Copper Dependent Modulation of α-Synuclein Phosphorylation in Differentiated SHSY5Y Neuroblastoma Cells
Abstract
1. Introduction
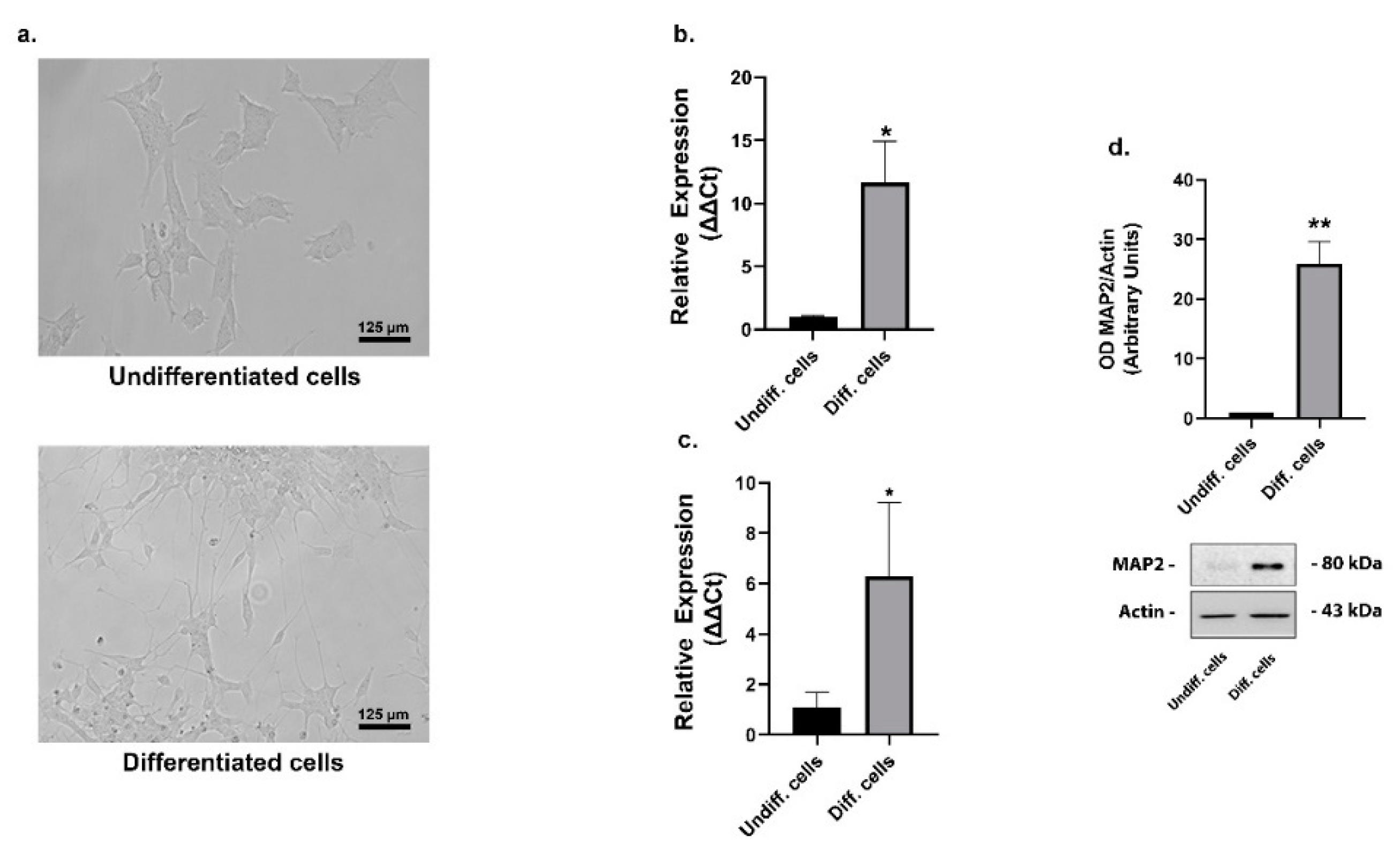
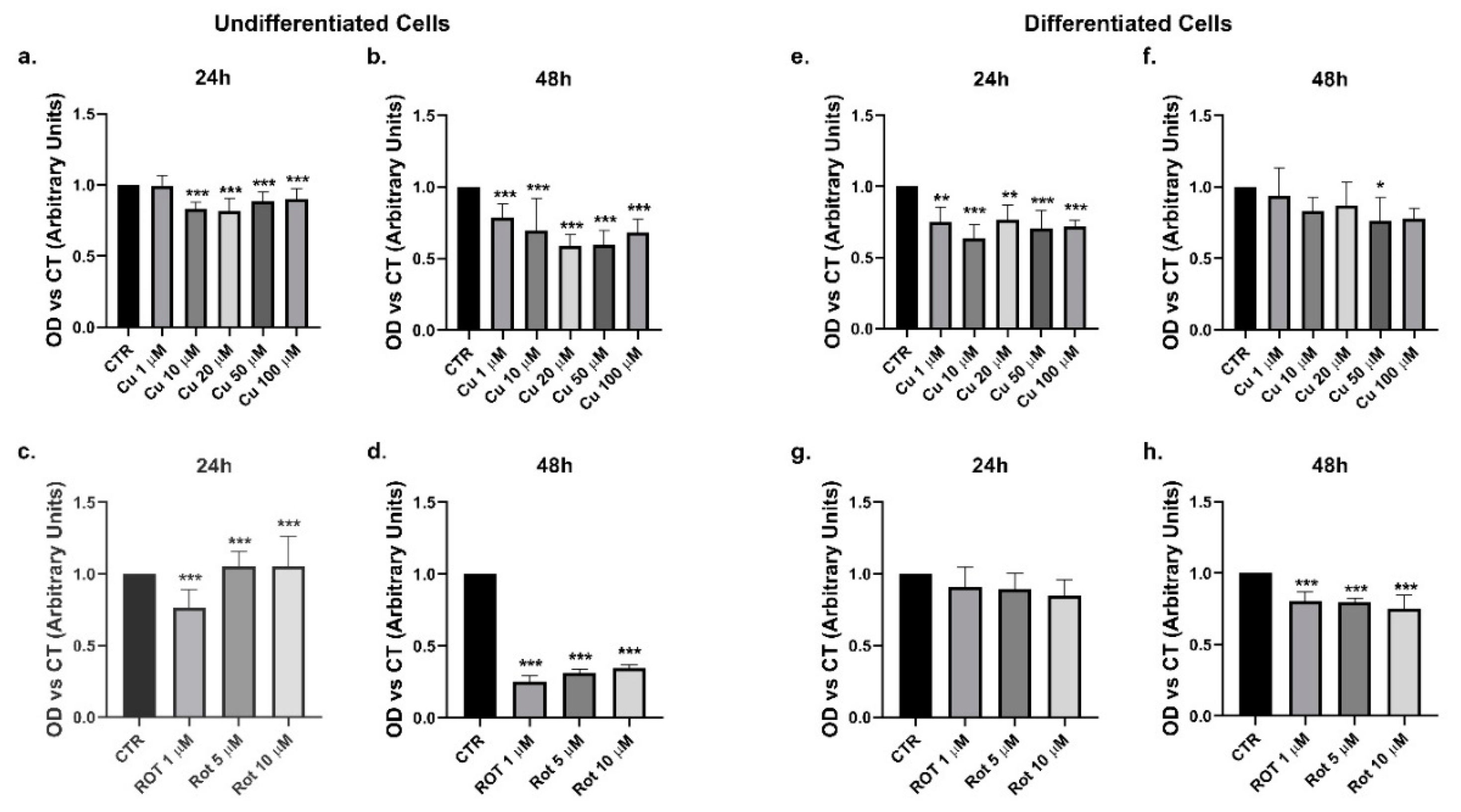
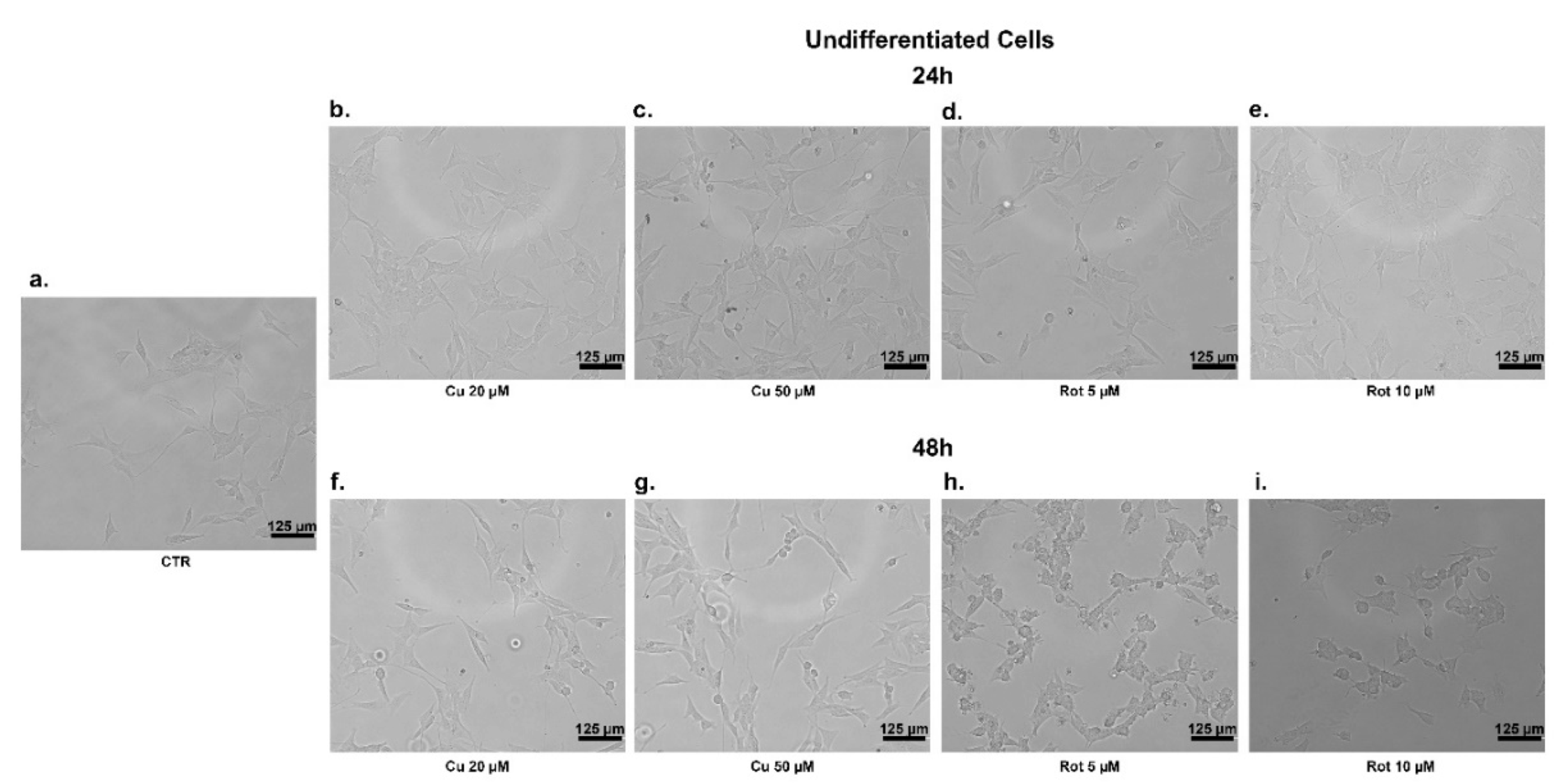
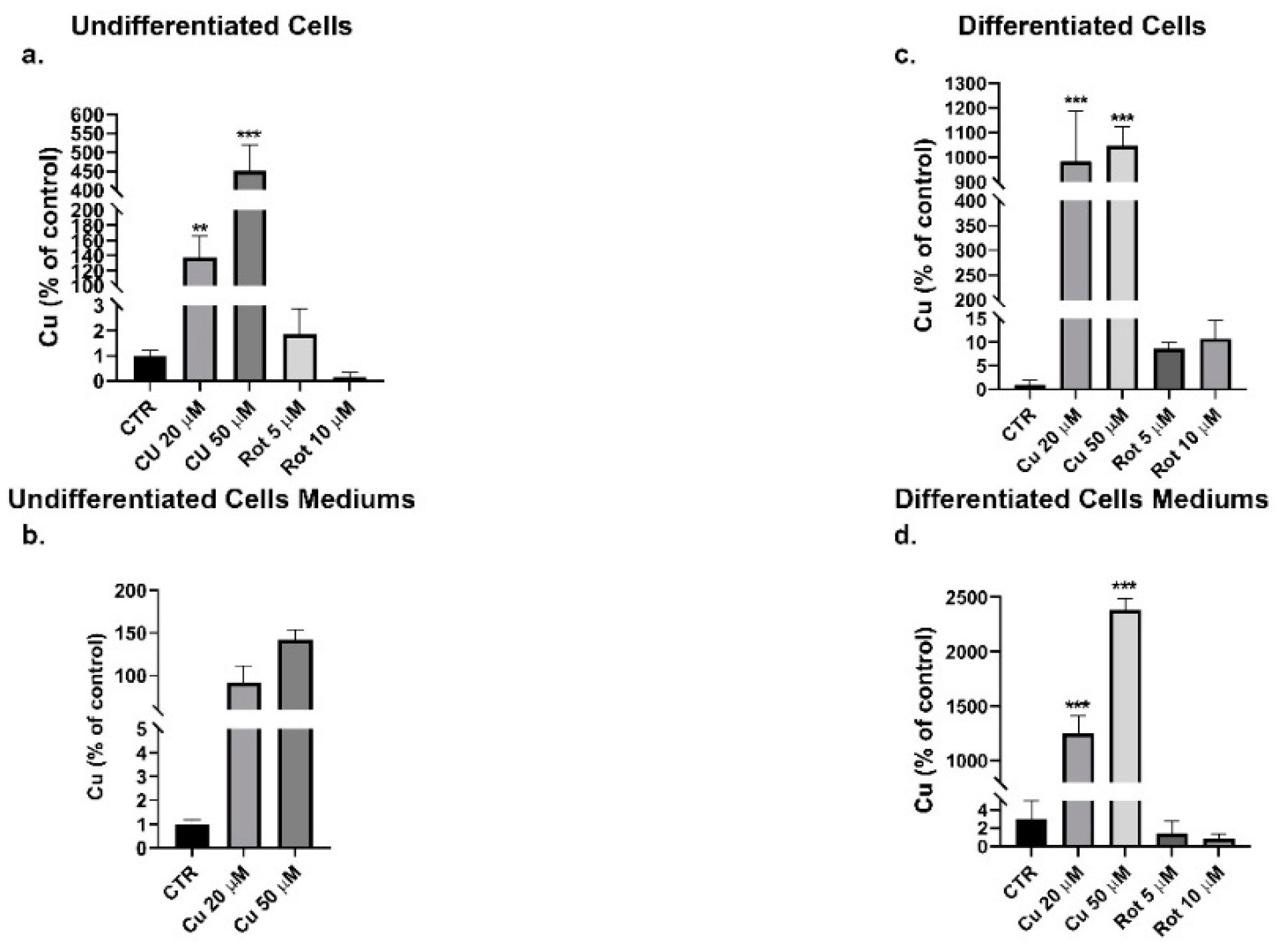
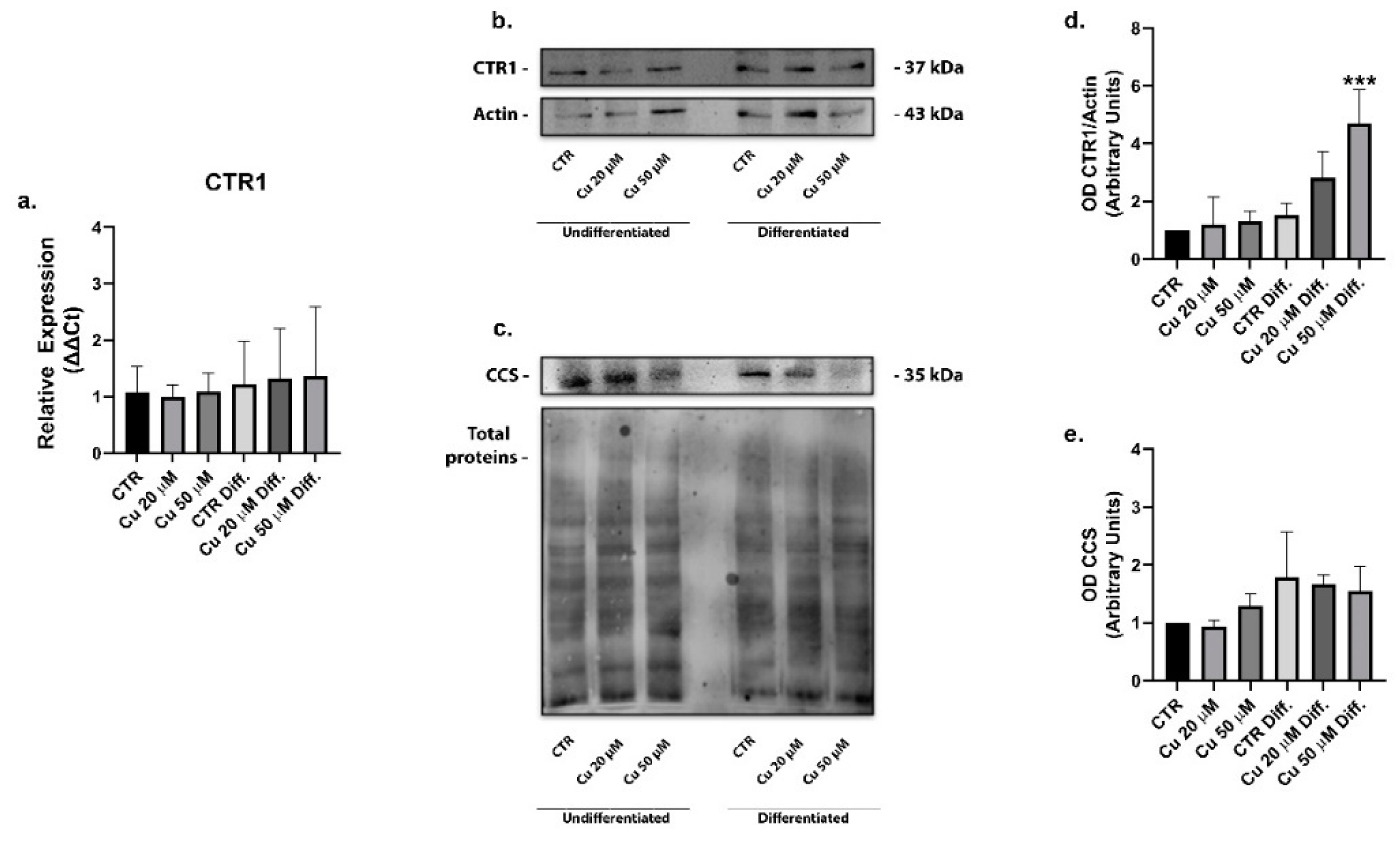
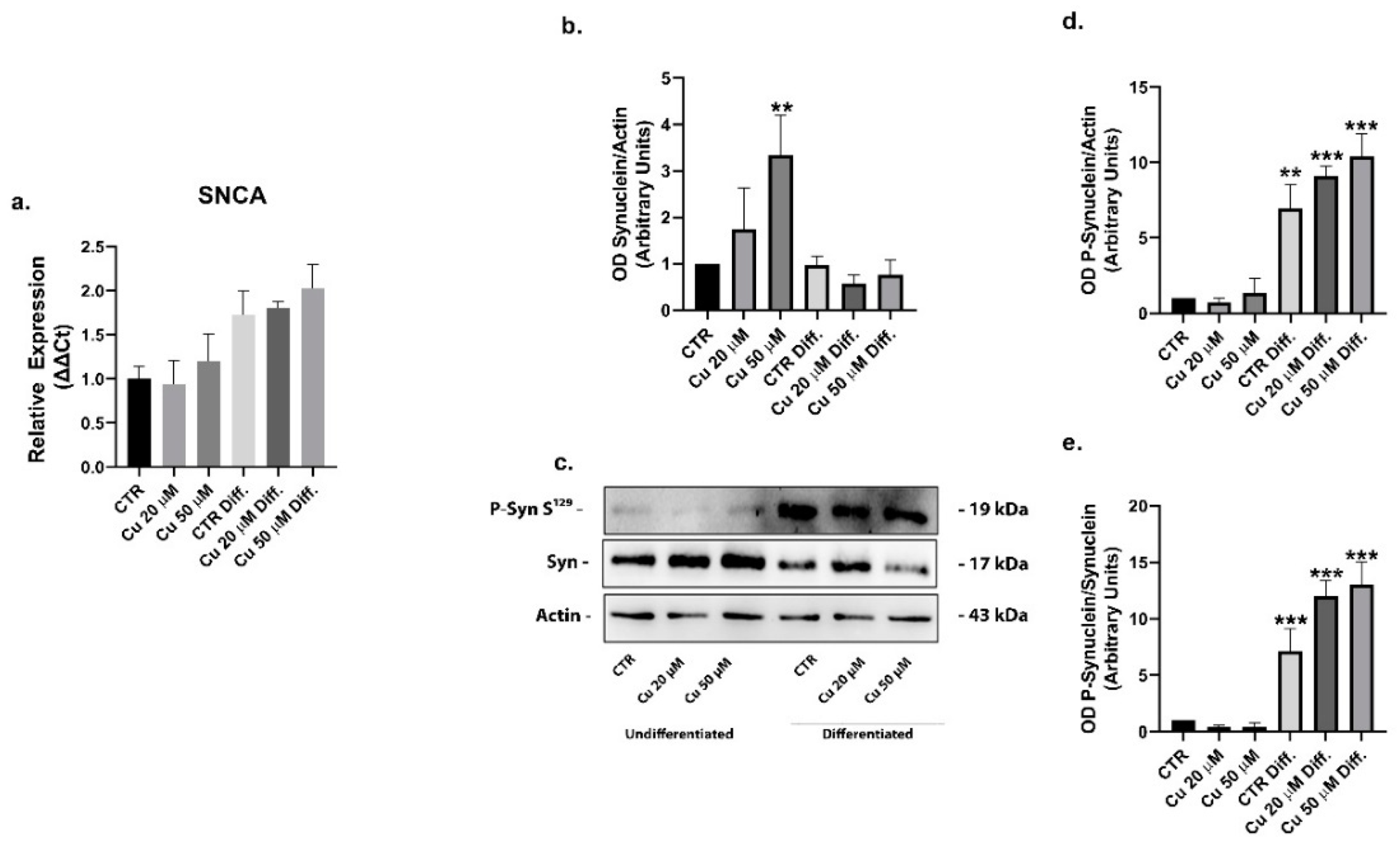
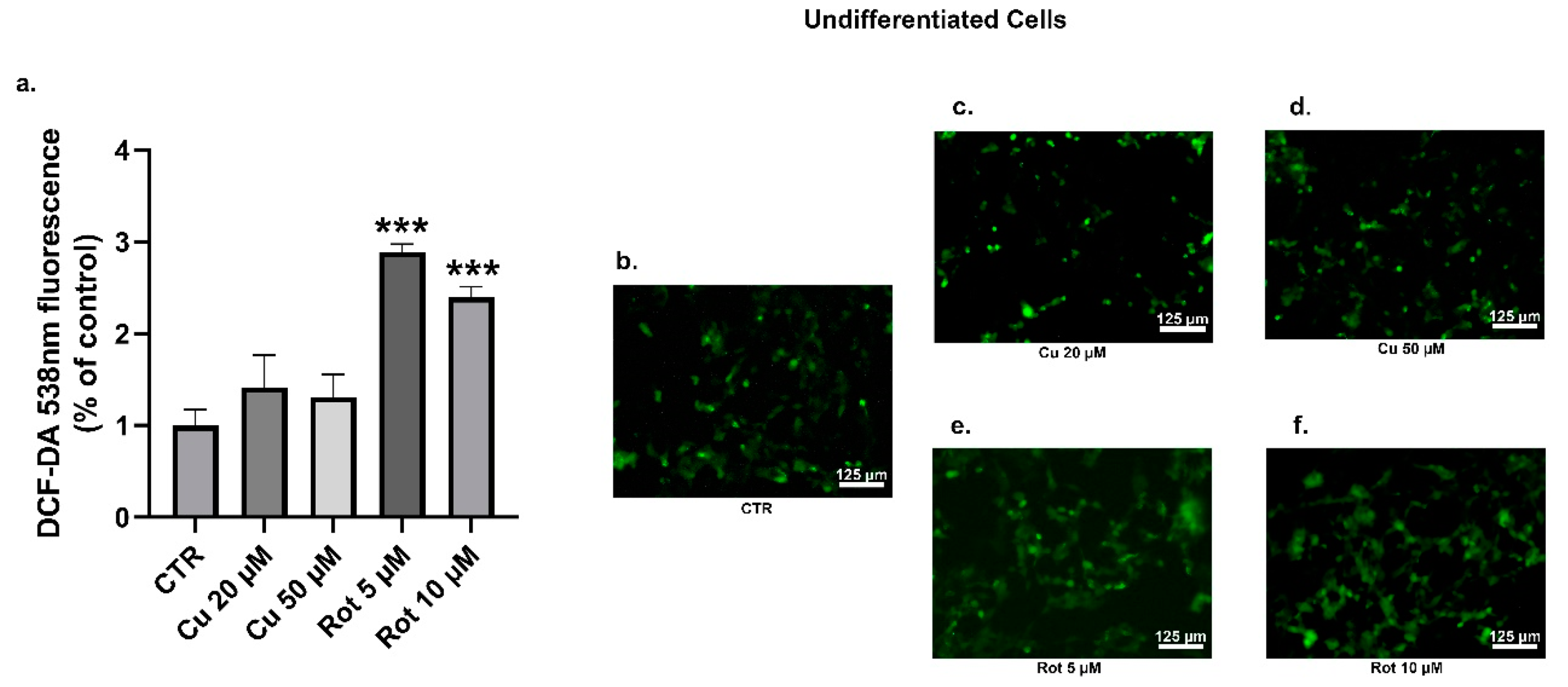
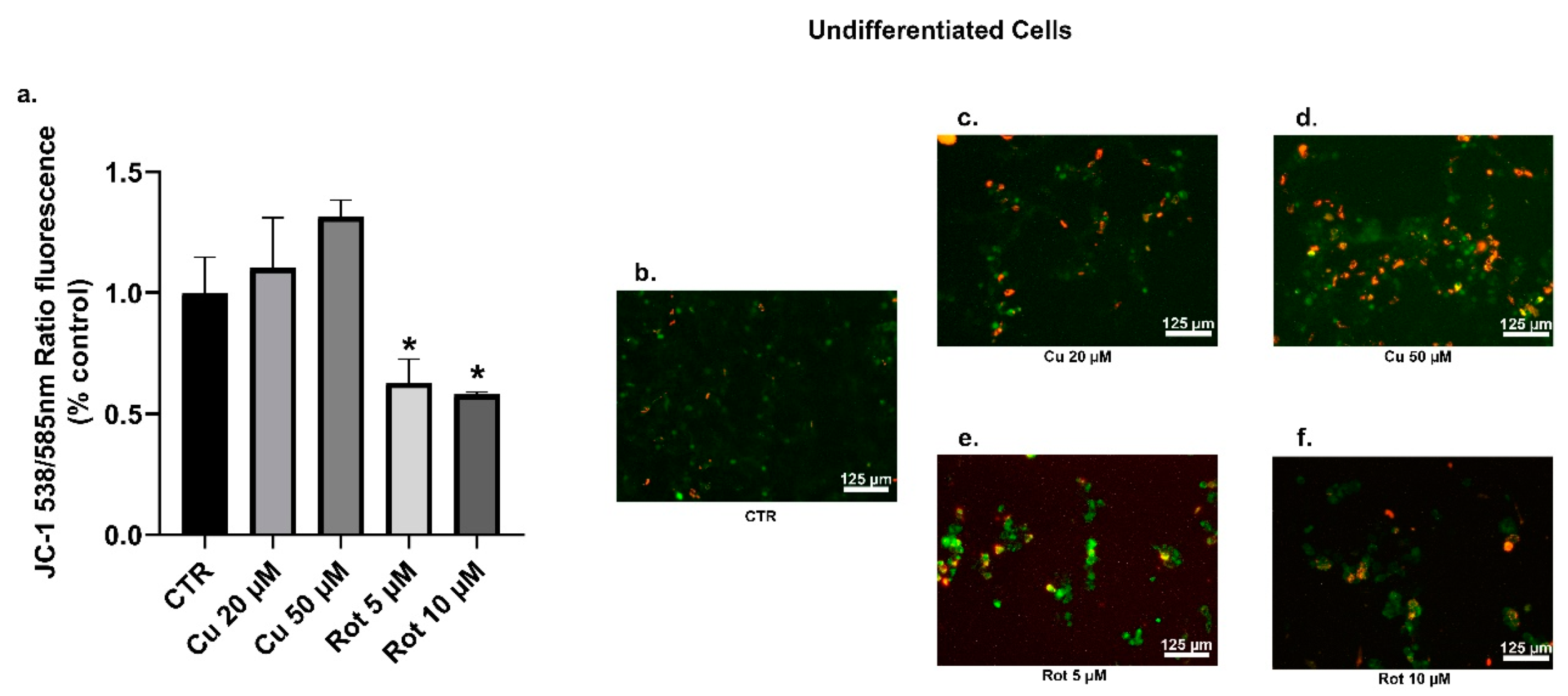
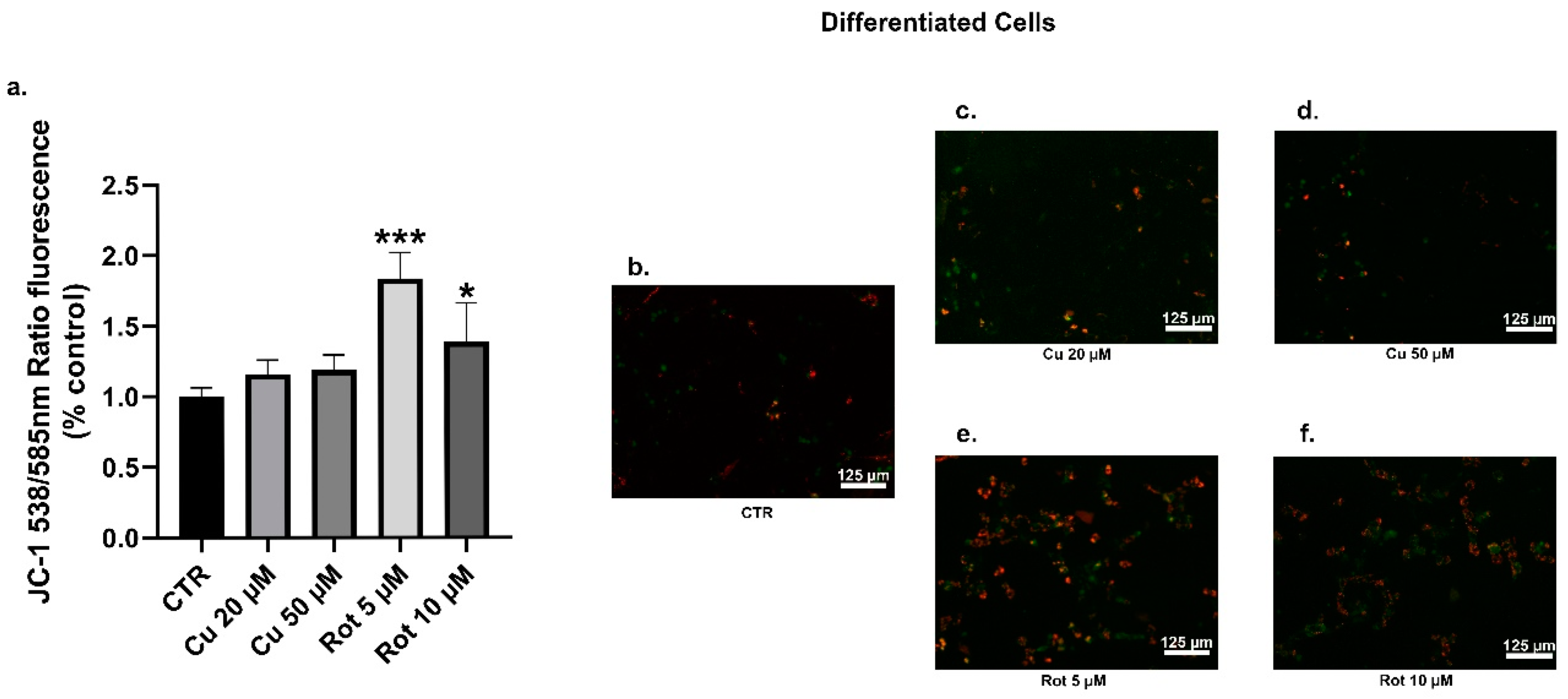
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Treatments
4.2. Cell Viability Assay
4.3. Analysis of Copper Content by Atomic Absorption
4.4. Western Blot Analyses
4.5. Analysis of Mitochondrial Membrane Potential and ROS Production
4.6. Expression Analysis by qPCR
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cu | copper |
| PD | Parkinson’s disease |
| CNS | central nervous system |
| ROS | reactive oxygen species |
| CTR1 | copper transporter protein 1 |
| PLK2 | polo like kinase 2 |
| PP2A | protein phosphatase 2A |
| LB | Lewy body |
| UPS | ubiquitin proteasome system |
| CTR2 | copper transporter protein 2 |
| Dmt1 | divalent metal transporter 1 |
| ATP7A | copper-transporting ATPases 1 |
| ATP7B | copper-transporting ATPases 2 |
| ATOX1 | copper transport protein |
| SOD3 | extracellular superoxide dismutase [Cu-Zn] |
| COX | cytochrome c oxidase |
| Cox17 | cytochrome c oxidase copper chaperone |
| Sco1 | cytochrome c oxidase assembly protein 1 chaperone |
| Sco2 | cytochrome c oxidase assembly protein 2 chaperone |
| CCS | copper chaperone for superoxide dismutase |
| SOD1 | superoxide dismutase [Cu-Zn] |
| TH | tyrosine hydroxylase |
| DAT | dopamine transporter |
| RA | retinoic acid |
| BDNF | brain-derived neurotrophic factor |
| MAP2 | microtubule-associated protein 2 |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| CTR | untreated cells |
| SNCA | α-synuclein gene |
| DCFDA | 2′,7′-dichlorofluorescin diacetate |
| MMP | mitochondrial membrane potential |
| JC-1 | 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbo-cyanine iodide |
| DMEM | Dulbecco’s Modified Eagle Medium |
| MMP | mitochondrial membrane potential |
| MPT | membrane permeability transition |
| ANT | nucleotide translocase adenine |
| OD | optical density |
| TBS | Tris-buffered saline |
References
