Repetitive Elements in Humans
Abstract
1. Introduction
- Single nucleotide polymorphisms (SNPs) (1 base pair exchanges);
- Microsatellites (1–10 base pair repeats);
- Small-scale insertion/inversion/deletion/duplication polymorphisms (invs/ins/indels/invdups) (1–50 base pairs in size);
- Minisatellites (10–100 base pairs in size);
- Small-scale repetitive elements (SSREs) (0.1–0.8 kilobase pairs in size);
- Submicroscopic copy number variants (CNVs) (in the megabase pair range);
- Chromosomal heteromorphisms (CHs) (in the several megabase pair range);
- Euchromatic variants (EVs) (in the several megabase pair range).
2. Repetitive Elements in Humans
2.1. Variable Number of Tandem Repeats (VNTRs)
Disease-Associated VNTRs
2.2. Small-Scale Repetitive Elements (SSREs)
- LINEs are formed by a group of mostly truncated retrotransposons and constitute >20% of the human genome. Three types of LINEs have been identified: LINE1 (~516,000 copies), LINE2 (~315,000 copies) and LINE3 (~37,000 copies). In fact, in humans, there are ~100 active LINEs per genome, which can still amplify and integrate at new genomic sites, as they comprise reverse transcriptase [26,27,28].
2.2.1. Disease-Associated SSREs
- To date, ~100 examples are known of diseases that are caused by LINE insertions and/or amplification, such as epithelial cell cancer or neurological disorders [45,46,47]. Additionally, the hypomethylation of LINES has been linked to chromosomal instability and altered gene expression in cancer and normal tissue types [48,49]. Similarly, >50 human diseases are associated with SINE activation [38]. As shown for ALU repeats, which are classified as SINEs, in an evolutionary context, their significance cannot be underestimated [42].
- For small NUMTs, the first hints of an association with disease were recently reported as the disruption of genes through their insertion [46,50]. Furthermore, mitochondrial diseases can be inherited via NUMTs not only via the maternal but also the paternal line: this has been shown in seven families [51].
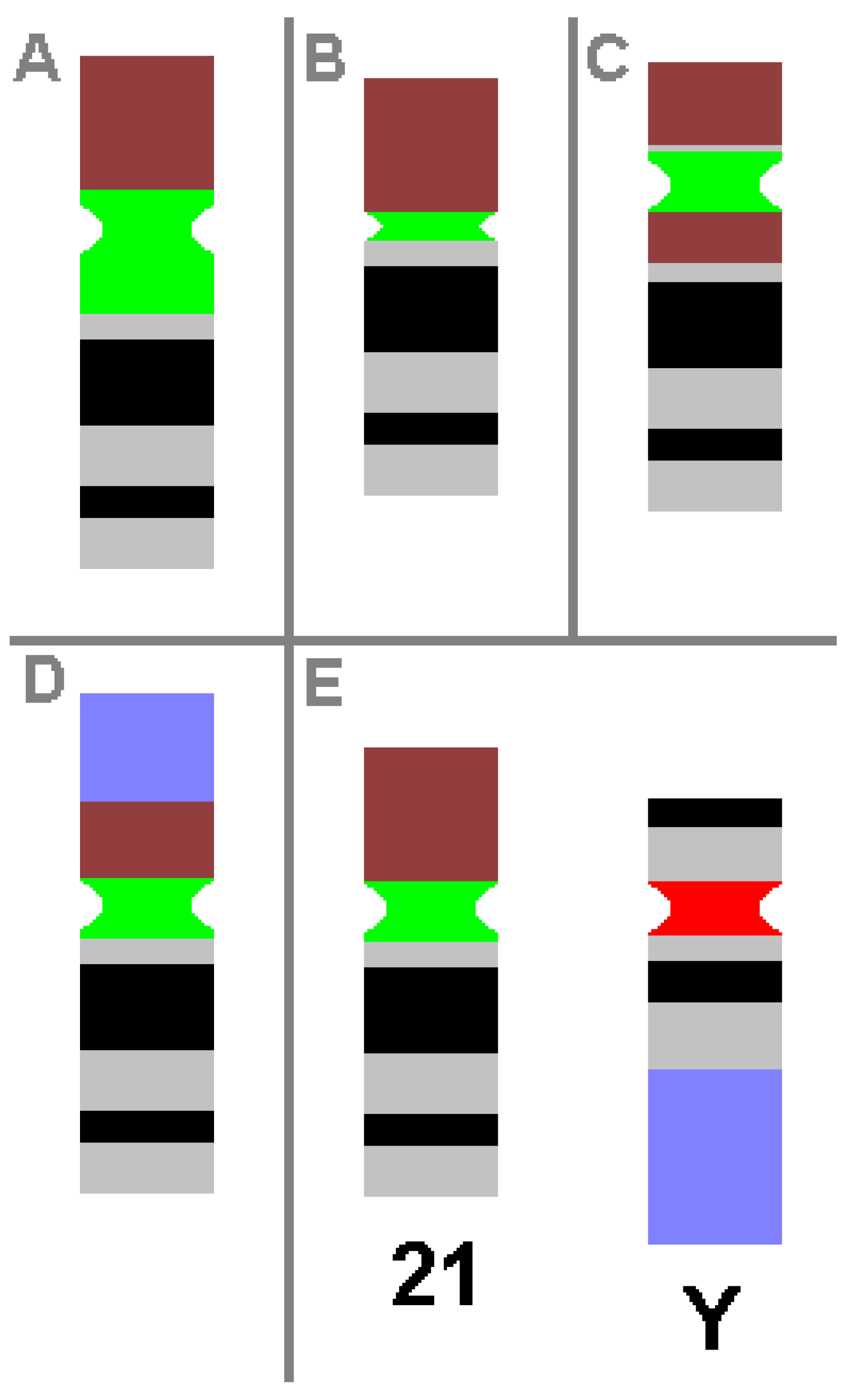
2.3. Chromosomal Heteromorphisms (CHs)
