Metabolic Requirements for Spermatogonial Stem Cell Establishment and Maintenance In Vivo and In Vitro
Abstract
1. Introduction
2. Development of the Male Gonad and Establishment of Spermatogonial Stem Cells (SSCs)
3. The Current State of Germ Cell Culture
4. Stem Cell Metabolism
4.1. Metabolism during Proliferation
4.2. Redox Homeostasis
4.3. Control of the Epigenetic Landscape
5. Metabolic Regulation within the Testis
5.1. The Metabolic Spermatogonial Stem Cell (SSC) Niche
5.2. The Role of Sertoli Cell Metabolism
6. Metabolism of Male Germ Cells
Regulation of Metabolic Transitions in the Germline
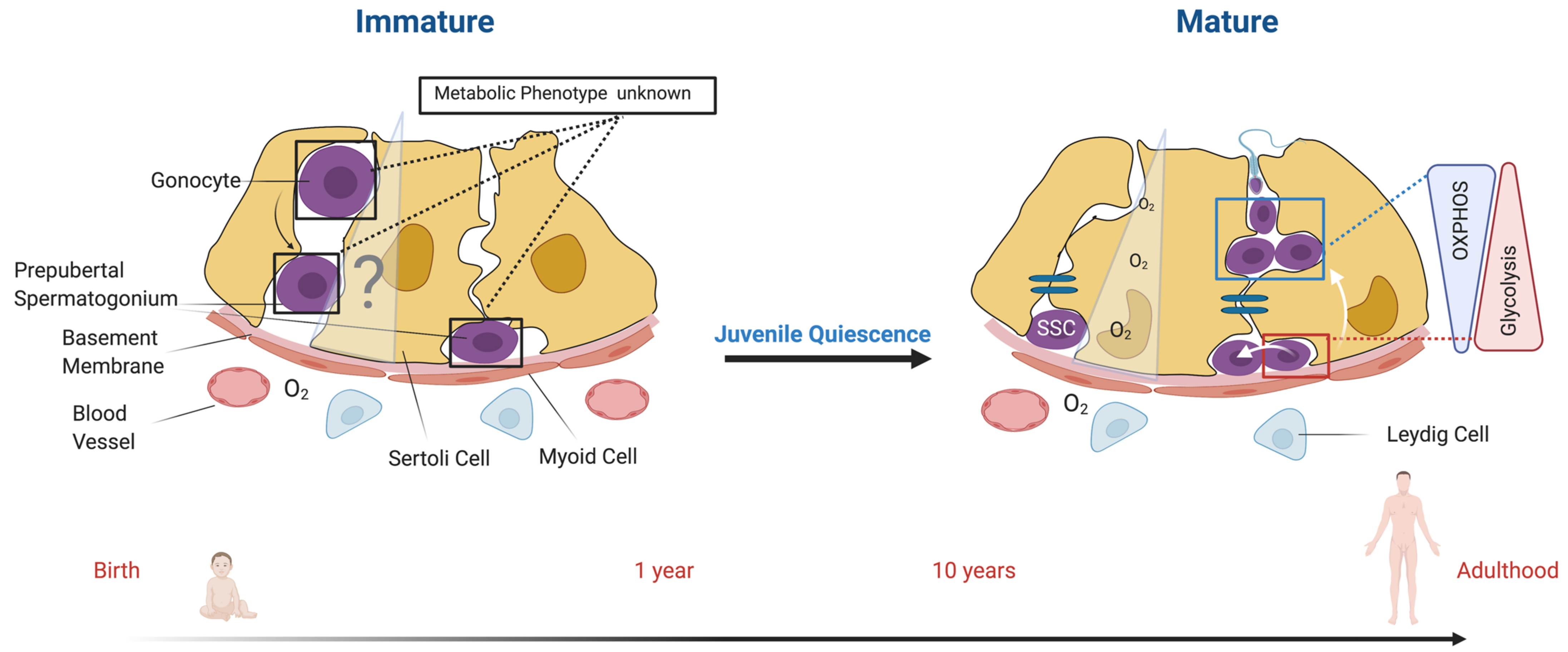
7. Missing Information and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brinster, R.L.; Zimmermann, J.W. Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. USA 1994, 91, 11298–11302. [Google Scholar] [CrossRef]
- Brinster, R.L.; Avarbock, M.R. Germline transmission of donor haplotype following spermatogonial transplantation. Proc. Natl. Acad. Sci. USA 1994, 91, 11303–11307. [Google Scholar] [CrossRef]
- Green, D.M.; Zhu, L.; Wang, M.; Chemaitilly, W.; Srivastava, D.K.; Kutteh, W.H.; Ke, R.W.; Sklar, C.A.; Pui, C.H.; Kun, L.E.; et al. Effect of cranial irradiation on sperm concentration of adult survivors of childhood acute lymphoblastic leukemia: A report from the St. Jude Lifetime Cohort Study. Hum. Reprod. 2017, 32, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.; Canada, A.; Stern, C.J. Fertility preservation in adolescents and young adults with cancer. J. Clin. Oncol. 2010, 28, 4831–4841. [Google Scholar] [CrossRef]
- Wallace, W.H.B.; Anderson, R.A.; Irvine, D.S. Fertility preservation for young patients with cancer: Who is at risk and what can be offered? Lancet Oncol. 2005, 6, 209–218. [Google Scholar] [CrossRef]
- Stukenborg, J.B.; Alves-Lopes, J.P.; Kurek, M.; Albalushi, H.; Reda, A.; Keros, V.; Töhönen, V.; Bjarnason, R.; Romerius, P.; Sundin, M.; et al. Spermatogonial quantity in human prepubertal testicular tissue collected for fertility preservation prior to potentially sterilizing therapy. Hum. Reprod. 2018, 33, 1677–1683. [Google Scholar] [CrossRef]
- Poganitsch-Korhonen, M.; Masliukaite, I.; Nurmio, M.; Lähteenmäki, P.; Van Wely, M.; Van Pelt, A.M.M.; Jahnukainen, K.; Stukenborg, J.B. Decreased spermatogonial quantity in prepubertal boys with leukaemia treated with alkylating agents. Leukemia 2017, 31, 1460–1463. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Orwig, K.E. Spermatogonial Stem Cell Culture in Oncofertility. Urol. Clin. North Am. 2020, 47, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Chiquoine, A.D. The Identification, Origin, and Migration of the Primordial Germ Cells in the Mouse Embryo. Anat. Rec. 1954, 118, 135–146. [Google Scholar] [CrossRef]
- Ross, A.J.; Capel, B. Signaling at the crossroads of gonad development. Trends Endocrinol. Metab. 2005, 16, 19–25. [Google Scholar] [CrossRef]
- Bendel-Stenzel, M.; Anderson, R.; Heasman, J.; Wylie, C. The origin and migration of primordial germ cells in the mouse. Cell Dev. Biol. 1998, 9, 393–400. [Google Scholar] [CrossRef]
- McLean, D.J.; Friel, P.J.; Johnston, D.S.; Griswold, M.D. Characterization of Spermatogonial Stem Cell Maturation and Differentiation in Neonatal Mice1. Biol. Reprod. 2003, 69, 2085–2091. [Google Scholar] [CrossRef]
- De Rooij, D.G.; Russell, L.D. All You Wanted to Know About Spermatogonia but Were Afraid to Ask. J. Androl. 2000, 21, 776–798. [Google Scholar]
- Picut, C.A.; Ziejewski, M.K.; Stanislaus, D. Comparative Aspects of Pre- and Postnatal Development of the Male Reproductive System. Birth Defects Res. 2018, 110, 190–227. [Google Scholar] [CrossRef]
- Tagelenbosch, R.A.J.; de Rooij, D.G. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1993, 290, 193–200. [Google Scholar] [CrossRef]
- Wanet, A.; Arnould, T.; Najimi, M.; Renard, P. Connecting Mitochondria, Metabolism, and Stem Cell Fate. Stem Cells Dev. 2015, 24, 1957–1971. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Kim, J.-H.; Chung, M.K.; Hong, Y.J.; Jang, H.S.; Seo, B.J.; Jung, T.H.; Kim, J.S.; Chung, H.-M.; Byun, S.J.; et al. Mitochondrial and metabolic remodeling during reprogramming and differentiation of the reprogrammed cells. Stem Cells Dev. 2015, 24, 1–23. [Google Scholar] [CrossRef]
- Burgess, R.J.; Agathocleous, M.; Morrison, S.J. Metabolic regulation of stem cell function. J. Intern. Med. 2014, 276, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Shyh-Chang, N.; Ng, H.H. The metabolic programming of stem cells. Genes Dev. 2017, 31, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Shyh-Chang, N.; Daley, G.Q.; Cantley, L.C. Stem cell metabolism in tissue development and aging. Development 2013, 140, 2535–2547. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Yamamoto, T.; Toh, H.; Kazuki, Y.; Kazuki, K.; Imoto, J.; Ikeo, K.; Oshima, M.; Shirahige, K.; Iwama, A.; et al. Aging of spermatogonial stem cells by Jnk-mediated glycolysis activation. Proc. Natl. Acad. Sci. USA 2019, 116, 16404–16409. [Google Scholar] [CrossRef]
- Lord, T.; Oatley, J.M. A revised Asingle model to explain stem cell dynamics in the mouse male germline. Reproduction 2017, 154, R55–R64. [Google Scholar] [CrossRef]
- Helsel, A.R.; Oatley, M.J.; Oatley, J.M. Glycolysis Optimized Conditions Enahnce Maintenance of REgenerative Integrity in Mouse Spermatogonial Stem cell during Long- Term Culture. Stem Cell Rep. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.A.; De Franca, L.R. Spermatogenesis and cycle of the seminiferous epithelium. Adv. Exp. Med. Biol. 2008, 636, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.D.; Ren, H.P.; Hikim, I.S.; Schulze, W.; Hikim, A.P.S. A comparative study in twelve mammalian species of volume densities, volumes, and numerical densities of selected testis components, emphasizing those related to the sertoli cell. Am. J. Anat. 1990, 188, 21–30. [Google Scholar] [CrossRef]
- Oatley, J.M.; Brinster, R.L. Spermatogonial Stem Cells. Methods Enzymol. 2006, 419, 259–282. [Google Scholar] [CrossRef]
- Oatley, J.M.; Brinster, R.L. Regulation of Spermatogonial Stem Cell Self-Renewal in Mammals. Annu. Rev. Cell Dev. Biol. 2008, 24, 263–286. [Google Scholar] [CrossRef]
- Oatley, J.M.; Brinster, R.L. The germline stem cell niche unit in mammalian testes. Physiol. Rev. 2012, 92, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.B.; Walker, W.H. The regulation of spermatogenesis by androgens. Semin. Cell Dev. Biol. 2014, 30, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Sharma, M.; Nabeshima, Y.I.; Braun, R.E.; Yoshida, S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science 2010, 328, 62–67. [Google Scholar] [CrossRef]
- Phillips, B.T.; Gassei, K.; Orwig, K.E. Spermatogonial stem cell regulation and spermatogenesis. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1663–1678. [Google Scholar] [CrossRef]
- De Rooij, D.G. The nature and dynamics of spermatogonial stem cells. Development 2017, 144, 3022–3030. [Google Scholar] [CrossRef] [PubMed]
