Abscopal Effect of Frozen Autograft Reconstruction Combined with an Immune Checkpoint Inhibitor Analyzed Using a Metastatic Bone Tumor Model
Abstract
:1. Introduction
2. Results
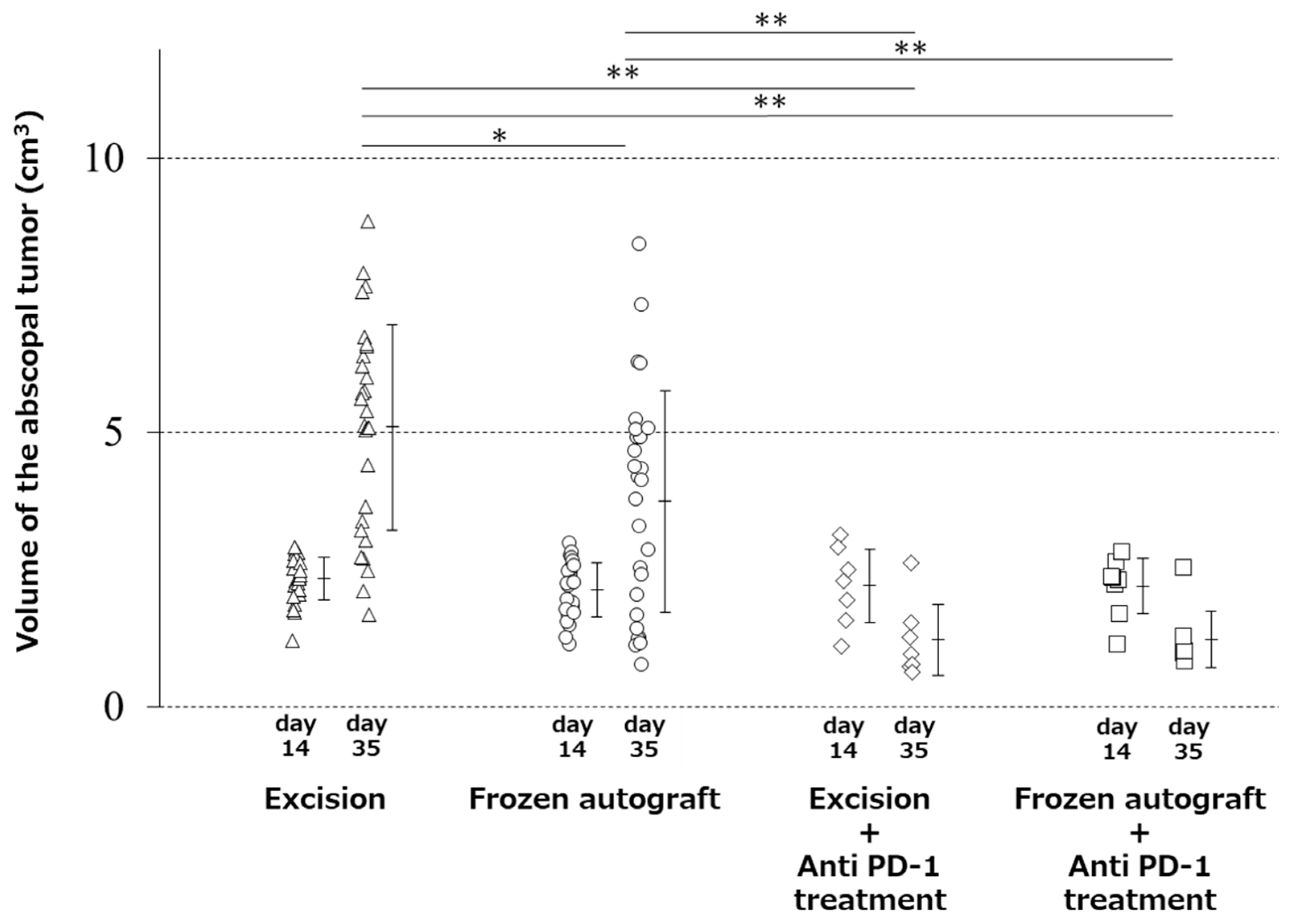
2.1. Abscopal Tumor Growth
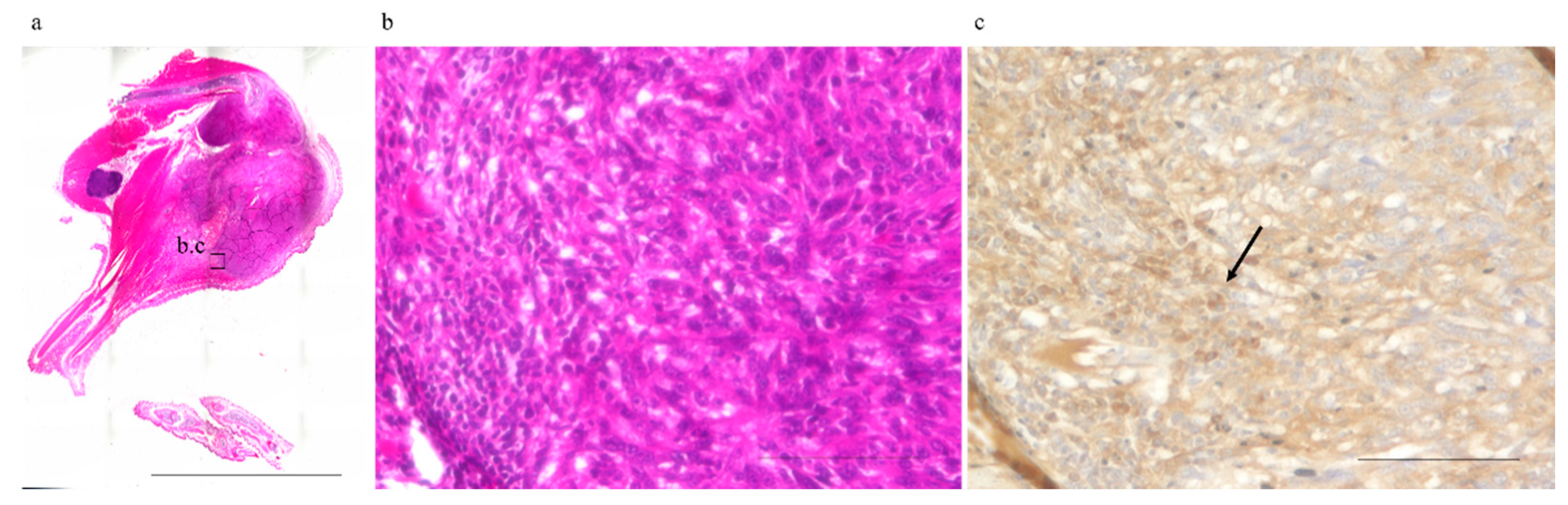
2.2. Abscopal Tumors from Anti-PD-1-Treated Mice Were Infiltrated by CD8+ T-Cells
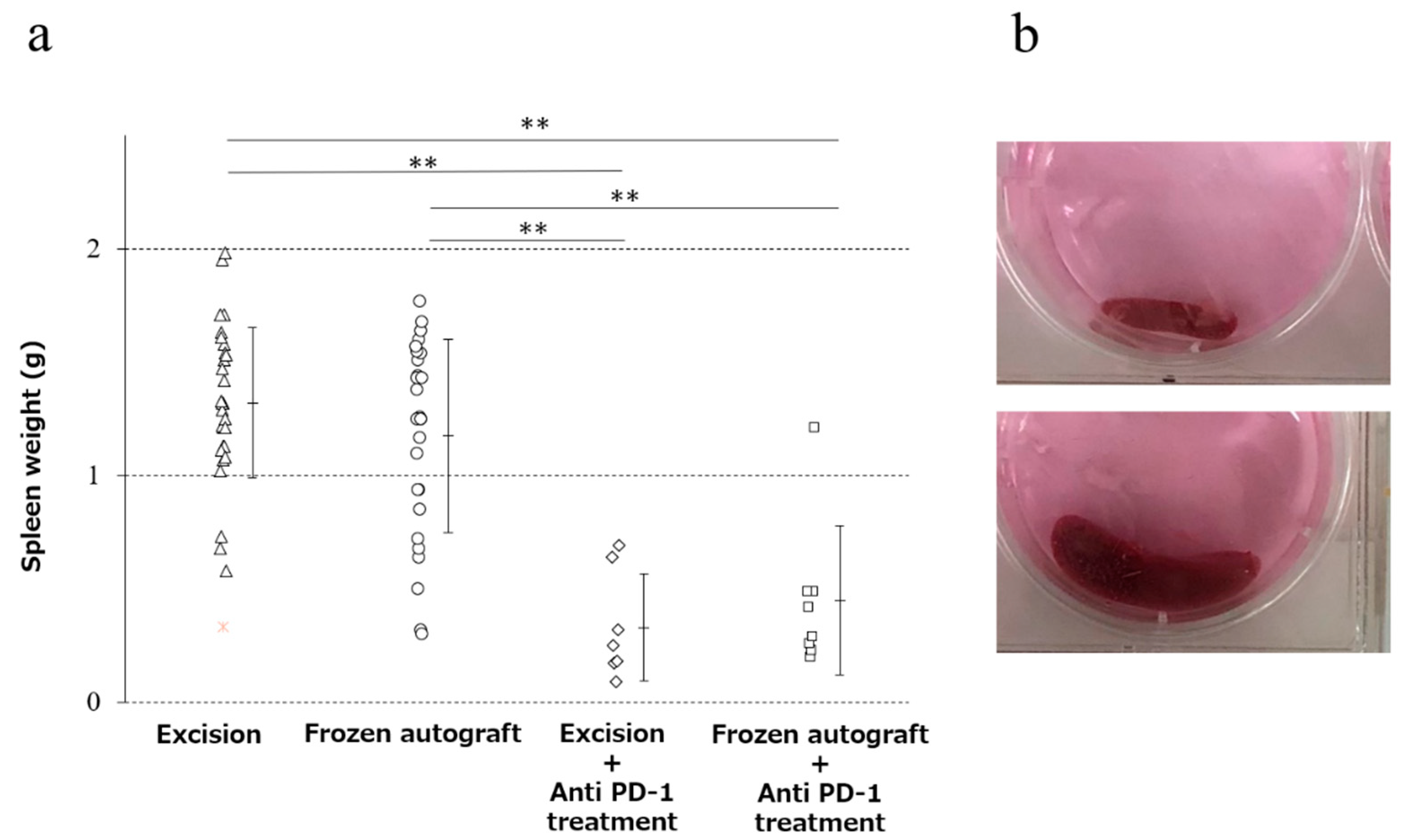
2.3. Anti-PD-1 Treatment Prevented Splenomegaly, and Cryotreatment Alone Did Not Significantly Prevent Splenomegaly
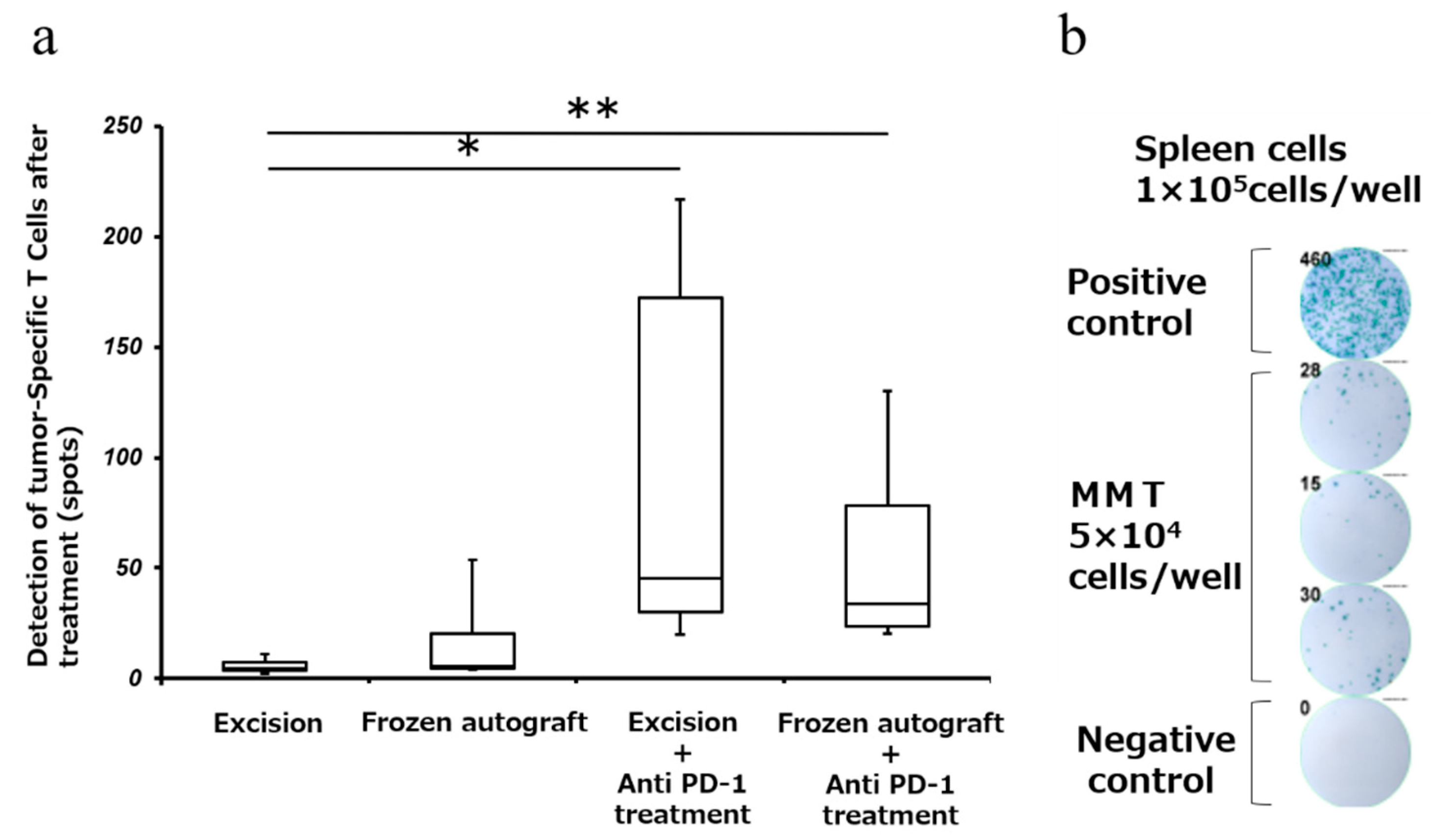
2.4. Anti-PD-1 Treatment Enhanced Tumor-Associated Antigen-Specific T Cell Responses
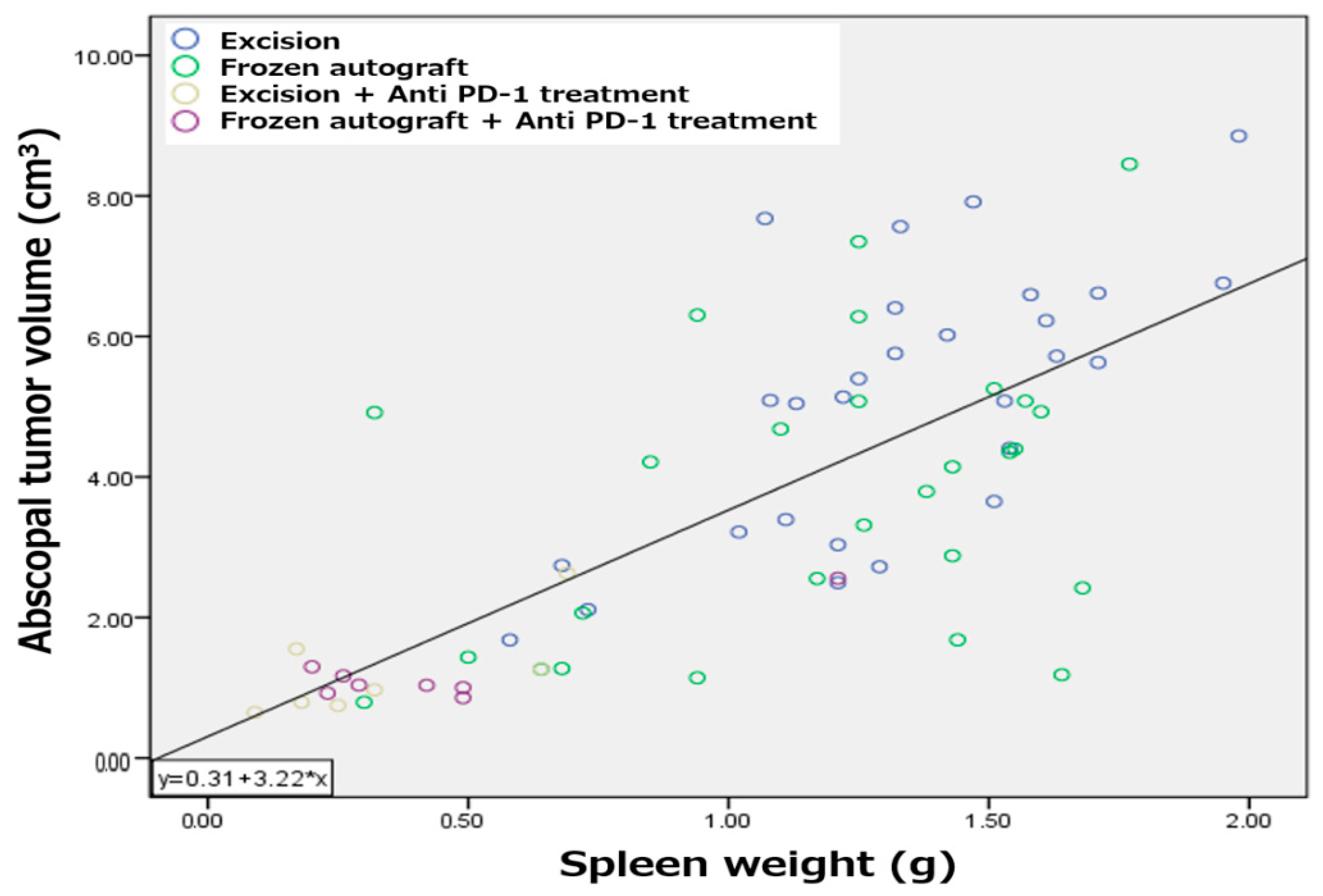
2.5. Splenomegaly Positively Correlated with Abscopal Tumor Development in a Metastatic Bone Tumor Model
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Tumors
4.3. Procedure
4.4. Immune Checkpoint Inhibitor for Adjuvant Treatment
4.5. Evaluation of Abscopal Effect
4.6. Infiltrating CD8+ T-Cells Surrounding the Abscopal Tumors
4.7. Evaluation of Spleen Weight
4.8. Tumor-Specific IFN-γELISPOT Assay
4.9. Selected Experimental Endpoints and the Large Tumor Volumes in This Study: Statement
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yantorno, C.; Soanes, W.A.; Gonder, M.J.; Shulman, S. Studies in cryo-immunology. I. The production of antibodies to uro-genital tissue in consequence of freezing treatment. Immunology 1967, 12, 395–410. [Google Scholar] [PubMed]
- Shulman, S.; Brandt, E.J.; Yantorno, C. Studies in cryo-immunology. II. Tissue and species specificity of the autoantibody response and comparison with iso-immunization. Immunology 1968, 14, 149–158. [Google Scholar]
- Tsuchiya, H.; Wan, S.L.; Sakayama, K.; Yamamoto, N.; Nishida, H.; Tomita, K. Reconstruction using an autograft containing tumour treated by liquid nitrogen. J. Bone Jt. Surg. Br. Vol. 2005, 87, 218–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, H.; Demura, S.; Kato, S.; Nishida, H.; Yoshioka, K.; Hayashi, H.; Inoue, K.; Ota, T.; Shinmura, K.; Yokogawa, N.; et al. Increase of IL-12 following Reconstruction for Total En Bloc Spondylectomy Using Frozen Autografts Treated with Liquid Nitrogen. PLoS ONE 2013, 8, e64818. [Google Scholar] [CrossRef]
