Human Milk Antibodies against S1 and S2 Subunits from SARS-CoV-2, HCoV-OC43, and HCoV-229E in Mothers with a Confirmed COVID-19 PCR, Viral SYMPTOMS, and Unexposed Mothers
Abstract
:1. Introduction
2. Results
2.1. Maternal Demographics
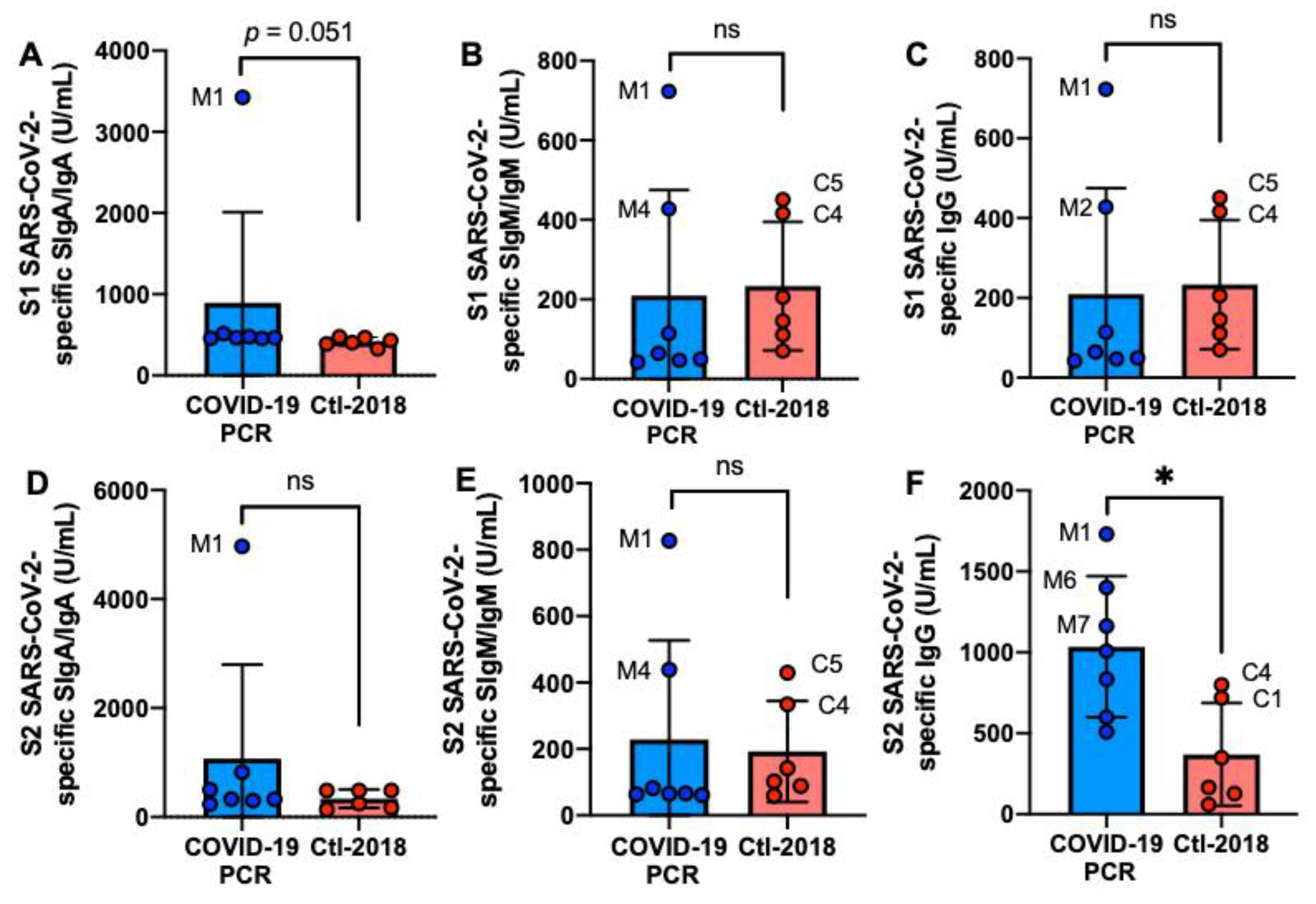
2.2. S1 or S2 Subunit SARS-CoV-2-Specific Human Milk Antibodies
2.3. S1- and S2 Subunits HCoVs-Reactive Human Milk Antibodies
2.4. S1 or S2 Subunit SARS-CoV-2-Reactive Human Milk Antibodies
2.5. S1- and S2 Subunits HCoVs-Reactive Human Milk Antibodies
2.6. Correlation Matrix between Antigens and Isotypes
2.6.1. S1 or S2 Subunit from SARS-CoV-2
2.6.2. S1 and S2 Subunits from SARS-CoV-2 and HCoVs
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Human Milk Collection
4.3. Human Milk Antibody Detection
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Demers-Mathieu, V.; Underwood, M.A.; Beverly, R.L.; Nielsen, S.D.; Dallas, D.C. Comparison of human milk immunoglobulin survival during gastric digestion between preterm and term infants. Nutrients 2018, 10, 631. [Google Scholar] [CrossRef] [Green Version]
- Demers-Mathieu, V.; Huston, R.K.; Markell, A.M.; McCulley, E.A.; Martin, R.L.; Spooner, M.; Dallas, D.C. Differences in maternal immunoglobulins within mother’s own breast milk and donor breast milk and across digestion in preterm infants. Nutrients 2019, 11, 920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demers-Mathieu, V.; Huston, R.K.; Markell, A.M.; McCulley, E.A.; Martin, R.L.; Dallas, D.C. Antenatal Influenza A-specific IgA, IgM, and IgG antibodies in mother’s own breast milk and donor breast milk, and gastric contents and stools from preterm infants. Nutrients 2019, 11, 1567. [Google Scholar] [CrossRef] [Green Version]
- Corthesy, B. Role of secretory IgA in infection and maintenance of homeostasis. Autoimmun. Rev. 2013, 12, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Bioley, G.; Monnerat, J.; Lötscher, M.; Vonarburg, C.; Zuercher, A.; Corthésy, B. Plasma-derived polyreactive secretory-like IgA and IgM opsonizing Salmonella enterica Typhimurium reduces invasion and gut tissue inflammation through agglutination. Front. Immunol. 2017, 8, 1043. [Google Scholar] [CrossRef] [Green Version]
- Longet, S.; Miled, S.; Lötscher, M.; Miescher, S.M.; Zuercher, A.W.; Corthésy, B. Human plasma-derived polymeric IgA and IgM antibodies associate with secretory component to yield biologically active secretory-like antibodies. J. Biol. Chem. 2013, 288, 4085–4094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammerschmidt, S.; Talay, S.R.; Brandtzaeg, P.; Chhatwal, G.S. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol. Microbiol. 1997, 25, 1113–1124. [Google Scholar] [CrossRef]
- Mazanec, M.B.; Nedrud, J.G.; Kaetzel, C.S.; Lamm, M.E. A three-tiered view of the role of IgA in mucosal defense. Immunol. Today 1993, 14, 430–435. [Google Scholar] [CrossRef]
- Demers-Mathieu, V.; Underwood, M.A.; Beverly, R.L.; Dallas, D.C. Survival of immunoglobulins from human milk to preterm infant gastric samples at 1, 2, and 3 hours postprandial. Neonatology 2018, 114, 242–250. [Google Scholar] [CrossRef]
