Selective Cytotoxicity of Complexes with N,N,N-Donor Dipodal Ligand in Tumor Cells
Abstract
:1. Introduction
2. Results
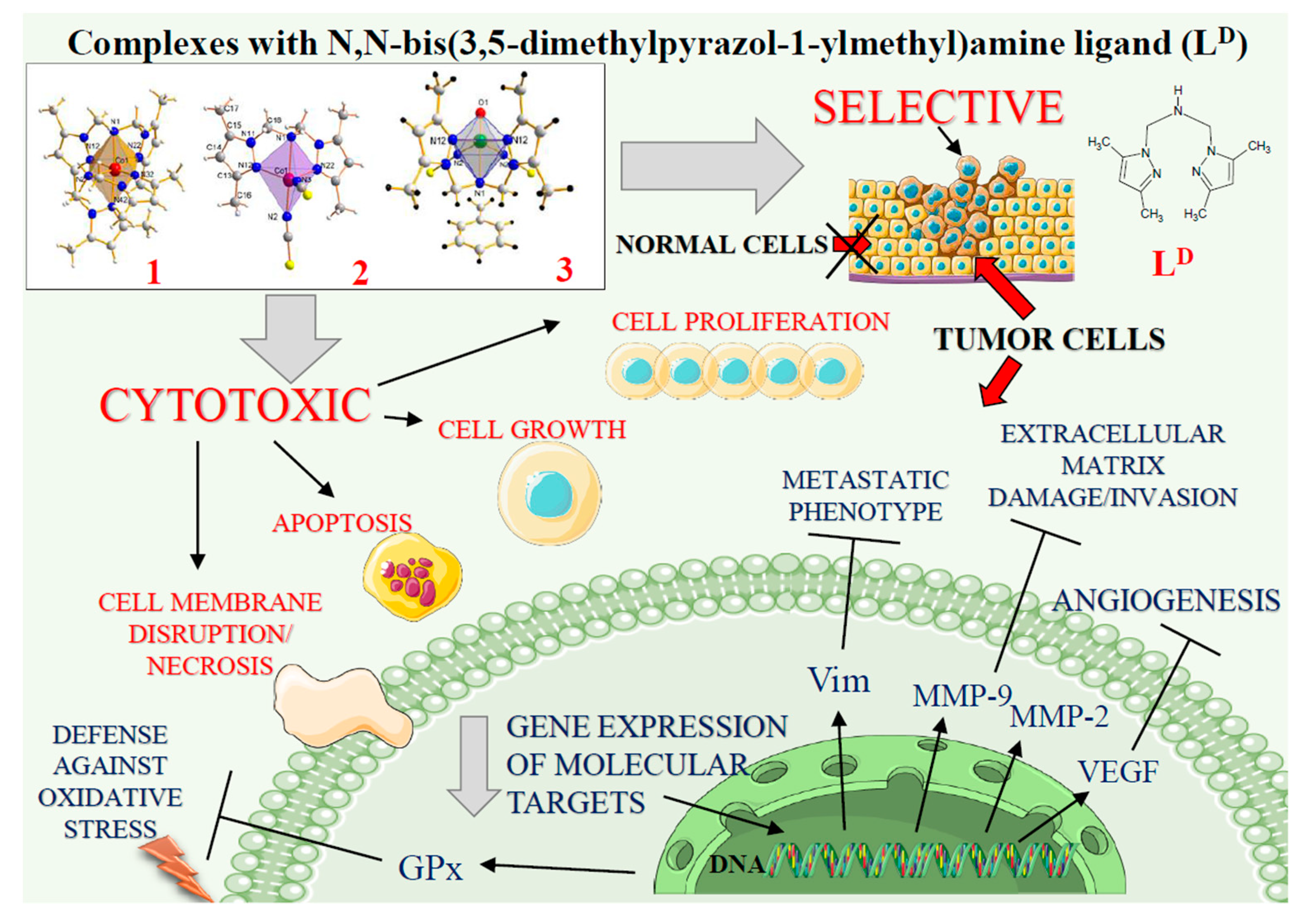
2.1. Cobalt and Vanadium Complexes Exert Toxic Effect on Tumour Cells but Not in Noncancerous Cells
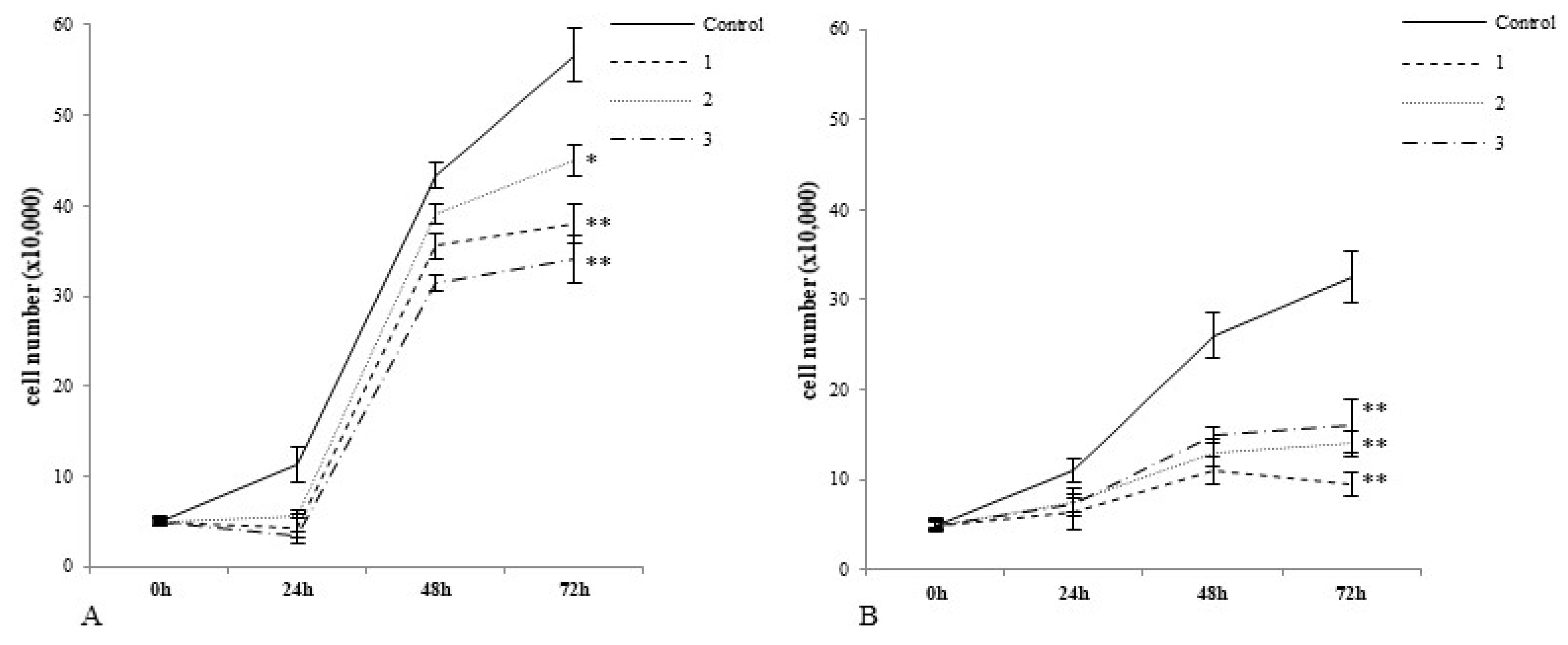
2.2. Cobalt and Vanadium Complexes Hamper Growth of Cancer Cells
2.3. Cobalt and Vanadium Complexes Exert Antiproliferative Effects on Tumour Cells
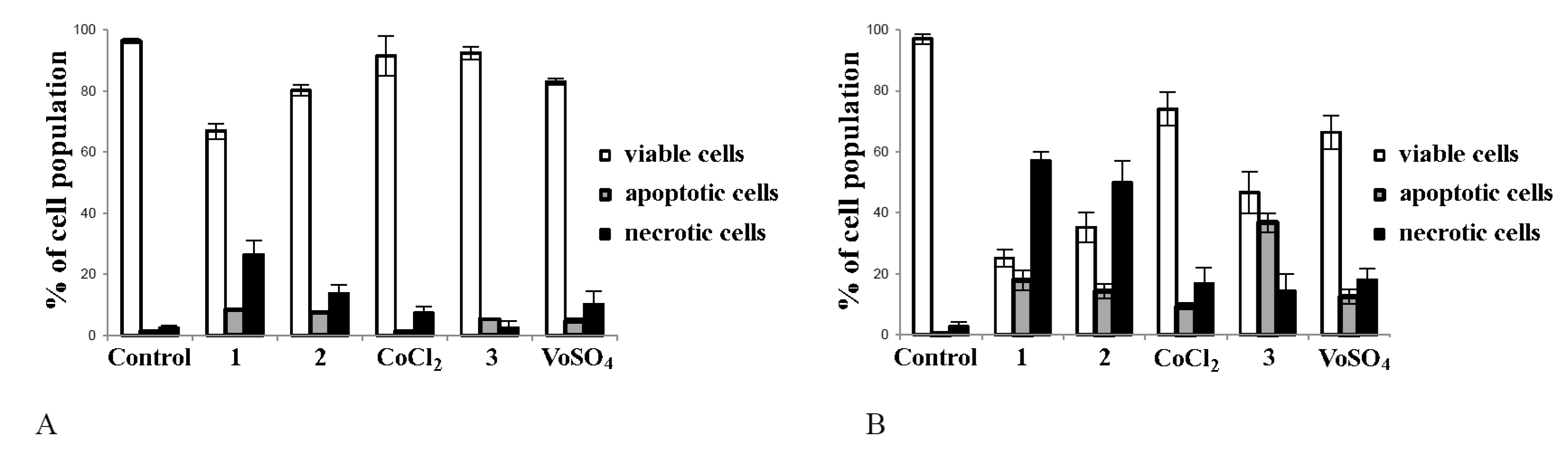
2.4. Cobalt and Vanadium Complexes Induce Apoptosis in Cancer Cells
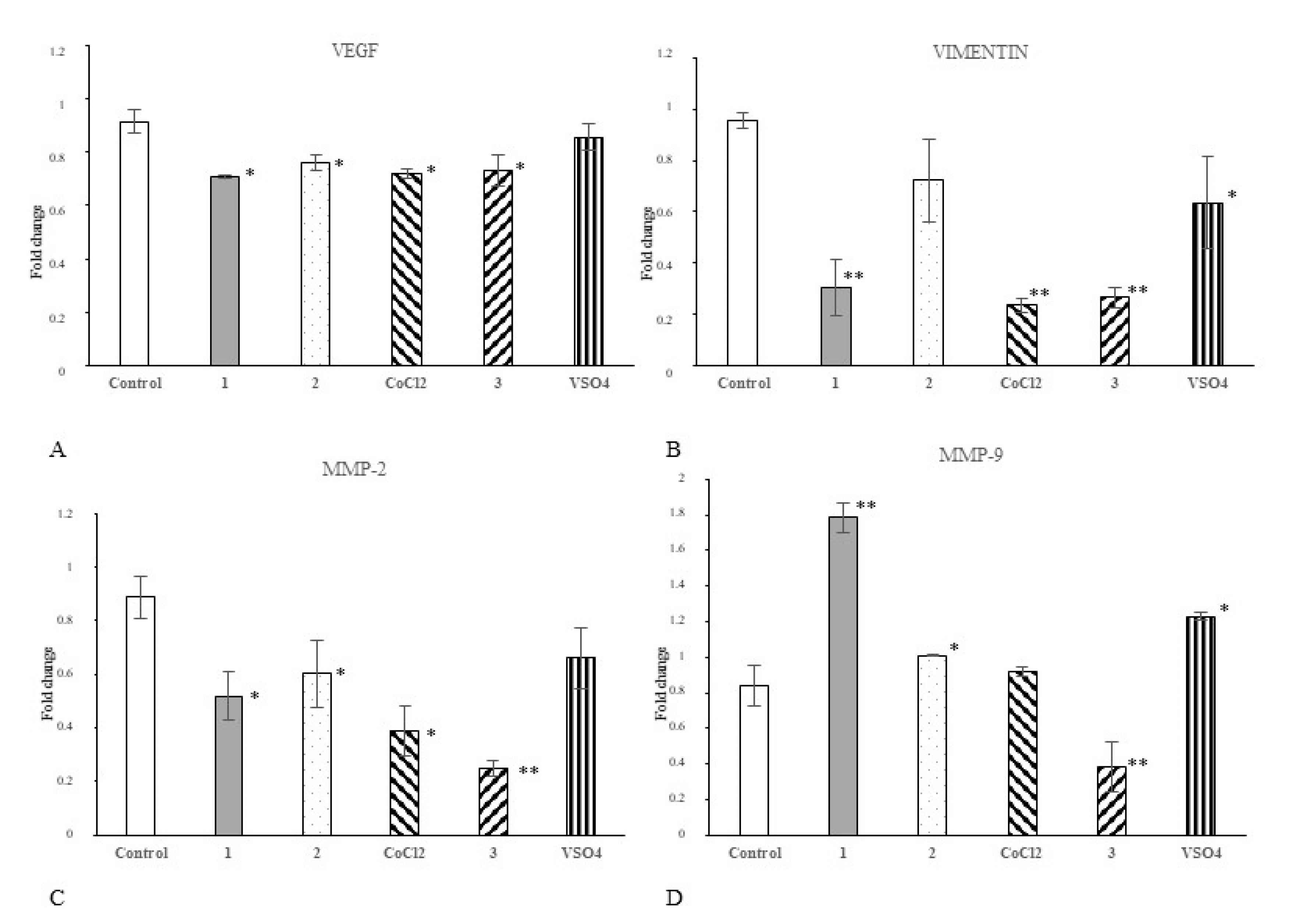
2.5. Cobalt and Vanadium Complexes Impair Expression of Genes Regulating Aggressive Phenotype of Cancer Cells
2.6. Cobalt Complexes Increase Glutathione Peroxidase (Gpx) Activity in Noncancerous Cells and Vanadium Complex Decreases the Enzyme Activity in Cancer Cells
2.7. Computational Prediction of Drugability of Cobalt and Vanadium Complexes
3. Discussion
4. Materials and Methods
4.1. Reagents
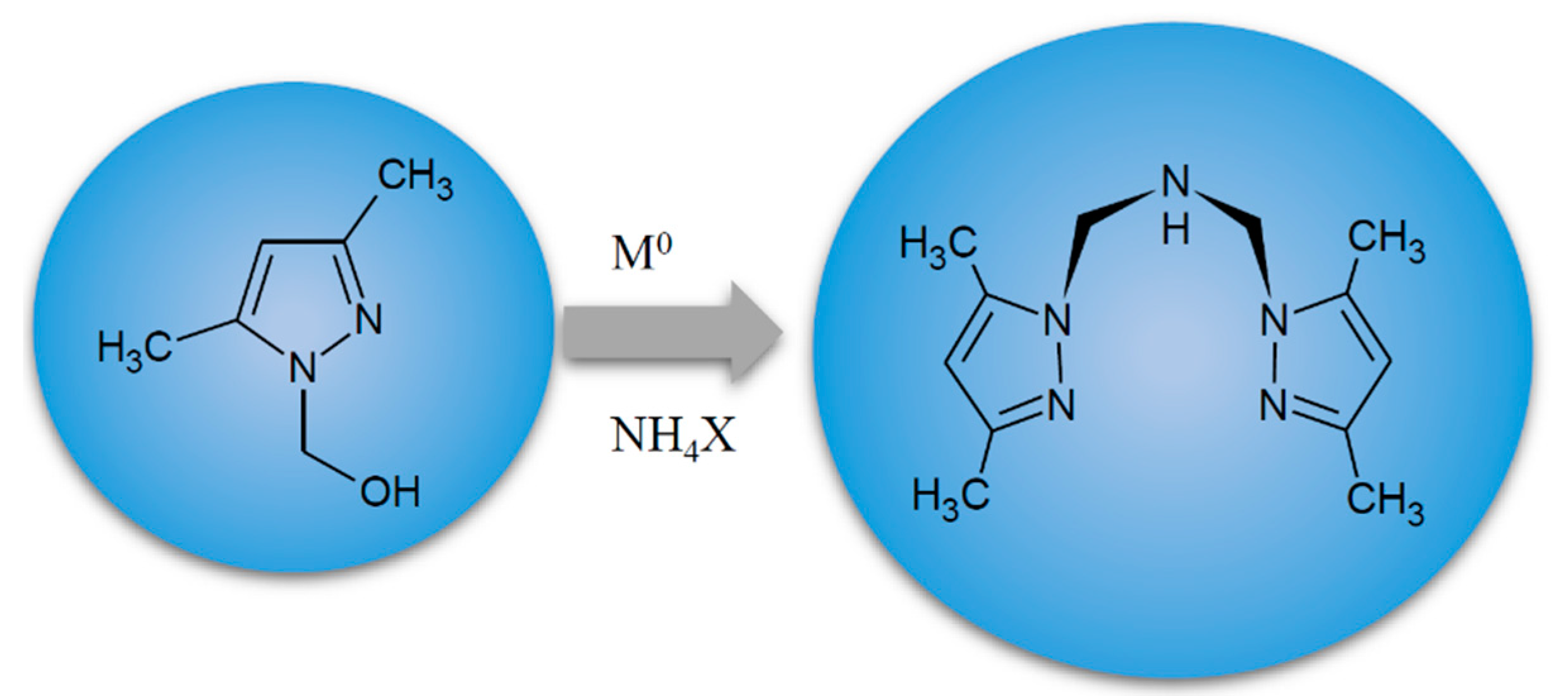
4.2. Metal Complexes and Their Synthesis
4.3. Cell Culture Experiments
4.4. Measurement of Cytotoxic Potency of Complexes with Trypan Blue Exclusion Test
4.5. Cell Growth Assessment
4.6. Antiproliferative MTT Assay
4.7. Apoptosis and Necrosis Detection
4.8. Measurement of Gene Expression With Real-Time PCR (Quantitative Polymerase Chain Reaction, qPCR)
4.9. Measurement of Extracellular Glutathione Peroxidase (GPx) Activity
4.10. Computational Prediction of Drugability
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mjos, K.D.; Orvig, C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Haas, K.L.; Franz, K.J. Application of metal coordination chemistry to explore and manipulate cell biology. Chem. Rev. 2009, 109, 4921–4960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wani, W.A.; Prashar, S.; Shreaz, S.; Gómez-Ruiz, S. Nanostructured materials functionalized with metal complexes: In search of alternatives for administering anticancer metallodrugs. Coord. Chem. Rev. 2016, 312, 67–98. [Google Scholar] [CrossRef]
