Increased Regulatory T Cells and Decreased Myeloid-Derived Suppressor Cells Induced by High CCL17 Levels May Account for Normal Incidence of Cancers among Patients with Atopic Dermatitis
Abstract
:1. Introduction
2. Results
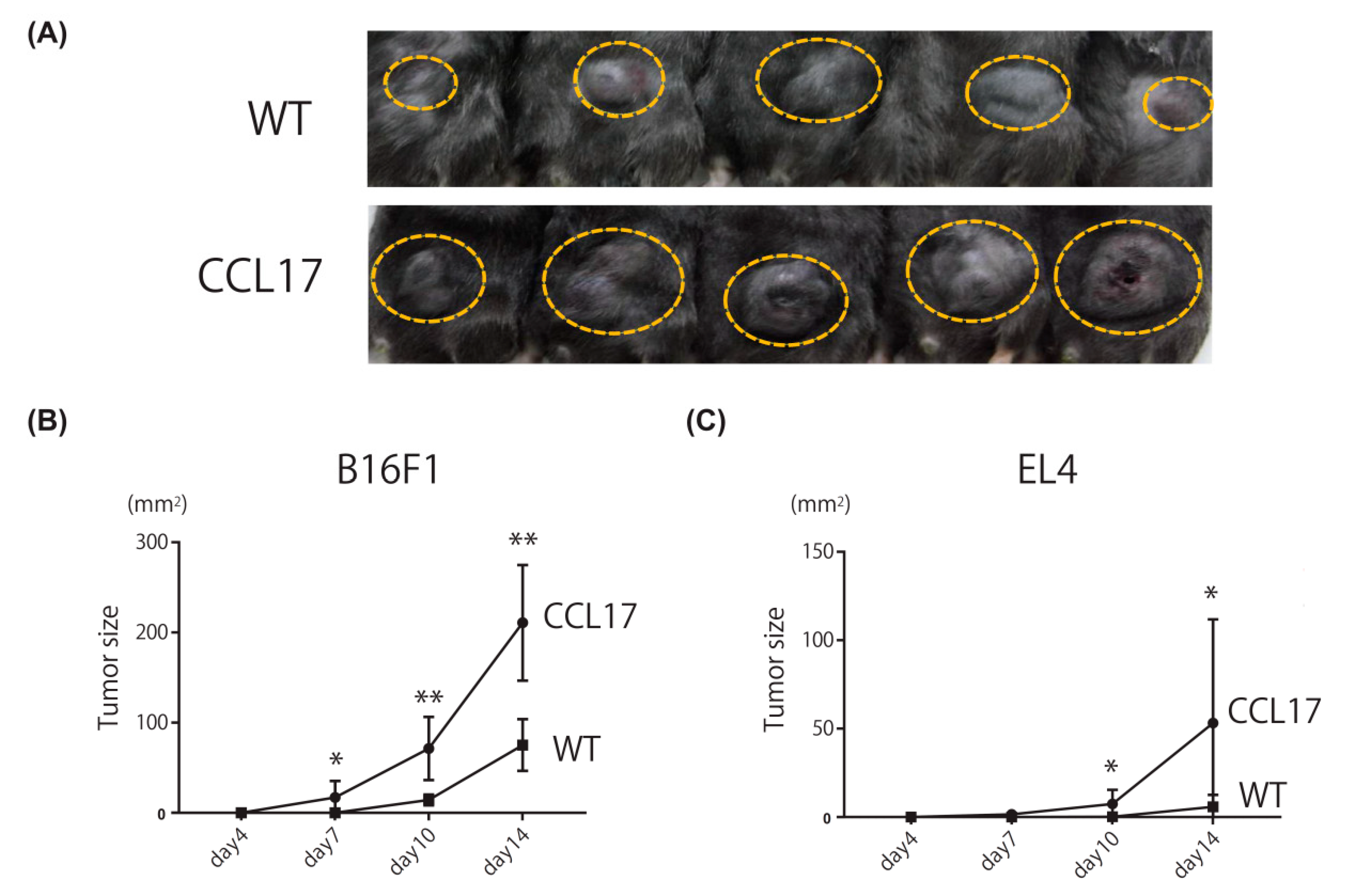
2.1. Enhanced Skin Tumor Formation in CCL17 TG Mice
2.2. Promotion of Lung Metastasis in CCL17 TG Mice
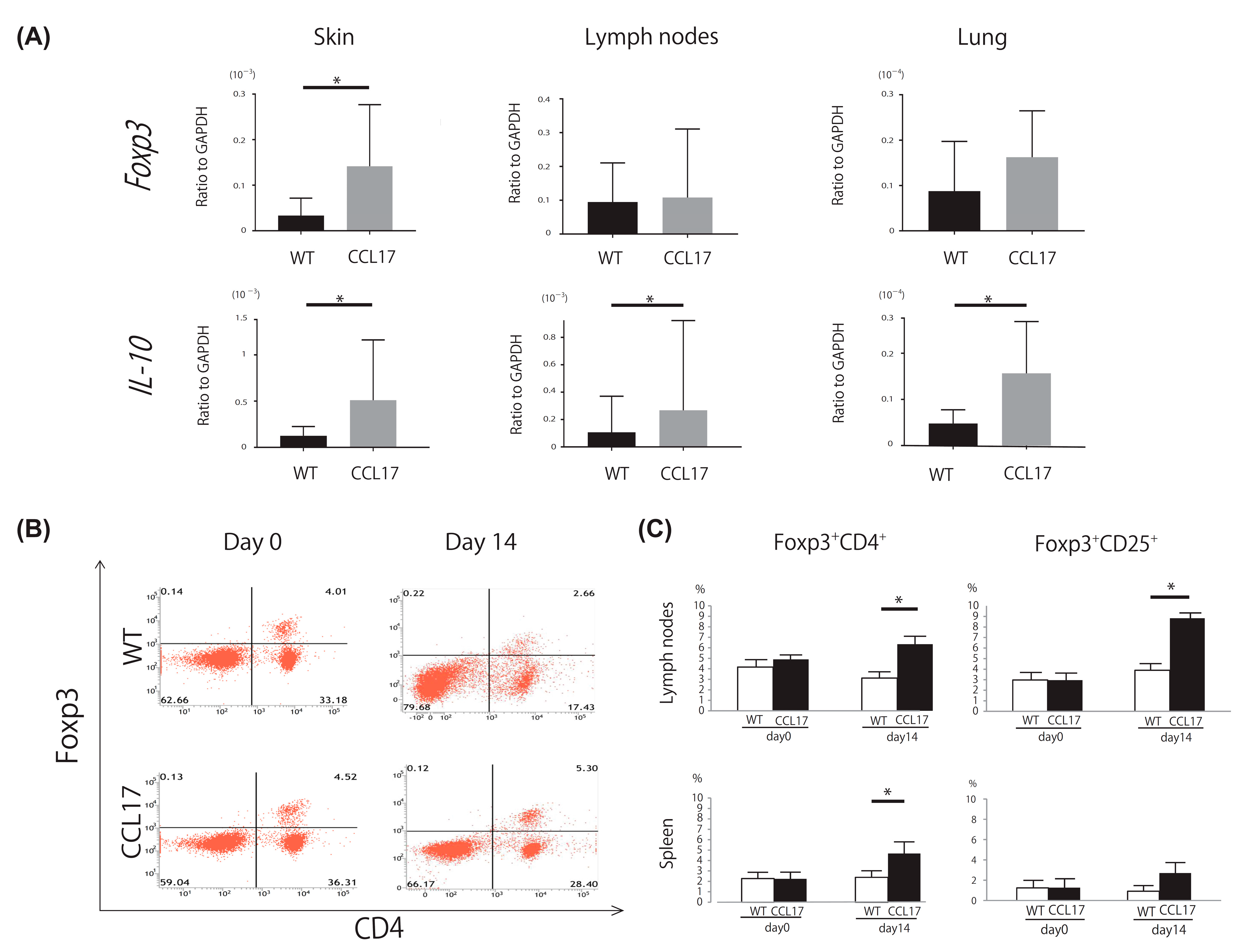
2.3. Increased Tregs in CCL17 TG Mice
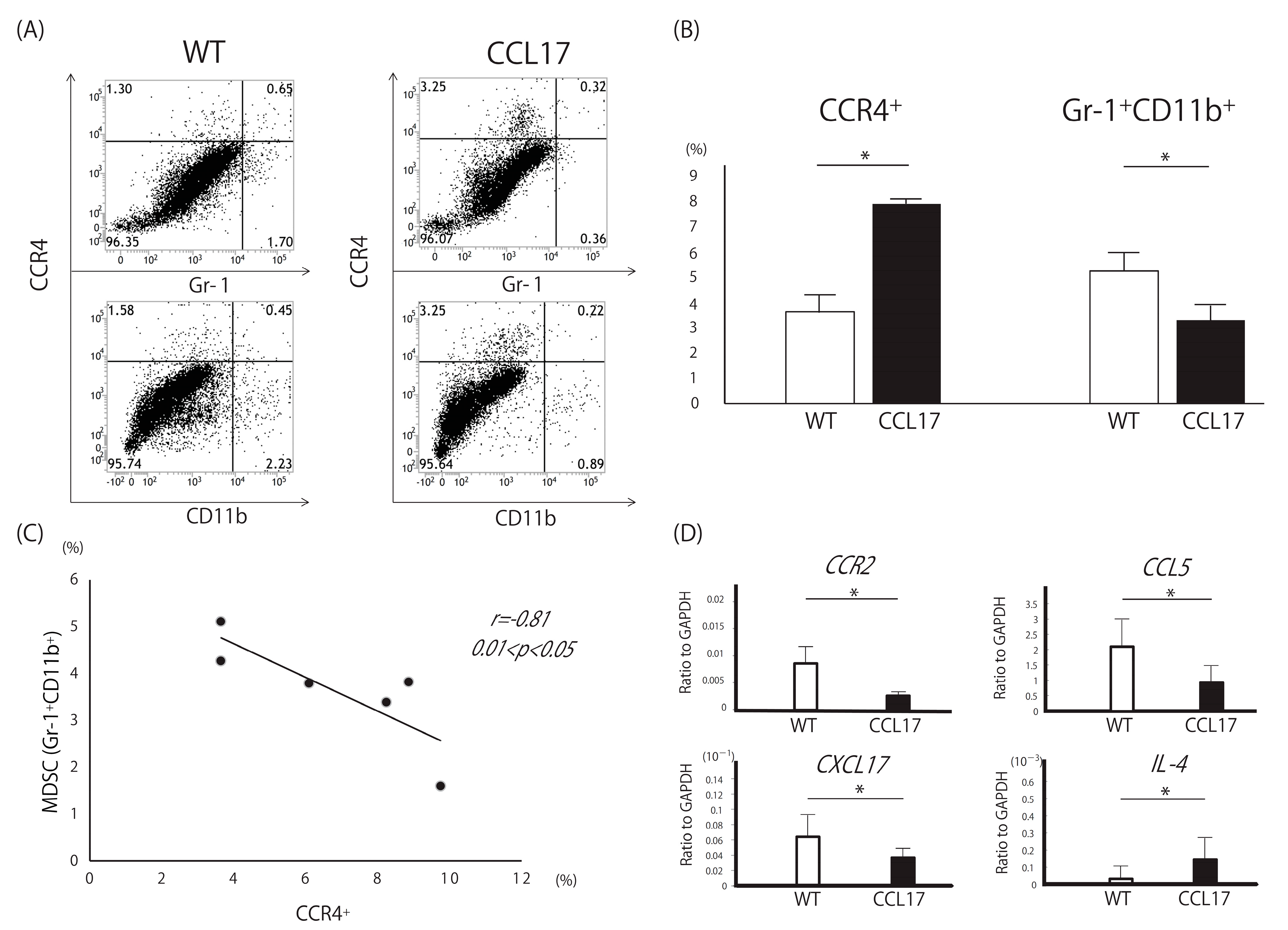
2.4. Decreased Myeloid-Derived Suppressor Cells in CCL17 TG Mice
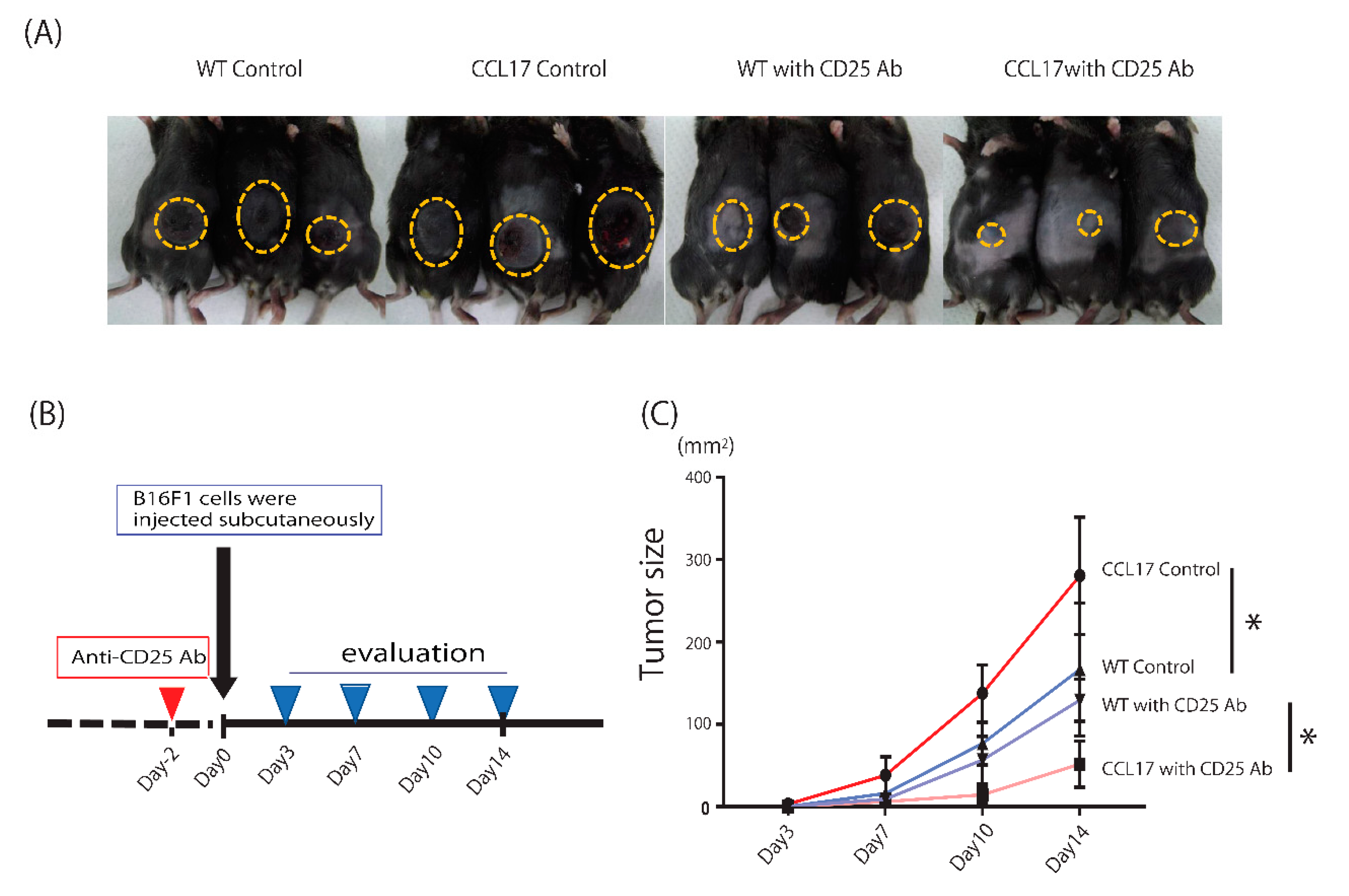
2.5. Enhanced Anti-Tumor Immunity after Depletion of Regulatory T Cells in CCL17 TG Mice
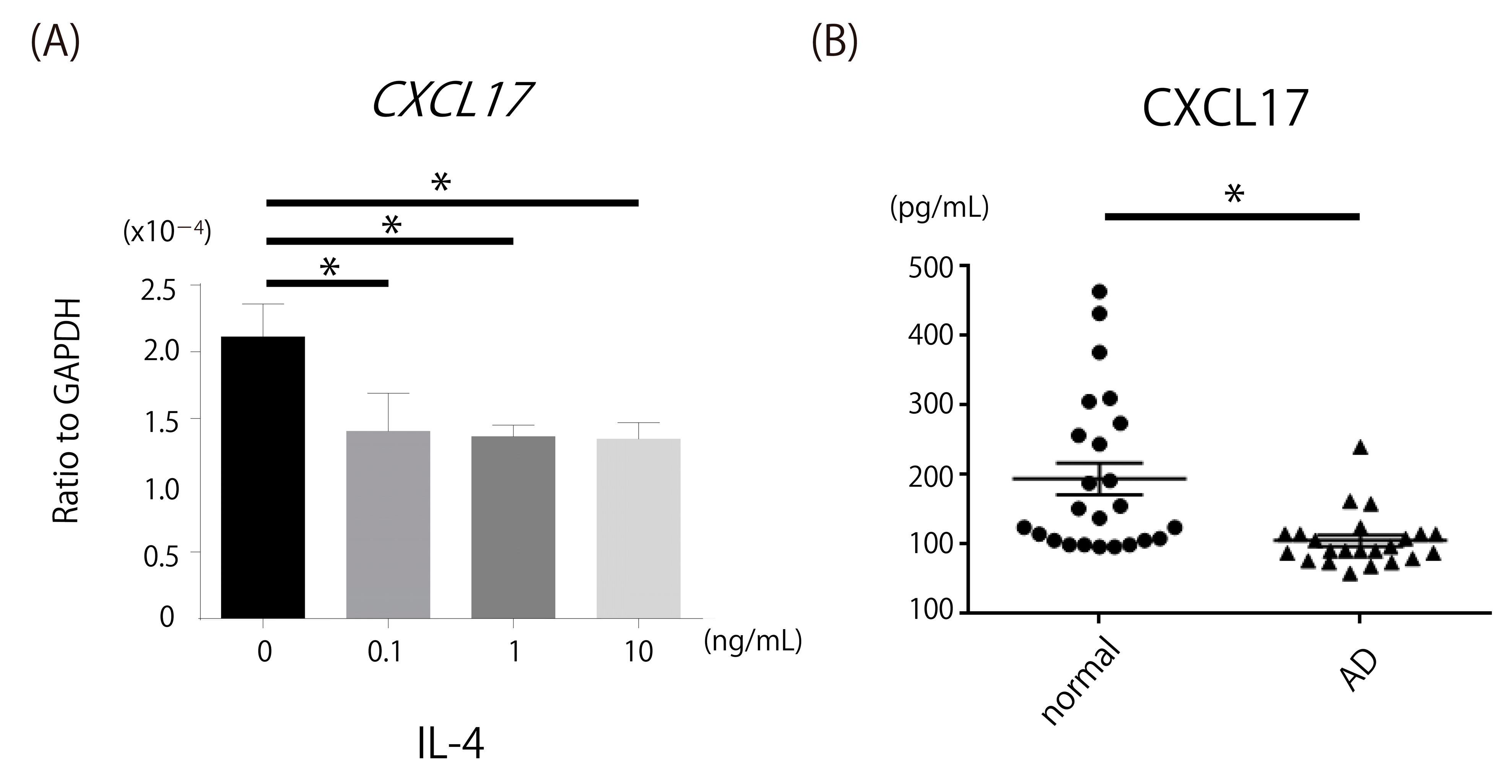
2.6. Decrease in CXCL17, a Chemoattractant of MDSCs, in Th2-Dominant Situation
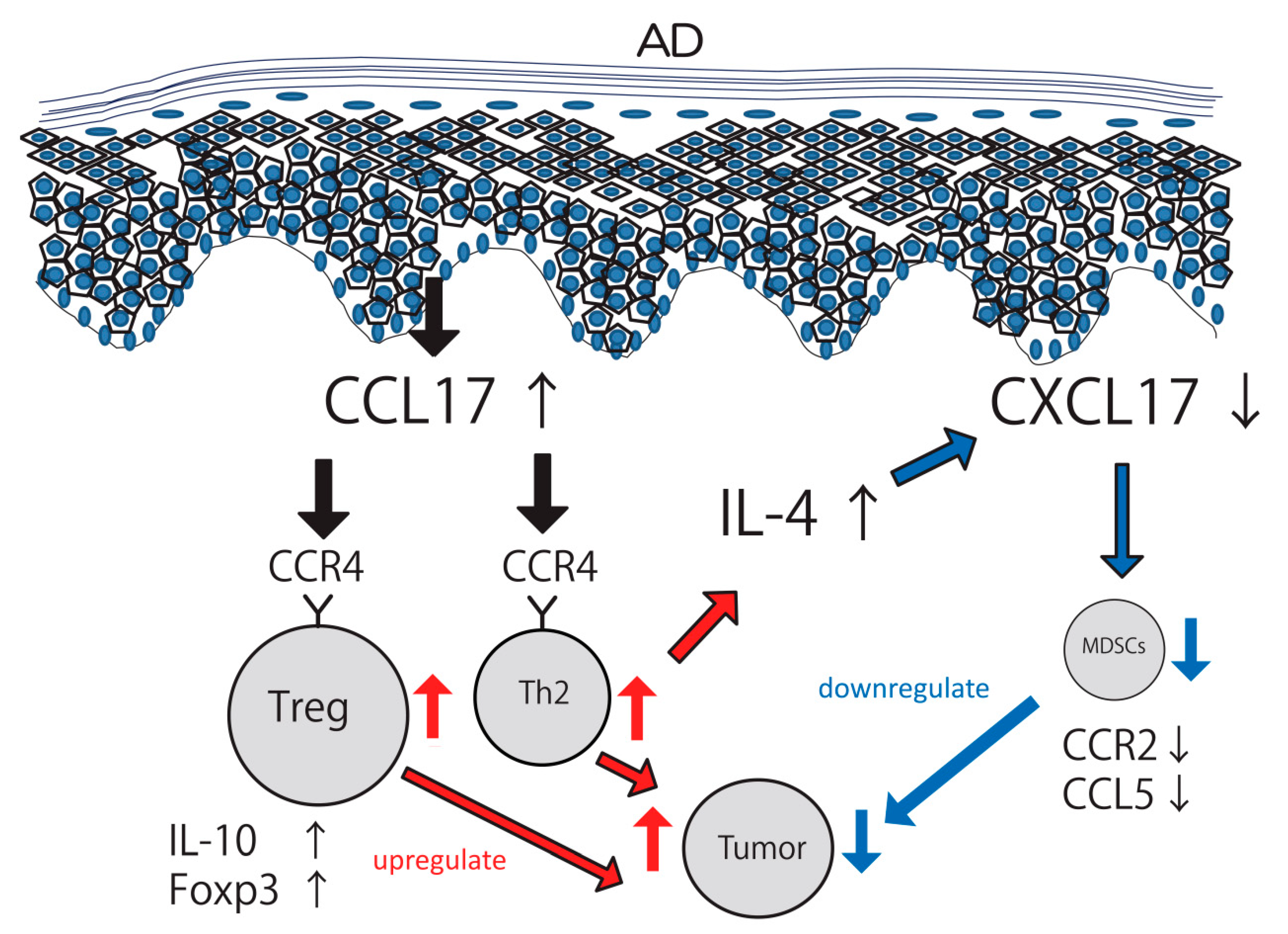
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. B16 Melanoma Cells and EL4 Lymphoma Cells
4.3. Primary Cutaneous Tumor Growth
4.4. Lung Metastasis
4.5. RNA Isolation and Quantitative Reverse Transcription-PCR
4.6. Histologic Examination
4.7. Flow Cytometric Analysis of Lymph Nodes
4.8. CXCL17 Expression Analysis of ELISA and In Vitro Cell Culture
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | atopic dermatitis |
| CTCL | cutaneous T-cell lymphoma |
| Tregs | regulatory T cells |
| TG | transgenic |
| MDSC | myeloid-derived suppressor cell |
| WT | wild-type |
| PBS | phosphate-buffered saline |
