noguchi, S.; Mori, T.; Otsuka, Y.; Yamada, N.; Yasui, Y.; Iwasaki, J.; Kumazaki, M.; Maruo, K.; Akao, Y. Anti-oncogenic microRNA-203 induces senescence by targeting E2F3 protein in human melanoma cells. J. Biol. Chem. 2012, 287, 11769–11777. [Google Scholar] [CrossRef] [PubMed]
- Glud, M.; Manfe, V.; Biskup, E.; Holst, L.; Dirksen, A.M.; Hastrup, N.; Nielsen, F.C.; Drzewiecki, K.T.; Gniadecki, R. MicroRNA miR-125b induces senescence in human melanoma cells. Melanoma Res. 2011, 21, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.A.; Majid, S.; de Semir, D.; Nosrati, M.; Bezrookove, V.; Kashani-Sabet, M. miRNA-205 suppresses melanoma cell proliferation and induces senescence via regulation of E2F1 protein. J. Biol. Chem. 2011, 286, 16606–16614. [Google Scholar] [CrossRef] [PubMed]
- Fattore, L.; Ruggiero, C.F.; Pisanu, M.E.; Liguoro, D.; Cerri, A.; Costantini, S.; Capone, F.; Acunzo, M.; Romano, G.; Nigita, G.; et al. Reprogramming miRNAs global expression orchestrates development of drug resistance in BRAF mutated melanoma. Cell Death Differ. 2019, 26, 1267–1282. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.K. Measurement of growth and viability of cells in culture. Methods Enzymol. 1979, 11, 141–152. [Google Scholar] [CrossRef]
- Pozarowski, P.; Darzynkiewicz, Z. Analysis of cell cycle by flow cytometry. Methods Mol. Biol. 2004, 281, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Debacq-Chainiaux, F.; Erusalimsky, J.D.; Campisi, J.; Toussaint, O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc. 2009, 4, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Haycock, J.W. Polyvinylpyrrolidone as a blocking agent in immunochemical studies. Anal. Biochem. 1993, 208, 397–399. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Ryu, B.; Kim, D.S.; Deluca, A.M.; Alani, R.M. Comprehensive expression profiling of tumor cell lines identifies molecular signatures of melanoma progression. PLoS ONE 2007, 2, e594. [Google Scholar] [CrossRef]
- Jolliffe, I. Principal Component Analysis. In Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.R.; Manduchi, E.; Stoeckert, C.J., Jr. Analysis and management of microarray gene expression data. Curr. Protoc. Mol. Biol. 2007, 77. [Google Scholar] [CrossRef] [PubMed]
- Cormack, R.M. A Review of Classification. J. R. Stat. Soc. Ser. A 1971, 134, 321–353. [Google Scholar] [CrossRef]
- Mächler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. Cluster: Cluster Analysis Basics and Extensions; 2012; Available online: https://cran.r-project.org/web/packages/available_packages_by_name.html (accessed on 8 April 2016).
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Schefe, J.H.; Lehmann, K.E.; Buschmann, I.R.; Unger, T.; Funke-Kaiser, H. Quantitative real-time RT-PCR data analysis: Current concepts and the novel “gene expression’s CT difference” formula. J. Mol. Med. 2006, 84, 901–910. [Google Scholar] [CrossRef]
- Leal, L.G.; Lopez, C.; Lopez-Kleine, L. Construction and comparison of gene co-expression networks shows complex plant immune responses. PeerJ 2014, 2, e610. [Google Scholar] [CrossRef]
- Numata, J.; Ebenhoh, O.; Knapp, E.W. Measuring correlations in metabolomic networks with mutual information. Genome Inform. 2008, 20, 112–122. [Google Scholar]
- Mendes, A.D.R.M.D.A. Mutual information: A dependence measure for nonlinear time series. Econometrics 2004, 344, 1–358. [Google Scholar]
- Henao, J.D. Coexnet: An R Package to Build CO-EXpression NETworks from Microarray Data; Version 1.8.0.; view on Bioconductor; 2019; Available online: https://rdrr.io/bioc/coexnet/ (accessed on 8 November 2020).
