sFasL—The Key to a Riddle: Immune Responses in Aging Lung and Disease
Abstract
:1. Introduction
2. Serum Levels of Soluble FasL Increase with Age
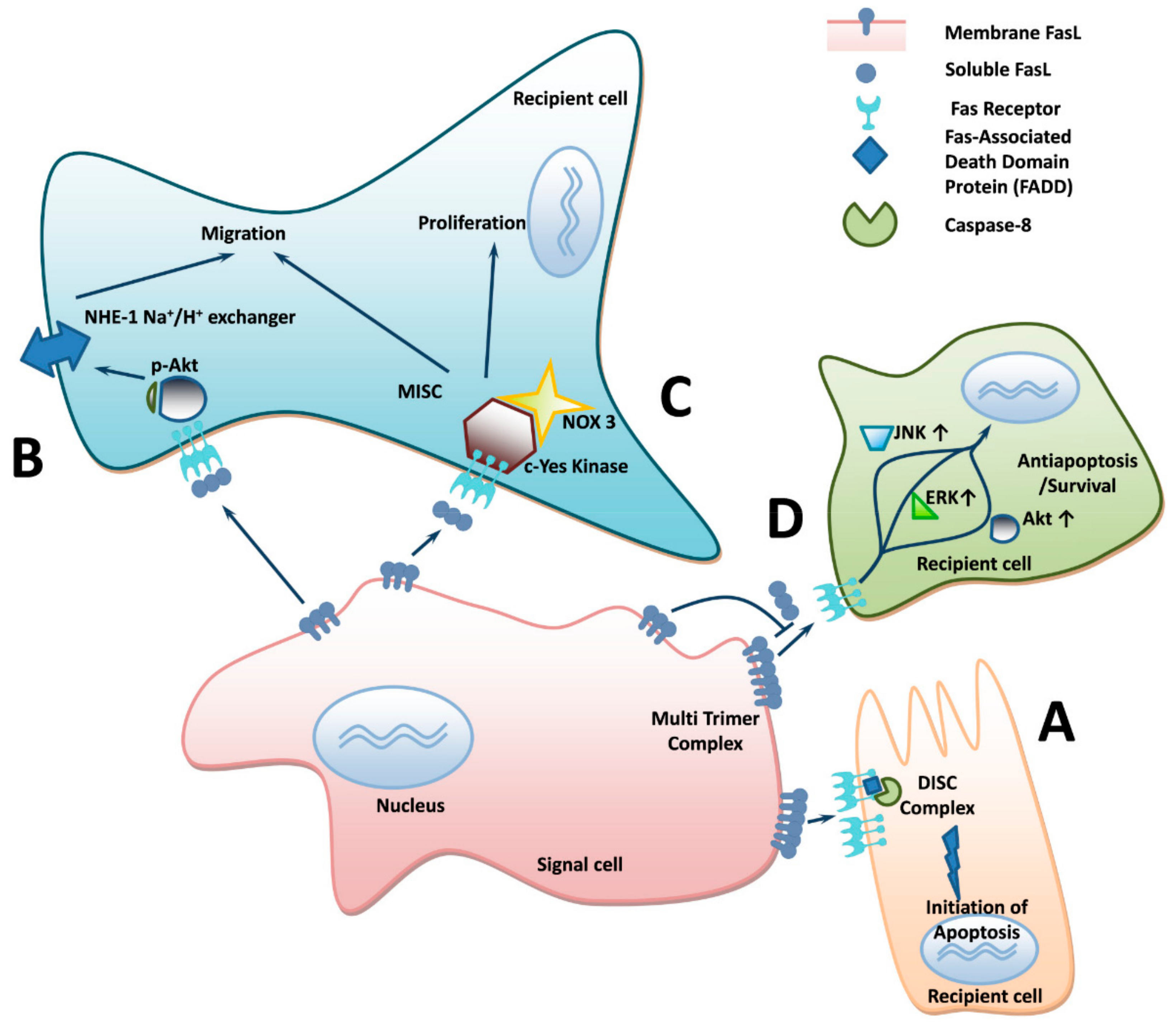
3. Molecular Signaling and Mechanism of Soluble FasL Increment with Age
4. Soluble FasL Increase in Serum of Patients with Pulmonary Disease
5. Immune Disorders and Viral Infection Increase the Levels of Soluble FasL in Blood
6. Discussion
Funding
Conflicts of Interest
References
- Lettau, M.; Paulsen, M.; Schmidt, H.; Janssen, O. Insights into the molecular regulation of FasL (CD178) biology. Eur. J. Cell Biol. 2011, 90, 456–466. [Google Scholar] [CrossRef]
- Guégan, J.-P.; Legembre, P. Nonapoptotic functions of Fas/CD95 in the immune response. FEBS J. 2018, 285, 809–827. [Google Scholar] [CrossRef] [PubMed]
- Schulte, M.; Reiss, K.; Lettau, M.; Maretzky, T.; Ludwig, A.; Hartmann, D.; de Strooper, B.; Janssen, O.; Saftig, P. ADAM10 regulates FasL cell surface expression and modulates FasL-induced cytotoxicity and activation-induced cell death. Cell Death Differ. 2007, 14, 1040–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuwano, K.; Maeyama, T.; Inoshima, I.; Ninomiya, K.; Hagimoto, N.; Yoshimi, M.; Fujita, M.; Nakamura, N.; Shirakawa, K.; Hara, N. Increased circulating levels of soluble Fas ligand are correlated with disease activity in patients with fibrosing lung diseases. Respirology 2002, 7, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.S.; Hackett, C.G.; Abernathy, E.F.; Lee, K.S.; Saff, R.R.; Hohlbaum, A.M.; Moody, K.-S.L.; Hobson, M.W.; Jones, A.; Kolovou, P.; et al. Opposing Roles for Membrane Bound and Soluble Fas Ligand in Glaucoma-Associated Retinal Ganglion Cell Death. PLoS ONE 2011, 6, e17659. [Google Scholar] [CrossRef] [PubMed]
- Wallach-Dayan, S.B.; Elkayam, L.; Golan-Gerstl, R.; Konikov, J.; Zisman, P.; Dayan, M.R.; Arish, N.; Breuer, R. Cutting edge: FasL(+) immune cells promote resolution of fibrosis. J. Autoimmun. 2015, 59, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Wallach-Dayan, S.B.; Golan-Gerstl, R.; Breuer, R. Evasion of myofibroblasts from immune surveillance: A mechanism for tissue fibrosis. Proc. Natl. Acad. Sci. USA 2007, 104, 20460–20465. [Google Scholar] [CrossRef] [Green Version]
- Golan-Gerstl, R.; Wallach-Dayan, S.B.; Amir, G.; Breuer, R. Epithelial Cell Apoptosis by Fas Ligand–Positive Myofibroblasts in Lung Fibrosis. Am. J. Respir. Cell Mol. Biol. 2007, 36, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Yamada, A.; Arakaki, R.; Saito, M.; Kudo, Y.; Ishimaru, N. Dual Role of Fas/FasL-Mediated Signal in Peripheral Immune Tolerance. Front. Immunol. 2017, 8, 403. [Google Scholar] [CrossRef] [Green Version]
- Kleber, S.; Sancho-Martinez, I.; Wiestler, B.; Beisel, A.; Gieffers, C.; Hill, O.; Thiemann, M.; Mueller, W.; Sykora, J.; Kuhn, A.; et al. Yes and PI3K Bind CD95 to Signal Invasion of Glioblastoma. Cancer Cell 2008, 13, 235–248. [Google Scholar] [CrossRef] [Green Version]
- Guégan, J.P.; Ginestier, C.; Charafe-Jauffret, E.; Ducret, T.; Quignard, J.-F.; Vacher, P.; Legembre, P. CD95/Fas and metastatic disease: What does not kill you makes you stronger. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef]
- Stock, C.; Schwab, A. Role of the Na+/H+ exchanger NHE1 in cell migration. Acta Physiol. 2006, 187, 149–157. [Google Scholar] [CrossRef]
- Okuda, R.; Aoshiba, K.; Matsushima, H.; Ogura, T.; Okudela, K.; Ohashi, K. Cellular senescence and senescence-associated secretory phenotype: Comparison of idiopathic pulmonary fibrosis, connective tissue disease-associated interstitial lung disease, and chronic obstructive pulmonary disease. J. Thorac. Dis. 2019, 11, 857–864. [Google Scholar] [CrossRef]