| NHEK | normal human epidermal keratinocytes |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
References
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Hanifin, J.M. Atopic dermatitis. J. Allergy Clin. Immunol. 1984, 73, 211–226. [Google Scholar] [CrossRef]
- Nettis, E.; Ortoncelli, M.; Pellacani, G.; Foti, C.; Di Leo, E.; Patruno, C.; Rongioletti, F.; Argenziano, G.; Ferrucci, S.M.; Macchia, L.; et al. A Multicenter Study on the Prevalence of Clinical Patterns and Clinical Phenotypes in Adult Atopic Dermatitis. J. Investig. Allergol. Clin. Immunol. 2020, 30, 448–450. [Google Scholar] [CrossRef]
- Schüz, J.; Morgan, G.; Böhler, E.; Kaatsch, P.; Michaelis, J.; Münster, E. Atopic disease and childhood acute lymphoblastic leukemia. Int. J. Cancer 2003, 105, 255–260. [Google Scholar] [CrossRef]
- Wen, W.; Shu, X.O.; Linet, M.S.; Neglia, J.P.; Potter, J.D.; Trigg, M.E.; Robison, L.L. Allergic disorders and the risk of childhood acute lymphoblastic leukemia (United States). Cancer Causes Control. 2000, 11, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Schlehofer, B.; Blettner, M.; Preston-Martin, S.; Niehoff, D.; Arslan, A.; Ahlbom, A.; Choi, W.N.; Giles, G.G.; Howe, G.R.; Little, J.; et al. Role of medical history in brain tumour development. Results from the international adult brain tumour study. Int. J. Cancer 1999, 82, 155–160. [Google Scholar] [CrossRef]
- Brenner, A.V.; Linet, M.S.; Fine, H.A.; Shapiro, W.R.; Selker, R.G.; Black, P.M.; Inskip, P.D. History of allergies and autoimmune diseases and risk of brain tumors in adults. Int. J. Cancer 2002, 99, 252–259. [Google Scholar] [CrossRef]