- Saitou, M.; Payer, B.; Lange, U.C.; Erhardt, S.; Barton, S.C.; Surani, M.A. Specification of germ cell fate in mice. Philos. Trans. R. Soc. B Biol. Sci. 2003, 358, 1363–1370. [Google Scholar] [CrossRef]
- Wilhelm, D.; Hiramatsu, R.; Mizusaki, H.; Widjaja, L.; Combes, A.N.; Kanai, Y.; Koopman, P. SOX9 regulates prostaglandin D synthase gene transcription in vivo to ensure testis development. J. Biol. Chem. 2007, 282, 10553–10560. [Google Scholar] [CrossRef]
- Yao, H.H.-C.; Ungewitter, E.; Franco, H.; Capel, B. Establishment of fetal Sertoli cells and their role in testis morphogenesis. In Sertoli Cell Biology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 57–79. ISBN 9780124170476. [Google Scholar]
- Kluin, P.M.; de Rooij, D.G. A comparison between the morphology and cell kinetics of gonocytes and adult type undifferentiated spermatogonia in the mouse. Int. J. Androl. 1981, 4, 475–493. [Google Scholar] [CrossRef]
- Nagano, R.; Tabata, S.; Nakanishi, Y.; Ohsako, S.; Kurohmaru, M.; Hayashi, Y. Reproliferation and relocation of mouse male germ cells (gonocytes) during prespermatogenesis. Anat. Rec. 2000, 258, 210–220. [Google Scholar] [CrossRef]
- Yoshida, S.; Sukeno, M.; Nakagawa, T.; Ohbo, K.; Nagamatsu, G.; Suda, T.; Nabeshima, Y.I. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development 2006, 133, 1495–1505. [Google Scholar] [CrossRef]
- Culty, M. Gonocytes, the forgotten cells of the germ cell lineage. Birth Defects Res. Part C Embryo Today Rev. 2009, 87, 1–26. [Google Scholar] [CrossRef]
- Culty, M. Gonocytes, from the fifties to the present: Is there a reason to change the name? Biol. Reprod. 2013, 89, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Manku, G.; Culty, M. Mammalian gonocyte and spermatogonia differentiation: Recent advances and remaining challenges. Reproduction 2015, 149, R139–R157. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.-E.; Oatley, J.M. Early postnatal interactions between Sertoli and germ cells. Sertoli Cell Biol. 2015, 81–98. [Google Scholar] [CrossRef]
- Law, N.C.; Oatley, J.M. Developmental underpinnings of spermatogonial stem cell establishment. Andrology 2020, 8, 852–861. [Google Scholar] [CrossRef]
- De Rooij, D.G. Stem cells in the testis. Int. J. Exp. Pathol. 1998, 79, 67–80. [Google Scholar] [CrossRef]
- Pui, H.P.; Saga, Y. Gonocytes-to-spermatogonia transition initiates prior to birth in murine testes and it requires FGF signaling. Mech. Dev. 2017, 144, 125–139. [Google Scholar] [CrossRef]
- Hermann, B.P.; Cheng, K.; Singh, A.; Roa-De La Cruz, L.; Mutoji, K.N.; Chen, I.-C.; Gildersleeve, H.; Lehle, J.D.; Mayo, M.; Westernströer, B.; et al. The Mammalian Spermatogenesis Single-Cell Transcriptome, from Spermatogonial Stem Cells to Spermatids. Cell Rep. 2018, 25, 1650–1667.e8. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Wilkinson, M.F. A single-cell view of spermatogonial stem cells. Curr. Opin. Cell Biol. 2020, 67, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Hadžiselimovič, F.; Zivkovic, D. Is the prohibition of hormonal treatment for cryptorchidism, as suggested by the Nordic consensus group, justifiable? Acta Paediatr. Int. J. Paediatr. 2007, 96, 1368. [Google Scholar] [CrossRef]
- Job, J.-C.; Toublanc, J.-E.; Chaussain, J.-L.; Gendrel, D.; Garnier, P.; Roger, M. Endocrine and Immunological Findings in Cryptorchid Infants. Hormones 1988, 30, 167–172. [Google Scholar] [CrossRef]
- Plant, T.M.; Terasawa, E.; Witchel, S.F. Puberty in Non-Human Primates and Man. In Knobil and Neill’s Physiology of Reproduction; Academic Press: Cambridge, MA, USA, 2015; Volume 2, ISBN 9780123977694. [Google Scholar]
- Foster, D.L.; Hileman, S.M. Puberty in the Sheep. In Knobil and Neill’s Physiology of Reproduction; Academic Press: Cambridge, MA, USA, 2015; Volume 2, ISBN 9780123977694. [Google Scholar]
- Huff, D.S.; Hadziselimovic, F.; Snyder, H.M.; Duckett, J.W.; Keating, M.A. Postnatal testicular maldevelopment in unilateral cryptorchidism. J. Urol. 1989, 142, 546–548. [Google Scholar] [CrossRef]
- Hadžiselimovič, F.; Thommen, L.; Girard, J.; Herzog, B. The Significance of Postnatal Gonadotropin Surge for Testicular Development in Normal and Cryptorchid Testes. J. Urol. 1986, 136, 274–276. [Google Scholar] [CrossRef]
- Drumond, A.L.; Meistrich, M.L.; Chiarini-Garcia, H. Spermatogonial morphology and kinetics during testis development in mice: A high-resolution light microscopy approach. Reproduction 2011, 142, 145–155. [Google Scholar] [CrossRef]
- Huff, D.S.; Snyder III, H.M.; Rusnack, S.L.; Zderic, S.A.; Carr, M.C.; Canning, D.A. Hormonal Therapy for the Subfertility of Cryptorchidism. Horm. Res. Paediatr. 2001, 55, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef]
- Asada, S.; Daitoku, H.; Matsuzaki, H.; Saito, T.; Sudo, T.; Mukai, H.; Iwashita, S.; Kako, K.; Kishi, T.; Kasuya, Y.; et al. Mitogen-activated protein kinases, Erk and p38, phosphorylate and regulate Foxo1. Cell. Signal. 2007, 19, 519–527. [Google Scholar] [CrossRef]
- Goertz, M.J.; Wu, Z.; Gallardo, T.D.; Hamra, F.K.; Castrillon, D.H. Foxo1 is required in mouse spermatogonial stem cells for their maintenance and the initiation of spermatogenesis. J. Clin. Invest. 2011, 121, 3456–3466. [Google Scholar] [CrossRef]
- Guo, J.; Sosa, E.; Chitiashvili, T.; Nie, X.; Rojas, E.J.; Oliver, E.; Connect, D.; Plath, K.; Hotaling, J.M.; Stukenborg, J.-B.; et al. Single-cell analysis of the developing human testis reveals somatic niche cell specification and fetal germline stem cell establishment. Cell Stem 2021, 28, 1–15. [Google Scholar] [CrossRef]
- Paniagua, R.; Nistal, M. Morphological and histometric study of human spermatogonia from birth to the onset of puberty. J. Anat. 1984, 139, 535–552. [Google Scholar]
- Guo, J.; Grow, E.J.; Mlcochova, H.; Maher, G.J.; Lindskog, C.; Nie, X.; Guo, Y.; Takei, Y.; Yun, J.; Cai, L.; et al. The adult human testis transcriptional cell atlas. Cell Res. 2018, 28, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Sohni, A.; Tan, K.; Song, H.W.; Burow, D.; de Rooij, D.G.; Laurent, L.; Hsieh, T.C.; Rabah, R.; Hammoud, S.S.; Vicini, E.; et al. The Neonatal and Adult Human Testis Defined at the Single-Cell Level. Cell Rep. 2019, 26, 1501–1517.e4. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Diaz, V.D.; Hermann, B.P. What has single-cell RNA-seq taught us about mammalian spermatogenesis? Biol. Reprod. 2019, 101, 617–634. [Google Scholar] [CrossRef] [PubMed]
- Voigt, A.L.; Kondro, D.A.; Powell, D.; Valli-Pulaski, H.; Ungrin, M.; Stukenborg, J.-B.; Klein, C.; Lewis, I.A.; Orwig, K.E.; Dobrinski, I. Unique Metabolic Phenotype and its Transition during Maturation of Juvenile Male Germ Cells. FASEB J. 2021. in revision. [Google Scholar]
- Valli, H.; Phillips, B.T.; Orwig, K.E.; Gassei, K.; Nagano, M.C. Spermatogonial stem cells and spermatogenesis. Knobil Neill’s Physiol. Reprod. 2015, 1, 595–635. [Google Scholar]
- Chiarini-Garcia, H.; Hornick, J.R.; Griswold, M.D.; Russell, L.D. Distribution of type A spermatogonia in the mouse is not random. Biol. Reprod. 2001, 65, 1179–1185. [Google Scholar] [CrossRef]