- Fan, Y.; Siklenka, K.; Arora, S.K.; Ribeiro, P.; Kimmins, S.; Xia, J. miRNet—Dissecting miRNA-target interactions and functional associations through network-based visual analysis. Nucleic Acids Res. 2016, 44, W135–W141. [Google Scholar] [CrossRef]
- Fan, Y.; Xia, J. miRNet-Functional Analysis and Visual Exploration of miRNA-Target Interactions in a Network Context. Methods Mol. Biol. 2018, 1819, 215–233. [Google Scholar] [CrossRef] [PubMed]

| L-Tyr vs. Respect to Unexposed B16 Cells | 5-Brd-2′-dU vs. Respect to Unexposed B16 cells | 5-Brd-2′-dU vs. Respect to L-Tyr | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| microRNA ID | log2 Fold Change | pValue | padj | BaseMean | microRNA ID | log2 Fold Change | pValue | padj | BaseMean | microRNA ID | log2 Fold Change | pValue | padj | BaseMean |
| mmu-let-7d-5p | −0.93 | 8.701 × 10−4 | 2.504 × 10−2 | 2014 | mmu-mir-3963 | −1.85 | 1804 × 10−9 | 1.401 × 10−6 | 29645 | mmu-mir-30d-5p | −1.04 | 8.70 × 10−6 | 6.50 × 10−4 | 2326 |
| mmu-let-7a-5p | −0.64 | 1.05 × 10−3 | 3.27 × 10−2 | 1648 | mmu-let-7a-5p | −0.96 | 6.47 × 10−4 | 2.093 × 10−2 | 1300 | mmu-mir-211-5p | −2.20 | 1.03 × 10−5 | 7.15×10−4 | 2024 |
| mmu-mir-151-5p | −0.94 | 3.567 × 10−4 | 1.375 × 10−2 | 859 | mmu-let-7d-5p | −0.89 | 1.425 × 10−3 | 3.882 × 10−2 | 1030 | mmu-mir-1249-3p | −2.23 | 6.09 × 10−5 | 3.02 × 10−3 | 705 |
| mmu-mir-29a-3p | −0.94 | 2.239 × 10−7 | 3.885 × 10−5 | 636 | mmu-mir-211-5p | −1.36 | 3.164 × 10−4 | 1.199 × 10−2 | 696 | mmu-mir-211-3p | −2.87 | 1.16 × 10−6 | 1.89 × 10−4 | 126 |
| mmu-let-7c-5p | −0.84 | 1.247 × 10−3 | 3.762 × 10−2 | 533 | mmu-mir-128-3p | −1.91 | 9.496 × 10−5 | 4.589 × 10−3 | 232 | mmu-mir-30a-3p | −5.10 | 5.56 × 10−9 | 2.70 × 10−6 | 45 |
| mmu-mir-27b-3p | −1.47 | 5.270 × 10−4 | 1.97 × 10−2 | 377 | mmu-mir-361-5p | −1.72 | 8.53 × 10−5 | 4.014 × 10−3 | 204 | mmu-mir-501-3p | −3.40 | 1.38 × 10−4 | 6.27 × 10−3 | 43 |
| mmu-mir-148b-3p | −2.00 | 2.046 × 10−5 | 1.775 × 10−3 | 118 | mmu-mir-138-5p | −3.26 | 1.889 × 10−5 | 2.000 × 10−4 | 65 | mmu-mir-130b-3p | −3.16 | 5.74 × 10−4 | 2.15 × 10−2 | 31 |
| mmu-mir-23a-3p | −3.05 | 1.413 × 10−4 | 7.519×10−3 | 49 | mmu-mir-671-3p | −2.35 | 1.686 × 10−3 | 4.292 × 10−2 | 27 | mmu-mir-671-3p | −2.76 | 1.30 × 10−4 | 6.12 × 10−3 | 29 |
| mmu-mir-99b-3p | −2.98 | 3.64 × 10−7 | 5.535 × 10−5 | 45 | mmu-mir-30a-3p | −4.20 | 1.719 × 10−6 | 2.670 × 10−4 | 26 | mmu-mir-351-5p | −4.35 | 9.37 × 10−6 | 9.10 × 10−4 | 25 |
| mmu-let-7g-5p | −4.44 | 8.90 × 10−9 | 4.118 × 10−6 | 45 | mmu-mir-23a-3p | −3.13 | 8.000 × 10−4 | 2.484 × 10−2 | 17 | mmu-mir-6236 | −4.22 | 1.52 × 10−6 | 2.02 × 10−4 | 22 |