- Fabbro-Peray, P.; Daures, J.-P.; Rossi, J.-F. Environmental risk factors for non-Hodgkin’s lymphoma: A population-based case-control study in Languedoc-Roussillon, France. Cancer Causes Control. 2001, 12, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Doody, M.M.; Linet, M.S.; Glass, A.G.; Friedman, G.D.; Pottern, L.M.; Boice, J.D.; Fraumeni, J.F. Leukemia, lymphoma, and multiple myeloma following selected medical conditions. Cancer Causes Control. 1992, 3, 449–456. [Google Scholar] [CrossRef]
- Cartwright, R.; McKinney, P.; O’Brien, C.; Richards, I.; Roberts, B.; Lauder, I.; Darwin, C.; Bernard, S.; Bird, C. Non-hodgkin’s lymphoma: Case control epidemiological study in Yorkshire. Leuk. Res. 1988, 12, 81–88. [Google Scholar] [CrossRef]
- Gandini, S.; Stanganell, I.; Palli, D.; Giorgi, V.D.; Masala, G.; Caini, S. Atopic dermatitis, naevi count and skin cancer risk: A meta-analysis. J. Dermatol. Sci. 2016, 84, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Baba, M.; Nishimura, M.; Kakizaki, M.; Takagi, S.; Yoshie, O. The T cell-directed CC chemokine TARC is a higly specific biological ligand for C chemokine receptor 4. J. Biol. Chem. 1997, 272, 15036–15042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zlotnik, A.; Yoshie, O. Chemokines: A new classification system and their role in immunity. Immunity 2000, 12, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Kakinuma, T.; Nakamura, K.; Wakugawa, M.; Mitsui, H.; Tada, Y.; Saeki, H.; Torii, H.; Asahina, A.; Onai, N.; Matsushima, K.; et al. Thymus and activation-regulated chemokine in atopic dermatitis: Serum thymus and activation-regulated chemokine level is closely related with disease activity. J. Allergy Clin. Immunol. 2001, 107, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Kakinuma, T.; Sugaya, M.; Nakamura, K.; Kaneko, F.; Wakugawa, M.; Matsushima, K.; Tamaki, K. Thymus and activa-tion-regulated chemokine (TARC/CCL17) in mycosis fungoides: Serum TARC levels reflect the disease activity of mycosis fungoides. J. Am. Acad. Dermatol. 2003, 48, 23–30. [Google Scholar] [CrossRef]
- Sekiya, T.; Yamada, H.; Yamaguchi, M.; Yamamoto, K.; Ishii, A.; Yoshie, O.; Sano, Y.; Morita, A.; Matsushima, K.; Hirai, K. Increased levels of a TH2-type CC chemokine thymus and activation-regulated chemokine (TARC) in serum and induced sputum of asthmatics. Allergy 2002, 57, 173–177. [Google Scholar] [CrossRef]
- Miyazaki, E.; Nureki, S.; Fukami, T.; Shigenaga, T.; Ando, M.; Ito, K.; Ando, H.; Sugisaki, K.; Kumamoto, T.; Tsuda, T. Elevated levels of thymus-and actication-regulated chemokine in brochoalveolar lavage fluid from patients with eosinophilic pneumonia. Am. J. Respir. Crit. Care Med. 2002, 165, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Li, X.; Chen, S.; Zeng, Q.; Zhao, Y.; Luo, F. Tumor-associated macrophage or chemokine ligand CCL17 positively regulates the tumorigenesis of hepatocellular carcinoma. Med. Oncol. 2016, 33, 17. [Google Scholar] [CrossRef]