- Chiarini-Garcia, H.; Raymer, A.M.; Russell, L.D. Non-random distribution of spermatogonia in rats: Evidence of niches in the seminiferous tubules. Reproduction 2003, 126, 669–680. [Google Scholar] [CrossRef]
- Yoshida, S.; Sukeno, M.; Nabeshima, Y.I. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science 2007, 317, 1722–1726. [Google Scholar] [CrossRef]
- Do Nascimento, H.F.; Drumond, A.L.; De França, L.R.; Chiarini-Garcia, H. Spermatogonial morphology, kinetics and niches in hamsters exposed to Short- and long-photoperiod. Int. J. Androl. 2009, 32, 486–497. [Google Scholar] [CrossRef]
- Costa, G.M.J.; Avelar, G.F.; Rezende-Neto, J.V.; Campos-Junior, P.H.A.; Lacerda, S.M.S.N.; Andrade, B.S.C.; Thomé, R.G.; Hofmann, M.C.; Franca, L.R. Spermatogonial Stem Cell Markers and Niche in Equids. PLoS ONE 2012, 7, 1–13. [Google Scholar] [CrossRef]
- Campos-Junior, P.H.A.; Costa, G.M.J.; Lacerda, S.M.S.N.; Rezende-Neto, J.V.; de Paula, A.M.; Hofmann, M.-C.; de França, L.R. The Spermatogonial Stem Cell Niche in the Collared Peccary (Tayassu tajacu). Biol. Reprod. 2012, 86, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.-C. Gdnf signaling pathways within the mammalian spermatognial stem cell niche. Mol. Cell. Endocrinol. 2008, 288, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wan, P.; Wang, M.; Zhang, J.; Gao, X.; Hu, B.; Han, J.; Chen, L.; Sun, K.; Wu, J.; et al. AIP1-mediated actin disassembly is required for postnatal germ cell migration and spermatogonial stem cell niche establishment. Cell Death Dis. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Caires, K.; Broady, J.; McLean, D. Maintaining the male germline: Regulation of spermatogonial stem cells. J. Endocrinol. 2010, 205, 133–145. [Google Scholar] [CrossRef] [PubMed]
- DeFalco, T.; Potter, S.J.; Williams, A.V.; Waller, B.; Kan, M.J.; Capel, B. Macrophages Contribute to the Spermatogonial Niche in the Adult Testis. Cell Rep. 2015, 12, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Bhang, D.H.; Kim, B.J.; Kim, B.G.; Schadler, K.; Baek, K.H.; Kim, Y.H.; Hsiao, W.; Ding, B.S.; Rafii, S.; Weiss, M.J.; et al. Testicular endothelial cells are a critical population in the germline stem cell niche. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- Heinrich, A.; DeFalco, T. Essential roles of interstitial cells in testicular development and function. Andrology 2020, 8, 903–914. [Google Scholar] [CrossRef]
- Huckins, C. The Spermatogonial Stem Cell Population in Adult Rats. Anat. Rec. 1971, 169, 533–557. [Google Scholar] [CrossRef]
- Caldeira-Brant, A.L.; Martinelli, L.M.; Marques, M.M.; Reis, A.B.; Martello, R.; Almeida, F.R.C.L.; Chiarini-Garcia, H. A subpopulation of human Adark spermatogonia behaves as the reserve stem cell. Reproduction 2020, 159, 437–451. [Google Scholar] [CrossRef]
- Vergouwen, R.P.F.A.; Huiskamp, R.; Bas, R.J.; Roepers-Gajadien, H.L.; Davids, J.A.G.; Rooij, D.G. De Postnatal development of testicular cell populations in mice. J. Reprod. Fertil. 1989, 99, 479–485. [Google Scholar] [CrossRef]
- Wu, X.; Schmidt, J.A.; Avarbock, M.R.; Tobias, J.W.; Carlson, C.A.; Kolon, T.F.; Ginsberg, J.P.; Brinster, R.L. Prepubertal human spermatogonia and mouse gonocytes share conserved gene expression of germline stem cell regulatory molecules. Proc. Natl. Acad. Sci. USA 2009, 106, 21672–21677. [Google Scholar] [CrossRef]
- Evans, E.; Hogarth, C.; Mitchell, D.; Griswold, M. Riding the spermatogenic wave: Profiling gene expression within neonatal germ: And sertoli cells during a synchronized initial wave of spermatogenesis in mice. Biol. Reprod. 2014, 90, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, T.; Orwig, K.E.; Avarbock, M.R.; Brinster, R.L. Remodeling of the postnatal mouse testis is accompanied by dramatic changes in stem cell number and niche accessibility. Proc. Natl. Acad. Sci. USA 2001, 98, 6186–6191. [Google Scholar] [CrossRef] [PubMed]
- Grumbach, M.M. The Neuroendocrinology of Human Puberty Revisited. Horm. Res. Paediatr. 2002, 57, 2–14. [Google Scholar] [CrossRef]
- Lara, N.L.M.; Costa, G.M.J.; Avelar, G.F.; Guimarães, D.A.; França, L.R. Postnatal testis development in the collared peccary (Tayassu tajacu), with emphasis on spermatogonial stem cells markers and niche. Gen. Comp. Endocrinol. 2019, 273, 98–107. [Google Scholar] [CrossRef]
- Plant, T.M.; Barker-Gibb, M.L. Neurobiological mechanisms of puberty in higher primates. Hum. Reprod. Update 2004, 10, 67–77. [Google Scholar] [CrossRef]
- Copeland, K.C.; Eichberg, J.W.; Parker, C.R., Jr.; Bartke, A. Puberty in the Chimpanzee: Somatomedin-C and Its Relationship to Somatic Growth and Steroid Hormone Concentrations. J. Clin. Endocrinol. Metab. 1985, 60, 1154–1160. [Google Scholar] [CrossRef]
- Kraemer, H.C.; Horvat, J.R.; Doering, C.; McGinnis, P.R. Male chimpanzee development focusing on adolescence: Integration of behavioral with physiological changes. Primates 1982, 23, 393–405. [Google Scholar] [CrossRef]
- Marson, J.; Meuris, S.; Cooper, R.W.; Jouannet, P. Puberty in the male chimpanzee: Time-related variations in luteinizing hormone, follicle-stimulating hormone, and testosterone. Biol. Reprod. 1991, 44, 456–460. [Google Scholar] [CrossRef]
- Marson, J.; Meuris, S.; Cooper, R.W.; Jouannet, P. Puberty in the Chimpanzee: Progressive Maturation of Semen Characteristics. Biol. Reprod. 1991, 44, 448–455. [Google Scholar] [CrossRef]
- Copeland, K.C.; Kuehl, T.J.; Reyes, P.; Castracane, V.D. The baboon as a model for puberty: Growth, testis size, plasma testosterone, and somatomedin-C. Pediatr. Res. 1981, 15, 1547. [Google Scholar] [CrossRef][Green Version]
- Steiner, R.A.; Bremner, W.J. Endocrine Correlates of Sexual Development in the Male Monkey, Macaca fascicularis. Endocrinology 1981, 109, 914–919. [Google Scholar] [CrossRef]
- Wickings, E.J.; Dixson, A.F. Testicular function, secondary sexual development, and social status in male mandrills (Mandrillus sphinx). Physiol. Behav. 1992, 52, 909–916. [Google Scholar] [CrossRef]
- Ojeda, S.R.; Advis, J.P.; Andrews, W.W. Neuroendocrine control of the onset of puberty in the rat. Fed. Proc. 1980, 39, 2365–2371. [Google Scholar]
- Cortes, D.; Müller, J.; Skakkebæk, N.E. Proliferation of Sertoli cells during development of the human testis assessed by stereological methods. Int. J. Androl. 1987, 10, 589–596. [Google Scholar] [CrossRef]
- Mäkelä, J.A.; Koskenniemi, J.J.; Virtanen, H.E.; Toppari, J. Testis Development. Endocr. Rev. 2019, 40, 857–905. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Wistuba, J.; Pock, T.; Schlatt, S.; Neuhaus, N. Spermatogonial stem cells: Updates from specification to clinical relevance. Hum. Reprod. Update 2019, 25, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Tarulli, G.A.; Stanton, P.G.; Meachem, S.J. Is the adult sertoli cell terminally differentiated? Biol. Reprod. 2012, 87, 13. [Google Scholar] [CrossRef] [PubMed]
