Involvement of Anoikis in Dissociated Optic Nerve Fiber Layer Appearance
Abstract
:1. Introduction
2. Anatomical Features of DONFL Appearance
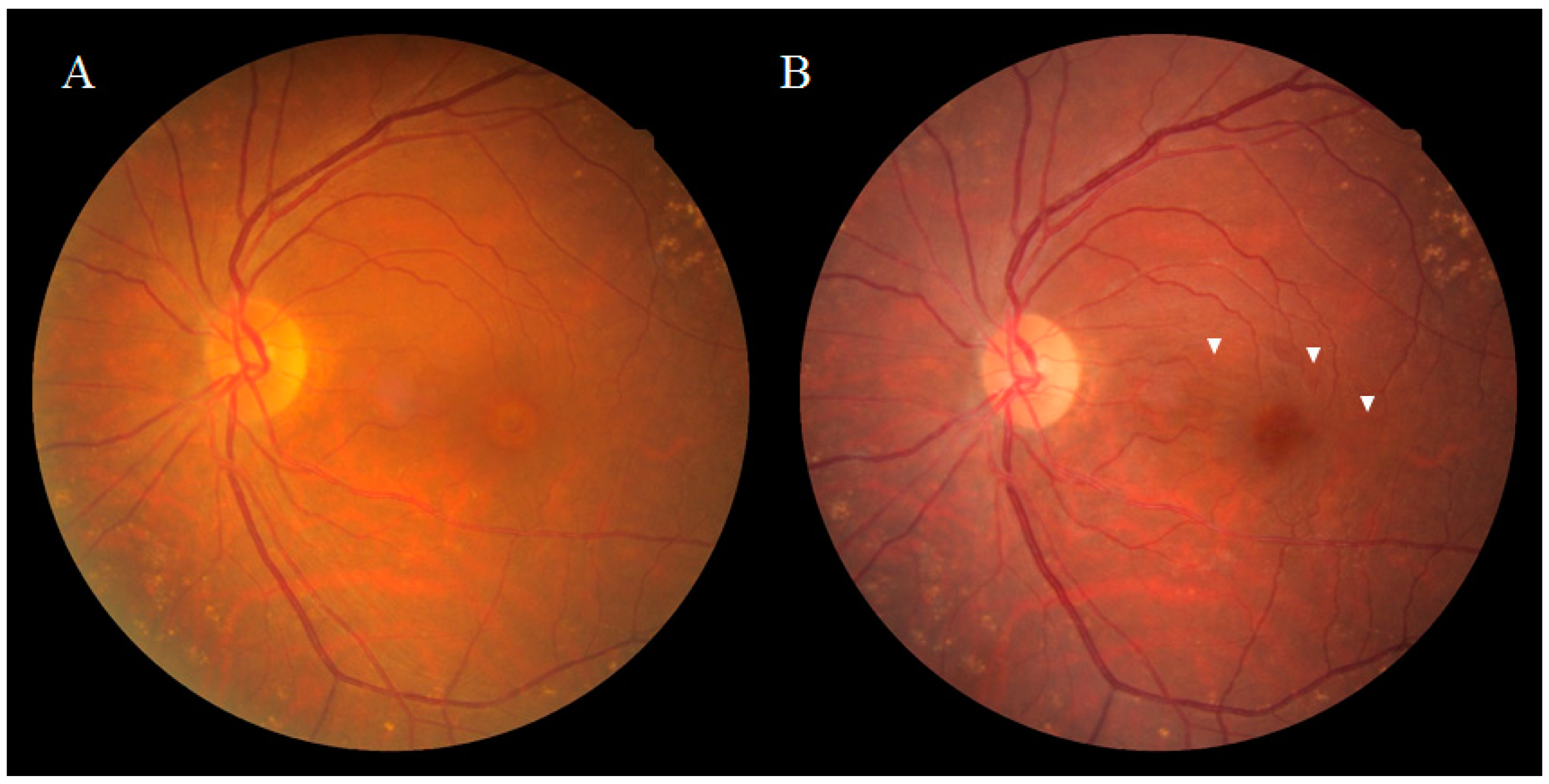
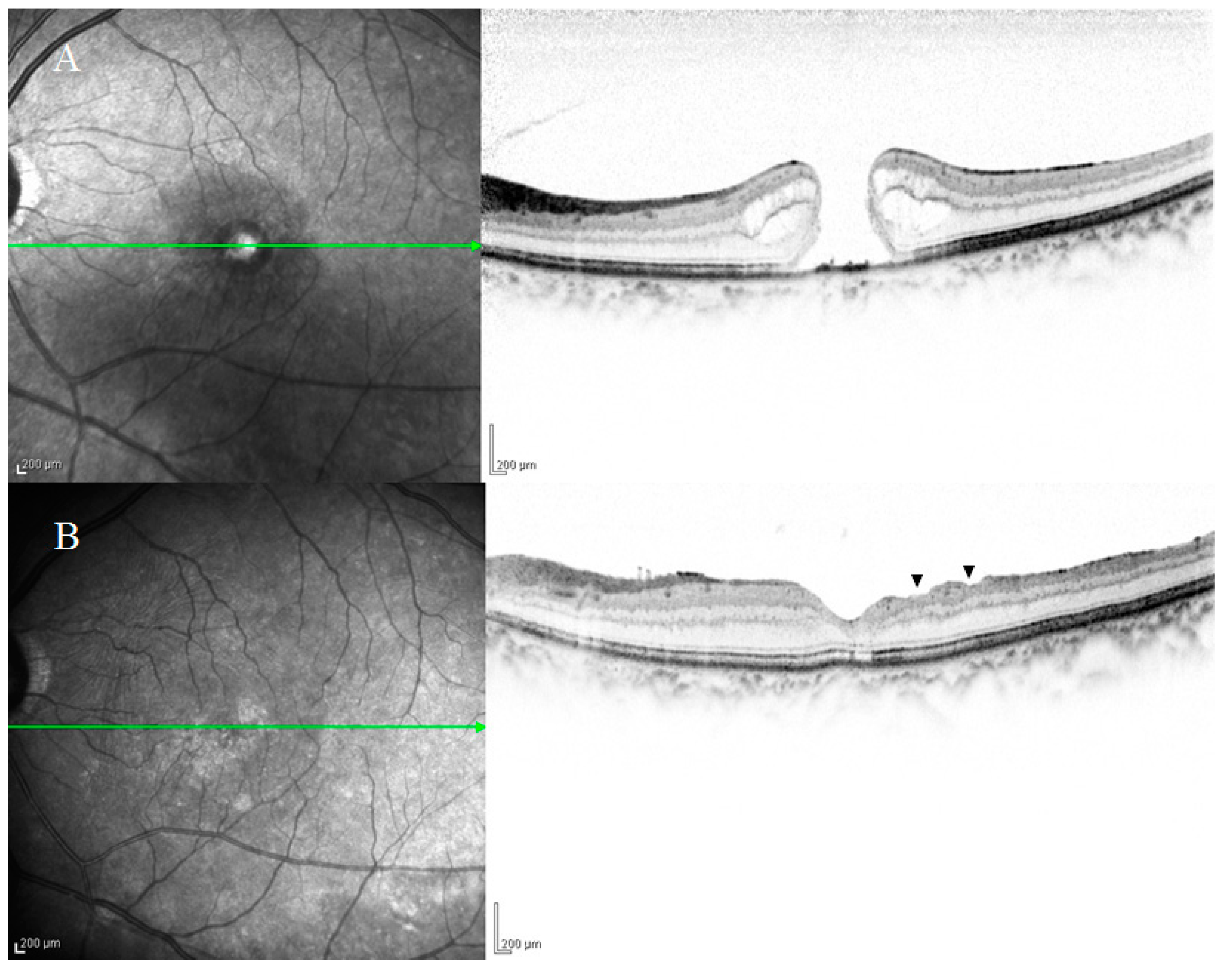
2.1. DONFL Appearance and Visual Function
2.2. Previous Studies on the Pathogenesis of DONFL Appearance
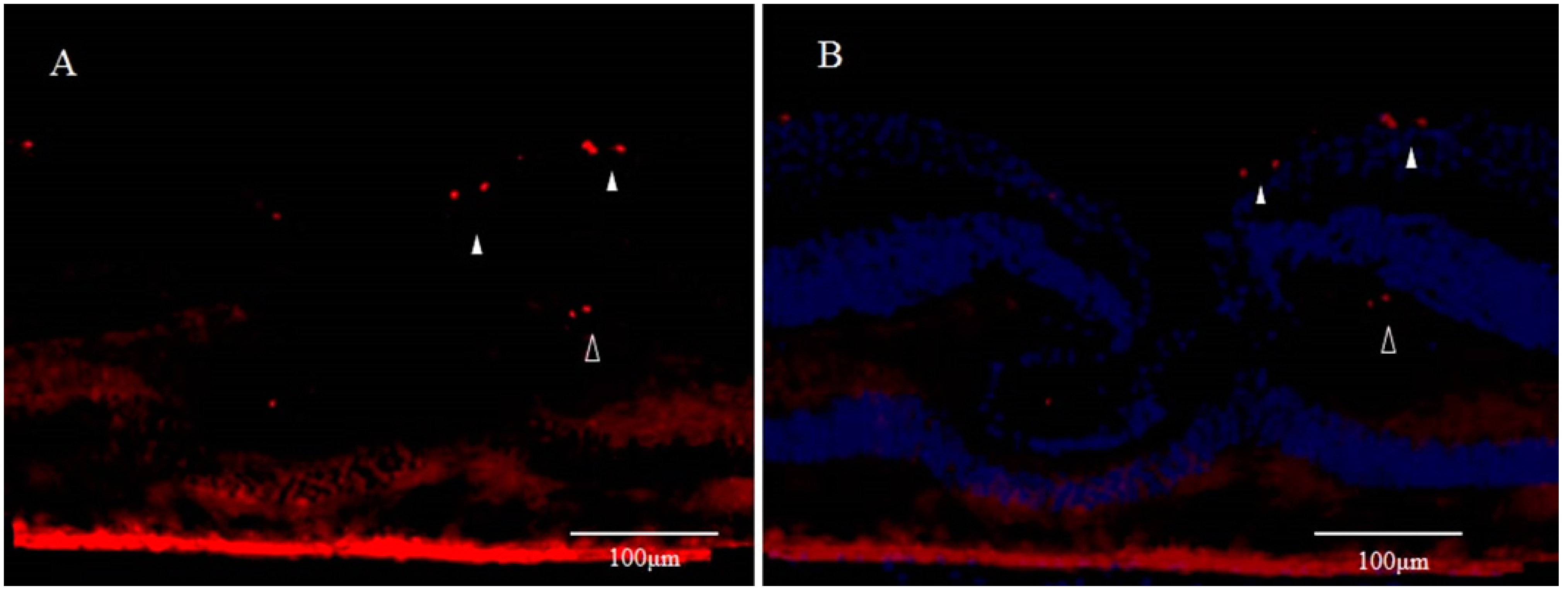
3. Involvement of Anoikis in the Pathogenesis of DONFL Appearance
3.1. Anoikis and Eye Diseases
3.2. Anoikis and Glial Cells
3.3. β. A3/A1-Crystallin and Anoikis
3.4. E-Cadherin and Anoikis
3.5. Anoikis and Retinal Ganglion Cells
3.6. Neurogenesis in the Macular Region
4. Reasons for the Frequent Occurrence of DONFL after Vitrectomy for an MH
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMD | age-related macular degeneration |
| CTGF | connective tissue growth factor |
| Cyr61 | cysteine-rich protein 61 |
| DONFL | dissociated optic nerve fiber layer |
| DR | diabetic retinopathy |
| E-cadherin | epithelial cadherin |
| ECM | extracellular matrix |
| ERM | epiretinal membrane |
| GFAP | glial fibrillary acidic protein |
| ILM | internal limiting membrane |
| MH | macular hole |
| MMP-2 | matrix metalloproteinase-2 |
| NFL | nerve fiber layer |
| OCT | optical coherence tomography |
| RNFL | retinal nerve fiber layer |
| RPE | retinal pigment epithelium |
| SLO | scanning laser ophthalmoscopy |
| TrkB | tropomyosin receptor kinase B |
References
- Tadayoni, R. Dissociated optic nerve fiber layer appearance of the fundus after idiopathic epiretinal membrane removal. Ophthalmology 2001, 108, 2279–2283. [Google Scholar] [CrossRef]
- Ito, Y.; Terasaki, H.; Takahashi, A.; Yamakoshi, T.; Kondo, M.; Nakamura, M. Dissociated Optic Nerve Fiber Layer Appearance after Internal Limiting Membrane Peeling for Idiopathic Macular Holes. Ophthalmology 2005, 112, 1415–1420. [Google Scholar] [CrossRef]
- Hisatomi, T.; Tachibana, T.; Notomi, S.; Koyanagi, Y.; Murakami, Y.; Takeda, A.; Ikeda, Y.; Yoshida, S.; Enaida, H.; Murata, T.; et al. Internal limiting membrane peeling-dependent retinal structural changes after vitrectomy in rhegmatogenous retinal detachment. Retina 2018, 38, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Dissociated Optic Nerve Fiber Layer in Subinternal Limiting Membrane Hemorrhage. Ophthalmology 2017, 124, 872. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, K.S.; Joe, S.G.; Kim, J.-G. Incidence and quantitative analysis of dissociated optic nerve fiber layer appearance: Real loss of retinal nerve fiber layer? Eur. J. Ophthalmol. 2018, 28, 317–323. [Google Scholar] [CrossRef]
- Kishimoto, H.; Kusuhara, S.; Matsumiya, W.; Nagai, T.; Negi, A. Retinal surface imaging provided by Cirrus high-definition optical coherence tomography prominently visualizes a dissociated optic nerve fiber layer appearance after macular hole surgery. Int. Ophthalmol. 2011, 31, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, S.; Matsumiya, W.; Imai, H.; Honda, S.; Tsukahara, Y.; Negi, A. Evaluating Dissociated Optic Nerve Fiber Layer Appearance Using En Face Layer Imaging Produced by Optical Coherence Tomography. Ophthalmology 2014, 232, 170–178. [Google Scholar] [CrossRef]
