In Search of Molecular Markers for Cerebellar Neurons
Abstract
1. Introduction
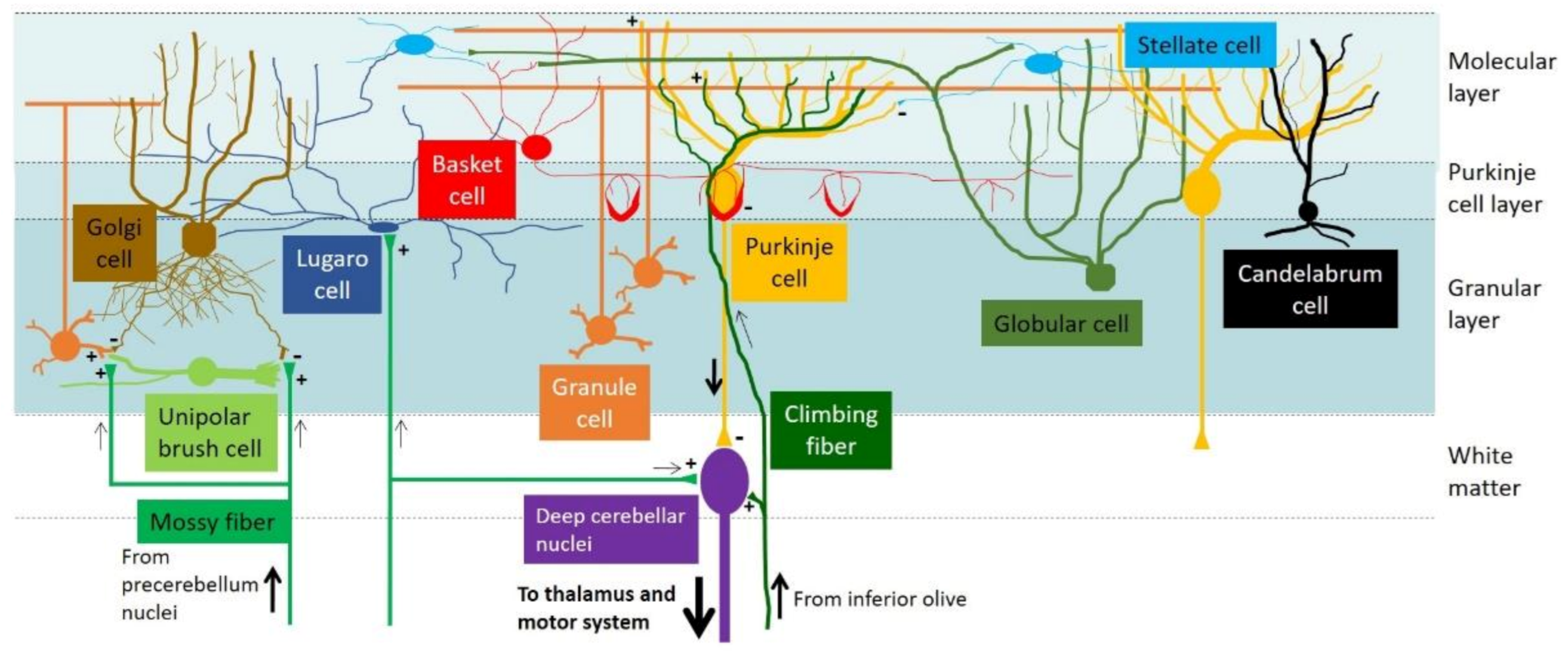
1.1. The Cerebellar Cortex
1.2. Development of the Mouse Cerebellum
1.3. Importance of Neuronal-Specific Markers in Cerebellum Research
2. Approaches to Discovering Molecular Markers of Cerebellar Neurons
2.1. Forward Genetics—From Phenotype to Genotype
2.2. Reverse Genetics—From Genotype to Phenotype
2.3. Global Screening
2.4. Laser-Capture Microdissection, qPCR, and Next-Generation Sequencing
2.5. Single-Cell Transcriptome
3. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIS | Axon initial segment |
| EGFP | Enhanced Green Fluorescent Protein |
| ENU | N-ethyl-N-nitrosourea |
| E## | Embryonic day ## |
| FFPE | Formalin-fixed-paraffin-embedded |
| GABA | γ-aminobutyric acid |
| GC(s) | Granule cell(s) |
| GIRKs | Potassium inwardly-rectifying channels |
| GL | Granular layer |
| iPSCs | Induced pluripotent stem cells |
| KI | Knockin |
| KO | Knockout |
| LCM | Laser-capture microdissection |
| ML | Molecular layer |
| P## | Postnatal day ## |
| PC(s) | Purkinje cell(s) |
| PCL | Purkinje cell layer |
References
- Klein, A.P.; Ulmer, J.L.; Quinet, S.A.; Mathews, V.; Mark, L.P. Nonmotor Functions of the Cerebellum: An Introduction. AJNR Am. J. Neuroradiol. 2016, 37, 1005–1009. [Google Scholar] [CrossRef]
- Mennink, L.M.; van Dijk, J.M.C.; van Dijk, P. The cerebellar (para)flocculus: A review on its auditory function and a possible role in tinnitus. Hear. Res. 2020, 398, 108081. [Google Scholar] [CrossRef]
- Wang, V.Y.; Zoghbi, H.Y. Genetic regulation of cerebellar development. Nat. Rev. Neurosci. 2001, 2, 484–491. [Google Scholar] [CrossRef]
- White, J.J.; Sillitoe, R.V. Development of the cerebellum: From gene expression patterns to circuit maps. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 149–164. [Google Scholar] [CrossRef]
- Beckinghausen, J.; Sillitoe, R.V. Insights into cerebellar development and connectivity. Neurosci. Lett. 2019, 688, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Leto, K.; Rolando, C.; Rossi, F. The genesis of cerebellar GABAergic neurons: Fate potential and specification mechanisms. Front. Neuroanat. 2012, 6, 6. [Google Scholar] [CrossRef]
- Zhang, L.; Goldman, J.E. Generation of cerebellar interneurons from dividing progenitors in white matter. Neuron 1996, 16, 47–54. [Google Scholar] [CrossRef]
- Brown, A.M.; Arancillo, M.; Lin, T.; Catt, D.R.; Zhou, J.; Lackey, E.P.; Stay, T.L.; Zuo, Z.; White, J.J.; Sillitoe, R.V. Molecular layer interneurons shape the spike activity of cerebellar Purkinje cells. Sci. Rep. 2019, 9, 1742. [Google Scholar] [CrossRef]
- Laine, J.; Axelrad, H. The candelabrum cell: A new interneuron in the cerebellar cortex. J. Comp. Neurol. 1994, 339, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Dino, M.R.; Schuerger, R.J.; Liu, Y.; Slater, N.T.; Mugnaini, E. Unipolar brush cell: A potential feedforward excitatory interneuron of the cerebellum. Neuroscience 2000, 98, 625–636. [Google Scholar] [CrossRef]
