Abstract
Erroneous conclusions may result from normalization of urine cadmium and N-acetyl-β-D-glucosaminidase concentrations ([Cd]u and [NAG]u) to the urine creatinine concentration ([cr]u). In theory, the sources of these errors are nullified by normalization of excretion rates (ECd and ENAG) to creatinine clearance (Ccr). We hypothesized that this alternate approach would clarify the contribution of Cd-induced tubular injury to nephron loss. We studied 931 Thai subjects with a wide range of environmental Cd exposure. For x = Cd or NAG, Ex/Ecr and Ex/Ccr were calculated as [x]u/[cr]u and [x]u[cr]p/[cr]u, respectively. Glomerular filtration rate (GFR) was estimated according to the Chronic Kidney Disease (CKD) Epidemiology Collaboration (eGFR), and CKD was defined as eGFR < 60 mL/min/1.73m2. In multivariable logistic regression analyses, prevalence odds ratios (PORs) for CKD were higher for log(ECd/Ccr) and log(ENAG/Ccr) than for log(ECd/Ecr) and log(ENAG/Ecr). Doubling of ECd/Ccr and ENAG/Ccr increased POR by 132% and 168%; doubling of ECd/Ecr and ENAG/Ecr increased POR by 64% and 54%. As log(ECd/Ccr) rose, associations of eGFR with log(ECd/Ccr) and log(ENAG/Ccr) became stronger, while associations of eGFR with log(ECd/Ecr) and log(ENAG/Ecr) became insignificant. In univariate regressions of eGFR on each of these logarithmic variables, R2 was consistently higher with normalization to Ccr. Our tabular and graphic analyses uniformly indicate that normalization to Ccr clarified relationships of ECd and ENAG to eGFR.
1. Introduction
Cadmium (Cd) is an important industrial toxin in several regions of the world [1]. It enters the human body in food, cigarette smoke, and polluted air, and is initially bound to the protein metallothionein (MT) in the liver. Complexes of CdMT are subsequently released to the circulation, filtered by renal glomeruli, and reabsorbed by proximal tubules. After Cd is separated from MT in lysosomes of tubular cells, it induces in situ synthesis of MT, which mitigates the toxicity of the free metal. Nevertheless, a small amount of unbound Cd inflicts injury that may eventuate in nephron loss and a reduction in the glomerular filtration rate (GFR) [2,3].
The lysosomal enzyme N-acetyl-β-D-glucosaminidase (NAG) is the most commonly employed marker of Cd-induced cell injury. Because this molecule is too large to undergo glomerular filtration, its appearance in urine signifies release by injured tubular cells [4]. The excretion rate of NAG (ENAG) typically correlates with that of Cd (ECd) [5,6,7,8,9,10,11], and the correlation holds at minimal Cd excretion rates [12,13,14]. Given these observations, we have argued that the two substances probably emanate from the same source [15]. We recently showed that ECd and ENAG were inversely related to estimated glomerular filtration rate (eGFR) in a sample of Thai subjects [15].
Historically, investigators of Cd nephrotoxicity have normalized the excretion rates of various substances (Ex) to that of creatinine (Ecr) [7,9,10,11,12,16,17]. Algebraically, Ex/Ecr simplifies to [x]u/[cr]u. Although normalization of [x]u to [cr]u corrects for the effect of urine flow rate (Vu) on [x]u, this convention introduces two other sources of error. First, because Ecr is primarily a function of muscle mass [18], [x]u/[cr]u may vary by a multiple among subjects with a given Ex. Second, if Cd and NAG emanate from tubular cells, their excretion rates may fall as nephrons are lost while Ecr remains relatively stable [19,20,21]. In this circumstance, ECd/Ecr and ENAG/Ecr may understate the severity of Cd-induced injury.
To avoid these potential errors, we recently adopted the practice of normalizing Ex to creatinine clearance (Ccr), a surrogate for the glomerular filtration rate (GFR) [15,22]. Ccr is the excretion rate divided by the plasma concentration of creatinine (Ecr/[cr]p); algebraically, Ex/Ccr simplifies to [x]u[cr]p/[cr]u in units of mass of x excreted per volume of filtrate [23]. Because the ratio [x]u/[cr]u remains in the calculation, Ex/Ccr, like Ex/Ecr, is unaffected by Vu. However, in contrast to Ex/Ecr, Ex/Ccr is also unaffected by muscle mass because [cr]p varies in proportion to Ecr at a given Ccr. Moreover, if x emanates from tubular cells, Ex may fall because of nephron loss; in that circumstance, Ex/Ccr depicts the excretion of x per surviving nephron because Ccr falls simultaneously [2,3].
Given the foregoing considerations, we hypothesized that normalization of ECd and ENAG to Ccr would clarify the contribution of Cd-induced tubular injury to nephron loss. We employed data from a large sample of Thai subjects to examine regressions of estimated GFR (eGFR) on log(ECd/Ecr), log(ENAG/Ecr), log(ECd/Ccr), and log(ENAG/Ccr). Normalization to Ccr increased coefficients of determination, effect sizes, and the strength of associations of eGFR with excretion rates.
2. Results
2.1. Tabular Analyses
Table 1 summarizes demographic features, renal function, and excretion rates of Cd and NAG in a cohort of 931 subjects. Data were organized in three subsets defined by gradations of log[(ECd/Ccr) × 105]. As log(ECd/Ccr) rose, age, [Cd]u, [NAG]u, ECd/Ecr, ENAG/Ecr, ECd/Ccr, and ENAG/Ccr also rose, and eGFR fell.

Table 1.
Study subjects grouped by urinary cadmium excretion rates normalized to creatinine clearance.
Table 2 presents two logistic regression models that quantified contributions of independent variables to the prevalence odds ratio (POR) for CKD (eGFR < 60 mL/min/1.73m2). Both models incorporated demographic factors and parameters of Cd and NAG excretion. ECd and ENAG were normalized to Ecr in model 1 and to Ccr in model 2.

Table 2.
Relationships of prevalence odds ratios for chronic kidney disease to demographic factors and parameters of Cd and NAG excretion.