- Pichi, F.; Lembo, A.; Morara, M.; Veronese, C.; Alkabes, M.; Nucci, P.; Ciardella, A.P. Early and late inner retinal changes after inner limiting membrane peeling. Int. Ophthalmol. 2013, 34, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, Y.; Suzuki, T.; Kinoshita, T.; Miyano, N.; Tashimo, A.; Ohtsuka, K. Optical coherence tomographic findings of dissociated optic nerve fiber layer appearance. Am. J. Ophthalmol. 2004, 137, 1155–1156. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Elsner, A.E.; Osako, M.; Iwasaki, T.; Okano, T.; Usui, M. Dissociated optic nerve fiber layer appearance after internal limiting membrane peeling for idiopathic macular hole. Retina 2003, 23, 561–563. [Google Scholar] [CrossRef]
- Kim, K.Y.; Yu, S.-Y.; Kim, M.; Kim, E.S.; Kwak, H.W. Morphological change of inner retinal layer on spectral-domain optical coherence tomography following macular hole surgery. Ophthalmology 2013, 230, 18–26. [Google Scholar] [CrossRef]
- Demirel, S.; Abdullayev, A.; Yanık, Ö; Batıoğlu, F.; Özmert, E. Evaluation of Ganglion Cell-Inner Plexiform Layer Thickness after Vitreoretinal Surgery with Internal Limiting Membrane Peeling in Cases with Idiopathic Macular Hole. Turk. J. Ophthalmol. 2017, 47, 138–143. [Google Scholar] [CrossRef]
- Runkle, A.P.; Srivastava, S.K.; Yuan, A.; Kaiser, P.K.; Singh, R.P.; Reese, J.L.; Ehlers, J.P. Factors associated with development of dissociated optic nerve fiber layer appearance in the pioneer intraoperative optical coherence tomography study. Retina 2018, 38, S103–S109. [Google Scholar] [CrossRef] [PubMed]
- Mitamura, Y.; Ohtsuka, K. Relationship of Dissociated Optic Nerve Fiber Layer Appearance to Internal Limiting Membrane Peeling. Ophthalmology 2005, 112, 1766–1770. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Ohta, K. Microperimetric determination of retinal sensitivity in areas of dissociated optic nerve fiber layer following internal limiting membrane peeling. Jpn. J. Ophthalmol. 2010, 54, 435–440. [Google Scholar] [CrossRef]
- Steel, D.H.W.; Dinah, C.; White, K.; Avery, P.J. The relationship between a dissociated optic nerve fibre layer appearance after macular hole surgery and Muller cell debris on peeled internal limiting membrane. Acta Ophthalmol. 2016, 95, 153–157. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, Y.J.; Lee, S.J. Incidence of and risk factors for dissociated optic nerve fiber layer after epiretinal membrane surgery. Retina 2016, 36, 1469–1473. [Google Scholar] [CrossRef] [Green Version]
- Spaide, R.F. “Dissociated optic nerve fiber layer appearance” after internal limiting membrane removal is inner retinal dimpling. Retina 2012, 32, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Hisatomi, T.; Tachibana, T.; Notomi, S.; Nakatake, S.; Fujiwara, K.; Murakami, Y.; Ikeda, Y.; Yoshida, S.; Enaida, H.; Murata, T.; et al. Incomplete repair of retinal structure after vitrectomy with internal limiting membrane peeling. Retina 2017, 37, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, J. Molecular mechanisms of “detachment-induced apoptosis—Anoikis”. Apoptosis 2002, 7, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Frisch, S.M.; Screaton, R.A. Anoikis mechanisms. Curr. Opin. Cell Biol. 2001, 13, 555–562. [Google Scholar] [CrossRef]