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; A Nalls, M.; Hallgrímsdóttir, I.B.; Hunkapiller, J.; Van Der Brug, M.; Cai, F.; A Kerchner, G.; Ayalon, G.; Bingol, B.; Sheng, M.; et al. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat. Genet. 2017, 49, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Cicero, C.E.; Mostile, G.; Vasta, R.; Rapisarda, V.; Signorelli, S.S.; Ferrante, M.; Zappia, M.; Nicoletti, A. Metals and neurodegenerative diseases. A systematic review. Environ. Res. 2017, 159, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, E.R.; Elbaz, A.; Nichols, E.; Abd-Allah, F.; Abdelalim, A.; Adsuar, J.C.; Ansha, M.G.; Brayne, C.; Choi, J.-Y.J.; Collado-Mateo, D.; et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef]
- Hu, C.-Y.; Fang, Y.; Li, F.-L.; Dong, B.; Hua, X.-G.; Jiang, W.; Zhang, H.; Lyu, Y.; Zhang, X.-J. Association between ambient air pollution and Parkinson’s disease: Systematic review and meta-analysis. Environ. Res. 2019, 168, 448–459. [Google Scholar] [CrossRef]
- Emamzadeh, F.N.; Surguchov, A. Parkinson’s Disease: Biomarkers, Treatment, and Risk Factors. Front. Neurosci. 2018, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, R.; Schapira, A.H. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Oueslati, A. Implication of Alpha-Synuclein Phosphorylation at S129 in Synucleinopathies: What Have We Learned in the Last Decade? J. Park. Dis. 2016, 6, 39–51. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Li, J.-D. The Roles of Post-translational Modifications on α-Synuclein in the Pathogenesis of Parkinson’s Diseases. Front. Neurosci. 2019, 13, 381. [Google Scholar] [CrossRef]
- Anandhan, A.; Rodriguez-Rocha, H.; Bohovych, I.; Griggs, A.M.; Zavala-Flores, L.; Reyes-Reyes, E.M.; Seravalli, J.; Stanciu, L.A.; Lee, J.; Rochet, J.-C.; et al. Overexpression of alpha-synuclein at non-toxic levels increases dopaminergic cell death induced by copper exposure via modulation of protein degradation pathways. Neurobiol. Dis. 2015, 81, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Raveendran, A.; Nagotu, S. Chaperones and Proteostasis: Role in Parkinson’s Disease. Diseases 2020, 8, 24. [Google Scholar] [CrossRef]
- Bisaglia, M.; Bubacco, L. Copper Ions and Parkinson’s Disease: Why Is Homeostasis So Relevant? Biomolecules 2020, 10, 195. [Google Scholar] [CrossRef]
- Ackerman, C.M.; Chang, C.J. Copper signaling in the brain and beyond. J. Biol. Chem. 2018, 293, 4628–4635. [Google Scholar] [CrossRef] [PubMed]