- Murakami, H.; Kato, S.; Ueda, Y.; Fujimaki, Y.; Tsuchiya, H. Reconstruction using a frozen tumor-bearing vertebra in total en bloc spondylectomy can enhance antitumor immunity. Eur. Spine J. 2013, 23, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Tsuchiya, H.; Tomita, K. Effects of liquid nitrogen treatment on the proliferation of osteosarcoma and the biomechanical properties of normal bone. J. Orthop. Sci. 2003, 8, 374–380. [Google Scholar] [CrossRef]
- Nishida, H.; Tsuchiya, H.; Tomita, K. Re-implantation of tumour tissue treated by cryotreatment with liquid nitrogen in-duces anti-tumour activity against murine osteosarcoma. J. Bone Jt. Surg. Br. 2008, 90, 1249–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Chen, G.; Liu, M.; Xu, Z.; Chen, H.; Yang, L.; Lv, Y. Scaffold strategies for modulating immune microenvironment during bone regeneration. Mater. Sci. Eng. C 2020, 108, 110411. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, X.; Wei, Y.; Zhang, X.; Meng, S.; Mo, X.; Liu, X.; Deng, X.; Zhang, L.; Deng, X. Attenuating Immune Response of Macro-phage by Enhancing Hydrophilicity of Ti Surface. J. Nanomater. 2015, 2015, 712810. [Google Scholar] [CrossRef] [Green Version]
- Lang, N.P.; Salvi, G.E.; Huynh-Ba, G.; Ivanovski, S.; Donos, N.; Bosshardt, D.D. Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin. Oral Implant. Res. 2011, 22, 349–356. [Google Scholar] [CrossRef]
- Shinmura, K.; Murakami, H.; Demura, S.; Kato, S.; Yoshioka, K.; Hayashi, H.; Inoue, K.; Ota, T.; Yokogawa, N.; Ishii, T.; et al. A histological examination of spinal reconstruction using a frozen bone autograft. PLoS ONE 2018, 13, e0191679. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic Correlates of the Abscopal Effect in a Patient with Melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demaria, S.; Bhardwaj, N.; McBride, W.H.; Formenti, S.C. Combining radiotherapy and immunotherapy: A revived part-nership. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 655–666. [Google Scholar] [CrossRef] [Green Version]