- Andrade, M.A.; Martins, L.M.D.R.S. Novel chemotherapeutic agents—The contribution of scorpionates. Curr. Med. Chem. 2019, 26, 7452–7475. [Google Scholar] [CrossRef]
- Munteanu, C.R.; Suntharalingam, K. Advances in cobalt complexes as anticancer agents. Dalt. Trans. 2015, 44, 13796–13808. [Google Scholar] [CrossRef] [PubMed]
- Adach, A.; Daszkiewicz, M.; Tyszka-Czochara, M. Comparative X-ray, vibrational, theoretical and biological studies of new in situ formed [CoLSX]2[CdX4] halogenocadmate(II) complexes containing N-scorpionate ligand. Polyhedron 2020, 175, 114229. [Google Scholar] [CrossRef]
- Jia, P.; Ouyang, R.; Cao, P.; Tong, X.; Zhou, X.; Lei, T.; Zhao, Y.; Guo, N.; Chang, H.; Miao, Y.; et al. Review: Recent advances and future development of metal complexes as anticancer agents. J. Coord. Chem. 2017, 70, 2175–2201. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Water-soluble C-scorpionate complexes—Catalytic and biological applications. Eur. J. Inorg. Chem. 2016, 2016, 2236–2252. [Google Scholar] [CrossRef]
- Pedrosa, P.; Carvalho, A.; Baptista, P.V.; Fernandes, A.R. Inorganic Coordination Chemistry: Where We Stand in Cancer Treatment? In Basic Concepts Viewed from Frontier in Inorganic Coordination Chemistry; IntechOpen: London, UK, 2018. [Google Scholar]
- Heffern, M.C.; Yamamoto, N.; Holbrook, R.J.; Eckermann, A.L.; Meade, T.J. Cobalt derivatives as promising therapeutic agents. Curr. Opin. Chem. Biol. 2013, 17, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Makinen, M.W.; Salehitazangi, M. The structural basis of action of vanadyl (VO2+) chelates in cells. Coord. Chem. Rev. 2014, 279, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Massoud, S.S.; Louka, F.R.; Ducharme, G.T.; Fischer, R.C.; Mautner, F.A.; Vančo, J.; Herchel, R.; Dvořák, Z.; Trávníček, Z. Copper(II) complexes based on tripodal pyrazolyl amines: Synthesis, structure, magnetic properties and anticancer activity. J. Inorg. Biochem. 2018, 180, 39–46. [Google Scholar] [CrossRef]
