Two 20-Residue-Long Peptides Derived from Plasmodium vivax Merozoite Surface Protein 10 EGF-Like Domains Are Involved in Binding to Human Reticulocytes
Abstract
:1. Introduction
2. Results
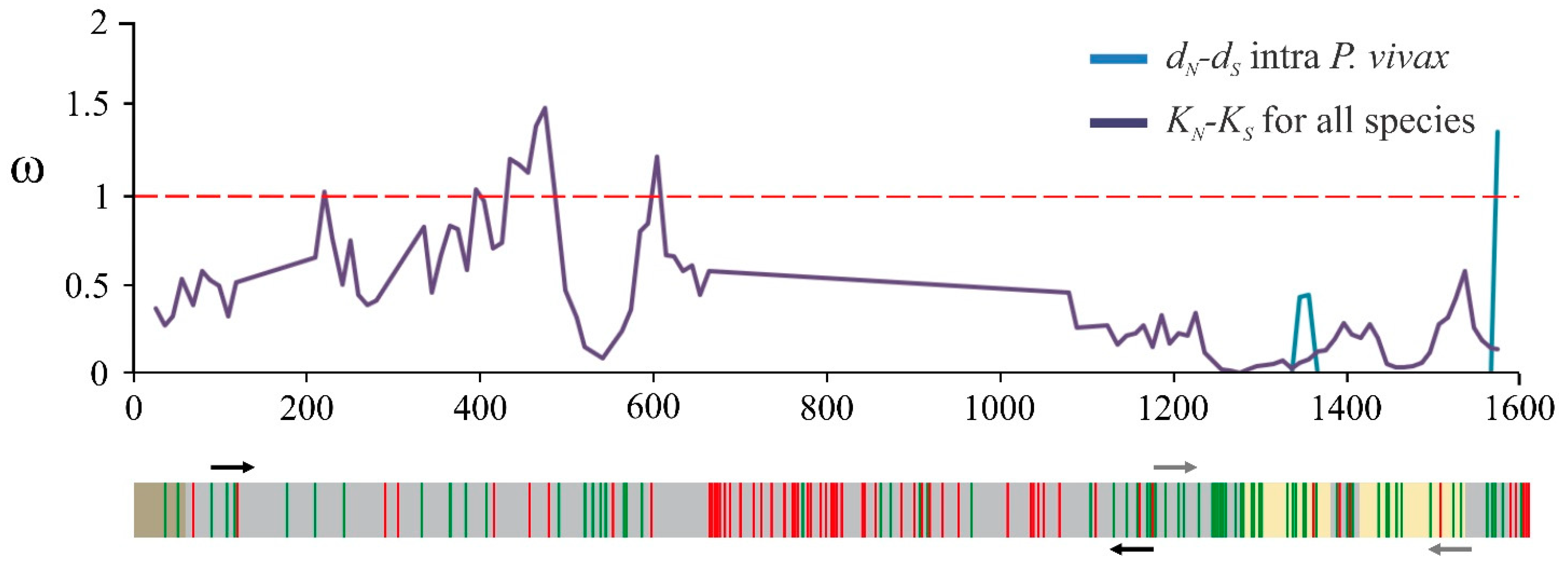
2.1. The msp10 Gene’s Limited Diversity Is a Worldwide Characteristic
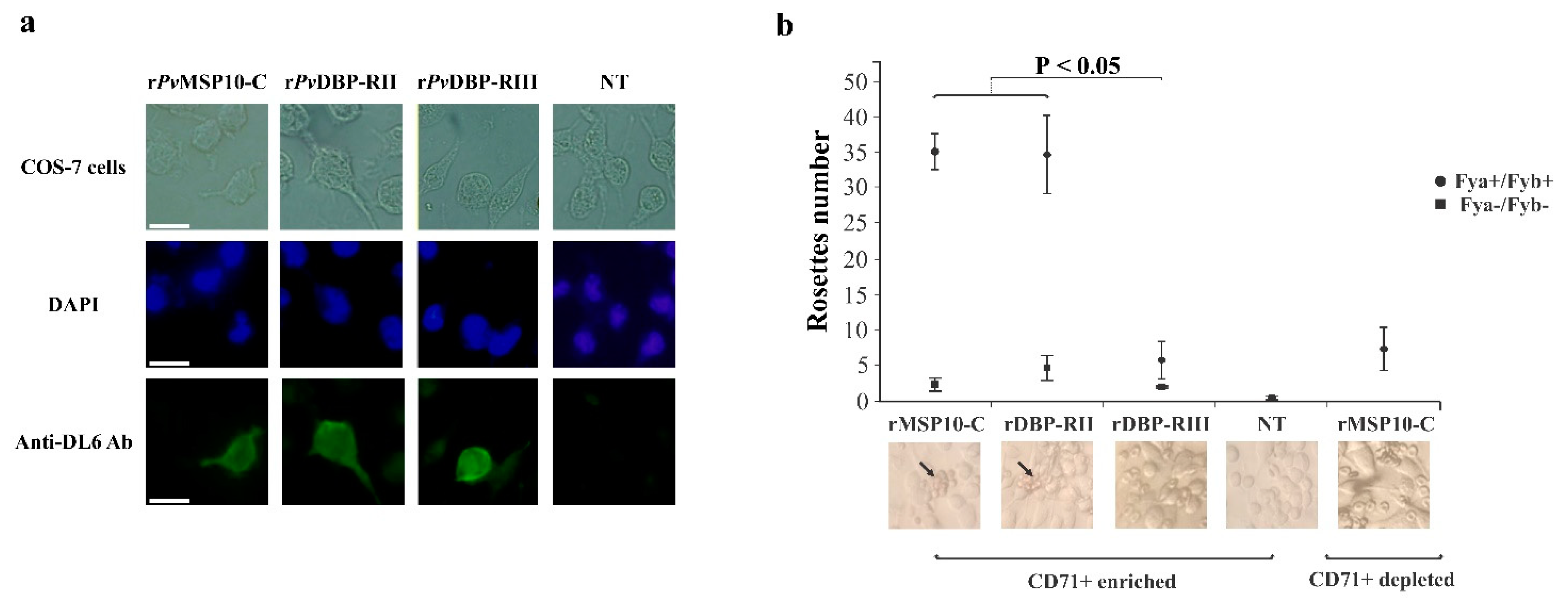
2.2. 3′-End Gene Region-Encoded PvMSP10-C Bound to Human Reticulocytes
2.3. PvMSP10 Binding Activity Was Governed by Two Small EGF-Like Domain-Derived Peptides
2.4. Peptides 42419 and 42420 Are Promising Vaccine Candidates
3. Discussion
4. Materials and Methods
4.1. msp10 Genetic Diversity and Natural Selection
4.2. Plasmid Construction
4.3. Obtaining and Purifying Recombinant Proteins
4.4. Ethics Committee and Reticulocyte Separation by Positive Selection
4.5. RBC Haemolysis Assay
4.6. Evaluating Protein-Cell Interaction by Flow Cytometry
4.7. COS-7 Cell Maintenance and Transfection
4.8. Erythrocyte Binding to COS-7 Transfected Cells Assay
4.9. Statistical Analysis
4.10. rPvMSP10-C350-478 3D Structural Modelling and Bioinformatics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| P. falciparum | Plasmodium falciparum |
| P. vivax | Plasmodium vivax |
| RBC | red blood cell |
| Mrz | merozoite |
| MSP1 | merozoite surface protein 1 |
| MSP1-P | MSP1 paralogue |
| RBSA | reticulocyte binding surface protein |
| TRAgs | tryptophan-rich antigen |
| GPI | glycosylphosphatidylinositol |
| EGF | epidermal growth factor |
| PvMSP10 | P. vivax merozoite surface protein 10 |
| PvMSP10-N | P. vivax merozoite surface protein 10 amino terminal region |
| PvMSP10-C | P. vivax merozoite surface protein 10 carboxyl terminal region |
| E. coli | Escherichia coli |
| PfMSP10 | Plasmodium falciparum merozoite surface protein 10 |
| CFU | colony forming unit |
| r | recombinant |
| IMAC | immobilised metal ion affinity chromatography |
| PBS | phosphate buffered saline |
| DAPI | 4′,6-diamidino-2-phenylindole |
| I-TASSER | iterative threading assembly refinement |
| BCE | B-cell epitope |
| TCE | T-cell epitope |
| aa | amino acid |
References
- Beeson, J.G.; Drew, D.R.; Boyle, M.J.; Feng, G.; Fowkes, F.J.; Richards, J.S. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol. Rev. 2016, 40, 343–372. [Google Scholar] [CrossRef] [Green Version]
- Mueller, I.; Galinski, M.R.; Baird, J.K.; Carlton, J.M.; Kochar, D.K.; Alonso, P.L.; del Portillo, H.A. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect. Dis. 2009, 9, 555–566. [Google Scholar] [CrossRef]