In model 1, Table 2, POR for CKD was associated with age, log2(ECd/Ecr), and log2(ENAG/Ecr), but not with diabetes, gender, hypertension, or smoking. Log2(ECd/Ecr) had the greatest effect size (β = 0.493), followed by log2(ENAG/Ecr) (β = 0.435) and age (β = 0.143). In model 2, results were qualitatively similar; POR was associated with age, log2(ECd/Ccr), and log2(ENAG/Ccr), but not with diabetes, gender, hypertension, or smoking. In contrast to model 1, log2(ENAG/Ccr had the greatest effect size (β = 0.985), followed by log2ECd/Ccr (β = 0.843) and age (β = 0.149). Effect size on POR was much greater for log2(ECd/Ccr) and log2(ENAG/Ccr) than for log2(ECd/Ecr) and log2(ENAG/Ecr). Doubling of ECd/Ecr and ENAG/Ecr increased POR for CKD by 63.7% and 54.4%, respectively; doubling of ECd/Ccr and ENAG/Ccr increased POR by 132% and 168%, respectively. In both models, POR for CKD rose by 14–15% with every 10-year increment above age 40.
Table 3 presents two multivariable linear regression models of eGFR. As in Table 2, the models incorporated demographic factors and parameters of Cd and NAG excretion. ECd and ENAG were normalized to Ecr in model 1 and to Ccr in model 2. Accordingly, subsets of subjects were defined by gradations of log[(ECd/Ecr) × 103] in model 1 and log[(ECd/Ccr) × 105] in model 2. In each model, age exhibited the greatest absolute value of standardized β (strength of association) at all three gradations of log[(ECd/Ecr) × 103] or log[(ECd/Ccr) × 105].

Table 3.
Relationships of eGFR to demographic factors and parameters of Cd and NAG excretion.
In model 1, Table 3, other demographic variables were also associated with eGFR, but only in the subset with the lowest log(ECd/Ecr). As log(ECd/Ecr) rose, standardized β became more negative and its absolute value increased for the regression of eGFR on log(ECd/Ecr). In contrast, standardized β remained positive for the regression of eGFR on log(ENAG/Ecr). In the subset with the highest log(ECd/Ecr), neither of the regressions reached statistical significance. Variation in eGFR accounted for by all independent variables (adjusted R2) fell as log(ECd/Ecr) rose and eGFR fell.
In model 2, Table 3, age was the only demographic factor associated with eGFR. As log(ECd/Ccr) rose, standardized β became more negative and its absolute value increased for regressions of eGFR on log(ECd/Ccr) and log(ENAG/Ccr). Both regressions were highly significant in the middle and highest subsets of log(ECd/Ccr). Variation in eGFR accounted for by all independent variables (adjusted R2) rose as log(ECd/Ccr) rose and GFR fell.
2.2. Graphic Analyses
Figure 1 compares linear and quadratic regressions of eGFR on log(ECd/Ecr) (graph (A)) and log(ECd/Ccr) (graph (C)). The graphs show that R2 was higher and p was lower for regressions of eGFR on log(ECd/Ccr). Tables (B) and (D) enumerate R2 and unstandardized and standardized β for linear regressions in subsets defined by increasing lower limits of log(ECd/Ecr) or log(ECd/Ccr). In all subsets and in the sample as a whole, R2 and absolute values of unstandardized and standardized β were higher for regressions of eGFR on log(ECd/Ccr).
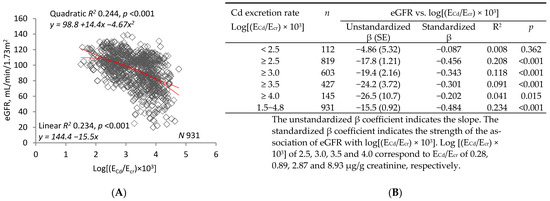
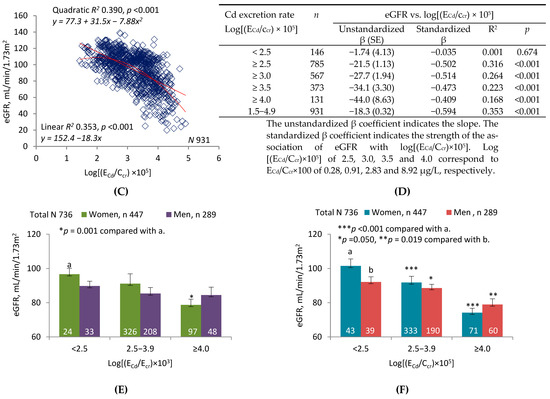
Figure 1.
Inverse relationships of eGFR to parameters of Cd excretion. Parameter abbreviations are summarized at the end of the text. Scatterplots in graphs (A,C) relate eGFR to log[(ECd/Ecr) × 103] and log[(ECd/Ccr) × 105] in all subjects. Linear and quadratic equations, their respective coefficients of determination (R2), and associated p-values are provided. In Table (B), linear relationships of eGFR to log[(ECd/Ecr) × 103] < 2.5, ≥2.5, ≥3.0, ≥3.5 and ≥4.0 are characterized with respective values of subject number (n), unstandardized and standardized β, R2, and p. In Table (D, linear relationships of eGFR to log[(ECd/Ccr) × 105] of <2.5, ≥2.5, ≥3.0, ≥3.5 and ≥4.0 are characterized with respective values of subject number (n), unstandardized and standardized β, R2, and p. In graphs (E,F), bars represent mean eGFR in women and men grouped by ranges of log[(ECd/Ecr) × 103] or log[(ECd/Ccr) × 105]. The subsets thus created are identical to those constituting Cd excretion levels 1, 2, and 3 in Table 3. The letters a and b identify reference values in women and men, respectively, at the lowest rates of Cd excretion. Where appropriate, statistical comparisons are made within each gender between mean eGFR in bars a and b and mean eGFR at higher rates of Cd excretion. Geometric mean (GM) values (standard deviation, SD) of ECd/Ecr are 0.15 (0.07) µg/g creatinine at level 1, 2.35 (2.42) µg/g creatinine at level 2, and 14.91(7.57) µg/g creatinine at level 3. GM (SD) of [(ECd/Ccr) × 100] are 0.15 (0.08) µg/L at level 1, 2.20 (2.25) µg/L at level 2, and 16.01 (11.05) µg/L at level 3.
Figure 1E depicts the mean eGFR of women and men in each of three subsets defined by ranges of log(ECd/Ecr). The ranges are those employed to denote levels of Cd excretion in Table 3, model 1. After adjustment for covariates and interactions, a significant difference in mean eGFR was demonstrated between women in the highest and lowest subsets. No differences were found among men. Figure 1F depicts the mean eGFR of women and men in each of three subsets defined by ranges of log(ECd/Ccr). The ranges are those employed to denote levels of Cd excretion in Table 3, model 2. After adjustment for covariates and interactions, significant differences in mean eGFR were demonstrated between both women and men in the lowest subset and their counterparts in the middle and highest subsets.