- Malavolta, M. Trace Elements and Minerals in Health and Longevity; Malavolta, M., Mocchegiani, E., Eds.; Springer International Publishing: Ancona, Italy, 2018. [Google Scholar] [CrossRef]
- Spasojević, I.; Mojović, M.; Stević, Z.; Spasić, S.D.; Jones, D.R.; Morina, A.; Spasić, M.B. Bioavailability and Catalytic Properties of Copper and Iron for Fenton Chemistry in Human Cerebrospinal Fluid. Redox Rep. 2010, 15, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Stuerenburg, H.J. CSF copper concentrations, blood-brain barrier function, and coeruloplasmin synthesis during the treatment of Wilson’s disease. J. Neural Transm. 2000, 107, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Huberts, D.H.; Van Der Klei, I.J. Moonlighting proteins: An intriguing mode of multitasking. Biochim. Biophys. Acta BBA Bioenergy 2010, 1803, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.H.; Maryon, E.B. How Mammalian Cells Acquire Copper: An Essential but Potentially Toxic Metal. Biophys. J. 2016, 110, 7–13. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M.; Kaplan, J.H. Copper transporters and copper chaperones: Roles in cardiovascular physiology and disease. Am. J. Physiol. Physiol. 2018, 315, C186–C201. [Google Scholar] [CrossRef] [PubMed]
- Prohaska, J.R. Role of copper transporters in copper homeostasis. Am. J. Clin. Nutr. 2008, 88, 826S–829S. [Google Scholar] [CrossRef]
- Davies, K.M.; Mercer, J.F.; Chen, N.; Double, K.L. Copper dyshomoeostasis in Parkinson’s disease: Implications for pathogenesis and indications for novel therapeutics. Clin. Sci. 2016, 130, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Muñoz, B.; Sepúlveda, F.J.; Urrutia, J.; Quiroz, M.; Luza, S.; De Ferrari, G.V.; Aguayo, L.G.; Opazo, C. Biphasic effects of copper on neurotransmission in rat hippocampal neurons. J. Neurochem. 2011, 119, 78–88. [Google Scholar] [CrossRef]
- Opazo, C.M.; Greenough, M.A.; Bush, A.I. Copper: From neurotransmission to neuroproteostasis. Front. Aging Neurosci. 2014, 6, 143. [Google Scholar] [CrossRef]
- Kepp, K.P.; Squitti, R. Copper imbalance in Alzheimer’s disease: Convergence of the chemistry and the clinic. Co-ord. Chem. Rev. 2019, 397, 168–187. [Google Scholar] [CrossRef]