- Wallach-Dayan, S.B.; Rojas, M. Senescence, the Janus of Lung Injury and Repair. Am. J. Respir. Cell Mol. Biol. 2020, 62, 548–549. [Google Scholar] [CrossRef] [PubMed]
- Baz-Martínez, M.; Da Silva-Álvarez, S.; Rodríguez, E.; Guerra, J.; El Motiam, A.; Vidal, A.; García-Caballero, T.; González-Barcia, M.; Sánchez, L.; Muñoz-Fontela, C.; et al. Cell senescence is an antiviral defense mechanism. Sci. Rep. 2016, 6, 37007. [Google Scholar] [CrossRef]
- Pinti, M. Development of real time PCR assays for the quantification of Fas and FasL mRNA levels in lymphocytes: Studies on centenarians. Mech. Ageing Dev. 2003, 124, 511–516. [Google Scholar] [CrossRef]
- Goto, M. Elevation of soluble Fas (APO-1, CD95) ligand in natural aging and Werner syndrome. Biosci. Trends 2008, 2, 124–127. [Google Scholar]
- Jiang, S.; Moriarty-Craige, S.E.; Li, C.; Lynn, M.J.; Cai, J.; Jones, D.P.; Sternberg, P. Associations of plasma-soluble fas ligand with aging and age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Gupta, S. Increased Apoptosis of T Cell Subsets in Aging Humans: Altered Expression of Fas (CD95), Fas Ligand, Bcl-2, and Bax. J. Immunol. 1998, 160, 1627–1637. [Google Scholar] [PubMed]
- Ichikura, T.; Majima, T.; Uchida, T.; Okura, E.; Ogawa, T.; Mochizuki, H. Plasma soluble Fas ligand concentration: Decrease in elderly men and increase in patients with gastric carcinoma. Oncol. Rep. 2001, 8, 311–314. [Google Scholar] [CrossRef]
- Kavathia, N.; Jain, A.; Walston, J.; Beamer, B.A.; Fedarko, N.S. Serum markers of apoptosis decrease with age and cancer stage. Aging 2009, 1, 652–663. [Google Scholar] [CrossRef] [Green Version]
- Kangas, R.; Törmäkangas, T.; Heinonen, A.; Alen, M.; Suominen, H.; Kovanen, V.; Laakkonen, E.K.; Korhonen, M.T. Declining Physical Performance Associates with Serum FasL, miR-21, and miR-146a in Aging Sprinters. Biomed Res. Int. 2017, 2017, 8468469. [Google Scholar] [CrossRef] [PubMed]
- Pahlavani, M.A.; Vargas, D.A. Aging but not dietary restriction alters the activation-induced apoptosis in rat T cells. FEBS Lett. 2001, 491, 114–118. [Google Scholar] [CrossRef] [Green Version]
- Duan, P.; Hu, C.; Butler, H.J.; Quan, C.; Chen, W.; Huang, W.; Tang, S.; Zhou, W.; Yuan, M.; Shi, Y.; et al. 4-Nonylphenol induces disruption of spermatogenesis associated with oxidative stress-related apoptosis by targeting p53-Bcl-2/Bax-Fas/FasL signaling. Environ. Toxicol. 2017, 32, 739–753. [Google Scholar] [CrossRef] [Green Version]
- Soni, H.; Kaminski, D.; Gangaraju, R.; Adebiyi, A. Cisplatin-induced oxidative stress stimulates renal Fas ligand shedding. Ren. Fail. 2018, 40, 314–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas-Rodríguez, S.; Folgueras, A.R.; López-Otín, C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2017, 1864, 2015–2025. [Google Scholar] [CrossRef]
- Zhou, T.; Prather, E.R.; Garrison, D.E.; Zuo, L. Interplay between ROS and Antioxidants during Ischemia-Reperfusion Injuries in Cardiac and Skeletal Muscle. Int. J. Mol. Sci. 2018, 19, 417. [Google Scholar] [CrossRef] [Green Version]
- Wallach-Dayan, S.B.; Izbicki, G.; Cohen, P.Y.; Gerstl-Golan, R.; Fine, A.; Breuer, R. Bleomycin initiates apoptosis of lung epithelial cells by ROS but not by Fas/FasL pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 290, L790–L796. [Google Scholar] [CrossRef]
- Sonveaux, P. ROS and radiotherapy: More we care. Oncotarget 2017, 8, 35482. [Google Scholar] [CrossRef]