- Valentijn, A.; Zouq, N.; Gilmore, A.P. Anoikis. Biochem. Soc. Trans. 2004, 32, 421–425. [Google Scholar] [CrossRef]
- Simpson, C.D.; Anyiwe, K.; Schimmer, A.D. Anoikis resistance and tumor metastasis. Cancer Lett. 2008, 272, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Taddei, M.L.; Giannoni, E.; Fiaschi, T.; Chiarugi, P. Anoikis: An emerging hallmark in health and diseases. J. Pathol. 2011, 226, 380–393. [Google Scholar] [CrossRef]
- Brem, R.B.; Robbins, S.G.; Wilson, D.J.; O’Rourke, L.M.; Mixon, R.N.; Robertson, J.E.; Planck, S.R.; Rosenbaum, J.T. Immu-nolocalization of integrins in the human retina. Invest. Ophthalmol. Vis. Sci. 1994, 35, 3466–3474. [Google Scholar]
- Lee, Y.-C.; Jin, J.-K.; Cheng, C.-J.; Huang, C.-F.; Song, J.H.; Huang, M.; Brown, W.S.; Zhang, S.; Yu-Lee, L.-Y.; Yeh, E.T.; et al. Targeting Constitutively Activated β1 Integrins Inhibits Prostate Cancer Metastasis. Mol. Cancer Res. 2013, 11, 405–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, A.R.C.; Corredor, R.G.; Obeso, B.A.; Trakhtenberg, E.F.; Wang, Y.; Ponmattam, J.; Dvoriantchikova, G.; Ivanov, D.; Shestopalov, V.I.; Goldberg, J.L.; et al. β1 Integrin-Focal Adhesion Kinase (FAK) Signaling Modulates Retinal Ganglion Cell (RGC) Survival. PLoS ONE 2012, 7, e48332. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, Y.-M.; Sun, M.-M.; Guo, X.-D.; Wang, Y.-C.; Zhang, Z.-Z. Inhibition on Apoptosis Induced by Elevated Hydrostatic Pressure in Retinal Ganglion Cell-5 via Laminin Upregulating β1-integrin/Focal Adhesion Kinase/Protein Kinase B Signaling Pathway. Chin. Med. J. 2016, 129, 976–983. [Google Scholar] [CrossRef]
- Ng, T.K.; Liang, X.Y.; Lu, F.; Liu, D.T.; Yam, G.H.; Ma, L.; Tam, P.O.; Chen, H.; Cen, L.-P.; Chen, L.J.; et al. Protective effects of an HTRA1 insertion–deletion variant against age-related macular degeneration in the Chinese populations. Lab. Investig. 2016, 97, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Albert, R.; Kristóf, E.; Zahuczky, G.; Szatmári-Tóth, M.; Veréb, Z.; Oláh, B.; Moe, M.C.; Facsko, A.; Fésüs, L.; Petrovski, G. Triamcinolone regulated apopto-phagocytic gene expression patterns in the clearance of dying retinal pigment epithelial cells. A key role of Mertk in the enhanced phagocytosis. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 435–446. [Google Scholar] [CrossRef]
- Petrovski, G.; Berényi, E.; Moe, M.C.; Vajas, A.; Fésüs, L.; Berta, A.; Facskó, A. Clearance of dying ARPE-19 cells by professional and nonprofessional phagocytesin vitro- implications for age-related macular degeneration (AMD). Acta Ophthalmol. 2010, 89, e30–e34. [Google Scholar] [CrossRef]
- Liu, H.; Yang, R.; Tinner, B.; Choudhry, A.; Schutze, N.; Chaqour, B. Cysteine-Rich Protein 61 and Connective Tissue Growth Factor Induce Deadhesion and Anoikis of Retinal Pericytes. Endocrinology 2008, 149, 1666–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.; Liu, H.; Williams, I.; Chaqour, B. Matrix Metalloproteinase-2 Expression and Apoptogenic Activity in Retinal Pericytes: Implications in Diabetic Retinopathy. Ann. N. Y. Acad. Sci. 2007, 1103, 196–201. [Google Scholar] [CrossRef] [PubMed]