- Laine, J.; Axelrad, H. Extending the cerebellar Lugaro cell class. Neuroscience 2002, 115, 363–374. [Google Scholar] [CrossRef]
- D’Angelo, E.; Solinas, S.; Mapelli, J.; Gandolfi, D.; Mapelli, L.; Prestori, F. The cerebellar Golgi cell and spatiotemporal organization of granular layer activity. Front. Neural Circuits 2013, 7, 93. [Google Scholar] [CrossRef]
- Schilling, K.; Oberdick, J.; Rossi, F.; Baader, S.L. Besides Purkinje cells and granule neurons: An appraisal of the cell biology of the interneurons of the cerebellar cortex. Histochem. Cell Biol. 2008, 130, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Cerminara, N.L.; Lang, E.J.; Sillitoe, R.V.; Apps, R. Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nature Rev. Neurosci. 2015, 16, 79–93. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E.; Antonietti, A.; Casali, S.; Casellato, C.; Garrido, J.A.; Luque, N.R.; Mapelli, L.; Masoli, S.; Pedrocchi, A.; Prestori, F.; et al. Modeling the Cerebellar Microcircuit: New Strategies for a Long-Standing Issue. Front. Cell Neurosci. 2016, 10, 176. [Google Scholar] [CrossRef]
- Prestori, F.; Mapelli, L.; D’Angelo, E. Diverse Neuron Properties and Complex Network Dynamics in the Cerebellar Cortical Inhibitory Circuit. Front. Mol. Neurosci. 2019, 12, 267. [Google Scholar] [CrossRef] [PubMed]
- Leto, K.; Arancillo, M.; Becker, E.B.; Buffo, A.; Chiang, C.; Ding, B.; Dobyns, W.B.; Dusart, I.; Haldipur, P.; Hatten, M.E.; et al. Consensus Paper: Cerebellar Development. Cerebellum 2016, 15, 789–828. [Google Scholar] [CrossRef] [PubMed]
- Rhinn, M.; Brand, M. The midbrain—Hindbrain boundary organizer. Curr. Opin. Neurobiol. 2001, 11, 34–42. [Google Scholar] [CrossRef]
- Haldipur, P.; Millen, K.J. What cerebellar malformations tell us about cerebellar development. Neurosci. Lett. 2019, 688, 14–25. [Google Scholar] [CrossRef]
- Van Essen, M.J.; Nayler, S.; Becker, E.B.E.; Jacob, J. Deconstructing cerebellar development cell by cell. PLoS Genet. 2020, 16. [Google Scholar] [CrossRef]
- Stephen, C.D.; Brizzi, K.T.; Bouffard, M.A.; Gomery, P.; Sullivan, S.L.; Mello, J.; MacLean, J.; Schmahmann, J.D. The Comprehensive Management of Cerebellar Ataxia in Adults. Curr. Treat. Options Neurol. 2019, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Depping, M.S.; Schmitgen, M.M.; Bach, C.; Listunova, L.; Kienzle, J.; Kubera, K.M.; Roesch-Ely, D.; Wolf, R.C. Abnormal Cerebellar Volume in Patients with Remitted Major Depression with Persistent Cognitive Deficits. Cerebellum 2020, 19, 762–770. [Google Scholar] [CrossRef]
- D’Angelo, E. The cerebellum gets social. Science 2019, 363, 229. [Google Scholar] [CrossRef]
- D’Angelo, E.; Casali, S. Seeking a unified framework for cerebellar function and dysfunction: From circuit operations to cognition. Front. Neural Circuits 2012, 6, 116. [Google Scholar] [CrossRef]