- Helmerhorst, H.J.F.; Schultz, M.J.; Van Der Voort, P.H.J.; De Jonge, E.; Van Westerloo, D.J. Bench-to-bedside review: The effects of hyperoxia during critical illness. Crit. Care 2015, 19, 284. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Aoshiba, K.; Nagai, A. Oxidative stress increases Fas ligand expression in endothelial cells. J. Inflamm. (Lond. Engl.) 2006, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Lorenzo, M.J.; Anel, A.; Gamen, S.; Monle n, I.; Lasierra, P.; Larrad, L.; Piñeiro, A.; Alava, M.A.; Naval, J. Activated human T cells release bioactive Fas ligand and APO2 ligand in microvesicles. J. Immunol. 1999, 163, 1274–1281. [Google Scholar] [PubMed]
- Picca, A.; Guerra, F.; Calvani, R.; Bucci, C.; Lo Monaco, M.; Bentivoglio, A.; Coelho-Júnior, H.; Landi, F.; Bernabei, R.; Marzetti, E. Mitochondrial Dysfunction and Aging: Insights from the Analysis of Extracellular Vesicles. Int. J. Mol. Sci. 2019, 20, 805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ståhl, A.-L.; Johansson, K.; Mossberg, M.; Kahn, R.; Karpman, D. Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatric Nephrol. (Berl. Ger.) 2019, 34, 11–30. [Google Scholar] [CrossRef] [Green Version]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Exosomal vesicles enhance immunosuppression in chronic inflammation: Impact in cellular senescence and the aging process. Cell. Signal. 2020, 75, 109771. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, E.; Blott, E.J.; Holt, O.; Sigismund, S.; Shaw, M.; Bossi, G.; Griffiths, G.M. Sorting of Fas ligand to secretory lysosomes is regulated by mono-ubiquitylation and phosphorylation. J. Cell Sci 2007, 120, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Wieckowski, E.; Taylor, D.D.; Reichert, T.E.; Watkins, S.; Whiteside, T.L. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 1010–1020. [Google Scholar]
- Klinker, M.W.; Lizzio, V.; Reed, T.J.; Fox, D.A.; Lundy, S.K. Human B Cell-Derived Lymphoblastoid Cell Lines Constitutively Produce Fas Ligand and Secrete MHCII(+)FasL(+) Killer Exosomes. Front. Immunol. 2014, 5, 144. [Google Scholar] [CrossRef] [Green Version]
- Saheera, S.; Potnuri, A.G.; Krishnamurthy, P. Nano-Vesicle (Mis)Communication in Senescence-Related Pathologies. Cells 2020, 9, 1974. [Google Scholar] [CrossRef]
- Volpe, E.; Sambucci, M.; Battistini, L.; Borsellino, G. Fas–Fas Ligand: Checkpoint of T Cell Functions in Multiple Sclerosis. Front. Immunol. 2016, 7, 382. [Google Scholar] [CrossRef] [Green Version]
- Morgan, M.J.; Liu, Z.-G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagata, S.; Golstein, P. The Fas death factor. Science 1995, 267, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Hug, H.; Strand, S.; Grambihler, A.; Galle, J.; Hack, V.; Stremmel, W.; Krammer, P.H.; Galle, P.R. Reactive Oxygen Intermediates Are Involved in the Induction of CD95 Ligand mRNA Expression by Cytostatic Drugs in Hepatoma Cells. J. Biol. Chem. 1997, 272, 28191–28193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Yang, C.; Sun, C.; Sun, Y.; Yang, Z.; Cheng, S.; Zhuge, B. miR-21-5p Suppressed the Sensitivity of Hepatocellular Carcinoma Cells to Cisplatin by Targeting FASLG. DNA Cell Biol. 2019, 38, 865–873. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Zhao, Z.; Jin, Z. Expression of miR-98 in myocarditis and its influence on transcription of the FAS/FASL gene pair. Genet. Mol. Res. GMR 2016, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kangas, R.; Pöllänen, E.; Rippo, M.R.; Lanzarini, C.; Prattichizzo, F.; Niskala, P.; Jylhävä, J.; Sipilä, S.; Kaprio, J.; Procopio, A.D.; et al. Circulating miR-21, miR-146a and Fas ligand respond to postmenopausal estrogen-based hormone replacement therapy—A study with monozygotic twin pairs. Mech. Ageing Dev. 2014, 143–144, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ayroldi, E.; D’Adamio, F.; Zollo, O.; Agostini, M.; Moraca, R.; Cannarile, L.; Migliorati, G.; Delfino, D.V.; Riccardi, C. Cloning and expression of a short Fas ligand: A new alternatively spliced product of the mouse Fas ligand gene. Blood 1999, 94, 3456–3467. [Google Scholar] [CrossRef]