- Mizukami, Y.; Kono, K.; Kawaguchi, Y.; Akaike, H.; Kamimura, K.; Sugai, H.; Fujii, H. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int. J. Cancer 2008, 122, 2286–2293. [Google Scholar] [CrossRef] [PubMed]
- Strauss, L.; Bergmann, C.; Szczepanski, M.; Gooding, W.; Johnson, J.T.; Whiteside, T.L. A unique subset of CD4+CD25highFoxp3+ T cells secreting interleukin-10 and transforming growth factor-beta1 mediates suppression in the tumor microenvironment. Clin. Cancer Res. 2007, 13, 4345–4354. [Google Scholar] [CrossRef] [Green Version]
- Ostrand-Rosenberg, S.; Sinha, P. Myeloid-Derived Suppressor Cells: Linking Inflammation and Cancer. J. Immunol. 2009, 182, 4499–4506. [Google Scholar] [CrossRef]
- Tsunemi, Y.; Saeki, H.; Nakamura, K.; Nagakubo, D.; Nakayama, T.; Yoshie, O.; Kagami, S.; Shimazu, K.; Kadono, T.; Sugaya, M.; et al. CCL17 transgenic mice show an enhanced Th2-type response to both allergic and non-allergic stimuli. Eur. J. Immunol. 2006, 36, 2116–2127. [Google Scholar] [CrossRef]
- Yamada, M.; Yanaba, K.; Hasegawa, M.; Matsushita, Y.; Horikawa, M.; Komura, K.; Matsushita, T.; Kawasuji, A.; Fujita, T.; Takehara, K.; et al. Regulation of local and metastatic host-mediated anti-tumour mechanisms by l-selectin and intercellular adhesion molecule-1. Clin. Exp. Immunol. 2005, 143, 216–227. [Google Scholar] [CrossRef]