- Lázaro, D.F.; Pavlou, M.A.S.; Outeiro, T.F. Cellular models as tools for the study of the role of alpha-synuclein in Parkinson’s disease. Exp. Neurol. 2017, 298, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Kunzler, A.; Zeidán-Chuliá, F.; Gasparotto, J.; Girardi, C.S.; Klafke, K.; Petiz, L.L.; Bortolin, R.C.; Rostirolla, D.C.; Zanotto-Filho, A.; Pasquali, M.A.D.B.; et al. Changes in Cell Cycle and Up-Regulation of Neuronal Markers During SH-SY5Y Neurodifferentiation by Retinoic Acid are Mediated by Reactive Species Production and Oxidative Stress. Mol. Neurobiol. 2017, 54, 6903–6916. [Google Scholar] [CrossRef] [PubMed]
- Shipley, M.M.; Mangold, C.A.; Szpara, M.L. Differentiation of the SH-SY5Y Human Neuroblastoma Cell Line. J. Vis. Exp. 2016, e53193. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, M.; Huh, Y.-J.; Lee, Y.-I. The Impairments of α-Synuclein and Mechanistic Target of Rapamycin in Rotenone-Induced SH-SY5Y Cells and Mice Model of Parkinson’s Disease. Front. Neurosci. 2019, 13, 1028. [Google Scholar] [CrossRef] [PubMed]
- Watt, N.T.; Hooper, N.M. The response of neurones and glial cells to elevated copper. Brain Res. Bull. 2001, 55, 219–224. [Google Scholar] [CrossRef]
- Chan, H.W.; Liu, T.; Verdile, G.; Bishop, G.; Haasl, R.J.; Smith, M.A.; Perry, G.; Martins, R.N.; Atwood, C.S. Copper Induces Apoptosis of Neuroblastoma Cells Via Post-translational Regulation of the Expression of Bcl-2-family Proteins and the txJ Mouse is a Better Model of Hepatic than Brain Cu Toxicity. Int. J. Clin. Exp. Med. 2008, 1, 76–88. [Google Scholar] [PubMed]
- Omar, S.H.; Kerr, P.G.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Olive (Olea europaea L.) Biophenols: A Nutriceutical against Oxidative Stress in SH-SY5Y Cells. Molecules 2017, 22, 1858. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.M.; Kamel, F.; Ross, G.W.; Hoppin, J.A.; Goldman, S.M.; Korell, M.; Marras, C.; Bhudhikanok, G.S.; Kasten, M.; Chade, A.R.; et al. Rotenone, Paraquat, and Parkinson’s Disease. Environ. Health Perspect. 2011, 119, 866–872. [Google Scholar] [CrossRef]
- Betarbet, R.; Sherer, T.B.; MacKenzie, G.; Garcia-Osuna, M.; Panov, A.V.; Greenamyre, J.T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 2000, 3, 1301–1306. [Google Scholar] [CrossRef]
- Hatori, Y.; Yan, Y.; Schmidt, K.; Furukawa, E.; Hasan, N.M.; Yang, N.; Liu, C.-N.; Sockanathan, S.; Lutsenko, S. Neuronal differentiation is associated with a redox-regulated increase of copper flow to the secretory pathway. Nat. Commun. 2016, 7, 10640. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-M.; Zhou, B.; Cosco, D.; Gitschier, J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc. Natl. Acad. Sci. USA 2001, 98, 6836–6841. [Google Scholar] [CrossRef] [PubMed]