- Masliukaite, I.; Hagen, J.M.; Jahnukainen, K.; Stukenborg, J.B.; Repping, S.; van der Veen, F.; van Wely, M.; van Pelt, A.M.M. Establishing reference values for age-related spermatogonial quantity in prepubertal human testes: A systematic review and meta-analysis. Fertil. Steril. 2016, 106, 1652–1657.e2. [Google Scholar] [CrossRef] [PubMed]
- Plant, T.M.; Ramaswamy, S.; Simorangkir, D.; Marshall, G.R. Postnatal and Pubertal Development of the Rhesus Monkey (Macaca mulatta) Testis. Ann. N. Y. Acad. Sci. 2005, 1061, 149–162. [Google Scholar] [CrossRef]
- Barrow, P.C.; Barbellion, S.; Stadler, J. Preclinical Evaluation of Juvenile Toxicity. In Drug Safety Evaluation: Methods and Protocols; Gautier, J.-C., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 17–35. ISBN 978-1-60761-849-2. [Google Scholar]
- Takashima, S.; Shinohara, T. Culture and transplantation of spermatogonial stem cells. Stem Cell Res. 2018, 29, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, T. Germ Line Stem Cell Competition in Postnatal Mouse Testes. Biol. Reprod. 2002, 66, 1491–1497. [Google Scholar] [CrossRef][Green Version]
- Honaramooz, A.; Megee, S.O.; Dobrinski, I. Germ Cell Transplantation in Pigs. Biol. Reprod. 2002, 28, 21–28. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.; Chang, G.; Chen, Y.; An, G.; Yan, L.; Gao, S.; Xu, Y.; Cui, Y.; Dong, J.; et al. Single-Cell RNA Sequencing Analysis Reveals Sequential Cell Fate Transition during Human Spermatogenesis. Cell Stem Cell 2018, 23, 599–614.e4. [Google Scholar] [CrossRef] [PubMed]
- Orwig, K.E.; Ryu, B.Y.; Avarbock, M.R.; Brinster, R.L. Male germ-line stem cell potential is predicted by morphology of cells in neonatal rat testes. Proc. Natl. Acad. Sci. USA 2002, 99, 11706–11711. [Google Scholar] [CrossRef]
- Hermann, B.P.; Mutoji, K.N.; Velte, E.K.; Ko, D.; Oatley, J.M.; Geyer, C.B.; McCarrey, J.R. Transcriptional and translational heterogeneity among neonatal mouse spermatogonia. Biol. Reprod. 2015, 92, 1–12. [Google Scholar] [CrossRef]
- Jan, S.Z.; Vormer, T.L.; Jongejan, A.; Röling, M.D.; Silber, S.J.; de Rooij, D.G.; Hamer, G.; Repping, S.; van Pelt, A.M.M. Unraveling transcriptome dynamics in human spermatogenesis. Development 2017, 144, 3659–3673. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, N.; Yoon, J.; Terwort, N.; Kliesch, S.; Seggewiss, J.; Huge, A.; Voss, R.; Schlatt, S.; Grindberg, R.V.; Scholer, H.R. Single-cell gene expression analysis reveals diversity among human spermatogonia. Mol. Hum. Reprod. 2017, 23, 79–90. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Ogonuki, N.; Inoue, K.; Miki, H.; Ogura, A.; Toyokuni, S.; Shinohara, T. Long-Term Proliferation in Culture and Germline Transmission of Mouse Male Germline Stem Cells. Biol. Reprod. 2003, 69, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Avarbock, M.R.; Brinster, R.L. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc. Natl. Acad. Sci. USA 2004, 101, 16489–16494. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H.; Avarbock, M.R.; Brinster, R.L. Culture Conditions and Single Growth Factors Affect Fate Determination of Mouse Spermatogonial Stem Cells. Biol. Reprod. 2004, 71, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Lindahl, M.; Hyvönen, M.E.; Parvinen, M.; de Rooij, D.G.; Hess, M.W.; Raatikainen-Ahokas, A.; Sainio, K.; Rauvala, H.; Lakso, M.; et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 2000, 287, 1489–1493. [Google Scholar] [CrossRef]
- Yomogida, K.; Yagura, Y.; Tadokoro, Y.; Nishimune, Y. Dramatic expansion of germinal stem cells by ectopically expressed human glial cell line-derived neurotrophic factor in mouse sertoli cells. Biol. Reprod. 2003, 69, 1303–1307. [Google Scholar] [CrossRef]
- Oatley, J.M.; Avarbock, M.R.; Brinster, R.L. Glial Cell Line-derived Neurotophic Factor Regulation of Genes Essential for Self-renewal of Mouse Spermatogonial Stem Cells Is Dependent of Src Family Kinase Signaling. J. Biol. Chem. 2007, 282, 25842–25851. [Google Scholar] [CrossRef]
- Lee, J.; Kanatsu-Shinohara, M.; Inoue, K.; Ogunuki, N.; Miki, H.; Toyokuni, S.; Kimura, T.; Nakano, T.; Ogura, A.; Shinohara, T. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development 2007, 134, 1853–1859. [Google Scholar] [CrossRef]
- Kubota, H.; Brinster, R.L. Culture of Rodent Spermatogonial Stem Cells, Male Germline Stem Cells of the Postnatal Animal. Methods Cell Biol. 2008, 86, 59–84. [Google Scholar] [PubMed]
- Godet, M.; Sabido, O.; Gilleron, J.; Durand, P. Meiotic progression of rat spermatocytes requires mitogen-activated protein kinases of Sertoli cells and close contacts between the germ cells and the Sertoli cells. Dev. Biol. 2008, 315, 173–188. [Google Scholar] [CrossRef]
- Garcia, T.X.; Hofmann, M.C. Regulation of germ line stem cell homeostasis. Anim. Reprod. 2015, 12, 35–45. [Google Scholar] [PubMed]
- Kostereva, N.; Hofmann, M.C. Regulation of the Spermatogonial Stem Cell Niche. Reprod. Domest. Anim. 2008, 43, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Meistrich, M.L.; van Beek, M. Spermatogonial Stem Cells. In Cell and Molecular Biology of the Testis; Oxford University Press: New York, NY, USA, 1993; pp. 266–295. [Google Scholar]
- De Rooij, D.G.; Lok, D.; Weenk, D. Feedback Regulation of the Proliferation of the Undifferentiated Spermatogonia in the Chinese Hamster by the Differentiating Spermatogonia. Cell Prolif. 1985, 18, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Bootsma, A.L.; Davids, J.A.G. The Cell Cycle of Spermatogonial Colony Forming Stem Cells in the Cba Mouse After Neutron Irradiation. Cell Prolif. 1988, 21, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Langenstroth, D.; Kossack, N.; Westernströer, B.; Wistuba, J.; Behr, R.; Gromoll, J.; Schlatt, S. Separation of somatic and germ cells is required to establish primate spermatogonial cultures. Hum. Reprod. 2014, 29, 2018–2031. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Llames, S.; García-Pérez, E.; Meana, Á.; Larcher, F.; Del Río, M. Feeder Layer Cell Actions and Applications. Tissue Eng. Part B Rev. 2015, 21, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Colman, M.J.; Schewe, M.; Meerlo, M.; Stigter, E.; Gerrits, J.; Pras-Raves, M.; Sacchetti, A.; Hornsveld, M.; Oost, K.C.; Snippert, H.J.; et al. Interplay between metabolic identities in the intestinal crypt supports stem cell function. Nature 2017, 543, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, L.K.; Dufresne, M.; Joppé, S.E.; Petryszyn, S.; Aumont, A.; Calon, F.; Barnabé-Heider, F.; Furtos, A.; Parent, M.; Chaurand, P.; et al. Aberrant Lipid Metabolism in the Forebrain Niche Suppresses Adult Neural Stem Cell Proliferation in an Animal Model of Alzheimer’s Disease. Cell Stem Cell 2015, 17, 397–411. [Google Scholar] [CrossRef]
- Suda, T.; Takubo, K.; Semenza, G.L. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 2011, 9, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Simsek, T.; Kocabas, F.; Zheng, J.; Deberardinis, R.J.; Mahmoud, A.I.; Olson, E.N.; Schneider, J.W.; Zhang, C.C.; Sadek, H.A. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 2010, 7, 380–390. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Miki, H.; Inoue, K.; Ogonuki, N.; Toyokuni, S.; Ogura, A.; Shinohara, T. Long-Term Culture of Mouse Male Germline Stem Cells Under Serum-or Feeder-Free Conditions1. Biol. Reprod. 2005, 72, 985–991. [Google Scholar] [CrossRef]