- López-Luppo, M.; Catita, J.; Ramos, D.; Navarro, M.; Carretero, A.; Mendes-Jorge, L.; Nacher, V.; Muñoz-Cánoves, P.; Rodriguez-Baeza, A.; Ruberte, J. Cellular Senescence Is Associated with Human Retinal Microaneurysm Formation During Aging. Investig. Opthalmol. Vis. Sci. 2017, 58, 2832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunk, E.C.; König, H.-G.; Bernas, T.; Engel, T.; Henshall, D.C.; Kirby, B.P.; Prehn, J.H.M. BH3-only proteins BIM and PUMA in the regulation of survival and neuronal differentiation of newly generated cells in the adult mouse hippocampus. Cell Death Dis. 2010, 1, e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhter, R.; Saleem, S.; Saha, A.; Biswas, S.C. The pro-apoptotic protein Bmf co-operates with Bim and Puma in neuron death induced by β-amyloid or NGF deprivation. Mol. Cell. Neurosci. 2018, 88, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Marchionini, D.M.; Collier, T.J.; Camargo, M.; McGuire, S.; Pitzer, M.; Sortwell, C. Interference with anoikis-induced cell death of dopamine neurons: Implications for augmenting embryonic graft survival in a rat model of Parkinson’s disease. J. Comp. Neurol. 2003, 464, 172–179. [Google Scholar] [CrossRef]
- Działo, J.; Tokarz-Deptuła, B.; Deptuła, W. Excitotoxicity and Wallerian degeneration as a process related to cell death in nervous system. Arch. Ital. Biol 2013, 151, 67–75. [Google Scholar]
- Miñambres, R.; Guasch, R.M.; Pérez-Aragó, A.; Guerri, C. The RhoA/ROCK-I/MLC pathway is involved in the ethanol-induced apoptosis by anoikis in astrocytes. J. Cell Sci. 2006, 119, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Ma, B.; Sen, T.; Asnaghi, L.; Valapala, M.; Yang, F.; Hose, S.; McLeod, D.S.; Lu, Y.; Eberhart, C.; Zigler, J.S.; et al. βA3/A1-Crystallin controls anoikis-mediated cell death in astrocytes by modulating PI3K/AKT/mTOR and ERK survival pathways through the PKD/Bit1-signaling axis. Cell Death Dis. 2011, 2, e217. [Google Scholar] [CrossRef]
- Abe, Y.; Yamamoto, T.; Sugiyama, Y.; Watanabe, T.; Saito, N.; Kayama, H.; Kumagai, T. “Anoikis” of Oligodendrocytes Induced by Wallerian Degeneration: Ultrastructural Observations. J. Neurotrauma 2004, 21, 119–124. [Google Scholar] [CrossRef]
- Plant, C.D.; Plant, G.W. Schwann Cell Transplantation Methods Using Biomaterials. Toxic. Assess. 2018, 1739, 439–453. [Google Scholar] [CrossRef]
- Koda, M.; Someya, Y.; Nishio, Y.; Kadota, R.; Mannoji, C.; Miyashita, T.; Okawa, A.; Murata, A.; Yamazaki, M. Brain-derived neurotrophic factor suppresses anoikis-induced death of Schwann cells. Neurosci. Lett. 2008, 444, 143–147. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Routhe, L.J.; Moos, T. The vascular basement membrane in the healthy and pathological brain. Br. J. Pharmacol. 2017, 37, 3300–3317. [Google Scholar] [CrossRef]
- Nuriya, M.; Hirase, H. Involvement of astrocytes in neurovascular communication. Prog. Brain Res. 2016, 225, 41–62. [Google Scholar] [CrossRef]
- Okada, M.; Matsumura, M.; Ogino, N.; Honda, Y. Müller cells in detached human retina express glial fibrillary acidic protein and vimentin. Graefe’s Arch. Clin. Exp. Ophthalmol. 1990, 228, 467–474. [Google Scholar] [CrossRef]