- Hsu, Y.-L.; Yen, M.-C.; Chang, W.-A.; Tsai, P.-H.; Pan, Y.-C.; Liao, S.-H.; Kuo, P.-L. CXCL17-derived CD11b+Gr-1+ myeloid-derived suppressor cells contribute to lung metastasis of breast cancer through platelet-derived growth factor-BB. Breast Cancer Res. 2019, 21, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Yan, J.; Xu, J.; Liu, C.-Q.; Zhen, Z.-J.; Chen, H.-W.; Ji, Y.; Wu, Z.-P.; Hu, J.-Y.; Zheng, L.; et al. CXCL17 Expression Predicts Poor Prognosis and Correlates with Adverse Immune Infiltration in Hepatocellular Carcinoma. PLoS ONE 2014, 9, e110064. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, L.; Hammarström, M.-L.; Lindmark, G.; Hammarström, S.; Sitohy, B. Ectopic expression of the chemokine CXCL17 in colon cancer cells. Br. J. Cancer 2016, 114, 697–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, S.; Kim, T.J.; Lee, H.; Min, Y.W.; Min, B.H.; Lee, J.H.; Son, H.J.; Rhee, P.L.; Beak, S.Y.; Kim, S.W.; et al. Association be-tween Atopic Dermatitis and Risk of Gastric Cancer: A Nationwide Population-based Study. Korean J. Gastroenterol. 2018, 71, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandini, S.; Lowenfels, A.B.; Jaffee, E.M.; Armstrong, T.D.; Maisonneuve, P. Allergies and the Risk of Pancreatic Cancer: A Meta-analysis with Review of Epidemiology and Biological Mechanisms. Cancer Epidemiol. Biomark. Prev. 2005, 14, 1908–1916. [Google Scholar] [CrossRef] [Green Version]
- Golumbek, P.T.; Lazenby, A.J.; I Levitsky, H.; Jaffee, L.M.; Karasuyama, H.; Baker, M.; Pardoll, D.M. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science 1991, 254, 713–716. [Google Scholar] [CrossRef]