- Han, H.J.; Park, S.G.; Kim, S.H.; Hwang, S.Y.; Han, J.; Traicoff, J.; Kho, W.G.; Chung, J.Y. Epidermal growth factor-like motifs 1 and 2 of Plasmodium vivax merozoite surface protein 1 are critical domains in erythrocyte invasion. Biochem. Biophys. Res. Commun. 2004, 320, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Valderrama-Aguirre, A.; Quintero, G.; Gomez, A.; Castellanos, A.; Perez, Y.; Mendez, F.; Arevalo-Herrera, M.; Herrera, S. Antigenicity, immunogenicity, and protective efficacy of Plasmodium vivax MSP1 PV200l: A potential malaria vaccine subunit. Am. J. Trop Med. Hyg 2005, 73 (Suppl. S5), 16–24. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, L.E.; Urquiza, M.; Ocampo, M.; Curtidor, H.; Suarez, J.; Garcia, J.; Vera, R.; Puentes, A.; Lopez, R.; Pinto, M.; et al. Plasmodium vivax MSP-1 peptides have high specific binding activity to human reticulocytes. Vaccine 2002, 20, 1331–1339. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Y.; Ito, D.; Kong, D.H.; Ha, K.S.; Chen, J.H.; Lu, F.; Li, J.; Wang, B.; Takashima, E.; et al. The Plasmodium vivax merozoite surface protein 1 paralog is a novel erythrocyte-binding ligand of P. vivax. Infect. Immun. 2013, 81, 1585–1595. [Google Scholar] [CrossRef] [Green Version]
- Camargo-Ayala, P.A.; Garzon-Ospina, D.; Moreno-Perez, D.A.; Ricaurte-Contreras, L.A.; Noya, O.; Patarroyo, M.A. On the Evolution and Function of Plasmodium vivax Reticulocyte Binding Surface Antigen (PvRBSA). Front. Genet. 2018, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Bozdech, Z.; Mok, S.; Hu, G.; Imwong, M.; Jaidee, A.; Russell, B.; Ginsburg, H.; Nosten, F.; Day, N.P.; White, N.J.; et al. The transcriptome of Plasmodium vivax reveals divergence and diversity of transcriptional regulation in malaria parasites. Proc. Natl. Acad. Sci. USA 2008, 105, 16290–16295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeeshan, M.; Tyagi, R.K.; Tyagi, K.; Alam, M.S.; Sharma, Y.D. Host-parasite interaction: Selective Pv-fam-a family proteins of Plasmodium vivax bind to a restricted number of human erythrocyte receptors. J. Infect. Dis 2015, 211, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Perez-Leal, O.; Sierra, A.Y.; Barrero, C.A.; Moncada, C.; Martinez, P.; Cortes, J.; Lopez, Y.; Salazar, L.M.; Hoebeke, J.; Patarroyo, M.A. Identifying and characterising the Plasmodium falciparum merozoite surface protein 10 Plasmodium vivax homologue. Biochem. Biophys. Res. Commun. 2005, 331, 1178–1184. [Google Scholar] [CrossRef]
- Garzon-Ospina, D.; Forero-Rodriguez, J.; Patarroyo, M.A. Inferring natural selection signals in Plasmodium vivax-encoded proteins having a potential role in merozoite invasion. Infect. Genet. Evol. 2015, 33, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.A.; Elango, A.P.; Rahman, A.A.; Fisher, D.; Collins, W.E.; Barnwell, J.W.; Escalante, A.A. Evidence of purifying selection on Merozoite Surface Protein 8 (MSP8) and 10 (MSP10) in Plasmodium spp. Infect. Genet. Evol. 2012, 12, 978–986. [Google Scholar] [CrossRef] [Green Version]
