Coumarins from Seseli devenyense Simonk.: Isolation by Liquid–Liquid Chromatography and Potential Anxiolytic Activity Using an In Vivo Zebrafish Larvae Model
Abstract
1. Introduction
2. Results
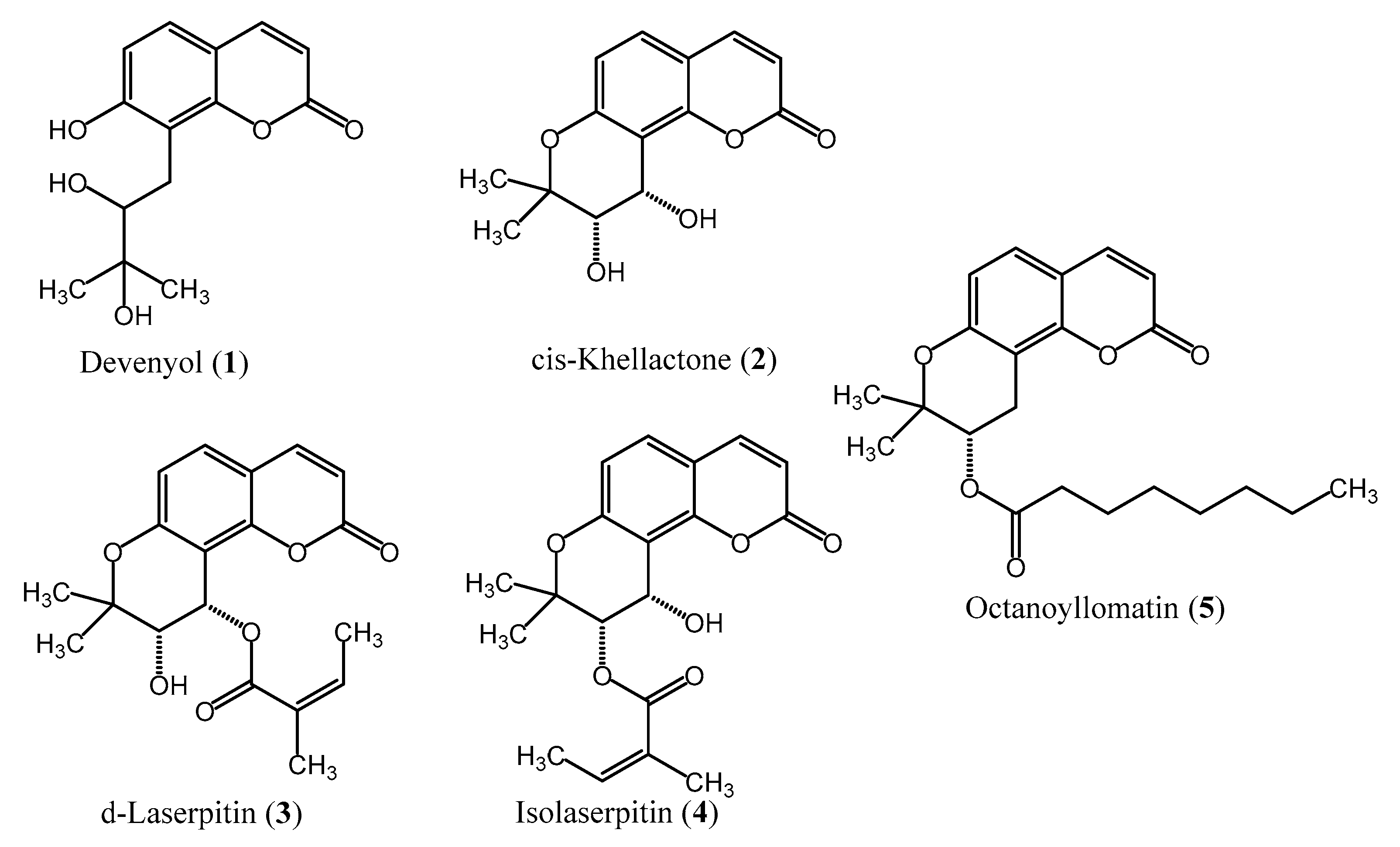
2.1. Liquid–Liquid Chromatography Isolation of Coumarins
2.2. The Effects of Seseli devenyense Crude Extract and Isolated Coumarins on Thigmotaxis Behaviours of the Zebrafish Larvae during Light-Dark Changes
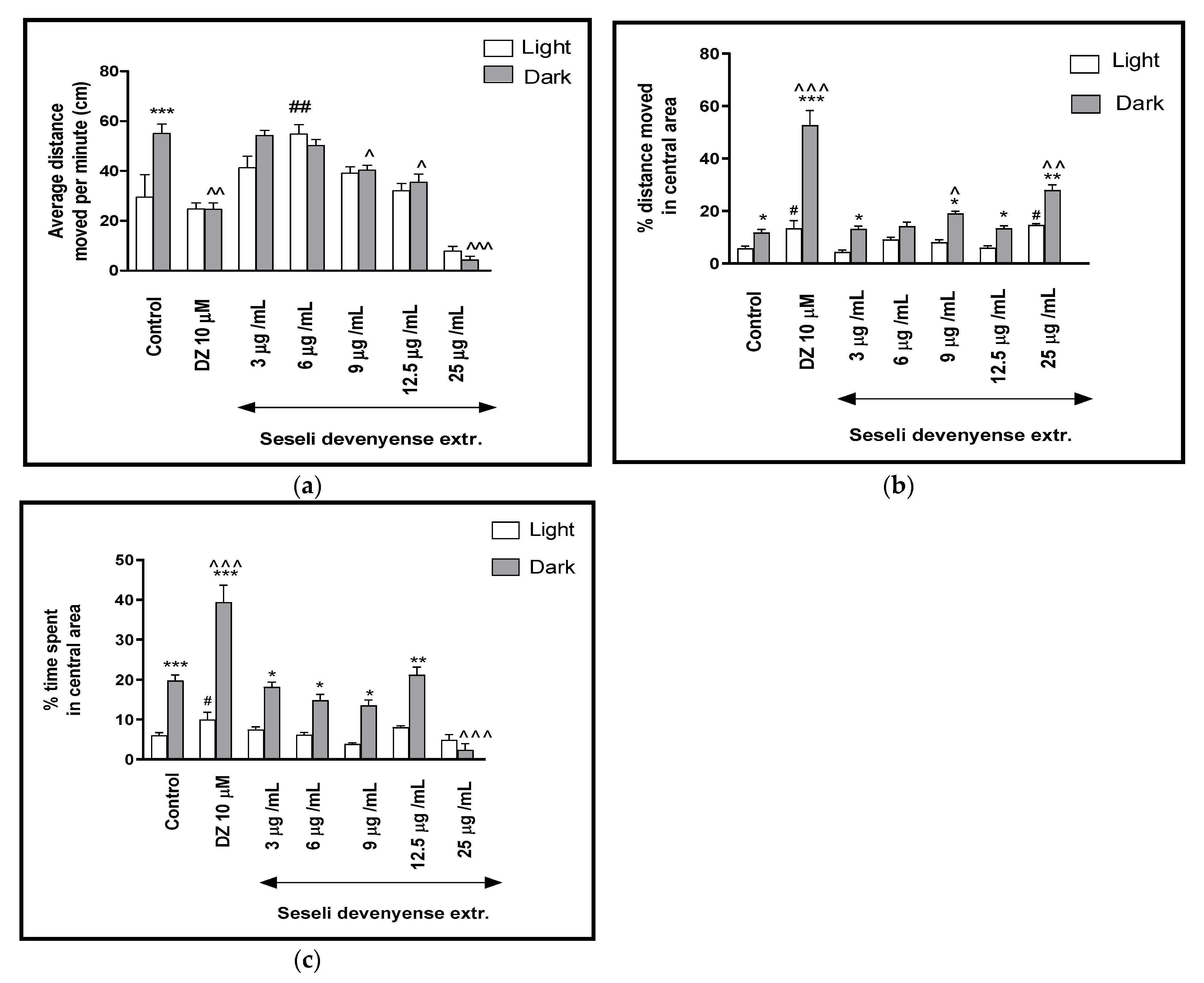
2.2.1. The Effects of SDMFE Extract on Thigmotaxis Behaviors of the Zebrafish Larvae during Light-Dark Changes