- Noda, S.; Suárez-Fariñas, M.; Ungar, B.; Kim, S.J.; Strong, C.D.G.; Xu, H.; Peng, X.; Estrada, Y.D.; Nakajima, S.; Honda, T.; et al. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J. Allergy Clin. Immunol. 2015, 136, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Gooderham, M.J.; Hong, H.C.-H.; Eshtiaghi, P.; Papp, K.A. Dupilumab: A review of its use in the treatment of atopic dermatitis. J. Am. Acad. Dermatol. 2018, 78, S28–S36. [Google Scholar] [CrossRef] [PubMed]
- Miyagaki, T.; Sugaya, M. Erythrodermic cutaneous T-cell lymphoma: How to differentiate this rare disease from atopic der-matitis. J. Dermatol. Sci. 2011, 64, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, M.L.; Nguyen, M.T.; Aguirre, A.S.; Martinez-Escala, M.E.; Kim, J.; Walker, C.J.; Pontes, D.S.; Silverberg, J.I.; Choi, J.; Pro, B.; et al. Progression of cutaneous T-cell lymphoma after dupilumab: Case review of 7 patients. J. Am. Acad. Dermatol. 2020, 83, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Hollins, L.C.; Wirth, P.; Fulchiero, G.J.; Foulke, G.T. Long-standing dermatitis treated with dupilumab with subsequent pro-gression to cutaneous T-cell lymphoma. Cutis 2020, 106, E8–E11. [Google Scholar] [CrossRef] [PubMed]