- Demers-Mathieu, V.; Huston, R.K.; Markell, A.M.; McCulley, E.A.; Martin, R.L.; Dallas, D.C. Impact of pertussis-specific IgA, IgM, and IgG antibodies in mother’s own breast milk and donor breast milk during preterm infant digestion. Pediatr. Res. 2020, 29, 1–8. [Google Scholar]
- Chang, T.H.; Wu, J.L.; Chang, L.Y. Clinical characteristics and diagnostic challenges of pediatric COVID-19: A systematic review and meta-analysis. J. Formos Med. Assoc. 2020, 119, 982–989. [Google Scholar] [CrossRef]
- Khan, J.; Vesel, L.; Bahl, R.; Martines, J.C. Timing of breastfeeding initiation and exclusivity of breastfeeding during the first month of life: Effects on neonatal mortality and morbidity—A systematic review and meta-analysis. Matern. Child. Health J. 2015, 19, 468–479. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Quadeer, A.A.; McKay, M.R. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses 2020, 12, 254. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Cheng, Y.; Wu, Y. Understanding SARS-CoV-2-mediated inflammatory responses: From mechanisms to potential therapeutic tools. Virol. Sin. 2020, 35, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Fox, A.; Marino, J.; Amanat, F.; Krammer, F.; Hahn-Holbrook, J.; Zolla-Pazner, S.; Powell, R.L. Robust and specific secretory IgA against SARS-CoV-2 detected in human milk. IScience 2020, 23, 101735. [Google Scholar] [CrossRef]
- Dong, Y.; Chi, X.; Huang, H.; Sun, L.; Zhang, M.; Xie, W.F.; Chen, W. Antibodies in the breast milk of a maternal woman with COVID-19. Emerg Microbes Infect. 2020, 9, 1467–1469. [Google Scholar] [CrossRef]
- Demers-Mathieu, V.; Do, D.M.; Mathijssen, G.B.; Sela, D.A.; Seppo, A.; Järvinen, K.M.; Medo, E. Difference in levels of SARS-CoV-2 S1 and S2 subunits- and nucleocapsid protein-reactive SIgM/IgM, IgG and SIgA/IgA antibodies in human milk. J. Perinatol. 2020, 1–10. [Google Scholar] [CrossRef]
- Dijkman, R.; Jebbink, M.F.; Gaunt, E.; Rossen, J.W.; Templeton, K.E.; Kuijpers, T.W.; van der Hoek, L. The dominance of human coronavirus OC43 and NL63 infections in infants. J. Clin. Virol. 2012, 53, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Jefferson, T.; Smith, S.; Demicheli, V.; Harnden, A.; Rivetti, A.; Di Pietrantonj, C. Assessment of the efficacy and effectiveness of influenza vaccines in healthy children: Systematic review. Lancet 2005, 365, 773–780. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 2020, 323, 1843–1844. [Google Scholar] [CrossRef] [Green Version]
- Walls, A.C.; Xiong, X.; Park, Y.J.; Tortorici, M.A.; Snijder, J.; Quispe, J.; Cameroni, E.; Gopal, R.; Dai, M.; Lanzavecchia, A.; et al. Unexpected receptor functional mimicry elucidates activation of coronavirus fusion. Cell 2019, 176, 1026–1039. [Google Scholar] [CrossRef] [Green Version]