- Baum, E.; Sattabongkot, J.; Sirichaisinthop, J.; Kiattibutr, K.; Davies, D.H.; Jain, A.; Lo, E.; Lee, M.C.; Randall, A.Z.; Molina, D.M.; et al. Submicroscopic and asymptomatic Plasmodium falciparum and Plasmodium vivax infections are common in western Thailand—Molecular and serological evidence. Malar. J. 2015, 14, 95. [Google Scholar] [CrossRef] [Green Version]
- Arevalo-Herrera, M.; Lopez-Perez, M.; Dotsey, E.; Jain, A.; Rubiano, K.; Felgner, P.L.; Davies, D.H.; Herrera, S. Antibody Profiling in Naive and Semi-immune Individuals Experimentally Challenged with Plasmodium vivax Sporozoites. PLoS Negl. Trop. Dis. 2016, 10, e0004563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Wang, B.; Sattabongkot, J.; Lim, C.S.; Tsuboi, T.; Han, E.T. Immunogenicity and antigenicity of Plasmodium vivax merozoite surface protein 10. Parasitol. Res. 2014, 113, 2559–2568. [Google Scholar] [CrossRef]
- Russell, B.; Suwanarusk, R.; Borlon, C.; Costa, F.T.; Chu, C.S.; Rijken, M.J.; Sriprawat, K.; Warter, L.; Koh, E.G.; Malleret, B.; et al. A reliable ex vivo invasion assay of human reticulocytes by Plasmodium vivax. Blood 2011, 118, e74–e81. [Google Scholar] [CrossRef] [PubMed]
- Malleret, B.; Xu, F.; Mohandas, N.; Suwanarusk, R.; Chu, C.; Leite, J.A.; Low, K.; Turner, C.; Sriprawat, K.; Zhang, R.; et al. Significant biochemical, biophysical and metabolic diversity in circulating human cord blood reticulocytes. PLoS ONE 2013, 8, e76062. [Google Scholar] [CrossRef] [Green Version]
- Drew, D.R.; O’Donnell, R.A.; Smith, B.J.; Crabb, B.S. A common cross-species function for the double epidermal growth factor-like modules of the highly divergent plasmodium surface proteins MSP-1 and MSP-8. J. Biol. Chem. 2004, 279, 20147–20153. [Google Scholar] [CrossRef] [Green Version]
- Wouters, M.A.; Rigoutsos, I.; Chu, C.K.; Feng, L.L.; Sparrow, D.B.; Dunwoodie, S.L. Evolution of distinct EGF domains with specific functions. Protein Sci. 2005, 14, 1091–1103. [Google Scholar] [CrossRef] [Green Version]
- Kanjee, U.; Rangel, G.W.; Clark, M.A.; Duraisingh, M.T. Molecular and cellular interactions defining the tropism of Plasmodium vivax for reticulocytes. Curr. Opin. Microbiol. 2018, 46, 109–115. [Google Scholar] [CrossRef]
- Kimura, M. The neutral theory of molecular evolution. Sci. Am. 1979, 241, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R. Molecular signatures of natural selection. Annu Rev. Genet. 2005, 39, 197–218. [Google Scholar] [CrossRef] [Green Version]