- Adach, A.; Daszkiewicz, M.; Barszcz, B.; Cieślak-Golonka, M.; Maciejewska, G. Redox processes as a route for the formation of an unusual structure of the Co(II)/Zn(II) complex isolated from the system: [Co(0)/Zn(II)-1-hydroxymethyl-3,5-dimethylpyrazole]. Inorg. Chem. Commun. 2010, 13, 361–364. [Google Scholar] [CrossRef]
- Adach, A.; Daszkiewicz, M.; Barszcz, B. Synthesis, X-ray structure, and spectroscopic investigation of new Co(II)/Cd(II) complexes formed via the reaction of 1-hydroxymethyl-3,5-dimethylpyrazole and Co0, CdCl2, NH4I. Struct. Chem. 2010, 21, 331–336. [Google Scholar] [CrossRef]
- Adach, A.; Daszkiewicz, M.; Tyszka-Czochara, M. A family of complexes with: N -scorpionate-type and other N -donor ligands obtained in situ from pyrazole derivative and zerovalent cobalt. Physicochemical and cytotoxicity studies. RSC Adv. 2016, 6, 44070–44079. [Google Scholar] [CrossRef]
- Adach, A. Review: An overview of recent developments in coordination chemistry of polypyrazolylmethylamines. Complexes with N-scorpionate ligands created in situ from pyrazole derivatives and zerovalent metals. J. Coord. Chem. 2017, 70, 757–779. [Google Scholar] [CrossRef]
- Trofimenko, S. Recent advances in Poly(pyrazolyl)borate (scorpionate) chemistry. Chem. Rev. 1993, 93, 943–980. [Google Scholar] [CrossRef]
- Adach, A.; Daszkiewicz, M.; Duczmal, M.; Staszak, Z. Cobalt(II) complex containing two-ring scorpionate-like ligands formed in situ. Studies on the [Co0-1-hydroxymethyl-3,5-dimethylpyrazole- MoO3-NH4I] system. Inorg. Chem. Commun. 2013, 35, 22–26. [Google Scholar] [CrossRef]