- DeMaria, S.; Kawashima, N.; Yang, A.M.; Devitt, M.L.; Babb, J.S.; Allison, J.P.; Formenti, S.C. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 2005, 11, 728–734. [Google Scholar]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; DeMaria, S. Fractionated but Not Single-Dose Radiotherapy Induces an Immune-Mediated Abscopal Effect when Combined with Anti–CTLA-4 Antibody. Clin. Cancer Res. 2009, 15, 5379–5388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, J.P.; Cohen-Inbar, O.; Bilsky, M.H.; Gerszten, P.C.; Sheehan, J.P. Stereotactic radiosurgery and immunotherapy for metastatic spinal melanoma. Neurosurg. Focus 2015, 38, E6. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; Guo, Z.; Si, T.; Xing, W.; Yu, W.; Wang, Y. Cryoablation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Oncotarget 2018, 10, 4180–4191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.V.; Patterson, S.G.; Plaza, M.J. Abscopal Effect following Cryoablation of Breast Cancer. J. Vasc. Interv. Radiol. 2019, 30, 466–469. [Google Scholar] [CrossRef]
- Sugita, S.; Murakami, H.; Kato, S.; Tanaka, S.; Tsuchiya, H. Disappearance of lung adenocarcinoma after total en bloc spon-dylectomy using frozen tumor-bearing vertebra for reconstruction. Eur. Spine J. 2016, 25, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Yonezawa, N.; Murakami, H.; Sangsin, A.; Mizukoshi, E.; Tsuchiya, H. Lung metastases regression with increased CD8+ T lymphocyte infiltration following preoperative spinal embolization and total en bloc spondylectomy using tumor-bearing frozen autograft in a patient with spinal metastatic leiomyosarcoma. Eur. Spine J. 2019, 28, 41–50. [Google Scholar] [CrossRef]
- Takahashi, Y.; Izumi, Y.; Matsutani, N.; Dejima, H.; Nakayama, T.; Okamura, R.; Uehara, H.; Kawamura, M. Optimized magnitude of cryosurgery facilitating anti-tumor immunoreaction in a mouse model of Lewis lung cancer. Cancer Immunol. Immunother. 2016, 65, 973–982. [Google Scholar] [CrossRef]
- Baust, J.G.; Gage, A.A. The molecular basis of cryosurgery. Bju Int. 2005, 95, 1187–1191. [Google Scholar] [CrossRef]
- Sabel, M.S. Cryo-immunology: A review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology 2009, 58, 1–11. [Google Scholar] [CrossRef]
