Abstract
Aurora kinases are serine/threonine kinases required for cell proliferation and are overexpressed in many human cancers. Targeting Aurora kinases has been a therapeutic strategy in cancer treatment. Here, we attempted to identify a deubiquitinase (DUB) that regulates Aurora kinase A (Aurora-A) protein stability and/or kinase activity as a potential cancer therapeutic target. Through pull-down assays with the human DUB library, we identified OTUD6A as an Aurora-A-specific DUB. OTUD6A interacts with Aurora-A through OTU and kinase domains, respectively, and deubiquitinates Aurora-A. Notably, OTUD6A promotes the protein half-life of Aurora-A and activates Aurora-A by increasing phosphorylation at threonine 288 of Aurora-A. From qPCR screening, we identified and validated that the cancer gene CKS2 encoding Cyclin-dependent kinases regulatory subunit 2 is the most upregulated cell cycle regulator when OTUD6A is overexpressed. The results suggest that OTUD6A may serve as a therapeutic target in human cancers.
1. Introduction
Aurora kinases are serine/threonine kinases required for cell proliferation. There are three human paralogues; Aurora kinase A (Aurora-A), Aurora kinase B (Aurora-B), and Aurora kinase C (Aurora-C). Chromosomal missegregation during cell division causes genomic instability, contributing to carcinogenesis [1], and Aurora kinases play a role in proper chromosomal segregation. Aurora kinases are overexpressed in many human cancers including colorectal cancer, glioma, breast cancer, ovarian cancer, and pancreatic cancer [2,3,4,5,6,7], which has been driving an extensive investigation on their molecular functions in the cancer field and their roles as cancer targets in various human cancers. Among the three Aurora kinases, Aurora kinase A encoded by AURKA is a critical regulator in mitosis and meiosis and it is the most activated during the G2-M phase [8]. Aurora-A helps spindle body development [9], completes centrosome formation and segregation [8], and organizes and aligns chromosomes in prometaphase [10]. Aurora-A is often dysregulated in many cancer types. In breast cancer, in particular, it is overexpressed in 94% of cancer tissues [9]. Aurora-A has been shown to function as an oncogene, as evidenced by that it causes genomic instability in multiple cancers [11,12,13], inhibits apoptosis [14,15], promotes migration, invasion, and metastasis of cancer cells by controlling epithelial-mesenchymal transition (EMT), and maintains cancer stem cell properties [16,17]. Aurora kinases are activated in the actively dividing cells, which enables the distinction between cancer cells and rarely dividing normal cells. Therefore, targeting Aurora kinases is potentially an effective therapeutic strategy by specifically eradicating cancer cells. Therefore, small-molecule inhibitors targeting Aurora kinases including Aurora-A have been developed and tested in pre-clinical or clinical trials [18,19].
Ubiquitin is a small 76-amino acid protein and modifies proteins post-translationally through ubiquitination, a process of covalent attachment of ubiquitin to a substrate protein via lysine residues. Deubiquitination, on the other hand, reverses the reaction by removing the ubiquitin or ubiquitin chain from the substrate proteins. Aurora-A is well-known to be ubiquitinated by the APC/C E3 ligase at the end of mitosis, which facilitates its protein degradation through proteasome [20]. However, the deubiquitinase (DUB) that specifically deubiquitinates Aurora-A is not clearly identified yet. Therefore, if Aurora-A-specific DUB is identified, we can delineate the ubiquitination-deubiquitination process of Aurora-A, which allows us to better understand the cell cycle machinery regulated by Aurora A, a pivotal cell cycle regulator during the mitosis. Moreover, in cancer therapy, we can possess an alternative cancer therapeutic strategy in addition to directly targeting Aurora-A.
In the present study, we aimed at finding a DUB specific to Aurora-A using the human DUB library and identified OTUD6A. OTUD6A (OTU domain-containing protein 6A) is a member of Ovarian tumor-associated proteases (OTUs) comprising sixteen DUB members [21]. The tumorigenic roles of OTUD6A have been demonstrated previously. OTUD6A enhances the protein levels of Snail, an EMT (epithelial-mesenchymal transition) inducer [22], and promotes tumorigenecity by deubiquitinating and stabilizing Drp1 [23]. We found that OTUD6A deubiquitinates Aurora-A to stabilize and activate by interacting through the kinase domain of Aurora-A. Moreover, OTUD6A induces the transcription of an oncogenic cell cycle-regulating gene, CKS2 (Cyclin-dependent kinases regulatory subunit 2) [24]. Thus, our study implicates the possibility of OTUD6A as a potential cancer therapeutic target.
2. Results
2.1. Identification of Aurora-A-Interacting Deubiquitinases (DUBs)
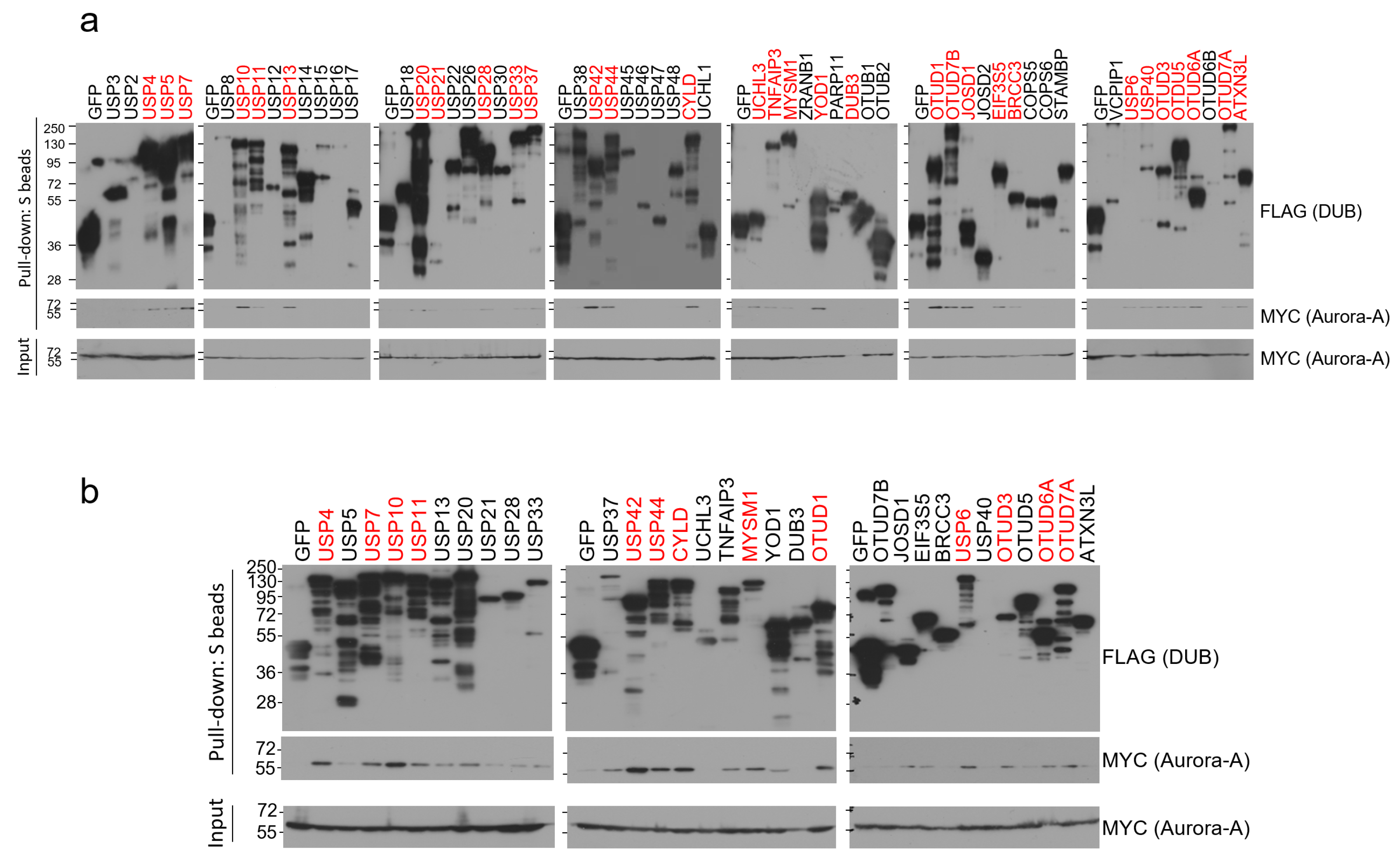
To identify Aurora-A-specific deubiquitinase(s), we conducted binding assays using the human DUB library. We cloned a total of 85 human DUB open reading frames (ORFs) to the expression vector with SFB tag (a triple-epitope tag containing S-protein, FLAG tag, and streptavidin-binding peptide) and checked their expression by western blotting. Sub-library including 59 DUBs with decent expression in HEK293T cells was chosen and implemented for the pull-down assays. We transiently co-transfected each SFB-tagged DUB and MYC-tagged Aurora-A plasmids into HEK293T cells and pulled down DUBs with S-protein beads. As shown in Figure 1a, about half of the DUBs (31 DUBs) interacted with Aurora-A when immuno-blotted with MYC antibody. Their interactions were compared again in the second binding assays and 13 DUBs (USP4, USP7, USP10, USP11, USP42, USP44, CYLD, MYSM1, OTUD1, USP6, OTUD3, OTUD6A, and OTUD7A) showed relatively strong interaction with Aurora-A (Figure 1b). Previously, USP2 was shown to bind to and deubiquitinate Aurora-A [25], but we could not detect their interaction in our initial binding assays (Figure 1a). Therefore, USP2 was excluded from our candidates.