- Adach, A.; Daszkiewicz, M.; Barszcz, B. Experimental and theoretical studies on the structure and spectroscopic properties of N-scorpionate complexes obtained from metallic cobalt in a one pot synthesis. Polyhedron 2015, 95, 60–68. [Google Scholar] [CrossRef]
- Adach, A.; Daszkiewicz, M.; Cieślak-Golonka, M. Cobalt(II) scorpionate-like complexes obtained from in situ synthesized ligand created in [Co(0)-1-hydroxymethyl-3,5-dimethylpyrazole-VOSO 4-NH 4SCN] system. Polyhedron 2012, 47, 104–111. [Google Scholar] [CrossRef]
- Masternak, J.; Zienkiewicz-Machnik, M.; Kowalik, M.; Jabłońska-Wawrzycka, A.; Rogala, P.; Adach, A.; Barszcz, B. Recent advances in coordination chemistry of metal complexes based on nitrogen heteroaromatic alcohols. Synthesis, structures and potential applications. Coord. Chem. Rev. 2016, 327–328, 242–270. [Google Scholar] [CrossRef]
- Adach, A.; Daszkiewicz, M.; Tyszka-Czochara, M.; Barszcz, B. New oxovanadium(IV) complexes with pincer ligand obtained in situ: Experimental and theoretical studies on the structure, spectroscopic properties and antitumour actIVity. RSC Adv. 2015, 5, 85470–85479. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Abou-Melha, K.S. Octahedral Co(II) and Ni(II) complexes of Schiff bases, semicarbazone and thiosemicarbazone, synthesis, biological, spectral, and thermal studies. J. Coord. Chem. 2008, 61, 2053–2067. [Google Scholar] [CrossRef]
- Harney, A.S.; Lee, J.; Manus, L.M.; Wang, P.; Ballweg, D.M.; LaBonne, C.; Meade, T.J. Targeted inhibition of Snail family zinc finger transcription factors by oligonucleotide-Co(III) Schiff base conjugate. Proc. Natl. Acad. Sci. USA 2009, 106, 13667–13672. [Google Scholar] [CrossRef] [Green Version]
- Hurtado, R.R.; Harney, A.S.; Heffern, M.C.; Holbrook, R.J.; Holmgren, R.A.; Meade, T.J. Specific inhibition of the transcription factor Ci by a cobalt(III) Schiff base-DNA conjugate. Mol. Pharm. 2012, 9, 325–333. [Google Scholar] [CrossRef] [Green Version]