- Chen, J.; Qian, W.; Mu, F.; Niu, L.; Du, D.; Xu, K. The future of cryoablation: An abscopal effect. Cryobiology 2020, 97, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Abdo, J.; Cornell, D.L.; Mittal, S.K.; Agrawal, D.K. Immunotherapy Plus Cryotherapy: Potential Augmented Abscopal Effect for Advanced Cancers. Front. Oncol. 2018, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Aarts, B.M.; Klompenhouwer, E.G.; Rice, S.L.; Imani, F.; Baetens, T.; Bex, A.; Horenblas, S.; Kok, M.; Haanen, J.B.A.G.; Beets-Tan, R.G.H.; et al. Cryoablation and immunotherapy: An overview of evidence on its synergy. Insights Imaging 2019, 10, 53. [Google Scholar] [CrossRef]
- Soule, E.; Bandyk, M.; Matteo, J. Percutaneous ablative cryoimmunotherapy for micrometastaic abscopal effect: No compli-cations. Cryobiology 2018, 82, 22–26. [Google Scholar] [CrossRef]
- Benzon, B.; Glavaris, S.A.; Simons, B.W.; Hughes, R.M.; Ghabili, K.; Mullane, P.; Miller, R.; Nugent, K.; Shinder, B.; Tosoian, J.; et al. Combining immune check-point blockade and cryoablation in an immunocompetent hormone sensitive murine model of prostate cancer. Prostate Cancer Prostatic Dis. 2018, 21, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Mu, F.; Zhang, C.; Li, Y.; Liu, W.; Jiang, F.; Li, L.; Liu, C.; Zeng, J.; Yao, F.; et al. Cryotherapy protocols for metastatic breast cancer after failure of radical surgery. Cryobiology 2013, 67, 17–22. [Google Scholar] [CrossRef]
- Li, F.; Guo, Z.; Yu, H.; Zhang, X.; Si, T.; Liu, C.; Yang, X.; Qi, L. Anti-tumor immunological response induced by cryoablation and anti-CTLA-4 antibody in an in vivo RM-1 cell prostate cancer murine model. Neoplasma 2014, 61, 659–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waitz, R.; Solomon, S.B.; Petre, E.N.; Trumble, A.E.; Fassò, M.; Norton, L.; Allison, J.P. Potent Induction of Tumor Immunity by Combining Tumor Cryoablation with Anti–CTLA-4 Therapy. Cancer Res. 2012, 72, 430–439. [Google Scholar] [CrossRef] [Green Version]