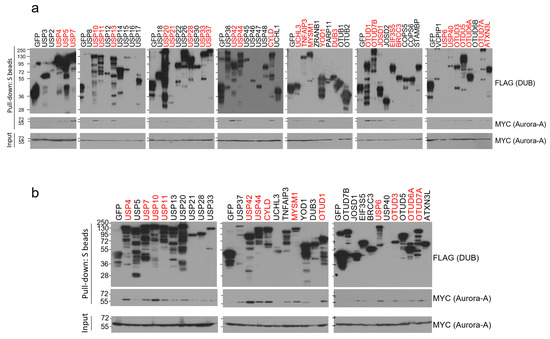
Figure 1.
Pull-down assays to identify Aurora-A-binding DUBs. (a) Each SFB-tagged DUB was co-transfected with MYC-tagged Aurora-A into HEK293T cells, followed by pulling down DUBs with S-protein beads and immunoblotting with antibodies against FLAG (to detect DUBs) and MYC (to detect Aurora-A). The first screening identified 31 binding DUBs (highlighted in red). (b) Pull-down assay was repeated with 31 binding DUBs identified from the first screening. 13 DUBs (highlighted in red) which display a relatively strong binding affinity to Aurora-A were selected in this second screening.
2.2. OTUD6A Deubiquitinates Aurora-A
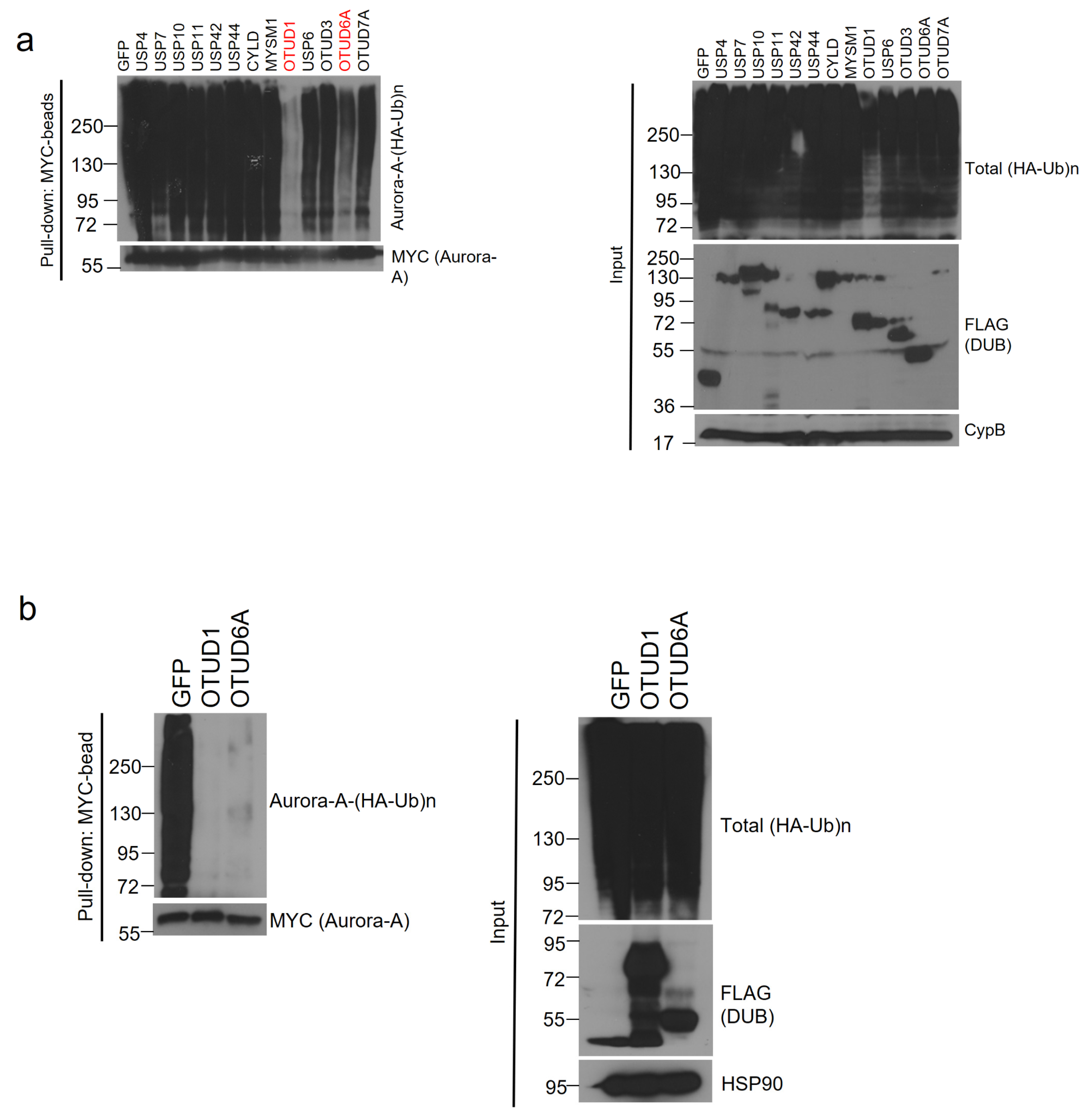
Using the 13 Aurora-A-interacting DUBs, we conducted a deubiquitination assay. Each SFB-tagged DUB, MYC-tagged Aurora-A, and HA (hemagglutinin)-tagged ubiquitin plasmids were transiently co-transfected into HEK293T cells and the cells were treated with proteasome inhibitor, MG132. Aurora-A was immunoprecipitated with MYC antibody-conjugated agarose beads (MYC-beads). Only OTUD1 and OTUD6A induced the deubiquitination of Aurora-A (Figure 2a). Then, we conducted a deubiquitination assay again and found that both OTUD1 and OTUD6A similarly deubiquitinated Aurora-A (Figure 2b).
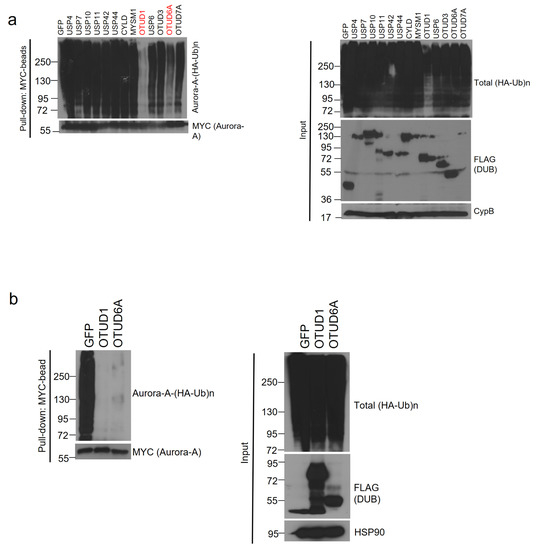
Figure 2.