- Satelli, A.; Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell. Mol. Life Sci. 2011, 68, 3033–3046. [Google Scholar] [CrossRef] [Green Version]
- Strouhalova, K.; Přechová, M.; Gandalovičová, A.; Brábek, J.; Gregor, M.; Rosel, D. Vimentin intermediate filaments as potential target for cancer treatment. Cancers 2020, 12, 184. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Kim, Y.J.; Seo, Y.R. An overview of carcinogenic heavy metal: Molecular toxicity mechanism and prevention. J. Cancer Prev. 2015, 20, 232–240. [Google Scholar] [CrossRef]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Dev. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef] [Green Version]
- Gałczyńska, K.; Ciepluch, K.; Madej, Ł.; Kurdziel, K.; Maciejewska, B.; Drulis-Kawa, Z.; Węgierek-Ciuk, A.; Lankoff, A.; Arabski, M. Selective cytotoxicity and antifungal properties of copper(II) and cobalt(II) complexes with imidazole-4-acetate anion or 1-allylimidazole. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Nair, R.S.; Kuriakose, M.; Somasundaram, V.; Shenoi, V.; Kurup, M.R.P.; Srinivas, P. The molecular response of vanadium complexes of nicotinoyl hydrazone in cervical cancers - A possible interference with HPV oncogenic markers. Life Sci. 2014, 116, 90–97. [Google Scholar] [CrossRef]
- Kongot, M.; Dohare, N.; Reddy, D.S.; Pereira, N.; Patel, R.; Subramanian, M.; Kumar, A. In vitro apoptosis-induction, antiproliferative and BSA binding studies of a oxidovanadium(V) complex. J. Trace Elem. Med. Biol. 2019, 51, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Waghray, A.; Patel, M.A.; Chatterjee, M. Vanadium in the detection, prevention and treatment of cancer: The in vivo evidence. Cancer Lett. 2010, 294, 1–12. [Google Scholar] [CrossRef]
- Evangelou, A.M. Vanadium in cancer treatment. Crit. Rev. Oncol. Hematol. 2002, 42, 249–265. [Google Scholar] [CrossRef]