- Ho, B.-Y.; Wu, Y.-M.; Chang, K.-J.; Pan, T.-M. Dimerumic Acid Inhibits SW620 Cell Invasion by Attenuating H2O2-Mediated MMP-7 Expression via JNK/C-Jun and ERK/C-Fos Activation in an AP-1-Dependent Manner. Int. J. Biol. Sci. 2011, 7, 869–880. [Google Scholar] [CrossRef] [Green Version]
- Kelly, P.J.; Morrow, J.D.; Ning, M.; Koroshetz, W.; Lo, E.H.; Terry, E.; Milne, G.L.; Hubbard, J.; Lee, H.; Stevenson, E.; et al. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: The Biomarker Evaluation for Antioxidant Therapies in Stroke (BEAT-Stroke) study. Stroke 2008, 39, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Haorah, J.; Ramirez, S.H.; Schall, K.; Smith, D.; Pandya, R.; Persidsky, Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J. Neurochem. 2007, 101, 566–576. [Google Scholar] [CrossRef]
- Demeule, M.; Brossard, M.; Pagé, M.; Gingras, D.; Béliveau, R. Matrix metalloproteinase inhibition by green tea catechins. Biochim. Biophys. Acta 2000, 1478, 51–60. [Google Scholar] [CrossRef]
- Weinlich, R.; Brunner, T.; Amarante-Mendes, G.P. Control of death receptor ligand activity by posttranslational modifications. Cell. Mol. Life Sci. CMLS 2010, 67, 1631–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voss, M.; Lettau, M.; Paulsen, M.; Janssen, O. Posttranslational regulation of Fas ligand function. Cell Commun. Signal. CCS 2008, 6, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrero, R.; Kajikawa, O.; Matute-Bello, G.; Wang, Y.; Hagimoto, N.; Mongovin, S.; Wong, V.; Park, D.R.; Brot, N.; Heinecke, J.W.; et al. The biological activity of FasL in human and mouse lungs is determined by the structure of its stalk region. J. Clin. Investig. 2011, 121, 1174–1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, A.J.; Tom, C.; Guemes, M.; Polanco, G.; Mayorga, M.E.; Wend, K.; Miranda-Carboni, G.A.; Krum, S.A. ERα signaling regulates MMP3 expression to induce FasL cleavage and osteoclast apoptosis. J. Bone Miner. Res. 2013, 28, 283–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krum, S.A.; Miranda-Carboni, G.A.; Hauschka, P.V.; Carroll, J.S.; Lane, T.F.; Freedman, L.P.; Brown, M. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J. 2008, 27, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Imai, Y.; Matsumoto, T.; Sato, S.; Takeuchi, K.; Igarashi, K.; Harada, Y.; Azuma, Y.; Krust, A.; Yamamoto, Y.; et al. Estrogen Prevents Bone Loss via Estrogen Receptor α and Induction of Fas Ligand in Osteoclasts. Cell 2007, 130, 811–823. [Google Scholar] [CrossRef]
- Wadsworth, S.J.; Atsuta, R.; McIntyre, J.O.; Hackett, T.-L.; Singhera, G.K.; Dorscheid, D.R. IL-13 and TH2 cytokine exposure triggers matrix metalloproteinase 7–mediated Fas ligand cleavage from bronchial epithelial cells. J. Allergy Clin. Immunol. 2010, 126, 366–374.e368. [Google Scholar] [CrossRef]
- Nareznoi, D.; Konikov-Rozenman, J.; Petukhov, D.; Breuer, R.; Wallach-Dayan, S.B. Matrix Metalloproteinases Retain Soluble FasL-mediated Resistance to Cell Death in Fibrotic-Lung Myofibroblasts. Cells 2020, 9, 411. [Google Scholar] [CrossRef] [Green Version]