- Hamra, F.K.; Chapman, K.M.; Nguyen, D.M.; Williams-Stephens, A.A.; Hammer, R.E.; Garbers, D.L. Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture. Proc. Natl. Acad. Sci. USA 2005, 102, 17430–17435. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Muneto, T.; Lee, J.; Takenaka, M.; Chuma, S.; Nakatsuji, N.; Horiuchi, T.; Shinohara, T. Long-Term Culture of Male Germline Stem Cells from Hamster Testes. Biol. Reprod. 2008, 78, 611–617. [Google Scholar] [CrossRef]
- Kubota, H.; Wu, X.; Goodyear, S.M.; Avarbock, M.R.; Brinster, R.L. Glial cell line-derived neurotrophic factor and endothelial cells promote self-renewal of rabbit germ cells with spermatogonial stem cell properties. FASEB J. 2011, 25, 2604–2614. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ryu, B.Y.; Kubota, H.; Avarbock, M.R.; Brinster, R.L. Conservation of spermatogonial stem cell self-renewal signaling between mouse and rat. Proc. Natl. Acad. Sci. USA 2005, 102, 14302–14307. [Google Scholar] [CrossRef] [PubMed]
- Kakiuchi, K.; Taniguchi, K.; Kubota, H. Conserved and non-conserved characteristics of porcine glial cell line-derived neurotrophic factor expressed in the testis. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, X.; Zheng, Y.; Zhu, J.; Qin, Y.; Lv, Y.; Zeng, W. Long-Term Propagation of Porcine Undifferentiated Spermatogonia. Stem Cells Dev. 2017, 26, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Oatley, M.J.; Kaucher, A.V.; Yang, Q.-E.; Waqas, M.S.; Oatley, J.M. Conditions for Long-Term Culture of Cattle Undifferentiated Spermatogonia. Biol. Reprod. 2016, 95, 14-14. [Google Scholar] [CrossRef] [PubMed]
- Pramod, R.K.; Mitra, A. In vitro culture and characterization of spermatogonial stem cells on Sertoli cell feeder layer in goat (Capra hircus). J. Assist. Reprod. Genet. 2014, 31, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Kanatsu-Shinohara, M.; Tanaka, T.; Ogonuki, N.; Ogura, A.; Morimoto, H.; Cheng, P.F.; Eisenman, R.N.; Trumpp, A.; Shinohara, T. Myc/Mycn-mediated glycolysis enhances mouse spermatogonial stem cell self-renewal. Genes Dev. 2016, 2637–2648. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.; Abramowitz, L.K.; Kubota, H.; Wu, X.; Niu, Z.; Avarbock, M.R.; Tobias, J.W.; Bartolomei, M.S.; Brinster, R.L. In Vivo and In Vitro Aging Is Detrimental to Mouse Spermatogonial Stem Cell Function. Biol. Reprod. 2011, 84, 698–706. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takashima, S.; Kanatsu-Shinohara, M.; Tanaka, T.; Morimoto, H.; Inoue, K.; Ogonuki, N.; Jijiwa, M.; Takahashi, M.; Ogura, A.; Shinohara, T. Functional differences between GDNF-dependent and FGF2-dependent mouse spermatogonial stem cell self-renewal. Stem Cell Rep. 2015, 4, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Kanatsu-Shinohara, M.; Inoue, K.; Ogonuki, N.; Morimoto, H.; Ogura, A.; Shinohara, T. Serum- and Feeder-Free Culture of Mouse Germline Stem Cells1. Biol. Reprod. 2011, 84, 97–105. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Ogonuki, N.; Matoba, S.; Morimoto, H.; Ogura, A.; Shinohara, T. Improved Serum- and Feeder-Free Culture of Mouse Germline Stem Cells. Biol. Reprod. 2014, 91, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K. Metabolic Regulation of a Bacterial Cell System with Emphasis on Escherichia coli Metabolism. ISRN Biochem. 2013, 2013, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Al-Ani, A.; Toms, D.; Kondro, D.; Thundathil, J.; Yu, Y.; Ungrin, M. Oxygenation in cell culture: Critical parameters for reproducibility are routinely not reported. PLoS ONE 2018, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.F.; Martins, A.D.; Moreira, A.C.; Cheng, C.Y.; Alves, M.G. The Warburg Effect Revisited-Lesson from the Sertoli Cell. Med. Res. Rev. 2015, 35, 126–151. [Google Scholar] [CrossRef]
- Rossignol, R.; Gilkerson, R.; Aggeler, R.; Yamagata, K.; Remington, S.J.; Capaldi, R.A. Energy Substrate Modulates Mitochondrial Structure and Oxidative Capacity in Cancer Cells. Cancer Res. 2004, 64, 985–993. [Google Scholar] [CrossRef]
- Smolková, K.; Bellance, N.; Scandurra, F.; Génot, E.; Gnaiger, E.; Plecitá-Hlavatá, L.; Jezek, P.; Rossignol, R. Mitochondrial bioenergetic adaptations of breast cancer cells to aglycemia and hypoxia. J. Bioenerg. Biomembr. 2010, 42, 55–67. [Google Scholar] [CrossRef]
- Morrison, S.J.; Spradling, A.C. Stem Cells and Niches: Mechanisms That Promote Stem Cell Maintenance throughout Life. Cell 2008, 132, 598–611. [Google Scholar] [CrossRef]
- Krisher, R.L.; Prather, R.S. A Role for the Warburg Effect in Preimplantation Embryo Development: Metabolic Modification to Support Rapid Cell Proliferation. Mol. Reprod. Dev. 2012, 79, 311–320. [Google Scholar] [CrossRef]
- Folmes, C.D.L.; Dzeja, P.P.; Nelson, T.J.; Terzic, A. Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell 2012, 11, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Moussaieff, A.; Rouleau, M.; Kitsberg, D.; Cohen, M.; Levy, G.; Barasch, D.; Nemirovski, A.; Shen-Orr, S.; Laevsky, I.; Amit, M.; et al. Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab. 2015, 21, 392–402. [Google Scholar] [CrossRef]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Formosa, L.E.; Ryan, M.T. Mitochondrial OXPHOS complex assembly lines. Nat. Cell Biol. 2018, 20, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Spangrude, G.J.; Johnson, G.R. Resting and activated subsets of mouse multipotent hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 1990, 87, 7433–7437. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Dzeja, P.P.; Faustino, R.S.; Perez-terzic, C.; Behfar, A.; Terzic, A. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Natl. Inst. Heal. 2011, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, H.; Lleonart, M.E.; Nakashima, Y.; Yokode, M.; Tanaka, M.; Bernard, D.; Gil, J.; Beach, D. A High Glycolytic Flux Supports the Proliferative Potential of Murine Embryonic Stem Cells. Antioxid. Redox Signal. 2006, 9, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-T.; Shih, Y.-R.V.; Kuo, T.K.; Lee, O.K.; Wei, Y.-H. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells 2008, 26, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Fillmore, N.; Huqi, A.; Jaswal, J.S.; Mori, J.; Paulin, R.; Haromy, A.; Onay-Besikci, A.; Ionescu, L.; Thébaud, B.; Michelakis, E.; et al. Effect of fatty acids on human bone marrow mesenchymal stem cell energy metabolism and survival. PLoS ONE 2015, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pietilä, M.; Palomäki, S.; Lehtonen, S.; Ritamo, I.; Valmu, L.; Nystedt, J.; Laitinen, S.; Leskelä, H.-V.; Sormunen, R.; Pesälä, J.; et al. Mitochondrial Function and Energy Metabolism in Umbilical Cord Blood- and Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2012, 21, 575–588. [Google Scholar] [CrossRef]