OTUD1 and OTUD6A deubiquitinate Aurora-A. (a) SFB-tagged 13 candidate DUBs were co-transfected with MYC-tagged Aurora-A and HA-tagged ubiquitin into HEK293T cells. After treating MG132 for 6 h, cells were lysed, followed by pulling down Aurora-A with MYC-beads and immunoblotting with antibodies against HA (to detect polyubiquitinated Aurora-A) and MYC (to detect Aurora-A). DUB assay identified OTUD1 and OTUD6A as Aurora-A-deubiquitinating DUBs. (b) DUB assay was repeated with OTUD1 and OTUD6A and a similar degree of deubiquitination of Aurora-A was observed.
2.3. Aurora-A and OTUD6A Interact with Each Other through the Kinase and OTU Domains, Respectively
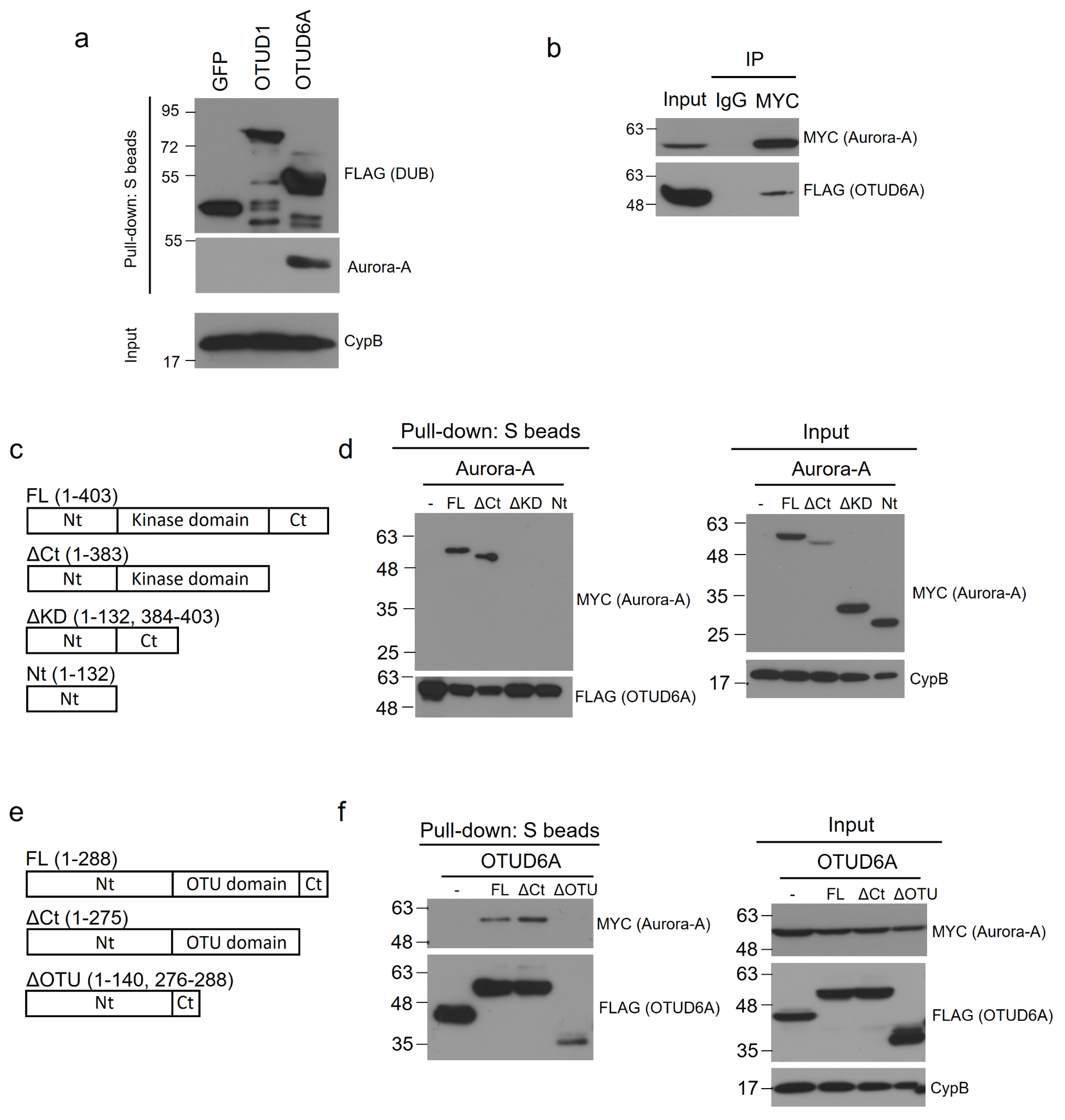
Next, we examined the interaction between endogenous Aurora-A and SFB-tagged OTUD6A. We transiently transfected SFB-tagged OTUD6A plasmid into HEK293T cells and pulled down OTUD6A with S-protein beads. Then, associated endogenous Aurora-A was blotted with Aurora-A-specific antibody. In this binding assay, Aurora-A exhibited a strong association with OTUD6A but weak or no interaction with GFP control protein and OTUD1 (Figure 3a). Based on this result, OTUD6A was chosen as a final candidate DUB and subjected to the subsequent experiments.
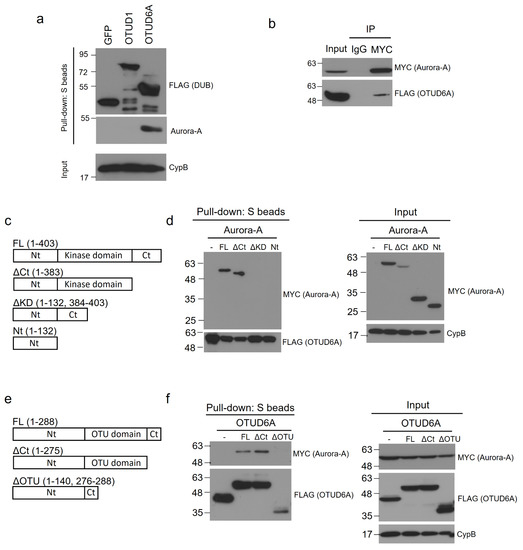
Figure 3.
OTUD6A interacts with Aurora-A through the OTU and kinase domains, respectively. (a) SFB-tagged OTUD1 and OTUD6A were transfected into HEK293T cells, followed by pulling down DUBs with S-protein beads and immunoblotting with antibodies against FLAG (to detect DUBs) and Aurora-A. (b) SFB-tagged OTUD6A was co-transfected with MYC-tagged Aurora-A into HEK293T cells, followed by pulling down Aurora-A with MYC-beads and immunoblotting with antibodies against FLAG (to detect OTUD6A) and MYC (to detect Aurora-A). (c) Schematic representation of full-length (FL) and truncated mutants of Aurora-A. (d) SFB-tagged OTUD6A was co-transfected with MYC-tagged full length or each mutant Aurora-A into HEK293T cells, followed by pulling down OTUD6A with S-protein beads and immunoblotting with antibodies against MYC (to detect Aurora-A (FL and mutants)) and FLAG (to detect OTUD6A). (e) Schematic representation of full-length (FL) and truncated mutants of OTUD6A. (f) MYC-tagged Aurora-A was co-transfected with SFB-tagged full-length or each mutant OTUD6A into HEK293T cells, followed by pulling down OTUD6A (FL and mutants) with S-protein beads and immunoblotting with antibodies against MYC (to detect Aurora-A) and FLAG (to detect OTUD6A (FL and mutants)).
To confirm the interaction between Aurora-A and OTUD6A, we conducted reverse co-immunoprecipitation by pulling down MYC-tagged Aurora-A from the transfected HEK293T cell lysate and subsequently immunoblotted SFB-tagged OTUD6A with FLAG antibody. As shown in Figure 3b, OTUD6A indeed interacted with Aurora-A.
