Tumor Accumulation and Off-Target Biodistribution of an Indocyanine-Green Fluorescent Nanotracer: An Ex Vivo Study on an Orthotopic Murine Model of Breast Cancer
Abstract
1. Introduction
2. Results and Discussion
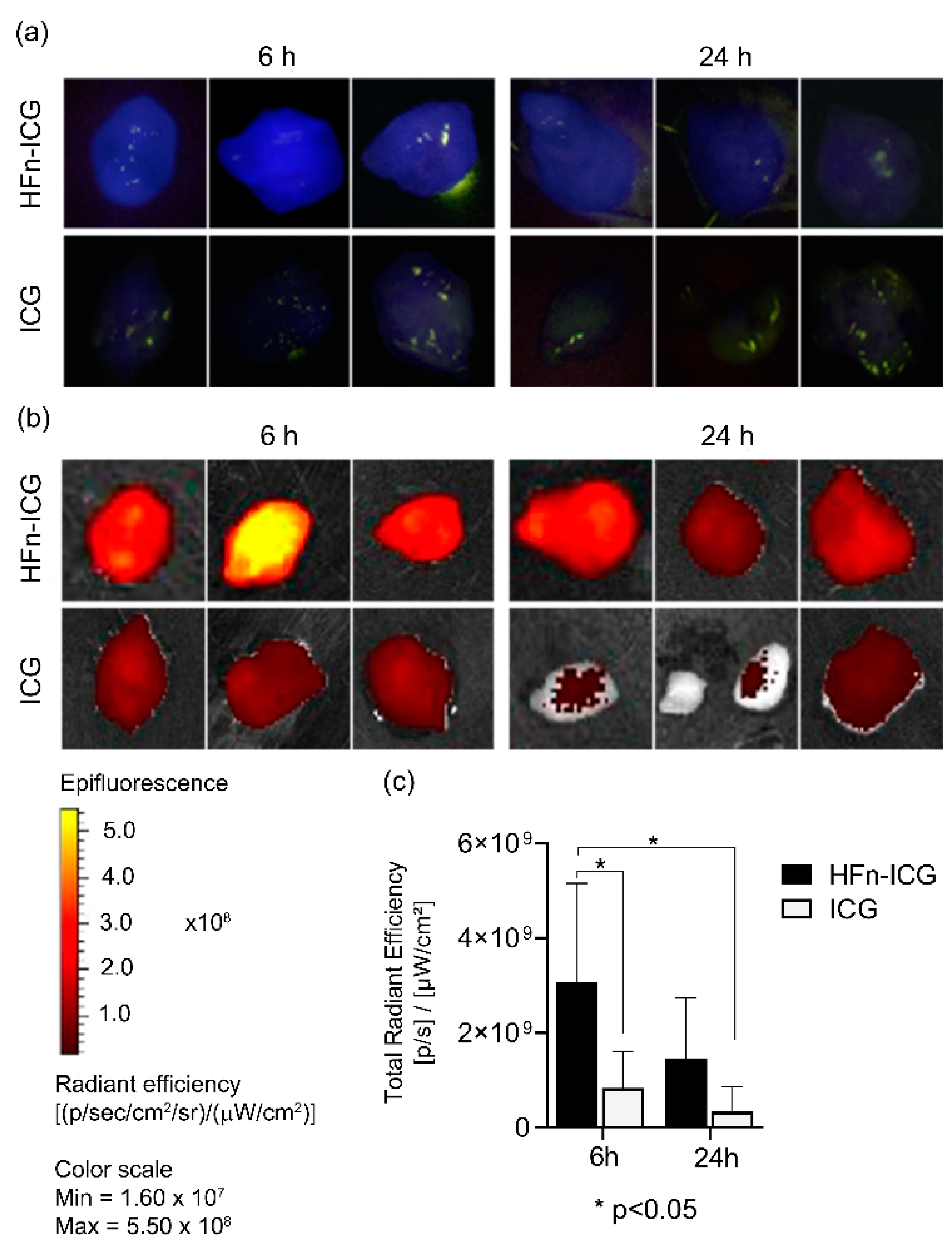
2.1. HFn-ICG Displayed a Higher Intratumor Accumulation Compared to Free ICG
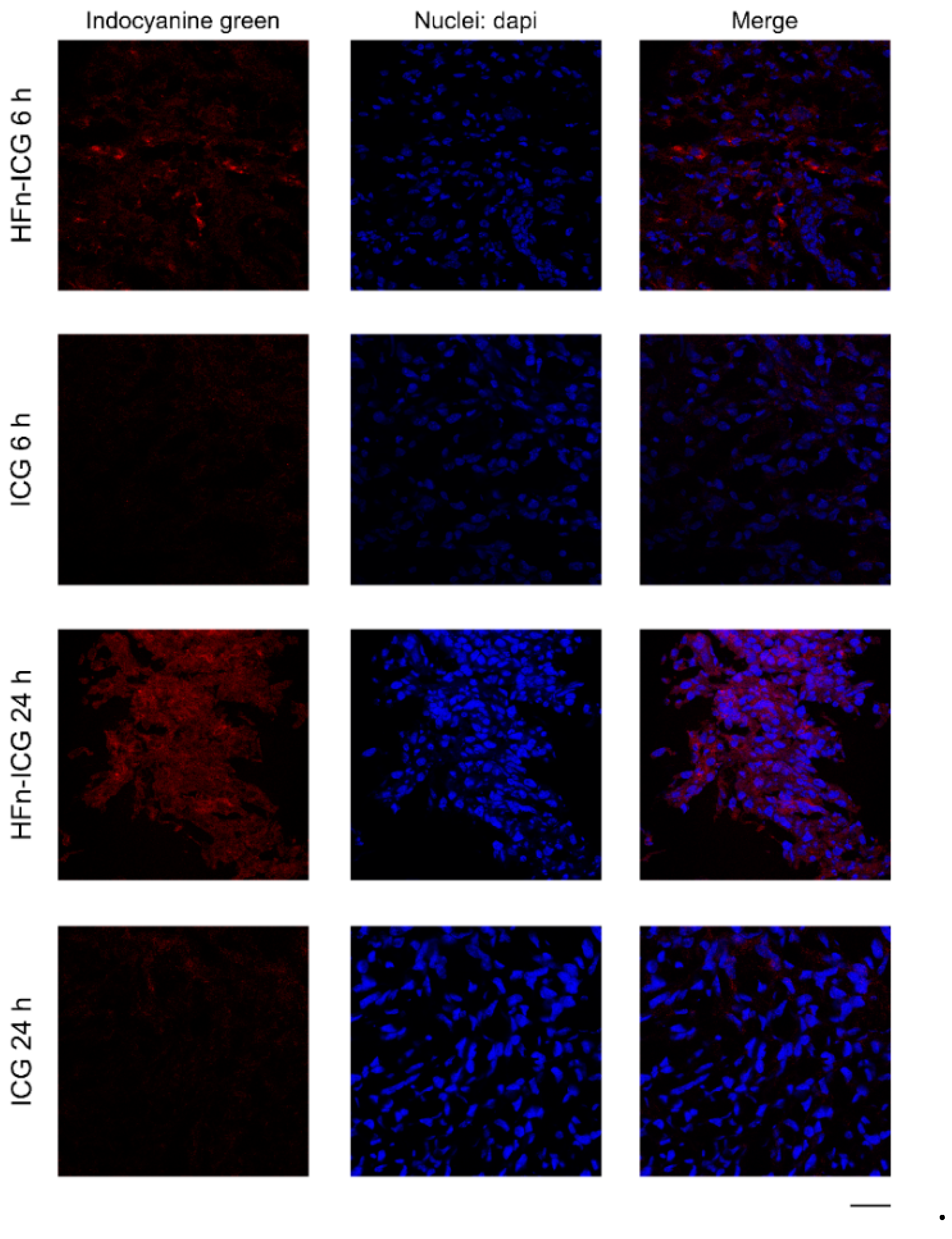
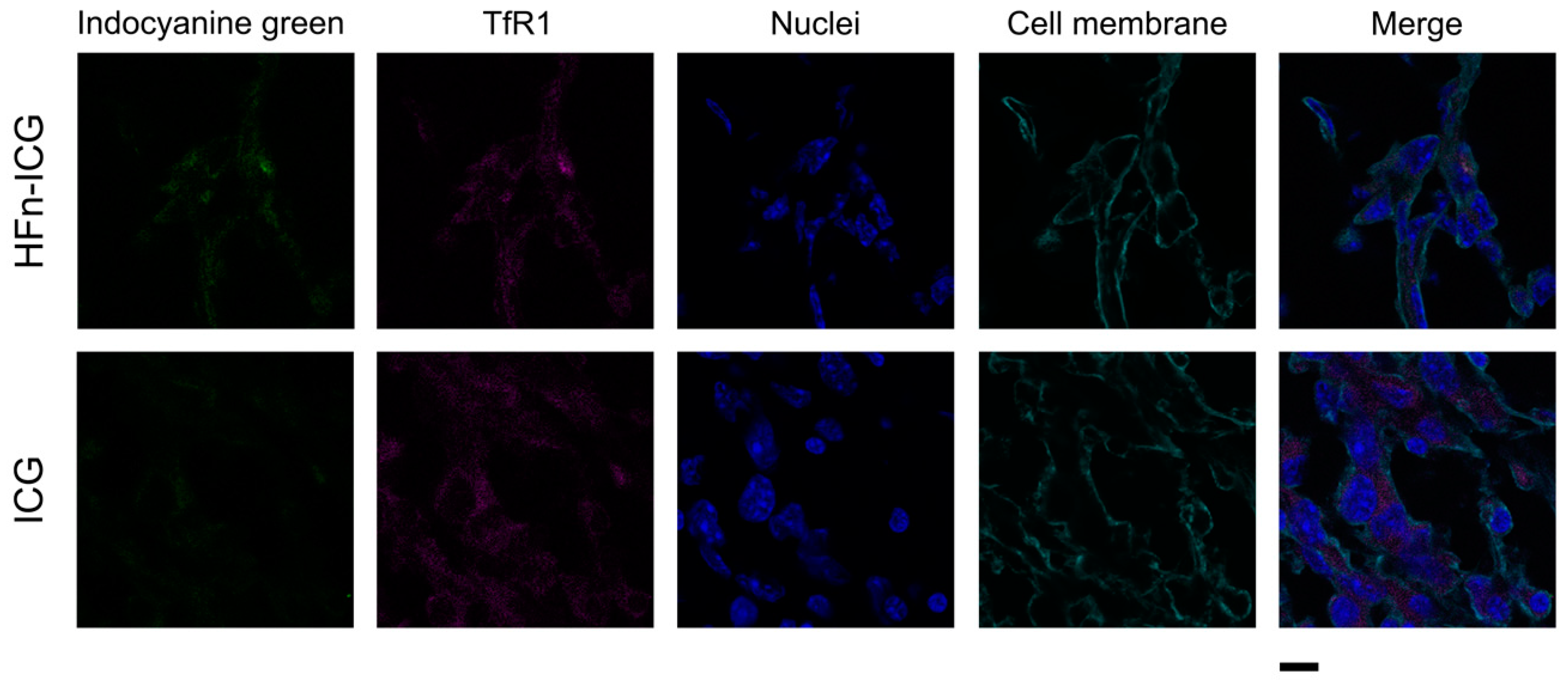
2.2. HFn Encapsulation Improves Tumor Uptake of ICG
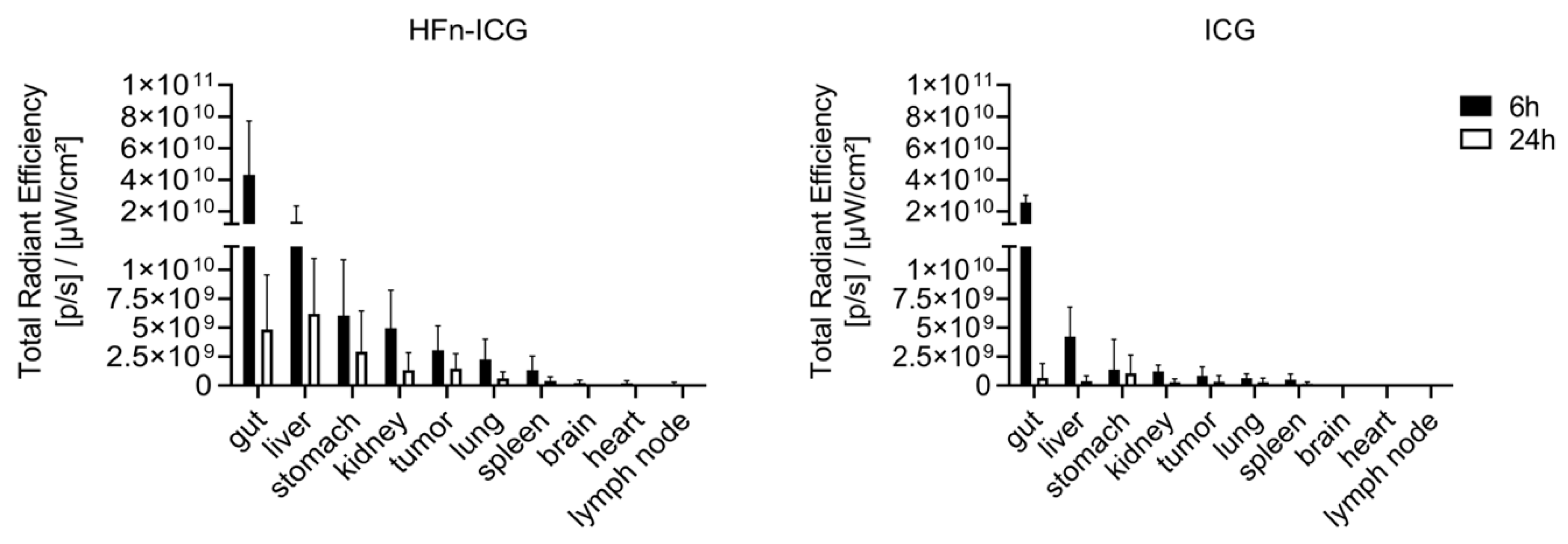
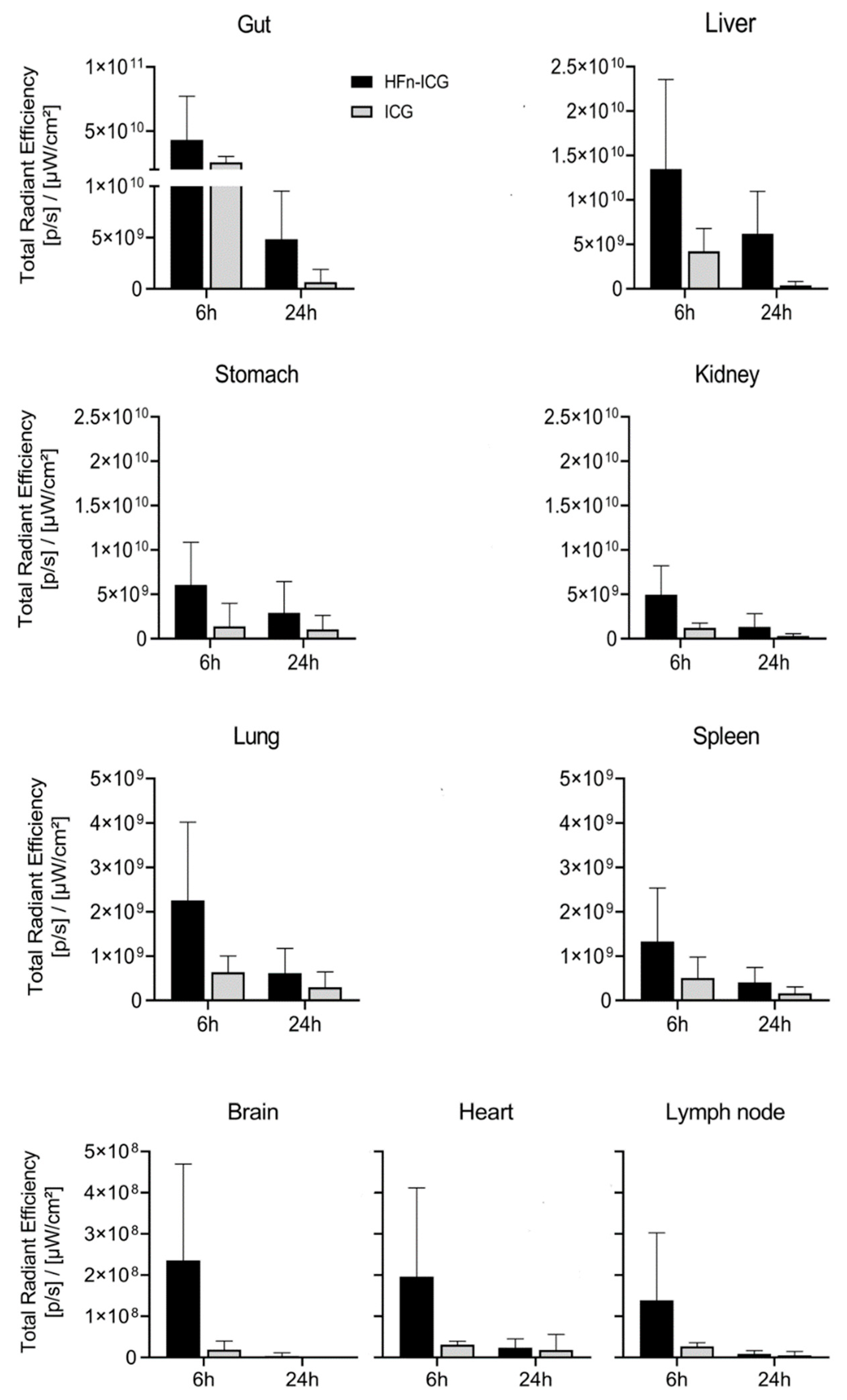
2.3. HFn Encapsulation Markedly Improved the ICG Kinetics of Biodistribution
3. Materials and Methods
3.1. Development of ICG-Loaded-HFn Nanoparticles
3.2. Animals
3.3. Tumor Targeting and Biodistribution
3.4. Confocal Laser Scanning Microscopy
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICG | Indocyanine Green |
| HFn | H-Ferritin |
| HFn−ICG | H-Ferritin loaded with ICG |
| FGS | Fluorescence-guided surgery |
| FDA | Food and Drug Administration |
| NIR | Near-infrared |
| SLN | Sentinel lymph node |
| TfR1 | Transferrin receptor 1 |
| DAPI | 4’,6-Diamidino-2-phenylindole dihydrochloride |
| ROI | Region of interest |
| SD | Standard deviation |
| PBS | Phosphate saline buffer |
| BSA | Bovine serum albumin |
| IgG | Immunoglobulin G |
| SNR | Signal to noise ratio |
| SBR | Signal to background ratio |
References
- Zheng, Y.; Yang, H.; Wang, H.; Kang, K.; Zhang, W.; Ma, G.; Du, S. Fluorescence-guided surgery in cancer treatment: Current status and future perspectives. Ann. Transl. Med. 2019, 7, S6. [Google Scholar] [CrossRef] [PubMed]
- Tringale, K.R.; Pang, J.; Nguyen, Q.T. Image-guided surgery in cancer: A strategy to reduce incidence of positive surgical margins. WIREs Syst. Biol. Med. 2018, 10, e1412. [Google Scholar] [CrossRef]