- Giza, J.; Urbanski, M.J.; Prestori, F.; Bandyopadhyay, B.; Yam, A.; Friedrich, V.; Kelley, K.; D’Angelo, E.; Goldfarb, M. Behavioral and cerebellar transmission deficits in mice lacking the autism-linked gene islet brain-2. J. Neurosci. 2010, 30, 14805–14816. [Google Scholar] [CrossRef] [PubMed]
- Hoche, F.; Guell, X.; Vangel, M.G.; Sherman, J.C.; Schmahmann, J.D. The cerebellar cognitive affective/Schmahmann syndrome scale. Brain 2018, 141, 248–270. [Google Scholar] [CrossRef]
- Schmahmann, J.D. The cerebellum and cognition. Neurosci. Lett. 2019, 688, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Soda, T.; Mapelli, L.; Locatelli, F.; Botta, L.; Goldfarb, M.; Prestori, F.; D’Angelo, E. Hyperexcitability and Hyperplasticity Disrupt Cerebellar Signal Transfer in the IB2 KO Mouse Model of Autism. J. Neurosci. 2019, 39, 2383–2397. [Google Scholar] [CrossRef]
- Slugocka, A.; Wiaderkiewicz, J.; Barski, J.J. Genetic Targeting in Cerebellar Purkinje Cells: An Update. Cerebellum 2017, 16, 191–202. [Google Scholar] [CrossRef]
- Zhang, X.M.; Ng, A.H.; Tanner, J.A.; Wu, W.T.; Copeland, N.G.; Jenkins, N.A.; Huang, J.D. Highly restricted expression of Cre recombinase in cerebellar Purkinje cells. Genesis 2004, 40, 45–51. [Google Scholar] [CrossRef]
- Ingram, M.; Wozniak, E.A.L.; Duvick, L.; Yang, R.; Bergmann, P.; Carson, R.; O’Callaghan, B.; Zoghbi, H.Y.; Henzler, C.; Orr, H.T. Cerebellar Transcriptome Profiles of ATXN1 Transgenic Mice Reveal SCA1 Disease Progression and Protection Pathways. Neuron 2016, 89, 1194–1207. [Google Scholar] [CrossRef] [PubMed]
- Celio, M.R.; Baier, W.; Scharer, L.; Gregersen, H.J.; de Viragh, P.A.; Norman, A.W. Monoclonal antibodies directed against the calcium binding protein Calbindin D-28k. Cell Calcium 1990, 11, 599–602. [Google Scholar] [CrossRef]
- Sequier, J.M.; Hunziker, W.; Andressen, C.; Celio, M.R. Calbindin D-28k Protein and mRNA Localization in the Rat Brain. Eur. J. Neurosci. 1990, 2, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Salero, E.; Hatten, M.E. Differentiation of ES cells into cerebellar neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 2997–3002. [Google Scholar] [CrossRef]
- Su, H.L.; Muguruma, K.; Matsuo-Takasaki, M.; Kengaku, M.; Watanabe, K.; Sasai, Y. Generation of cerebellar neuron precursors from embryonic stem cells. Dev. Biol. 2006, 290, 287–296. [Google Scholar] [CrossRef]
- Alexander, C.J.; Hammer, J.A. An Improved Method for Differentiating Mouse Embryonic Stem Cells into Cerebellar Purkinje Neurons. Cerebellum 2019, 18, 406–421. [Google Scholar] [CrossRef]
- Tam, W.Y.; Cheung, K.K. Phenotypic characteristics of commonly used inbred mouse strains. J. Mol. Med. 2020, 98, 1215–1234. [Google Scholar] [CrossRef] [PubMed]
- Sidman, R.L.; Lane, P.W.; Dickie, M.M. Staggerer, a new mutation in the mouse affecting the cerebellum. Science 1962, 137, 610–612. [Google Scholar] [CrossRef]