| mmu-mir-30c-3p | −2.09 | 6.679 × 10−4 | 2.377 × 10−2 | 35 | mmu-mir-532-5p | −3.10 | 1.498 × 10−3 | 3.976 × 10−2 | 11 | mmu-mir-103-3p | −3.20 | 1.32 × 10−3 | 4.38 × 10−2 | 11 |
| mmu-mir-103-3p | −2.87 | 5.338 × 10−5 | 4.116 × 10−3 | 32 | mmu-mir-99b-5p | 1.14 | 2.312 × 10−4 | 7.241 × 10−3 | 10602 | mmu-mir-26a-5p | 0.73 | 6.91 × 10−5 | 3.63 × 10−3 | 1879 |
| mmu-mir-23b-3p | −2.70 | 1.346 × 10−4 | 7.473 × 10−3 | 28 | mmu-mir-129-5p | 1.6 | 2.962 × 10−5 | 1.550 × 10−3 | 1955 | mmu-mir-21a-5p | 1.67 | 6.68 × 10−5 | 3.02 × 10−3 | 518 |
| mmu-mir-361-5p | −3.39 | 1.787 × 10−4 | 8.670 × 10−3 | 23 | mmu-mir-22-3p | 1.32 | 1.698 × 10−4 | 6.194 × 10−3 | 764 | mmu-let-7b-3p | 1.78 | 9.39 × 10−5 | 4.57 × 10−3 | 203 |
| mmu-mir-31-5p | −3.01 | 1.881 × 10−4 | 8.704 × 10−3 | 22 | mmu-mir-30d-5p | 1.20 | 2.214 × 10−5 | 1.719 × 10−3 | 618 | mmu-mir-335-5p | 4.00 | 3.35 × 10−4 | 1.14 × 10−2 | 141 |
| mmu-mir-101b-3p | −4.12 | 3.302 × 10−6 | 3.554 × 10−4 | 17 | mmu-mir-191-5p | 1.48 | 3.663 × 10−4 | 1.326 × 10−3 | 574 | mmu-mir-193b-3p | 6.14 | 1.23 × 10−14 | 8.95 × 10−12 | 68 |
| mmu-mir-320-3p | −3.37 | 1.658 × 10−4 | 8.670 × 10−3 | 14 | mmu-mir-21a-5p | 2.17 | 2.679 × 10−4 | 7.450 × 10−3 | 560 | mmu-mir-484 | 2.88 | 1.89 × 10−5 | 1.44 × 10−3 | 65 |
| mmu-mir-181b-3p | −3.11 | 7.574 × 10−4 | 2.564 × 10−2 | 12 | mmu-mir-335-5p | 3.32 | 1.029 × 10−4 | 3.727 × 10−3 | 329 | mmu-mir-99b-3p | 2.89 | 3.87 × 10−4 | 1.52 × 10−2 | 54 |
| mmu-mir-872-5p | −3.37 | 2.226 × 10−4 | 9.848 × 10−3 | 12 | mmu-let-7b-3p | 1.77 | 1.009 × 10−3 | 2.909 × 10−2 | 228 | mmu-mir-335-3p | 4.75 | 1.05 × 10−5 | 6.80 × 10−4 | 51 |
| mmu-mir-29a-3p | −3.51 | 1.083 × 10−4 | 6.592 × 10−3 | 11 | mmu-mir-129-3p | 2.10 | 2.947 × 10−4 | 1.149 × 10−2 | 191 | mmu-mir-365-3p | 4.30 | 9.47 × 10−3 | 7.20 × 10−4 | 42 |
| mmu-mir-339-5p | −3.15 | 6.087 × 10−4 | 2.283 × 10−2 | 11 | mmu-mir-335-3p | 3.46 | 4.762 × 10−5 | 2.917 × 10−3 | 171 | mmu-miR-144-3p | 3.54 | 4.52 × 10−4 | 1.74 × 10−2 | 35 |
| mmu-mir-93-5p | −2.84 | 1.31 × 10−3 | 3.868 × 10−2 | 10 | mmu-mir-455-3p | 2.75 | 2.150 × 10−4 | 7.299 × 10−3 | 153 | mmu-mir-27b-3p | 3.46 | 6.08 × 10−5 | 3.17 × 10−3 | 32 |
| mmu-mir-30d-5p | 1.02 | 8.078 × 10−6 | 7.626 × 10−4 | 5118 | mmu-mir-378a-3p | 2.07 | 5.965 × 10−5 | 3.431 × 10−3 | 104 | mmu-mir-320-3p | 3.65 | 2.18 × 10−4 | 9.65 × 10−3 | 23 |
| mmu-mir-211-5p | 1.1 | 6.743 × 10−4 | 2.318 × 10−2 | 1391 | mmu-mir-193b-3p | 3.64 | 1.059 × 10−4 | 4.269 × 10−3 | 97 | mmu-mir-27a | 4.21 | 1.61 × 10−5 | 1.38 × 10−3 | 21 |
| mmu-mir-211-3p | 1.26 | 8.183 × 10−5 | 5.408 × 10−3 | 159 | mmu-miR-144-3p | 3.79 | 9.074 × 10−5 | 4.145 × 10−3 | 42 | mmu-mir-193b | 4.03 | 4.33 × 10−5 | 2.52 × 10−3 | 20 |
