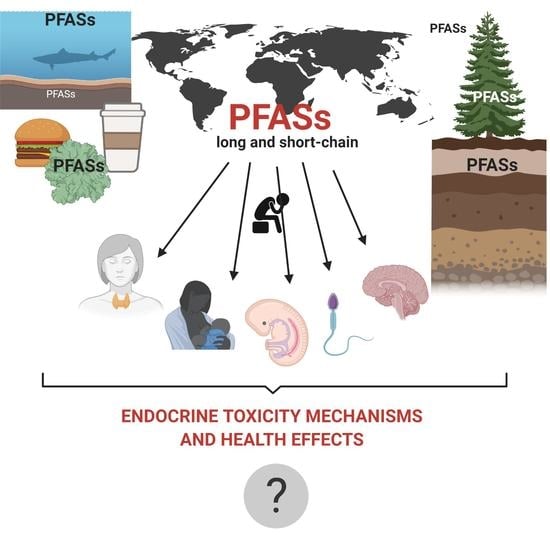
Endocrine Disruptor Potential of Short- and Long-Chain Perfluoroalkyl Substances (PFASs)—A Synthesis of Current Knowledge with Proposal of Molecular Mechanism
Abstract
:Highlights
- Short-chain PFASs, similar to long-chain PFASs, are widely distributed in biotic and abiotic components of the environment.
- Health effects caused by PFASs exposure are related to endocrine disruption and varied according to gender and age of development.
- Obtained data showed that both long- and short-chain PFASs exhibit potential impacts on steroid hormone precursor (DHEA, aldosterone) level.
- In many cases short-chain PFASs exhibit similar or even higher endocrine disrupting potential than long-chain PFASs (especially PFHxS).
- In vivo and in vitro studies have reported that PFASs can bind to nuclear receptors, such as estrogen receptors (ERs), androgen receptor (ARs) and thyroid hormone receptor (TRs); therefore, they are can alter steroidogenesis.
1. Introduction
1.1. PFASs Chemical Structure and Classification
1.2. PFASs Laws and Regulations
1.3. Long-Chain PFASs Alternatives
1.4. Health Effects Caused by PFASs
1.5. The Aim of the Review
2. Physicochemical Properties
3. Sources of Exposure and Transformation in Living Organisms
3.1. Sources of Exposure
3.2. Biotransformation and Accumulation
3.3. Excretion
4. PFASs in Environment
4.1. Abiotic
4.2. Biotic (Human)
5. Endocrine Disruption Caused by PFASs
5.1. Influence on Steroidogenesis
Cholesterol Homeostasis Alterations
5.2. Hormones Disturbance
5.3. Fetus and Newborn
5.4. Reproductive Toxicity
5.5. Obesity
5.6. Neuroendocrine Toxicity
5.7. Genotoxicity, Cancerogenicity and Mutagenicity
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| AR | androgen receptor |
| BMI | body mass index |
| BPA | bisphenol A |
| BPAF | bisphenol AF |
| DHEA | dehydroepiandrosterone |
| DHEAS | dehydroepiandrosterone-sulfate |
| DHT | 5α-dihydrotestosterone |
| EDCs | endocrine disrupting chemicals |
| ER | estrogen receptors |
| Erα | estrogen receptor α |
| Erβ | estrogen receptor β |
| FSH | follicle stimulating hormone |
| FT4 | free thyroxine |
| FXR | farnesoid X receptor |
| GluR2 | glutamate receptor 2 |
| HDL | high-density lipoprotein |
| IARC | International Agency for Research on Cancer |
| LH | luteinizing hormone |
| LPL | lipoprotein lipase |
| LTP | long-term potentiation |
| LXR | liver X receptor |
| NMDAR | N-methyl-d-aspartate receptor |
| PBMCs | peripheral blood mononuclear cells |
| PFAA | perfluoroalkyl acids |
| PFASs | per- and polyfluoroalkyl substances |
| PFBS | perfluorobutane sulfonic acid |
| PFCAs | perfluoroalkyl carboxylic acids |
| PFDA | perfluorodecanoic acid |
| PFHxA | perfluorohexanoic acid |
| PFHxS | perfluorohexane sulfonic acid |
| PFOA | perfluorooctanoic acid |
| PFOS | perfluorooctanesulfonic acid |
| PFOSA | perfluoroalkane sulfonamide acids |
| PFSAs | perfluoroalkane sulfonic acids |
| POPs | persistent organic pollutants |
| PPARα | proliferator-activated receptor alfa |
| PPARγ | proliferator-activated receptor gamma |
| ROS | reactive oxygen species |
| RXR | retinoid x receptor |
| T3 | free triiodothyronine |
| T4 | total thyroxine |
| TDI | tolerable daily intake |
| THs | thyroid hormones |
| TR | thyroid hormone receptor |
| TSH | thyroid stimulating hormone |
| TTE | transplacental transfer efficiency |
| TWI | tolerated weekly intake |
| 8-OHdG | 8-hydroxy-2’-deoxyguanozine |
References
- WHO (World Health Organization). Global Assessment of the State-of-the-Science of Endocrine Disruptors. WHO/PCS/EDC/02.2. International Programme on Chemical Safety. 2002. Available online: http://www.who.int/ipcs/publications/new_issues/endocrine_disruptors/en (accessed on 6 November 2020).
- Conneely, O.M. Perspective: Female steroid hormone action. Endocrinology 2001, 142, 2194–2199. [Google Scholar] [CrossRef] [PubMed]
- OECD. Toward a New Comprehensive Global Database of per and Polyfuoroalkyl Substances (PFASs): Summary on Updating the OECD 2007 List of per-and Polyfuoroalkyl Substances (PFASs). 2018. Available online: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV-JM-MONO(2018)7&doclanguage=en (accessed on 1 October 2020).
- Grandjean, P.; Clapp, R. Changing Interpretation of Human Health Risks from Perfluorinated Compounds. Public Health Rep. 2014, 129, 482–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- US EPA. Fact Sheet PFOA & PFOS Drinking Water Health Advisories. EPA 800-F-16-003. Drinking Water Health Advisories for PFOA and PFOS (epa.gov). 2016. Available online: https://www.epa.gov/sites/production/files/2016-06/documents/drinkingwaterhealthadvisories_pfoa_pfos_updated_5.31.16.pdf (accessed on 3 October 2020).
- Wang, Z.; Cousins, I.T.; Scheringer, M.; Buck, R.C.; Hungerbühler, K. Global emission inventories for C4–C14 perfluoroalkyl carboxylic acid homologues from 1951 to 2030, part I: Production and emissions from quantifiable sources. Environ. Int. 2014, 70, 62–75. [Google Scholar] [CrossRef]
- Bartell, S.M.; Calafat, A.M.; Lyu, C.; Kato, K.; Ryan, P.B.; Steenland, K. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ. Health Persp. 2010, 118, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Vierke, L.; Berger, U.; Cousins, I.T. Estimation of the acid dissociation constant of perfluoroalkyl carboxylic acids through an experimental investigation of their water-to-air transport. Environ. Sci. Technol. 2013, 47, 11032–11039. [Google Scholar] [CrossRef] [PubMed]
- Armitage, J.M.; Schenker, U.; Scheringer, M.; Martin, J.W.; MacLeod, M.; Cousins, I.T. Modeling the global fate and transport of perfluorooctane sulfonate (PFOS) and precursor compounds in relation to temporal trends in wildlife exposure. Environ. Sci. Technol. 2009, 43, 9274–9280. [Google Scholar] [CrossRef]
- Toms, L.M.; Thompson, J.; Rotander, A.; Hobson, P.; Calafat, A.M.; Kato, K.; Ye, X.; Broomhall, S.; Harden, F.; Mueller, J.F. Decline in perfluorooctane sulfonate and perfluorooctanoate serum concentrations in an Australian population from 2002 to 2011. Environ. Int. 2014, 71, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Okada, E.; Kashino, I.; Matsuura, H.; Sasaki, S.; Miyashita, C.; Yamamoto, J.; Ikeno, T.; Ito, Y.M.; Matsumura, T.; Tamakoshi, A.; et al. Temporal trends of perfluoroalkyl acids in plasma samples of pregnant women in Hokkaido, Japan, 2003–2011. Environ. Int. 2013, 60, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, J.; Hu, J.; Liu, C.; Guo, W.; Wang, Q.; Wang, H. The inventory of sources, environmental releases and risk assessment for perfluorooctane sulfonate in China. Environ. Pollut. 2012, 165, 193–198. [Google Scholar] [CrossRef]
- The World Bank. China Reduction and Phase-Out of PFOS in Priority Sectors Project. Report No: PAD1742. 2017. Available online: http://documents1.worldbank.org/curated/en/715131491789645333/pdf/China-Reduction-PFOS-PAD-03242017.pdf (accessed on 17 October 2020).
- Li, L.; Zhai, Z.H.; Liu, J.G.; Hu, J.X. Estimating industrial and domestic environmental releases of perfluorooctanoic acid and its salts in China from 2004 to 2012. Chemosphere 2015, 129, 100–109. [Google Scholar] [CrossRef]
- Xie, S.W.; Wang, T.Y.; Liu, S.J.; Jones, K.C.; Sweetman, A.J.; Lu, Y.L. Industrial source identification and emission estimation of perfluorooctane sulfonate in China. Environ. Int. 2013, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.W.; Lu, Y.L.; Wang, T.Y.; Liu, S.J.; Jones, K.; Sweetman, A. Estimation of PFOS emission from domestic sources in the eastern coastal region of China. Environ. Int. 2013, 59, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J. Sources, Transport and Fate of Perfluoroalkyl Acids in the Atmosphere. Ph.D. Thesis, Stockholm University, Stockholm, Sweden, 2017. [Google Scholar]
- Wang, T.; Vestergren, R.; Herzke, D.; Yu, J.C.; Cousins, I.T. Levels, isomer profiles, and estimated riverine mass discharges of perfluoroalkyl acids and fluorinated alternatives at the mouths of Chinese Rivers. Environ. Sci. Technol. 2016, 50, 11584–11592. [Google Scholar] [CrossRef]
- Danish EPA. Ministry of Environmental Agency, Alternatives to Perfluoroalkyl and Polyfluoro-Alkyl Substances (PFAS) in Textiles LOUS Survey of Chemical Substances in Consumer Products No. 137, 2015. ISBN 978-87-93352-16-2. 2015. Available online: https://www2.mst.dk/Udgiv/publications/2015/05/978-87-93352-16-2.pdf (accessed on 19 October 2020).