- Flores, A.; Schell, J.; Krall, A.S.; Jelinek, D.; Miranda, M.; Grigorian, M.; Braas, D.; White, A.C.; Zhou, J.L.; Graham, N.A.; et al. Lactate dehydrogenase activity drives hair follicle stem cell activation. Nat. Cell Biol. 2017, 19, 1017. [Google Scholar] [CrossRef]
- Shyh-Chang, N.; Daley, G.Q. Metabolic switches linked to pluripotency and embryonic stem cell differentiation. Cell Metab. 2015, 21, 349–350. [Google Scholar] [CrossRef]
- Zheng, X.; Boyer, L.; Jin, M.; Mertens, J.; Kim, Y.; Ma, L.; Ma, L.; Hamm, M.; Gage, F.H.; Hunter, T. Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation. eLife 2016, 1–25. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Chang, C.; Yang, Z.; Wang, P.; Fu, H.; Wei, X.; Chen, E.; Tan, S.; Huang, W.; et al. A bioenergetic shift is required for spermatogonial differentiation. Cell Discov. 2020, 6. [Google Scholar] [CrossRef]
- Butcher, L.; Coates, A.; Martin, K.L.; Rutherford, A.J.; Leese, H.J. Metabolism of pyruvate by the early human embryo. Biol. Reprod. 1998, 58, 1054–1056. [Google Scholar] [CrossRef]
- Brinster, R.L.; Troike, D.E. Requirements for blastocyst development in vitro. J. Anim. Sci. 1979, 49, 26–34. [Google Scholar] [CrossRef]
- Gardner, D.K.; Lane, M.; Stevens, J.; Schoolcraft, W.B. Noninvasive assessment of human embryo nutrient consumption as a measure of developmental potential. Fertil. Steril. 2001, 76, 1175–1180. [Google Scholar] [CrossRef]
- Leese, H.J.; Barton, A.M. Pyruvate and glucose uptake by mouse ova and preimplantation embryos. J. Reprod. Fertil. 1984, 2, 1–5. [Google Scholar] [CrossRef]
- Leese, H.J. Metabolism of the preimplantation embryo: 40 Years on. Reproduction 2012, 143, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Varum, S.; Rodrigues, A.S.; Moura, M.B.; Momcilovic, O.; Easley IV, C.A.; Ramalho-Santos, J.; van Houten, B.; Schatten, G. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Choi, M.; Margineantu, D.; Margaretha, L.; Hesson, J.; Cavanaugh, C.; Blau, C.A.; Horwitz, M.S.; Hockenbery, D.; Ware, C.; et al. HIF1α induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. EMBO J. 2012, 31, 2103–2116. [Google Scholar] [CrossRef]
- Sperber, H.; Mathieu, J.; Wang, Y.; Ferreccio, A.; Hesson, J.; Xu, Z.; Fischer, K.A.; Devi, A.; Detraux, D.; Gu, H.; et al. The metabolome regulates the epigenetic landscape during naive-to-primed human embryonic stem cell transition. Nat. Cell Biol. 2015, 17, 1523–1535. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ocampo, A.; Belmonte, J.C.I. Cellular Metabolism and Induced Pluripotency. Cell 2016, 166, 1371–1385. [Google Scholar] [CrossRef]
- Zhang, J.; Nuebel, E.; Daley, G.Q.; Koehler, C.M.; Teitell, M.A. Metabolic regulation in pluripotent stem cells during reprogramming and self-renewal. Cell Stem Cell 2012, 11, 589–595. [Google Scholar] [CrossRef]
- Vozza, A.; Parisi, G.; De Leonardis, F.; Lasorsa, F.M.; Castegna, A.; Amorese, D.; Marmo, R.; Calcagnile, V.M.; Palmieri, L.; Ricquier, D.; et al. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc. Natl. Acad. Sci. USA 2014, 111, 960–965. [Google Scholar] [CrossRef]
- Folmes, C.D.L.; Nelson, T.J.; Martinez-Fernandez, A.; Arrell, D.K.; Lindor, J.Z.; Dzeja, P.P.; Ikeda, Y.; Perez-Terzic, C.; Terzic, A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011, 14, 264–271. [Google Scholar] [CrossRef]
- Sone, M.; Morone, N.; Nakamura, T.; Tanaka, A.; Okita, K.; Woltjen, K.; Nakagawa, M.; Heuser, J.E.; Yamada, Y.; Yamanaka, S.; et al. Hybrid Cellular Metabolism Coordinated by Zic3 and Esrrb Synergistically Enhances Induction of Naive Pluripotency. Cell Metab. 2017, 25, 1103–1117.e6. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Wind, F.; Negelein, E. The metabolism of tumors in the body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef]
- Guppy, M.; Greiner, E.; Brand, K. The role of the Crabtree effect and an endogenous fuel in the energy metabolism of resting and proliferating thymocytes. Eur. J. Biochem. 1993, 212, 95–99. [Google Scholar] [CrossRef]
- Anastasiou, D.; Poulogiannis, G.; Asara, J.M.; Boxer, M.B.; Jiang, J.; Shen, M.; Bellinger, G.; Sasaki, A.T.; Locasale, J.W.; Auld, D.S.; et al. Inhibition of Pyruvate Kinase M2 by Reactive Oxygen Species Contributes to Cellular Antioxidant Responses. Science 2011, 334, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Bigarella, C.L.; Liang, R.; Ghaffari, S. Stem cells and the impact of ROS signaling. Development 2014, 141, 4206–4218. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.P.; Price, N.L.; Ling, A.J.Y.; Moslehi, J.J.; Montgomery, M.K.; Rajman, L.; White, J.P.; Teodoro, J.S.; Wrann, C.D.; Hubbard, B.P.; et al. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013, 155, 1624–1638. [Google Scholar] [CrossRef]
- Hemberger, M.; Dean, W.; Reik, W. Epigenetic dynamics of stem cells and cell lineage commitment: Digging Waddington’s canal. Nat. Rev. Mol. Cell Biol. 2009, 10, 526–537. [Google Scholar] [CrossRef]
- Waddington, C.H. Organisers and Genes; Cambridge Biological Studies; Cambridge University Press: Cambridge, UK, 1940. [Google Scholar]
- Ryall, J.G.; Dell’Orso, S.; Derfoul, A.; Juan, A.; Zare, H.; Feng, X.; Clermont, D.; Koulnis, M.; Gutierrez-Cruz, G.; Fulco, M.; et al. The NAD+-dependent SIRT1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell 2015, 16, 171–183. [Google Scholar] [CrossRef]
- Harvey, A.; Caretti, G.; Moresi, V.; Renzini, A.; Adamo, S. Interplay between Metabolites and the Epigenome in Regulating Embryonic and Adult Stem Cell Potency and Maintenance. Stem Cell Rep. 2019, 13, 573–589. [Google Scholar] [CrossRef]
- Ryall, J.G.; Cliff, T.; Dalton, S.; Sartorelli, V. Metabolic Reprogramming of Stem Cell Epigenetics. Cell Stem Cell 2015, 17, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Shyh-Chang, N.; Locasale, J.W.; Lyssiotis, C.A.; Zheng, Y.; Teo, R.Y.; Ratanasirintrawoot, S.; Zhang, J.; Onder, T.; Unternaehrer, J.J.; Zhu, H.; et al. Influence of Threonine Metabolism on S-adenosyl-methionine and Histone Methylation. Science 2013, 393, 222–226. [Google Scholar] [CrossRef]
- Carey, B.W.; Finley, L.W.S.; Cross, J.R.; Allis, C.D.; Thompson, C.B. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature 2015, 518, 413–416. [Google Scholar] [CrossRef]
- Wellen, K.E.; Hatzivassiliou, G.; Sachdeva, U.M.; Bui, T.V.; Cross, J.R.; Thompson, C.B. ATP-citrate lyase links cellular metabolism to histone acetylation. Science 2009, 324, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.D.; Allis, C.D.; Bernstein, E. Epigenetics: A Landscape Takes Shape. Cell 2007, 128, 635–638. [Google Scholar] [CrossRef]
- Lu, V.; Teitell, M.A. Alpha-ketoglutarate: A “magic” metabolite in early germ cell development. EMBO J. 2019, 38, 2018–2019. [Google Scholar] [CrossRef]
- Yoshida, S. Open niche regulation of mouse spermatogenic stem cells. Dev. Growth Differ. 2018, 60, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Fayomi, A.P.; Peters, K.; Sukhwani, M.; Valli-Pulaski, H.; Shetty, G.; Meistrich, M.L.; Houser, L.; Robertson, N.; Roberts, V.; Ramsey, C.; et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science 2019, 363, 1314–1319. [Google Scholar] [CrossRef]