- Field, L.S.; Luk, E.; Culotta, V.C. Copper Chaperones: Personal Escorts for Metal Ions. J. Bioenergy Biomembr. 2002, 34, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.M.; Gupta, A.; Polishchuk, E.; Yu, C.H.; Polishchuk, R.; Dmitriev, O.Y.; Lutsenko, S. Molecular Events Initiating Exit of a Copper-transporting ATPase ATP7B from the Trans-Golgi Network. J. Biol. Chem. 2012, 287, 36041–36050. [Google Scholar] [CrossRef] [PubMed]
- Bohlken, A.; Cheung, B.B.; Bell, J.L.; Koach, J.; Smith, S.; Sekyere, E.; Thomas, W.; Norris, M.; Haber, M.; Lovejoy, D.B.; et al. ATP7A is a novel target of retinoic acid receptor β2 in neuroblastoma cells. Br. J. Cancer 2009, 100, 96–105. [Google Scholar] [CrossRef]
- Perfeito, R.; Lázaro, D.F.; Outeiro, T.F.; Rego, A.C. Linking alpha-synuclein phosphorylation to reactive oxygen species formation and mitochondrial dysfunction in SH-SY5Y cells. Mol. Cell. Neurosci. 2014, 62, 51–59. [Google Scholar] [CrossRef]
- Smith, W.W.; Margolis, R.L.; Li, X.; Troncoso, J.C.; Lee, M.K.; Dawson, V.L.; Dawson, T.M.; Iwatsubo, T.; Ross, C.A. Synuclein Phosphorylation Enhances Eosinophilic Cytoplasmic Inclusion Formation in SH-SY5Y Cells. J. Neurosci. 2005, 25, 5544–5552. [Google Scholar] [CrossRef]
- Xun, Z.; Lee, D.-Y.; Lim, J.; Canaria, C.A.; Barnebey, A.; Yanonne, S.M.; McMurray, C.T. Retinoic acid-induced differentiation increases the rate of oxygen consumption and enhances the spare respiratory capacity of mitochondria in SH-SY5Y cells. Mech. Ageing Dev. 2012, 133, 176–185. [Google Scholar] [CrossRef]
- Forkink, M.; Manjeri, G.R.; Liemburg-Apers, D.C.; Nibbeling, E.; Blanchard, M.; Wojtala, A.; Smeitink, J.A.; Wieckowski, M.R.; Willems, P.H.; Koopman, W.J. Mitochondrial hyperpolarization during chronic complex I inhibition is sustained by low activity of complex II, III, IV and V. Biochim. Biophys. Acta BBA Bioenergy 2014, 1837, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Spletter, M.L.; Johnson, D.A.; Wright, L.S.; Svendsen, C.N.; Johnson, J.A. Rotenone-induced caspase 9/3-independent and -dependent cell death in undifferentiated and differentiated human neural stem cells. J. Neurochem. 2005, 92, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Petit, P.; Zamzami, N.; Vayssière, J.; Mignotte, B. The biochemistry of programmed cell death. FASEB J. 1995, 9, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; E Klaunig, J. Role of the mitochondrial membrane permeability transition (MPT) in rotenone-induced apoptosis in liver cells. Toxicol. Sci. 2000, 53, 340–351. [Google Scholar] [CrossRef]