- Wang, F.; Shih, K.M.; Ma, R.; Li, X. Influence of cations on the partition behavior of perfluoroheptanoate (PFHpA) and perfluorohexanesulfonate (PFHxS) on waste water sludge. Chemosphere 2015, 131, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Olsen, G.W.; Mair, D.C.; Reagen, W.K.; Ellefson, M.E.; Ehresman, D.J.; Butenhoff, J.L.; Zobel, L.R. Preliminary evidence of a decline in perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations in American Red Cross blood donors. Chemosphere 2007, 68, 105–111. [Google Scholar] [CrossRef]
- Zhang, Y.; Beesoon, S.; Zhu, L.; Martin, J.W. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ. Sci. Technol. 2013, 47, 10619–10627. [Google Scholar] [CrossRef]
- Lieder, P.H.; Chang, S.C.; York, R.G.; Butenhoff, J.L. Toxicological evaluation of potassium perfluorobutanesulfonate in a 90-day oral gavage study with Sprague-Dawley rats. Toxicology 2009, 255, 45–52. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Z.; Krebs, K.A.; Pope, R.H.; Roache, N.F. Concentrations and trends of perfluorinated chemicals in potential indoor sources from 2007 through 2011 in the US. Chemosphere 2014, 98, 51–57. [Google Scholar] [CrossRef]
- Glynn, A.; Berger, U.; Bignert, A.; Ullah, S.; Aune, M.; Lignell, S.; Darnerud, P.O. Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: Serial sampling during pregnancy and nursing, and temporal trends 1996-2010. Environ. Sci. Technol. 2012, 46, 9071–9079. [Google Scholar] [CrossRef]
- Kato, H.; Fujii, S.; Takahashi, M.; Matsumoto, M.; Koizumi, H.M.; Ono, A.; Hirose, A. Repeated dose and reproductive/developmental toxicity of perfluorododecanoic acid in rats. Environ. Toxicol. 2015, 30, 1244–1263. [Google Scholar] [CrossRef]
- Crawford, N.M.; Fenton, S.E.; Strynar, M.; Hines, E.P.; Pritchard, D.A.; Steiner, A.Z. Effects of perfluorinated chemicals on thyroid function, markers of ovarian reserve and natural fertility. Reprod. Toxicol. 2017, 104, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kranthi Kumar, K.; Uma Devi, B.; Neeraja, P. Integration of in silico approaches to determination of endocrine-disrupting perfluorinated chemicals binding potency with steroidogenic acute regulatory protein. Biochem. Biophys. Res. Commun. 2017, 491, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Sepulveda, M.S.; Roy, K.; Leszczynski, J. Endocrine-disrupting activity of per- and polyfluoroalkyl substances: Exploring combined approaches of ligand and structure based modeling. Chemosphere 2017, 184, 514–523. [Google Scholar] [CrossRef]
- Kang, J.; Choi, J.; Park, J. Transcriptional changes in steroidogenesis by perfluoroalkyl acids (PFOA and PFOS) regulate the synthesis of sex hormones in H295R cells. Chemosphere 2016, 155, 436–443. [Google Scholar] [CrossRef]
- Chen, L.; Lam, J.; Hu, C.; Tsui, M.; Lam, P.; Zhou, B. Perfluorobutanesulfonate exposure skews sex ratio in fish and transgenerationally impairs reproduction. Environ. Sci. Technol. 2019, 53, 8389–8397. [Google Scholar] [CrossRef]
- Feng, X.; Cao, X.; Zhao, S.; Wang, X.; Hua, X.; Chen, L. Exposure of Pregnant Mice to Perfluorobutanesulfonate Causes Hypothyroxinemia and Developmental Abnormalities in Female Offspring. Toxicol. Sci. 2017, 155, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Ramhøj, L.; Hass, U.; Boberg, J.; Scholze, M.; Christiansen, S.; Nielsen, F.; Axelstad, M. Perfluorohexane sulfonate (PFHxS) and a mixture of endocrine disrupters reduce thyroxine levels and cause antiandrogenic effects in rats. Toxicol. Sci. 2018, 163, 579–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, L.; Liu, M.; Song, S.; Hu, C.; Lam, P.; Lam, J.; Chen, L. Interaction between hypoxia and perfluorobutane sulfonate on developmental toxicity and endocrine disruption in marine medaka embryos. Aquat. Toxicol. 2020, 222, 105466. [Google Scholar] [CrossRef]
- Cao, X.; Liu, J.; Zhang, Y.; Wang, Y.; Xiong, J.; Wu, J.; Chen, L. Exposure of adult mice to perfluorobutanesulfonate impacts ovarian functions through hypothyroxinemia leading to down-regulation of Akt-mTOR signaling. Chemosphere 2020, 244, 5497. [Google Scholar] [CrossRef]
- Zhao, B.; Chuc, Y.; Hardyb, D.; Li, X.K.; Ge, R.R. Inhibition of 3β and 17β-hydroxysteroid dehydrogenase activities in rat Leydig cells by perfluorooctane acid. J. Steroid. Biochem. Mol. Biol. 2010, 118, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Governini, L.; Orvieto, R.; Guerranti, C.; Gambera, L.; De Leo, V.; Piomboni, P. The impact of environmental exposure to perfluorinated compounds on oocyte fertilization capacity. J. Assist. Reprod. Genet. 2011, 28, 415–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.G.; Liu, W.; Jin, Y.H. Effects of perfluorooctane sulfonate on rat thyroid hormone biosynthesis and metabolism. Environ. Toxicol. Chem. 2009, 28, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Luebker, D.J.; Case, M.T.; York, R.G.; Moore, J.A.; Hansen, K.J.; Butenhoff, J.L. Two-generation reproduction and cross-foster studies of perfluorooctanesulfonate (PFOS) in rats. Toxicology 2015, 215, 126–148. [Google Scholar] [CrossRef] [PubMed]
- Blake, B.; Pinney, S.M.; Hines, E.; Fenton, S.; Ferguson, K. Associations between longitudinal serum perfluoroalkyl substance (PFAS) levels and measures of thyroid hormone, kidney function, and body mass index in the Fernald Community Cohort. Environ. Pollut. 2018, 242, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Byrne, S.; Miller, P.; Medina, S.S.; Waghiyi, V.; Hippel, B.L.F.; Carpenter, D.O. Exposure to perfluoroalkyl substances and associations with serum thyroid hormones in a remote population of Alaska Natives. Environ. Res. 2018, 166, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zeng, X.; Bloom, M.; Lin, S.; Wang, S.; Yim, S.; Chu, Y.C.; Gurram, N.; Hu, L.; Liu, K.; et al. Isomers of perfluoroalkyl substances and overweight status among Chinese by sex status: Isomers of C8 Health Project in China. Environ. Int. 2019, 124, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, H.; Larose, T.; Øien, T.; Sandanger, T.; Odland, J.; van de Bor, M.; Jacobsen, G. Prenatal exposure to persistent organic pollutants and child overweight/obesity at 5-year follow-up: A prospective cohort study. Environ. Health Persp. 2018, 17, 9. [Google Scholar] [CrossRef] [Green Version]
- Hartman, T.; Calafat, A.; Holmes, A.; Marcus, M.; Northstone, K.; Flanders, W.; Kato, K.; Taylor, E.V. Prenatal Exposure to Perfluoroalkyl Substances and Body Fatness in Girls. Child Obes. 2017, 13, 222–230. [Google Scholar] [CrossRef]
- He, X.; Liu, Y.; Xu, B.; Gu, L.; Tang, W. PFOA is associated with diabetes and metabolic alteration in US men: National Health and Nutrition Examination Survey 2003–2012. Sci. Total Environ. 2018, 625, 566–574. [Google Scholar] [CrossRef]
- Karnes, C.; Winquist, A.; Steenland, K. Incidence of type II diabetes in a cohort with substantial exposure to perfluorooctanoic acid. Environ. Res. 2014, 128, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, W.; Wu, S.; Liang, F.; Li, Y.; Zhang, J.; Cui, L.; Feng, Y.; Wang, Y. Perfluoroalkyl substances exposure and risk of polycystic ovarian syndrome related infertility in Chinese women. Environ. Pollut. 2019, 247, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, W.; Li, W.; Liu, C.; Chen, Y.; Yang, Q.; Wang, Y.; Sun, K. Inhibition of 11β-HSD2 expression by triclosan via induction of apoptosis in human placental syncytiotrophoblasts. J. Clin. Endoc. Metab. 2015, 100, E542–E549. [Google Scholar] [CrossRef] [Green Version]
- Lum, K.J.R.; Sundaram, D.B.; Barr, T.A.; Louis, G.M.; Buck, L. Perfluoroalkyl Chemicals, Menstrual Cycle Length, and Fecundity: Findings from a Prospective Pregnancy Study. Epidemiology 2017, 28, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.R.; Sancho, C.G.; Gambaretti, J.; Marchand, P.; Ruault, B.M.C.; Severi, G.; Arveux, P.; Antignac, J.; Kvaskoff, M. Perfluorinated alkylated substances serum concentration and breast cancer risk: Evidence from a nested case-control study in the French E3N cohort. Int. J. Cancer 2020, 146, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Nian, M.; Li, Q.; Bloom, M.; Qian, Z.; Syberg, K.; Vaughn, M.; Wang, S.; Wei, Q.; Zeeshan, M.; Gurram, N.; et al. Liver function biomarkers disorder is associated with exposure to perfluoroalkyl acids in adults: Isomers of C8 Health Project in China. Environ. Res. 2019, 172, 81–88. [Google Scholar] [CrossRef]
- Salihovica, S.; Stubleskib, J.; Kärrmanb, A.; Larssonc, A.; Falla, T.; Lindd, L.; Lind, P. Changes in markers of liver function in relation to changes in perfluoroalkylsubstances-A longitudinal study. Environ. Int. 2018, 117, 196–203. [Google Scholar] [CrossRef]
- Darrow, L.; Groth, A.; Winquist, A.; Shin, H.; Bartell, S.; Steenland, K. Modeled Perfluorooctanoic Acid (PFOA) Exposure and Liver Function in a Mid-Ohio Valley Community. Environ. Health Persp. 2016, 124, 1227–1233. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zeng, X.; Bloom, M.; Qian, Z.; Hinyard, L.; Belue, R.; Lin, S.; Wang, S.; Tian, Y.; Yang, M.; et al. Renal function and isomers of perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS): Isomers of C8 Health Project in China. Chemosphere 2019, 218, 1042–1049. [Google Scholar] [CrossRef]
- Zhou, Y.; Bao, W.; Qian, Z.; Geiger, D.S.; Parrish, K.; Yang, B.; Lee, Y.; Dong, G. Perfluoroalkyl substance exposure and urine CC16 levels among asthmatics: A case-control study of children. Environ. Res. 2017, 159, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Qian, Z.; Dharmage, S.; Perret, J.; Geiger, S.; Rigdon, S.; Howard, S.; Zeng, X.; Hu, L.; Yang, B.; et al. Association of perfluoroalkyl substances exposure with impaired lung function in children. Environ. Res. 2017, 155, 15–21. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Q.; He, X.; Wu, Y.; Xu, C. Association between polyfluoroalkyl chemical concentrations and leucocyte telomere length in US adults. Sci. Total Environ. 2018, 653, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Heilmann, C.; Weihe, P.; Nielsen, F.; Mogensen, U.; Timmermann, A.; Jørgensen, B.E. Estimated exposures to perfluorinated compounds in infancy predict attenuated vaccine antibody concentrations at age 5-years. J. Immunotoxicol. 2017, 14, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Michałowicz, J.; Mokra, K.; Bąk, A. Bisphenol A and its analogs induce morphological and biochemical alterations in human peripheral blood mononuclear cells (in vitro study). Toxicol. In Vitro 2015, 29, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Mokra, K.; Surowaniec, K.A.; Woźniak, K.; Michałowicz, J. Evaluation of DNA-damaging potential of bisphenol A and its selected analogs in human peripheral blood mononuclear cells (in vitro study). Food Chem. Toxicol. 2017, 100, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Mokra, K.; Kocia, M.; Michałowicz, J. Bisphenol A and its analogs exhibit different apoptotic potential in peripheral blood mononuclear cells (in vitro study). Food Chem. Toxicol. 2015, 84, 79–88. [Google Scholar] [CrossRef]
- Maćczak, A.; Cyrkler, M.; Bukowska, B.; Michałowicz, J. Eryptosis-inducing activity of bisphenol A and its analogs in human red blood cells (in vitro study). J. Hazard. Mat. 2016, 307, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Prevedouros, K.; Cousins, I.; Buck, R.; Korzeniowski, S. Sources, fate and transport of perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. [Google Scholar] [CrossRef] [PubMed]
- De Vos, M.; Huijbregts, M.; van den Greve, H.M.; Vethaak, A.; Van de Vijver, K.; Leonards, P.; van Leeuwen, S.; de Voogt, P.; Hendriks, A. Accumulation of perfluorooctane sulfonate (PFOS) in the food chain of the Western Scheldt estuary: Comparing field measurements with kinetic modeling. Chemosphere 2008, 70, 1766–1773. [Google Scholar] [CrossRef]
- Kissa, E. Fluorinated Surfactants and Repellents, Revised and Expanded, Surfactant Science Series, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2001; Volume 97, ISBN 0-8247-0472-X. [Google Scholar]
- Benford, D.; de Boer, J.; Carere, A.; di Domenico, A.; Johansson, N.; Schrenk, D.; Schoeters, P.; de Voogt, P.; Dellatte, E. Opinion of the scientific panel on contaminants in the food chain on perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts. EFSA J. 2008, 653, 1–131. [Google Scholar]
- Martin, J.; Asher, B.; Beesoon, S.; Benskin, J.; Ross, M. PFOS or PreFOS? Are perfluorooctane sulfonate precursors (PreFOS) important determinants of human and environmental perfluorooctane sulfonate (PFOS) exposure? J. Environ. Monit. 2010, 12, 1979–2004. [Google Scholar] [CrossRef] [PubMed]
- ATSDR (Agency for Toxic Substances and Disease Registry). Draft Toxicological Profile for Perfluoroalkyls. 2015. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf (accessed on 19 October 2020).