- Nguyen-Contant, P.; Embong, A.K.; Kanagaiah, P.; Chaves, F.A.; Yang, H.; Branche, A.R.; Topham, D.J.; Sangster, M.Y. S protein-reactive IgG and memory B cell production after human SARS-CoV-2 infection includes broad reactivity to the S2 subunit. mBio Am. Soc. Microbiol. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Elshabrawy, H.A.; Coughlin, M.M.; Baker, S.C.; Prabhakar, B.S. Human monoclonal antibodies against highly conserved HR1 and HR2 domains of the SARS-CoV spike protein are more broadly neutralizing. PLoS ONE 2012, 7, e50366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhang, H.N.; Jiang, H.W.; Tian, X.; Ma, M.L.; Qi, H.; Meng, Q.F.; Guo, S.J.; Wu, Y.; Wang, W. Linear epitopes of SARS-CoV-2 spike protein elicit neutralizing antibodies in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 1095–1097. [Google Scholar] [CrossRef]
- Wang, L.; Shi, W.; Chappell, J.D.; Joyce, M.G.; Zhang, Y.; Kanekiyo, M.; Becker, M.M.; van Doremalen, N.; Fischer, R.; Wang, N.; et al. Importance of neutralizing monoclonal antibodies targeting multiple antigenic sites on the Middle East respiratory syndrome coronavirus spike glycoprotein to avoid neutralization escape. J. Virol. 2018, 92, 1095–1097. [Google Scholar] [CrossRef] [Green Version]
- Taylor, H.P.; Dimmock, N.J. Mechanism of neutralization of influenza virus by secretory IgA is different from that of monomeric IgA or IgG. J. Exp. Med. 1985, 161, 198–209. [Google Scholar] [CrossRef]
- Stubbe, H.; Berdoz, J.; Kraehenbuhl, J.P.; Corthésy, B. Polymeric IgA is superior to monomeric IgA and IgG carrying the same variable domain in preventing Clostridium difficile toxin A damaging of T84 monolayers. J. Immunol. 2000, 164, 1952–1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, A.T.; Garcia-Carreras, B.; Hitchings, M.D.; Yang, B.; Katzelnick, L.C.; Rattigan, S.M.; Borgert, B.A.; Moreno, C.A.; Solomon, B.D.; Trimmer-Smith, L.; et al. A systematic review of antibody mediated immunity to coronaviruses: Kinetics, correlates of protection, and association with severity. Nat. Commun. 2020, 11, 1–6. [Google Scholar] [CrossRef]
- Demers-Mathieu, V.; Mathijssen, G.; Dapra, C.; Do, D.M.; Medo, E. Active free secretory component and secretory IgA in human milk: Do maternal vaccination, allergy, infection, mode of delivery, nutrition and active lifestyle change their concentrations? Pediatr. Res. 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.; Loyal, L.; Frentsch, M.; Wendisch, D.; Georg, P.; Kurth, F.; Hippenstiel, S.; Dingeldey, M.; Kruse, B.; Fauchere, F.; et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature 2020, 587, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Mateus, J.; Grifoni, A.; Tarke, A.; Sidney, J.; Ramirez, S.I.; Dan, J.M.; Burger, Z.C.; Rawlings, S.A.; Smith, D.M.; Phillips, E.; et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 2020, 370, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020, 181, 1489–1501. [Google Scholar] [CrossRef]