- Webb, A.H.; Gao, B.T.; Goldsmith, Z.K.; Irvine, A.S.; Saleh, N.; Lee, R.P.; Lendermon, J.B.; Bheemreddy, R.; Zhang, Q.; Brennan, R.C.; et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer 2017, 17, 434. [Google Scholar] [CrossRef]
- Dufour, A.; Sampson, N.S.; Li, J.; Kuscu, C.; Rizzo, R.C.; DeLeon, J.L.; Zhi, J.; Jaber, N.; Liu, E.; Zucker, S.; et al. Small-molecule anticancer compounds selectively target the hemopexin domain of matrix metalloproteinase. Cancer Res. 2011, 71, 4977–4988. [Google Scholar] [CrossRef] [Green Version]
- Deryugina, E.I.; Quigley, J.P. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol. 2015, 44–46, 94–112. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, D.E. What has polar surface area ever done for drug discovery? Future Med. Chem. 2011, 3, 469–484. [Google Scholar] [CrossRef]
- Nettleton, D.O.; Einolf, H.J. Assessment of cytochrome P450 enzyme inhibition and inactivation in drug discovery and development. Curr. Top. Med. Chem. 2011, 11, 382–403. [Google Scholar] [CrossRef]
- Arnott, J.A.; Planey, S.L. The influence of lipophilicity in drug discovery and design. Expert Opin. Drug Discov. 2012, 7, 863–875. [Google Scholar] [CrossRef]
- Waring, M.J. Lipophilicity in drug discovery. Expert Opin. Drug Discov. 2010, 5, 235–248. [Google Scholar] [CrossRef]
- Tyszka-Czochara, M.; Bukowska-Strakova, K.; Kocemba-Pilarczyk, K.A.; Majka, M. Caffeic acid targets AMPK signaling and regulates tricarboxylic acid cycle anaplerosis while metformin downregulates HIF-1α-induced glycolytic enzymes in human cervical squamous cell carcinoma lines. Nutrients 2018, 10, 841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyszka-Czochara, M.; Bukowska-Strakova, K.; Majka, M. Metformin and caffeic acid regulate metabolic reprogramming in human cervical carcinoma SiHa/HTB-35 cells and augment anticancer activity of Cisplatin via cell cycle regulation. Food Chem. Toxicol. 2017, 106, 260–272. [Google Scholar] [CrossRef]