Figure 2 compares linear and quadratic regressions of eGFR on log(ENAG/Ecr) (graph (A)) and log(ENAG/Ccr) (graph (C)). The graphs show that R2 was higher and p was lower for regressions of eGFR on log(ENAG/Ccr). Tables (B) and (D) enumerate R2 and unstandardized and standardized β for linear regressions in subsets defined by increasing lower limits of log(ENAG/Ecr) or log(ENAG/Ccr). In all subsets and in the sample as a whole, R2 and absolute values of unstandardized and standardized β were higher for regressions of eGFR on log(ENAG/Ccr). In two subsets, only the regressions of eGFR on log(ENAG/Ccr) were significant.
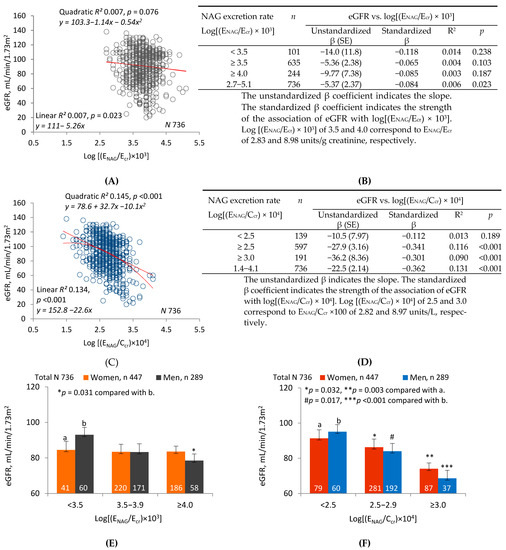
Figure 2.
Inverse relationships of eGFR to parameters of NAG excretion. Parameter abbreviations are summarized at the end of the text. Scatterplots in graphs (A,C) relate eGFR to log[(ENAG/Ecr) × 103] and log[(ENAG/Ccr) × 104] in all subjects. Linear and quadratic equations, their respective coefficients of determination (R2), and associated p-values are provided. In Table (B), linear relationships of eGFR to log [(ENAG)/Ecr) × 103] < 3.5, ≥3.5 and ≥4.0 are characterized with respective values of subject number (n), unstandardized and standardized β, R2, and p. In Table (D), linear relationships of eGFR to log[(ENAG/Ccr) × 104] < 2.5, ≥2.5 and ≥3.0 are characterized with respective values of n, unstandardized and standardized β, R2, and p. In graphs (E,F), the bars represent the mean eGFR in women and men grouped by ranges of log[(ENAG/Ecr) × 103] or log[(ENAG/Ccr) × 104] (NAG excretion levels 1, 2, and 3 from lowest to highest). Numbers of women and men within each NAG excretion level are provided. The letters a and b identify reference values in women and men, respectively, at the lowest rates of Cd excretion. Where appropriate, statistical comparisons are made within each gender between mean eGFR in bars a and b and mean eGFR at higher rates of Cd excretion. The geometric mean (GM) (SD) of ENAG/Ecr in groups 1, 2 and 3 is 1.75 (0.65), 5.36 (1.68) and 14.50 (11.98) units/g creatinine, respectively. The GM (SD) of [(ENAG/Ccr) × 100] in groups 1, 2 and 3 is 1.73 (0.66), 5.56 (2.25) and 17.52 (17.16) units/L, respectively.
Figure 2E depicts the mean eGFR of women and men in each of three subsets defined by ranges of log(ENAG/Ecr). After adjustment for covariates and interactions, a significant difference was demonstrated between men in the highest and lowest subsets. No differences in eGFR were seen among women. Figure 2F depicts the mean eGFR of women and men in each of three subsets defined by log(ENAG/Ccr). After adjustment for covariates and interactions, significant differences were demonstrated between both women and men in the lowest subset and their counterparts in the middle and highest subsets.
Figure 3 compares linear and quadratic regressions of log(ENAG/Ecr) on log(ECd/Ecr) (graph (A)) or log(ENAG/Ccr) on log(ECd/Ccr) (graph (C)). The graphs show that R2 was higher and p was lower for regressions of log(ENAG/Ccr) on log(ECd/Ccr). Tables (B) and (D) examine R2 and unstandardized and standardized β in subsets defined by progressively increasing lower limits of log(ECd/Ecr) or log(ECd/Ccr). In all but one of the subsets and in the sample as a whole, R2 and the absolute values of unstandardized and standardized β were higher for regressions of log(ENAG/Ccr) on log(ECd/Ccr). Regressions of log(ENAG/Ccr) on log(ECd/Ccr) were significant in three of the subsets, but regressions of log(ENAG/Ecr) on log(ECd/Ecr) were significant in only one.
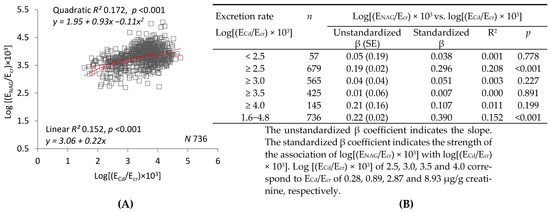
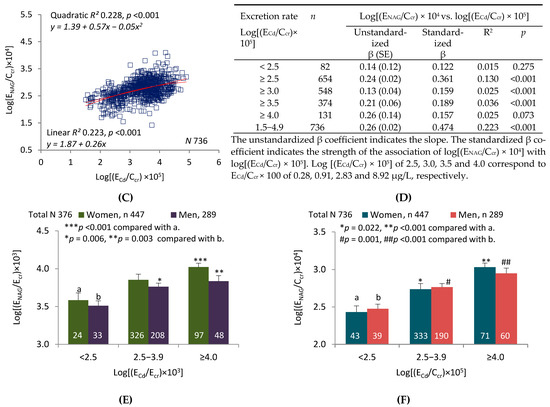
Figure 3.