- Harvey, M. Prism, Version 7; GraphPad: San Diego, CA, USA, 1994. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morimura, S.; Sugaya, M.; Oka, T.; Suga, H.; Miyagaki, T.; Tsunemi, Y.; Asano, Y.; Sato, S. Increased Regulatory T Cells and Decreased Myeloid-Derived Suppressor Cells Induced by High CCL17 Levels May Account for Normal Incidence of Cancers among Patients with Atopic Dermatitis. Int. J. Mol. Sci. 2021, 22, 2025. https://doi.org/10.3390/ijms22042025
Morimura S, Sugaya M, Oka T, Suga H, Miyagaki T, Tsunemi Y, Asano Y, Sato S. Increased Regulatory T Cells and Decreased Myeloid-Derived Suppressor Cells Induced by High CCL17 Levels May Account for Normal Incidence of Cancers among Patients with Atopic Dermatitis. International Journal of Molecular Sciences. 2021; 22(4):2025. https://doi.org/10.3390/ijms22042025
Chicago/Turabian StyleMorimura, Sohshi, Makoto Sugaya, Tomonori Oka, Hiraku Suga, Tomomitsu Miyagaki, Yuichiro Tsunemi, Yoshihide Asano, and Shinichi Sato. 2021. "Increased Regulatory T Cells and Decreased Myeloid-Derived Suppressor Cells Induced by High CCL17 Levels May Account for Normal Incidence of Cancers among Patients with Atopic Dermatitis" International Journal of Molecular Sciences 22, no. 4: 2025. https://doi.org/10.3390/ijms22042025
APA StyleMorimura, S., Sugaya, M., Oka, T., Suga, H., Miyagaki, T., Tsunemi, Y., Asano, Y., & Sato, S. (2021). Increased Regulatory T Cells and Decreased Myeloid-Derived Suppressor Cells Induced by High CCL17 Levels May Account for Normal Incidence of Cancers among Patients with Atopic Dermatitis. International Journal of Molecular Sciences, 22(4), 2025. https://doi.org/10.3390/ijms22042025