- Cao, Z.; Livas, T.; Kyprianou, N. Anoikis and EMT: Lethal “Liaisons” during Cancer Progression. Crit. Rev. Oncog. 2016, 21, 155–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, W.-K.; Tang, X.-C.; Yi, M.; Chen, P.-Q.; Liu, F.-Y.; Hu, X.-H.; Hu, W.-F.; Fu, S.-J.; Liu, J.-F.; Wu, K.; et al. p53 Directly Regulates αA- and βA3/A1-Crystallin Genes to Modulate Lens Differentiation. Curr. Mol. Med. 2013, 13, 968–978. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, O.; Srivastava, K. Characterization of a sodium deoxycholate-activatable proteinase activity associated with βA3/A1-crystallin of human lenses. Biochim. Biophys. Acta Protein Struct. Mol. Enzym. 1999, 1434, 331–346. [Google Scholar] [CrossRef]
- Srivastava, O.; Srivastava, K.; Harrington, V. Age-Related Degradation of βA3/A1-Crystallin in Human Lenses. Biochem. Biophys. Res. Commun. 1999, 258, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Werten, P.J.; Vos, E.; de Jong, W.W. Truncation of βA3/A1-crystallin during Aging of the Bovine Lens; Possible Implications for Lens Optical Quality. Exp. Eye Res. 1999, 68, 99–103. [Google Scholar] [CrossRef]
- Ma, Z.; Yao, W.; Chan, C.-C.; Kannabiran, C.; Wawrousek, E.; Hejtmancik, J.F. Human βA3/A1-crystallin splicing mutation causes cataracts by activating the unfolded protein response and inducing apoptosis in differentiating lens fiber cells. Biochim. Biophys. Acta Bioenerg. 2016, 1862, 1214–1227. [Google Scholar] [CrossRef]
- Kannabiran, C.; Rogan, P.K.; Olmos, L.; Basti, S.; Rao, G.N.; Kaiser-Kupfer, M.; Hejtmancik, J.F. Autosomal dominant zonular cataract with sutural opacities is associated with a splice mutation in the betaA3/A1-crystallin gene. Mol. Vis. 1998, 4, 21. [Google Scholar]
- Zigler, J.S.; Sinha, D. βA3/A1-crystallin: More than a lens protein. Prog. Retin. Eye Res. 2015, 44, 62–85. [Google Scholar] [CrossRef]
- Hegde, S.; Kesterson, R.A.; Srivastava, O.P. CRYβA3/A1-Crystallin Knockout Develops Nuclear Cataract and Causes Impaired Lysosomal Cargo Clearance and Calpain Activation. PLoS ONE 2016, 11, e0149027. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Ma, B.; Zhang, C.; Gongora, C.; Zigler, J.S.; Duncan, M.K.; Sinha, D. Expression of βA3/A1-crystallin in the developing and adult rat eye. J. Mol. Histol. 2011, 42, 59–69. [Google Scholar] [CrossRef]
- Ghosh, S.; Shang, P.; Terasaki, H.; Stepicheva, N.; Hose, S.; Yazdankhah, M.; Weiss, J.; Sakamoto, T.; Bhutto, I.A.; Xia, S.; et al. A Role for βA3/A1-Crystallin in Type 2 EMT of RPE Cells Occurring in Dry Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2018, 59, AMD104–AMD113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, P.; Valapala, M.; Grebe, R.; Hose, S.; Ghosh, S.; Bhutto, I.A.; Handa, J.T.; Lutty, G.A.; Lu, L.; Wan, J.; et al. The amino acid transporter SLC36A4 regulates the amino acid pool in retinal pigmented epithelial cells and mediates the mechanistic target of rapamycin, complex 1 signaling. Aging Cell 2017, 16, 349–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valapala, M.; Sergeev, Y.; Wawrousek, E.; Hose, S.; Zigler, J.S.; Sinha, D. Modulation of V-ATPase by βA3/A1-Crystallin in Retinal Pigment Epithelial Cells. Taurine 6 2015, 854, 779–784. [Google Scholar] [CrossRef]