Direct relationships of parameters of NAG excretion to parameters of Cd excretion. Parameter abbreviations are summarized at the end of the text. Scatterplots in graphs (A,C) relate log[(ENAG/Ecr) × 103] to log[(ECd/Ecr) × 103], and log[(ENAG/Ccr) × 104] to log[(ECd/Ccr) × 105] in all subjects. Linear and quadratic equations, their respective coefficients of determination (R2), and associated p-values are provided. In Table (B), linear relationships of log[(ENAG/Ecr) × 103] to log [(ECd/Ecr) × 103] < 2.5, ≥2.5, ≥3.0, ≥3.5 and ≥4.0 are characterized with respective values of subject number (n), unstandardized and standardized β, R2, and p. In Table (D), linear relationships of log[(ENAG/Ccr) × 104] to log [(ECd/Ccr) × 105] < 2.5, ≥2.5, ≥3.0, ≥3.5 and ≥4.0 are characterized with respective values of subject number (n), unstandardized and standardized β, R2, and p. In graphs (E,F), the bars represent mean log[(ENAG/Ecr) × 103] or mean log[(ENAG/Ccr) × 105] in women and men grouped by ranges of log[(ECd/Ecr) × 103] or log[(ECd/Ccr) × 105]. The subsets thus created are identical to those constituting Cd excretion levels in Table 3 and in Figure 1E,F. Numbers of subjects within each Cd excretion level and corresponding values of GM (SD) of ECd/Ecr and [(ECd/Ccr) × 100] are as described in the legend for Figure 1. The letters a and b identify reference values in women and men, respectively, at the lowest rates of Cd excretion. Where appropriate, statistical comparisons are made within each gender between mean eGFR in bars a and b and mean eGFR at higher rates of Cd excretion.
Figure 3E depicts log(ENAG/Ccr) of women and men in each of three subsets defined by ranges of log(ECd/Ecr). After adjustment for covariates and interactions, a significant difference in mean log(ENAG/Ecr) was demonstrated between women in the highest and lowest subsets, and between men in the lowest and the middle and highest subsets. Figure 3F depicts log(ENAG/Ccr) of women and men in each of three subsets defined by ranges of log(ECd/Ccr). After adjustment for covariates and interactions, significant differences in mean log(ENAG/Ccr) were demonstrated between both women and men in the lowest subset and their counterparts in the middle and highest subsets.
3. Discussions
We have previously argued that regressions of eGFR on parameters of Cd and NAG excretion provide insight into the pathogenesis of Cd nephropathy [15]. Heretofore, investigators of this issue have normalized [Cd]u and [NAG]u to [cr]u [7,8,16,17]. The resulting ratios adjust [Cd]u and [NAG]u for variation in Vu, but muscle mass affects [cr]u, and nephron number affects excretion rates of substances that emanate from tubular cells. In theory, normalization of ECd and ENAG to Ccr resolves these issues for reasons summarized in the Introduction. We therefore hypothesized that this methodological modification would add clarity to relationships among eGFR, ECd, and ENAG.
3.1. Interpretation of Tabular Analyses
We created the study sample to encompass a broad range of probability that asymptomatic participants had developed Cd nephropathy. To highlight the relevance of previous Cd accumulation to GFR, we divided the sample into three subsets defined by gradations of log(ECd/Ccr). As log(ECd/Ccr) rose, age, ECd/Ecr, ENAG/Ecr, ECd/Ccr, and ENAG/Ccr also rose, and eGFR fell (Table 1).
We performed two multivariable logistic regression analyses to quantify effects of demographic features and excretory parameters on the probability of CKD (eGFR < 60 mL/min/1.73m2) (Table 2). The unit of probability was the ratio of odds of having CKD to odds of not having it, i.e., the probability odds ratio (POR). POR was associated with log2(ECd/Ecr) and log2(ENAG/Ecr) in model 1 and with log2(ECd/Ccr) and log2(ENAG/Ccr) in model 2. POR was higher for logs of the ratios in model 2, as were the percentage increases in POR per doubling of ratios.
We also performed two regression model analyses of eGFR on demographic variables and parameters of Cd and NAG excretion (Table 3). Each regression was examined in three subsets defined by progressively higher ranges of log(ECd/Ecr) (model 1) or log(ECd/Ccr) (model 2). The ranges were designated Cd excretion levels 1, 2, and 3. In model 1, associations of eGFR with log(ECd/Ecr) and log(ENAG/Ecr) became less significant as log(ECd/Ecr) rose in the subsets. Moreover, at excretion level 3, standardized β for the regression of eGFR on ENAG/Ecr was positive rather than negative, and thus implied the absence of an inverse relationship between eGFR and the severity of tubular injury. In model 2, as log(ECd/Ccr) rose in the Cd excretion subsets, associations of eGFR with ECd/Ccr and ENAG/Ccr became more significant, the absolute value of standardized β (strength of association) increased, the slope implied by standardized β became more negative, the inverse relationship of eGFR to tubular injury was thus enhanced, and R2 for the entire analysis rose.
Collectively, our tabular data demonstrate multiple benefits of normalizing excretion rates to Ccr rather than Ecr. This approach substantially magnified the effects of ECd and ENAG on POR for CKD; exposed the inverse relationship between eGFR and ENAG at high levels of Cd excretion; demonstrated qualitatively similar regressions of eGFR on log(ECd/Ccr) and log(ENAG/Ccr); increased the size of effects of ECd and ENAG on eGFR and the strength of associations among these variables; and produced a multilinear regression model that accounted for more variation in eGFR.
3.2. Interpretation of Graphic Analyses
The univariate analyses in Figure 1 and Figure 2 confirm the concepts imparted by Table 3. Figure 1 depicts linear and quadratic regressions of eGFR on log(ECd/Ecr) or log(ECd/Ccr). R2 was higher and p was lower for both types of regression when eGFR was plotted against log(ECd/Ccr); moreover, dispersion was visibly reduced and a curvilinear relationship between eGFR and ECd was more evident (Figure 1A,C). Slope analyses were performed over ranges of log(ECd/Ecr) or log(ECd/Ccr) that were progressively reduced by raising lower limits. Within each range, effect size (unstandardized β) and strength of association (standardized β) were greater for relationships of eGFR to log(ECd/Ccr). Subsets created in Table 3 according to Cd excretion level were employed in Figure 1E,F, and differences in mean eGFR were more pronounced among subsets defined by log(ECd/Ccr).