- Mondal, S.B.; Gao, S.; Zhu, N.; Liang, R.; Gruev, V.; Achilefu, S. Real-time fluorescence image-guided oncologic surgery. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 124, pp. 171–211. ISBN 978-0-12-411638-2. [Google Scholar]
- Sorrentino, L.; Sartani, A.; Pietropaolo, G.; Bossi, D.; Mazzucchelli, S.; Truffi, M.; Foschi, D.; Corsi, F. A novel indocyanine green fluorescence-guided video-assisted technique for sentinel node biopsy in breast cancer. World J. Surg. 2018, 42, 2815–2824. [Google Scholar] [CrossRef]
- Okusanya, O.T.; Madajewski, B.; Segal, E.; Judy, B.F.; Venegas, O.G.; Judy, R.P.; Quatromoni, J.G.; Wang, M.D.; Nie, S.; Singhal, S. Small portable interchangeable imager of fluorescence for fluorescence guided surgery and research. Technol. Cancer Res. Treat. 2015, 14, 213–220. [Google Scholar] [CrossRef]
- Chi, C.; Du, Y.; Ye, J.; Kou, D.; Qiu, J.; Wang, J.; Tian, J.; Chen, X. Intraoperative imaging-guided cancer surgery: From current fluorescence molecular imaging methods to future multi-modality imaging technology. Theranostics 2014, 4, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A review of indocyanine green fluorescent imaging in surgery. Int. J. Biomed. Imaging 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Schaafsma, B.E.; Mieog, J.S.D.; Hutteman, M.; van der Vorst, J.R.; Kuppen, P.J.K.; Löwik, C.W.G.M.; Frangioni, J.V.; van de Velde, C.J.H.; Vahrmeijer, A.L. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J. Surg. Oncol. 2011, 104, 323–332. [Google Scholar] [CrossRef]
- DSouza, A.V.; Lin, H.; Henderson, E.R.; Samkoe, K.S.; Pogue, B.W. Review of fluorescence guided surgery systems: Identification of key performance capabilities beyond indocyanine green imaging. J. Biomed. Opt. 2016, 21, 80901. [Google Scholar] [CrossRef] [PubMed]
- Barth, C.W.; Gibbs, S. Fluorescence image-guided surgery: A perspective on contrast agent development. Proc. SPIE 2020, 11222. [Google Scholar] [CrossRef]