- US EPA. Perfluorooctanoic Acid (PFOA) and Fluorinated Telomers. U.S. Environmental Protection Agency. 2008. Available online: http://www.epa.gov/oppt/pfoa (accessed on 17 October 2020).
- OECD. Hazard Assessment of Perfluorooctane Sulfonate (PFOS) and Its Salts. Organisation for Economic Co-Operation and Development. ENV/JM/RD (2002)17/FINAL. 2002. Available online: https://fluoridealert.org/news/oecd-report-hazard-assessment-of-perfluorooctane-sulfonate-pfos-and-its-salts (accessed on 17 October 2020).
- OECD. Results of the 2006 Survey on Production and Use of PFOS, PFAS, PFOA, PFCA, Their Related Substances and Products/Mixtures Containing These Substances. Organisation for Economic Co-Operation and Development. 2006. Available online: http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?doclanguage=en&cote=env/jm/mono(2006)36 (accessed on 19 October 2020).
- OECD. Report of an OECD Workshop on Perfluorocarboxylic Acids (PFCAs) and Precursors. Organisation for Economic Co-Operation and Development. 2007. Available online: http://www.olis.oecd.org/olis/2007doc.nsf/LinkTo/NT00002AB6/$FILE/JT03229256.PDF (accessed on 19 October 2020).
- Schultz, M.; Barofsky, D.; Field, J. Fluorinated alkyl surfactants. Environ. Eng. Sci. 2003, 20, 487–501. [Google Scholar] [CrossRef]
- Khalil, N.; Chen, A.; Lee, M.; Czerwinski, S.; Ebert, J.; DeWitt, J.; Kannan, K. Association of Perfluoroalkyl Substances, Bone Mineral Density, and Osteoporosis in the U.S. Population in NHANES 2009–2010. Environ. Health Persp. 2016, 124, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Kaboré, H.; Duy, V.S.; Munoz, G.; Méité, L.; Desrosiers, M.; Liu, J.; Sory, T.; Sauvé, S. Worldwide drinking water occurrence and levels of newly-identified perfluoroalkyl and polyfluoroalkyl substances. Sci. Total Environ. 2018, 616–617, 1089–1100. [Google Scholar] [CrossRef]
- Li, Y.J.; Li, L.; Zhang, Z.; Huang, Y.; Liu, N.; Wuc, J.; He, Z.; Zhang, Y.; Niu, Z. Perfluoroalkyl acids in drinking water of China in 2017: Distribution characteristics, influencing factors and potential risk. Environ. Int. 2019, 123, 87–95. [Google Scholar] [CrossRef] [PubMed]
- De Silva, A.; Allard, C.; Spencer, C.; Webster, G.; Shoeib, M. Phosphorus-containing fluorinated organics: Polyfluoroalkyl phosphoric acid diesters (diPAPs), perfluorophosphonates (PFPAs), and perfluorophosphinates (PFPIAs) in residential indoor dust. Environ. Sci. Technol. 2012, 46, 12575–12582. [Google Scholar] [CrossRef]
- Li, L.; Liu, J.; Hu, J.; Wania, F. Degradation of fluorotelomer-based polymers contributes to the global occurrence of fluorotelomer alcohol and perfluoroalkyl carboxylates: A combined dynamic substance flow and environmental fate modeling analysis. Environ. Sci. Technol. 2017, 51, 4461–4470. [Google Scholar] [CrossRef]
- Winkens, K.; Giovanoulis, G.; Koponen, J.; Vestergren, R.; Berger, U.; Karvonen, A.; Pekkanen, J.; Kiviranta, H.; Cousins, I. Perfluoroalkyl acids and their precursors in floor dust of children’s bedrooms-Implications for indoor exposure. Environ. Int. 2018, 119, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Cai, D.; Guo, M.; Li, M.; Li, X. Per- and polyfluorinated compounds in saleswomen’s urine linked to indoor dust in clothing shops. Sci. Total Environ. 2019, 667, 594–600. [Google Scholar] [CrossRef]
- Ehresman, D.; Froehlich, J.; Olsen, G.; Chang, S.; Butenhoff, J. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ. Res. 2007, 103, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Shiman, R.S.; Calafat, A.; Ye, X.; Mora, A.; Webster, T.F.; Oken, T.; Sagiv, S. Predictors of per- and polyfluoroalkyl substance (PFAS) plasma concentrations in 6–10 year old American children. Environ. Sci. Technol. 2017, 51, 5193–5204. [Google Scholar] [CrossRef] [PubMed]
- Boronow, K.E.; Brody, J.G.; Schaider, L.A.; Peaslee, G.F.; Havas, L.; Cohn, B.A. Serum concentrations of PFASs and exposure-related behaviors in African American and non-Hispanic white women. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 206–217. [Google Scholar] [CrossRef] [Green Version]
- Nelson, J.W.; Scammell, M.K.; Hatch, E.E.; Webster, T.F. Social disparities in exposures to bisphenol A and polyfluoroalkyl chemicals: A cross-sectional study within NHANES 2003–2006. Environ. Health 2012, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.B. Contribution of diet and other factors to the levels of selected polyfluorinated compounds: Data from NHANES 2003–2008. Int. J. Hygen Environ. Health 2014, 217, 52–61. [Google Scholar] [CrossRef]
- Siebenaler, R.; Cameron, R.; Butt, C.; Hoffman, K.; Higgins, C.; Stapleton, H. Serum perfluoroalkyl acids (PFAAs) and associations with behavioral attributes. Chemosphere 2017, 184, 687–693. [Google Scholar] [CrossRef]
- Franko, J.; Meade, B.; Frasch, H.; Barbero, A.; Anderson, S. Dermal penetration potential of perfluorooctanoic acid (PFOA) in human and mouse skin. J. Toxicol. Environ. Health Part A 2012, 75, 50–62. [Google Scholar] [CrossRef]
- Harada, K.; Hashida, S.; Kaneko, T.; Takenaka, K.; Minata, M.; Inoue, K.; Saito, N.; Koizumi, A. Biliary excretion and cerebrospinal fluid partition of perfluorooctanoate and perfluorooctane sulfonate in humans. Environ. Toxicol. Pharmacol. 2007, 24, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Tsuyama, Y.; Sanoh, S.; Ohta, S.; Kotake, Y. Perfluorooctane sulfonate induces neuronal vulnerability by decreasing GluR2 expression. Arch. Toxicol. 2017, 91, 885–895. [Google Scholar] [CrossRef]
- Borg, D.; Bogdanska, J.; Sundstrom, M.; Nobel, S.; Hakansson, H.; Bergman, A.; Depierre, J.; Halldin, K.; Bergstrom, U. Tissue distribution of (35)S-labelled perfluorooctane sulfonate (PFOS) in C57Bl/6 mice following late gestational exposure. Rep. Toxicol. 2010, 30, 558–565. [Google Scholar] [CrossRef]
- Bischel, H.N.; Spencer, M.L.A.; Zhang, C.; Luthy, R.G. Strong associations of short-chain perfluoroalkyl acids with serum albumin and investigation of binding mechanisms. Environ. Toxicol. Chem. 2011, 30, 2423–2430. [Google Scholar] [CrossRef] [PubMed]
- Kucharzyk, K.H.; Darlington, R.; Benotti, M.; Deeb, R.; Hawley, E. Novel treatment technologies for PFAS compounds: A critical review. J. Environ. Manag. 2017, 204, 757–764. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Z.; Zong, W.; Liu, R. Interaction rule and mechanism of perfluoroalkyl sulfonates containing different carbon chains with human serum albumin. RSC Adv. 2017, 7, 24781–24788. [Google Scholar] [CrossRef] [Green Version]
- Maestri, L.; Negri, S.; Ferrari, M.; Ghittori, S.; Fabris, F.; Danesino, P.; Imbriani, M. Determination of perfluorooctanoic acid and perfluorooctanesulfonate in human tissues by liquid chromatography/single quadrupole mass spectrometry. Rapid Commun. Mass. Spec. 2006, 20, 2728–2734. [Google Scholar] [CrossRef]
- Peng, H.; Wei, Q.; Wan, Y.; Giesy, J.; Li, L.; Hu, J. Tissue distribution and maternal transfer of poly- and perfluorinated compounds in Chinese sturgeon (Acipenser sinensis): Implications for reproductive risk. Environ. Sci. Technol. 2010, 44, 1868–1874. [Google Scholar] [CrossRef]
- Pérez, F.; Nadal, M.; Ortega, N.A.; Fàbrega, F.; Domingo, J.; Barceló, D.; Farré, M. Accumulation of perfluoroalkyl substances in human tissues. Environ. Int. 2013, 59, 354–362. [Google Scholar] [CrossRef]
- Koskela, A.; Koponen, J.; Lehenkari, P.; Viluksela, M.; Korkalainen, M.; Tuukkanen, J. Perfluoroalkyl substances in human bone: Concentrations in bones and effects on bone cell differentiation. Sci. Rep. 2017, 7, 6841. [Google Scholar] [CrossRef]
- Bogdanska, J.; Sundström, M.; Bergström, U.; Borg, D.; Valugerdi, A.M.; Bergman, Å.; DePierre, J.; Nobel, S. Tissue distribution of 35S-labelled perfluorobutanesulfonic acid in adult mice following dietary exposure for 1–5 days. Chemosphere 2014, 98, 28–36. [Google Scholar] [CrossRef]
- Kim, S.; Shin, H.; Lee, Y.; Cho, H. Sex-specific risk assessment of PFHxS using a physiologically based pharmacokinetic model. Arch. Toxicol. 2018, 92, 1113–1131. [Google Scholar] [CrossRef]
- Sundstrom, M.; Chang, S.; Noker, P.; Gorman, G.; Hart, J.; Ehresman, D.; Bergman, A.; Butenhoff, J. Comparative pharmacokinetics of perfluorohexanesulfonate (PFHxS) in rats, mice, and monkeys. Rep. Toxicol. 2012, 33, 441–451. [Google Scholar] [CrossRef]
- Weaver, Y.; Ehresman, D.; Butenhoff, J.; Hagenbuch, B. Roles of rat renal organic anion transporters in transporting perfluorinated carboxylates with different chain lengths. Toxicol. Sci. 2010, 113, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Wong, F.; MacLeod, M.; Mueller, J.; Cousins, I. Enhanced elimination of perfluorooctane sulfonic acid by menstruating women: Evidence from population-based pharmacokinetic modeling. Environ. Sci. Technol. 2014, 48, 8807–8814. [Google Scholar] [CrossRef]
- Mondal, D.; Weldon, R.; Armstrong, B.; Gibson, L.; Espinosa, L.M.; Shin, H.; Fletcher, T. Breastfeeding: A potential excretion route for mothers and implications for infant exposure to perfluoroalkyl acids. Environ. Health Persp. 2014, 122, 187–192. [Google Scholar] [CrossRef]
- Rickard, R. Toxicology–Perfluorocarboxylates -PFOA -PFHxA -PFBA. 2009. Available online: https://cfpub.epa.gov/ncer_abstracts/index.cfm/fuseaction/display.files/fileID/14214 (accessed on 5 December 2020).