- Valderrama-Aguirre, A.; Zuniga-Soto, E.; Marino-Ramirez, L.; Moreno, L.A.; Escalante, A.A.; Arevalo-Herrera, M.; Herrera, S. Polymorphism of the Pv200L fragment of Merozoite Surface Protein-1 of Plasmodium vivax in clinical isolates from the Pacific coast of Colombia. Am. J. Trop. Med. Hyg. 2011, 84 (Suppl. S2), 64–70. [Google Scholar] [CrossRef] [Green Version]
- Mascorro, C.N.; Zhao, K.; Khuntirat, B.; Sattabongkot, J.; Yan, G.; Escalante, A.A.; Cui, L. Molecular evolution and intragenic recombination of the merozoite surface protein MSP-3alpha from the malaria parasite Plasmodium vivax in Thailand. Parasitology 2005, 131 Pt 1, 25–35. [Google Scholar] [CrossRef]
- Garzon-Ospina, D.; Lopez, C.; Forero-Rodriguez, J.; Patarroyo, M.A. Genetic diversity and selection in three Plasmodium vivax merozoite surface protein 7 (pvmsp-7) genes in a Colombian population. PLoS ONE 2012, 7, e45962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garzon-Ospina, D.; Forero-Rodriguez, J.; Patarroyo, M.A. Heterogeneous genetic diversity pattern in Plasmodium vivax genes encoding merozoite surface proteins (MSP) -7E, -7F and -7L. Malar. J. 2014, 13, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, M.C.; Trakarnsanga, K.; Heesom, K.J.; Cogan, N.; Green, C.; Toye, A.M.; Parsons, S.F.; Anstee, D.J.; Frayne, J. Comparison of the Proteome of Adult and Cord Erythroid Cells, and Changes in the Proteome Following Reticulocyte Maturation. Mol. Cell Proteom. 2016, 15, 1938–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaoka, H.; Kanoi, B.N.; Jinoka, K.; Morita, M.; Arumugam, T.U.; Palacpac, N.M.Q.; Egwang, T.G.; Horii, T.; Tsuboi, T.; Takashima, E. The N-Terminal Region of Plasmodium falciparum MSP10 Is a Target of Protective Antibodies in Malaria and Is Important for PfGAMA/PfMSP10 Interaction. Front. Immunol. 2019, 10, 2669. [Google Scholar] [CrossRef]
- Puentes, A.; Ocampo, M.; Rodriguez, L.E.; Vera, R.; Valbuena, J.; Curtidor, H.; Garcia, J.; Lopez, R.; Tovar, D.; Cortes, J.; et al. Identifying Plasmodium falciparum Merozoite Surface Protein-10 human erythrocyte specific binding regions. Biochimie 2005, 87, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.R.; Mackey, M.D. Electrostatic Complementarity as a Fast and Effective Tool to Optimize Binding and Selectivity of Protein-Ligand Complexes. J. Med. Chem. 2019, 62, 3036–3050. [Google Scholar] [CrossRef] [PubMed]
- Naray-Szabo, G. Electrostatic complementarity in molecular associations. J. Mol. Graph. 1989, 7, 76–81. [Google Scholar] [CrossRef]