To determine the Aurora-A domain(s) that OTUD6A interacts with, we generated three truncated mutants of Aurora-A; Aurora-AΔCt (C-terminal-deleted, aa 1–383), Aurora-AΔKD (kinase-domain-deleted, aa 1–132 & 384–403), and Aurora-ANt (N-terminal only, aa 1–132) (Figure 3c). With the Aurora-A mutants, we conducted a binding assay with OTUD6A. We transiently co-transfected SFB-tagged OTUD6A and each MYC-tagged truncated Aurora-A mutant plasmid along with full-length wild-type Aurora-AFL (aa 1–403) into HEK293T cells and pulled down OTUD6A with S-protein beads. As shown in Figure 3d, Aurora-AFL and Aurora-AΔCt interacted but Aurora-AΔKD and Aurora-ANt did not interact with OTUD6A, which implies that OTUD6A binds to the kinase domain (KD) of Aurora-A.
Next, we examined which domain(s) on OTUD6A interact(s) with Aurora-A. We generated two truncated mutants of OTUD6A: OTUD6AΔCt (C-terminal-deleted, aa 1–275) and OTUD6AΔOTU (OTU-domain-deleted, aa 1–140 & 276–288) (Figure 3e). With the OTUD6A mutants, we conducted a binding assay with Aurora-A. We transiently co-transfected MYC-tagged Aurora-A plasmid and each SFB-tagged truncated OTUD6A mutant plasmid along with full-length wild-type OTUD6AFL (aa 1–288) into HEK293T cells and pulled down each OTUD6A with S-protein beads. As shown in Figure 3f, OTUD6AFL and OTUD6AΔCt interacted but OTUD6AΔOTU did not interact with Aurora-A, which implies that Aurora-A binds to the OTU domain of OTUD6A.
2.4. OTUD6A Promotes Protein Stability and Kinase Activity of Aurora-A
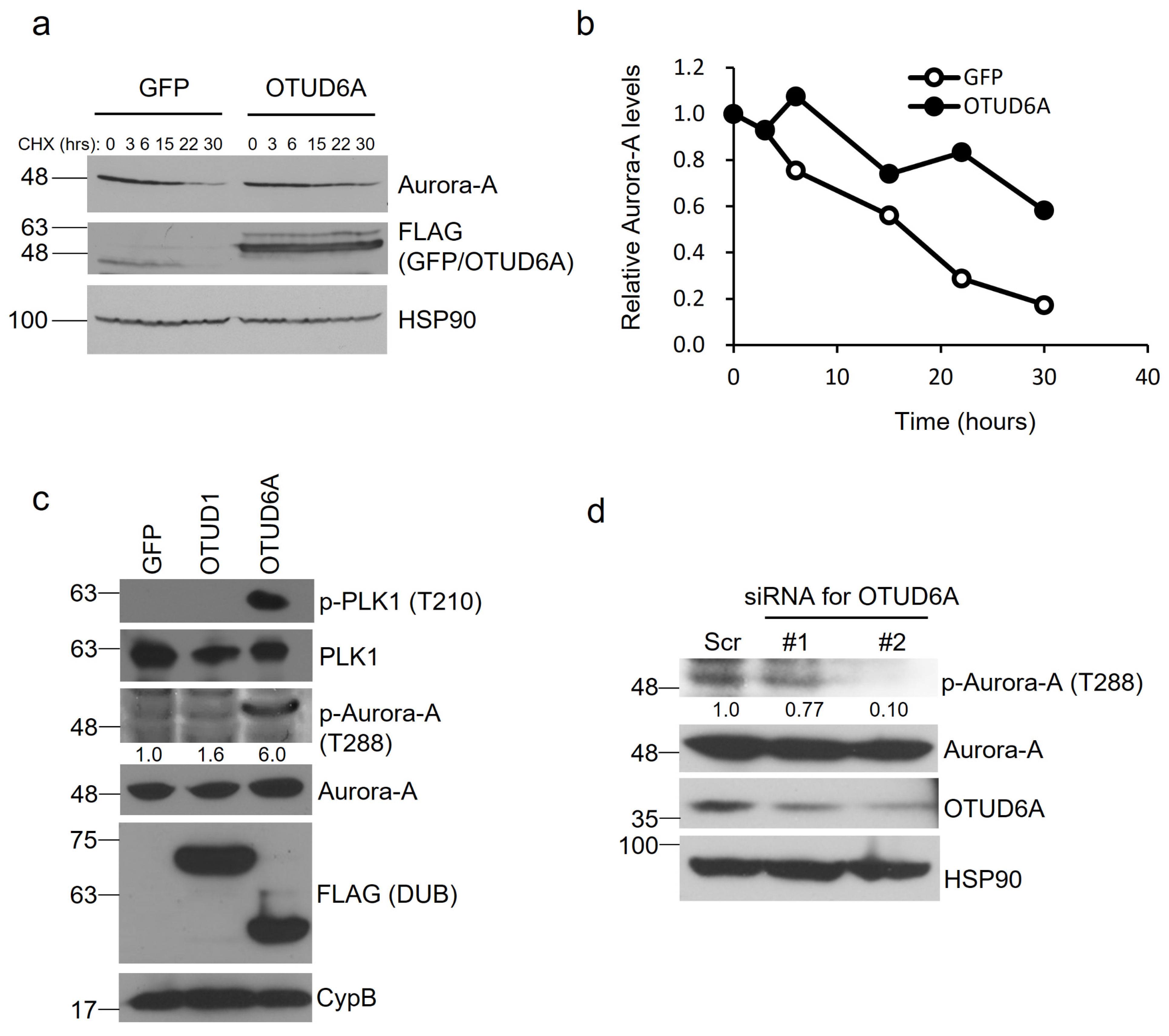
To determine whether OTUD6A-mediated deubiquitination increases protein stability of Aurora-A, we transfected SFB-tagged OTUD6A into HEK293T cells and treated the cells with cycloheximide at 50 μg/mL for the indicated period. When normalized following quantitation, the protein half-life of Aurora-A was improved from ~17 to ~33 h by OTUD6A (Figure 4a,b).
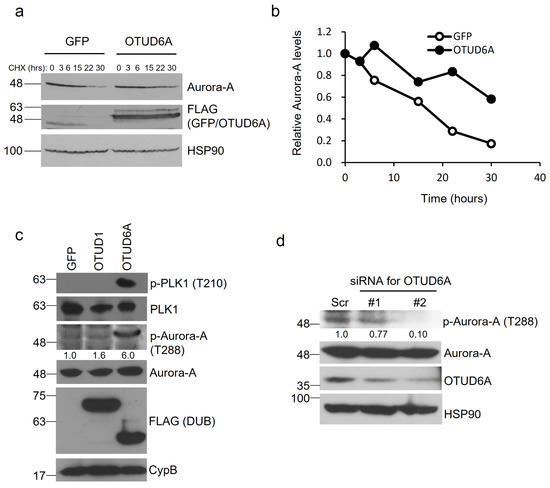
Figure 4.
OTUD6A induces protein stability and enzymatic activity of Aurora-A. (a) SFB-tagged OTUD6A or GFP was transfected into HEK293T cells and translation inhibitor cycloheximide (50 μg/mL) was treated for the indicated period. The protein stability of endogenous Aurora-A was examined with a specific antibody. (b) Relative protein stability of Aurora-A tested in (a) was quantitated by normalizing with the expression levels of loading control HSP90. (c) The levels of phosphorylated Aurora-A at threonine 288 were induced by OTUD6A expression, which in turn increased phospho-PLK1 levels (at threonine 210). (d) Silencing OTUD6A with siRNAs suppressed Aurora-A phosphorylation at threonine 288.
Aurora-A is mainly activated after threonine 288 within the activation loop of the catalytic domain is autophosphorylated [26]. We examined whether OTUD6A promotes the kinase activity of Aurora-A by analyzing phosphorylation at threonine 288. When we overexpressed SFB-tagged OTUD6A in HEK293T cells, we could observe a lot more increase of phospho-Thr 288 by OTUD6A (+500%) than by OTUD1 (+60%) compared to the control. Moreover, activated Aurora-A by OTUD6A induced phosphorylation at threonine 210 of Polo-like kinase 1 (PLK1), a key substrate of Aurora-A, which is known to be a prerequisite for PLK1 activation in promoting mitotic entry [27,28] (Figure 4c). When we silenced OTUD6A with siRNAs, on the other hand, we could see 23–90% reduction in phospho-Thr 288 of Aurora-A (Figure 4d). Collectively, OTUD6A stabilizes Aurora-A and activates its enzymatic activity.
2.5. OTUD6A Promotes CKS2 Gene Expression
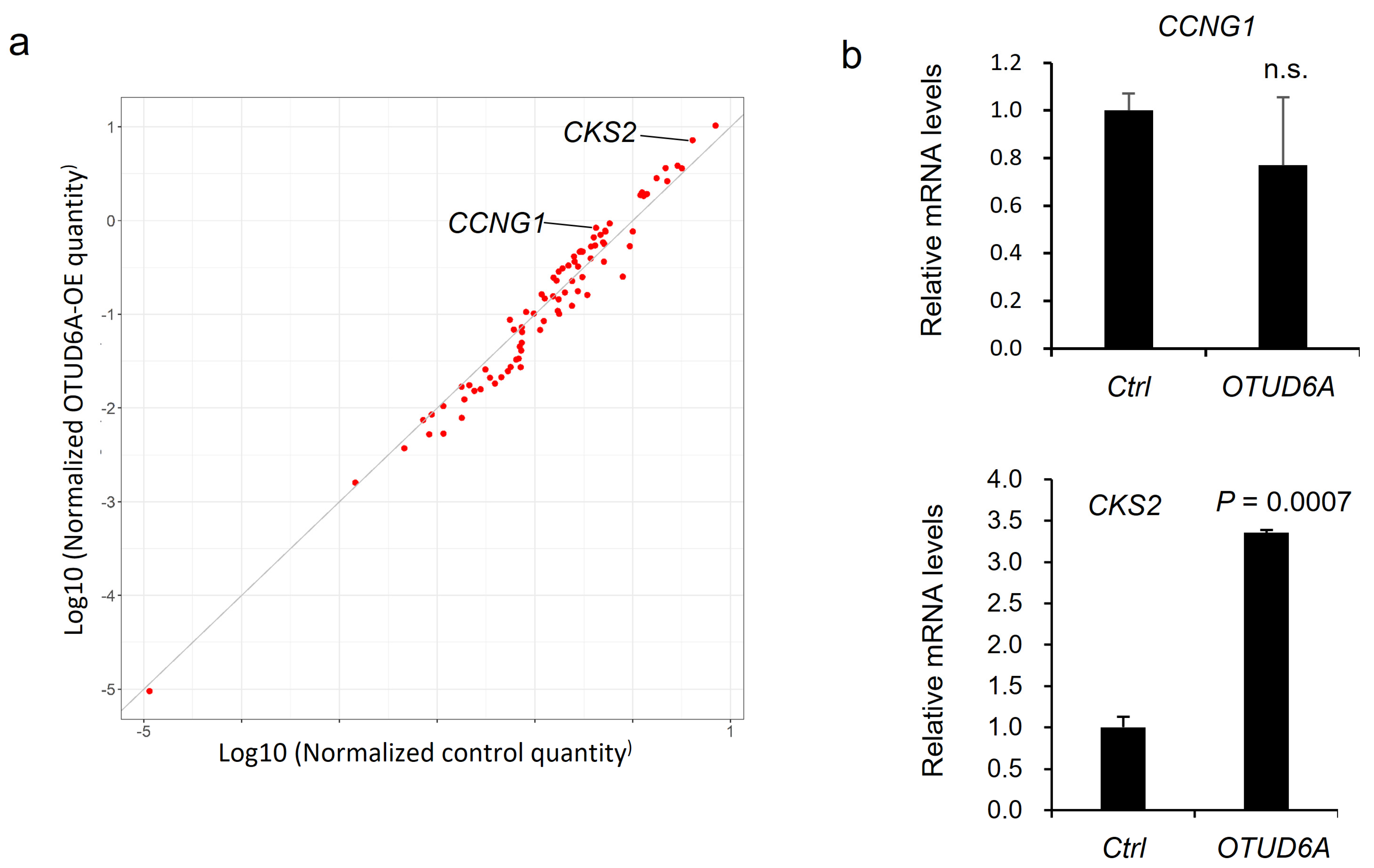
Aurora-A is an important cell cycle regulator during mitosis and is reported to regulate target gene transcription in a kinase activity-dependent manner. For example, Aurora-A affects target gene expression by stabilizing YAP transcription coactivator [29], and by phosphorylating at Serine 10 of histone H3 and the Runt-related transcription factors (RUNX) [30,31]. Therefore, we asked what cell cycle-regulating gene is the most upregulated by OTUD6A-mediated deubiquitination of Aurora-A. To address this question, we utilized qPCR screening kit which profiles cell cycle-regulating genes. Compared to the control, CCNG1 (Cyclin G1) and CKS2 (Cyclin-dependent kinases regulatory subunit 2) were two of the most upregulated genes in OTUD6A-transfected HEK293T cells in the screening (Figure 5a). We conducted an independent validation and found that CKS2 was significantly upregulated by OTUD6A overexpression (Figure 5b).
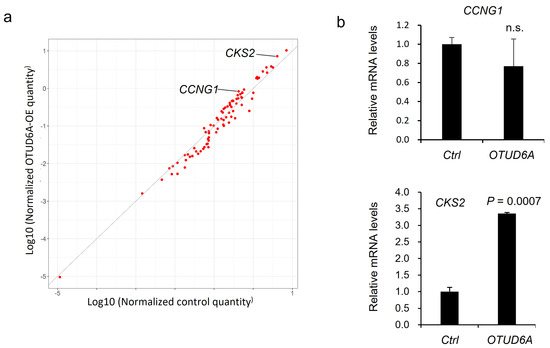
Figure 5.
qPCR screening to identify cell cycle-regulating genes regulated by OTUD6A. (a) Scatterplot describes the relative expression of cell cycle-regulating genes in OTUD6A-overexpressing cells following qPCR screening. CCNG1 and CKS2 are indicated as two of the most upregulated genes. (b) qPCR validates CKS2 expression is significantly upregulated by OTUD6A. Statistical significance in (b) was determined by an unpaired t-test. Error bars are s.e.m.
3. Discussion
In the present study, we screened human DUBs that bind to and deubiquitinate Aurora-A in an unbiased manner. Previously, USP2 was identified as a DUB for Aurora-A but it did not show decent physical association with its substrate protein, Aurora-A in our screening, which prompted us to exclude it from the subsequent studies. OTUD1, one of the final candidate DUBs for Aurora-A, bound to and deubiquitinated Aurora-A but its interaction with endogenous Aurora-A was much weaker than OTUD6A. Moreover, OTUD1 was not able to increase the enzymatic activity of Aurora-A as much as OTUD6A. Therefore, OTUD6A was selected as a DUB that binds to, deubiquitinates, and activates Aurora-A. PLK1 is a key substrate of Aurora-A and phosphorylation at threonine 210 by Aurora-A occurs during G2 phase of the cell cycle, which is a prerequisite for PLK1 activation [27,28]. The activated PLK1 then phosphorylates substrates regulating mitotic entry such as CDC25C, promoting mitosis [32]. Consequently, our data suggest that Aurora-A, a critical mitotic regulator, is deubiquitinated, stabilized, and activated by OTUD6A, which in turn activates PLK1 to drive cells into mitosis.
In addition to PLK1, many Aurora-A downstream substrates have been identified and the functional roles of Aurora-A-mediated phosphorylation of the substrates have been extensively investigated in tumorigenesis [33]. For example, p53 is phosphorylated at multiple serine residues, which regulates its protein stability and transcriptional activity [34,35,36]. Phosphorylated Twist promotes epithelial-mesenchymal transition (EMT) and chemoresistance [37], and phosphorylated β-catenin becomes resistant to degradation and is promoted to localize into the nucleus, which results in increased transcriptional activity [38]. Phosphorylation of ERα (Estrogen receptor alpha) enhances its DNA-binding potential, which facilitates transcription of cyclin D1 [39]. Therefore, it is meaningful to examine whether such oncogenic pathways are also regulated by OTUD6A if OTUD6A is a bona fide DUB for Aurora-A.
We found that OTUD6A and Aurora-A interact with each other through their enzymatic domains, OTU and kinase domains, respectively. Moreover, Aurora-A is known to regulate target gene transcription by stabilizing YAP transcription coactivator [29], and by phosphorylating at Serine 10 of histone H3 and RUNX transcription factors [30,31], so we scrutinized how OTUD6A affects transcriptional programs by analyzing differentially expressed cell cycle-regulating genes. According to the qPCR screening, a cancer gene CKS2 was validated as the most induced cell cycle regulator when OTUD6A was overexpressed. Cyclin-dependent kinases regulatory subunit 2 encoded by CKS2 is known to regulate in vitro tumorigenecity of tongue squamous cell carcinoma, ovarian, cancer, esophageal cancer, breast cancer, and hepatocellular carcinoma [40,41,42,43,44]. One possible mechanism is that OTUD6A-mediated deubiquitination and stabilization of Aurora-A may increase YAP stability, which in turn activates CKS2 transcription as a TEAD/YAP target gene. This idea is supported by the fact that the promoter region of CKS2 genomic locus is bound by YAP according to the ChIP-seq analysis [45]. On the other hand, the expression of Aurora-A itself is directly regulated by transcription factors including FOXM1, ARID3A, E4TF1, and SIX3 [46,47,48,49] and non-DUB molecules such as Twist, YBX1, and ALDH1A1 have been shown to inhibit proteolysis of Aurora-A through the positive feedback loop with Aurora-A. However, the molecular mechanism by which Aurora-A is stabilized is unclear [37,50,51].
There are only a limited number of studies on OTUD6A. However, it has been demonstrated that OTUD6A enhances the protein levels of one of the crucial EMT inducers, Snail [22], and promotes colorectal cancer by deubiquitinating and stabilizing DRP1 [23]. Therefore, the present study and the previous reports suggest that OTUD6A could be a feasible therapeutic target in human cancers.
4. Materials and Methods
4.1. Cell Culture
The HEK293T cell line was obtained from American Type Culture Collection (ATCC) (Manassas, VA, USA). The cell line was cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (GenDepot, Houston, TX, USA) at the humidified incubator with 5% CO2 at 37 °C.
4.2. Plasmids and siRNAs
Fifty-nine human DUB ORFs in Gateway Entry vector were obtained from DNASU Plasmid Repository (Manassas, AZ, USA) and DF/HCC DNA Resource Core (Boston, MA, USA) and were individually subcloned into a pBabe-SFB expression vector using the Gateway system (Thermofisher, Waltham, MA, USA). Aurora-A ORF in the Entry vector was subcloned into a MYC-tagged expression vector using the Gateway system. HA-ubiquitin vector was obtained from Addgene (Addgene #17608) (Watertown, MA, USA). siRNAs targeting OTUD6A were purchased from Genepharma (Shanghai, China) and their sense sequences are as followed: siRNA #1, GCUGGAGAAGUUCCAAGACTT; siRNA #2, GCACUACAACUCCGUGACATT.
4.3. Site-Directed Mutagenesis
We conducted mutagenesis to generate three Aurora-A and two OTUD6A truncated mutants with EZchange site-direct mutagenesis kit (Enzynomics, Daejeon, Korea) according to the manufacturer’s protocol.
4.4. Western Blotting
Western blotting was performed as described [52]. The cells were lysed in RIPA buffer supplemented with protease and phosphatase inhibitors (GenDepot, Houston, TX, USA). The proteins were separated by SDS-PAGE and transferred onto a PVDF membrane. The membranes were blocked in 5% non-fat milk in Tris-buffered saline-Tween 20 (TBS-T) and then incubated with the specific primary antibodies. After washed in TBS-T, the membranes were incubated with an HRP-conjugated secondary antibody. The bands were visualized by chemiluminescence. The following antibodies were used: antibodies against OTUD6A (1:1000, Proteintech, Rosemont, IL, USA), MYC (1:10,000, Proteintech, Rosemont, IL, USA), HSP90 (1:5000, Santa Cruz, Dallas, TX, USA), HA (1:1000, Santa Cruz, Dallas, TX, USA), FLAG (1:2000, Proteintech, Rosemont, IL, USA), Cyclophilin B (1:5000, Thermofisher, Waltham, MA, USA), Aurora-A (1:2000, Cell Signaling, Danvers, MA, USA), phospho-Aurora-A (Thr 288) (1:500, Abclonal, Woburn, MA, USA), PLK1 (1:1000, Abclonal, Woburn, MA, USA), and phospho-PLK1 (Thr 210) (1:1000, Cell Signaling, Danvers, MA, USA).
4.5. Immunoprecipitation and Pull-Down Assays
The cells were lysed in NETN buffer (200 mM Tris-HCl, pH 8.0, 100 mM NaCl, 0.05% Nonidet P-40, 1 mM EDTA) containing protease and phosphatase inhibitors (GenDepot, Houston, TX, USA). To pull down SFB-tagged proteins, cell lysates were incubated with S-protein beads (Merck, Darmstadt, Germany). To pull down MYC-tagged protein (Aurora-A), the cell lysates were incubated with MYC-beads (Thermofisher, Waltham, MA, USA) or normal mouse lgG-conjugated beads (Santa Cruz, Dallas, TX, USA). After inverting at 4 °C overnight, the precipitated protein complexes were washed three times with NETN buffer and the bound proteins were eluted by boiling in 2X Laemmli buffer and vortexing for 30 s and subjected to immunoblotting with the indicated antibodies.
4.6. Deubiquitination Assay
The HEK293T cells were co-transfected with MYC-tagged Aurora-A, SFB-tagged DUBs, and HA-tagged ubiquitin, and were treated with the proteasome inhibitor MG132 (10 μM) (Cayman, Ann Arbor, MI, USA) for 6 h. After cell lysis, MYC-tagged Aurora-A was immunoprecipitated with MYC-beads (Thermofisher, Waltham, MA, USA), and then polyubiquitinated Aurora-A was immunoblotted with HA antibody.
4.7. Total RNA Isolation, Reverse Transcription, and Quantitative PCR
Total RNA was extracted using MagListo RNA extraction kit (Bioneer, Daejeon, Korea) and RNase-free DNase I (Thermofisher, Waltham, MA, USA) and then reverse-transcribed with an iScript cDNA synthesis Kit (Bio-rad, Hercules, CA, USA). qPCR was performed using SYBR Green reagent (Enzynomics, Daejeon, Korea) and specific primer pairs. Real-time PCR and data collection were performed on a CFX Connect instrument (Bio-rad, Hercules, CA, USA). Gene expression was quantitated by the ΔΔCt method and was normalized to the expression levels of internal control (β-Actin). All qPCR reactions were performed in three replicates. Primer sequences used in the qPCR analysis are as followed; CCNG1-Forward, TCTTGCCTACGAGTCCCC; CCNG1-Reverse, GAGAGTCAGTTGTTGTCAGTACC; CKS2-Forward, CGCTCTCGTTTCATTTTCTGC; CKS2-Reverse, CTTGTTTGGAAAGTTCTCTGGG; β-Actin-Forward, AGGCACCAGGGCGTGAT; β-Actin-Reverse, GCCCACATAGGAATCCTTCTGAC.
4.8. qPCR Screening
qPCR screening kit for cell cycle-regulating genes was purchased from Bioneer (Daejeon, Korea). Gene list is available at https://www.bioneer.co.kr/20-sh-0001-10-cfg.html (accessed on 1 November 2020). Control or OTUD6A expression vector was transfected into HEK293T cells, and total RNA extraction and cDNA synthesis were performed as described above. 20 ng cDNA mixed with SYBR Green was distributed to each well of the 96-well format assay plate containing gene-specific primer sets. Real-time PCR and data collection were performed as described above.
4.9. Statistical Analysis
The data are presented as mean ± s.e.m., and a two-tailed unpaired t-test was used to compare two groups of independent samples. p < 0.05 was considered statistically significant.
Author Contributions
J.K. conceived and designed the study. H.J.K. performed the experiments. J.K. contributed to funding acquisition and data analysis and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Korea Foundation for the Advancement of Science & Creativity (KOFAC), and funded by the Korea Government (MOE), by the Sogang University Research Grant of 2019 (grant No. 201910004.01 and 201915003.01) and by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIT) (grant No. 2020R1F1A1065643).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon reasonable request from the corresponding author.