- Sinha, D.; Valapala, M.; Shang, P.; Hose, S.; Grebe, R.; Lutty, G.A.; Zigler, J.S.; Kaarniranta, K.; Handa, J.T. Lysosomes: Regulators of autophagy in the retinal pigmented epithelium. Exp. Eye Res. 2016, 144, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Valapala, M.; Wilson, C.; Hose, S.; Bhutto, I.A.; Grebe, R.; Dong, A.; Greenbaum, S.; Gu, L.; Sengupta, S.; Cano, M.; et al. Lysosomal-mediated waste clearance in retinal pigment epithelial cells is regulated by CRYBA1/βA3/A1-crystallin via V-ATPase-MTORC1 signaling. Autophagy 2014, 10, 480–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zigler, J.S.; Zhang, C.; Grebe, R.; Sehrawat, G.; Hackler, L.; Adhya, S.; Hose, S.; McLeod, D.S.; Bhutto, I.; Barbour, W.; et al. Mutation in the A3/A1-crystallin gene impairs phagosome degradation in the retinal pigmented epithelium of the rat. J. Cell Sci. 2011, 124, 523–531. [Google Scholar] [CrossRef] [Green Version]
- Valapala, M.; Hose, S.; Gongora, C.; Dong, L.; Wawrousek, E.F.; Zigler, J.S.; Sinha, D. Impaired endolysosomal function disrupts Notch signalling in optic nerve astrocytes. Nat. Commun. 2013, 4, 1629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, D.; Valapala, M.; Bhutto, I.; Patek, B.; Zhang, C.; Hose, S.; Yang, F.; Cano, M.; Stark, W.J.; Lutty, G.A.; et al. βA3/A1-crystallin is required for proper astrocyte template formation and vascular remodeling in the retina. Transgenic Res. 2012, 21, 1033–1042. [Google Scholar] [CrossRef] [Green Version]
- Sinha, D.; Klise, A.; Sergeev, Y.; Hose, S.; Bhutto, I.A.; Hackler, L.; Malpic-Llanos, T.; Samtani, S.; Grebe, R.; Goldberg, M.F.; et al. βA3/A1-crystallin in astroglial cells regulates retinal vascular remodeling during development. Mol. Cell. Neurosci. 2008, 37, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valapala, M.; Edwards, M.; Hose, S.; Hu, J.; Wawrousek, E.; Lutty, G.A.; Zigler, J.S.; Qian, J.; Sinha, D. βA3/A1-crystallin is a critical mediator of STAT3 signaling in optic nerve astrocytes. Sci. Rep. 2015, 5, 8755. [Google Scholar] [CrossRef] [Green Version]
- Zigler, J.S.; Valapala, M.; Shang, P.; Hose, S.; Goldberg, M.F.; Sinha, D. βA3/A1-crystallin and persistent fetal vasculature (PFV) disease of the eye. Biochim. Biophys. Acta Gen. Subj. 2016, 1860, 287–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, S.; Takeichi, M. Cadherins in Brain Morphogenesis and Wiring. Physiol. Rev. 2012, 92, 597–634. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, M. Morphogenetic roles of classic cadherins. Curr. Opin. Cell Biol. 1995, 7, 619–627. [Google Scholar] [CrossRef]
- Kumar, S.; Park, S.H.; Cieply, B.; Schupp, J.; Killiam, E.; Zhang, F.; Rimm, D.L.; Frisch, S.M. A Pathway for the Control of Anoikis Sensitivity by E-Cadherin and Epithelial-to-Mesenchymal Transition. Mol. Cell. Biol. 2011, 31, 4036–4051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, X.; Pham, T.; Temple, B.; Gray, S.; Cannon, C.; Chen, R.; Abdel-Mageed, A.B.; Biliran, H. The Anoikis Effector Bit1 Inhibits EMT through Attenuation of TLE1-Mediated Repression of E-Cadherin in Lung Cancer Cells. PLoS ONE 2016, 11, e0163228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, S.C.; Takeichi, M. Cadherins in neuronal morphogenesis and function. Dev. Growth Differ. 2008, 50, S119–S130. [Google Scholar] [CrossRef]