- Dagliyan, O.; Proctor, E.A.; D’Auria, K.M.; Ding, F.; Dokholyan, N.V. Structural and dynamic determinants of protein-peptide recognition. Structure 2011, 19, 1837–1845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokumasu, F.; Ostera, G.R.; Amaratunga, C.; Fairhurst, R.M. Modifications in erythrocyte membrane zeta potential by Plasmodium falciparum infection. Exp. Parasitol. 2012, 131, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, H.P.; Cesar, C.L.; Barjas-Castro Mde, L. Electrical properties of the red blood cell membrane and immunohematological investigation. Rev. Bras. Hematol. Hemoter. 2011, 33, 297–301. [Google Scholar] [CrossRef] [Green Version]
- Moumaris, M.; Bretagne, J.; Abuaf, N. Biological Membranes and Malaria-Parasites. Open Parasitol. J. 2019, 7, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Giraldo, M.A.; Arevalo-Pinzon, G.; Rojas-Caraballo, J.; Mongui, A.; Rodriguez, R.; Patarroyo, M.A. Vaccination with recombinant Plasmodium vivax MSP-10 formulated in different adjuvants induces strong immunogenicity but no protection. Vaccine 2009, 28, 7–13. [Google Scholar] [CrossRef]
- Aurrecoechea, C.; Brestelli, J.; Brunk, B.P.; Dommer, J.; Fischer, S.; Gajria, B.; Gao, X.; Gingle, A.; Grant, G.; Harb, O.S.; et al. PlasmoDB: A functional genomic database for malaria parasites. Nucleic Acids Res. 2009, 37, D539–D543. [Google Scholar] [CrossRef] [Green Version]
- Abascal, F.; Zardoya, R.; Telford, M.J. TranslatorX: Multiple alignment of nucleotide sequences guided by amino acid translations. Nucleic Acids Res. 2010, 38, W7–W13. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. Muscle: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pond, S.L.; Frost, S.D.; Grossman, Z.; Gravenor, M.B.; Richman, D.D.; Brown, A.J. Adaptation to different human populations by HIV-1 revealed by codon-based analyses. PLoS Comput. Biol. 2006, 2, e62. [Google Scholar]
- Kosakovsky Pond, S.L.; Frost, S.D. Not so different after all: A comparison of methods for detecting amino acid sites under selection. Mol. Biol. Evol. 2005, 22, 1208–1222. [Google Scholar] [CrossRef] [Green Version]
- Murrell, B.; Wertheim, J.O.; Moola, S.; Weighill, T.; Scheffler, K.; Kosakovsky Pond, S.L. Detecting individual sites subject to episodic diversifying selection. PLoS Genet. 2012, 8, e1002764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murrell, B.; Moola, S.; Mabona, A.; Weighill, T.; Sheward, D.; Kosakovsky Pond, S.L.; Scheffler, K. FUBAR: A fast, unconstrained bayesian approximation for inferring selection. Mol. Biol. Evol. 2013, 30, 1196–1205. [Google Scholar] [CrossRef] [Green Version]
- Kosakovsky Pond, S.L.; Posada, D.; Gravenor, M.B.; Woelk, C.H.; Frost, S.D. Automated phylogenetic detection of recombination using a genetic algorithm. Mol. Biol. Evol. 2006, 23, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Anisimova, M.; Nielsen, R.; Yang, Z. Effect of recombination on the accuracy of the likelihood method for detecting positive selection at amino acid sites. Genetics 2003, 164, 1229–1236. [Google Scholar]