- Worley, R.; Moore, S.; Tierney, B.; Ye, X.; Calafat, A.; Campbell, S.; Woudneh, M.; Fisher, J. Per- and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community. Environ. Int. 2017, 106, 135–423. [Google Scholar] [CrossRef]
- Li, Y.; Fletcher, T.; Mucs, D.; Scott, K.; Lindh, C.; Tallving, P.; Jakobsson, K. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occ. Environ. Med. 2018, 75, 46–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, M.; Nilsson, H.; Buck, R. Elimination kinetics of perfluorohexanoic acid in humans and comparison with mouse, rat and monkey. Chemosphere 2013, 10, 2419–2425. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. EFSA J. 2018, 16, e05194. [Google Scholar] [CrossRef] [PubMed]
- Rankin, K.; Mabury, S.; Jenkins, A.J.; Washington, W. A North American and global survey of perfluoroalkyl substances in surface soils: Distribution patterns and mode of occurrence. Chemosphere 2016, 161, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Lee, D.; Jeong, D.; Kuppusamy, S.; Lee, Y.; Park, B.; Kim, J. Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) concentrations in the South Korean agricultural environment: A national survey. J. Int. Agricul. 2017, 16, 1841–1851. [Google Scholar] [CrossRef] [Green Version]
- Gawor, A.; Shunthirasingham, C.; Hayward, S.; Lei, Y.; Gouin, T.; Mmereki, B.; Masamba, W.; Ruepert, C.; Castillo, L.; Shoeib, M.; et al. Neutral polyfluoroalkyl substances in the global atmosphere. Environ. Sci. Process. Impacts 2014, 16, 404–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, F.; Schoeib, M.; Katsoyiannis, A.; Eckhardt, S.; Stohl, A.; Nizzetto, B.P.; Li, H.; Fellin, P.; Su, Y.; Hung, H. Assessing temporal trends and source regions of per- and polyfluoroalkyl substances (PFASs) in air under the Arctic Monitoring and Assessment Programme (AMAP). Atmos. Environ. 2018, 172, 65–73. [Google Scholar] [CrossRef]
- Pellizzaro, A.; Zaggia, A.; Fanta, M.; Conte, L.; Falletti, L. Identification and quantification of linear and branched isomers ofperfluorooctanoic and perfluorooctane sulfonic acids in contaminated groundwater in the veneto region. J. Chromatogr. A 2018, 1533, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhu, L.; Liu, Z. Occurrence and partition of perfluorinated compounds in water and sediment from Liao river and Taihu Lake, China. Chemosphere 2011, 83, 806–814. [Google Scholar] [CrossRef]
- Casal, P.; Zhang, Y.; Martin, J.; Pizarro, M.; Jimenez, B.; Dachs, J. Role of snow deposition of perfluoroalkylated substances at Coastal Livingston Island (Maritime Antarctica). Environ. Sci. Technol. 2017, 51, 8460–8470. [Google Scholar] [CrossRef]
- Ahrens, L.; Bundschuh, M. Fate and effects of poly- and perfluoroalkyl substances in the aquatic environment: A review. Environ. Toxicol. Chem. 2014, 33, 1921–1929. [Google Scholar] [CrossRef]
- Wang, X.; Halsall, C.; Codling, G.; Xie, Z.; Xu, B.; Zhao, Z.; Xue, Y.; Ebinghaus, R.; Jones, K. Accumulation of perfluoroalkyl compounds in tibetan mountain snow: Temporal patterns from 1980 to 2010. Environ. Sci. Technol. 2014, 48, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Kwok, K.; Yamazaki, E.; Yamashita, N.; Taniyasu, S.; Murphy, M.; Horii, Y.; Lam, P. Transport of Perfluoroalkyl substances (PFAS) from an arctic glacier to downstream locations: Implications for sources. Sci. Total Environ. 2013, 447, 46–55. [Google Scholar] [CrossRef]
- Zhao, Z.; Tang, J.; Mi, L.; Tian, C.; Zhong, G.; Zhang, G.; Wang, S.; Li, Q.; Ebinghaus, R.; Xie, Z.; et al. Perfluoroalkyl and polyfluoroalkyl substances in the lower atmosphere and surface waters of the Chinese Bohai Sea, Yellow Sea, and Yangtze River estuary. Sci. Total Environ. 2017, 599–600, 114–223. [Google Scholar] [CrossRef]
- Chen, H.; Sun, R.; Zhang, C.; Han, J.; Wang, X.; Han, G.; He, X. Occurrence, spatial and temporal distributions of perfluoroalkyl substances in wastewater, seawater and sediment from Bohai sea, China. Environ. Pollut. 2017, 221, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, W.; Xu, Y.; Zhao, Y.; Wang, P.; Wang, X.; Li, X.; Cai, C.; Liu, Y.; Xiong, G.; et al. Characteristics of perfluoroalkyl acids in atmospheric PM10 from the coastal cities of the Bohai and Yellow Seas, Northern China. Environ. Pollut. 2018, 243, 1894–1903. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, H.; Yao, D.; Li, J.; Wang, X.; Dong, W. Spatial Distribution of Perfluorinated Compounds in Atmosphere of the Pearl River Delta, China. Arch. Environ. Contam. Toxicol. 2019, 7, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Son, M.; Shin, E.; Choi, S.; Chang, Y. Matrix-specific distribution and compositional profiles of perfluoroalkyl substances (PFASs) in multimedia environments. J. Hazard. Mater. 2019, 364, 19–27. [Google Scholar] [CrossRef]
- Bräunig, J.; Baduel, C.; Barnes, C.; Mueller, J. Leaching and bioavailability of selected perfluoroalkyl acids (PFAAs) from soil contaminated by firefighting activities. Sci. Total Environ. 2019, 646, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Skaar, J.; Ræder, E.; Lyche, J.; Ahrens, L.; Kallenborn, R. Elucidation of contamination sources for poly- and perfluoroalkyl substances (PFASs) on Svalbard (Norwegian Arctic). Environ. Sci. Pollut. Res. 2019, 26, 7356–7363. [Google Scholar] [CrossRef] [PubMed]
- Høisæter, Å.; Pfaff, A.; Breedveld, G. Leaching and transport of PFAS from aqueous film-forming foam (AFFF) in the unsaturated soil at a firefighting training facility under cold climatic conditions. J. Contam. Hydrol. 2019, 222, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Kim, S.; Shoeib, M.; Oh, J.; Park, J. Human exposure to per- and polyfluoroalkyl substances (PFASs) via house dust in Korea: Implication to exposure pathway. Sci. Total Environ. 2016, 553, 266–275. [Google Scholar] [CrossRef]
- Xu, Z.; Fiedler, S.; Pfister, G.; Henkelmann, B.; Mosch, C.; Volkel, W.; Fromme, H.; Schramm, K. Human exposure to fluorotelomer alcohols, perfluorooctane sulfonate and perfluorooctanoate via house dust in Bavaria. Sci. Total Environ. 2013, 443, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Jogsten, I.E.; Nadal, M.; van Bavel, B.; Lindström, G.; Domingo, J. Per- and polyfluorinated compounds (PFCs) in house dust and indoor air in Catalonia, Spain: Implications for human exposure. Environ. Int. 2012, 39, 172–180. [Google Scholar] [CrossRef]
- Kannan, K.; Corsolini, S.; Falandysz, J.; Fillmann, G.; Kumar, K.; Loganathan, B.; Mohd, M.; Olivero, J.; Van Wouwe, N.; Yang, J.; et al. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ. Sci. Technol. 2004, 38, 4489–4495. [Google Scholar] [CrossRef]
- Zhang, H.; Yolton, K.; Webster, G.; Ye, X.; Calafat, A.; Dietrich, K.; Xu, Y.; Xie, C.; Braun, J.; Lanphear, B.; et al. Prenatal and childhood perfluoroalkyl substances exposures and children’s reading skills at ages 5 and 8 years. Environ. Int. 2018, 111, 224–231. [Google Scholar] [CrossRef]
- Gebbink, W.; Berger, U.; Cousins, I. Estimating human exposure to PFOS isomers and PFCA homologues: The relative importance of direct and indirect (precursor) exposure. Environ. Int. 2015, 74, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fromme, H.; Wöckner, M.; Roscher, E.; Völkel, W. ADONA and perfluoroalkylated substances in plasma samples of German blood donors living in South Germany. Int. J. Hygen Environ. Health 2017, 220, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Tanner, E.; Bloom, M.; Wu, Q.; Kannan, K.; Yucel, R.; Shrestha, S.; Fitzgerald, E. Occupational exposure to perfluoroalkyl substances and serum levels of perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) in an aging population from upstate New York: A retrospective cohort study. Int. Arch. Occupat. Environ. Health 2018, 91, 145–154. [Google Scholar] [CrossRef]
- Ye, X.; Kato, K.; Wong, L.; Jia, T.; Kalathil, A.; Latremouille, J.; Calafat, A. Per- and polyfluoroalkyl substances in sera from children 3 to 11 years of age participating in the National Health and Nutrition Examination Survey 2013–2014. Int. J. Hygen Environ. Health 2018, 221, 9–16. [Google Scholar] [CrossRef]
- Daly, E.; Chan, B.; Talbot, E.; Nassif, J.; Bean, C.; Cavallo, S.; Metcalf, E.; Simone, K.; Woolf, A. Per- and polyfluoroalkyl substance (PFAS) exposure assessment in a community exposed to contaminated drinking water, New Hampshire, 2015. Int. J. Hygen Environ. Health 2018, 221, 569–577. [Google Scholar] [CrossRef]
- Richterová, D.; Fábelová, H.; Patayová, J.; Pulkrabová, D.; Lanková, K.; Rausová, E.; Šovčíková, J.; Štencl, J.; Hajšlová, T.; Murínová, P.T.Ľ. Determinants of prenatal exposure to perfluoroalkyl substances in the Slovak birth cohort. Environ. Int. 2018, 121, 1304–1310. [Google Scholar] [CrossRef]