Figure 2 depicts linear and quadratic regressions of eGFR on log(ENAG/Ecr) or log(ENAG/Ccr). R2 was much higher for both regressions when eGFR was plotted against log(ENAG/Ccr); simultaneously, dispersion was reduced, and both linear and quadratic relationships of eGFR to ENAG were more visually evident. As in Figure 1, slope analyses were performed over ranges of log(ECd/Ecr) or log(ECd/Ccr) that were progressively reduced by raising lower limits. Within each range, effect size (unstandardized β) and strength of association (standardized β) were much greater for relationships of eGFR to log(ENAG/Ccr). Linear regressions relevant to each range were significant only for the highest range of ENAG/Ecr, but they were highly significant for all but the lowest range of ENAG/Ccr. Three subsets were created within the sample according to low, medium, or high ranges of log(ENAG/Ecr) or log(ENAG/Ccr); as in Figure 1, differences in mean eGFR were more pronounced among subsets defined by log(ENAG/Ccr).
Figure 3 depicts linear and quadratic regressions of log(ENAG/Ecr) on log(ECd/Ecr), and log(ENAG/Ccr) on log(ECd/Ccr). Although the graphs are visually similar, a reduction in dispersion was demonstrated in the plot of ENAG/Ccr against ECd/Ccr, and R2 was higher for both linear and quadratic regressions of log(ENAG/Ccr) on log(ECd/Ccr). In the slope analyses, R2 and unstandardized and standardized β and were higher for each individual linear regression of log(ENAG/Ccr) on log(ECd/Ccr). As in Figure 1, subsets created in Table 3 according to Cd excretion level were employed in Figure 3E,F. Differences in NAG excretion were more pronounced when subsets were defined by log(ECd/Ccr). Thus, in all three figures, normalization of excretion rates to Ccr rather than Ecr increased coefficients of determination, effect size, and strength of association for each possible bivariate relationship among eGFR, ECd, and ENAG. Within each gender, normalization to Ccr also accentuated differences in eGFR and NAG excretion among subsets defined by Cd excretion.
3.3. Creatinine Excretion, Creatinine Clearance, and GFR
Creatinine, a small nitrogenous waste product (m.w. 113 Da), is synthesized from creatine phosphate in skeletal muscle. It can enter plasma from the gut if meat is ingested, but most of its flux into plasma results from endogenous production in muscle cells. A small fraction of the influx is diverted to the colonic lumen for bacterial metabolism, and that fraction increases as [cr]p rises. Nevertheless, at all but the most severely reduced values of GFR, renal excretion is the principal avenue of creatinine elimination [25,26].
When plasma is in equilibrium with respect to creatinine, the principal determinant of Ecr is muscle mass, which is highly variable in the population [18]. Most excreted creatinine is filtered, and the remainder is secreted by proximal tubules. The secreted fraction is small when GFR is normal; however, as GFR falls, the secreted fraction rises, Ccr (=Ecr/[cr]p) increasingly overestimates GFR [26,27], and in theory, ECd/Ccr and ENAG/Ccr increasingly underestimate the excretion of Cd and NAG per volume of filtrate. If Ccr were more uniformly representative of GFR, the relationships of eGFR to log(ECd/Ccr) and log(ENAG/Ccr) would likely be less quadratic and more linear than Figure 1 and Figure 2 indicate. In any case, the inverse nature of these relationships is indisputable.
Estimated GFR approximates the radionuclide-based gold standard of GFR determination more closely than Ccr does [24]. One could argue, therefore, that we should normalize ECd and ENAG to eGFR in our work. In theory, this method would offer the same advantages as normalization to Ccr, and at low GFR, it would prevent underestimation of ECd and ENAG per volume of filtrate. The practical obstacle to this approach is that determinations of ECd/eGFR and ENAG/eGFR would require timed urine collections for the measurement of ECd and ENAG. Normalization to Ccr, though possibly less accurate, is more convenient and less susceptible to procedural error because it is accomplished with single aliquots of serum and urine.
3.4. Tubular Release of Cd and NAG Necessitates Normalization of Excretion Rates to Ccr
Excreted NAG emanates exclusively from injured tubular cells because the molecule is too large to undergo glomerular filtration [4]. The source of excreted Cd is more debatable, but several considerations suggest that Cd is also released from injured cells [15]. In multiple studies, ECd varied directly, not inversely, with GFR (nephron number) [19,20,21]. Similarly, ECd varied directly with the Cd content of kidneys sampled at autopsy or transplantation [28,29]. Because animal studies indicated that the tubular reabsorptive capacity for filtered Cd is quite high [30], it is unlikely that a typically intoxicated human excretes unreabsorbed Cd immediately after filtration. Previously reported correlations of ECd with ENAG suggest that Cd and NAG emanate from a common source [5,6,7,8,9,10,11,12,13,14,15]; consequently, we have argued that both Cd and NAG are released into glomerular filtrate from tubular cells. If this inference is correct, then ECd, like ENAG, is an indicator of Cd-induced tubular injury [15]. Tubulointerstitial nephritis, destruction of nephrons, and a reduction in GFR are logical sequelae of such injury [3,31].
If urinary Cd and NAG emanate from tubules, then we should expect the excretion of these substances to vary directly with the number of intact nephrons and the severity of cellular injury. In the absence of renal hypoperfusion, we assume a proportional relationship between GFR and nephron number [3]. Consequently, to focus on the severity of injury as a determinant of eGFR, we nullify the simultaneous contribution of nephron number to ECd and ENAG by normalizing these excretion rates to Ccr. Whereas the relationship of Ecr to nephron number is indirect and highly variable among subjects, the relationship of Ccr to nephron number is relatively direct and consistent. On theoretical grounds, we expect eGFR to be more closely associated with ECd/Ccr and ENAG/Ccr than with ECd/Ecr and ENAG/Ecr, and the data reported herein confirm that expectation. We recommend the adoption of Ccr as the optimal denominator for the normalization of excretion rates in studies of Cd nephropathy.
4. Materials and Methods
4.1. Study Population
To develop a diverse sample with a wide range of environmental exposure to Cd, we assembled archived data drawn from multiple sites in Thailand. At the time of recruitment, all participants had lived at their current addresses for at least 30 years, and all gave informed consent to participate. Exclusion criteria were pregnancy, breast-feeding, a history of metal work, and a hospital record or physician’s diagnosis of an advanced chronic disease. Smoking, diabetes, hypertension, regular use of medications, educational level, occupation, and family health history were ascertained by questionnaire. Diabetes was defined as fasting plasma glucose levels ≥126 mg/dL or a physician’s prescription of anti-diabetic medications. Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, a physician’s diagnosis, or prescription of anti-hypertensive medications.