- Tyszka-Czochara, M.; Konieczny, P.; Majka, M. Caffeic acid expands anti-tumor effect of metformin in human metastatic cervical carcinoma HTB-34 cells: Implications of AMPK activation and impairment of fatty acids de novo biosynthesis. Int. J. Mol. Sci. 2017, 18, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyszka-Czochara, M.; Lasota, M.; Majka, M. Caffeic acid and metformin inhibit invasive phenotype induced by TGF-β1 in C-4I and HTB-35/SiHa human cervical squamous carcinoma cells by acting on different molecular targets. Int. J. Mol. Sci. 2018, 19, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adachi, T.; Wang, J.; Wang, X.L. Age-related change of plasma extracellular-superoxide dismutase. Clin. Chim. Acta 2000, 290, 169–178. [Google Scholar] [CrossRef]
- Comhair, S.A.A.; Bhathena, P.R.; Farver, C.; Thunnissen, F.B.J.M.; Erzurum, S.C. Extracellular glutathione peroxidase induction in asthmatic lungs: Evidence for redox regulation of expression in human airway epithelial cells. FASEB J. 2001, 15, 70–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howie, A.F.; Walker, S.W.; Akesson, B.; Arthur, J.R.; Beckett, G.J. Thyroidal extracellular glutathione peroxidase: A potential regulator of thyroid-hormone synthesis. Biochem. J. 1995, 308, 713–717. [Google Scholar] [CrossRef]
- Zagrodzki, P.; Joniec, A.; Gawlik, M.; Gawlik, M.; Krosniak, M.; Fołta, M.; Barton, H.; Pasko, P.; Chlopicka, J.; Zachwieja, Z. High fructose model of oxidative stress and metabolic disturbances in rats. Part, I. Antioxidant status of rats’ tissues. Bull. Vet. Inst. Pulawy 2007, 51, 407–412. [Google Scholar]
- Daina, A.; Zoete, V. Application of the swissdrugdesign online resources in virtual screening. Int. J. Mol. Sci. 2019, 20, 4612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manchester, J.; Walkup, G.; Rivin, O.; You, Z. Evaluation of p K a estimation methods on 211 druglike compounds. J. Chem. Inf. Model. 2010, 50, 565–571. [Google Scholar] [CrossRef] [PubMed]


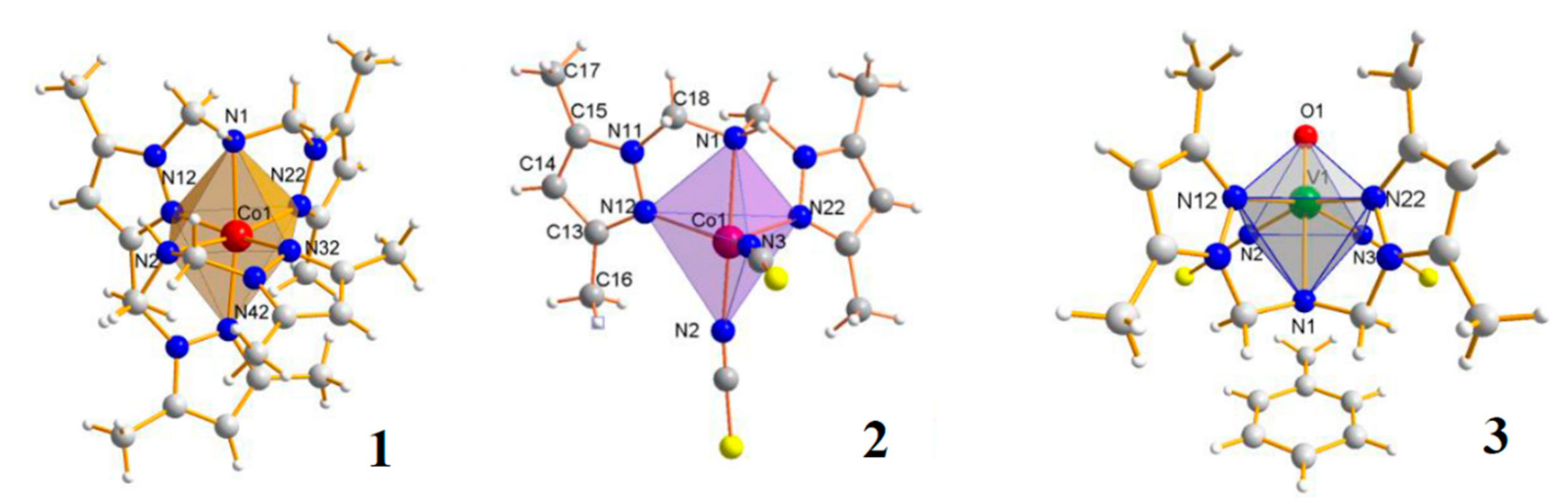
| Compound | IC50 [μM] for Noncancerous CHO-K1 Cells | IC50 [μM] for Cancer HEP G2 Cells | Antiproliferative Index IC50 CHO-K1/IC50 HEP G2 |
|---|---|---|---|
| 1 | 121.8 ± 10 | 22.0 ± 4 | 5.5 |
| 2 | 268.2 ± 15 | 38.2 ± 5 | 7.0 |
| 3 | 123.1 ± 3 | 45.6 ± 5 | 2.7 |
| VOSO4 | 98.3 ± 9 [22] | 75.6 ± 8 | 1.3 |
| CoCl2 | 175.6 ± 28 [15] | 160.2 ± 18 [15] | 1.1 |
| Cisplatin | 19.1 ± 2 | 21.3 ± 2 [15] | 0.9 |
| Compound | tPSA [Å2] | XLOGP3 | CYPinh | Lipinskiviol |
|---|---|---|---|---|
| 1 | 45.36 | 14.43 | No | 3 |
| 2 | 37.95 | 9.03 | No | 2 |
| 3 | 146.9 | 6.3 | No | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyszka-Czochara, M.; Adach, A.; Grabowski, T.; Konieczny, P.; Pasko, P.; Ortyl, J.; Świergosz, T.; Majka, M. Selective Cytotoxicity of Complexes with N,N,N-Donor Dipodal Ligand in Tumor Cells. Int. J. Mol. Sci. 2021, 22, 1802. https://doi.org/10.3390/ijms22041802
Tyszka-Czochara M, Adach A, Grabowski T, Konieczny P, Pasko P, Ortyl J, Świergosz T, Majka M. Selective Cytotoxicity of Complexes with N,N,N-Donor Dipodal Ligand in Tumor Cells. International Journal of Molecular Sciences. 2021; 22(4):1802. https://doi.org/10.3390/ijms22041802
Chicago/Turabian StyleTyszka-Czochara, Malgorzata, Anna Adach, Tomasz Grabowski, Paweł Konieczny, Paweł Pasko, Joanna Ortyl, Tomasz Świergosz, and Marcin Majka. 2021. "Selective Cytotoxicity of Complexes with N,N,N-Donor Dipodal Ligand in Tumor Cells" International Journal of Molecular Sciences 22, no. 4: 1802. https://doi.org/10.3390/ijms22041802
APA StyleTyszka-Czochara, M., Adach, A., Grabowski, T., Konieczny, P., Pasko, P., Ortyl, J., Świergosz, T., & Majka, M. (2021). Selective Cytotoxicity of Complexes with N,N,N-Donor Dipodal Ligand in Tumor Cells. International Journal of Molecular Sciences, 22(4), 1802. https://doi.org/10.3390/ijms22041802