- Fingleton, B.; Vargo-Gogola, T.; Crawford, H.C.; Matrisian, L.M. Matrilysin [MMP-7] Expression Selects for Cells with Reduced Sensitivity to Apoptosis. Neoplasia 2001, 3, 459–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsiades, N.; Yu, W.H.; Poulaki, V.; Tsokos, M.; Stamenkovic, I. Matrix metalloproteinase-7-mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res. 2001, 61, 577–581. [Google Scholar]
- Margaryan, S.; Witkowicz, A.; Arakelyan, A.; Partyka, A.; Karabon, L.; Manukyan, G. sFasL-mediated induction of neutrophil activation in patients with type 2 diabetes mellitus. PLoS ONE 2018, 13, e0201087. [Google Scholar] [CrossRef]
- Fois, A.G.; Paliogiannis, P.; Sotgia, S.; Mangoni, A.A.; Zinellu, E.; Pirina, P.; Carru, C.; Zinellu, A. Evaluation of oxidative stress biomarkers in idiopathic pulmonary fibrosis and therapeutic applications: A systematic review. Respir. Res. 2018, 19, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kliment, C.R.; Oury, T.D. Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Radic. Biol. Med. 2010, 49, 707–717. [Google Scholar] [CrossRef]
- Han, M.K.; Murray, S.; Fell, C.D.; Flaherty, K.R.; Toews, G.B.; Myers, J.; Colby, T.V.; Travis, W.D.; Kazerooni, E.A.; Gross, B.H.; et al. Sex differences in physiological progression of idiopathic pulmonary fibrosis. Eur. Respir. J. 2008, 31, 1183–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuwano, K.; Hagimoto, N.; Kawasaki, M.; Nakamura, N.; Shirakawa, K.; Maeyama, T.; Hara, N. Expression of FasL and Fas protein and their soluble form in patients with hypersensitivity pneumonitis. Int. Arch. Allergy Immunol. 2000, 122, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Kuwano, K.; Kawasaki, M.; Maeyama, T.; Hagimoto, N.; Nakamura, N.; Shirakawa, K.; Hara, N. Soluble form of fas and fas ligand in BAL fluid from patients with pulmonary fibrosis and bronchiolitis obliterans organizing pneumonia. Chest 2000, 118, 451–458. [Google Scholar] [CrossRef] [Green Version]
- Kopiński, P.; Balicka-Ślusarczyk, B.; Dyczek, A.; Szpechciński, A.; Przybylski, G.; Jarzemska, A.; Wandtke, T.; Jankowski, M.; Iwaniec, T.; Chorostowska-Wynimko, J. Enhanced expression of Fas Ligand (FasL) in the lower airways of patients with fibrotic interstitial lung diseases (ILDs). Folia Histochem. Cytobiol. 2011, 49, 636–645. [Google Scholar] [CrossRef] [Green Version]
- Erdoğan, B.; Uzaslan, E.; Budak, F.; Karadağ, M.; Ediger, D.; Oral, B.; Göral, G.; Ege, E.; Gözü, O. The evaluation of soluble Fas and soluble Fas ligand levels of bronchoalveolar lavage fluid in lung cancer patients. Tuberk. Toraks 2005, 53, 127–131. [Google Scholar]
- Shimizu, M.; Kondo, M.; Ito, Y.; Kume, H.; Suzuki, R.; Yamaki, K. Soluble Fas and Fas ligand provide new information on metastasis and response to chemotherapy in SCLC patients. Cancer Detect. Prev. 2005, 29, 175–180. [Google Scholar] [CrossRef]
- Yoshimura, C.; Nomura, S.; Kanazawa, S.; Kuwana, M.; Muramatsu, M.; Yamaguchi, K.; Fukuhara, S. Analysis of cytotoxic T lymphocytes and Fas/FasL in Japanese patients with non-small cell lung cancer associated with HLA-A2. J. Cancer Res. Clin. Oncol. 2002, 128, 581–588. [Google Scholar] [CrossRef]
- Shikuwa, C.; Kadota, J.-I.; Mukae, H.; Iwashita, T.; Kaida, H.; Ishii, H.; Ishimatsu, Y.; Kohno, S. High concentrations of soluble Fas ligand in bronchoalveolar lavage fluid of patients with pulmonary sarcoidosis. Respir. Int. Rev. Thorac. Dis. 2002, 69, 242–246. [Google Scholar] [CrossRef]
- Albertine, K.H.; Soulier, M.F.; Wang, Z.; Ishizaka, A.; Hashimoto, S.; Zimmerman, G.A.; Matthay, M.A.; Ware, L.B. Fas and Fas Ligand Are Up-Regulated in Pulmonary Edema Fluid and Lung Tissue of Patients with Acute Lung Injury and the Acute Respiratory Distress Syndrome. Am. J. Pathol. 2002, 161, 1783–1796. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.S.; Chen, Y.M.; Hsieh, Y.L.; Yu, C.F.; Tsai, C.M.; Perng, R.P. Pleural effusion and serum soluble fas-ligand levels are elevated in different clinical conditions. Lung 2002, 180, 25–32. [Google Scholar] [CrossRef]