- Kuo, T.T.; Baker, K.; Yoshida, M.; Qiao, S.-W.; Aveson, V.G.; Lencer, W.I.; Blumberg, R.S. Neonatal Fc receptor: From immunity to therapeutics. J. Clin. Immunol. 2010, 30, 777–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhi, R.T.; Wurcel, A.; Lee, H.; McGovern, B.; Shopis, J.; Geary, M.; Sivamurthy, R.; Sax, P.E.; Ukomadu, C. Response to hepatitis B vaccine in HIV-1–positive subjects who test positive for isolated antibody to hepatitis B core antigen: Implications for hepatitis B vaccine strategies. J. Infect. Dis. 2005, 191, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Demers-Mathieu, V.; Mathijssen, G.B.; DaPra, C.; Medo, E. The effects of probiotic supplementation on the gene expressions of immune cell surface markers and levels of antibodies and pro-inflammatory cytokines in human milk. J. Perinatol. 2020, 18, 1–9. [Google Scholar] [CrossRef]





| Demographics | COVID-19 PCR (n = 7) b | Ctl1-2018 (n = 6) |
|---|---|---|
| Postpartum time, months a | 6 ± 1 (5–8) | 6 ± 1 (5–8) |
| Infant gender, n | 4 males: 3 females | 3 males: 3 females |
| Maternal age, years a | 31 ± 4 (26–37) | 31 ± 4 (27–37) |
| Influenza vaccine during pregnancy, n (%) | 2 (28.6) | 5 (83.3) |
| Time from infection to collection, days | 47 ± 24 (16–84) | - |
| Demographics | Viral Symptoms (n = 20) b | Ctl2-2018 (n = 16) |
|---|---|---|
| Postpartum time, months a | 5 ± 2 (2–11) | 5 ± 3 (5–8) |
| Infant gender, n | 8 males: 12 females | 8 males: 8 females |
| Maternal age, years a | 32 ± 4 (27–41) | 32 ± 4 (25–37) |
| Influenza vaccine during pregnancy, n (%) | 7 (35) | 6 (37.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demers-Mathieu, V.; DaPra, C.; Mathijssen, G.; Sela, D.A.; Järvinen, K.M.; Seppo, A.; Fels, S.; Medo, E. Human Milk Antibodies against S1 and S2 Subunits from SARS-CoV-2, HCoV-OC43, and HCoV-229E in Mothers with a Confirmed COVID-19 PCR, Viral SYMPTOMS, and Unexposed Mothers. Int. J. Mol. Sci. 2021, 22, 1749. https://doi.org/10.3390/ijms22041749
Demers-Mathieu V, DaPra C, Mathijssen G, Sela DA, Järvinen KM, Seppo A, Fels S, Medo E. Human Milk Antibodies against S1 and S2 Subunits from SARS-CoV-2, HCoV-OC43, and HCoV-229E in Mothers with a Confirmed COVID-19 PCR, Viral SYMPTOMS, and Unexposed Mothers. International Journal of Molecular Sciences. 2021; 22(4):1749. https://doi.org/10.3390/ijms22041749
Chicago/Turabian StyleDemers-Mathieu, Veronique, Ciera DaPra, Gabrielle Mathijssen, David A. Sela, Kirsi M. Järvinen, Antti Seppo, Shawn Fels, and Elena Medo. 2021. "Human Milk Antibodies against S1 and S2 Subunits from SARS-CoV-2, HCoV-OC43, and HCoV-229E in Mothers with a Confirmed COVID-19 PCR, Viral SYMPTOMS, and Unexposed Mothers" International Journal of Molecular Sciences 22, no. 4: 1749. https://doi.org/10.3390/ijms22041749
APA StyleDemers-Mathieu, V., DaPra, C., Mathijssen, G., Sela, D. A., Järvinen, K. M., Seppo, A., Fels, S., & Medo, E. (2021). Human Milk Antibodies against S1 and S2 Subunits from SARS-CoV-2, HCoV-OC43, and HCoV-229E in Mothers with a Confirmed COVID-19 PCR, Viral SYMPTOMS, and Unexposed Mothers. International Journal of Molecular Sciences, 22(4), 1749. https://doi.org/10.3390/ijms22041749