| mmu-mir-129-5p | 1.35 | 4.746 × 10−4 | 1.553 × 10−2 | 677 | mmu-mir-29c-3p | 3.42 | 2.991 × 10−4 | 1.161 × 10−2 | 23 | mmu-mir-221-3p | 3.39 | 7.87 × 10−4 | 2.80 × 10−2 | 15 |
| mmu-mir-99b-5p | 0.85 | 2.270 × 10−4 | 9.848 × 10−3 | 670 | mmu-mir-365-3p | 3.64 | 7.318 × 10−5 | 3.788 × 10−3 | 22 | mmu-let-7e-5p | 3.66 | 2.37 × 10−4 | 1.02 × 10−2 | 13 |
| mmu-mir-1249-3p | 2.11 | 6.548 × 10−8 | 1.515 × 10−5 | 524 | mmu-mir-26a-2-5p | 3.03 | 1.511 × 10−3 | 3.976 × 10−2 | 22 | mmu-mir-455-3p | 3.36 | 8.42 × 10−4 | 2.90 × 10−2 | 12 |
| mmu-mir-191-5p | 1.00 | 3.067 × 10−4 | 1.228 × 10−2 | 355 | mmu-let-7e-5p | 3.35 | 4.058 × 10−4 | 1.500 × 10−2 | 20 | |||||
| mmu-mir-129-3p | 2.10 | 3.328 × 10−6 | 3.554 × 10−4 | 322 | mmu-mir-328-3p | 4.18 | 8.692 × 10−6 | 1.038 × 10−3 | 16 | |||||
| mmu-mir-328-3p | 2.50 | 6.864 × 10−5 | 5.014 × 10−3 | 81 | mmu-mir-542-3p | 3.37 | 5.392 × 10−4 | 1.820 × 10−2 | 16 | |||||
| mmu-mir-151-3p | 2.38 | 1.237 × 10−4 | 7.153 × 10−3 | 61 | mmu-mir-23b-5p | 3.13 | 1.360 × 10−3 | 3.770 × 10−2 | 15 | |||||
| mmu-mir-143-3p | 4.01 | 6.562 × 10−7 | 8.280 × 10−5 | 40 | ||||||||||
| mmu-mir-149-5p | 3.03 | 9.253 × 10−4 | 2.987 × 10−2 | 26 | ||||||||||
| mmu-mir-6236 | 4.38 | 1.794 × 10−7 | 3.556 × 10−5 | 21 | ||||||||||
| mmu-mir-423-3p | 2.79 | 1.246 × 10−3 | 3.762 × 10−2 | 20 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, H.M.; Muñoz, E.N.; Osuna, D.; Florez, M.; Carvajal, M.; Gómez, L.A. Reciprocal Changes in miRNA Expression with Pigmentation and Decreased Proliferation Induced in Mouse B16F1 Melanoma Cells by l-Tyrosine and 5-Bromo-2′-Deoxyuridine. Int. J. Mol. Sci. 2021, 22, 1591. https://doi.org/10.3390/ijms22041591
Rivera HM, Muñoz EN, Osuna D, Florez M, Carvajal M, Gómez LA. Reciprocal Changes in miRNA Expression with Pigmentation and Decreased Proliferation Induced in Mouse B16F1 Melanoma Cells by l-Tyrosine and 5-Bromo-2′-Deoxyuridine. International Journal of Molecular Sciences. 2021; 22(4):1591. https://doi.org/10.3390/ijms22041591
Chicago/Turabian StyleRivera, Hernán Mauricio, Esther Natalia Muñoz, Daniel Osuna, Mauro Florez, Michael Carvajal, and Luis Alberto Gómez. 2021. "Reciprocal Changes in miRNA Expression with Pigmentation and Decreased Proliferation Induced in Mouse B16F1 Melanoma Cells by l-Tyrosine and 5-Bromo-2′-Deoxyuridine" International Journal of Molecular Sciences 22, no. 4: 1591. https://doi.org/10.3390/ijms22041591
APA StyleRivera, H. M., Muñoz, E. N., Osuna, D., Florez, M., Carvajal, M., & Gómez, L. A. (2021). Reciprocal Changes in miRNA Expression with Pigmentation and Decreased Proliferation Induced in Mouse B16F1 Melanoma Cells by l-Tyrosine and 5-Bromo-2′-Deoxyuridine. International Journal of Molecular Sciences, 22(4), 1591. https://doi.org/10.3390/ijms22041591