- Low, P.S.; Singhal, S.; Srinivasarao, M. Fluorescence-guided surgery of cancer: Applications, tools and perspectives. Curr. Opin. Chem. Biol. 2018, 45, 64–72. [Google Scholar] [CrossRef]
- Hill, T.K.; Abdulahad, A.; Kelkar, S.S.; Marini, F.C.; Long, T.E.; Provenzale, J.M.; Mohs, A.M. Indocyanine green-loaded nanoparticles for image-guided tumor surgery. Bioconjugate Chem. 2015, 26, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zheng, M.; Yue, C.; Luo, Z.; Gong, P.; Gao, G.; Sheng, Z.; Zheng, C.; Cai, L. Improving drug accumulation and photothermal efficacy in tumor depending on size of ICG loaded lipid-polymer nanoparticles. Biomaterials 2014, 35, 6037–6046. [Google Scholar] [CrossRef]
- Nagaya, T.; Nakamura, Y.A.; Choyke, P.L.; Kobayashi, H. Fluorescence-guided surgery. Front. Oncol. 2017, 7, 314. [Google Scholar] [CrossRef]
- Muhanna, N.; Chan, H.H.L.; Douglas, C.M.; Daly, M.J.; Jaidka, A.; Eu, D.; Bernstein, J.; Townson, J.L.; Irish, J.C. Sentinel lymph node mapping using ICG fluorescence and cone beam CT—A feasibility study in a rabbit model of oral cancer. BMC Med. Imaging 2020, 20, 106. [Google Scholar] [CrossRef] [PubMed]
- Starosolski, Z.; Bhavane, R.; Ghaghada, K.B.; Vasudevan, S.A.; Kaay, A.; Annapragada, A. Indocyanine green fluorescence in second near-infrared (NIR-II) window. PLoS ONE 2017, 12, e0187563. [Google Scholar] [CrossRef]
- Alius, C.; Oprescu, S.; Balalau, C.; Elena Nica, A. Indocyanine green enhanced surgery; principle, clinical applications and future research directions. J. Clin. Investig. Surg. 2018, 3, 1–8. [Google Scholar] [CrossRef]
- Verbeek, F.P.R.; Troyan, S.L.; Mieog, J.S.D.; Liefers, G.-J.; Moffitt, L.A.; Rosenberg, M.; Hirshfield-Bartek, J.; Gioux, S.; van de Velde, C.J.H.; Vahrmeijer, A.L.; et al. Near-infrared fluorescence sentinel lymph node mapping in breast cancer: A multicenter experience. Breast Cancer Res. Treat. 2014, 143, 333–342. [Google Scholar] [CrossRef]
