Expression of Lymphatic Markers in the Berger’s Space and Bursa Premacularis
Abstract
:1. Introduction
2. Results
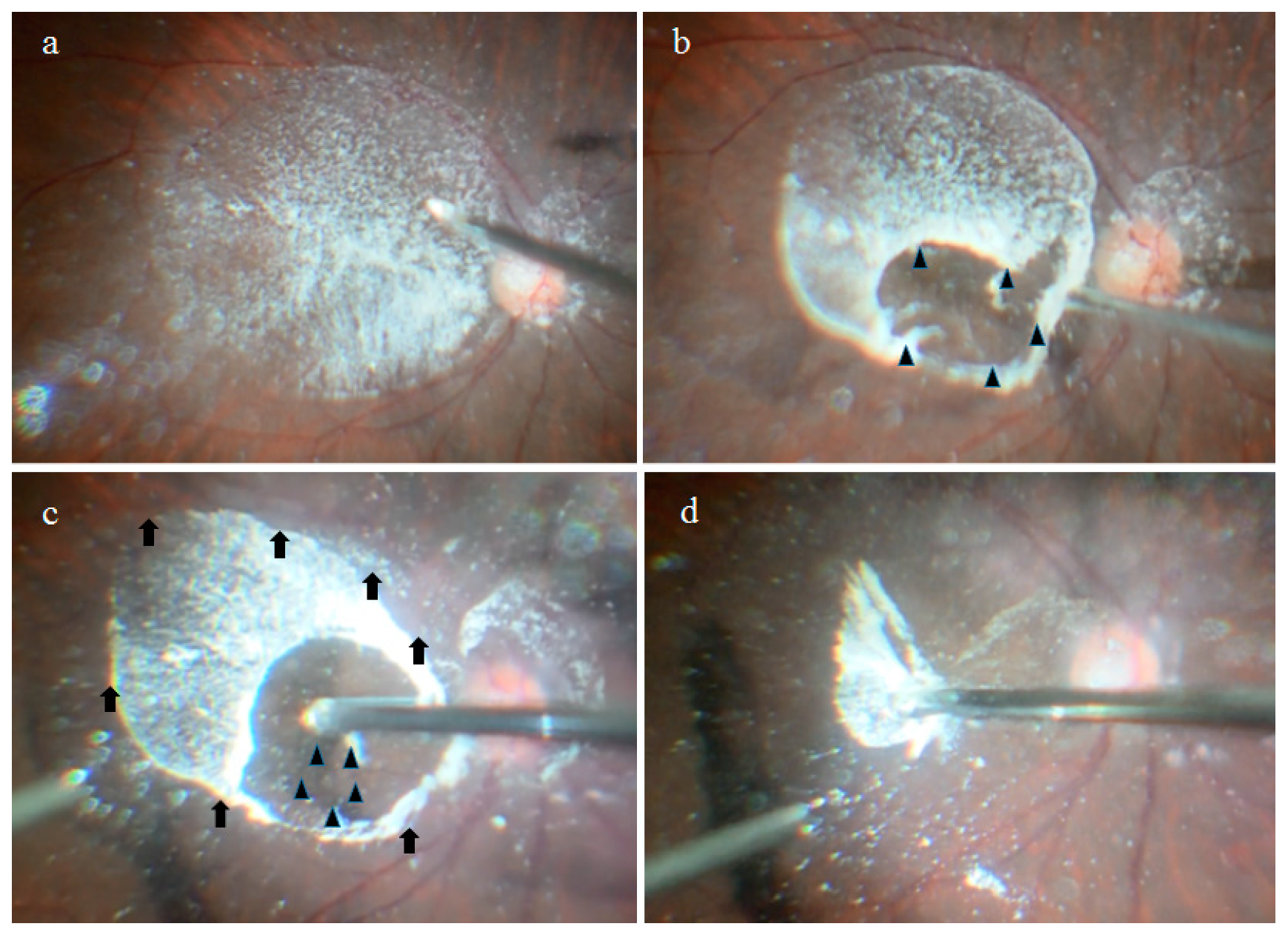
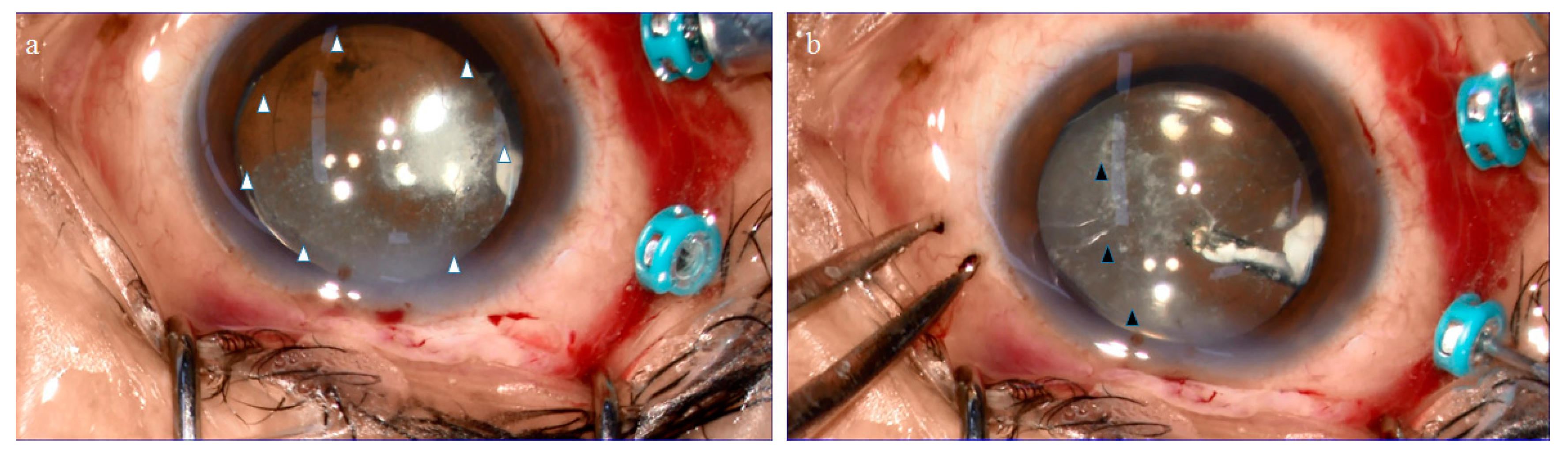
2.1. Anatomical Similarities between the BS and the BPM Observed during Specimen Collection
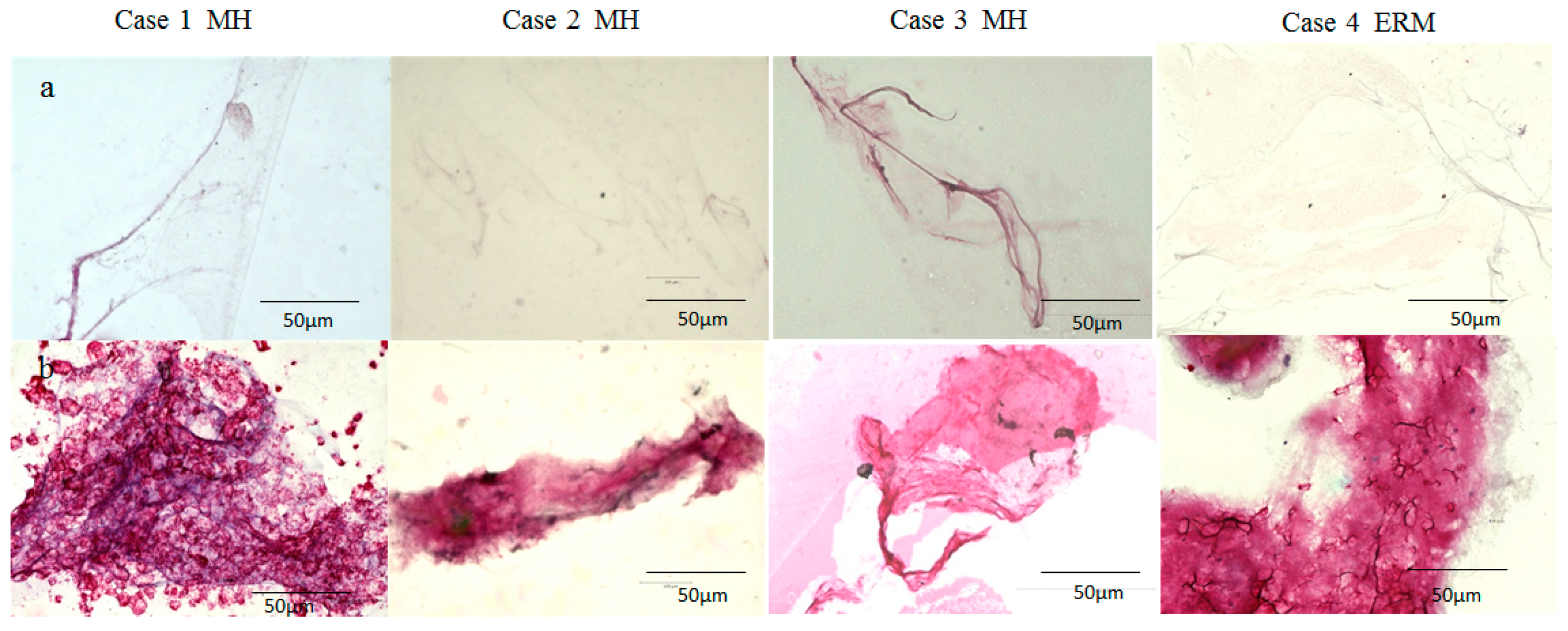
2.2. Immunostaining of the BPM and the Vitreous Core (VC) with Antibodies for Fibrillin-1 and -2
2.3. Immunostaining of the BS, BPM, and VC with Antibodies for Fibrillin-1 and -2, Podoplanin, and LYVE-1
3. Discussion
4. Materials and Methods
4.1. Collection of the BS, BPM, and VC Specimens
4.2. Immunostaining of the BPM and VC with Antibodies for Fibrillin-1 and -2
4.3. Immunostaining of the BS, BPM, and VC Specimens with Antibodies for Fibrillin-1 and -2, Podoplanin, and LYVE-1
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BPM | bursa premacularis |
| PVD | posterior vitreous detachment |
| LYVE-1 | lymphatic vessel endothelial hyaluronan receptor 1 |
| BS | Berger’s space |
| AHM | anterior hyaloid membrane |
| TA | triamcinolone acetonide |
| VC | vitreous core |
| SS-OCT | swept-source optical coherence tomography |
| ERM | epiretinal membrane |
| MH | macular hole |
| PDR | proliferative diabetic retinopathy |
| 3D-OCT | three-dimensional optical coherence tomography |
| ABA | Agaricus bisporus agglutinin |
| AQP4 | aquaporin-4 |
| SD-OCT | spectral domain optical coherence tomography |
| YAG | yttrium aluminum garnet |
| IOP | intraocular pressure |
| RPE | retinal pigment epithelial |
| GFAP | glial fibrillary acidic protein |
| CME | cystoid macular edema |
| PBS | phosphate buffered saline |
References
- Sebag, J. Structure of the vitreous. In The Vitreous-Structure, Function, and Pathobiology; Sebag, J., Ed.; Springer: New York, NY, USA, 1989; Volume 4, pp. 36–37, 133–135. ISBN 978-1-4613-8908-8. [Google Scholar]
- Swindle-Reilly, K.E.; Shah, M.; Hamilton, P.D.; Eskin, T.A.; Kaushal, S.; Ravi, N. Rabbit study of an in situ forming hydrogel vitreous substitute. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4840–4846. [Google Scholar] [CrossRef]
- Sebag, J.S. Surgical anatomy of vitreous and the vitreoretinal interface. In Duane’s Ophthalmology; Sebag, J.S., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; Volume 6, Chapter 51; ISBN 978-0781753296. [Google Scholar]
- Eisner, G. Postmortem slitlamp study of the vitreous body I-III. Graefe’s Arch. Klin. Exp. Ophthalmol. 1971, 182, 1–40. [Google Scholar] [CrossRef]
- Worst, J.G.; Los, L.I. Comparative anatomy of the vitreous body in rhesus monkeys and man. Doc. Ophthalmol. 1992, 82, 169–178. [Google Scholar] [CrossRef]
- Worst, J.G. Cisternal systems of the fully developed vitreous body in the young adult. Trans. Ophthalmol. Soc. UK 1977, 97, 550–554. [Google Scholar] [PubMed]
- Worst, J.G.F. The bursa intravitrealis premacularis. In New Developments in Ophthalmology Nijmegen 16–18 October 1975; Duetman, A.F., Ed.; Springer: New York, NY, USA, 1975; pp. 275–279. ISBN 978-9061941478. [Google Scholar]
- Worst, J. Extracapsular surgery in lens implantation (Binkhorst lecture). Part iv. Some anatomical and pathophysiological implications. J. Am. Intraocul. Implant Soc. 1978, 4, 7–14. [Google Scholar] [CrossRef]
- Sato, T.; Morishita, S.; Horie, T.; Fukumoto, M.; Kida, T.; Oku, H.; Nakamura, K.; Takai, S.; Jin, D.; Ikeda, T. Involvement of premacular mast cells in the pathogenesis of macular diseases. PLoS ONE 2019, 14, e0211438. [Google Scholar] [CrossRef]
- Santos-Bueso, E. Berger’s space. Arch. Soc. Esp. Oftalmol. 2019, 94, 471–477. [Google Scholar] [CrossRef]
- Li, S.T.; Yiu, E.P.; Wong, A.H.; Yeung, J.C.; Yu, L.W. Management of traumatic haemorrhage in the Berger’s space of a 4-year-old child. Int. Ophthalmol. 2017, 37, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Turgut, B.; Türkçüoğlu, P.; Deniz, N.; Catak, O. Annular and central heavy pigment deposition on the posterior lens capsule in the pigment dispersion syndrome: Pigment deposition on the posterior lens capsule in the pigment dispersion syndrome. Int. Ophthalmol. 2008, 28, 441–445. [Google Scholar] [CrossRef]
- Tanaka, H.; Ohara, K.; Shiwa, T.; Minami, M. Idiopathic opacification of Berger’s space. J. Cataract Refract. Surg. 2004, 30, 2232–2234. [Google Scholar] [CrossRef]
- Mares, V.; Nehemy, M.B.; Salomao, D.R.; Goddard, S.; Tesmer, J.; Pulido, J.S. Multimodal imaging and histopathological evaluation of Berger’s Space. Ocul. Oncol. Pathol. 2020, 6, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Mansukhani, S.A.; Pulido, J.S.; Khanna, S.S. Nd:YAG capsulotomy for the management of posterior capsular amyloidosis. Am. J. Ophthalmol. Case Rep. 2018, 13, 50–52. [Google Scholar] [CrossRef]
- Kislitsyna, N.; Novikov, S.; Kolesnik, A.; Kolesnik, S.; Veselkova, M. On the inner side of lens. Anatomic and topographic features of anterior vitreous. In Proceedings of the 2019 EVRS Congress, Lisbon, Portugal, 27–30 June 2019. [Google Scholar]
- Kislitsyna, N.; Novikov, S.; Kolesnik, A.; Kolesnik, S.; Veselkova, M. Anatomical topographical features of anterior vitreous cortex. Fyodrov J. Ophthalmic Surg. 2017, 1, 66–71. [Google Scholar] [CrossRef]
- Sebag, J. Vitreous: The resplendent enigma. Br. J. Ophthalmol. 2009, 93, 989–991. [Google Scholar] [CrossRef] [PubMed]
- Kishi, S. Impact of swept source optical coherence tomography on ophthalmology. Taiwan J. Ophthalmol. 2016, 6, 58–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishi, S.; Shimizu, K. Oval defect in detached posterior hyaloid membrane in idiopathic preretinal macular fibrosis. Am. J. Ophthalmol. 1994, 118, 451–456. [Google Scholar] [CrossRef]
- Kishi, S.; Shimizu, K. Posterior precortical vitreous pocket. Arch. Ophthalmol. 1990, 108, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Engelbert, M. A new understanding of vitreous structure. Rev. Ophthalmol. 2016, 1, 60–64. [Google Scholar]
- Itakura, H.; Kishi, S.; Li, D.; Akiyama, H. Observation of posterior precortical vitreous pocket using swept-source optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3102–3107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worst, J.G.F.; Sebag, J.; Kishi, S. Posterior precortical vitreous pocket. Arch. Ophthalmol. 1991, 109, 1058–1060. [Google Scholar] [CrossRef]
- Kakehashi, A.; Kado, M.; Akiba, J.; Hirokawa, H. Variations of posterior vitreous detachment. Br. J. Ophthalmol. 1997, 81, 527–532. [Google Scholar] [CrossRef]
- Kakehashi, A.; Kado, M.; Akiba, J.; Hirokawa, H. Biomicroscopic findings of posterior vitreous. Nippon Ganka Gakkai Zasshi 1995, 99, 323–328. [Google Scholar]
- She, X.; Ye, X.; Chen, R.; Pan, D.; Shen, L. Characteristics of posterior precortical vitreous pockets and Cloquet’s canal in patients with myopia by optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4882–4888. [Google Scholar] [CrossRef] [Green Version]
- Polak, B.C.P.; Ringens, P.J.; Worst, J.G.F. Physiological vitreous changes may contribute to the pathogenesis of macular degeneration. Acta Ophthalmol. 2012, 90, e652–e653. [Google Scholar] [CrossRef] [PubMed]
- Kishi, S. The vitreous and the macula. Nippon Ganka Gakkai Zasshi 2015, 119, 117–143. (In Japanese) [Google Scholar] [PubMed]
- Okada, M.; Ogino, N.; Matsumura, M.; Honda, Y.; Nagai, Y. Histological and immunohistochemical study of idiopathic epiretinal membrane. Ophthalmic Res. 1995, 27, 118–128. [Google Scholar] [CrossRef]
- Sebag, J. Vitreoschisis. Graefes Arch Clin Exp Ophthalmol. 2008, 246, 329–332. [Google Scholar] [CrossRef] [Green Version]
- Sebag, J.; Niemeyer, M.; Koss, M.J. Anomalous posterior vitreous detachment and vitreoschisis. In Vitreous: In Health and Disease; Sebag, J., Ed.; Springer Publishing Company: New York, NY, USA, 2014; pp. 241–263. ISBN 978-1-4939-1086-1. [Google Scholar]
- Gupta, P.; Yee, K.M.; Garcia, P.; Rosen, R.B.; Parikh, J.; Hageman, G.S.; Sadun, A.A.; Sebag, J. Vitreoschisis in macular diseases. Br. J. Ophthalmol. 2011, 95, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Eisner, G. Gross anatomy of the vitreous body (author’s trans). Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 1975, 193, 33–56. [Google Scholar] [CrossRef]
- Kishi, S.; Hagimura, N.; Shimizu, K. The role of the premacular liquified pocket and premacular vitreous cortex in idiopathic macular hole development. Am. J. Ophthalmol. 1996, 122, 622–628. [Google Scholar] [CrossRef]
- Itakura, H.; Kishi, S. Aging changes of vitreomacular interface. Retina 2011, 31, 1400–1404. [Google Scholar] [CrossRef] [PubMed]
- Kesler, C.T.; Liao, S.; Munn, L.L.; Padera, T.P. Lymphatic vessels in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Isogai, S.; Hitomi, J.; Yaniv, K.; Weinstein, B.M. Zebrafish as a new animal model to study lymphangiogenesis. Anat. Sci. Int. 2009, 84, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Alderfer, L.; Wei, A.; Hanjaya-Putra, D. Lymphatic Tissue Engineering and Regeneration. J. Biol. Eng. 2018, 17, 12–32. [Google Scholar] [CrossRef]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burfeind, K.G.; Murchison, C.F.; Westaway, S.K.; Simon, M.J.; Erten-Lyons, D.; Kaye, J.A.; Quinn, J.F.; Iliff, J.J. The effects of noncoding aquaporin-4 single-nucleotide polymorphisms on cognition and functional progression of Alzheimer’s disease. Alzheimers Dement (N.Y.) 2017, 3, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Bucchieri, F.; Farina, F.; Zummo, G.; Cappello, F. Lymphatic vessels of the dura mater: A new discovery? J. Anat. 2015, 227, 702–703. [Google Scholar] [CrossRef] [Green Version]
- Raper, D.; Louveau, A.; Kipnis, J. How do meningeal lymphatic vessels drain the CNS? Trends Neurosci. 2016, 39, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Ford, M.L. How brains are drained: Discovery of lymphatics within the CNS. Am. J. Transpl. 2016, 3, 735. [Google Scholar] [CrossRef] [Green Version]
- London, A.; Benhar, I.; Schwartz, M. The retina as a window to the brain-from eye research to CNS disorders. Nat. Rev. Neurol. 2013, 9, 44–53. [Google Scholar] [CrossRef]
- Nakao, S.; Hafezi-Moghadam, A.; Ishibashi, T. Lymphatics and lymphangiogenesis in the eye. J. Ophthalmol. 2012, 2012, 783163. [Google Scholar] [CrossRef] [PubMed]
- Wostyn, P.; van Dam, D.; Audenaert, K.; Killer, H.E.; de Deyn, P.P.; de Groot, V. A new glaucoma hypothesis: A role of glymphatic system dysfunction. Fluids Barriers CNS 2015, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Denniston, A.K.; Keane, P.A. Paravascular Pathways in the Eye: Is There an ‘Ocular Glymphatic System’? Investig. Ophthalmol. Vis. Sci. 2015, 56, 3955–3956. [Google Scholar] [CrossRef]
- Wostyn, P.; De Groot, V.; Van Dam, D.; Audenaert, K.; Killer, H.E.; De Deyn, P.P. Age-related macular degeneration, glaucoma and Alzheimer’s disease: Amyloidogenic diseases with the same glymphatic background? Cell Mol. Life Sci. 2016, 3, 4299–4301. [Google Scholar] [CrossRef] [PubMed]
- Wostyn, P.; de Groot, V.; van Dam, D.; Audenaert, K.; de Deyn, P.P. The Glymphatic System: A New Player in Ocular Diseases? Investig. Ophthalmol. Vis. Sci. 2016, 57, 5426–5427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gausas, R.E.; Daly, T.; Fogt, F. D2-40 expression demonstrates lymphatic vessel characteristics in the dural portion of the optic nerve sheath. Ophthalmic Plast. Reconstr. Surg. 2007, 23, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gimotty, P.A.; Guerry, D.; Karakousis, G.; Elder, D.E. Lymphatic invasion as a prognostic biomarker in primary cutaneous melanoma. Methods Mol. Biol. 2014, 1102, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Morgado, F.N.; da Silva, A.V.A.; Porrozzi, R. Infectious diseases and the lymphoid extracellular matrix remodeling: A focus on conduit system. Cells 2020, 9, 725. [Google Scholar] [CrossRef] [Green Version]
- Palframan, R.T.; Jung, S.; Cheng, G.; Weninger, W.; Luo, Y.; Dorf, M.; Littman, D.R.; Rollins, B.J.; Zweerink, H.; Rot, A.; et al. Inflammatory chemokine transport and presentation in HEV: A remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 2001, 194, 1361–1373. [Google Scholar] [CrossRef] [Green Version]
- Roozendaal, R.; Mempel, T.R.; Pitcher, L.A.; Gonzalez, S.F.; Verschoor, A.; Mebius, R.E.; von Andrian, U.H.; Carroll, M.C. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity. 2009, 30, 264–276. [Google Scholar] [CrossRef] [Green Version]
- Drumea-Mirancea, M.; Wessels, J.T.; Müller, C.A.; Essl, M.; Eble, J.A.; Tolosa, E.; Koch, M.; Reinhardt, D.P.; Sixt, M.; Sorokin, L.; et al. Characterization of a conduit system containing laminin-5 in the human thymus: A potential transport system for small molecules. J. Cell Sci. 2006, 119, 1396–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolte, M.A.; Beliën, J.A.M.; Schadee-Eestermans, I.; Jansen, W.; Unger, W.W.J.; van Rooijen, N.; Kraal, G.; Mebius, R.E. A conduit system distributes chemokines and small blood-borne molecules through the splenic white pulp. J. Exp. Med. 2003, 198, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Pegu, A.; Flynn, J.L.; Reinhart, T.A. Afferent and efferent interfaces of lymph nodes are distinguished by expression of lymphatic endothelial markers and chemokines. Lymphat. Res. Biol. 2007, 5, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Amano, I.; Hata, M.; Kojima, H.; Sawa, Y. Immunohistochemical examination on the distribution of cells expressed lymphatic endothelial marker podoplanin and LYVE-1 in the mouse tongue tissue. Acta. Histochem. Cytochem. 2010, 43, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.W.; Tedla, N.; Lloyd, A.R.; Wakefield, D.; McNeil, P.H. Mast cell activation and migration to lymph nodes during induction of an immune response in mice. J. Clin. Investig. 1998, 102, 1617–1626. [Google Scholar] [CrossRef] [Green Version]
- Lobov, G.I.; Pan’kova, M.N. Mechanical properties of lymph node capsule. Bull. Exp. Biol. Med. 2011, 151, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Bouta, E.M.; Ju, Y.; Rahimi, H.; de Mesy-Bentley, K.L.; Wood, R.W.; Xing, L.; Schwarz, E.M. Power Doppler ultrasound phenotyping of expanding versus collapsed popliteal lymph nodes in murine inflammatory arthritis. PLoS ONE 2013, 8, e73766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scelsi, R.; Scelsi, L.; Cortinovis, R.; Poggi, P. Morphological changes of dermal blood and lymphatic vessels in chronic venous insufficiency of the leg. Int. Angiol. 1994, 13, 308–311. [Google Scholar]
- Bouta, E.M.; Wood, R.W.; Brown, E.B.; Rahimi, H.; Ritchlin, C.T.; Schwarz, E.M. In vivo quantification of lymph viscosity and pressure in lymphatic vessels and draining lymph nodes of arthritic joints in mice. J. Physiol. 2014, 592, 1213–1223. [Google Scholar] [CrossRef]
- Jongebloed, W.L.; Humalda, D.; Worst, J.F. A SEM-correlation of the anatomy of the vitreous body: Making visible the invisible. Doc. Ophthalmol. 1986, 64, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Bogoslowski, A.; Kubes, P. Lymph nodes: The unrecognized barrier against pathogens. ACS Infect. Dis. 2018, 4, 1158–1161. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.M.; Nossal, G.J. Ontogeny of the immune response. I. The development of the follicular antigen-trapping mechanism. J. Exp. Med. 1966, 124, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Tassignon, M.J.; Dhubhghaill, S.N. Real-time intraoperative optical coherence tomography imaging confirms older concepts about the Berger space. Ophthalmic Res. 2016, 56, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Cradock, K.A.; Liu, Z.G.; Ray, P.S.; Boppart, S.A. Intraoperative optical coherence tomography for assessing human lymph nodes for metastatic cancer. BMC Cancer 2016, 23, 16–144. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, N.; Abbas, M.; Ishaque, N.; Chaudhry, M.M.; Hameed, M. Neodymium: YAG laser treatment for dense premacular subhyaloid hemorrhage. JLUMHS 2008, 1, 34–36. [Google Scholar] [CrossRef]
- Kumar, V.; Goel, N. “Arcus retinalis”: A novel clinical marker of sub-internal limiting membrane hemorrhage. Eur. J. Ophthalmol. 2020, 22, 1120672120934958. [Google Scholar] [CrossRef]
- Morris, R.; Kuhn, F.; Witherspoon, C.D.; Mester, V.; Dooner, J. Hemorrhagic macular cysts in Terson’s syndrome and its implications for macular surgery. Dev. Ophthalmol. 1997, 29, 44–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, M.; Nakamura, K.; Shibata, M.; Yokoyama, K.; Matsuki, M.; Ikeda, T. Magnetic resonance imaging findings of Terson’s syndrome suggesting a possible vitreous hemorrhage mechanism. Jpn. J. Ophthalmol. 2010, 54, 135–139. [Google Scholar] [CrossRef]
- Meyer, C.H.; Mennel, S.; Rodrigues, E.B.; Schmidt, J.C. Is the location of valsalva hemorrhages submembranous or subhyaloidal? Am. J. Ophthalmol. 2006, 41, 231. [Google Scholar] [CrossRef] [PubMed]
- Jongebloed, W.L.; Worst, J.F. The cisternal anatomy of the vitreous body. Doc Ophthalmol. 1987, 67, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Sato, K.; Katano, T.; Hayashi, Y. Surgically induced detachment of the anterior hyaloid membrane from the posterior lens capsule. Arch. Ophthalmol. 1999, 117, 408–409. [Google Scholar] [CrossRef] [Green Version]
- Fine, H.F.; Spaide, R.F. Visualization of the posterior precortical vitreous pocket in vivo with triamcinolone. Arch. Ophthalmol. 2006, 124, 1663. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Nakashizuka, H.; Hattori, T.; Mori, R.; Mizutani, Y.; Yuzawa, M. Three-dimensional depiction of the vitreous pocket using triamcinolone acetonide. Eur. J. Ophthalmol. 2009, 19, 1102–1105. [Google Scholar] [CrossRef] [PubMed]
- Streeten, B.W.; Pulaski, J.P. Posterior zonules and lens extraction. Arch. Ophthalmol. 1978, 96, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Bergua, A.; Küchle, M. Visualization of the hyalo-capsular ligament in the living eye. Graefes Arch. Clin. Exp. Ophthalmol. 2002, 240, 503–505. [Google Scholar] [CrossRef]
- Kawagoe, M.; Tsuruga, E.; Oka, K.; Sawa, Y.; Ishikawa, H. Matrix metalloproteinase-2 degrades fibrillin-1 and fibrillin-2 of oxytalan fibers in the human eye and periodontal ligaments in vitro. Acta Histochem. Cytochem. 2013, 46, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Worst, J.F.G.; Los, L.I. Cisternal Anatomy of the Vitreous; Kugler Publications: Amsterdam, The Netherlands, 1995; pp. 5, 26. ISBN 978-9062991105. [Google Scholar]
- Oliver, G.; Detmar, M. The rediscovery of the lymphatic system: Old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002, 16, 773–783. [Google Scholar] [CrossRef] [Green Version]
- Van de Pavert, S.A.; Mebius, R.E. Development of secondary lymphoid organs in relation to lymphatic vasculature. Adv. Anat. Embryol. Cell Biol. 2014, 214, 81–91. [Google Scholar] [CrossRef]
- Díaz-Flores, L.; Gutiérrez, R.; García, M.P.; González-Gómez, M.; Díaz-Flores, L., Jr.; Carrasco, J.L. Intussusceptive lymphangiogenesis in the sinuses of developing human foetal lymph nodes. Ann. Anat. 2019, 226, 73–83. [Google Scholar] [CrossRef]
- Los, L.I. The rabbit as an animal model for post-natal vitreous matrix differentiation and degeneration. Eye (Lond.) 2008, 22, 1223–1232. [Google Scholar] [CrossRef] [Green Version]
- Breslin, J.W.; Yang, Y.; Scallan, J.P.; Sweat, R.S.; Adderley, S.P.; Murfee, W.L. Lymphatic vessel network structure and physiology. Compr. Physiol. 2018, 9, 207–299. [Google Scholar] [CrossRef] [PubMed]
- Scavelli, C.; Weber, E.; Aglianò, M.; Cirulli, T.; Nico, B.; Vacca, A.; Ribatti, D. Lymphatics at the crossroads of angiogenesis and lymphangiogenesis. J. Anat. 2004, 204, 433–449. [Google Scholar] [CrossRef]
- Bazigou, E.; Wilson, J.T.; Moore, J.E., Jr. Primary and secondary lymphatic valve development: Molecular, functional and mechanical insights. Microvasc. Res. 2014, 96, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Gerli, R.; Solito, R.; Weber, E.; Aglianó, M. Specific adhesion molecules bind anchoring filaments and endothelial cells in human skin initial lymphatics. Lymphology 2000, 33, 148–157. [Google Scholar]
- Weber, E.; Rossi, A.; Solito, R.; Sacchi, G.; Agliano, M.; Gerli, R. Focal adhesion molecules expression and fibrillin deposition by lymphatic and blood vessel endothelial cells in culture. Microvasc. Res. 2002, 64, 47–55. [Google Scholar] [CrossRef]
- Quondamatteo, F.; Reinhardt, D.P.; Charbonneau, N.L.; Pophal, G.; Sakai, L.Y.; Herken, R. Fibrillin-1 and fibrillin-2 in human embryonic and early fetal development. Matrix Biol. 2002, 21, 637–646. [Google Scholar] [CrossRef]
- Paniagua, D.; Vergara, I.; Boyer, L.; Alagón, A. Role of lymphatic system on snake venome absorption. In Snake Venoms. Toxinology; Gopalakrishnakone, P., Inagaki, H., Vogel, C.W., Mukherjee, A.K., Rahmy, T.R., Eds.; Springer Science + Business Media: Dordrecht, The Netherlands, 2017; Volume 19, pp. 453–477. ISBN 978-94-007-6409-5. [Google Scholar]
- Grüntzig, J.; Huth, F. Studies on the lymphatic drainage of the eye. 3. Observations of the drainage of Indian ink from the vitreous after bilateral cervical lymph node dissection (author’s transl). Klin. Monbl. Augenheilkd. 1977, 171, 774–779, (Article in German). [Google Scholar] [PubMed]
- Grüntzig, J.; Schicha, H.; Becker, V.; Keim, J.; Feinendegen, L.E. Studies of the lymph drainage of the eye. 4. Drainage of lymphotropic radioactive tracers (99mTc-Microcolloid) after intravitreal injection. Klin. Monbl. Augenheilk. 1978, 172, 87–94. (In German) [Google Scholar]
- Grüntzig, J.; Schicha, H.; Huth, F. Eye and lymph drainage. Z. Lymphol. 1979, 3, 35–45. (In German) [Google Scholar] [PubMed]
- Camelo, S.; Lajavardi, L.; Bochot, A.; Goldenberg, B.; Naud, M.C.; Fattal, E.; Behar-Cohen, F.; de Kozak, Y. Drainage of fluorescent liposomes from the vitreous to cervical lymph nodes via conjunctival lymphatics. Ophthalmic Res. 2008, 40, 145. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous humor dynamics: A review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yücel, Y.H.; Johnston, M.G.; Ly, T.; Patel, M.; Drake, B.; Gümüş, E.; Fraenkl, S.A.; Moore, S.; Tobbia, D.; Armstrong, D.; et al. Identification of lymphatics in the ciliary body of the human eye: A novel “uveolymphatic” outflow pathway. Exp. Eye Res. 2009, 89, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Grüntzig, J.; Hollmann, F. Lymphatic vessels of the eye—old questions—new insights. Ann. Anat. 2019, 221, 1–16. [Google Scholar] [CrossRef]
- Caglı, B.; Tuncel, S.A.; Yılmaz, E.; Tekatas, A.; Ermıs, V. Vitreous humor diffusion measurements from diffusion weighted imaging in idiopathic intracranial hypertension. Ideggyogy. Szle. 2016, 69, 405–410. [Google Scholar] [CrossRef]
- Hayreh, S.S. Posterior drainage of the intraocular fluid from the vitreous. Exp. Eye Res. 1966, 5, 123–144. [Google Scholar] [CrossRef]
- Kuhnt, H. L’oedema papillaire. Ann. Ocul. 1879, 82, 180. [Google Scholar]
- Ulrich, R. Studien über die Pathogenese des Glaucoms. Graefe’s Arch. Fur Ophthalmol. 1884, 30, 235–288. [Google Scholar] [CrossRef] [Green Version]
- Algvere, P.; Bill, A. Drainage of microspheres and RBCs from the vitreous of aphakic and phakic eyes. Arch. Ophthalmol. 1979, 97, 1333–1336. [Google Scholar] [CrossRef]
- Faghih, M.M.; Sharp, M.K. Is bulk flow plausible in perivascular, paravascular and paravenous channels? Fluids Barriers CNS 2018, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Behar-Cohen, F.F.; Moulin, A.; Jonet, L.; Gelize, E.; Sellam, A.; Crisanti, P. Aquaporin 4 and glymphatic drainage in the human macula. Investig. Ophthalmol. Vis. Sci. 2018, 59, 388. [Google Scholar]
- Ikeda, T.; Nakamura, K.; Oku, H.; Horie, T.; Kida, T.; Takai, S. Immunohistological study of monkey foveal retina. Sci. Rep. 2019, 9, 5258. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, K.; Khamsy, L.; Kowalczuk, L.; Moulin, A.; Nicolas, M.; Zografos, L.; Lassiaz, P.; Behar-Cohen, F. Glial cells of the human fovea. Mol. Vis. 2020, 26, 235–245. [Google Scholar] [PubMed]
- Mader, S.; Brimberg, L. Aquaporin-4 water channel in the brain and its implication for health and disease. Cells 2019, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Frisina, R.; Pinackatt, S.J.; Sartore, M.; Monfardini, A.; Baldi, A.; Cesana, B.M.; Semerato, C.F.; Bratu, A.; Parolini, B. Cystoid macular edema after pars plana vitrectomy for idiopathic epiretinal membrane. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morishita, S.; Sato, T.; Oosuka, S.; Horie, T.; Kida, T.; Oku, H.; Nakamura, K.; Takai, S.; Jin, D.; Ikeda, T. Expression of Lymphatic Markers in the Berger’s Space and Bursa Premacularis. Int. J. Mol. Sci. 2021, 22, 2086. https://doi.org/10.3390/ijms22042086
Morishita S, Sato T, Oosuka S, Horie T, Kida T, Oku H, Nakamura K, Takai S, Jin D, Ikeda T. Expression of Lymphatic Markers in the Berger’s Space and Bursa Premacularis. International Journal of Molecular Sciences. 2021; 22(4):2086. https://doi.org/10.3390/ijms22042086
Chicago/Turabian StyleMorishita, Seita, Takaki Sato, Shou Oosuka, Taeko Horie, Teruyo Kida, Hidehiro Oku, Kimitoshi Nakamura, Shinji Takai, Denan Jin, and Tsunehiko Ikeda. 2021. "Expression of Lymphatic Markers in the Berger’s Space and Bursa Premacularis" International Journal of Molecular Sciences 22, no. 4: 2086. https://doi.org/10.3390/ijms22042086