- Toms, L.; Bräunig, J.; Vijayasarathy, S.; Phillips, S.; Hobson, P.; Aylward, L.; Kirk, M.; Mueller, J. Per- and polyfluoroalkyl substances (PFAS) in Australia: Current levels and estimated population reference values for selected compounds. Int. J. Hygen Environ. Health 2019, 222, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Qian, Z.; Vaughn, M.; Huang, J.; Ward, P.; Zeng, X.; Zhou, Y.; Zhu, Y.; Yuan, P.; Li, M.; et al. Positive associations of serum perfluoroalkyl substances with uric acid and hyperuricemia in children from Taiwan. Environ. Pollut. 2016, 212, 519–524. [Google Scholar] [CrossRef] [Green Version]
- Arrebola, J.; Castaño, A.; Esteban, M.; Bartolomé, M.; Gómez, P.B.; Ramos, J. BIOAMBIENT ES: Differential contribution of animal and vegetable food items on persistent organic pollutant serum concentrations in Spanish adults. Data from BIOAMBIENT.ES project. Sci. Total Environ. 2018, 634, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhong, Y.; Li, J.; Zhang, J.; Lyu, B.; Zhao, Y.; Wu, Y. Occurrence of perfluoroalkyl substances in matched human serum, urine, hair and nail (China). J. Environ. Sci. 2018, 67, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Buhrke, T.; Krüger, E.; Pevny, S.; Rößler, M.; Bitter, K.; Lampen, A. Perfluorooctanoic acid (PFOA) affects distinct molecular signaling pathways in human primary hepatocytes. Toxicology 2015, 333, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Huang, H.; Hu, J.; Qin, Y.; Wu, D.; Song, L.; Xia, Y.; Wang, X. Endocrine related effects of perfluorooctanoic acid (PFOA) in zebrafish, H295R steroidogenesis andreceptor reporter gene assays. Chemosphere 2013, 91, 1099–1106. [Google Scholar] [CrossRef]
- Behr, A.; Lichtenstein, D.; Braeuning, A.; Lampen, A.; Buhrke, T. Perfluoroalkylated substances (PFAS) affect neither estrogen and androgen receptor activity nor steroidogenesis in human cells in vitro. Toxicol. Lett. 2018, 291, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.; Glintborg, D.; Gade, C.; Timmermann, N.F.; Kyhl, H.; Frederiksen, H.; Andersson, A.; Juul, A.; Sidelmann, J.; Andersen, H.; et al. Prenatal exposure to perfluorodecanoic acid is associated with lower circulating concentration of adrenal steroid metabolites during mini puberty in human female infants. The Odense Child Cohort. Environ. Res. 2020, 182, 109101. [Google Scholar] [CrossRef] [PubMed]
- Traish, A.M.; Kang, H.P.; Saad, F.; Guay, A.T. Dehydroepiandrosterone (DHEA)--a precursor steroid or an active hormone in human physiology. J. Sex Med. 2011, 8, 2960–2982. [Google Scholar] [CrossRef] [PubMed]
- Gaby, A.R. Dehydroepiandrosterone: Biological effects and clinical significance. Altern. Med. Rev. 1996, 1, 60–69. [Google Scholar]
- Becher, G.; Haug, L.; Toft, G. Long-term effects of prenatal exposure to perfluoroalkyl substances on female reproduction. Hum. Reprod. 2013, 28, 3337–3348. [Google Scholar] [CrossRef] [Green Version]
- Vested, A.; Hansen, R.C.; Olsen, S.; Bonde, J.; Kristensen, S.; Halldorsson, T.; Becher, G.; Haug, L.; Ernst, E.; Toft, G. Associations of in utero exposure to perfluorinated alkyl acids with human semen quality and reproductive hormones in adult men. Environ. Health Persp. 2013, 121, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Ernst, A.; Brix, N.; Lauridsen, L.; Olsen, J.; Parner, E.; Liew, Z.; Olsen, L.; Hansen, R.C. Exposure to Perfluoroalkyl Substances during Fetal Life and Pubertal Development in Boys and Girls from the Danish National Birth Cohort. Environ. Health Persp. 2019, 127, 17004. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, A.; Wasada Ochi, H.; Yoshioka, M.; Yamanaka, N.; Ikezawa, M.; Guruge, K. Changes in hepato-renal gene expression in microminipigs following a single exposure to a mixture of perfluoroalkyl acids. PLoS ONE 2019, 14, 0210110. [Google Scholar] [CrossRef]
- Fei, C.; McLaughlin, J.; Tarone, R.; Olsen, J. Fetal growth indicators and perfluorinated chemicals: A study in the Danish national birth cohort. Am. J. Epidemiol. 2008, 168, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Brisbois, T.; Farmer, A.; McCargar, L. Early markers of adult obesity: A review. Obes. Rev. 2012, 13, 347–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajaratnam, R.; Gylling, H.; Miettinen, T.A. Cholesterol Absorption, Synthesis, and Fecal Output in Postmenopausal Women with and Without Coronary Artery Disease. Arteriosclerosis. Thromb. Vasc. Biol. 2001, 21, 1650–1655. [Google Scholar] [CrossRef] [Green Version]
- Kanayama, T.; Kobayashi, N.; Mamiya, S.; Nakanishi, T.; Nishikawa, J. Organotin compounds promote adipocyte differentiation as agonists of the peroxisome proliferator-activated receptor gamma/retinoid X receptor pathway. Mol. Pharmacol. 2005, 67, 766–774. [Google Scholar] [CrossRef]
- Arevalo, G.M.; Magdalena, A.P.; Dos Santos, R.J.; Quesada, I.; Carneiro, E.; Nadal, A. Exposure to bisphenol-A during pregnancy partially mimics the effects of a high-fat diet altering glucose homeostasis and gene expression in adult male mice. PLoS ONE 2014, 9, e0100214. [Google Scholar] [CrossRef] [Green Version]
- Cortes, V.; Quezada, N.; Rigotti, A.; Maiz, A. New heterodimeric nuclear receptors: Key metabolic regulators with relevance in the pathophysiology and therapy of dyslipidemias and diabetes mellitus. Rev. Médica Chile 2005, 133, 1483–1492. [Google Scholar] [CrossRef] [Green Version]
- Steenland, K.; Tinker, S.; Frisbee, S.; Ducatman, A.; Vaccarino, V. Association of Perfluorooctanoic Acid and Perfluorooctane Sulfonate with Serum Lipids Among Adults Living Near a Chemical Plant. Am. J. Epidemiol. 2009, 170, 1268–1278. [Google Scholar] [CrossRef] [Green Version]
- Nelson, J.; Hatch, W.E.; Webster, T. Exposure to Polyfluoroalkyl Chemicals and Cholesterol, Body Weight, and Insulin Resistance in the General, U.S. Population. Environ. Health Persp. 2009, 118, 197–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksen, K.; Nielsen, R.O.; McLaughlin, J.; Lipworth, L.; Tjønneland, A.; Overvad, K.; Sørensen, M. Association between Plasma PFOA and PFOS Levels and Total Cholesterol in a Middle-Aged Danish Population. PLoS ONE 2013, 8, e0056969. [Google Scholar] [CrossRef] [PubMed]
- Seacat, A.; Thomford, P.; Hansen, K.; Olsen, G.; Case, M.; Butenhof, J. Subchronic toxicity studies on perfuorooctanesulfonate potassium salt in cynomolgus monkeys. Toxicol. Sci. 2002, 68, 249–264. [Google Scholar] [CrossRef] [Green Version]
- Pouwer, M.; Pieterman, E.; Chang, S.; Olsen, G.; Caspers, M.; Verschuren, L.; Jukema, J.; Princen, H. Dose effects of ammonium perfuorooctanoate on lipoprotein metabolism in APOE*3-Leiden.CETP mice. Toxicol. Sci. 2019, 168, 519–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louisse, J.; Rijkers, D.; Stoopen, G.; Janssen, A.; Staats, M.; Hoogenboom, R.; Kersten, S.; Peijnenburg, A. Perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), and perfluorononanoic acid (PFNA) increase triglyceride levels and decrease cholesterogenic gene expression in human HepaRG liver cells. Arch. Toxicol. 2020, 943137–943155. [Google Scholar] [CrossRef] [PubMed]
- Florentin, A.; Deblonde, D.N.; Hautemaniere, A.; Hartemann, P. Impacts of two perfluorinated compounds (PFOS and PFOA) on human hepatoma cells: Cytotoxicity but no genotoxicity? Int. J. Hygen Environ. Health 2011, 214, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Wood, C.; Lin, M.; Starkov, A.; Lau, C.; Wallace, K.; Corton, J.; Abbott, B. Perfluoroalkyl acids-induced liver steatosis: Effects on genes controlling lipid homeostasis. Toxicology 2017, 378, 37–52. [Google Scholar] [CrossRef]
- Rosen, M.; Das, K.; Rooney, J.; Abbott, B.; Lau, C.; Corton, J. PPARα-independent transcriptional targets of perfluoroalkyl acids revealed by transcript profiling. Toxicology 2017, 387, 95–107. [Google Scholar] [CrossRef]
- Chinetti, G.; Gbaguidi, F.; Griglio, S.; Mallat, Z.; Antonucci, M.; Poulain, P.; Chapman, J.; Fruchart, J.; Tedgui, A.; Najib-Fruchart, J.; et al. CLA-1/SR-BI is expressed in atherosclerotic lesion macrophages and regulated by activators of peroxisome proliferator-activated receptors. Circulation 2000, 101, 2411–2417. [Google Scholar] [CrossRef] [Green Version]
- Watkins, A.; Wood, C.; Lin, M.; Abbott, B. The effects of perfluorinated chemicals on adipocyte differentiation in vitro. Mol. Cell. Endocrinol. 2015, 400, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Clark, J.; Laragy, T.M.; Park, Y. Perfluorobutanesulfonic acid (PFBS) potentiates adipogenesis of 3T3-L1 adipocytes. Food Chem. Toxicol. 2018, 120, 340–345. [Google Scholar] [CrossRef]