2.2.2. The Effects of Devenyol on Locomotor Activity and Thigmotaxis Behaviors of the Zebrafish Larvae during Light-Dark Changes
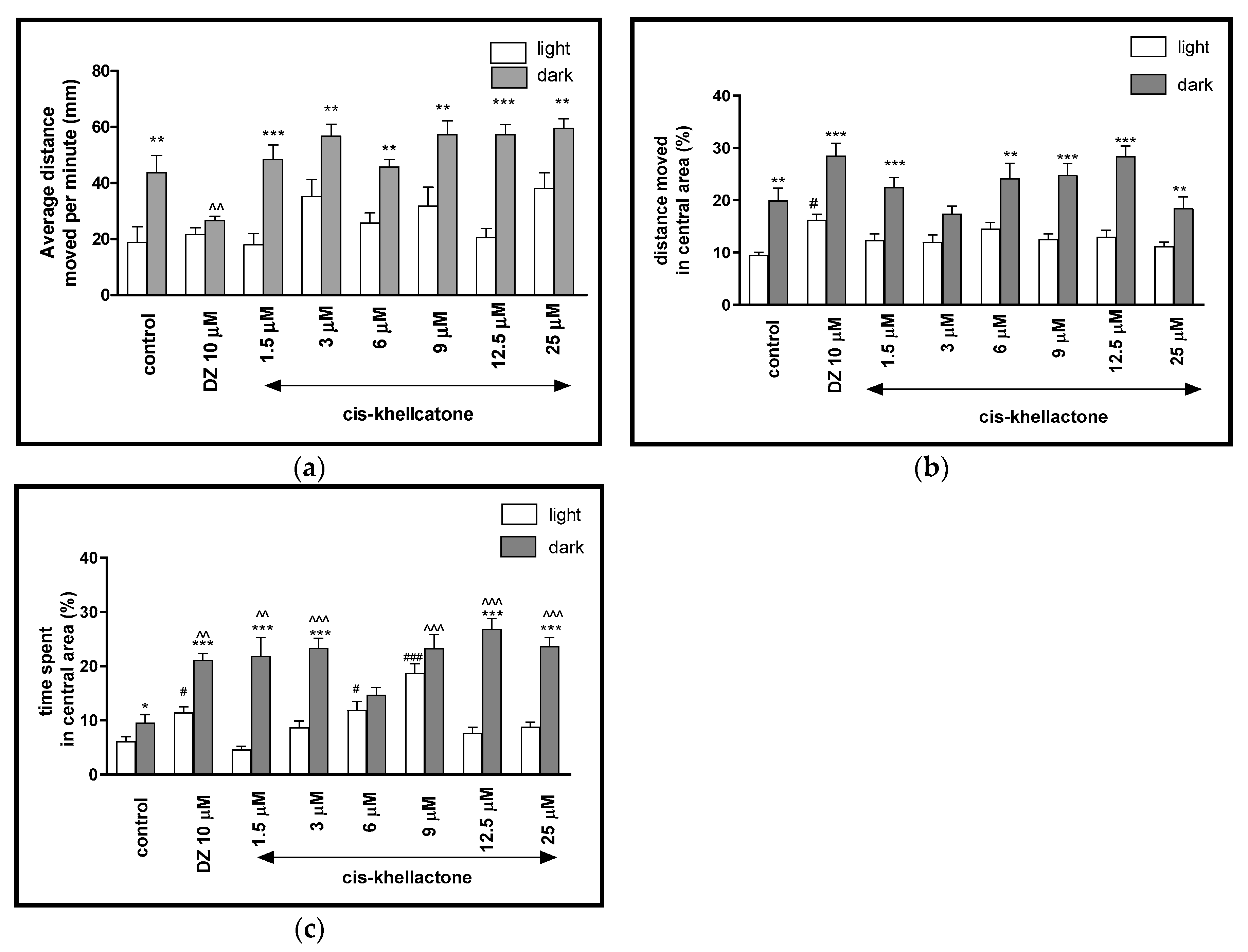
2.2.3. The Effects of cis-Khellactone on Locomotor Activity and Thigmotaxis Behaviors of the Zebrafish Larvae during Light-Dark Changes
2.2.4. The Effects of D-Laserpitin on Locomotor Activity and Thigmotaxis Behaviors of the Zebrafish Larvae during Light-Dark Changes
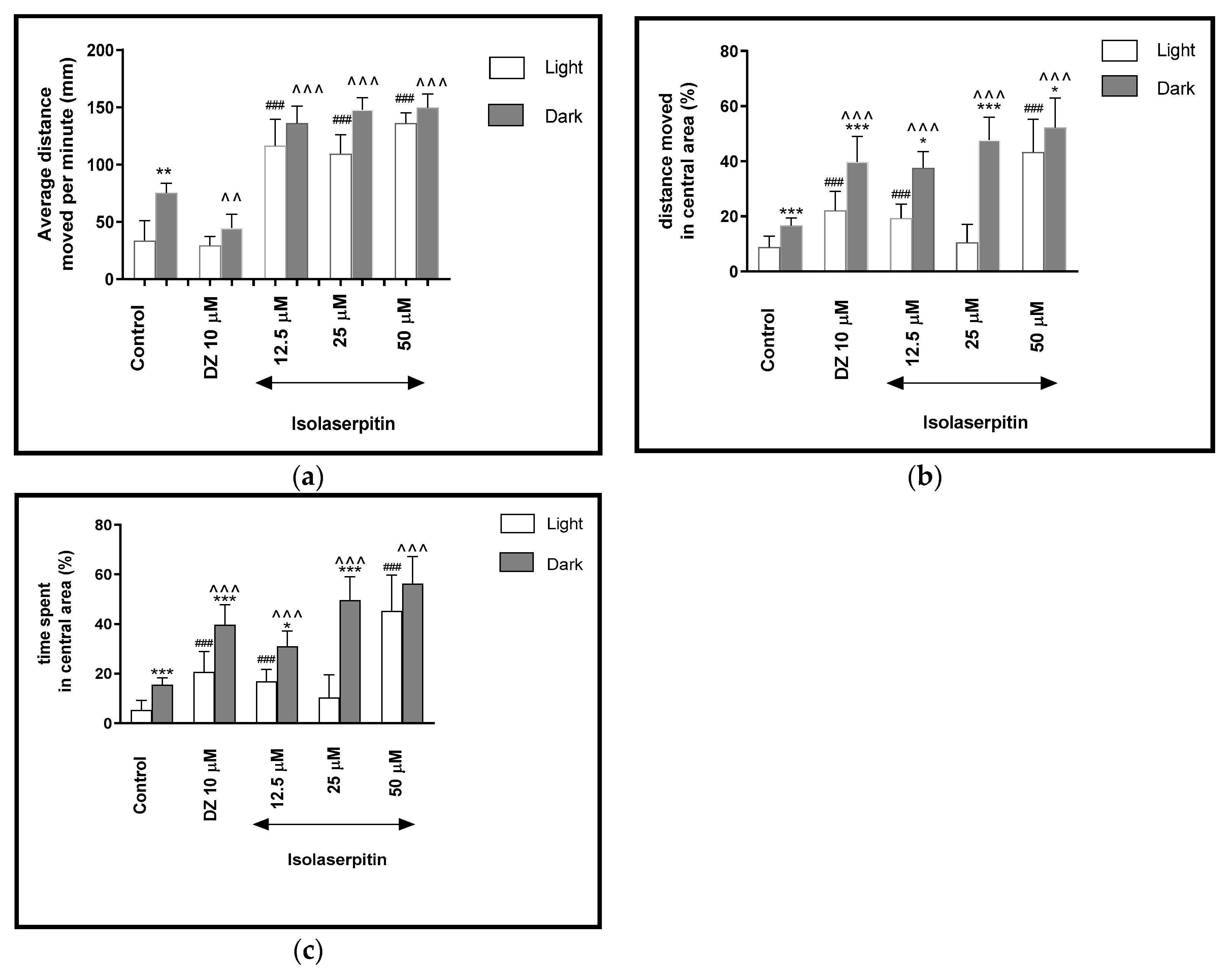