- Vogel, M.W.; Caston, J.; Yuzaki, M.; Mariani, J. The Lurcher mouse: Fresh insights from an old mutant. Brain Res. 2007, 1140, 4–18. [Google Scholar] [CrossRef]
- Phillips, R.J.S. “Lurcher”, a new gene in linkage group XI of the house mouse. J. Genet. 1960, 57, 35–42. [Google Scholar] [CrossRef]
- Hamilton, B.A.; Frankel, W.N.; Kerrebrock, A.W.; Hawkins, T.L.; FitzHugh, W.; Kusumi, K.; Russell, L.B.; Mueller, K.L.; van Berkel, V.; Birren, B.W.; et al. Disruption of the nuclear hormone receptor RORalpha in staggerer mice. Nature 1996, 379, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; De Jager, P.L.; Takahashi, K.A.; Jiang, W.; Linden, D.J.; Heintz, N. Neurodegeneration in Lurcher mice caused by mutation in delta2 glutamate receptor gene. Nature 1997, 388, 769–773. [Google Scholar] [CrossRef]
- Selimi, F.; Lohof, A.M.; Heitz, S.; Lalouette, A.; Jarvis, C.I.; Bailly, Y.; Mariani, J. Lurcher GRID2-induced death and depolarization can be dissociated in cerebellar Purkinje cells. Neuron 2003, 37, 813–819. [Google Scholar] [CrossRef]
- Araki, K.; Meguro, H.; Kushiya, E.; Takayama, C.; Inoue, Y.; Mishina, M. Selective expression of the glutamate receptor channel delta 2 subunit in cerebellar Purkinje cells. Biochem. Biophys. Res. Commun. 1993, 197, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, R.; Sakimura, K.; Watanabe, M. GluD2 Endows Parallel Fiber-Purkinje Cell Synapses with a High Regenerative Capacity. J. Neurosci. 2016, 36, 4846–4858. [Google Scholar] [CrossRef]
- Stottmann, R.W.; Moran, J.L.; Turbe-Doan, A.; Driver, E.; Kelley, M.; Beier, D.R. Focusing forward genetics: A tripartite ENU screen for neurodevelopmental mutations in the mouse. Genetics 2011, 188, 615–624. [Google Scholar] [CrossRef]
- Ding, S.; Wu, X.; Li, G.; Han, M.; Zhuang, Y.; Xu, T. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell 2005, 122, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Skarnes, W.C.; Auerbach, B.A.; Joyner, A.L. A Gene Trap Approach in Mouse Embryonic Stem-Cells—The Lacz Reporter Is Activated by Splicing, Reflects Endogenous Gene-Expression, and Is Mutagenic in Mice. Genes Dev. 1992, 6, 903–918. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, A.J.; Akagi, K.; Largaespada, D.A.; Copeland, N.G.; Jenkins, N.A. Mammalian mutagenesis using a highly mobile somatic Sleeping Beauty transposon system. Nature 2005, 436, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Nolan, P.M.; Peters, J.; Strivens, M.; Rogers, D.; Hagan, J.; Spurr, N.; Gray, I.C.; Vizor, L.; Brooker, D.; Whitehill, E.; et al. A systematic, genome-wide, phenotype-driven mutagenesis programme for gene function studies in the mouse. Nat. Genet. 2000, 25, 440–443. [Google Scholar] [CrossRef]
- Oliver, P.L.; Davies, K.E. New insights into behaviour using mouse ENU mutagenesis. Hum. Mol. Genet. 2012, 21, R72–R81. [Google Scholar] [CrossRef]