- Arenas, M.; Posada, D. Coalescent simulation of intracodon recombination. Genetics 2010, 184, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Rozas, J.; Ferrer-Mata, A.; Sanchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sanchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.H.; Wilcox, W.C.; Sodora, D.L.; Long, D.; Levin, J.Z.; Eisenberg, R.J. Expression of herpes simplex virus type 1 glycoprotein D deletion mutants in mammalian cells. J. Virol. 1988, 62, 1932–1940. [Google Scholar] [CrossRef] [Green Version]
- Chitnis, C.E.; Miller, L.H. Identification of the erythrocyte binding domains of Plasmodium vivax and Plasmodium knowlesi proteins involved in erythrocyte invasion. J. Exp. Med. 1994, 180, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Houghten, R.A. General method for the rapid solid-phase synthesis of large numbers of peptides: Specificity of antigen-antibody interaction at the level of individual amino acids. Proc. Natl. Acad. Sci. USA 1985, 82, 5131–5135. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhang, Y. I-TASSER server: New development for protein structure and function predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef] [Green Version]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kale, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; MacKerell, A.D., Jr. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef] [Green Version]
- Jorgensen, W.; Chandrasekhar, J.; Madura, J. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926. [Google Scholar] [CrossRef]
- Sondergaard, C.R.; Olsson, M.H.; Rostkowski, M.; Jensen, J.H. Improved Treatment of Ligands and Coupling Effects in Empirical Calculation and Rationalization of pKa Values. J. Chem. Theory Comput. 2011, 7, 2284–2295. [Google Scholar] [CrossRef]
- Benkert, P.; Biasini, M.; Schwede, T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 2011, 27, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B.; et al. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 2018, 27, 293–315. [Google Scholar] [CrossRef]
- Dolinsky, T.J.; Nielsen, J.E.; McCammon, J.A.; Baker, N.A. PDB2PQR: An automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 2004, 32, W665–W667. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef] [Green Version]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Porollo, A.A.; Adamczak, R.; Meller, J. POLYVIEW: A flexible visualization tool for structural and functional annotations of proteins. Bioinformatics 2004, 20, 2460–2462. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.; Apweiler, R.; Attwood, T.K.; Bairoch, A.; Bateman, A.; Binns, D.; Bork, P.; Das, U.; Daugherty, L.; Duquenne, L.; et al. InterPro: The integrative protein signature database. Nucleic Acids Res. 2009, 37, D211–D215. [Google Scholar] [CrossRef] [Green Version]