- Bernardi, P.; Di Lisa, F. The mitochondrial permeability transition pore: Molecular nature and role as a target in cardioprotection. J. Mol. Cell. Cardiol. 2015, 78, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Muntané, G.; Ferrer, I.; Martinez-Vicente, M. α-synuclein phosphorylation and truncation are normal events in the adult human brain. Neuroscience 2012, 200, 106–119. [Google Scholar] [CrossRef]
- Pronin, A.N.; Morris, A.J.; Surguchov, A.; Benovic, J.L. Synucleins Are a Novel Class of Substrates for G Protein-coupled Receptor Kinases. J. Biol. Chem. 2000, 275, 26515–26522. [Google Scholar] [CrossRef]
- Fujiwara, H.; Hasegawa, M.; Dohmae, N.; Kawashima, A.; Masliah, E.; Goldberg, M.S.; Shen, J.; Takio, K.; Iwatsubo, T. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 2002, 4, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.P.; Walker, D.E.; Goldstein, J.M.; De Laat, R.; Banducci, K.; Caccavello, R.J.; Barbour, R.; Huang, J.; Kling, K.; Lee, M.; et al. Phosphorylation of Ser-129 Is the Dominant Pathological Modification of α-Synuclein in Familial and Sporadic Lewy Body Disease. J. Biol. Chem. 2006, 281, 29739–29752. [Google Scholar] [CrossRef] [PubMed]
- Rasia, R.M.; Bertoncini, C.W.; Marsh, D.; Hoyer, W.; Cherny, D.; Zweckstetter, M.; Griesinger, C.; Jovin, T.M.; Fernández, C.O. Structural characterization of copper(II) binding to -synuclein: Insights into the bioinorganic chemistry of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2005, 102, 4294–4299. [Google Scholar] [CrossRef] [PubMed]
- Lucas, H.R.; Lee, J.C. Copper(ii) enhances membrane-bound α-synuclein helix formation. Metallomics 2011, 3, 280–283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Castillo-Gonzalez, J.A.; Loera-Arias, M.D.J.; Saucedo-Cardenas, O.; Montes-De-Oca-Luna, R.; Garcia-Garcia, A.; Rodriguez-Rocha, H. Phosphorylatedα-Synuclein-Copper Complex Formation in the Pathogenesis of Parkinson’s Disease. Park. Dis. 2017, 2017, 9164754. [Google Scholar] [CrossRef]
- González, N.; Arcos-López, T.; König, A.; Quintanar, L.; Márquez, M.M.; Outeiro, T.F.; Fernández, C.O. Effects of alpha-synuclein post-translational modifications on metal binding. J. Neurochem. 2019, 150, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, J.D.; Dykes-Hoberg, M.; Corson, L.B.; Becker, M.; Cleveland, D.W.; Price, N.L.; Culotta, V.C.; Wong, P.C. The copper chaperone CCS is abundant in neurons and astrocytes in human and rodent brain. J. Neurochem. 1999, 72, 422–429. [Google Scholar] [CrossRef]
- Bertinato, J.; L’Abbé, M.R. Copper Modulates the Degradation of Copper Chaperone for Cu, Zn Superoxide Dismutase by the 26 S Proteosome. J. Biol. Chem. 2003, 278, 35071–35078. [Google Scholar] [CrossRef]
- Mbefo, M.K.; Paleologou, K.E.; Boucharaba, A.; Oueslati, A.; Schell, H.; Fournier, M.; Olschewski, D.; Yin, G.; Zweckstetter, M.; Masliah, E.; et al. Phosphorylation of Synucleins by Members of the Polo-like Kinase Family. J. Biol. Chem. 2010, 285, 2807–2822. [Google Scholar] [CrossRef]
- McCormack, A.L.; Mak, S.K.; A Di Monte, D. Increased α-synuclein phosphorylation and nitration in the aging primate substantia nigra. Cell Death Dis. 2012, 3, e315. [Google Scholar] [CrossRef] [PubMed]
- Oueslati, A.; Schneider, B.L.; Aebischer, P.; Lashuel, H.A. Polo-like kinase 2 regulates selective autophagic -synuclein clearance and suppresses its toxicity in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, E3945–E3954. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Nagpure, B.V.; Wong, P.T.-H.; Bian, J.-S. Hydrogen sulfide protects SH-SY5Y neuronal cells against d-galactose induced cell injury by suppression of advanced glycation end products formation and oxidative stress. Neurochem. Int. 2013, 62, 603–609. [Google Scholar] [CrossRef]
- Kelner, G.S.; Lee, M.; Clark, M.E.; Maciejewski, D.; McGrath, D.; Rabizadeh, S.; Lyons, T.; Bredesen, D.; Jenner, P.; Maki, R.A. The Copper Transport Protein Atox1 Promotes Neuronal Survival. J. Biol. Chem. 2000, 275, 580–584. [Google Scholar] [CrossRef]
- Inglis, K.J.; Chereau, D.; Brigham, E.F.; Chiou, S.-S.; Schöbel, S.; Frigon, N.L.; Yu, M.; Caccavello, R.J.; Nelson, S.; Motter, R.; et al. Polo-like Kinase 2 (PLK2) Phosphorylates α-Synuclein at Serine 129 in Central Nervous System. J. Biol. Chem. 2009, 284, 2598–2602. [Google Scholar] [CrossRef]