- Xie, G.; Harrison, J.; Clapcote, S.J.; Huang, Y.; Zhang, J.Y.; Wang, L.Y.; Roder, J.C. A new Kv1.2 channelopathy underlying cerebellar ataxia. J. Biol. Chem. 2010, 285, 32160–32173. [Google Scholar] [CrossRef]
- Robbins, C.A.; Tempel, B.L. Kv1.1 and Kv1.2: Similar channels, different seizure models. Epilepsia 2012, 53, 134–141. [Google Scholar] [CrossRef]
- McNamara, N.M.C.; Averill, S.; Wilkin, G.P.; Dolly, J.O.; Priestley, J.V. Ultrastructural localization of a voltage-gated K+ channel alpha subunit (K(v)1.2) in the rat cerebellum. Eur. J. Neurosci. 1996, 8, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kunkel, D.D.; Martin, T.M.; Schwartzkroin, P.A.; Tempel, B.L. Heteromultimeric K+ Channels in Terminal and Juxtaparanodal Regions of Neurons. Nature 1993, 365, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Kunkel, D.D.; Schwartzkroin, P.A.; Tempel, B.L. Localization of Kv1.1 and Kv1.2, two K channel proteins, to synaptic terminals, somata, and dendrites in the mouse brain. J. Neurosci. 1994, 14, 4588–4599. [Google Scholar] [CrossRef]
- Isaacs, A.M.; Oliver, P.L.; Jones, E.L.; Jeans, A.; Potter, A.; Hovik, B.H.; Nolan, P.M.; Vizor, L.; Glenister, P.; Simon, A.K.; et al. A mutation in Af4 is predicted to cause cerebellar ataxia and cataracts in the robotic mouse. J. Neurosci. 2003, 23, 1631–1637. [Google Scholar] [CrossRef][Green Version]
- Isnard, P.; Core, N.; Naquet, P.; Djabali, M. Altered lymphoid development in mice deficient for the mAF4 proto-oncogene. Blood 2000, 96, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Bitoun, E.; Finelli, M.J.; Oliver, P.L.; Lee, S.; Davies, K.E. AF4 Is a Critical Regulator of the IGF-1 Signaling Pathway during Purkinje Cell Development. J. Neurosci. 2009, 29, 15366–15374. [Google Scholar] [CrossRef]
- Shima, Y.; Sugino, K.; Hempel, C.M.; Shima, M.; Taneja, P.; Bullis, J.B.; Mehta, S.; Lois, C.; Nelson, S.B. A Mammalian enhancer trap resource for discovering and manipulating neuronal cell types. eLife 2016, 5, e13503. [Google Scholar] [CrossRef]
- Gurumurthy, C.B.; Grati, M.; Ohtsuka, M.; Schilit, S.L.P.; Quadros, R.M.; Liu, X.Z. CRISPR: A versatile tool for both forward and reverse genetics research. Hum. Genet. 2016, 135, 971–976. [Google Scholar] [CrossRef]
- Shalem, O.; Sanjana, N.E.; Zhang, F. High-throughput functional genomics using CRISPR-Cas9. Nat. Rev. Genet. 2015, 16, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Majeed, S.; Hoque, M.Z.; Ahmad, I. Latest Developed Strategies to Minimize the Off-Target Effects in CRISPR-Cas-Mediated Genome Editing. Cells 2020, 9, 1608. [Google Scholar] [CrossRef] [PubMed]
- Bae, T.; Hur, J.W.; Kim, D.; Hur, J.K. Recent trends in CRISPR-Cas system: Genome, epigenome, and transcriptome editing and CRISPR delivery systems. Genes Genom. 2019, 41, 871–877. [Google Scholar] [CrossRef]
- Thomas, K.R.; Capecchi, M.R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell 1987, 51, 503–512. [Google Scholar] [CrossRef]