- Saxena, V.; Sadoqi, M.; Shao, J. Degradation kinetics of indocyanine green in aqueous solution. J. Pharm. Sci. 2003, 92, 2090–2097. [Google Scholar] [CrossRef]
- Muckle, T.J. Plasma proteins binding of indocyanine green. Biochem. Med. 1976, 15, 17–21. [Google Scholar] [CrossRef]
- Veys, I.; Pop, C.-F.; Barbieux, R.; Moreau, M.; Noterman, D.; De Neubourg, F.; Nogaret, J.-M.; Liberale, G.; Larsimont, D.; Bourgeois, P. ICG fluorescence imaging as a new tool for optimization of pathological evaluation in breast cancer tumors after neoadjuvant chemotherapy. PLoS ONE 2018, 13, e0197857. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Tse, B.W.-C.; Yang, H.; Thorling, C.A.; Liu, Y.; Touraud, M.; Chouane, J.B.; Liu, X.; Roberts, M.S.; et al. Indocyanine green-incorporating nanoparticles for cancer theranostics. Theranostics 2018, 8, 1227–1242. [Google Scholar] [CrossRef]
- Sevieri, M.; Silva, F.; Bonizzi, A.; Sitia, L.; Truffi, M.; Mazzucchelli, S.; Corsi, F. Indocyanine green nanoparticles: Are they compelling for cancer treatment? Front. Chem. 2020, 8, 535. [Google Scholar] [CrossRef]
- Egloff-Juras, C.; Bezdetnaya, L.; Dolivet, G.; Lassalle, H.-P. NIR fluorescence-guided tumor surgery: New strategies for the use of indocyanine green. IJN 2019, 14, 7823–7838. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Jiang, H. Image-guided surgery using multimodality strategy and molecular probes: Image-guided surgery using multimodality strategy and molecular probes. WIREs Nanomed. Nanobiotechnol. 2016, 8, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Truffi, M.; Fiandra, L.; Sorrentino, L.; Monieri, M.; Corsi, F.; Mazzucchelli, S. Ferritin nanocages: A biological platform for drug delivery, imaging and theranostics in cancer. Pharmacol. Res. 2016, 107, 57–65. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, H.; Zhang, Y.; Liu, G.; Niu, G.; Chen, X. Functional ferritin nanoparticles for biomedical applications. Front. Chem. Sci. Eng. 2017, 11, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-R.; Ko, H.K.; Ryu, J.H.; Ahn, K.Y.; Lee, Y.-H.; Oh, S.J.; Na, J.H.; Kim, T.W.; Byun, Y.; Kwon, I.C.; et al. Engineered human ferritin nanoparticles for direct delivery of tumor antigens to lymph node and cancer immunotherapy. Sci. Rep. 2016, 6, 35182. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fang, C.J.; Ryan, J.C.; Niemi, E.C.; Lebrón, J.A.; Björkman, P.J.; Arase, H.; Torti, F.M.; Torti, S.V.; Nakamura, M.C.; et al. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc. Natl. Acad. Sci. USA 2010, 107, 3505–3510. [Google Scholar] [CrossRef]
- Fan, K.; Cao, C.; Pan, Y.; Lu, D.; Yang, D.; Feng, J.; Song, L.; Liang, M.; Yan, X. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat. Nanotechnol. 2012, 7, 459–464. [Google Scholar] [CrossRef]
- Cheng, X.; Fan, K.; Wang, L.; Ying, X.; Sanders, A.J.; Guo, T.; Xing, X.; Zhou, M.; Du, H.; Hu, Y.; et al. TfR1 binding with H-ferritin nanocarrier achieves prognostic diagnosis and enhances the therapeutic efficacy in clinical gastric cancer. Cell Death Dis. 2020, 11, 92. [Google Scholar] [CrossRef]
- Fan, K.; Yan, X. Bioengineered ferritin nanoprobes for cancer theranostics. In Handbook of Nanomaterials for Cancer Theranostics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 143–175. ISBN 978-0-12-813339-2. [Google Scholar]
- Liang, M.; Fan, K.; Zhou, M.; Duan, D.; Zheng, J.; Yang, D.; Feng, J.; Yan, X. H-ferritin-nanocaged doxorubicin nanoparticles specifically target and kill tumors with a single-dose injection. Proc. Natl. Acad. Sci. USA 2014, 111, 14900–14905. [Google Scholar] [CrossRef]