2.2.5. The Effects of Isolasepitin on Locomotor Activity and Thigmotaxis Behaviors of the Zebrafish Larvae during Light-Dark Changes
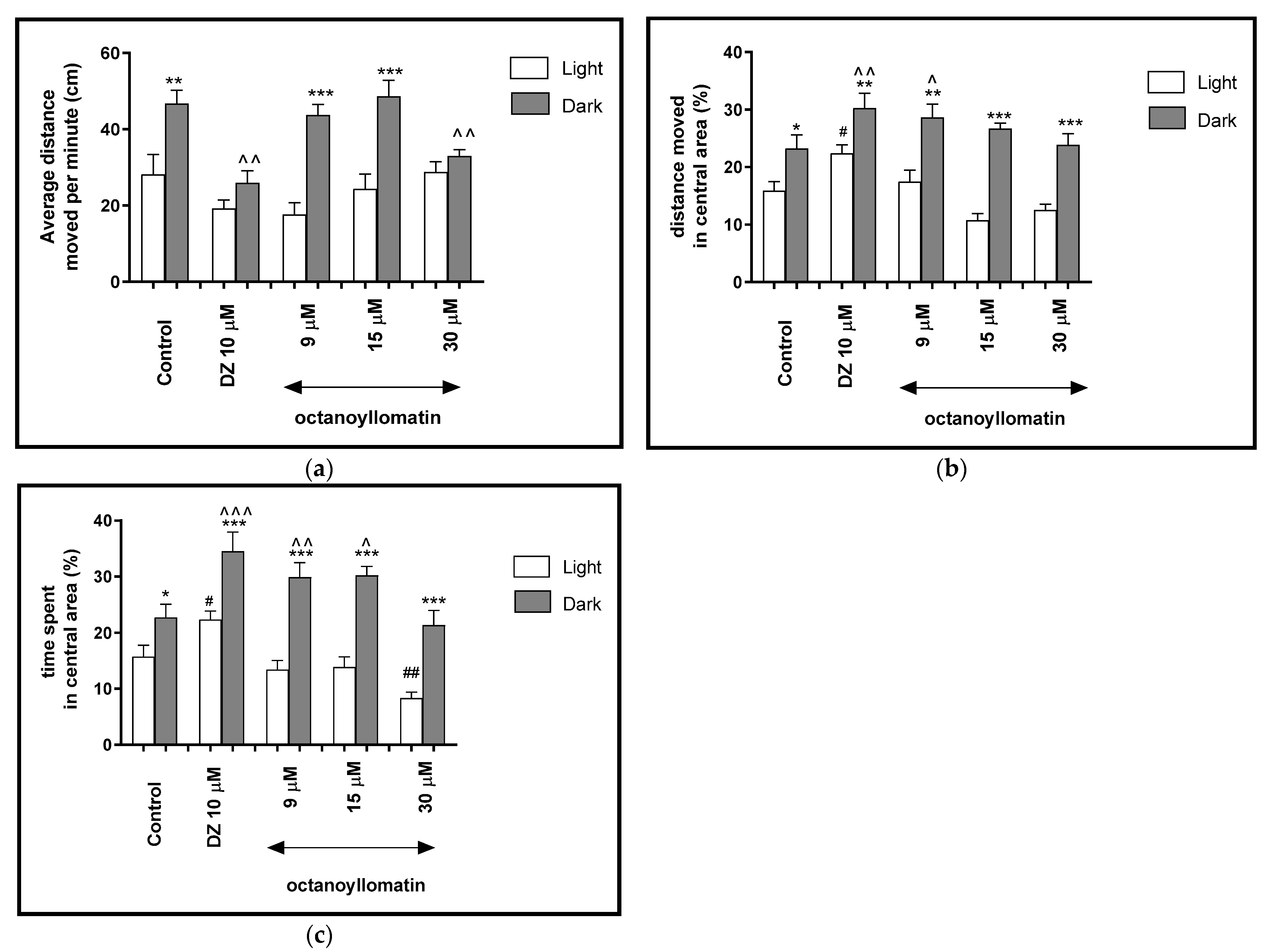
2.2.6. The Effects of Ocatonoyllomatin on Locomotor Activity and Thigmotaxis Behaviors of the Zebrafish Larvae during Light-Dark Changes
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Extraction
4.3. Liquid–Liquid Chromatography
4.3.1. Shake-Flask Experiments
4.3.2. Preparation of Biphasic Solvent Systems
4.3.3. Liquid–Liquid Chromatography Separations
4.4. HPLC-DAD Analysis
4.5. Structural Elucidation
4.6. Zebrafish Model for Evaluation Anxiety-Like Behaviors
4.6.1. Zebrafish Husbandry and Egg Collection
4.6.2. Determination of the Maximum Tolerated Concentration
4.6.3. Anxiolytic Activity Assay
- (a)
- Preincubation: larvae were pre-incubated in a 1500 μL solution of tested compounds in individual wells of a 24-well plate at 28.5 °C, in dark, for 30 min.
- (b)
- Acclimatization (min 0–10): plates were placed in an automated video recording bench station (ViewPoint, Lyon, France). Larvae were allowed to acclimatize to the test apparatus for 10 min. During this period, lights were kept on.
- (c)
- Visual motor challenge (min 11–95): this stage experiment was divided into two phases:Phase 1—continuous illumination: larvae were subjected to continuous light for 40 min, with the purpose to determine the spontaneous locomotor activity and thigmotaxis behavior (before applying the factor causing the anxiety).Phase 2—light/dark transition: larvae were subjected to three cycles, each involving the 10-min lighting phase (continuous light) and the 5-min dark phase (anxiogenic factor).