- Nakagawa, T.; Nabeshima, Y.I.; Yoshida, S. Functional Identification of the Actual and Potential Stem Cell Compartments in Mouse Spermatogenesis. Dev. Cell 2007, 12, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S. Elucidating the identity and behavior of spermatogenic stem cells in the mouse testis. Reproduction 2012, 144, 293–302. [Google Scholar] [CrossRef]
- Yoshida, S.; Nabeshima, Y.I.; Nakagawa, T. Stem cell heterogeneity: Actual and potential stem cell compartments in mouse spermatogenesis. Ann. New York Acad. Sci. 2007, 1120, 47–58. [Google Scholar] [CrossRef]
- Chan, F.; Oatley, M.J.; Kaucher, A.V.; Yang, Q.-E.; Bieberich, C.J.; Shashikant, C.S.; Oatley, J.M. Functional and molecular features of the Id4+ germline stem cell population in mouse testes. Genes Dev. 2014, 28, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Nakagawa, T.; Enomoto, H.; Suzuki, M.; Yamamoto, M.; Simons, B.D.; Yoshida, S. Mouse spermatogenic stem cells continually interconvert between equipotent singly isolated and syncytial states. Cell Stem Cell 2014, 14, 658–672. [Google Scholar] [CrossRef]
- Stine, R.R.; Matunis, E.L. Stem cell competition: Finding balance in the niche. Trends Cell Biol. 2013, 23, 357–364. [Google Scholar] [CrossRef]
- Thayer, K.A.; Ruhlen, R.L.; Howdeshell, K.L.; Buchanan, D.L.; Cooke, P.S.; Preziosi, D.; Welshons, W.V.; Haseman, J.; Vom Saal, F.S. Altered prostate growth and daily sperm production in male mice exposed prenatally to subclinical doses of 17α-ethinyl oestradiol. Hum. Reprod. 2001, 16, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Wenger, R.H.; Katschinski, M. The hypoxic testis and post-meiotic expression of PAS domain proteins. Semin. Cell Dev. Biol. 2005, 16, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Free, M.J.; Schluntz, G.A.; Jaffe, R.A. Respiratory gas tensions in tissues and fluids of the male rat reproductive tract. Biol. Reprod. 1976, 14, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.F.; Alves, M.G. Sertoli Cell Metabolism and Spermatogenesis; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9783319197906. [Google Scholar]
- Robinson, R.; Fritz, I.B. Metabolism of Glucose by Sertoli Cells in Culture. Biol. Reprod. 1981, 24, 1032–1041. [Google Scholar] [CrossRef]
- Mita, M.; Hall, P.F. Metabolism of Round Spermatids from Rats: Lactate as the Preferred Substrate1. Biol. Reprod. 1982, 26, 445–448. [Google Scholar] [CrossRef]
- Nakamura, M.; Kamachi, T.; Okinaga, S.; Arai, K. Metabolism of Round Spermatids: Pyruvate cannot Maintain the ATP Level: ATP synthesis/α-ketoacid/rat spermatids. Dev. Growth Differ. 1986, 28, 489–498. [Google Scholar] [CrossRef]
- Bajpai, M.; Gupta, G.; Setty, B.S. Changes in carbohydrate metabolism of testicular germ cells during meiosis in the rat. Eur. J. Endocrinol. 1998, 138, 322–327. [Google Scholar] [CrossRef]
- Nakamura, M.; Okinaga, S.; Arai, K. Metabolism of round spermatids: Evidence that lactate is preferred substrate. Am. J. Physiol. Endocrinol. Metab. 1984, 10. [Google Scholar] [CrossRef]
- Grootegoed, J.A.; Oonk, R.B.; Jansen, R.; Van Der Molen, H.J. Metabolism of radiolabelled energy-yielding substrates by rat Sertoli cells. J. Reprod. Fertil. 1986, 77, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.D. The blood-testis barrier and its formation relative to spermatocyte maturation in the adult rat: A lanthanum tracer study. Anat. Rec. 1978, 190, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.; Park, M.; Yun, J.W.; Lee, J.; Kim, J.S.; Cho, S.J.; Lee, Y.M.; Lee, I.; Choi, Y.; Park, K. Oncogenic KRAS signaling activates mTORC1 through COUP-TFII-mediated lactate production. EMBO Rep. 2019, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Galardo, M.N.; Regueira, M.; Riera, M.F.; Pellizzari, E.H.; Cigorraga, S.B.; Meroni, S.B. Lactate regulates rat male germ cell function through reactive oxygen species. PLoS ONE 2014, 9, 1–11. [Google Scholar] [CrossRef]
- Aito, H.; Aalto, K.; Pentika, V.; Dunkel, L. Lactate inhibits germ cell apoptosis in the human testis. Mol. Human Reprod. 2002, 8, 109–117. [Google Scholar]
- Jutte, N.H.P.M.; Grootegoed, J.A.; Rommerts, F.F.G.; Van der Molen, H.J. Exogenous lactate is essential for metabolic activities in isolated rat spermatocytes and spermatids. Reproduction 1981, 62, 399–405. [Google Scholar] [CrossRef]
- Sasaki, H.; Matsui, Y. Epigenetic events in mammalian germ-cell development: Reprogramming and beyond. Nat. Rev. Genet. 2008, 9, 129–140. [Google Scholar] [CrossRef]
- Boussouar, F.; Benahmed, M. Lactate and energy metabolism in male germ cells. Trends Endocrinol. Metab. 2004, 15, 345–350. [Google Scholar] [CrossRef]
- Lord, T.; Nixon, B. Metabolic Changes Accompanying Spermatogonial Stem Cell Differentiation. Dev. Cell 2020, 52, 399–411. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, L.; Sun, M.; Hai, Y.; Li, Z.; He, Z. Expansion and long-term culture of human spermatogonial stem cells via the activation of SMAD3 and AKT pathways. Exp. Biol. Med. 2015, 240, 1112–1122. [Google Scholar] [CrossRef]
- Nakamura, M. Studies of metabolism of round spermatids: Glucose as unfavorable substrate. Biol. Reprod. 1986, 35, 927–935. [Google Scholar] [CrossRef]
- Rathke, C.; Baarends, W.M.; Awe, S.; Renkawitz-Pohl, R. Chromatin dynamics during spermiogenesis. Biochim. Biophys. Acta Gene Regul. Mech. 2014, 1839, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Boussouar, F.; Goudarzi, A.; Buchou, T.; Shiota, H.; Barral, S.; Debernardi, A.; Guardiola, P.; Brindle, P.; Martinez, G.; Arnoult, C.; et al. A specific CBP/p300-dependent gene expression programme drives the metabolic remodelling in late stages of spermatogenesis. Andrology 2014, 2, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Okinaga, S.; Arai, K. Metabolism of Pachytene Primary Spermatocytes from Rat Testes: Pyruvate Maintenance of Adenosine Triphosphate Level. Biol. Reprod. 1984, 30, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Grootegoed, J.A.; Jansen, R.; Van Der Molen, H.J. The role of glucose, pyruvate and lactate in ATP production by rat spermatocytes and spermatids. BBA Bioenerg. 1984, 767, 248–256. [Google Scholar] [CrossRef]
- Nakamura, J.; Hino, A.; Yasumasu, I. Stimulation of Protein Synthesis in Round Spermatids from Rat Testes by Lactate. Biochem. J. 1981, 89, 1309–1315. [Google Scholar]
- Ford, W.C.L. Glycolysis and sperm motility: Does a spoonful of sugar help the flagellum go round? Hum. Reprod. Update 2006, 12, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Krisfalusi, M.; Miki, K.; Magyar, P.L.; O’Brien, D.A. Multiple Glycolytic Enzymes Are Tightly Bound to the Fibrous Sheath of Mouse Spermatozoa. Biol. Reprod. 2006, 75, 270–278. [Google Scholar] [CrossRef]
- Hereng, T.H.; Elgstøen, K.B.P.; Cederkvist, F.H.; Eide, L.; Jahnsen, T.; Sklhegg, B.S.; Rosendal, K.R. Exogenous pyruvate accelerates glycolysis and promotes capacitation in human spermatozoa. Hum. Reprod. 2011, 26, 3249–3263. [Google Scholar] [CrossRef] [PubMed]