- Duarte, J.; Perrière, G.; Laudet, V.; Rechavi, R.M. NUREBASE: Database of nuclear hormone receptors. Nucleic Acids Res. 2002, 30, 364–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Ding, Z.; Shi, Q.; Ge, X.; Wang, H.; Li, M.; Chen, G.; Wang, Q.; Ju, Q.; Zhang, J.; et al. Anti-androgenic mechanisms of Bisphenol A involve androgen receptor signaling pathway. Toxicology 2017, 387, 10–16. [Google Scholar] [CrossRef]
- Carmeci, C.; Thompson, D.; Ring, H.; Francke, U.; Weigel, R. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics 1997, 45, 607–617. [Google Scholar] [CrossRef]
- Nohynek, G.; Borgert, C.; Dietrich, D.; Rozman, K. Endocrine disruption: Fact or urban legend? Toxicol. Lett. 2013, 223, 295–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiboutot, D.; Gilliland, K.; Cong, Z.; Jabara, S.; McAllister, J.; Sivarajah, A.; Clawson, G. Human skin is a steroidogenic tissue: Steroidogenic enzymes and cofactors are expressed in epidermis, normal sebocytes, and an immortalized sebocyte cell line (SEB-1). J. Invest. Dermatol. 2003, 120, 905–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.; Zbytek, B.; Nikolakis, G.; Manna, P.; Skobowiat, C.; Zmijewski, M.; Li, W.; Janjetovic, Z.; Postlethwaite, A.; Zouboulis, C.; et al. Steroidogenesis in the skin: Implications for local immune functions. J. Steroid Biochem. Mol. Biol. 2013, 137, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, S.; Gustafsson, J. Biological role of estrogen and estrogen receptors. Crit. Rev. Biochem. Mol. Biol. 2002, 37, 1–28. [Google Scholar] [CrossRef]
- Du, G.; Hu, J.; Huang, H.; Qin, Y.; Han, X.; Wu, D.; Song, L.; Xia, Y.; Wang, X. Perfluorooctane sulfonate (PFOS) affects hormone receptor activity, steroidogenesis, and expression of endocrine-related genes in vitro and in vivo. Environ. Toxicol. Chem. 2013, 353–360. [Google Scholar] [CrossRef]
- Benninghoff, A.; Bisson, W.; Koch, D.; Ehresman, D.; Kolluri, S.; Williams, D. Estrogen-like activity of perfluoroalkyl acids in vivo and interaction with human and rainbow trout estrogen receptors in vitro. Toxicol. Sci. 2011, 120, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Ortega, C.A.; Betancourt, M.; Rosas, P.; Cuevas, V.F.; Chavira, R.; Bonilla, E.; Casas, E.; Ducolomb, Y. Endocrine disruptor effect of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) on porcine ovarian cell steroidogenesis. Toxicol. In Vitro 2018, 46, 86–93. [Google Scholar] [CrossRef]
- Di Nisio, A.; Sabovic, I.; Valente, U.; Tescari, S.; Rocca, M.S.; Guidolin, D.; Acqua, D.S.; Acquasaliente, L.; Pozzi, N.; Plebani, M.; et al. Endocrine Disruption of Androgenic Activity by Perfluoroalkyl Substances: Clinical and Experimental Evidence. J. Clin. Endocr. Metab. 2019, 104, 1259–1271. [Google Scholar] [CrossRef] [Green Version]
- Kjeldsen, L.; Jørgensen, B.E. Perfluorinated compounds affect the function of sex hormone receptors. Environ. Sci. Pollut. Res. 2013, 20, 8031–8044. [Google Scholar] [CrossRef]
- Olsen, G.; Burris, J.; Burlew, M.; Mandel, J. Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations. J. Occup. Environ. Med. 2003, 45, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Qu, K.; Luan, F.; Liu, Y.; Zhu, Y.; Yuan, Y.; Li, H.; Zhang, H.; Hai, Y.; Zhao, C. Binding specificities of estrogen receptor with perfluorinated compounds: A cross species comparison. Environ. Int. 2020, 134, 5284. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Y.; Xie, Z.; Zeng, F. Perfluorinated alkyl substances in serum of the southern Chinese general population and potential impact on thyroid hormones. Sci. Rep. 2017, 7, 3380. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, S.; Bloom, M.; Yucel, R.; Seegal, R.; Rej, R.; McCaffrey, R.; Wu, Q.; Kannan, K.; Fitzgerald, E. Perfluoroalkyl substances, thyroid hormones, and neuropsychological status in older adults. Int. J. Hygen Environ. Health 2017, 220, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.; Lin, C.; Chen, M.; Hsieh, W.; Chen, P. Perfluoroalkyl substances and thyroid hormones in cord blood. Environ. Pollut. 2017, 222, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Bloom, M.; Yucel, R.; Seegal, R.; Wu, Q.; Kannan, K.; Rej, R.; Fitzgerald, E. Perfluoroalkyl substances and thyroid function in older adults. Environ. Int. 2015, 75, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Lee, H.; Moon, H.; Kim, S.; Lee, J.; Ha, M.; Hong, S.; Kim, S.; Choi, K. Perfluoroalkyl acids in serum of Korean children: Occurrences, related sources, and associated health outcomes. Sci. Total Environ. 2018, 645, 958–965. [Google Scholar] [CrossRef]
- Preston, E.; Webster, T.; Oken, E.; Henn, B.; McClean, M.; Shiman, R.S.; Pearce, E.; Braverman, L.; Calafat, A.; Ye, X.; et al. Maternal Plasma per- and Polyfluoroalkyl Substance Concentrations in Early Pregnancy and Maternal and Neonatal Thyroid Function in a Prospective Birth Cohort: Project Viva (USA). Environ. Health Persp. 2018, 126, 7013. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Moon, S.; Oh, B.; Jung, D.; Ji, K.; Choi, K.; Park, Y. Association between perfluoroalkyl substances exposure and thyroid function in adults: A meta-analysis. PLoS ONE 2018, 13, 7244. [Google Scholar] [CrossRef] [PubMed]
- Reardon, A.; Moez, K.E.; Dinu, I.; Goruk, S.; Field, C.; Kinniburgh, D.; MacDonald, A.; Martin, J.; APrON Study. Longitudinal analysis reveals early-pregnancy associations between perfluoroalkyl sulfonates and thyroid hormone status in a Canadian prospective birth cohort. Environ. Int. 2019, 129, 389–399. [Google Scholar] [CrossRef]
- Chen, F.; Yin, S.; Kelly, B.; Liu, W. Isomer-Specific Transplacental Transfer of Perfluoroalkyl Acids: Results from a Survey of Paired Maternal, Cord Sera, and Placentas. Environ. Sci. Technol. 2017, 51, 5756–5763. [Google Scholar] [CrossRef]
- Kim, S.; Choi, K.; Ji, J.; Seo, Y.; Kho, J.; Park, S.; Kim, S.; Park, I.; Hwang, J.; Jeon, H.; et al. Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environ. Sci. Technol. 2011, 45, 7465–7472. [Google Scholar] [CrossRef]
- Beesoon, S.; Webster, G.; Shoeib, M.; Harner, T.; Benskin, J.; Martin, J. Isomer profiles of perfluorochemicals in matched maternal, cord, and house dust samples: Manufacturing sources and transplacental transfer. Environ. Health Persp. 2011, 119, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Syme, M.; Paxton, J.; Keelan, J. Drug transfer and metabolism by the human placenta. Clin. Pharmacol. 2004, 43, 487–514. [Google Scholar] [CrossRef]
- Philipson, E.; Kuhnert, B.; Syracuse, C. Fetal acidosis, 2-chloroprocaine, and epidural anesthesia for cesarean section. Am. J. Obst. Gynecol. 1985, 151, 322–324. [Google Scholar] [CrossRef]
- Biehl, D.; Shnider, S.; Levinson, G.; Callender, K. Placental transfer of lidocaine: Effects of fetal acidosis. Anesthesiology 1978, 48, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Cariou, R.; Veyrand, B.; Yamada, A.; Berrebi, A.; Zalko, D.; Durand, S.; Pollono, C.; Marchand, P.; Leblanc, J.; Antignac, J.; et al. Perfluoroalkyl acid (PFAA) levels and profiles in breast milk, maternal and cord serum of French women and their newborns. Environ. Int. 2015, 84, 71–81. [Google Scholar] [CrossRef]
- Kato, K.; Wong, L.; Chen, A.; Dunbar, C.; Webster, G.; Lanphear, B.; Calafat, A. Changes in serum concentrations of maternal poly- and perfluoroalkyl substances over the course of pregnancy and predictors of exposure in a multiethnic cohort of Cincinnati, Ohio pregnant women during 2003–2006. Environ. Sci. Technol. 2014, 48, 9600–9608. [Google Scholar] [CrossRef]
- Gyllenhammar, I.; Benskin, J.; Sandblom, O.; Berger, U.; Ahrens, L.; Lignell, S.; Wiberg, K.; Glynn, A. Perfluoroalkyl acids (PFAAs) in serum from 2–4-month-old infants-influence of maternal serum concentrations, gestational age, breastfeeding and contaminated drinking water. Environ. Sci. Technol. 2018, 52, 7101–7110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgado, M.C.; Casas, M.; Espinosa, L.M.; Ballester, F.; Basterrechea, M.; Grimalt, J.; Jimenez, A.; Kraus, T.; Schettgen, T.; Sunyer, J.; et al. Transfer of perfluoroalkyl substances from mother to fetus in a Spanish birth cohort. Environ. Res. 2015, 142, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Spratlen, M.; Perera, F.; Lederman, S.; Robinson, M.; Kannan, K.; Trasande, L.; Herbstman, J. Cord blood perfluoroalkyl substances in mothers exposed to the World Trade Center disaster during pregnancy. Environ. Pollut. 2019, 246, 482–490. [Google Scholar] [CrossRef]
- Buekers, J.; Colles, A.; Cornelis, C.; Morrens, B.; Govarts, E.; Schoeters, G. Socio-Economic Status and Health: Evaluation of Human Biomonitored Chemical Exposure to Per- and Polyfluorinated Substances across Status. Int. J. Environ. Res. Public Health 2018, 15, 2818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Ha, E.; Wen, T.; Su, Y.; Lien, G.; Chen, C.; Chen, P.; Hsieh, W. Perfluorinated compounds in umbilical cord blood and adverse birth outcomes. PLoS ONE 2012, 7, 2474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, C.; Fei, C.; Gamborg, M.; Nohr, E.; Sorensen, T.; Olsen, J. Prenatal exposures to perfluorinated chemicals and anthropometric measures in infancy. Am. J. Epidemiol. 2010, 172, 1230–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Washino, N.; Saijo, Y.; Kato, S.; Ban, S.; Konishi, K.; Ito, R.; Nakata, A.; Iwasaki, Y.; Saito, K.; Nakazawa, H.; et al. Correlations between prenatal exposure to perfluorinated chemicals and reduced fetal growth. Environ. Health Persp. 2009, 117, 660–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emerce, E.; Çetin, Ö. Genotoxicity assessment of perfluoroalkyl substances on human sperm. Toxicol. Indust. 2018, 16, 9191. [Google Scholar] [CrossRef]
- Steves, A.; Turry, A.; Gill, B.; Townsend, C.D.; Bradner, J.; Bachli, I.; Caudle, W.; Miller, G.; Chan, A.; Easley, C. Per- and polyfluoroalkyl substances impact human spermatogenesis in a stem-cell-derived model. Syst. Biol. Reprod. Med. 2018, 64, 225–239. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Niu, X.; Wang, X.; Yin, H.; Zeng, F.; He, C. Biomonitoring PFAAs in blood and semen samples: Investigation of a potential link between PFAAs exposure and semen mobility in China. Environ. Int. 2018, 113, 50–54. [Google Scholar] [CrossRef]
- Butenhoff, J.; Chang, S.; Ehresman, D.; York, R. Evaluation of potential reproductive and developmental toxicity of potassium perfluorohexanesulfonate in Sprague Dawley rats. Reprod. Toxicol. 2009, 27, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.; Oken, E.; Shiman, R.S.; Webster, T.; Gillman, M.; Calafat, A.; Ye, X.; Sagiv, S. Prenatal exposure to perfluoroalkyl substances and adiposity in early and mid-childhood. Environ. Health Persp. 2017, 125, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Braun, J.; Chen, A.; Romano, M.; Calafat, A.; Webster, G.; Yolton, K.; Lanphear, B. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The HOME study. Obesity (Silver Spring) 2016, 24, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halldorsson, T.; Rytter, D.; Haug, L.; Bech, B.; Danielsen, I.; Becher, G.; Henriksen, T.; Olsen, S. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: A prospective cohort study. Environ. Health Persp. 2012, 120, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Martinsson, M.; Nielsen, C.; Björk, J.; Rylander, L.; Malmqvist, E.; Lindh, C.; Hydbom, R.A. Intrauterine exposure to perfluorinated compounds and overweight at age 4: A case-control study. PLoS ONE 2020, 15, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alderete, T.; Jin, R.; Walker, D.; Valvi, D.; Chen, Z.; Jones, D.; Peng, C.; Gilliland, F.; Berhane, K.; Conti, D.; et al. Perfluoroalkyl substances, metabolomic profiling, and alterations in glucose homeostasis among overweight and obese Hispanic children: A proof-of-concept analysis. Environ. Int. 2019, 126, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Ducatman, A. Associations between lipid/lipoprotein levels and perfluoroalkyl substances among US children aged 6–11 years. Environ. Pollut. 2018, 243, 1–8. [Google Scholar] [CrossRef]
- Patisaul, H.; Polston, E. Influence of endocrine active compounds on the developing rodent brain. Brain Res. Rev. 2008, 57, 352–362. [Google Scholar] [CrossRef]
- Bean, L.; Ianov, L.; Foster, T. Estrogen receptors, the hippocampus, and memory. Neuroscientist 2014, 20, 534–545. [Google Scholar] [CrossRef] [Green Version]
- Bornehag, C.; Lindh, C.; Reichenberg, A.; Wikström, S.; Unenge Hallerback, M.; Evans, S.; Sathyanarayana, S.; Barrett, E.; Nguyen, R.; Bush, N.; et al. Association of prenatal phthalate exposure with language development in early childhood. JAMA Pediatrics 2018, 172, 1169. [Google Scholar] [CrossRef]
- Braun, J.; Bellinger, D.; Hauser, R.; Wright, R.; Chen, A.; Calafat, A.; Yolton, K.; Lanphear, P. Prenatal phthalate, triclosan, and bisphenol A exposures and child visual-spatial abilities. Neurotoxicology 2017, 58, 75–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braun, J.; Yolton, K.; Stacy, S.; Erar, B.; Papandonatos, G.; Bellinger, D.; Lanphear, B.; Chen, A. Prenatal environmental chemical exposures and longitudinal patterns of child neurobehavior. Neurotoxicology 2017, 62, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Vuong, A.; Braun, J.; Yolton, K.; Wang, Z.; Xie, C.; Webster, G.; Ye, X.; Calafat, A.; Dietrich, K.; Lanphear, B.; et al. Prenatal and childhood exposure to perfluoroalkyl substances (PFAS) and measures of attention, impulse control, and visual spatial abilities. Environ. Int. 2018, 119, 413–420. [Google Scholar] [CrossRef]
- Vuong, A.; Yolton, K.; Wang, Z.; Xie, C.; Webster, G.; Ye, X.; Calafat, A.; Braun, J.; Dietrich, K.; Lanphear, B.; et al. Childhood perfluoroalkyl substance exposure and executive function in children at 8 years. Environ. Int. 2018, 119, 212–219. [Google Scholar] [CrossRef]
- Lien, G.; Huang, C.; Shiu, J.; Chen, M.; Hsieh, W.; Guo, Y.; Chen, P. Perfluoroalkyl substances in cord blood and attention deficit/hyperactivity disorder symptoms in seven-year-old children. Chemosphere 2016, 156, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Oulhote, Y.; Steuerwald, U.; Debes, F.; Weihe, P.; Grandjean, P. Behavioral difficulties in 7-year old children in relation to developmental exposure to perfluorinated alkyl substances. Environ. Int. 2016, 97, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Harris, M.; Oken, E.; Shiman, R.S.; Calafat, A.; Ye, X.X.; Bellinger, D.; Webster, T.; White, R.; Sagiv, S. Prenatal and childhood exposure to per- and polyfluoroalkyl substances (PFASs) and child cognition. Environ. Int. 2018, 115, 358–369. [Google Scholar] [CrossRef]
- Liew, Z.; Ritz, B.; von Ehrenstein, O.; Bech, B.; Nohr, E.; Fei, C.; Bossi, R.; Henriksen, T.; Jørgensen, B.E.; Olsen, J. Attention deficit/hyperactivity disorder and childhood autism in association with prenatal exposure to perfluoroalkyl substances: A nested case-control study in the Danish National Birth Cohort. Environ. Health Persp. 2015, 123, 367–373. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Q.; Liu, C.; Li, C.; Li, Y.; Li, S.; Liu, X.; Shao, J. Evaluation of PFOS-mediated neurotoxicity in rat primary neurons and astrocytes cultured separately or in co-culture. Toxicol. In Vitro 2017, 38, 77–90. [Google Scholar] [CrossRef]
- Wang, R.; Wang, X.; Niu, Y.; Cheng, X.; Shang, Y.; Li, S.; Li, X.; Liu, S.J. Role of astrocytes-derived d-serine in PFOS-induced neurotoxicity through NMDARs in the rat primary hippocampal neurons. Toxicology 2019, 422, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, W.; Niu, Q.; Wang, Y.; Zhao, H.; Zhang, H.; Song, J.; Tsuda, S.; Saito, N. Effects of perfluorooctane sulfonate and its alternatives on long-term potentiation in the hippocampus CA1 region of adult rats in vivo. Toxicol. Res. 2016, 5, 539–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, B.H.; Olsen, G.W. Bladder cancer in perfluorooctanesulfonyl fluoride manufacturing workers. Ann. Epidemiol. 2007, 17, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Hurley, S.; Goldberg, D.; Wang, M.; Park, J.; Petreas, M.; Bernstein, L.; Culver, A.H.; Nelson, D.; Reynolds, P. Breast cancer risk and serum levels of per- and poly-fluoroalkyl substances: A case-control study nested in the California Teachers Study. Environ. Health 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wielsøe, M.; Long, M.; Ghisari, M.; Jørgensen, B.E. Perfluoroalkylated substances (PFAS) affect oxidative stress biomarkers in vitro. Chemosphere 2014, 129, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Yahia, D.; Haruka, I.; Kagashi, Y.; Tsuda, S. 8-Hydroxy-2′-deoxyguanosine as a biomarker of oxidative DNA damage induced by perfluorinated compounds in TK6 cells. Environ. Toxicol. 2016, 31, 192–200. [Google Scholar] [CrossRef] [PubMed]
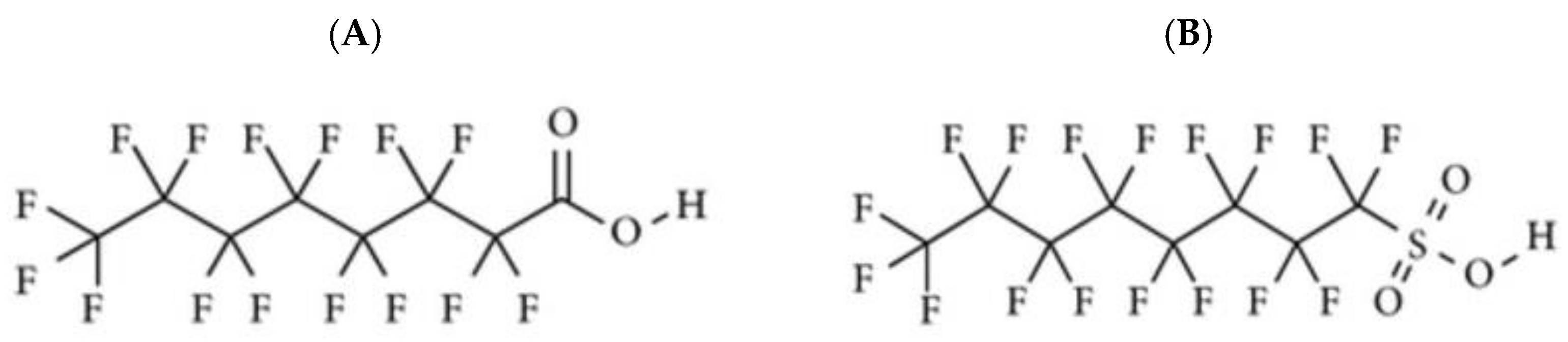




| Abbreviation | Number of Carbons in Fluorinated Chain | Chemical Names | Chemical Formula | |
|---|---|---|---|---|
| PFASs | PFCAs | |||
| PFOA | 7 | perfluorooctanoic acid | C8HF15O2 | |
| PFHxA | 5 | perfluorohexanoic acid | C6HF11O2 | |
| PFNA | 8 | perfluorononanoic acid | C9HF17O2 | |
| PFDA | 9 | perfluorodecanoic acid | C10H19O2 | |
| PFSAs | ||||
| PFOS | 8 | perfluorooctane sulfonic acid | C8F17SO3K | |
| PFHxS | 6 | perfluorohexane sulfonic acid | C6F13SO3K | |
| PFBS | 4 | perfluorobutane sulfonic acid | C4F9SO3K | |