- Hodgson, A.; Wier, E.M.; Fu, K.; Sun, X.; Wan, F. Ultrasound imaging of splenomegaly as a proxy to monitor colon tumor development in Apcmin716/+mice. Cancer Med. 2016, 5, 2469–2476. [Google Scholar] [CrossRef] [PubMed]
- Joosten, J.; Muijen, G.V.; Wobbes, T.; Ruers, T. In Vivo Destruction of Tumor Tissue by Cryoablation Can Induce Inhibition of Secondary Tumor Growth: An Experimental Study. Cryobiology 2001, 42, 49–58. [Google Scholar] [CrossRef]
- Sabel, M.S.; Arora, A.; Su, G.; Chang, A.E. Adoptive immunotherapy of breast cancer with lymph node cells primed by cry-oablation of the primary tumor. Cryobiology 2006, 53, 360–366. [Google Scholar] [CrossRef]
- Sabel, M.S.; Nehs, M.A.; Su, G.; Lowler, K.P.; Ferrara, J.L.; Chang, A.E. Immunologic response to cryoablation of breast cancer. Breast Cancer Res. Treat. 2005, 90, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urano, M.; Tanaka, C.; Sugiyama, Y.; Miya, K.; Saji, S. Antitumor effects of residual tumor after cryoablation: The combined effect of residual tumor and a protein-bound polysaccharide on multiple liver metastases in a murine model. Cryobiology 2003, 46, 238–245. [Google Scholar] [CrossRef]
- Hoffmann, N.E.; Coad, J.E.; Huot, C.S.; Swanlund, D.J.; Bischof, J.C. Investigation of the mechanism and the effect of cryo-immunology in the copenhagen rat. Cryobiology 2001, 42, 59–68. [Google Scholar] [CrossRef]
- Udagawa, M.; Kudo-Saito, C.; Hasegawa, G.; Yano, K.; Yamamoto, A.; Yaguchi, M.; Toda, M.; Azuma, I.; Iwai, T.; Kawakami, Y. Enhancement of Immunologic Tumor Regression by Intratumoral Administration of Dendritic Cells in Combination with Cryoablative Tumor Pretreatment and Bacillus Calmette-Guerin Cell Wall Skeleton Stimulation. Clin. Cancer Res. 2006, 12, 7465–7475. [Google Scholar] [CrossRef] [Green Version]
- Friedman, E.J.; Orth, C.R.; Brewton, K.A.; Ponniah, S.; Alexander, R.B. Cryosurgical ablation of the normal ventral prostate plus adjuvant does not protect Copenhagen rats from dunning prostatic adenocarcinoma challenge. J. Urol. 1997, 158, 1585–1588. [Google Scholar] [CrossRef]
- Hayakawa, K.; Yamashita, T.; Suzuki, K.; Tomita, K.; Hosokawa, M.; Kodama, T.; Kobayashi, H. Comparative immunologi-cal studies in rats following cryosurgery and surgical excision of 3-methylcholanthrene-induced primary autochthonous tumors. Gan 1982, 73, 462–469. [Google Scholar]