- Budak, F.; Uzaslan, E.K.; Cangür, S.; Göral, G.; Oral, H.B. Increased pleural soluble fas ligand (sFasL) levels in tuberculosis pleurisy and its relation with T-helper type 1 cytokines. Lung 2008, 186, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Chu, J.J.; Chiang, C.D. Increased soluble Fas ligand concentration in tuberculous pleural effusion. J. Formos. Med Assoc. Taiwan Yi Zhi 2001, 100, 32–34. [Google Scholar] [PubMed]
- Naumnik, W.; Izycki, T.; Ossolinska, M.; Chyczewska, E. Serum levels of sFas and sFasL during chemotherapy of lung cancer. Exp. Oncol. 2007, 29, 132–136. [Google Scholar]
- Kosacka, M.; Porębska, I.; Korzeniewska, A.; Rubinsztajn, R.; Grabicki, M.; Jankowska, R.; Batura-Gabryel, H.; Chazan, R. Serum levels of apoptosis-related markers (sFasL, TNF-a, p53 and bcl-2) in COPD patients. Pneumonol. I Alergol. Pol. 2016, 84, 11–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takabatake, N.; Nakamura, H.; Inoue, S.; Terashita, K.; Yuki, H.; Kato, S.; Yasumura, S.; Tomoike, H. Circulating levels of soluble Fas ligand and soluble Fas in patients with chronic obstructive pulmonary disease. Respir. Med. 2000, 94, 1215–1220. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, N.; Gotoh, K.; Minatoguchi, S.; Asano, K.; Nishigaki, K.; Nomura, M.; Ohno, A.; Watanabe, M.; Sano, H.; Kumada, H.; et al. An increase of soluble Fas, an inhibitor of apoptosis, associated with progression of COPD. Respir. Med. 1998, 92, 993–999. [Google Scholar] [CrossRef] [Green Version]
- Kao, S.-L.; Yu, H.-R.; Kuo, H.-C.; Tsui, K.-Y.; Wu, C.-C.; Chang, L.-S.; Liang, C.-D.; Chung, Y.-H.; Yang, K.D. Higher levels of soluble Fas ligand and transforming growth factor-β after omalizumab treatment: A case report. J. Microbiol. Immunol. Infect. Wei Mian Yu Gan Ran Za Zhi 2012, 45, 69–71. [Google Scholar] [CrossRef] [Green Version]
- Mezei, G.; Lévay, M.; Sepler, Z.; Héninger, E.; Kozma, G.T.; Cserháti, E. Seasonal changes of proapoptotic soluble Fas ligand level in allergic rhinitis combined with asthma. Pediatric Allergy Immunol. 2006, 17, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Daneshmandi, S.; Pourfath Elah, A.A.; Pourpak, Z.; Heydarnezhad, H. Soluble form of FasL (sFasL) in adult asthma. Iran. Red Crescent Med. J. (IRCMJ) 2009, 11, 301–305. [Google Scholar]
- Kuwano, K.; Hagimoto, N.; Kawasaki, M.; Yatomi, T.; Nakamura, N.; Nagata, S.; Suda, T.; Kunitake, R.; Maeyama, T.; Miyazaki, H.; et al. Essential roles of the Fas-Fas ligand pathway in the development of pulmonary fibrosis. J. Clin. Investig. 1999, 104, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Matute-Bello, G.; Liles, W.C.; Steinberg, K.P.; Kiener, P.A.; Mongovin, S.; Chi, E.Y.; Jonas, M.; Martin, T.R. Soluble Fas Ligand Induces Epithelial Cell Apoptosis in Humans with Acute Lung Injury (ARDS). J. Immunol. 1999, 163, 2217–2225. [Google Scholar]
- Sakamoto, N.; Mukae, H.; Fujii, T.; Kakugawa, T.; Kaida, H.; Kadota, J.-I.; Kohno, S. Soluble form of Fas and Fas ligand in serum and bronchoalveolar lavage fluid of individuals infected with human T-lymphotropic virus type 1. Respir. Med. 2004, 98, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Janssen, O.; Qian, J.; Linkermann, A.; Kabelitz, D. CD95 ligand—Death factor and costimulatory molecule? Cell Death Differ. 2003, 10, 1215–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohana-Kashtan, O.; Civin, C.I. Fas ligand as a tool for immunosuppression and generation of immune tolerance. Stem Cells 2004, 22, 908–924. [Google Scholar] [CrossRef] [PubMed]