Blood and urine were obtained in 2001 and 2002 from control subjects in Bangkok, and in 2004 and 2005 from subjects in subsistence farming areas of Mae Sot District. As judged by the Cd content of rice, a dietary staple in Thailand, exposure to Cd was low in Bangkok and moderate or high in Mae Sot [17,32,33,34]. Because occupational exposure was an exclusion criterion, we presumed that all participants had acquired Cd from the environment. After exclusion of participants with incomplete datasets, the study sample included 545 women and 386 men. Within the sample, urinary Cd varied by a factor >1000; age ranged from 16 to 87 years, and eGFR from 20 to 139 mL/min/1.73 m2. The Institutional Ethical Committees of Chulalongkorn University, Chiang Mai University, and the Mae Sot Hospital approved the study protocol [33].
4.2. Specimen Collection and Analysis
Second morning-void urine samples were collected after an overnight fast. Within the ensuing 3 h, specimens of whole blood were obtained and serum samples were prepared. Aliquots of urine, whole blood, and serum were transported on ice from a mobile clinic to a laboratory and stored at −20 or −80 °C for later analysis. Assays of creatinine in urine and serum ([cr]u, [cr]p]) were based on the Jaffe reaction. The assay of NAG in urine ([NAG]u) was based on colorimetry (NAG test kit, Shionogi Pharmaceuticals, Sapporo, Japan).
For the Bangkok group, [Cd]u was determined by inductively-coupled plasma mass spectrometry (ICP/MS, Agilent 7500, Agilent Technologies, Santa Clara, CA, USA) because this method was sufficiently sensitive to measure Cd concentrations below the detectable limit of atomic absorption spectrophotometry. Multi-element standards (EM Science, EM Industries, Inc., Newark, NJ, USA) were used to calibrate Cd analyses, and accuracy and precision of those analyses were evaluated with reference urine (Lyphochek®, Bio-Rad, Gladesville, New South Wales, Australia). When [Cd]u was less than the detection limit, 0.05 μg/L, the concentration assigned was the detection limit divided by the square root of 2. Fifty-eight subjects (14.7%) in the Bangkok group had [Cd]u < 0.05 μg/L.
For the Mae Sot groups, [Cd]u was determined by atomic absorption spectrophotometry (Shimadzu Model AA-6300, Kyoto, Japan). Urine standard reference material No. 2670 (National Institute of Standards, Washington, DC, USA) was used for quality assurance and control purposes. None of the urine samples from the Mae Sot groups were found to have [Cd]u below the detection limit.
4.3. Normalization of Excretion Rates to Ecr and Ccr
Excretion rates of Cd and NAG (ECd and ENAG) were normalized to Ecr or to Ccr, a surrogate for GFR. ECd/Ecr and ENAG/Ecr were expressed as mass excreted per g of creatinine; ECd/Ccr and ENAG/Ccr were expressed as mass excreted per volume of filtrate. For x = Cd or NAG, Ex/Ecr was calculated as [x]u/[cr]u, and Ex/Ccr was calculated as [x]u[cr]p/[cr]u [23].
4.4. Estimated Glomerular Filtration Rates (eGFR)
The glomerular filtration rate was estimated with equations from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [27]. CKD stages 1, 2, 3, 4, and 5 corresponded to eGFR of 90–119, 60–89, 30–59, 15–29 and <15 mL/min/1.73 m2, respectively. For dichotomous comparisons, CKD was defined as eGFR < 60 mL/min/1.73 m2.
4.5. Statistical Analysis
Data were analyzed with SPSS 17.0 (SPSS Inc., Chicago, IL, USA). The Kruskal–Wallis test was used to assess differences in means among three subsets, and the Pearson chi-squared test was used to assess differences in percentages. The one-sample Kolmogorov–Smirnov test was used to identify departures of continuous variables from a normal distribution, and a base-10 or base-2 logarithmic transformation was applied to variables that showed rightward skewing before they were subjected to parametric statistical analysis. Throughout the text, base-10 and base-2 logarithms are denoted as log(x) and log2(x), respectively.
In our regression analyses (Table 2 and Table 3), independent variables included log(ECd/Ecr), log(ECd/Ccr), log(ENAG/Ecr), log(ENAG/Ccr), age, diabetes, gender, hypertension, and smoking. For each independent variable (x), we used multivariable logistic regression analyses to ascertain the prevalence odds ratio (POR) for CKD and the corresponding β-coefficient (Table 2). The β-coefficient—i.e., the slope of a line relating the natural log of POR to x—thus depicted the effect of a one-unit change in x on POR while other independent variables remained constant. Log base-2 transformation of ECd/Ecr, ENAG/Ecr, ECd/Ccr, and ENAG/Ccr permitted estimation of the factor by which POR increased as each ratio was doubled. We employed two models in each logistic regression analysis: model 1 incorporated log2(ECd/Ecr) and log2(ENAG/Ecr); model 2 incorporated log2(ECd/Ccr) and log2(ENAG/Ccr).
We performed multivariable linear regression analyses in three subsets of the study sample (Table 3). The subsets were defined by gradations of log[(ECd/Ecr) × 103] (model 1) or log[(ECd/Ccr) × 105] (model 2). We examined associations of eGFR with log10(ECd/Ecr), log10(ENAG/Ecr), log10(ECd/Ccr), log10(ENAG/Ccr), and the aforementioned demographic variables. For each model, an adjusted coefficient of determination (R2) and standardized β were obtained to indicate, respectively, the total variation in eGFR that was explained by all independent variables, and the strength of association between eGFR and an individual independent variable.
Polynomial regression was used to fit lines and curves to the following scatterplots: eGFR against log(ECd/Ecr), log(ENAG/Ecr), log(ECd/Ccr), and log(ENAG/Ccr); log(ENAG/Ecr) against log(ECd/Ecr); and log(ENAG/Ccr) against log(ECd/Ccr). A linear model, y = a + bx, was adopted if the relationship was monotonic. A quadratic model (second-order polynomial), y = a + b1x + b2 x2, was used if there was a significant change in the direction of the slope (b1 to b2) for prediction of dependent variable y. In both types of equations, a represented the y-intercept.
Relationships between x and y were assessed with R2 (the coefficient of determination) and with unstandardized and standardized β coefficients. In linear and quadratic models, R2 is the fraction of variation in y that is explained by variation in x. In linear models, the unstandardized β coefficient is the slope of the linear regression, and the standardized β coefficient indicates the strength of the association between y and x on a uniform scale. A linear regression method was used to perform slope analyses of quadratic curves relating eGFR to log(ECd/Ecr), log(ENAG/Ecr), log(ECd/Ccr), and log(ENAG/Ccr).
In Figure 1E,F, Figure 2E,F and Figure 3E,F, a univariate model analysis was used to derive mean eGFR (Figure 1 and Figure 2), mean log(ENAG/Ecr) (Figure 3), and mean log(ENAG/Ccr) (Figure 3) for men and women separately with adjustment for covariates (including age) and interactions among independent variables. The Cd-excretion levels in subsets of Figure 1E,F and Figure 3E,F are identical to those depicted in subsets of Table 3. Raw data for eGFR were employed in Table 1 and Table 2 and in Figure 1A,C and Figure 2A,C. In all analyses, two-sided p-Values ≤ 0.05 were assumed to indicate statistical significance.
5. Conclusions
Excretion rates of Cd and NAG elucidate reductions in GFR that result from renal accumulation of Cd. The conventional method for expressing these excretion rates, normalization of urine concentrations to [cr]u, incorporates conceptual flaws that are eliminated if the rates are normalized to Ccr. In a large and diverse sample of Thai subjects, the alternate approach strengthened all identifiable relationships between eGFR and conventionally quantified urine components, and exposed additional relationships that were obscured by the conventional method. Normalization to Ccr should replace normalization to [cr]u in studies that relate urine composition to Cd-induced diminution of eGFR.
Author Contributions
S.S. and K.R.P. conceptualized the comparison of two methods for the normalization of excretion rates. K.R.P. proposed the normalization of excretion rates to creatinine clearance (Ccr). In collaboration with G.C.G. and D.A.V., S.S. organized and analyzed the data and created the tables and figures. K.R.P. wrote initial and subsequent drafts of the manuscript, and S.S. reviewed and edited all drafts. W.R. and M.N. supervised the recruitment of participants and the collection of biologic specimens in Thailand. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study analyzed archived data taken from published reports [17,33,34]. Ethical review and approval were not applicable.
Informed Consent Statement
All participants took part in the study after giving informed consent.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This work was partially supported by the Commission for Higher Education, Thailand Ministry of Education in partnership with the National Research Centre for Environmental Toxicology and Department of Nephrology, Princess Alexandra Hospital, and the Centre for Kidney Disease Research, The University of Queensland Faculty of Medicine and Translational Research Institute. Additionally, this work was supported with resources of the Stratton Veterans Affairs Medical Center, Albany, NY, USA, and was made possible by facilities at that institution. Opinions expressed in this paper are those of the authors and do not represent the official position of the United States Department of Veterans’ Affairs.
Conflicts of Interest
The authors have declared no potential conflict of interest.
Abbreviations
| GFR | Glomerular filtration rate, mL/min |
| eGFR | Estimated glomerular filtration rate, mL/min/1.73m2 (of body surface area) |
| CKD-EPI | Chronic kidney disease epidemiology collaboration |
| Cd | Cadmium |
| NAG | N-acetyl-β-D-glucosaminidase |
| Cr | Creatinine |
| Ccr | Creatinine clearance (a surrogate for GFR), mL/min |
| Vu | Rate of urine flow, volume/time |
| [x]u | Urine concentration of substance x (cr, Cd, or NAG), mass/volume |
| Ex | Excretion rate of substance x (cr, Cd, or NAG), mass/time |
| Ex/Ecr | Amount of x excreted per gram of creatinine excreted, mass/mass |
| [x]u/[cr]u | Ratio of urine concentration of x to urine concentration of cr, mass/mass |
| Ex/Ccr | Amount of x excreted per volume of filtrate, mass/volume |
| MT | Metallothionein |
| CdMT | Cd-metallothionein complex |
| POR | Probability odds ratio |
References
- Satarug, S. Dietary cadmium intake and its effects on kidneys. Toxics 2018, 6, 15. [Google Scholar] [CrossRef]
- Satarug, S.; Phelps, K.R. Cadmium Exposure and Toxicity. In Metal Toxicology Handbook; Bagchi, D., Bagchi, M., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 219–274. [Google Scholar]
- Satarug, S.; Gobe, G.C.; Vesey, D.A.; Phelps, K.R. Cadmium and lead exposure, nephrotoxicity, and mortality. Toxics 2020, 8, 86. [Google Scholar] [CrossRef]
- Price, R.G. Measurement of N-acetyl-beta-glucosaminidase and its isoenzymes in urine: Methods and clinical applications. Eur. J. Clin. Chem. Clin. Biochem. 1992, 30, 693–705. [Google Scholar] [PubMed]
- Kawada, T.; Koyama, H.; Suzuki, S. Cadmium, NAG activity, and β2-microglobulin in the urine of cadmium pigment workers. Br. J. Ind. Med. 1989, 46, 52–55. [Google Scholar] [PubMed]
- Kawada, T.; Shinmyo, R.R.; Suzuki, S. Urinary cadmium and N-acetyl-β-D-glucosaminidase excretion of inhabitants living in a cadmium-polluted area. Int. Arch. Occup. Environ. Health 1992, 63, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Swaddiwudhipong, W.; Limpatanachote, P.; Nishijo, M.; Honda, R.; Mahasakpan, P.; Krintratun, S. Cadmium-exposed population in Mae Sot District, Tak Province: 3. Associations between urinary cadmium and renal dysfunction, hypertension, diabetes, and urinary stones. J. Med. Assoc. Thai. 2010, 93, 231–238. [Google Scholar] [PubMed]
- Zhang, Y.R.; Wang, P.; Liang, X.X.; Tan, C.S.; Tan, J.B.; Wang, J.; Huang, Q.; Huang, R.; Li, Z.X.; Chen, W.C.; et al. Associations between urinary excretion of cadmium and renal biomarkers in non-smoking females: A cross-sectional study in rural areas of South China. Int. J. Environ. Res. Public Health 2015, 12, 11988–12001. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.D.; Yim, D.H.; Eom, S.Y.; Moon, S.I.; Park, C.H.; Kim, G.B.; Yu, S.D.; Choi, B.S.; Park, J.D.; Kim, H. Temporal changes in urinary levels of cadmium, N-acetyl-beta-d-glucosaminidase and beta2-microglobulin in individuals in a cadmium-contaminated area. Environ. Toxicol. Pharmacol. 2015, 39, 35–41. [Google Scholar] [CrossRef]