- Mazzucchelli, S.; Bellini, M.; Fiandra, L.; Truffi, M.; Rizzuto, M.A.; Sorrentino, L.; Longhi, E.; Nebuloni, M.; Prosperi, D.; Corsi, F. Nanometronomic treatment of 4T1 breast cancer with nanocaged doxorubicin prevents drug resistance and circumvents cardiotoxicity. Oncotarget 2017, 8, 8383–8396. [Google Scholar] [CrossRef] [PubMed]
- Andreata, F.; Bonizzi, A.; Sevieri, M.; Truffi, M.; Monieri, M.; Sitia, L.; Silva, F.; Sorrentino, L.; Allevi, R.; Zerbi, P.; et al. Co-administration of H-ferritin-doxorubicin and trastuzumab in neoadjuvant setting improves efficacy and prevents cardiotoxicity in HER2 + murine breast cancer model. Sci. Rep. 2020, 10, 11425. [Google Scholar] [CrossRef]
- Ruggiero, M.; Alberti, D.; Bitonto, V.; Geninatti Crich, S. Ferritin: A platform for MRI contrast agents delivery. Inorganics 2019, 7, 33. [Google Scholar] [CrossRef]
- Zhen, Z.; Tang, W.; Todd, T.; Xie, J. Ferritins as nanoplatforms for imaging and drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Sitia, L.; Sevieri, M.; Bonizzi, A.; Allevi, R.; Morasso, C.; Foschi, D.; Corsi, F.; Mazzucchelli, S. Development of tumor-targeted indocyanine green-loaded ferritin nanoparticles for intraoperative detection of cancers. ACS Omega 2020, 5, 12035–12045. [Google Scholar] [CrossRef]
- Mazzucchelli, S.; Truffi, M.; Baccarini, F.; Beretta, M.; Sorrentino, L.; Bellini, M.; Rizzuto, M.A.; Ottria, R.; Ravelli, A.; Ciuffreda, P.; et al. H-ferritin-nanocaged olaparib: A promising choice for both BRCA-mutated and sporadic triple negative breast cancer. Sci. Rep. 2017, 7, 7505. [Google Scholar] [CrossRef]
- Bonizzi, A.; Truffi, M.; Sevieri, M.; Allevi, R.; Sitia, L.; Ottria, R.; Sorrentino, L.; Sottani, C.; Negri, S.; Grignani, E.; et al. Everolimus nanoformulation in biological nanoparticles increases drug responsiveness in resistant and low-responsive breast cancer cell lines. Pharmaceutics 2019, 11, 384. [Google Scholar] [CrossRef]