- Muschen, M.; Moers, C.; Warskulat, U.; Even, J.; Niederacher, D.; Beckmann, M.W. CD95 ligand expression as a mechanism of immune escape in breast cancer. Immunology 2000, 99, 69–77. [Google Scholar] [CrossRef]
- Calmon-Hamaty, F.; Audo, R.; Combe, B.; Morel, J.; Hahne, M. Targeting the Fas/FasL system in Rheumatoid Arthritis therapy: Promising or risky? Cytokine 2015, 75, 228–233. [Google Scholar] [CrossRef]
- Audo, R.; Calmon-Hamaty, F.; Papon, L.; Combe, B.; Morel, J.; Hahne, M. Distinct Effects of Soluble and Membrane-Bound Fas Ligand on Fibroblast-like Synoviocytes From Rheumatoid Arthritis Patients. Arthritis Rheumatol. 2014, 66, 3289–3299. [Google Scholar] [CrossRef] [PubMed]
- Serrao, K.L.; Fortenberry, J.D.; Owens, M.L.; Harris, F.L.; Brown, L.A. Neutrophils induce apoptosis of lung epithelial cells via release of soluble Fas ligand. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, L298–L305. [Google Scholar] [CrossRef] [Green Version]
- Sharma, K.; Wang, R.X.; Zhang, L.Y.; Yin, D.L.; Luo, X.Y.; Solomon, J.C.; Jiang, R.F.; Markos, K.; Davidson, W.; Scott, D.W.; et al. Death the Fas way: Regulation and pathophysiology of CD95 and its ligand. Pharmacol. Ther. 2000, 88, 333–347. [Google Scholar] [CrossRef]
- Salton, F.; Volpe, M.C.; Confalonieri, M. Epithelial–Mesenchymal Transition in the Pathogenesis of Idiopathic Pulmonary Fibrosis. Medicina 2019, 55, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotti, R.; Shu, E.; Petrachi, T.; Marconi, A.; Palazzo, E.; Quadri, M.; Lin, A.; O’Reilly, L.A.; Pincelli, C. Soluble Fas Ligand Is Essential for Blister Formation in Pemphigus. Front. Immunol. 2018, 9, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieux-Laucat, F.; Magerus-Chatinet, A.; Neven, B. The Autoimmune Lymphoproliferative Syndrome with Defective FAS or FAS-Ligand Functions. J. Clin. Immunol. 2018, 38, 558–568. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, L.A.; Hughes, P.; Lin, A.; Waring, P.; Siebenlist, U.; Jain, R.; Gray, D.H.D.; Gerondakis, S.; Strasser, A. Loss of c-REL but not NF-κB2 prevents autoimmune disease driven by FasL mutation. Cell Death Differ. 2015, 22, 767–778. [Google Scholar] [CrossRef] [Green Version]
- Lau, C.-Y.; Mihalek, A.D.; Wang, J.; Dodd, L.E.; Perkins, K.; Price, S.; Webster, S.; Pittaluga, S.; Folio, L.R.; Rao, V.K.; et al. Pulmonary Manifestations of the Autoimmune Lymphoproliferative Syndrome. A Retrospective Study of a Unique Patient Cohort. Ann. Am. Thorac. Soc. 2016, 13, 1279–1288. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Y.; Yu, B.; Qian, H.; He, Y.; Shi, G. A Potential of sFasL in Preventing Gland Injury in Sjogren’s Syndrome. Biomed. Res. Int. 2017, 5981432. [Google Scholar] [CrossRef]
- Bohana-Kashtan, O.; Morisot, S.; Hildreth, R.; Brayton, C.; Levitsky, H.I.; Civin, C.I. Selective Reduction of Graft-versus-Host Disease-Mediating Human T Cells by Ex Vivo Treatment with Soluble Fas Ligand. J. Immunol. (Baltim. Md. 1950) 2009, 183, 696–705,. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linkermann, A.; Qian, J.; Lettau, M.; Kabelitz, D.; Janssen, O. Considering Fas ligand as a target for therapy. Expert Opin. Ther. Targets 2005, 9, 119–134. [Google Scholar] [CrossRef]
- Marfè, G.; Tafani, M.; Fiorito, F.; Pagnini, U.; Iovane, G.; De Martino, L. Involvement of FOXO Transcription Factors, TRAIL-FasL/Fas, and Sirtuin Proteins Family in Canine Coronavirus Type II-Induced Apoptosis. PLoS ONE 2011, 6, e27313. [Google Scholar] [CrossRef] [Green Version]