- Zhao, Y.; Kwan, K.M.; Mailloux, C.M.; Lee, W.K.; Grinberg, A.; Wurst, W.; Behringer, R.R.; Westphal, H. LIM-homeodomain proteins Lhx1 and Lhx5, and their cofactor Ldb1, control Purkinje cell differentiation in the developing cerebellum. Proc. Natl. Acad. Sci. USA 2007, 104, 13182–13186. [Google Scholar] [CrossRef]
- Shawlot, W.; Behringer, R.R. Requirement for Lim1 in head-organizer function. Nature 1995, 374, 425–430. [Google Scholar] [CrossRef]
- Zhao, Y.; Sheng, H.Z.; Amini, R.; Grinberg, A.; Lee, E.; Huang, S.; Taira, M.; Westphal, H. Control of hippocampal morphogenesis and neuronal differentiation by the LIM homeobox gene Lhx5. Science 1999, 284, 1155–1158. [Google Scholar] [CrossRef]
- Kwan, K.M. Conditional alleles in mice: Practical considerations for tissue-specific knockouts. Genesis 2002, 32, 49–62. [Google Scholar] [CrossRef]
- Lui, N.C.; Tam, W.Y.; Gao, C.; Huang, J.D.; Wang, C.C.; Jiang, L.; Yung, W.H.; Kwan, K.M. Lhx1/5 control dendritogenesis and spine morphogenesis of Purkinje cells via regulation of Espin. Nat. Commun. 2017, 8, 15079. [Google Scholar] [CrossRef]
- Madisen, L.; Zwingman, T.A.; Sunkin, S.M.; Oh, S.W.; Zariwala, H.A.; Gu, H.; Ng, L.L.; Palmiter, R.D.; Hawrylycz, M.J.; Jones, A.R.; et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010, 13, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Nordquist, D.T.; Kozak, C.A.; Orr, H.T. cDNA cloning and characterization of three genes uniquely expressed in cerebellum by Purkinje neurons. J. Neurosci. 1988, 8, 4780–4789. [Google Scholar] [CrossRef]
- Rong, Y.Q.; Wang, T.Y.; Morgan, J.I. Identification of candidate Purkinje cell-specific markers by gene expression profiling in wild-type and pcd(3J) mice. Mol. Brain Res. 2004, 132, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Schuller, U.; Kho, A.T.; Zhao, Q.; Ma, Q.F.; Rowitch, D.H. Cerebellar “transcriptome” reveals cell-type and stage-specific expression during postnatal development and tumorigenesis. Mol. Cell Neurosci. 2006, 33, 247–259. [Google Scholar] [CrossRef]
- Chen, X.R.; Heck, N.; Lohof, A.M.; Rochefort, C.; Morel, M.P.; Wehrle, R.; Doulazmi, M.; Marty, S.; Cannaya, V.; Avci, H.X.; et al. Mature Purkinje cells require the retinoic acid-related orphan receptor-alpha (RORalpha) to maintain climbing fiber mono-innervation and other adult characteristics. J. Neurosci. 2013, 33, 9546–9562. [Google Scholar] [CrossRef]
- Ino, H. Immunohistochemical characterization of the orphan nuclear receptor ROR alpha in the mouse nervous system. J. Histochem. Cytochem. 2004, 52, 311–323. [Google Scholar] [CrossRef]
- Jones, A.R.; Overly, C.C.; Sunkin, S.M. The Allen Brain Atlas: 5 years and beyond. Nat. Rev. Neurosci. 2009, 10, 821–828. [Google Scholar] [CrossRef]
- Sunkin, S.M.; Ng, L.; Lau, C.; Dolbeare, T.; Gilbert, T.L.; Thompson, C.L.; Hawrylycz, M.; Dang, C. Allen Brain Atlas: An integrated spatio-temporal portal for exploring the central nervous system. Nucleic Acids Res. 2013, 41, D996–D1008. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.; Hatten, M.E. Molecular markers of neuronal progenitors in the embryonic cerebellar anlage. J. Neurosci. 2006, 26, 12226–12236. [Google Scholar] [CrossRef]
- Gong, S.; Zheng, C.; Doughty, M.L.; Losos, K.; Didkovsky, N.; Schambra, U.B.; Nowak, N.J.; Joyner, A.; Leblanc, G.; Hatten, M.E.; et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 2003, 425, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Condie, B.G. The untapped potential of the GENSAT mice-A valuable resource for developmental biology. Genesis 2016, 54, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Gerfen, C.R.; Paletzki, R.; Heintz, N. GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron 2013, 80, 1368–1383. [Google Scholar] [CrossRef]
- Demchuk, A.M.; Dube, S.T.; Mesina, L.; McNaughton, B.L. Limitations of the GENSAT Egr1-EGFP transgenic mouse strain for neural circuit activity mapping. Neurosci. Lett. 2020, 732, 135072. [Google Scholar] [CrossRef]
- Sjostedt, E.; Zhong, W.; Fagerberg, L.; Karlsson, M.; Mitsios, N.; Adori, C.; Oksvold, P.; Edfors, F.; Limiszewska, A.; Hikmet, F.; et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science 2020, 367. [Google Scholar] [CrossRef]
- Thul, P.J.; Lindskog, C. The human protein atlas: A spatial map of the human proteome. Protein Sci. 2018, 27, 233–244. [Google Scholar] [CrossRef]
- Bevilacqua, C.; Ducos, B. Laser microdissection: A powerful tool for genomics at cell level. Mol. Aspects Med. 2018, 59, 5–27. [Google Scholar] [CrossRef] [PubMed]
- Kerman, I.A.; Buck, B.J.; Evans, S.J.; Akil, H.; Watson, S.J. Combining laser capture microdissection with quantitative real-time PCR: Effects of tissue manipulation on RNA quality and gene expression. J. Neurosci. Methods 2006, 153, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Pieczora, L.; Stracke, L.; Vorgerd, M.; Hahn, S.; Theiss, C.; Theis, V. Unveiling of miRNA Expression Patterns in Purkinje Cells During Development. Cerebellum 2017, 16, 376–387. [Google Scholar] [CrossRef]
- Haldipur, P.; Aldinger, K.A.; Bernardo, S.; Deng, M.; Timms, A.E.; Overman, L.M.; Winter, C.; Lisgo, S.N.; Razavi, F.; Silvestri, E.; et al. Spatiotemporal expansion of primary progenitor zones in the developing human cerebellum. Science 2019, 366, 454–460. [Google Scholar] [CrossRef]
- Davis, S.; Scott, C.; Ansorge, O.; Fischer, R. Development of a Sensitive, Scalable Method for Spatial, Cell-Type-Resolved Proteomics of the Human Brain. J. Proteome Res. 2019, 18, 1787–1795. [Google Scholar] [CrossRef]
- Zhang, P.G.Y.; Yeung, J.; Gupta, I.; Ramirez, M.; Ha, T.; Swanson, D.J.; Nagao-Sato, S.; Itoh, M.; Kawaji, H.; Lassmann, T.; et al. Discovery of Transcription Factors Novel to Mouse Cerebellar Granule Cell Development Through Laser-Capture Microdissection. Cerebellum 2018, 17, 308–325. [Google Scholar] [CrossRef]
- Aguado, C.; Colon, J.; Ciruela, F.; Schlaudraff, F.; Cabanero, M.J.; Perry, C.; Watanabe, M.; Liss, B.; Wickman, K.; Lujan, R. Cell type-specific subunit composition of G protein-gated potassium channels in the cerebellum. J. Neurochem. 2008, 105, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Von Ahlfen, S.; Missel, A.; Bendrat, K.; Schlumpberger, M. Determinants of RNA quality from FFPE samples. PLoS ONE 2007, 2, e1261. [Google Scholar] [CrossRef]
- Carter, R.A.; Bihannic, L.; Rosencrance, C.; Hadley, J.L.; Tong, Y.; Phoenix, T.N.; Natarajan, S.; Easton, J.; Northcott, P.A.; Gawad, C. A Single-Cell Transcriptional Atlas of the Developing Murine Cerebellum. Curr. Biol. 2018, 28, 2910–2920. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Sheng, A.L.; Xiao, Q.; Shen, L.; Ju, X.C.; Zhang, M.; He, S.T.; Wu, C.; Luo, Z.G. Single-cell transcriptomes reveal molecular specializations of neuronal cell types in the developing cerebellum. J. Mol. Cell Biol. 2019, 11, 636–648. [Google Scholar] [CrossRef]
- Gupta, I.; Collier, P.G.; Haase, B.; Mahfouz, A.; Joglekar, A.; Floyd, T.; Koopmans, F.; Barres, B.; Smit, A.B.; Sloan, S.A.; et al. Single-cell isoform RNA sequencing characterizes isoforms in thousands of cerebellar cells. Nat. Biotechnol. 2018, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.J.; Zhang, P.G.Y.; Robert, R.; Yeung, J.; Swanson, D.J.; Mathelier, A.; Wasserman, W.W.; Im, S.; Itoh, M.; Kawaji, H.; et al. Identification of novel cerebellar developmental transcriptional regulators with motif activity analysis. BMC Genom. 2019, 20, 718. [Google Scholar] [CrossRef]
| Methodology | Neuronal Type and Specific Marker | References |
|---|---|---|
| Forward and reverse genetics | Purkinje cell: Grid2, Pcp2 Basket cell: Kcna2 | [29,30,42,52,53,54,55,56] |
| Global screening (in situ hybridization) | Purkinje cell: Esrrb, Nr2f2, Foxp2, Foxp4 Granule cell: Zic1, Neurod1, Etv1, Nfia | [74] |
| Laser-capture microdissection | Stellate cell: GIRK3 | [92] |
| Single-cell transcriptome | Purkinje cell: Adcy1, Itpka, Csdc2 Granule cell: Cntn2, Tubb3 | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tam, W.Y.; Wang, X.; Cheng, A.S.K.; Cheung, K.-K. In Search of Molecular Markers for Cerebellar Neurons. Int. J. Mol. Sci. 2021, 22, 1850. https://doi.org/10.3390/ijms22041850
Tam WY, Wang X, Cheng ASK, Cheung K-K. In Search of Molecular Markers for Cerebellar Neurons. International Journal of Molecular Sciences. 2021; 22(4):1850. https://doi.org/10.3390/ijms22041850
Chicago/Turabian StyleTam, Wing Yip, Xia Wang, Andy S. K. Cheng, and Kwok-Kuen Cheung. 2021. "In Search of Molecular Markers for Cerebellar Neurons" International Journal of Molecular Sciences 22, no. 4: 1850. https://doi.org/10.3390/ijms22041850
APA StyleTam, W. Y., Wang, X., Cheng, A. S. K., & Cheung, K.-K. (2021). In Search of Molecular Markers for Cerebellar Neurons. International Journal of Molecular Sciences, 22(4), 1850. https://doi.org/10.3390/ijms22041850