- Dahmene, M.; Bérard, M.; Oueslati, A. Dissecting the Molecular Pathway Involved in PLK2 Kinase-mediated α-Synuclein-selective Autophagic Degradation. J. Biol. Chem. 2017, 292, 3919–3928. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Xiaolei, L.; Weiwei, Y.; Hao, W.; Yuangang, Z.; Dongmei, L.; Yazhuo, Z.; Hui, Y. Protein Phosphatase 2A is Involved in the Tyrosine Hydroxylase Phosphorylation Regulated by α-Synuclein. Neurochem. Res. 2015, 40, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Javadpour, P.; Dargahi, L.; Ahmadiani, A.; Ghasemi, R. To Be or Not to Be: PP2A as a Dual Player in CNS Functions, Its Role in Neurodegeneration, and Its Interaction with Brain Insulin Signaling. Cell. Mol. Life Sci. 2019, 76, 2277–2297. [Google Scholar] [CrossRef]
- Liu, Y.; Peterson, D.A.; Kimura, H.; Schubert, D. Mechanism of Cellular 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Reduction. J. Neurochem. 2002, 69, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Salvioli, S.; Ardizzoni, A.; Franceschi, C.; Cossarizza, A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess ΔΨchanges in intact cells: Implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997, 411, 77–82. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, M.; Spinelli, C.C.; De Riccardis, L.; Buccolieri, A.; Di Giulio, S.; Musarò, D.; Pagano, C.; Manno, D.; Maffia, M. Copper Dependent Modulation of α-Synuclein Phosphorylation in Differentiated SHSY5Y Neuroblastoma Cells. Int. J. Mol. Sci. 2021, 22, 2038. https://doi.org/10.3390/ijms22042038
Greco M, Spinelli CC, De Riccardis L, Buccolieri A, Di Giulio S, Musarò D, Pagano C, Manno D, Maffia M. Copper Dependent Modulation of α-Synuclein Phosphorylation in Differentiated SHSY5Y Neuroblastoma Cells. International Journal of Molecular Sciences. 2021; 22(4):2038. https://doi.org/10.3390/ijms22042038
Chicago/Turabian StyleGreco, Marco, Chiara Carmela Spinelli, Lidia De Riccardis, Alessandro Buccolieri, Simona Di Giulio, Debora Musarò, Claudia Pagano, Daniela Manno, and Michele Maffia. 2021. "Copper Dependent Modulation of α-Synuclein Phosphorylation in Differentiated SHSY5Y Neuroblastoma Cells" International Journal of Molecular Sciences 22, no. 4: 2038. https://doi.org/10.3390/ijms22042038
APA StyleGreco, M., Spinelli, C. C., De Riccardis, L., Buccolieri, A., Di Giulio, S., Musarò, D., Pagano, C., Manno, D., & Maffia, M. (2021). Copper Dependent Modulation of α-Synuclein Phosphorylation in Differentiated SHSY5Y Neuroblastoma Cells. International Journal of Molecular Sciences, 22(4), 2038. https://doi.org/10.3390/ijms22042038