- Paoli, D.; Pelloni, M.; Gallo, M.; Coltrinari, G.; Lombardo, F.; Lenzi, A.; Gandini, L. Sperm glyceraldehyde 3-phosphate dehydrogenase gene expression in asthenozoospermic spermatozoa. Asian J. Androl. 2016, 18, 409–413. [Google Scholar] [CrossRef]
- Du Plessis, S.; Agarwal, A.; Mohanty, G.; van der Linde, M. Oxidative phosphorylation versus glycolysis: What fuel do spermatozoa use? Asian J. Androl. 2015, 17, 230. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Bedford, M.T. Epigenetic regulation of the histone-to-protamine transition during spermiogenesis. Reproduction 2016, 151, R55–R70. [Google Scholar] [CrossRef]
- Venkatesh, S.; Workman, J.L. Histone exchange, chromatin structure and the regulation of transcription. Nat. Rev. Mol. Cell Biol. 2015, 16, 178–189. [Google Scholar] [CrossRef]
- Lettieri, G.; D’agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimmino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, S.; Notari, T.; et al. Discovery of the involvement in DNA oxidative damage of human sperm nuclear basic proteins of healthy young men living in polluted areas. Int. J. Mol. Sci. 2020, 21, 4198. [Google Scholar] [CrossRef]
- Hobbs, R.M.; Seandel, M.; Falciatori, I.; Rafii, S.; Pandolfi, P.P. Plzf regulates the germline progenitor self-renewal by opposing mTORC1. Cell 2010, 142, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Carnevalli, L.S.; Trumpp, A. Tuning mTORC1 activity for balanced self-renewal and differentiation. Dev. Cell 2010, 19, 187–188. [Google Scholar] [CrossRef]
- Ho, T.T.; Warr, M.R.; Adelman, E.R.; Lansinger, O.M.; Flach, J.; Verovskaya, E.V.; Figueroa, M.E.; Passegué, E. Autophagy maintains the metabolism and function of young and old stem cells. Nature 2017, 543, 205–210. [Google Scholar] [CrossRef]
- Sahin, P.; Sahin, Z.; Gungor-Ordueri, N.E.; Donmez, B.O.; Celik-Ozenci, C. Inhibition of mammalian target of rapamycin signaling pathway decreases retinoic acid stimulated gene 8 expression in adult mouse testis. Fertil. Steril. 2014, 102, 1482–1490.e3. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Z.; Xiong, Z.; Dai, H.; Zou, Z.; Jia, C.; Bai, X.; Chen, Z. mTORC1 activation promotes spermatogonial differentiation and causes subfertility in mice. Biol. Reprod. 2016, 95, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Moreira, B.P.; Oliveira, P.F.; Alves, M.G. Molecular mechanisms controlled by mTOR in male reproductive system. Int. J. Mol. Sci. 2019, 20, 1633. [Google Scholar] [CrossRef]
- Nakamura, M.; Fujiwara, A.; Yasumasu, I.; Okinaga, S.; Arai, K. Regulation of glucose metabolism by adenine nucleotides in round spermatids from rat testes. J. Biol. Chem. 1982, 257, 13945–13950. [Google Scholar] [CrossRef]
- Gandhi, K.K.; Anand, S.R. Regulation of Glycolysis/Fructolysis in buffalo spermatozoa. J. Reprod. Fertil. 1982, 64, 145–150. [Google Scholar] [CrossRef]
- Peterson, R.N.; Freund, M. ATP synthesis and oxidative metabolism in human spermatozoa. Biol. Reprod. 1970, 3, 47–54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iguchi, N.; Tobias, J.W.; Hecht, N.B. Expression profiling reveals meiotic male germ cell mRNAs that are translationally up- and down-regulated. Proc. Natl. Acad. Sci. USA 2006, 103, 7712–7717. [Google Scholar] [CrossRef]
- Danshina, P.V.; Geyer, C.B.; Dai, Q.; Goulding, E.H.; Willis, W.D.; Kitto, G.B.; McCarrey, J.R.; Eddy, E.M.; O’Brien, D.A. Phosphoglycerate Kinase 2 (PGK2) Is Essential for Sperm Function and Male Fertility in Mice. Biol. Reprod. 2010, 82, 136–145. [Google Scholar] [CrossRef] [PubMed]
- De Mateo, S.; Sassone-Corsi, P. Regulation of spermatogenesis by small non-coding RNAs: Role of the germ granule. Semin. Cell Dev. Biol. 2014, 29, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lv, C.; Guo, Y.; Yuan, S. Mitochondria Associated Germinal Structures in Spermatogenesis: piRNA Pathway Regulation and Beyond. Cells 2020, 9, 399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Q.; Wang, M.; Jiang, M.; Wang, Y.; Sun, Y.; Wang, J.; Xie, T.; Tang, C.; Tang, N.; et al. GASZ and mitofusin-mediated mitochondrial functions are crucial for spermatogenesis. EMBO Rep. 2016, 17, 220–234. [Google Scholar] [CrossRef]
- Paniagua, R.; Nistal, M.; Amat, P.; Rodriguez, M.C. Presence of ribonucleoproteins and basic proteins in the nuage and intermitochondrial bars of human spermatogonia. J. Anat. 1985, 143, 201–206. [Google Scholar]
- Lee, W.Y.; Park, H.J.; Lee, R.; Lee, K.H.; Kim, Y.H.; Ryu, B.Y.; Kim, N.H.; Kim, J.H.; Kim, J.H.; Moon, S.H.; et al. Establishment and in vitro culture of porcine spermatogonial germ cells in low temperature culture conditions. Stem Cell Res. 2013, 11, 1234–1249. [Google Scholar] [CrossRef]
- Su, S.; Szarek, M.; Vooght, A.; Hutson, J.; Li, R. Gonocyte transformation to spermatogonial stem cells occurs earlier in patients with undervirilisation syndromes. J. Pediatr. Surg. 2014, 49, 323–327. [Google Scholar] [CrossRef]
- Hayashi, Y.; Otsuka, K.; Ebina, M.; Igarashi, K.; Takehara, A.; Matsumoto, M.; Kanai, A.; Igarashi, K.; Soga, T.; Matsui, Y. Distinct requirements for energy metabolism in mouse primordial germ cells and their reprogramming to embryonic germ cells. Proc. Natl. Acad. Sci. USA 2017, 114, 8289–8294. [Google Scholar] [CrossRef]
- Tischler, J.; Gruhn, W.H.; Reid, J.; Allgeyer, E.; Buettner, F.; Marr, C.; Theis, F.; Simons, B.D.; Wernisch, L.; Surani, M.A. Metabolic regulation of pluripotency and germ cell fate through α-ketoglutarate. EMBO J. 2019, 38, 1–15. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C.; Boisvert, A.; Manku, G.; Culty, M. Protective role of peroxiredoxins against reactive oxygen species in neonatal rat testicular gonocytes. Antioxidants 2020, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Folmes, C.D.; Nelson, T.J.; Terzic, A. Energy metabolism in nuclear reprogramming. Biomark. Med. 2011, 5, 715–729. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voigt, A.L.; Thiageswaran, S.; de Lima e Martins Lara, N.; Dobrinski, I. Metabolic Requirements for Spermatogonial Stem Cell Establishment and Maintenance In Vivo and In Vitro. Int. J. Mol. Sci. 2021, 22, 1998. https://doi.org/10.3390/ijms22041998
Voigt AL, Thiageswaran S, de Lima e Martins Lara N, Dobrinski I. Metabolic Requirements for Spermatogonial Stem Cell Establishment and Maintenance In Vivo and In Vitro. International Journal of Molecular Sciences. 2021; 22(4):1998. https://doi.org/10.3390/ijms22041998
Chicago/Turabian StyleVoigt, Anna Laura, Shiama Thiageswaran, Nathalia de Lima e Martins Lara, and Ina Dobrinski. 2021. "Metabolic Requirements for Spermatogonial Stem Cell Establishment and Maintenance In Vivo and In Vitro" International Journal of Molecular Sciences 22, no. 4: 1998. https://doi.org/10.3390/ijms22041998
APA StyleVoigt, A. L., Thiageswaran, S., de Lima e Martins Lara, N., & Dobrinski, I. (2021). Metabolic Requirements for Spermatogonial Stem Cell Establishment and Maintenance In Vivo and In Vitro. International Journal of Molecular Sciences, 22(4), 1998. https://doi.org/10.3390/ijms22041998