| Category | Study Type | PFASs | Results | References |
|---|---|---|---|---|
| Endocrine disruption | Odense Child Cohort (adults, n = 210) | PFOS, PFOA, PFHxS, PFNA, PFDeA | association between serum PFOS level and increased thyroid stimulating hormone; positive association between repeated measures of serum PFNA and total T4 level in women | [41] |
| Cross-sectional (children, n = 85) | PFOA, PFNA, PFUnA, PFDA | disturbance of thyroid hormone homeostasis (differs between sexes) | [42] | |
| Obesity | Cross-sectional (adults, n = 1612) | PFOA, PFOS and other PFASs | positive association between PFASs exposure with overweight and increased waist circumference (with particular emphasis on the effect of PFOA on selected obesity parameters) | [43] |
| Odense Child Cohort (mother–child, n = 412) | PFOS, PFOA | each ln-unit increase in maternal serum PFOS and PFOA levels during pregnancy increased odds for overweight or/and obesity in children | [44] | |
| Odense Child Cohort (mother–daughter, n = 359) | PFOA, PFOS, PFNA, PFHxS | prenatal exposure to PFOA and PFOS was associated with girls % body fatness (except PFHxS and PFNA) | [45] | |
| Diabetes | Cross-sectional (adults, n = 7904) | PFOA | serum PFOA was positively associated with diabetes in men; PFOA disrupt cholesterol metabolism (at environmental relevant level) | [46] |
| Odense Child Cohort (adults, n = 4129) | PFOA | no association between PFOA exposure and incidence of diabetes | [47] | |
| Reproductive disorders | Case-control (adult women, n = 367) | PFOS, PFOA, PFBS, PFHxS, PFNA, PFDA and other | association between plasma PFDA level and significantly increased risk of PCOS-related infertility | [48] |
| In vitro (primary human placental cytotrophoblasts) | PFOS | apoptosis of human placental syncytiotrophoblasts) | [49] | |
| Odense Child Cohort (couples, n = 501) | PFOA, PFOS, PFNA, PFOSA, PFDeA and other | associations between two perfluoroalkyl substances and menstrual cycle length changes (2–5% shorter menstrual cycles during PFOA exposure and 3% longer during PFDeA exposure); association between selected perfluoroalkyl substances and lower pregnancy probability | [50] | |
| Breast cancer | Odense Child Cohort (adult women, n = 388) | PFOS, PFOA | positive association between high concentrations of PFOS and breast cancer risk (for analyses that were restricted to expression of estrogen receptors: ER+/PR+ tumors) | [51] |
| Hepatotoxicity | Odense Child Cohort (adults, n = 1605) | PFOA, PFOS, their isomers and other | clinically significant hepatic cell dysfunction (abnormal liver function biomarkers: prealbumin and ALT level) | [52] |
| Cross-sectional (adults, n = 1016) | PFOA, PFOS, PFOSA, PFNA, PFDA, PFHxS and other | positive association between the changes in activity of ALT, ALP, and GGT after PFASs exposure and changes in circulating bilirubin level | [53] | |
| Cross-sectional (adults, n = 30,723) | PFOA | association between PFOA and ALT, a marker of hepatocellular damage but no evidence that PFOA increases the risk of clinically diagnosed liver disease | [54] | |
| Nephrotoxicity | Odense Child Cohort (adults, n = 1612) | PFOA, PFOS | negative association between PFASs exposure (except for PFOA and PFDA) and estimated glomerular filtration rate (eGFR) and positive association with chronic kidney disease (CKD) | [55] |
| Asthma | Cross-sectional (children, n = 456) | PFOS, PFOA, PFBS, PFDA, PFNA, PFHxS and other | significant inverse association between serum PFASs and CC16 (club cell secretory protein; biomarker of asthma) levels in asthmatics | [56] |
| Cross-sectional (children, n = 300) | PFOA, PFOS | positive association between serum PFASs level and impaired lung function in children (association was significant only in asthmatic children) | [57] | |
| Immunotoxicity | Cross-sectional (adult, n = 733) | PFOA, PFOS, PFHxS, PFNA, PFDA and other | strong positive associations between blood PFOS level and leucocyte telomere length | [58] |
| Odense Child Cohort(mother–child, n = 349) | PFOS, PFHxS, PFOA, PFNA, PFDA | deficient antibody responses in children prenatally exposed to PFASs | [59] |
| PFOA | PFOS | PFBS | |
|---|---|---|---|
| Chemical Properties | |||
| Chemical Abstracts Services Number (CAS. No.) | 335-7-1 | 2795-39-3 | 375-73-5 |
| Physical state (at 20–25 °C) | white powder | white powder | liquid |
| Molecular weight (g/mol) | 414 | 538 | 338 |
| Solubility in water (at 25 °C; g/L) | 9.5 | 0.550–0.570 | Fully miscible |
| Physical Properties | |||
| Melting point (°C) | 45–54 | >400 | −21 |
| Boiling point (°C) | 188–192 | not measurable | not measurable |
| Organic-carbon partition coefficient (log Koc) | 2.06 | 2.57 | 2.7–3.6 |
| Biochemical half-life | water: >92 years (at 25 °C) atmospheric: 90 days | water: >41 years (at 25 °C) atmospheric: 114 days | water:> 1 year (at 25 °C) atmospheric: 76.4 days |
| Elimination (t1/2, Days) | PFOA | PFHxA | PFBA | PFOS | PFHxS | PFBS |
|---|---|---|---|---|---|---|
| Human | 2.1–3.9 y | 14–49 d * | 3–4 d | 3.3–27 y | 7.7–15.5 y | 26 d |
| Monkey | 21 d | 1 d | 2 d | 45 d | 100–141 d | 4 d |
| Rat | 5 d | 0.2–0.05 d | 0.3 d | 24–82 d | 0.9–34 d | 0.02–0.3 d |
| PFASs | Place of Study | Year of Samples Collection | Level | Reference |
|---|---|---|---|---|
| Serum | ||||
| PFOA | New York, USA (occupational exposure) | 2000–2002 | 8.1 ng/L | [135] |
| USA (children serum, 3–11 year) | 2013–2014 | 1.92 ng/mL | [136] | |
| New Hampshire, USA | 2015 | 3.09 μg/L | [137] | |
| Slovakia (cord study) | 2010–2012 | 0.79 ng/mL | [138] | |
| Australia (cohort study) | 2014–2015 | 2.03 ng/mL | [139] | |
| PFOS | New York, USA (occupational exposure) | 2000–2002 | 34.3 ng/L | [135] |
| Taipei, Taiwan | 2009–2010 | 28.9 ng/mL | [140] | |
| Spain (cohort study) | 2009–2010 | 7.61 ng/mL | [141] | |
| USA (children 3–11 year) | 2013–2014 | 3.88 ng/mL | [136] | |
| New Hampshire, USA | 2015 | 8.59 μg/L | [137] | |
| Slovakia (cord blood) | 2010–2012 | 0.36 ng/mL | [138] | |
| Australia (cohort study) | 2014–2015 | 5.24 ng/mL | [139] | |
| PFBS | Serum (Taipei, Taiwan) | 2009–2010 | 0.5 ng/mL | [140] |
| Taipei, Taiwan | 2009–2010 | 1.3 ng/mL | [140] | |
| USA (children serum, 3–11 year) | 2013–2014 | 0.843 ng/mL | [136] | |
| Spain (cohort study) | 2009–2010 | 0.836 ng/mL | [141] | |
| Australia (cohort study) | 2014–2015 | 2.05 ng/mL | [139] | |
| Slovakia (cord blood) | 2010–2012 | 0.07 ng/mL | [138] | |
| PFNA | Taipei, Taiwan | 2009–2010 | 0.8 ng/mL | [140] |
| USA (children 3–11 year) | 2013–2014 | 0.794 ng/mL | [136] | |
| Spain (cohort study) | 2009–2010 | 0.954 ng/mL | [141] | |
| Slovakia (cord blood) | 2010–2012 | 0.20 ng/mL | [138] | |
| Australia (cohort study) | 2014–2015 | 0.49 ng/mL | [139] | |
| Urine | ||||
| PFOA | China | 2015 | 4.61 ng/L | [142] |
| China | 2011 | 12.9 ng/L | [143] | |
| Decatur, USA | 2016 | 0.027 µg/L | [106] | |
| PFOS | China | 2015 | 18.71 ng/L | [142] |
| China | 2011 | 49.6 ng/L | [143] | |
| PFHxS | China | 2015 | <1.41 ng/L | [142] |
| PFNA | China | 2015 | 0.46 ng/L | [142] |
| PFASs | Maternal Serum Mean (ng/mL) (max/min) | Cord Serum Mean (ng/mL) (max/min) | References |
|---|---|---|---|
| PFOA | 1.22 (1.045/7.31) | 0.919 (0.311/7.06) | [199] |
| 1.560 (1.045/7.31) | 1.237 (0.237/2.878) | [193] | |
| 2.8 (1.2/6.7) | 3.10 * | [200,201] | |
| 4.80 * | 0.6/10.56 | [200,202] | |
| PFOS | 3.67 (3.064/24.5) | 1.28 (0/8.04) | [199] |
| 8.670 (1.728/22.857) | 3.668 (0.535/12.674) | [193] | |
| 12.70 * | 3.5 * | [200] | |
| 3.35 | 0.53/4.71 | [202] | |
| PFHxS | 2.28 (0.619/31) | 1.19 (0/16) | [199] |
| 0.528 (BQL/1.149) | 0.331 (BQL/1.070) | [193] | |
| 1.20 * | 0.60 * | [200] | |
| 2.24 | 0.05–1.93 | [202] | |
| PFNA | 0.519 (0.430/3.29) | 0.266 (0/2.25) | [199] |
| 0.41 (0.08/1.4) | 0.41 * | [199] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokra, K. Endocrine Disruptor Potential of Short- and Long-Chain Perfluoroalkyl Substances (PFASs)—A Synthesis of Current Knowledge with Proposal of Molecular Mechanism. Int. J. Mol. Sci. 2021, 22, 2148. https://doi.org/10.3390/ijms22042148
Mokra K. Endocrine Disruptor Potential of Short- and Long-Chain Perfluoroalkyl Substances (PFASs)—A Synthesis of Current Knowledge with Proposal of Molecular Mechanism. International Journal of Molecular Sciences. 2021; 22(4):2148. https://doi.org/10.3390/ijms22042148
Chicago/Turabian StyleMokra, Katarzyna. 2021. "Endocrine Disruptor Potential of Short- and Long-Chain Perfluoroalkyl Substances (PFASs)—A Synthesis of Current Knowledge with Proposal of Molecular Mechanism" International Journal of Molecular Sciences 22, no. 4: 2148. https://doi.org/10.3390/ijms22042148