- Pla, P.; Moore, R.; Morali, O.G.; Grille, S.; Martinozzi, S.; LaRue, L. Cadherins in neural crest cell development and transformation. J. Cell. Physiol. 2001, 189, 121–132. [Google Scholar] [CrossRef]
- Pal, M.; Bhattacharya, S.; Kalyan, G.; Hazra, S. Cadherin profiling for therapeutic interventions in Epithelial Mesenchymal Transition (EMT) and tumorigenesis. Exp. Cell Res. 2018, 368, 137–146. [Google Scholar] [CrossRef]
- Riehl, R.; Johnson, K.; Bradley, R.; Grunwald, G.B.; Cornel, E.; Lilienbaum, A.; Holt, C.E. Cadherin Function Is Required for Axon Outgrowth in Retinal Ganglion Cells In Vivo. Neuron 1996, 17, 837–848. [Google Scholar] [CrossRef] [Green Version]
- Oblander, S.A.; Ensslen-Craig, S.E.; Longo, F.M.; Brady-Kalnay, S.M. E-cadherin promotes retinal ganglion cell neurite outgrowth in a protein tyrosine phosphatase-mu-dependent manner. Mol. Cell. Neurosci. 2007, 34, 481–492. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, T.; Nakamura, K.; Oku, H.; Horie, T.; Kida, T.; Takai, S. Immunohistological Study of Monkey Foveal Retina. Sci. Rep. 2019, 9, 5258. [Google Scholar] [CrossRef]
- Lepousez, G.; Lledo, P.-M. Life and death decision in adult neurogenesis: In praise of napping. Neuron 2011, 71, 768–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biebl, M.; Cooper, C.M.; Winkler, J.; Kuhn, H.G. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci. Lett. 2000, 291, 17–20. [Google Scholar] [CrossRef]
- Ehninger, D.; Kempermann, G. Neurogenesis in the adult hippocampus. Cell Tissue Res. 2007, 331, 243–250. [Google Scholar] [CrossRef]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and Functional Implications of Adult Neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitman, M.C.; Greer, C.A. Adult neurogenesis and the olfactory system. Prog. Neurobiol. 2009, 89, 162–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cullen, D.K.; O’Donnell, J.C.; Katiyar, K.S.; Panzer, K.V. A tissue-engineered rostral migratory stream for directed neuronal replacement. Neural Regen. Res. 2018, 13, 1327–1331. [Google Scholar] [CrossRef]
- Woźniak, W.; Bruska, M. Sources and chain migration of neurons to the olfactory bulb. Folia Morphol. 1999, 58, 21–27. [Google Scholar]
- Oleñik, A.; Rios, J.; Mateo, C. Inverted internal limiting membrane flap technique for macular holes in high myopia with axial length ≥30 mm. Retina 2016, 36, 1688–1693. [Google Scholar] [CrossRef]
- Maruichi, M.; Oku, H.; Takai, S.; Muramatsu, M.; Sugiyama, T.; Imamura, Y.; Minami, M.; Ueki, M.; Satoh, B.; Sakaguchi, M.; et al. Measurement of activities in two different angiotensin II generating systems, chymase and angiotensin-converting enzyme, in the vitreous fluid of vitreoretinal diseases: A possible involvement of chymase in the pathogenesis of macular hole patients. Curr. Eye Res. 2004, 29, 321–325. [Google Scholar] [CrossRef]
- Sato, T.; Morishita, S.; Horie, T.; Fukumoto, M.; Kida, T.; Oku, H.; Nakamura, K.; Takai, S.; Jin, D.; Ikeda, T. Involvement of premacular mast cells in the pathogenesis of macular diseases. PLoS ONE 2019, 14, e0211438. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef] [Green Version]
- Kambe, N.; Hiramatsu, H.; Shimonaka, M.; Fujino, H.; Nishikomori, R.; Heike, T.; Ito, M.; Kobayashi, K.; Ueyama, Y.; Matsuyoshi, N.; et al. Development of both human connective tissue-type and mucosal-type mast cells in mice from hematopoietic stem cells with identical distribution pattern to human body. Blood 2004, 103, 860–867. [Google Scholar] [CrossRef] [Green Version]
- Gruber, B.L. Mast cells in the pathogenesis of fibrosis. Curr. Rheumatol. Rep. 2003, 5, 147–153. [Google Scholar] [CrossRef]
- Ribatti, D.; Crivellato, E. Mast cells, angiogenesis, and tumour growth. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 2–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Dong, H.; Li, N.; Zhang, S.; Sun, J.; Zhang, S.; Qian, Y. Activated brain mast cells contribute to postoperative cognitive dysfunction by evoking microglia activation and neuronal apoptosis. J. Neuroinflamm. 2016, 13, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Galli, S.J.; Tsai, M. Mast cells: Versatile regulators of inflammation, tissue remodeling, host defense and homeostasis. J. Dermatol. Sci. 2008, 49, 7–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebihara, N.; Takai, S.; Miyazaki, M.; Murakami, A. Mast Cell Chymase Induces Conjunctival Epithelial Cell Apoptosis by a Mechanism Involving Degradation of Fibronectin. Curr. Eye Res. 2005, 30, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.; Ooto, S.; Hangai, M.; Arakawa, N.; Oshima, S.; Shibata, N.; Hanebuchi, M.; Inoue, T.; Yoshimura, N. High-Resolution Imaging of the Retinal Nerve Fiber Layer in Normal Eyes Using Adaptive Optics Scanning Laser Ophthalmoscopy. PLoS ONE 2012, 7, e33158. [Google Scholar] [CrossRef]
- Fujikawa, H.; Tanaka, K.; Toiyama, Y.; Saigusa, S.; Inoue, Y.; Uchida, K.; Kusunoki, M. High TrkB expression levels are associated with poor prognosis and EMT induction in colorectal cancer cells. J. Gastroenterol. 2012, 47, 775–784. [Google Scholar] [CrossRef]
- Yu, X.; Liu, L.; Cai, B.; He, Y.; Wan, X. Suppression of anoikis by the neurotrophic receptor TrkB in human ovarian cancer. Cancer Sci. 2008, 99, 543–552. [Google Scholar] [CrossRef]
- Oku, H.; Ikeda, T.; Honma, Y.; Sotozono, C.; Nishida, K.; Nakamura, Y.; Kida, T.; Kinoshita, S. Gene expression of neurotrophins and their high-affinity Trk receptors in cultured human Müller cells. Ophthalmic Res. 2002, 34, 38–42. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, T.; Nakamura, K.; Sato, T.; Kida, T.; Oku, H. Involvement of Anoikis in Dissociated Optic Nerve Fiber Layer Appearance. Int. J. Mol. Sci. 2021, 22, 1724. https://doi.org/10.3390/ijms22041724
Ikeda T, Nakamura K, Sato T, Kida T, Oku H. Involvement of Anoikis in Dissociated Optic Nerve Fiber Layer Appearance. International Journal of Molecular Sciences. 2021; 22(4):1724. https://doi.org/10.3390/ijms22041724
Chicago/Turabian StyleIkeda, Tsunehiko, Kimitoshi Nakamura, Takaki Sato, Teruyo Kida, and Hidehiro Oku. 2021. "Involvement of Anoikis in Dissociated Optic Nerve Fiber Layer Appearance" International Journal of Molecular Sciences 22, no. 4: 1724. https://doi.org/10.3390/ijms22041724
APA StyleIkeda, T., Nakamura, K., Sato, T., Kida, T., & Oku, H. (2021). Involvement of Anoikis in Dissociated Optic Nerve Fiber Layer Appearance. International Journal of Molecular Sciences, 22(4), 1724. https://doi.org/10.3390/ijms22041724