- Nakamoto, Y.; Mizukoshi, E.; Kitahara, M.; Arihara, F.; Sakai, Y.; Kakinoki, K.; Fujita, Y.; Marukawa, Y.; Arai, K.; Yamashita, T.; et al. Prolonged recurrence-free survival following OK432-stimulated dendritic cell transfer into hepatocellular carcinoma during transarterial embolization. Clin. Exp. Immunol. 2010, 163, 165–177. [Google Scholar] [CrossRef]
- Marconi, R.; Strolin, S.; Bossi, G.; Strigari, L. A meta-analysis of the abscopal effect in preclinical models: Is the biologically effective dose a relevant physical trigger? PLoS ONE 2017, 12, e0171559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.-X. Irradiation and anti–PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, K.; Ishiwata, Y.; Nakagawa, K.; Yokochi, S.; Taruki, C.; Akuta, T.; Ohtomo, K.; Matsushima, K.; Tamatani, T.; Kanegasaki, S. Enhancement of Antitumor Radiation Efficacy and Consistent Induction of the Abscopal Effect in Mice by ECI301, an Active Variant of Macrophage Inflammatory Protein-1α. Clin. Cancer Res. 2008, 14, 1159–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, W.; Wolchok, J.D.; Chen, L.; Weiping, Z.; Jedd, D.W.; Lieping, C. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy. Sci. Transl. Med. 2016, 8, 1–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutzky, J. Checkpoint inhibitors in the treatment of cutaneous malignant melanoma. Chin. Clin. Oncol. 2014, 3, 1–12. [Google Scholar]
- Doyle, C. Combination Immunotherapy Superior to Monotherapy in Patients with Melanoma. Am. Health Drug Benefits 2015, 8, 41. [Google Scholar]
- Cen, O.; Kannan, K.; Huck Sappal, J.; Yu, J.; Zhang, M.; Arikan, M.; Ucur, A.; Ustek, D.; Cen, Y.; Gordon, L.; et al. Spleen Ty-rosine Kinase Inhibitor TAK-659 Prevents Splenomegaly and Tumor Development in a Murine Model of Epstein-Barr Vi-rus-Associated Lymphoma. mSphere 2018, 22, e00378-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dargart, J.L.; Fish, K.; Gordon, L.I.; Longnecker, R.; Cen, O. Dasatinib therapy results in decreased B cell proliferation, splenomegaly, and tumor growth in a murine model of lymphoma expressing Myc and Epstein-Barr virus LMP2A. Antivir. Res. 2012, 95, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, W.-C.; Lin, S.-Y.; Lan, C.-W.; Huang, Y.-C.; Lin, C.-Y.; Hsiao, P.-W.; Chen, Y.-R.; Yang, W.-C.; Yang, N.-S. Inhibiting MDSC differentiation from bone marrow with phytochemical polyacetylenes drastically impairs tumor metastasis. Sci. Rep. 2016, 6, 36663. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, H.; Minato, N.; Nakano, T.; Honjo, T. Immunological studies on PD-1 deficient mice: Implication of PD-1 as a negative regulator for B cell responses. Int. Immunol. 1998, 10, 1563–1572. [Google Scholar] [CrossRef] [Green Version]
- Schaue, D.; Ratikan, J.A.; Iwamoto, K.S.; McBride, W.H. Maximizing Tumor Immunity with Fractionated Radiation. Int. J. Radiat. Oncol. 2012, 83, 1306–1310. [Google Scholar] [CrossRef] [Green Version]
- Matsumine, A.; Kusuzaki, K.; Matsubara, T.; Shintani, K.; Satonaka, H.; Wakabayashi, T.; Miyazaki, S.; Morita, K.; Takegami, K.; Uchida, A. Novel hyperthermia for metastatic bone tumors with magnetic materials by generating an alternating elec-tromagnetic field. Clin. Exp. Metastasis 2007, 24, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Lodi, M.B.; Fanti, A.; Muntoni, G.; Mazzarella, G. A Multiphysic Model for the Hyperthermia Treatment of Residual Osteo-sarcoma Cells in Upper Limbs Using Magnetic Scaffolds. IEEE J. Multiscale Multiphys. Comput. Tech. 2019, 4, 337–347. [Google Scholar] [CrossRef]
- Akatsu, T.; Ono, K.; Katayama, Y.; Tamura, T.; Nishikawa, M.; Kugai, N.; Yamamoto, M.; Nagata, N. The Mouse Mammary Tumor Cell Line, MMT060562, Produces Prostaglandin E2 and Leukemia Inhibitory Factor and Supports Osteoclast Formation In Vitro Via a Stromal Cell-Dependent Pathway. J. Bone Min. Res. 1998, 13, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Akatsu, T.; Murakami, T.; Wada, S.; Nishikawa, M.; Kugai, N.; Yamamoto, M.; Matsuura, N.; Nagata, N. Mouse Mammary Carcinoma Cell Line (BALB/c-MC) Stimulates Osteoclast Formation From Mouse Bone Marrow Cells Through Cell-to-Cell Contact. Bone 1998, 23, 27–32. [Google Scholar] [CrossRef]