- Falvo, E.; Tremante, E.; Fraioli, R.; Leonetti, C.; Zamparelli, C.; Boffi, A.; Morea, V.; Ceci, P.; Giacomini, P. Antibody-drug conjugates: Targeting melanoma with cisplatin encapsulated in protein-cage nanoparticles based on human ferritin. Nanoscale 2013, 5, 12278–12285. [Google Scholar] [CrossRef]
- Pandolfi, L.; Bellini, M.; Vanna, R.; Morasso, C.; Zago, A.; Carcano, S.; Avvakumova, S.; Bertolini, J.A.; Rizzuto, M.A.; Colombo, M.; et al. H-ferritin enriches the curcumin uptake and improves the therapeutic efficacy in triple negative breast cancer cells. Biomacromolecules 2017, 18, 3318–3330. [Google Scholar] [CrossRef]
- Falvo, E.; Malagrinò, F.; Arcovito, A.; Fazi, F.; Colotti, G.; Tremante, E.; Di Micco, P.; Braca, A.; Opri, R.; Giuffrè, A.; et al. The presence of glutamate residues on the PAS sequence of the stimuli-sensitive nano-ferritin improves in vivo biodistribution and mitoxantrone encapsulation homogeneity. J. Control. Release 2018, 275, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Damiani, V.; Falvo, E.; Fracasso, G.; Federici, L.; Pitea, M.; De Laurenzi, V.; Sala, G.; Ceci, P. Therapeutic efficacy of the novel stimuli-sensitive nano-ferritins containing doxorubicin in a head and neck cancer model. Int. J. Mol. Sci. 2017, 18, 1555. [Google Scholar] [CrossRef] [PubMed]
- Fracasso, G.; Falvo, E.; Colotti, G.; Fazi, F.; Ingegnere, T.; Amalfitano, A.; Doglietto, G.B.; Alfieri, S.; Boffi, A.; Morea, V.; et al. Selective delivery of doxorubicin by novel stimuli-sensitive nano-ferritins overcomes tumor refractoriness. J. Control. Release 2016, 239, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, L.; Di Penta, A.; Carmona, U.; Yang, F.; Schöps, R.; Brandsch, M.; Zugaza, J.L.; Knez, M. H-chain ferritin: A natural nuclei targeting and bioactive delivery nanovector. Adv. Healthcare Mater. 