- determination of paternity;
- differentiation of mono- and dizygotic twins;
- determination of the parental origin of derivative chromosomes or of haploid sets in polyploidy or chimera;
- detection of maternal cell contamination in amniotic fluid cell cultures;
- follow-up of bone marrow transplantations; or
- identification of some genetic linkages [1].
Disease-Associated CHs
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CHs | chromosomal heteromorphisms |
| CNVs | submicroscopic copy number variants |
| EVs | euchromatic variants |
| HOR | higher-order repeat |
| invs/ins/indels/invdups | small-scale insertion/inversion/deletion/duplication polymorphisms |
| ITSs | interstitial telomeric sequences |
| LINEs | long interspersed nuclear elements |
| lncRNAs | long non-coding RNAs |
| ncRNAs | non-coding RNAs |
| NumtS | Polymorphic mitochondrial insertions |
| pir | PIWI-interacting RNA |
| PTGs | protein-coding genes |
| SINEs | short interspersed nuclear elements |
| SNPs | single nucleotide polymorphisms |
| sSMC | small supernumerary marker chromosomes |
| SSREs | small-scale repetitive elements |
| VNTRs | variable number of tandem repeats |
References
- Liehr, T. Benign & Pathological Chromosomal Imbalances; Microscopic and Submicroscopic Copy Number Variations (CNVs) in Genetics and Counseling, 1st ed.; Academic Press: Berlin, Germany, 2014. [Google Scholar]
- Mkrtchyan, H.; Gross, M.; Hinreiner, S.; Polytiko, A.; Manvelyan, M.; Mrasek, K.; Kosyakova, N.; Ewers, E.; Nelle, H.; Liehr, T.; et al. The human genome puzzle—The role of copy number variation in somatic mosaicism. Curr. Genom. 2010, 11, 426–431. [Google Scholar] [CrossRef] [PubMed]
- McGowan-Jordan, J.; Hastings, R.; Moore, S. (Eds.) ISCN 2020: An International System for Human Cytogenomic Nomenclature (2020); Karger: Basel, Switzerland, 2020. [Google Scholar]
- Salzberg, S.L. Open questions: How many genes do we have? BMC Biol. 2018, 16, 94. [Google Scholar] [CrossRef]
- Statistics about the Current GENCODE Release (Version 37). Available online: https://www.gencodegenes.org/human/stats.html (accessed on 18 January 2021).
- NCBI Gene Statistics. Available online: https://www.ncbi.nlm.nih.gov/genome/annotation_euk/Homo_sapiens/109/ (accessed on 18 January 2021).
- Human Assembly and Gene Annotation. Available online: https://www.ensembl.org/Homo_sapiens/Info/Annotation (accessed on 18 January 2021).
- GeneCards®: The Human Gene Database. Available online: https://www.genecards.org/ (accessed on 18 January 2021).
- Bessenyei, B.; Márka, M.; Urbán, L.; Zeher, M.; Semsei, I. Single nucleotide polymorphisms: Aging and diseases. Biogerontology 2004, 5, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Liehr, T. Human Genetics—Edition 2020: A Basic Training Package, 1st ed.; Epubli: Berlin, Germany, 2020. [Google Scholar]
- Zhao, T.; Duan, Z.; Genchev, G.Z.; Lu, H. Closing human reference genome gaps: Identifying and characterizing gap-closing sequences. G3 (Bethesda) 2020, 10, 2801–2809. [Google Scholar] [CrossRef]
- De Bustos, A.; Cuadrado, A.; Jouve, N. Sequencing of long stretches of repetitive DNA. Sci. Rep. 2016, 6, 36665. [Google Scholar] [CrossRef] [PubMed]
- Liehr, T. Repetitive elements, heteromorphisms and copy number variants. In Chromosomics, 1st ed.; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Lee, H.; Zhang, Z.; Krause, H.M. Long noncoding RNAs and repetitive elements: Junk or intimate evolutionary partners? Trends Genet. 2019, 35, 892–902. [Google Scholar] [CrossRef]
- Lopez-Pajares, V. Long non-coding RNA regulation of gene expression during differentiation. Pflug. Arch. 2016, 468, 971–981. [Google Scholar] [CrossRef]
- Zheng, D.; Huo, M.; Li, B.; Wang, W.; Piao, H.; Wang, Y.; Zhu, Z.; Li, D.; Wang, T.; Liu, K. The Role of exosomes and exosomal microRNA in cardiovascular disease. Front. Cell Dev. Biol. 2021, 8, 616161. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, X.; Wang, M.; Qin, R. The patterns of antisense long non-coding RNAs regulating corresponding sense genes in human cancers. J. Cancer 2021, 12, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.X.; Wang, J.; Gu, X.Y.; Huang, C.H.; Chen, C.; Hong, M.; Chen, Z. TTN-AS1 as a potential diagnostic and prognostic biomarker for multiple cancers. Biomed. Pharmacother. 2021, 135, 111169. [Google Scholar] [CrossRef]
- Tian, D.; Sun, S.; Lee, J.T. The long noncoding RNA, Jpx, is a molecular switch for X chromosome inactivation. Cell 2010, 143, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Nunez, E.; Fu, X.D.; Rosenfeld, M.G. Nuclear organization in the 3D space of the nucleus—Cause or consequence? Curr. Opin. Genet. Dev. 2009, 19, 424–436. [Google Scholar] [CrossRef]
- Sassi, F.M.C.; Oliveira, E.A.; Bertollo, L.A.C.; Nirchio, M.; Hatanaka, T.; Marinho, M.M.F.; Moreira-Filho, O.; Aroutiounian, R.; Liehr, T.; Al-Rikabi, A.B.H.; et al. Chromosomal Evolution and Evolutionary Relationships of Lebiasina Species (Characiformes, Lebiasinidae). Int. J. Mol. Sci. 2019, 20, 2944. [Google Scholar] [CrossRef] [PubMed]
- Liehr, T. Uniparental Disomy (UPD) in Clinical Genetics. A Guide for Clinicians and Patients, 1st ed.; Springer: Berlin, Germany, 2014. [Google Scholar]
- Goyns, M.H.; Lavery, W.L. Telomerase and mammalian ageing: A critical appraisal. Mech. Ageing Dev. 2000, 114, 69–77. [Google Scholar] [CrossRef]
- Araújo Carvalho, A.C.; Tavares Mendes, M.L.; da Silva Reis, M.C.; Santos, V.S.; Tanajura, D.M.; Martins-Filho, P.R.S. Telomere length and frailty in older adults-A systematic review and meta-analysis. Ageing Res. Rev. 2019, 54, 100914. [Google Scholar] [CrossRef]
- Ye, J.; Renault, V.M.; Jamet, K.; Gilson, E. Transcriptional outcome of telomere signalling. Nat. Rev. Genet. 2014, 15, 491–503. [Google Scholar] [CrossRef]
- Khokhlov, A.N. Does aging need its own program, or is the program of development quite sufficient for it? Stationary cell cultures as a tool to search for anti-aging factors. Curr. Aging Sci. 2013, 6, 14–20. [Google Scholar] [CrossRef]
- Liehr, T.; Buleu, O.; Karamysheva, T.; Bugrov, A.; Rubtsov, N. New insights into Phasmatodea chromosomes. Genes 2017, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Vergnaud, G.; Denoeud, F. Minisatellites: Mutability and genome architecture. Genome Res. 2000, 10, 899–907. [Google Scholar] [CrossRef]
- Chao, T.K.; Hu, J.; Pringsheim, T. Risk factors for the onset and progression of Huntington disease. Neurotoxicology 2017, 61, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, M.; Tang, D.; Calligaris, R.; Vlachouli, C.; Finaurini, S.; Sanges, R.; Goldwurm, S.; Catalan, M.; Antonutti, L.; Manganotti, P.; et al. A human minisatellite hosts an alternative transcription start site for NPRL3 driving its expression in a repeat number-dependent manner. Hum. Mutat. 2020, 41, 807–824. [Google Scholar] [CrossRef]
- Linthorst, J.; Meert, W.; Hestand, M.S.; Korlach, J.; Vermeesch, J.R.; Reinders, M.J.T.; Holstege, H. Extreme enrichment of VNTR-associated polymorphicity in human subtelomeres: Genes with most VNTRs are predominantly expressed in the brain. Transl. Psychiatry 2020, 10, 369. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, S.; Das, A.; Sengupta, P.; Dutta, S.; Roychoudhury, S.; Choudhury, A.P.; Ahmed, A.B.F.; Bhattacharjee, S.; Slama, P. Viral Pandemics of the Last Four Decades: Pathophysiology, Health Impacts and Perspectives. Int. J. Environ. Res. Public Health 2020, 17, 9411. [Google Scholar] [CrossRef]
- Holmes, E.C. The evolution of endogenous viral elements. Cell Host Microbe 2011, 10, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Long Terminal Repeat. Available online: https://de.wikipedia.org/wiki/Long_Terminal_Repeat (accessed on 18 January 2021).
- Richardson, S.R.; Faulkner, G.J. Heritable L1 retrotransposition events during development: Understanding their origins: Examination of heritable, endogenous L1 retrotransposition in mice opens up exciting new questions and research directions. Bioessays 2018, 40, e1700189. [Google Scholar] [CrossRef] [PubMed]
- Streva, V.A.; Jordan, V.E.; Linker, S.; Hedges, D.J.; Batzer, M.A.; Deininger, P.L. Sequencing, identification and mapping of primed L1 elements (SIMPLE) reveals significant variation in full length L1 elements between individuals. BMC Genom. 2015, 16, 220. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Long Interspersed Nuclear Element. Available online: https://en.wikipedia.org/wiki/Long_interspersed_nuclear_element (accessed on 18 January 2021).
- Tucker, J.M.; Glaunsinger, B.A. Host noncoding retrotransposons induced by DNA viruses: A SINE of infection? J. Virol. 2017, 91, e00982-17. [Google Scholar] [CrossRef] [PubMed]
- Dayama, G.; Emery, S.B.; Kidd, J.M.; Mills, R.E. The genomic landscape of polymorphic human nuclear mitochondrial insertions. Nucleic Acids Res. 2014, 42, 12640–12649. [Google Scholar] [CrossRef] [PubMed]
- Hoser, S.M.; Hoffmann, A.; Meindl, A.; Gamper, M.; Fallmann, J.; Bernhart, S.H.; Müller, L.; Ploner, M.; Misslinger, M.; Kremser, L.; et al. Intronic tRNAs of mitochondrial origin regulate constitutive and alternative splicing. Genome Biol. 2020, 21, 299. [Google Scholar] [CrossRef] [PubMed]
- Lutz-Bonengel, S.; Niederstätter, H.; Naue, J.; Koziel, R.; Yang, F.; Sänger, T.; Huber, G.; Berger, C.; Pflugradt, R.; Strobl, C.; et al. Evidence for multi-copy MEGA-NUMTs in the human genome. Nucleic Acids Res. 2021, gkaa1271. [Google Scholar] [CrossRef]
- Matylla-Kulinska, K.; Tafer, H.; Weiss, A.; Schroeder, R. Functional repeat-derived RNAs often originate from retrotransposon-propagated ncRNAs. Wiley Interdiscip. Rev. RNA 2014, 5, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Ramos, M.A. Satellite DNA: An evolving topic. Genes 2017, 8, 230. [Google Scholar] [CrossRef]
- Liehr, T. Benign and pathological gain or loss of genetic material—About microscopic and submicroscopic copy number variations (CNVs) in human genetics. Tsitologiia 2016, 58, 476–477. [Google Scholar]
- Rawal, L.; Kumar, S.; Mishra, S.R.; Lal, V.; Bhattacharya, S.K. Clinical Manifestations of chromosomal anomalies and polymorphic variations in patients suffering from reproductive failure. J. Hum. Reprod. Sci. 2020, 13, 209–215. [Google Scholar] [PubMed]
- Chen, J.M.; Chuzhanova, N.; Stenson, P.D.; Férec, C.; Cooper, D.N. Meta-analysis of gross insertions causing human genetic disease: Novel mutational mechanisms and the role of replication slippage. Hum. Mutat. 2005, 25, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Solyom, S.; Kazazian, H.H., Jr. Mobile elements in the human genome: Implications for disease. Genome Med. 2012, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Carreira, P.E.; Richardson, S.R.; Faulkner, G.J. L1 retrotransposons, cancer stem cells and oncogenesis. FEBS J. 2014, 281, 63–73. [Google Scholar] [CrossRef]
- Kitkumthorn, N.; Mutirangura, A. Long interspersed nuclear element-1 hypomethylation in cancer: Biology and clinical applications. Clin. Epigenetics 2011, 2, 315–330. [Google Scholar] [CrossRef]
- Wei, W.; Chinnery, P.F. Inheritance of mitochondrial DNA in humans: Implications for rare and common diseases. J. Intern. Med. 2020, 287, 634–644. [Google Scholar] [CrossRef]
- Wei, W.; Pagnamenta, A.T.; Gleadall, N.; Sanchis-Juan, A.; Stephens, J.; Broxholme, J.; Tuna, S.; Odhams, C.A.; Genomics England Research Consortium; NIHR BioResource; et al. Nuclear-mitochondrial DNA segments resemble paternally inherited mitochondrial DNA in humans. Nat. Commun. 2020, 11, 1740. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Singchat, W.; Jehangir, M.; Suntronpong, A.; Panthum, T.; Malaivijitnond, S.; Srikulnath, K. Dark matter of primate genomes: Satellite DNA repeats and their evolutionary dynamics. Cells 2020, 9, 2714. [Google Scholar] [CrossRef] [PubMed]
- Liehr, T. Cases with Heteromorphisms. 2021. Available online: http://cs-tl.de/DB/CA/HCM/0-Start.html (accessed on 18 January 2021).
- Ahmad, S.F.; Martins, C. The modern view of B chromosomes under the impact of high scale omics analyses. Cells 2019, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Liehr, T. A guide for human geneticists and clinicians. In Small Supernumerary Marker Chromosomes (sSMC), 1st ed.; Springer: Berlin, Germany, 2012. [Google Scholar]
- Liehr, T. Small Supernumerary Marker Chromosomes. 2021. Available online: http://cs-tl.de/DB/CA/sSMC/0-Start.html (accessed on 18 January 2021).
- Ershova, E.S.; Malinovskaya, E.M.; Golimbet, V.E.; Lezheiko, T.V.; Zakharova, N.V.; Shmarina, G.V.; Veiko, R.V.; Umriukhin, P.E.; Kostyuk, G.P.; Kutsev, S.I.; et al. Copy number variations of satellite III (1q12) and ribosomal repeats in health and schizophrenia. Schizophr. Res. 2020, 223, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Storry, J.R.; Olsson, M.L. The ABO blood group system revisited: A review and update. Immunohematology 2009, 25, 48–59. [Google Scholar]
- Chen, Z.; Yang, S.H.; Xu, H.; Li, J.J. ABO blood group system and the coronary artery disease: An updated systematic review and meta-analysis. Sci. Rep. 2016, 6, 23250. [Google Scholar] [CrossRef]
| Type | GENCODE [5] | NCBI [6] | Ensemble [7] | Genecards [8] |
|---|---|---|---|---|
| Total number of genes | 60,660 | 54,644 | 59,662 | 270,168 |
| Protein-coding genes | 19,962 | 20,203 | 20,448 | 20,916 |
| Genes that have more than one distinct translation | 13,685 * | 20,110 * | n.a. | n.a. |
| Non-coding genes | n.a. | 17,871 | 23,997 | 219,587 |
| lncRNA genes | 17,958 | n.a. | n.a. | 75,839 * |
| Small ncRNA/pir ncRNA genes | 7569 | n.a. | n.a. | 109,820 * |
| Pseudogenes | 14,761 | 15,067 | 15,217 | 21,888 |
| Others | 350 | 1503 | n.a. | 7777 |
| Type | Length of Basic Units (bp) | Class of Genetic Polymorphisms |
|---|---|---|
| Satellite I DNA | 17–25 | 4 |
| Satellite II DNA | 5 | 2 |
| Satellite III DNA | 5 interspersed with ~10 bp of definite sequence | 2 |
| α-satellite DNA | 171 | 5 |
| β-satellite DNA | 68–69 | 4 |
| γ-satellite DNA | 220 | 5 |
| Centromeric Amplification or Diminution | Pericentric Inversion | Others (e.g., Insertions or Translocations) | |
|---|---|---|---|
| Number of types found | 129 | 23 | 70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liehr, T. Repetitive Elements in Humans. Int. J. Mol. Sci. 2021, 22, 2072. https://doi.org/10.3390/ijms22042072
Liehr T. Repetitive Elements in Humans. International Journal of Molecular Sciences. 2021; 22(4):2072. https://doi.org/10.3390/ijms22042072
Chicago/Turabian StyleLiehr, Thomas. 2021. "Repetitive Elements in Humans" International Journal of Molecular Sciences 22, no. 4: 2072. https://doi.org/10.3390/ijms22042072
APA StyleLiehr, T. (2021). Repetitive Elements in Humans. International Journal of Molecular Sciences, 22(4), 2072. https://doi.org/10.3390/ijms22042072