- Carlsson, G.; Gullberg, B. Estimation of liver tumor volume using different formulas?An experimental study in rats. J. Cancer Res. Clin. Oncol. 1983, 105, 20–23. [Google Scholar] [CrossRef] [PubMed]


| Group | Number of CD8+ T-Cells Infiltrating the Abscopal Tumor |
|---|---|
| Excision | 13.9 ± 11.0 |
| Frozen autograft | 18.8 ± 15.2 |
| Excision plus anti-PD-1 treatment | 40.9 ± 25.5 * |
| Frozen autograft plus anti-PD-1 treatment | 45.7 ± 21.0 **,# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yonezawa, N.; Murakami, H.; Demura, S.; Kato, S.; Miwa, S.; Yoshioka, K.; Shinmura, K.; Yokogawa, N.; Shimizu, T.; Oku, N.; et al. Abscopal Effect of Frozen Autograft Reconstruction Combined with an Immune Checkpoint Inhibitor Analyzed Using a Metastatic Bone Tumor Model. Int. J. Mol. Sci. 2021, 22, 1973. https://doi.org/10.3390/ijms22041973
Yonezawa N, Murakami H, Demura S, Kato S, Miwa S, Yoshioka K, Shinmura K, Yokogawa N, Shimizu T, Oku N, et al. Abscopal Effect of Frozen Autograft Reconstruction Combined with an Immune Checkpoint Inhibitor Analyzed Using a Metastatic Bone Tumor Model. International Journal of Molecular Sciences. 2021; 22(4):1973. https://doi.org/10.3390/ijms22041973
Chicago/Turabian StyleYonezawa, Noritaka, Hideki Murakami, Satoru Demura, Satoshi Kato, Shinji Miwa, Katsuhito Yoshioka, Kazuya Shinmura, Noriaki Yokogawa, Takaki Shimizu, Norihiro Oku, and et al. 2021. "Abscopal Effect of Frozen Autograft Reconstruction Combined with an Immune Checkpoint Inhibitor Analyzed Using a Metastatic Bone Tumor Model" International Journal of Molecular Sciences 22, no. 4: 1973. https://doi.org/10.3390/ijms22041973
APA StyleYonezawa, N., Murakami, H., Demura, S., Kato, S., Miwa, S., Yoshioka, K., Shinmura, K., Yokogawa, N., Shimizu, T., Oku, N., Kitagawa, R., Handa, M., Annen, R., Kurokawa, Y., Fushimi, K., Mizukoshi, E., & Tsuchiya, H. (2021). Abscopal Effect of Frozen Autograft Reconstruction Combined with an Immune Checkpoint Inhibitor Analyzed Using a Metastatic Bone Tumor Model. International Journal of Molecular Sciences, 22(4), 1973. https://doi.org/10.3390/ijms22041973