Acknowledgments
We acknowledge Junkyu Kang, Jaehyuk Yang, and Insu Koh for their technical support. We also thank Bong-Gun Ju for his intellectual input.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| SFB | A triple-epitope tag containing S-protein, FLAG tag, and streptavidin-binding peptide |
| DUB | Deubiquitinase |
| HA | Hemagglutinin |
| CypB | Cyclophilin B |
| GFP | Green fluorescent protein |
| HSP90 | Heat shock protein 90 |
| PLK1 | Polo-like kinase 1 |
| IP | Immunoprecipitation |
| IgG | Immunoglobulin G |
| FL | Full-length |
| Nt | Amino terminal |
| Ct | Carboxyl terminal |
| KD | Kinase domain |
| CHX | Cycloheximide |
| siRNA | Small interfering RNA |
References
- Bolanos-Garcia, V.M. Aurora kinases. Int. J. Biochem. Cell Biol. 2005, 37, 1572–1577. [Google Scholar] [CrossRef]
- Bischoff, J.R.; Anderson, L.; Zhu, Y.; Mossie, K.; Ng, L.; Souza, B.; Schryver, B.; Flanagan, P.; Clairvoyant, F.; Ginther, C.; et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 1998, 17, 3052–3065. [Google Scholar] [CrossRef]
- Giet, R.; Prigent, C. Aurora/Ipl1p-related kinases, a new oncogenic family of mitotic serine-threonine kinases. J. Cell Sci. 1999, 112 Pt 21, 3591–3601. [Google Scholar]
- Gritsko, T.M.; Coppola, D.; Paciga, J.E.; Yang, L.; Sun, M.; Shelley, S.A.; Fiorica, J.V.; Nicosia, S.V.; Cheng, J.Q. Activation and overexpression of centrosome kinase BTAK/Aurora-A in human ovarian cancer. Clin. Cancer Res. 2003, 9, 1420–1426. [Google Scholar]
- Reichardt, W.; Jung, V.; Brunner, C.; Klein, A.; Wemmert, S.; Romeike, B.F.; Zang, K.D.; Urbschat, S. The putative serine/threonine kinase gene STK15 on chromosome 20q13.2 is amplified in human gliomas. Oncol. Rep. 2003, 10, 1275–1279. [Google Scholar] [CrossRef]
- Tanaka, T.; Kimura, M.; Matsunaga, K.; Fukada, D.; Mori, H.; Okano, Y. Centrosomal kinase AIK1 is overexpressed in invasive ductal carcinoma of the breast. Cancer Res. 1999, 59, 2041–2044. [Google Scholar] [PubMed]
- Zhou, H.; Kuang, J.; Zhong, L.; Kuo, W.L.; Gray, J.W.; Sahin, A.; Brinkley, B.R.; Sen, S. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat. Genet. 1998, 20, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Hannak, E.; Kirkham, M.; Hyman, A.A.; Oegema, K. Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J. Cell Biol. 2001, 155, 1109–1116. [Google Scholar] [CrossRef]
- Crane, R.; Gadea, B.; Littlepage, L.; Wu, H.; Ruderman, J.V. Aurora A, meiosis and mitosis. Biol. Cell 2004, 96, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Katayama, H.; Sasai, K.; Kloc, M.; Brinkley, B.R.; Sen, S. Aurora kinase-A regulates kinetochore/chromatin associated microtubule assembly in human cells. Cell Cycle 2008, 7, 2691–2704. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Nosho, K.; Shima, K.; Irahara, N.; Kure, S.; Toyoda, S.; Kirkner, G.J.; Goel, A.; Fuchs, C.S.; Ogino, S. Aurora-A expression is independently associated with chromosomal instability in colorectal cancer. Neoplasia 2009, 11, 418–425. [Google Scholar] [CrossRef]
- Li, J.J.; Weroha, S.J.; Lingle, W.L.; Papa, D.; Salisbury, J.L.; Li, S.A. Estrogen mediates Aurora-A overexpression, centrosome amplification, chromosomal instability, and breast cancer in female ACI rats. Proc. Natl. Acad. Sci. USA 2004, 101, 18123–18128. [Google Scholar] [CrossRef] [PubMed]
- Torchia, E.C.; Chen, Y.; Sheng, H.; Katayama, H.; Fitzpatrick, J.; Brinkley, W.R.; Caulin, C.; Sen, S.; Roop, D.R. A genetic variant of Aurora kinase A promotes genomic instability leading to highly malignant skin tumors. Cancer Res. 2009, 69, 7207–7215. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.; Naber, C.; Steffler, T.; Checkland, T.; Keats, J.; Maxwell, C.; Perry, T.; Chau, H.; Belch, A.; Pilarski, L.; et al. Aurora A kinase RNAi and small molecule inhibition of Aurora kinases with VE-465 induce apoptotic death in multiple myeloma cells. Leuk. Lymphoma 2008, 49, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.R.; Huang, S.; Long, Z.J.; Chen, J.J.; Zou, Z.Z.; Li, J.; Lin, D.J.; Liu, Q. Inhibition of mitotic kinase Aurora suppresses Akt-1 activation and induces apoptotic cell death in all-trans retinoid acid-resistant acute promyelocytic leukemia cells. J. Transl. Med. 2011, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Chefetz, I.; Holmberg, J.C.; Alvero, A.B.; Visintin, I.; Mor, G. Inhibition of Aurora-A kinase induces cell cycle arrest in epithelial ovarian cancer stem cells by affecting NFkB pathway. Cell Cycle 2011, 10, 2206–2214. [Google Scholar] [CrossRef] [PubMed]
- Mannino, M.; Gomez-Roman, N.; Hochegger, H.; Chalmers, A.J. Differential sensitivity of Glioma stem cells to Aurora kinase A inhibitors: Implications for stem cell mitosis and centrosome dynamics. Stem Cell Res. 2014, 13, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Bavetsias, V.; Linardopoulos, S. Aurora Kinase Inhibitors: Current Status and Outlook. Front. Oncol. 2015, 5, 278. [Google Scholar] [CrossRef]
- Moore, A.S.; Blagg, J.; Linardopoulos, S.; Pearson, A.D. Aurora kinase inhibitors: Novel small molecules with promising activity in acute myeloid and Philadelphia-positive leukemias. Leukemia 2010, 24, 671–678. [Google Scholar] [CrossRef]
- Min, M.; Mevissen, T.E.; De Luca, M.; Komander, D.; Lindon, C. Efficient APC/C substrate degradation in cells undergoing mitotic exit depends on K11 ubiquitin linkages. Mol. Biol. Cell 2015, 26, 4325–4332. [Google Scholar] [CrossRef] [PubMed]
- Mevissen, T.E.; Hospenthal, M.K.; Geurink, P.P.; Elliott, P.R.; Akutsu, M.; Arnaudo, N.; Ekkebus, R.; Kulathu, Y.; Wauer, T.; El Oualid, F.; et al. OTU deubiquitinases reveal mechanisms of linkage specificity and enable ubiquitin chain restriction analysis. Cell 2013, 154, 169–184. [Google Scholar] [CrossRef]
- Qian, W.; Li, Q.; Wu, X.; Li, W.; Li, Q.; Zhang, J.; Li, M.; Zhang, D.; Zhao, H.; Zou, X.; et al. Deubiquitinase USP29 promotes gastric cancer cell migration by cooperating with phosphatase SCP1 to stabilize Snail protein. Oncogene 2020, 39, 6802–6815. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Liu, J.; Peng, Y.; Zhang, J.; Dai, X.; Zhang, S.; Wang, Y.; Liu, J.; Long, J. Deubiquitinase OTUD6A promotes proliferation of cancer cells via regulating Drp1 stability and mitochondrial fission. Mol. Oncol. 2020, 14, 3169–3183. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Lin, H.; Zhang, Z. CKS2 in human cancers: Clinical roles and current perspectives (Review). Mol. Clin. Oncol. 2015, 3, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Solomon, L.R.; Pereda-Lopez, A.; Giranda, V.L.; Luo, Y.; Johnson, E.F.; Shoemaker, A.R.; Leverson, J.; Liu, X. Ubiquitin-specific cysteine protease 2a (USP2a) regulates the stability of Aurora-A. J. Biol. Chem. 2011, 286, 38960–38968. [Google Scholar] [CrossRef] [PubMed]
- Willems, E.; Dedobbeleer, M.; Digregorio, M.; Lombard, A.; Lumapat, P.N.; Rogister, B. The functional diversity of Aurora kinases: A comprehensive review. Cell Div. 2018, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, W.; Macurek, L.; Freire, R.; Lindqvist, A.; Medema, R.H. Bora and Aurora-A continue to activate Plk1 in mitosis. J. Cell Sci. 2014, 127 Pt 4, 801–811. [Google Scholar] [CrossRef]
- Macurek, L.; Lindqvist, A.; Lim, D.; Lampson, M.A.; Klompmaker, R.; Freire, R.; Clouin, C.; Taylor, S.S.; Yaffe, M.B.; Medema, R.H. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature 2008, 455, 119–123. [Google Scholar] [CrossRef]
- Wang, P.; Gong, Y.; Guo, T.; Li, M.; Fang, L.; Yin, S.; Kamran, M.; Liu, Y.; Xu, J.; Xu, L.; et al. Activation of Aurora A kinase increases YAP stability via blockage of autophagy. Cell Death Dis. 2019, 10, 432. [Google Scholar] [CrossRef]
- Chuang, L.S.; Khor, J.M.; Lai, S.K.; Garg, S.; Krishnan, V.; Koh, C.G.; Lee, S.H.; Ito, Y. Aurora kinase-induced phosphorylation excludes transcription factor RUNX from the chromatin to facilitate proper mitotic progression. Proc. Natl. Acad. Sci. USA 2016, 113, 6490–6495. [Google Scholar] [CrossRef]
- Kim, S.R.; Kim, K.B.; Chae, Y.C.; Park, J.W.; Seo, S.B. H3S10 phosphorylation-mediated transcriptional regulation by Aurora kinase A. Biochem. Biophys. Res. Commun. 2016, 469, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Joukov, V.; De Nicolo, A. Aurora-PLK1 cascades as key signaling modules in the regulation of mitosis. Sci. Signal. 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Huang, C.; Liu, K.; Li, X.; Dong, Z. Targeting AURKA in Cancer: Molecular mechanisms and opportunities for Cancer therapy. Mol. Cancer 2021, 20, 15. [Google Scholar] [CrossRef]
- Hsueh, K.W.; Fu, S.L.; Chang, C.B.; Chang, Y.L.; Lin, C.H. A novel Aurora-A-mediated phosphorylation of p53 inhibits its interaction with MDM2. Biochim. Biophys. Acta 2013, 1834, 508–515. [Google Scholar] [CrossRef]
- Katayama, H.; Sasai, K.; Kawai, H.; Yuan, Z.M.; Bondaruk, J.; Suzuki, F.; Fujii, S.; Arlinghaus, R.B.; Czerniak, B.A.; Sen, S. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat. Genet. 2004, 36, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kaneko, S.; Yang, L.; Feldman, R.I.; Nicosia, S.V.; Chen, J.; Cheng, J.Q. Aurora-A abrogation of p53 DNA binding and transactivation activity by phosphorylation of serine 215. J. Biol. Chem. 2004, 279, 52175–52182. [Google Scholar] [CrossRef]
- Wang, J.; Nikhil, K.; Viccaro, K.; Chang, L.; Jacobsen, M.; Sandusky, G.; Shah, K. The Aurora-A-Twist1 axis promotes highly aggressive phenotypes in pancreatic carcinoma. J. Cell Sci. 2017, 130, 1078–1093. [Google Scholar] [CrossRef]
- Jin, S.; Wang, X.; Tong, T.; Zhang, D.; Shi, J.; Chen, J.; Zhan, Q. Aurora-A enhances malignant development of esophageal squamous cell carcinoma (ESCC) by phosphorylating beta-catenin. Mol. Oncol. 2015, 9, 249–259. [Google Scholar] [CrossRef]
- Zheng, X.Q.; Guo, J.P.; Yang, H.; Kanai, M.; He, L.L.; Li, Y.Y.; Koomen, J.M.; Minton, S.; Gao, M.; Ren, X.B.; et al. Aurora-A is a determinant of tamoxifen sensitivity through phosphorylation of ERalpha in breast cancer. Oncogene 2014, 33, 4985–4996. [Google Scholar] [CrossRef]
- Gao, F.; Li, C.; Zhao, X.; Xie, J.; Fang, G.; Li, Y. CKS2 modulates cell-cycle progression of tongue squamous cell carcinoma cells partly via modulating the cellular distribution of DUTPase. J. Oral Pathol. Med. 2020. [Google Scholar] [CrossRef]
- Huang, N.; Wu, Z.; Hong, H.; Wang, X.; Yang, F.; Li, H. Overexpression of CKS2 is associated with a poor prognosis and promotes cell proliferation and invasion in breast cancer. Mol. Med. Rep. 2019, 19, 4761–4769. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.H.; Wang, Y.; Xu, D. CKS2 promotes tumor progression and metastasis and is an independent predictor of poor prognosis in epithelial ovarian cancer. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3225–3234. [Google Scholar] [PubMed]
- Zhang, J.; Song, Q.; Liu, J.; Lu, L.; Xu, Y.; Zheng, W. Cyclin-Dependent Kinase Regulatory Subunit 2 Indicated Poor Prognosis and Facilitated Aggressive Phenotype of Hepatocellular Carcinoma. Dis. Markers 2019, 2019, 8964015. [Google Scholar] [CrossRef]
- Zhang, X.M.; Wang, J.; Liu, Z.L.; Liu, H.; Cheng, Y.F.; Wang, T. LINC00657/miR-26a-5p/CKS2 ceRNA network promotes the growth of esophageal cancer cells via the MDM2/p53/Bcl2/Bax pathway. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef]
- Lian, I.; Kim, J.; Okazawa, H.; Zhao, J.; Zhao, B.; Yu, J.; Chinnaiyan, A.; Israel, M.A.; Goldstein, L.S.; Abujarour, R.; et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010, 24, 1106–1118. [Google Scholar] [CrossRef]
- Tanaka, M.; Ueda, A.; Kanamori, H.; Ideguchi, H.; Yang, J.; Kitajima, S.; Ishigatsubo, Y. Cell-cycle-dependent regulation of human aurora A transcription is mediated by periodic repression of E4TF1. J. Biol. Chem. 2002, 277, 10719–10726. [Google Scholar] [CrossRef]
- Tang, J.; Yang, L.; Li, Y.; Ning, X.; Chaulagain, A.; Wang, T.; Wang, D. ARID3A promotes the development of colorectal cancer by upregulating AURKA. Carcinogenesis 2020. [Google Scholar] [CrossRef]
- Yang, N.; Wang, C.; Wang, Z.; Zona, S.; Lin, S.X.; Wang, X.; Yan, M.; Zheng, F.M.; Li, S.S.; Xu, B.; et al. FOXM1 recruits nuclear Aurora kinase A to participate in a positive feedback loop essential for the self-renewal of breast cancer stem cells. Oncogene 2017, 36, 3428–3440. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Sun, Y.; She, X.; Wang, Z.; Chen, S.; Deng, Z.; Zhang, Y.; Liu, Q.; Liu, Q.; Zhao, C.; et al. SIX3, a tumor suppressor, inhibits astrocytoma tumorigenesis by transcriptional repression of AURKA/B. J. Hematol. Oncol. 2017, 10, 115. [Google Scholar] [CrossRef]
- Nikhil, K.; Raza, A.; Haymour, H.S.; Flueckiger, B.V.; Chu, J.; Shah, K. Aurora Kinase A-YBX1 Synergy Fuels Aggressive Oncogenic Phenotypes and Chemoresistance in Castration-Resistant Prostate Cancer. Cancers 2020, 12, 660. [Google Scholar] [CrossRef]
- Wang, J.; Nikhil, K.; Viccaro, K.; Chang, L.; White, J.; Shah, K. Phosphorylation-dependent regulation of ALDH1A1 by Aurora kinase A: Insights on their synergistic relationship in pancreatic cancer. BMC Biol. 2017, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lee, S.J.; Kwon, Y.; Ma, L.; Kim, J. Tumor suppressive function of Matrin 3 in the basal-like breast cancer. Biol. Res. 2020, 53, 42. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).