- Jespersen, M.C.; Peters, B.; Nielsen, M.; Marcatili, P. BepiPred-2.0: Improving sequence-based B-cell epitope prediction using conformational epitopes. Nucleic Acids Res. 2017, 45, W24–W29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurtz, V.; Paul, S.; Andreatta, M.; Marcatili, P.; Peters, B.; Nielsen, M. NetMHCpan-4.0: Improved Peptide-MHC Class I Interaction Predictions Integrating Eluted Ligand and Peptide Binding Affinity Data. J. Immunol. 2017, 199, 3360–3368. [Google Scholar] [CrossRef]
- Jensen, K.K.; Andreatta, M.; Marcatili, P.; Buus, S.; Greenbaum, J.A.; Yan, Z.; Sette, A.; Peters, B.; Nielsen, M. Improved methods for predicting peptide binding affinity to MHC class II molecules. Immunology 2018, 154, 394–406. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricaurte-Contreras, L.A.; Lovera, A.; Moreno-Pérez, D.A.; Bohórquez, M.D.; Suárez, C.F.; Gutiérrez-Vásquez, E.; Cuy-Chaparro, L.; Garzón-Ospina, D.; Patarroyo, M.A. Two 20-Residue-Long Peptides Derived from Plasmodium vivax Merozoite Surface Protein 10 EGF-Like Domains Are Involved in Binding to Human Reticulocytes. Int. J. Mol. Sci. 2021, 22, 1609. https://doi.org/10.3390/ijms22041609
Ricaurte-Contreras LA, Lovera A, Moreno-Pérez DA, Bohórquez MD, Suárez CF, Gutiérrez-Vásquez E, Cuy-Chaparro L, Garzón-Ospina D, Patarroyo MA. Two 20-Residue-Long Peptides Derived from Plasmodium vivax Merozoite Surface Protein 10 EGF-Like Domains Are Involved in Binding to Human Reticulocytes. International Journal of Molecular Sciences. 2021; 22(4):1609. https://doi.org/10.3390/ijms22041609
Chicago/Turabian StyleRicaurte-Contreras, Laura Alejandra, Andrea Lovera, Darwin Andrés Moreno-Pérez, Michel David Bohórquez, Carlos Fernando Suárez, Elizabeth Gutiérrez-Vásquez, Laura Cuy-Chaparro, Diego Garzón-Ospina, and Manuel Alfonso Patarroyo. 2021. "Two 20-Residue-Long Peptides Derived from Plasmodium vivax Merozoite Surface Protein 10 EGF-Like Domains Are Involved in Binding to Human Reticulocytes" International Journal of Molecular Sciences 22, no. 4: 1609. https://doi.org/10.3390/ijms22041609
APA StyleRicaurte-Contreras, L. A., Lovera, A., Moreno-Pérez, D. A., Bohórquez, M. D., Suárez, C. F., Gutiérrez-Vásquez, E., Cuy-Chaparro, L., Garzón-Ospina, D., & Patarroyo, M. A. (2021). Two 20-Residue-Long Peptides Derived from Plasmodium vivax Merozoite Surface Protein 10 EGF-Like Domains Are Involved in Binding to Human Reticulocytes. International Journal of Molecular Sciences, 22(4), 1609. https://doi.org/10.3390/ijms22041609