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baxter, A.J.; Scott, K.M.; Vos, T.; Whiteford, H.A. Global prevalence of anxiety disorders: A systematic review and meta-regression. Psychol. Med. 2012, 43, 897–910. [Google Scholar] [CrossRef] [PubMed]
- WHO. Depression and Other Common Mental Disorders; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Lydiard, R.B. The role of GABA in anxiety disorders. J. Clin. Psychiatry 2003, 64, 21–27. [Google Scholar]
- Muniandy, Y. The use of larval zebrafish (Danio rerio) model for identifying new anxiolytic drugs from herbal medicine. Zebrafish 2018, 15, 321–339. [Google Scholar] [CrossRef]
- Brandao, M.L.; Vianna, D.M.; Masson, S.; Santos, J. Neural organization of different types of fear: Implication for the understanding of anxiety. Rev. Bras. Psiquiatr. 2003, 25, 36–41. [Google Scholar] [CrossRef][Green Version]
- Shader, R.I.; Greenblatt, D.J. Use of benzodiazepines in anxiety disorders. N. Engl. J. Med. 1993, 328, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Cicek, S.S. Structure-dependent activity of natural GABA (A) receptor modulators. Molecules 2018, 23, 1512. [Google Scholar] [CrossRef]
- Gelfuso, E.A.; Rosa, D.S.; Fachin, A.L.; Mortari, M.R.; Cuhna, A.O.S.; Beleboni, R.O. Anxiety: A systematic review of neurobiology, traditional pharmaceuticals and novel alternatives from medicinal plants. CNS Neurol. Disord. Drug Targets 2014, 13, 150–165. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Orhan, I.E.; Cordell, G.A.; Nabavi, S.M.; Budzyńska, B. Implication of coumarins towards central nervous system disorders. Pharmacol. Res. 2016, 103, 188–203. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Nahar, L. Progress in the chemistry of naturally occurring coumarins. Prog. Chem. Org. Nat. Prod. 2017, 106, 241–304. [Google Scholar] [CrossRef] [PubMed]
- Sahranavard, S.; Ghafari, S.; Mosaddegh, M. Medicinal plants used in Iranian traditional medicine to treat epilepsy. Seizure 2014, 23, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, K.S.; Sethi, O.P.; Chopra, I.C. Pharmacodynamic effects of a volatile fraction from Seseli sibiricum. Arch. Int. Pharmacodyn. Ther. 1963, 143, 41–51. [Google Scholar]
- Stankov-Jovanovic, V.P.; Ilic, M.D.; Mitic, V.D.; Mihajilov-Krstev, T.M.; Simonovic, S.R.; Nikolic Mandic, S.D.; Tabet, J.C.; Colee, R.B. Secondary metabolites of Seseli rigidum: Chemical composition plus antioxidant, antimicrobial and cholinesterase inhibition activity. J. Pharm. Biomed. Anal. 2015, 111, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Widelski, J.; Melliou, E.; Fokialakis, N.; Magiatis, P.; Głowniak, K.; Chinou, I. Coumarins from the fruits of Seseli devenyense. J. Nat. Prod. 2005, 68, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Barbazuk, W.B. The syntenic relationship of the zebrafish and human genomes. Genome Res. 2000, 10, 1351–1358. [Google Scholar] [CrossRef]
- Barros, T.P.; Alderton, W.K.; Reynolds, H.M.; Roach, A.G.; Berghmans, S. Zebrafish: An emerging technology for in vivo pharmacological assessment to identify potential safety liabilities in early drug discovery. Br. J. Pharmacol. 2008, 154, 1400–1413. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Guo, S. Use of zebrafish as a model to understand mechanisms of addiction and complex neurobehavioral phenotypes. Neurobiol. Dis. 2010, 40, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Friesen, J.B.; Pauli, G.F. G.U.E.S.S—A generally useful estimate of solvent systems for CCC. J. Liq. Chromatogr. Rel. Technol. 2005, 28, 2777–2806. [Google Scholar] [CrossRef]
- Swager, T.M.; Cardellina, J.H., II. Coumarins from musineon divaricatum. Phytochemistry 1985, 24, 805–813. [Google Scholar] [CrossRef]
- Lemmich, J.; Pedersen, A.P.; Nielsen, E.B. Revised absolute configurations of natural khellactone esters. Tetrahedron Lett. 1969, 10, 3365–3366. [Google Scholar] [CrossRef]
- Berthod, A. Series Editor’ S Preface, Countercurrent Chromatography; Elsevier B.V.: London, UK, 2002. [Google Scholar]
- Foucault, A.P. Centrifugal Partition Chromatography; Marcel Dekker: New York, NY, USA, 1995. [Google Scholar]