2015, 4, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Fiandra, L.; Mazzucchelli, S.; De Palma, C.; Colombo, M.; Allevi, R.; Sommaruga, S.; Clementi, E.; Bellini, M.; Prosperi, D.; Corsi, F. Assessing the in vivo targeting efficiency of multifunctional nanoconstructs bearing antibody-derived ligands. ACS Nano 2013, 7, 6092–6102. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevieri, M.; Sitia, L.; Bonizzi, A.; Truffi, M.; Mazzucchelli, S.; Corsi, F. Tumor Accumulation and Off-Target Biodistribution of an Indocyanine-Green Fluorescent Nanotracer: An Ex Vivo Study on an Orthotopic Murine Model of Breast Cancer. Int. J. Mol. Sci. 2021, 22, 1601. https://doi.org/10.3390/ijms22041601
Sevieri M, Sitia L, Bonizzi A, Truffi M, Mazzucchelli S, Corsi F. Tumor Accumulation and Off-Target Biodistribution of an Indocyanine-Green Fluorescent Nanotracer: An Ex Vivo Study on an Orthotopic Murine Model of Breast Cancer. International Journal of Molecular Sciences. 2021; 22(4):1601. https://doi.org/10.3390/ijms22041601
Chicago/Turabian StyleSevieri, Marta, Leopoldo Sitia, Arianna Bonizzi, Marta Truffi, Serena Mazzucchelli, and Fabio Corsi. 2021. "Tumor Accumulation and Off-Target Biodistribution of an Indocyanine-Green Fluorescent Nanotracer: An Ex Vivo Study on an Orthotopic Murine Model of Breast Cancer" International Journal of Molecular Sciences 22, no. 4: 1601. https://doi.org/10.3390/ijms22041601
APA StyleSevieri, M., Sitia, L., Bonizzi, A., Truffi, M., Mazzucchelli, S., & Corsi, F. (2021). Tumor Accumulation and Off-Target Biodistribution of an Indocyanine-Green Fluorescent Nanotracer: An Ex Vivo Study on an Orthotopic Murine Model of Breast Cancer. International Journal of Molecular Sciences, 22(4), 1601. https://doi.org/10.3390/ijms22041601