- Eom, S.Y.; Seo, M.N.; Lee, Y.S.; Park, K.S.; Hong, Y.S.; Sohn, S.J.; Kim, Y.D.; Choi, B.S.; Lim, J.A.; Kwon, H.J.; et al. Low-level environmental cadmium exposure induces kidney tubule damage in the general population of Korean adults. Arch. Environ. Contam. Toxicol. 2017, 73, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lu, Y.; Li, Y.; Zhao, H.; Wang, X.; Shen, Y.; Kuang, X. Correlation between environmental low-dose cadmium exposure and early kidney damage: A comparative study in an industrial zone vs. a living quarter in Shanghai, China. Environ. Toxicol. Pharmacol. 2020, 79, 103381. [Google Scholar] [CrossRef]
- Bernard, A.; Thielemans, N.; Roels, H.; Lauwerys, R. Association between NAG-B and cadmium in urine with no evidence of a threshold. Occup. Environ. Med. 1995, 52, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Noonan, C.W.; Sarasua, S.M.; Campagna, D.; Kathman, S.J.; Lybarger, J.A.; Mueller, P.W. Effects of exposure to low levels of environmental cadmium on renal biomarkers. Environ. Health Perspect. 2002, 110, 151–155. [Google Scholar] [CrossRef]
- Thomas, D.K.; Hodgson, S.; Nieuwenhuijsen, M.; Jarup, L. Early kidney damage in a population exposed to cadmium and other heavy metals. Environ. Health Perspect. 2009, 117, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Ruangyuttikarn, W.; Nishijo, M.; Gobe, G.C.; Phelps, K.R. The source and pathophysiologic significance of excreted cadmium. Toxics 2019, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Jarup, L.; Persson, B.; Elinder, C.G. Decreased glomerular filtration rate in solderers exposed to cadmium. Occup. Environ. Med. 1995, 52, 818–822. [Google Scholar] [CrossRef]
- Honda, R.; Swaddiwudhipong, W.; Nishijo, M.; Mahasakpan, P.; Teeyakasem, W.; Ruangyuttikarn, W.; Satarug, S.; Padungtod, C.; Nakagawa, H. Cadmium induced renal dysfunction among residents of rice farming area downstream from a zinc-mineralized belt in Thailand. Toxicol. Lett. 2010, 198, 26–32. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Arteaga, C.; McManus, C.; Smith, J.; Moffitt, S. Measurement of muscle mass in humans: Validity of the 24-hour urinary creatinine method. Am. J. Clin. Nutr. 1983, 37, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Weaver, V.M.; Garcia Vargas, G.; Silbergeld, E.K.; Rothenberg, S.J.; Fadrowski, J.J.; Rubio-Andrade, M.; Parsons, P.J.; Steuerwald, A.J.; Navas-Acien, A.; Guallar, E. Impact of urine concentration adjustment method on associations between urine metals and estimated glomerular filtration rates (eGFR) in adolescents. Environ. Res. 2014, 132, 226–232. [Google Scholar] [CrossRef]
- Buser, M.C.; Ingber, S.Z.; Raines, N.; Fowler, D.A.; Scinicariello, F. Urinary and blood cadmium and lead and kidney function: NHANES 2007-2012. Int. J. Hyg. Environ. Health 2016, 219, 261–267. [Google Scholar] [CrossRef]
- Jin, R.; Zhu, X.; Shrubsole, M.J.; Yu, C.; Xia, Z.; Dai, Q. Associations of renal function with urinary excretion of metals: Evidence from NHANES 2003-2012. Environ. Int. 2018, 121, 1355–1362. [Google Scholar] [CrossRef]
- Satarug, S.; Boonprasert, K.; Gobe, G.C.; Ruenweerayut, R.; Johnson, D.W.; Na-Bangchang, K.; Vesey, D.A. Chronic exposure to cadmium is associated with a marked reduction in glomerular filtration rate. Clin. Kidney J. 2018, 12, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Phelps, K.R.; Gosmanova, E.O. A generic method for analysis of plasma concentrations. Clin. Nephrol. 2020, 94, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Scmid, C.H.; Zhang, Y.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Mitch, W.E.; Walser, M. A proposed mechanism for reduced creatinine excretion in severe chronic renal failure. Nephron 1978, 21, 248–254. [Google Scholar] [CrossRef]
- Mitch, W.E.; Collier, V.U.; Walser, M. Creatinine metabolism in chronic renal failure. Clin. Sci. 1980, 58, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Soveri, I.; Berg, U.B.; Bjork, J.; Elinder, C.-G.; Grubb, A.; Mejare, I.; Sterner, G.; Back, S.-E. Measuring GFR: A systematic review. Am. J. Kidney Dis. 2014, 64, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Baker, J.R.; Reilly, P.E.B.; Moore, M.R.; Williams, D.J. Cadmium levels in the lung, liver, kidney cortex, and urine samples from Australians without occupational exposure to metals. Arch. Environ. Health 2002, 57, 69–77. [Google Scholar] [CrossRef]
- Akerstrom, M.; Barregard, L.; Lundh, T.; Sallsten, G. The relationship between cadmium in kidney and cadmium in urine and blood in an environmentally exposed population. Toxicol. Appl. Pharmacol. 2013, 268, 286–293. [Google Scholar] [CrossRef]
- Nomiyama, K.; Foulkes, C. Reabsorption of filtered cadmium-metallothionein in the rabbit kidney. Proc. Soc. Exp. Biol. Med. 1977, 156, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Schnaper, H.W. The tubulointerstitial pathophysiology of progressive kidney disease. Adv. Chron. Kidney Dis. 2017, 24, 107–116. [Google Scholar] [CrossRef]
- Simmons, R.W.; Pongsakul, P.; Saiyasitpanich, D.; Klinphoklap, S. Elevated levels of cadmium and zinc in paddy soils and elevated levels of cadmium in rice grain downstream of a zinc mineralized area in Thailand: Implications for public health. Environ. Geochem. Health 2005, 27, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Swaddiwudhipong, W.; Ruangyuttikarn, W.; Nishijo, M.; Ruiz, P. Modeling cadmium exposures in low- and high-exposure areas in Thailand. Environ. Health Perspect. 2013, 121, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Swaddiwudhipong, W.; Nguntra, P.; Kaewnate, Y.; Mahasakpan, P.; Limpatanachote, P.; Aunjai, T.; Jeekeeree, W.; Punta, B.; Funkhiew, T.; Phopueng, I. Human health effects from cadmium exposure: Comparison between persons living in cadmium-contaminated and non-contaminated areas in northwestern Thailand. Southeast Asian J. Trop. Med. Public Health 2015, 46, 133–142. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).