- Friesen, J.B.; McAlpine, J.B.; Chen, S.N.; Pauli, G.F. Countercurrent separation of natural products: An update. J. Nat. Prod. 2015, 78, 1765–1796. [Google Scholar] [CrossRef]
- Luca, S.V.; Miron, A.; Ignatova, S.; Skalicka-Wozniak, K. An overview of the two-phase solvent systems used in the countercurrent separation of phenylethanoid glycosides and iridoids and their biological relevance. Phytochem. Rev. 2019, 18, 377–403. [Google Scholar] [CrossRef]
- Morley, R.; Minceva, M. Operating mode and parameter selection in liquid-liquid chromatography. J. Chromatogr. A 2020, 1617, 460479. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Mroczek, T.; Garrard, I.; Głowniak, K. Isolation of the minor and rare constituents from the fruits of Peucedanum alsaticum L. using high-performance counter-current chromatography. J. Sep. Sci. 2012, 35, 790–797. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Garrard, I.A. A comprehensive classification of solvent system used for natural products purification in countercurrent and centrifugal partition chromatography. Nat. Prod. Rep. 2015, 32, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Skalicka-Woźniak, K.; Mroczek, T.; Walasek, M.; Głowniak, K. Efficient isolation of dihydropyranocoumarins and simple coumarins from Mutellina purpurea fruits. Planta. Med. 2016, 82, 1105–1109. [Google Scholar] [CrossRef]
- Kozioł, E.; Deniz, F.S.S.; Orhan, I.E.; Marcourt, L.; Budzyńska, B.; Wolfender, J.L.; Crawford, A.D.; Skalicka-Woźniak, K. High-performance counter-current chromatography isolation and initial neuroactivity characterization of furanocoumarin derivatives from Peucedanum alsaticum L. (Apiaceae). Phytomedicine 2019, 54, 259–264. [Google Scholar] [CrossRef]
- Kozioł, E.; Luca, S.V.; Agalar, H.G.; Saglik, B.N.; Demirci, F.; Marcourt, L.; Wolfender, J.L.; Jóźwiak, K.; Skalicka-Woźniak, K. Rutamarin: Efficient liquid–liquid chromatographic isolation from ruta graveolens L. and evaluation of its in vitro and in silico MAO-B inhibitory activity. Molecules 2020, 25, 2678. [Google Scholar] [CrossRef] [PubMed]
- Kozioł, E.; Skalicka-Woźniak, K.; Michalak, A.; Kaszubska, K.; Budzyńska, B. Xanthotoxin reverses Parkinson’s disease-like symptoms in zebrafish larvae and mice models: A comparative study. Pharmacol. Rep. 2020, in press. [Google Scholar]
- Widelski, J.; Luca, S.V.; Skiba, A.; Chinou, I.; Marcourt, L.; Wolfender, J.L.; Skalicka-Woźniak, K. Isolation and antimicrobial activity o coumarin derivatives from fruits of Peucedanum luxurians Tamamsch. Molecules 2018, 23, 1222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J. Therapeutic effects of herbal extracts and constituents in animal models of psychiatric disorders. Life Sci. 2004, 75, 1659–1699. [Google Scholar] [CrossRef]
- Kumar, D.; Bhat, Z.A.; Kumar, V.; Shah, M.Y. Nature: Anxiolytic in the lap of nature. Webmed. Cent. Pharm. Sci. 2011, 2, WMC002140. [Google Scholar]
- Budzynska, B.; Kruk-Slomka, M.; Skalicka-Wozniak, K.; Biala, G.; Glowniak, K. The effects of imperatorin on anxiety and memory-related behavior in male Swiss mice. Exp. Clin. Psychopharmacol. 2012, 20, 325–332. [Google Scholar] [CrossRef]
- Kumar, D.; Bhat, Z.A.; Kumar, V.; Shah, M.Y. Coumarins from Angelica archangelica Linn and their effects on anxiety-like behavior. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 40, 180–186. [Google Scholar] [CrossRef]
- Singhhuber, J.; Baburin, I.; Ecker, G.F.; Kopp, B.; Hering, S. Insight into structure-activity relationship of GABAA receptor modulating coumarins and furanocoumarins. Eur. J. Pharmacol. 2011, 668, 57–64. [Google Scholar] [CrossRef]
- Bergendorf, O.; Dekermendjian, K.; Nielsen, M.; Shan, R.; Witt, R.; AI, J.; Sterner, O. Furanocouamrins with affinity to brain benzodiazepine receptors in vitro. Phytochemistry 1997, 44, 1121–1124. [Google Scholar] [CrossRef]
- Dekermendjian, K.; Ai, J.; Nielsen, M.; Sterner, O.; Shan, R.; Witt, M.R. Characterization of furanocoumarin phellopterin as a rat brain benzodiazepine receptor partial agonist in vitro. Neurosci. Lett. 1996, 219, 151–154. [Google Scholar] [CrossRef]
- Bourin, M. Animal models for screening anxiolytic-like drugs: A perspective. Dialogues Clin. Neurosci. 2015, 17, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.R.; Noyes, P.D.; Tanguay, R.L. Advancements in zebrafish applications for 21st century toxicology. Pharmacol. Ther. 2016, 161, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Maciąg, M.; Michalak, A.; Skalicka-Woźniak, K.; Zykubek, M.; Ciszewski, A.; Budzyńska, B. Zebrafish and mouse model for anxiety evaluation—A comparative study with xanthotoxin as a model compund. Brain. Res. Bull. 2020, 165, 139–145. [Google Scholar] [CrossRef]
- Schnorr, S.J.; Steenberger, P.J.; Richardson, M.K.; Champagne, D.L. Measuring thigmotaxis in larval zebrafish. Behav. Brain Res. 2012, 228, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Thuy, T.T.T.; Thanh, L.T.; Okada, M. Antidepressive and anxiolytic effects of ostruthis, a TREK-1 channel activator. PLoS ONE 2018, 13, e0201092. [Google Scholar] [CrossRef] [PubMed]
- Monsef-Esfahani, H.R.; Amini, M.; Goodarzi, N.; Saiedmohammadi, F.; Hajiaghaee, R.; Faramarzi, M.A.; Tofighi, Z.; Ghahremani, M.H. Coumarin compounds of Biebesteinia multifida roots show potential anxiolytic effects in mice. DARU 2013, 21, 51. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Ruiz, M.; Gonzales-Carranza, A.; Zamilpa, A.; Jimenez-Ferrer, E.; Huerta-Reyes, M.; Navarro-Garcia, V.M. The standardized extract of Leoselia mexicana possesses anxiolytic activity through the γ-amino butyric acid mechanism. J. Ethnopharmacol. 2011, 138, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Urbagarova, B.M.; Radnaeva, L.D.; Shults, E.E.; Taraskin, V.V.; Petrova, T.N.; Rybalova, T.V.; Frolova, T.S.; Pokrovskii, A.G.; Ganbaatar, J. Chromones and coumarins from Saposhnikovia divaricata (Turcz.) Schischk growing in Buryatia and Mongolia and their cytotoxicity. J. Ethnopharmacol. 2020, 261, 112517. [Google Scholar] [CrossRef]


| Hexane/Ethyl Acetate/Methanol/Water (v/v/v/v) | Partition Coefficients (Pi) | |||||
|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | ||
| I | 2/5/2/5 | 0.80 | 2.50 | – | – | – |
| II | 6/5/6/5 | – | – | 0.84 | 1.01 | |
| III | 5/2/5/2 | – | – | – | – | 2.01 |
| Technique | Mass of Processed Sample | Mass of Isolated Coumarin (Yield §) | Total Separation Time $ | Total Solvent Consumption # | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||||
| Liquid–liquid chromatography | 240 mg | 5.5 mg (2.292%) | 9.2 mg (3.833%) | 1.4 mg (0.583%) | 1.4 mg (0.583%) | 3.1 mg (1.292%) | ~3.5 h | ~1.3 L |
| Solid–liquid chromatography * | 1.6 g | 7 mg (0.437%) | 3.6 mg (0.225%) | 2 mg (0.125%) | 3 mg (0.187%) | 13 mg (0.812%) | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widelski, J.; Luca, S.V.; Skiba, A.; Maciąg, M.; Budzyńska, B.; Marcourt, L.; Wolfender, J.-L.; Skalicka-Woźniak, K. Coumarins from Seseli devenyense Simonk.: Isolation by Liquid–Liquid Chromatography and Potential Anxiolytic Activity Using an In Vivo Zebrafish Larvae Model. Int. J. Mol. Sci. 2021, 22, 1829. https://doi.org/10.3390/ijms22041829
Widelski J, Luca SV, Skiba A, Maciąg M, Budzyńska B, Marcourt L, Wolfender J-L, Skalicka-Woźniak K. Coumarins from Seseli devenyense Simonk.: Isolation by Liquid–Liquid Chromatography and Potential Anxiolytic Activity Using an In Vivo Zebrafish Larvae Model. International Journal of Molecular Sciences. 2021; 22(4):1829. https://doi.org/10.3390/ijms22041829
Chicago/Turabian StyleWidelski, Jarosław, Simon Vlad Luca, Adrianna Skiba, Monika Maciąg, Barbara Budzyńska, Laurence Marcourt, Jean-Luc Wolfender, and Krystyna Skalicka-Woźniak. 2021. "Coumarins from Seseli devenyense Simonk.: Isolation by Liquid–Liquid Chromatography and Potential Anxiolytic Activity Using an In Vivo Zebrafish Larvae Model" International Journal of Molecular Sciences 22, no. 4: 1829. https://doi.org/10.3390/ijms22041829
APA StyleWidelski, J., Luca, S. V., Skiba, A., Maciąg, M., Budzyńska, B., Marcourt, L., Wolfender, J.-L., & Skalicka-Woźniak, K. (2021). Coumarins from Seseli devenyense Simonk.: Isolation by Liquid–Liquid Chromatography and Potential Anxiolytic Activity Using an In Vivo Zebrafish Larvae Model. International Journal of Molecular Sciences, 22(4), 1829. https://doi.org/10.3390/ijms22041829