- Law, H.K.; Cheung, C.; Sia, S.; Chan, Y.; Peiris, J.M.; Lau, Y. Toll-like receptors, chemokine receptors and death receptor ligands responses in SARS coronavirus infected human monocyte derived dendritic cells. BMC Immunol. 2009, 10, 35. [Google Scholar] [CrossRef] [Green Version]
- Mesel-Lemoine, M.; Millet, J.; Vidalain, P.O.; Law, H.; Vabret, A.; Lorin, V.; Escriou, N.; Albert, M.L.; Nal, B.; Tangy, F. A Human Coronavirus Responsible for the Common Cold Massively Kills Dendritic Cells but Not Monocytes. J. Virol. 2012, 86, 7577–7587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Gallo, M.; Poissonnier, A.; Blanco, P.; Legembre, P. CD95/Fas, Non-Apoptotic Signaling Pathways, and Kinases. Front. Immunol. 2017, 8, 1216. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, J. Immune privilege or inflammation? The paradoxical effects of Fas ligand. Arch. Immunol Exp. (Warsz.) 2000, 48, 73–79. [Google Scholar] [PubMed]
- Kuwano, K.; Hagimoto, N.; Maeyama, T.; Fujita, M.; Yoshimi, M.; Inoshima, I.; Nakashima, N.; Hamada, N.; Watanabe, K.; Hara, N. Mitochondria-mediated apoptosis of lung epithelial cells in idiopathic interstitial pneumonias. Lab. Investig. 2002, 82, 1695–1706. [Google Scholar] [CrossRef] [Green Version]
- Strasser, A.; Jost, P.J.; Nagata, S. The Many Roles of FAS Receptor Signaling in the Immune System. Immunity 2009, 30, 180–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, M.-H.; Chang, W.-M.; Lee, W.-J.; Chang, Y.-C.; Lai, T.-C.; Chan, D.V.; Sharma, R.; Lin, Y.-F.; Hsiao, M. A Fas Ligand (FasL)-Fused Humanized Antibody Against Tumor-Associated Glycoprotein 72 Selectively Exhibits the Cytotoxic Effect Against Oral Cancer Cells with a Low FasL/Fas Ratio. Mol. Cancer Ther. 2017, 16, 1102–1113. [Google Scholar] [CrossRef] [Green Version]
- Muraki, M. Sensitization to cell death induced by soluble Fas ligand and agonistic antibodies with exogenous agents: A review. AIMS Med Sci. 2020, 7, 122–203. [Google Scholar] [CrossRef]
- Krishnan, A.; Fei, F.; Jones, A.; Ksander, B.R.; Rothstein, A.M.; Gregory-Ksander, M.S. Gene therapy treatment with AAV-soluble Fas ligand protects retinal ganglion cells during development of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2594. [Google Scholar]
- Krishnan, A.; Fei, F.; Jones, A.; Busto, P.; Marshak-Rothstein, A.; Ksander, B.R.; Gregory-Ksander, M. Overexpression of Soluble Fas Ligand following Adeno-Associated Virus Gene Therapy Prevents Retinal Ganglion Cell Death in Chronic and Acute Murine Models of Glaucoma. J. Immunol. 2016, 197, 4626–4638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putzbach, W.; Haluck-Kangas, A.; Gao, Q.Q.; Sarshad, A.A.; Bartom, E.T.; Stults, A.; Qadir, A.S.; Hafner, M.; Peter, M.E. CD95/Fas ligand mRNA is toxic to cells. eLife 2018, 7, e38621. [Google Scholar] [CrossRef] [PubMed]
- Su, K.-W.; Yeh, K.-W.; Huang, J.L. Cord Blood Soluble Fas Ligand Predicts Allergic Rhinitis, Obstructive-type Lung Function, and Mite Sensitization at Seven Year-old Children: The PATCH study. J. Allergy Clin. Immunol. 2018, 141, AB203. [Google Scholar] [CrossRef]
- Szymona, K.; Karakula-Juchnowicz, H.; Zdzisinska, B.; Flis, M.; Kaławaj, K.; Rosa, W.; Kandefer-Szerszeń, M. Soluble Fas ligand (sFasL) as a predictor of reduction of general psychopathology in schizophrenia after antipsychotic treatment. Eur. Psychiatry 2016, 33, S108. [Google Scholar] [CrossRef]

| Condition | Direction of Change | Notes | References |
|---|---|---|---|
| Aging | |||
| Normal aging | ↑ | Serum | [17,18] |
| Aging-associated chronic inflammation | ↓ | Serum | [20,21] |
| Werner syndrome | ↑ | Serum | [17] |
| Age-related macular degeneration | ↑ | