6,7,4′-Trihydroxyflavanone Protects against Dextran Sulfate Sodium-Induced Colitis by Regulating the Activity of T Cells and Colon Cells In Vivo
Abstract
1. Introduction
2. Results
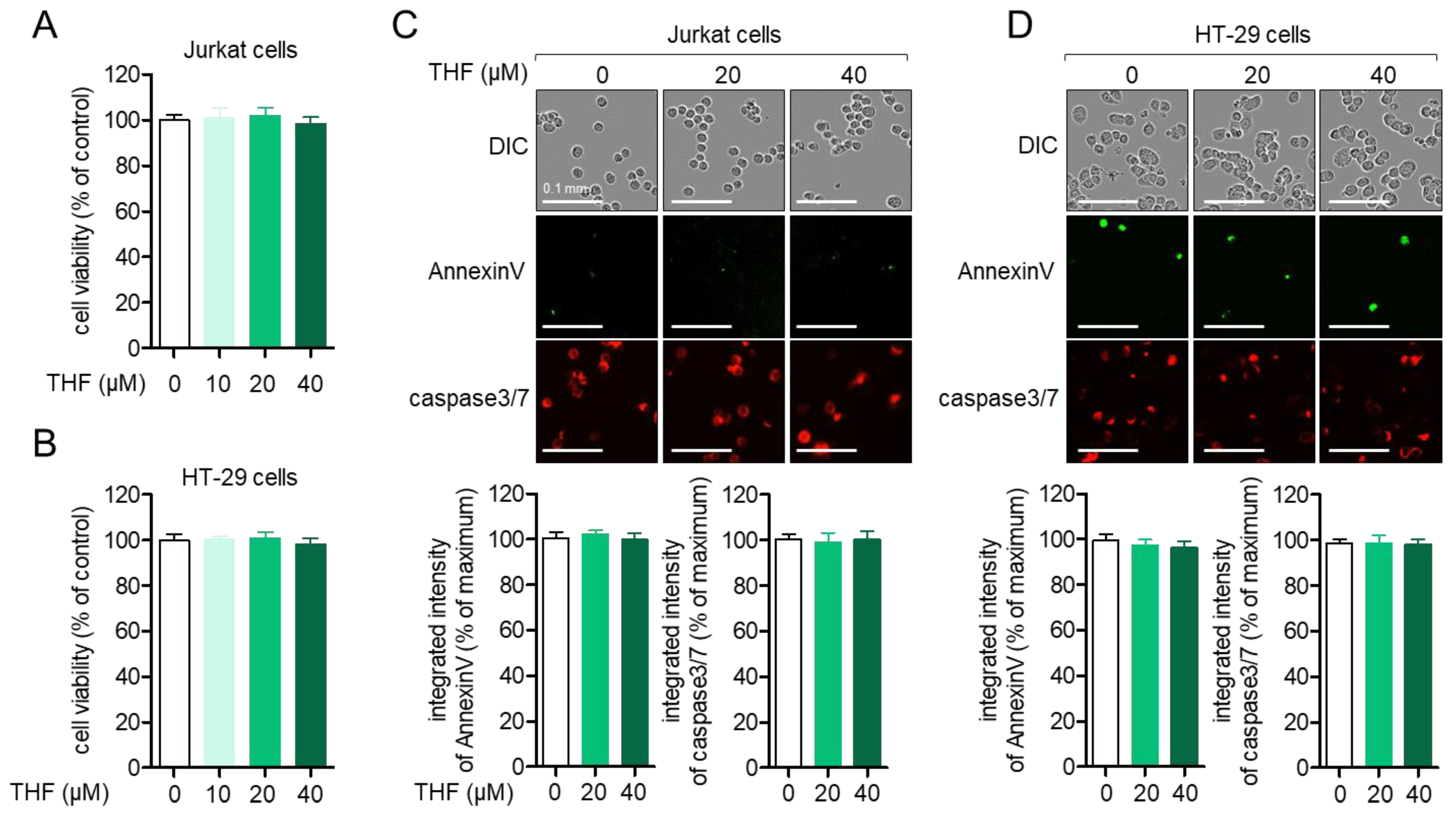
2.1. THF Does Not Have a Negative Effect on the Viability of Jurkat Cells and HT-29 Cells
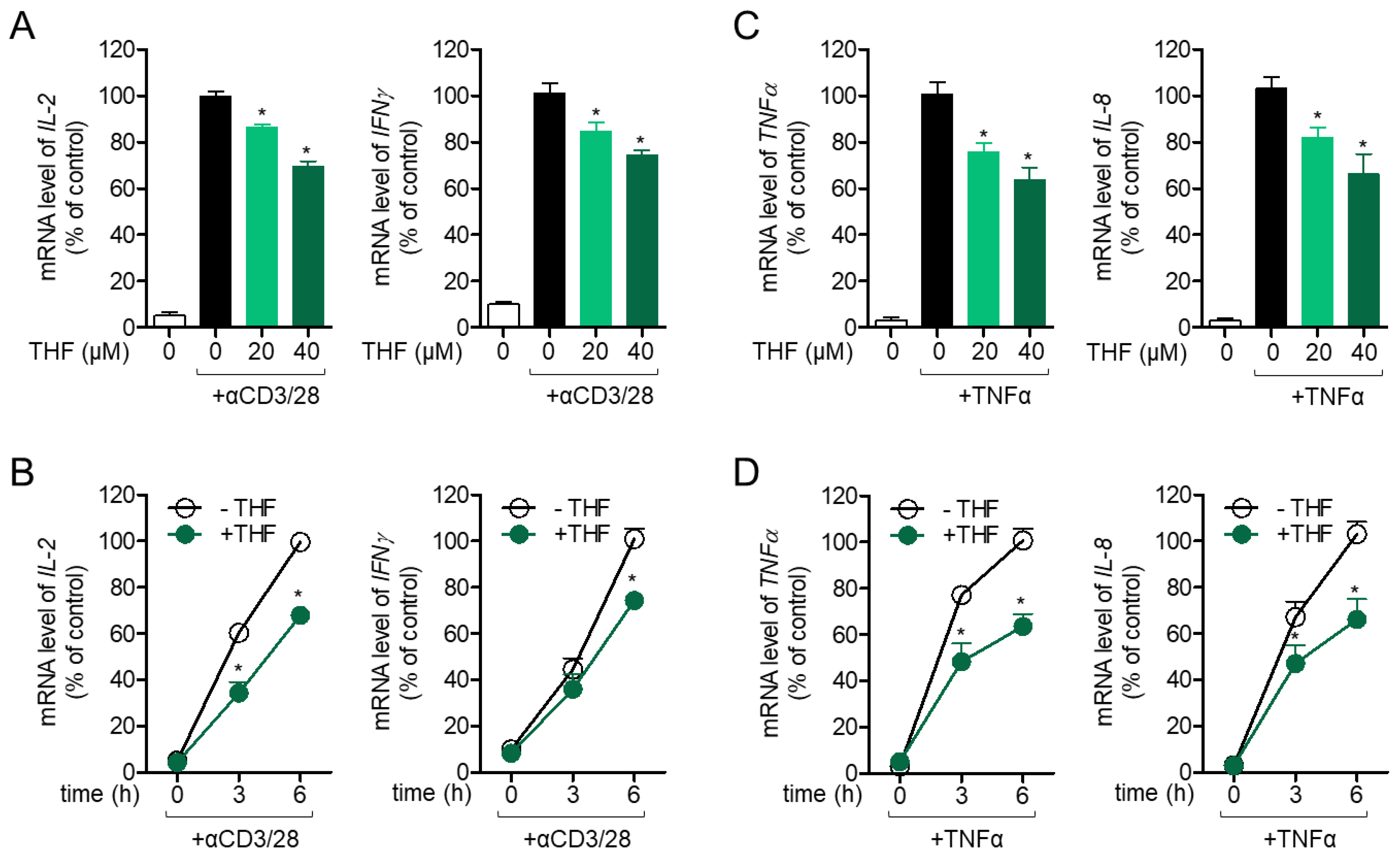
2.2. THF Regulates the Activity of Jurkat T Cells and HT-29 Cells in Stimulated Conditions
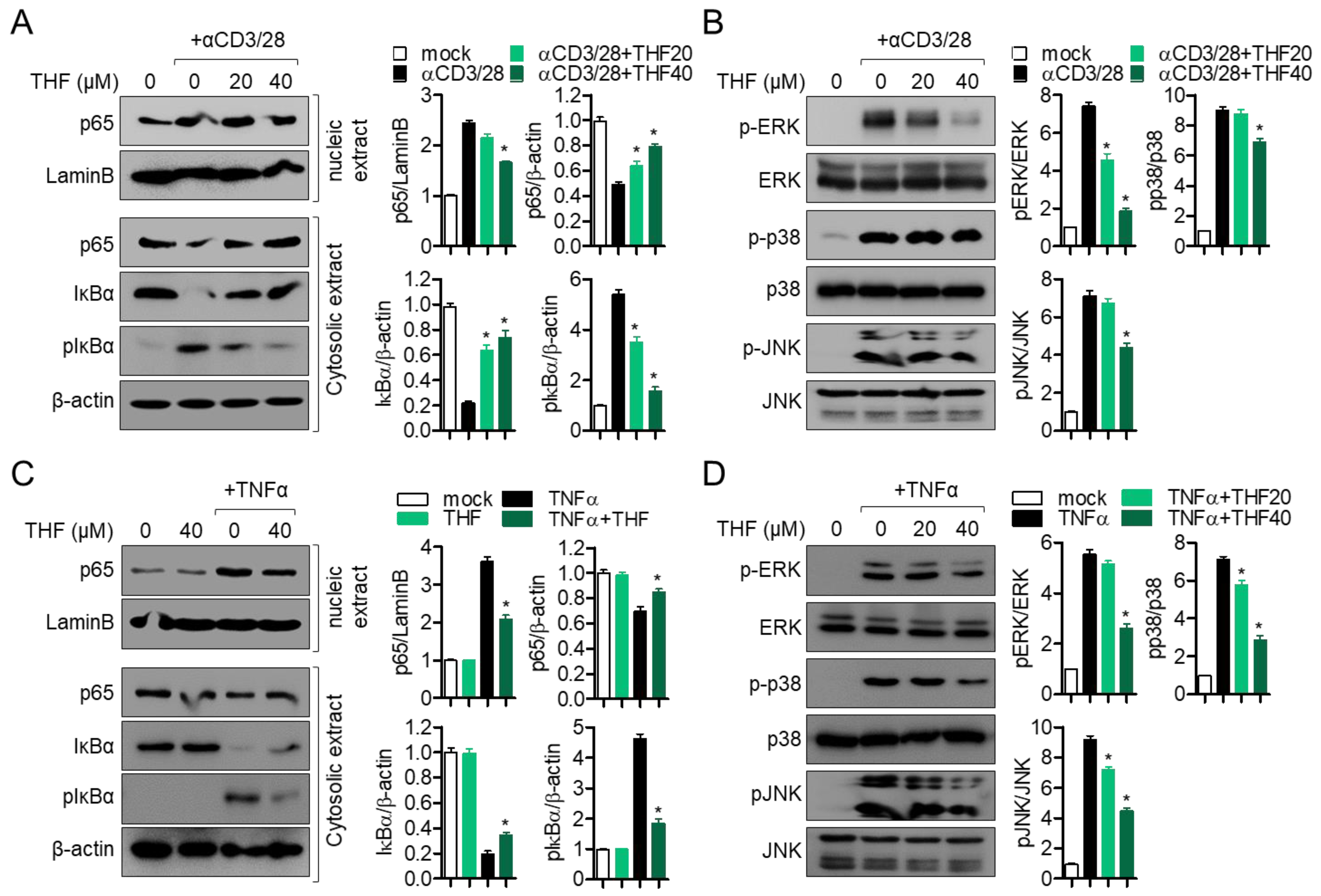
2.3. Pre-Treatment with THF Suppresses MAPK Signaling Pathway via p65 Translocation in Activated Jurkat and HT-29 Cells
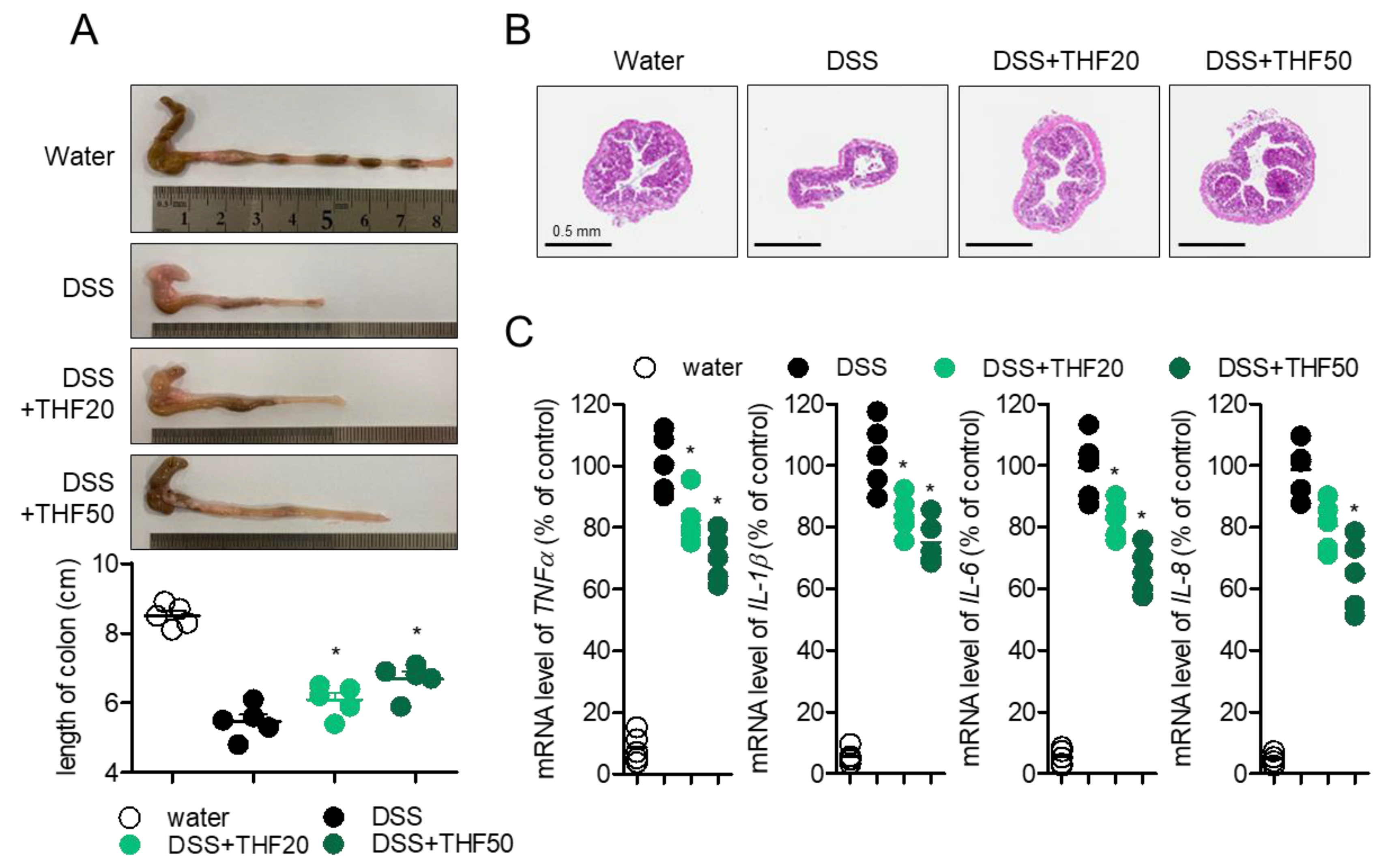
2.4. Oral Administration of THF Attenuates DSS-Induced Colitis in a Mouse Model
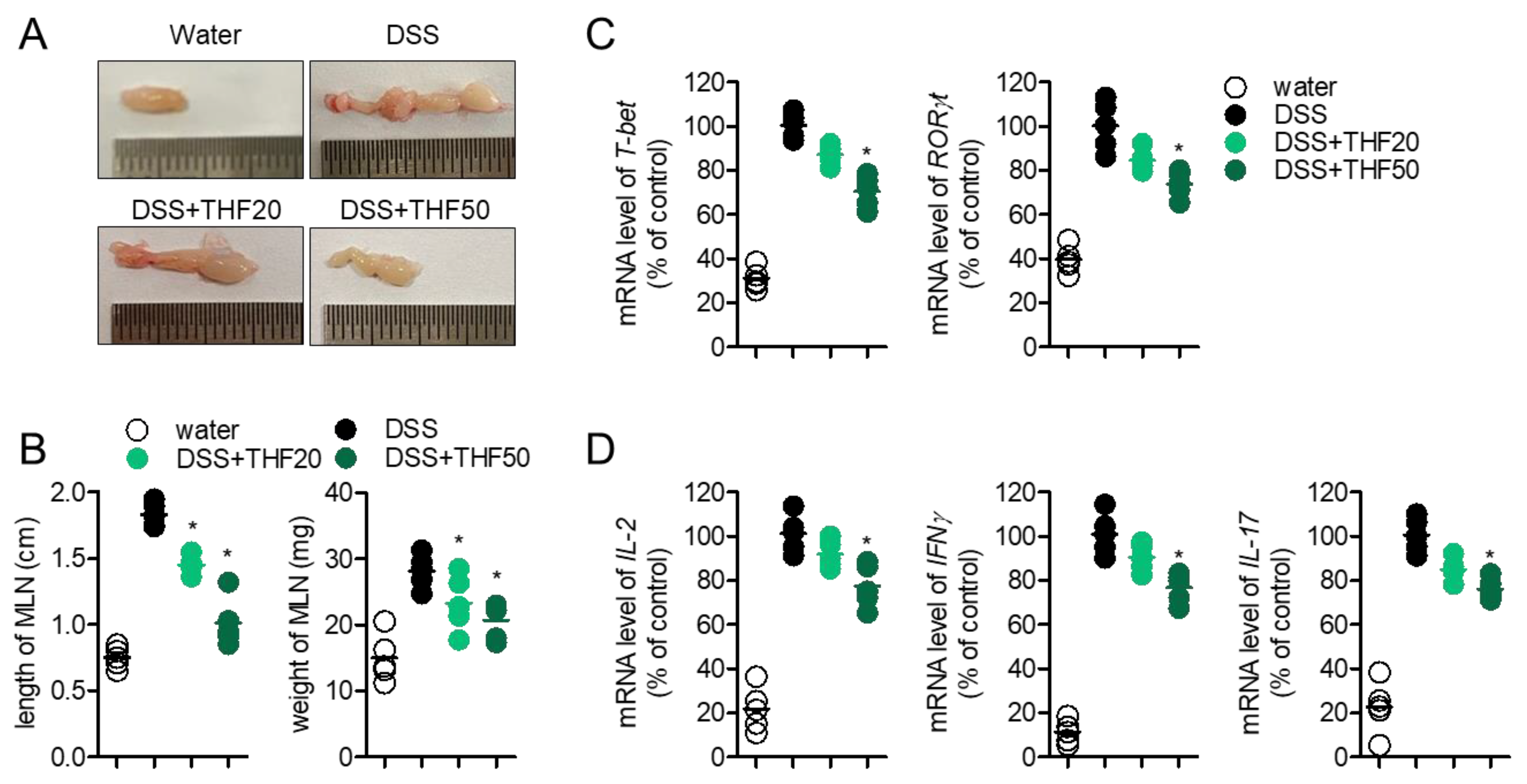
2.5. Oral Administration of THF Suppresses DSS-Induced Intestinal Inflammation
2.6. Oral Administration of THF Blocks Th1/Th17 Cells to Produce the Effector Cytokines in a DSS-Induced Colitis Model
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Mice
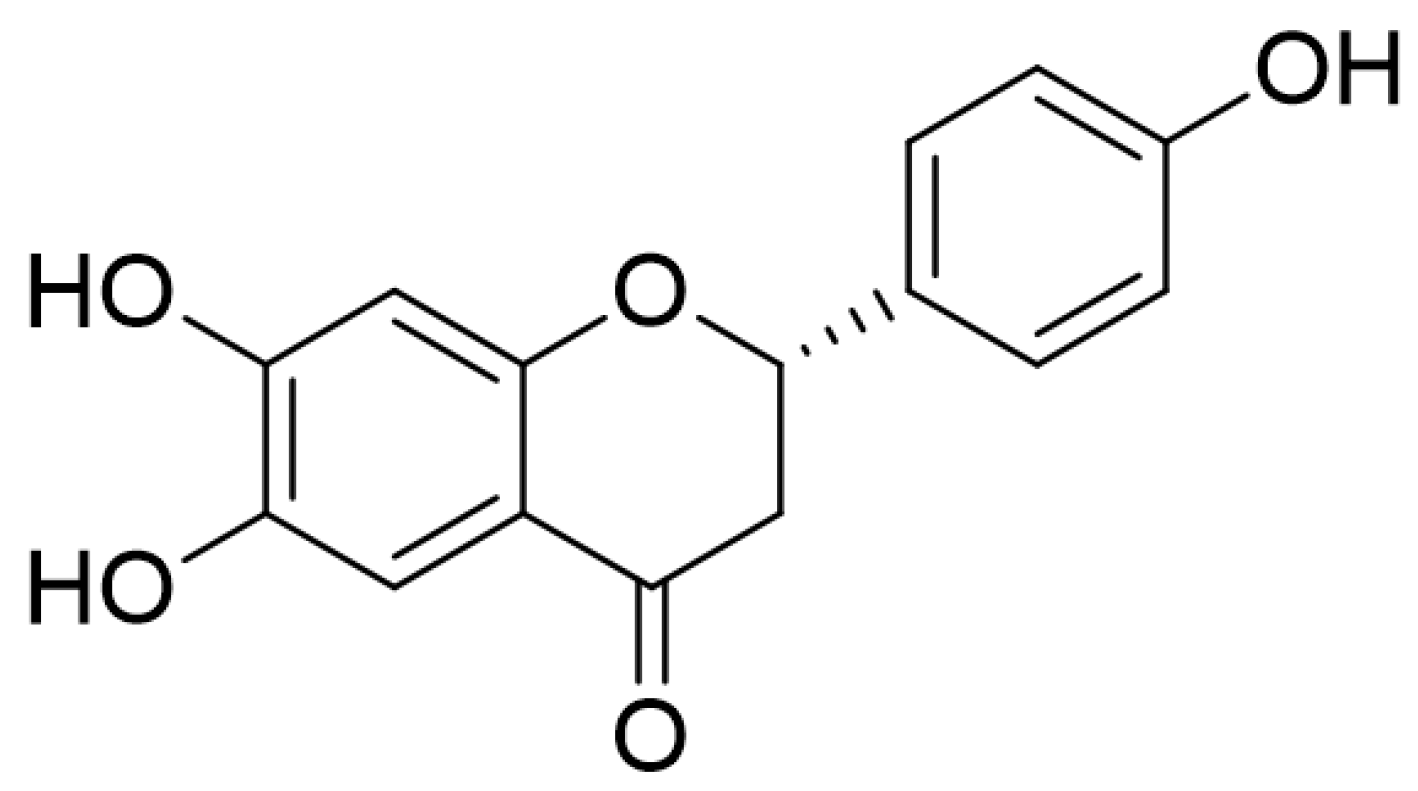
4.3. Isolation of THF from D. odorifera
4.4. Reagents and Antibodies
4.5. MTT Assay
4.6. Measurement of AnnexinV and Caspase3/7 by the IncuCyte Imaging System
4.7. Real-Time Quantitative PCR
4.8. Western Blot Analysis
4.9. DSS-Induced Colitis Model
4.10. H&E Staining
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| THF | 6,7,4′-tryhydroxyflavanone |
| TCR | T cell receptor |
| NF-κB | Nuclear factor kappa B |
| IBD | Immune bowel disease |
References
- Baumgart, D.C.; Sandborn, W.J. Crohn’s disease. Lancet 2012, 380, 1590–1605. [Google Scholar] [CrossRef]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Kaser, A.; Zeissig, S.; Blumberg, R.S. Inflammatory Bowel Disease. Annu. Rev. Immunol. 2010, 28, 573–621. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Sundrud, M.S. Cytokine networks and T-cell subsets in inflammatory bowel diseases. Inflamm. Bowel Dis. 2016, 22, 1157–1167. [Google Scholar] [CrossRef]
- Sakuraba, A.; Sato, T.; Kamada, N.; Kitazume, M.; Sugita, A.; Hibi, T. Th1/Th17 Immune Response Is Induced by Mesenteric Lymph Node Dendritic Cells in Crohn’s Disease. Gastroenterology 2009, 137, 1736–1745. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, T.; Suzuki, N.; Yamaki, S.; Sun, S.; Asao, A.; Okuyama, Y.; So, T.; Iwakura, Y.; Ishii, N. Mesenteric lymph nodes contribute to proinflammatory Th17-cell generation during inflammation of the small intestine in mice. Eur. J. Immunol. 2016, 46, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Onizawa, M.; Nagaishi, T.; Kanai, T.; Nagano, K.; Oshima, S.; Nemoto, Y.; Yoshioka, A.; Totsuka, T.; Okamoto, R.; Nakamura, T.; et al. Signaling pathway via TNF-α/NF-κB in intestinal epithelial cells may be directly involved in colitis-associated carcinogenesis. Am. J. Physiol. Liver Physiol. 2009, 296, G850–G859. [Google Scholar] [CrossRef]
- Xu, P.; Elamin, E.; Elizalde, M.; Bours, P.P.H.A.; Pierik, M.J.; Masclee, A.A.M.; Jonkers, D.M.A.E. Modulation of Intestinal Epithelial Permeability by Plasma from Patients with Crohn’s Disease in a Three-dimensional Cell Culture Model. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Kim, E.N.; Kim, Y.G.; Lee, J.H.; Min, B.S.; Jeong, G.S. 6,7,4′-Trihydroxyflavone inhibits osteoclast formation and bone resorption in vitro and in vivo. Phyther. Res. 2019, 33, 2948–2959. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Kim, K.S.; Ko, W.; Li, B.; Keo, S.; Jeong, G.S.; Oh, H.; Kim, Y.C. The neoflavonoid latifolin isolated from meoh extract of Dalbergia odorifera attenuates inflammatory responses by inhibiting NF-κB activation via Nrf2-mediated heme oxygenase-1 expression. Phyther. Res. 2014, 28, 1216–1223. [Google Scholar] [CrossRef]
- Lee, H.S.; Jeong, G.S. 6,7,4′-Trihydroxyflavanone Prevents Methamphetamine-Induced T Cell Deactivation by Protecting the Activated T Cells from Apoptosis. Am. J. Chin. Med. 2021, 49, 95–111. [Google Scholar] [CrossRef]
- Hisamatsu, T.; Erben, U.; Kühl, A.A. The Role of T-Cell Subsets in Chronic Inflammation in Celiac Disease and Inflammatory Bowel Disease Patients: More Common Mechanisms or More Differences? Inflamm. Intest. Dis. 2016, 1, 52–62. [Google Scholar] [CrossRef]
- Dias, A.M.; Correia, A.; Pereira, M.S.; Almeida, C.R.; Alves, I.; Pinto, V.; Catarino, T.A.; Mendes, N.; Leander, M.; Teresa Oliva-Teles, M.; et al. Metabolic control of T cell immune response through glycans in inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 2018, 115, E4651–E4660. [Google Scholar] [CrossRef]
- Imam, T.; Park, S.; Kaplan, M.H.; Olson, M.R. Effector T helper cell subsets in inflammatory bowel diseases. Front. Immunol. 2018, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, F.; Aune, T.M. Either IL-2 or IL-12 Is Sufficient to Direct Th1 Differentiation by Nonobese Diabetic T Cells. J. Immunol. 2003, 170, 735–740. [Google Scholar] [CrossRef]
- Naito, Y.; Takagi, T.; Uchiyama, K.; Kuroda, M.; Kokura, S.; Ichikawa, H.; Yanagisawa, R.; Inoue, K.I.; Takano, H.; Satoh, M.; et al. Reduced intestinal inflammation induced by dextran sodium sulfate in interleukin-6-deficient mice. Int. J. Mol. Med. 2004, 14, 191–196. [Google Scholar] [CrossRef]
- Zhao, M.; Tan, Y.; Peng, Q.; Huang, C.; Guo, Y.; Liang, G.; Zhu, B.; Huang, Y.; Liu, A.; Wang, Z.; et al. IL-6/STAT3 pathway induced deficiency of RFX1 contributes to Th17-dependent autoimmune diseases via epigenetic regulation. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Renaude, E.; Kroemer, M.; Loyon, R.; Binda, D.; Borg, C.; Guittaut, M.; Hervouet, E.; Peixoto, P. The fate of Th17 cells is shaped by epigenetic modifications and remodeled by the tumor microenvironment. Int. J. Mol. Sci. 2020, 21, 1673. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.S.; Rajmane, A.R.; Adil, M.; Kandhare, A.D.; Ghosh, P.; Bodhankar, S.L. Naringin ameliorates acetic acid induced colitis through modulation of endogenous oxido-nitrosative balance and DNA damage in rats. J. Biomed. Res. 2014, 28, 132–145. [Google Scholar] [PubMed]
- Vochyánová, Z.; Bartošová, L.; Bujdáková, V.; Fictum, P.; Husník, R.; Suchý, P.; Šmejkal, K.; Hošek, J. Diplacone and mimulone ameliorate dextran sulfate sodium-induced colitis in rats. Fitoterapia 2015, 101, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Yue, B.; Ren, J.; Yu, Z.; Luo, X.; Ren, Y.; Zhang, J.; Mani, S.; Wang, Z.; Dou, W. Pinocembrin alleviates ulcerative colitis in mice via regulating gut microbiota, suppressing TLR4/MD2/NF-κB pathway and promoting intestinal barrier. Biosci. Rep. 2020, 40, 40. [Google Scholar] [CrossRef]
- Abraham, R.T.; Weiss, A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat. Rev. Immunol. 2004, 4, 301–308. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Rajasekaran, N.; Choi, J.S.; Kim, J.H.; Jang, M.H.; Shin, Y.K.; Suh, P.G.; Ryu, S.H. Intestinal epithelial cell-specific deletion of PLD2 alleviates DSS-induced colitis by regulating occludin. Sci. Rep. 2017, 7, 1573. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.E.C.; Kaetzel, C.S. Long-Term Exposure of the HT-29 Human Intestinal Epithelial Cell Line to TNF Causes Sustained Up-Regulation of the Polymeric Ig Receptor and Proinflammatory Genes through Transcriptional and Posttranscriptional Mechanisms. J. Immunol. 2005, 174, 7278–7284. [Google Scholar] [CrossRef] [PubMed]
- Gioia, L.; Siddique, A.; Head, S.R.; Salomon, D.R.; Su, A.I. A genome-wide survey of mutations in the Jurkat cell line. BMC Genom. 2018, 19, 334. [Google Scholar] [CrossRef]
- Bundscherer, A.; Bundscherer, A.C.; Malsy, M.; Gruber, M.A.; Graf, B.M.; Sinner, B. Acetaminophen and Metamizole Induce Apoptosis in HT 29 and SW 480 Colon Carcinoma Cell Lines In Vitro. Anticancer Res. 2018, 38, 745–751. [Google Scholar] [PubMed]
- Shen, C.C.; Cheng, J.J.; Lay, H.L.; Wu, S.Y.; Ni, C.L.; Teng, C.M.; Chen, C.C. Cytotoxic apigenin derivatives from Chrysopogon aciculatis. J. Nat. Prod. 2012, 75, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.Y.; Pu, Y.Q.; Xu, B.L.; Zhang, T.; Wang, B. Study on the physicochemical properties and anti-inflammatory effects of paeonol in rats with TNBS-induced ulcerative colitis. Int. Immunopharmacol. 2017, 42, 32–38. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-S.; Jeong, G.-S. 6,7,4′-Trihydroxyflavanone Protects against Dextran Sulfate Sodium-Induced Colitis by Regulating the Activity of T Cells and Colon Cells In Vivo. Int. J. Mol. Sci. 2021, 22, 2083. https://doi.org/10.3390/ijms22042083
Lee H-S, Jeong G-S. 6,7,4′-Trihydroxyflavanone Protects against Dextran Sulfate Sodium-Induced Colitis by Regulating the Activity of T Cells and Colon Cells In Vivo. International Journal of Molecular Sciences. 2021; 22(4):2083. https://doi.org/10.3390/ijms22042083
Chicago/Turabian StyleLee, Hyun-Su, and Gil-Saeng Jeong. 2021. "6,7,4′-Trihydroxyflavanone Protects against Dextran Sulfate Sodium-Induced Colitis by Regulating the Activity of T Cells and Colon Cells In Vivo" International Journal of Molecular Sciences 22, no. 4: 2083. https://doi.org/10.3390/ijms22042083
APA StyleLee, H.-S., & Jeong, G.-S. (2021). 6,7,4′-Trihydroxyflavanone Protects against Dextran Sulfate Sodium-Induced Colitis by Regulating the Activity of T Cells and Colon Cells In Vivo. International Journal of Molecular Sciences, 22(4), 2083. https://doi.org/10.3390/ijms22042083




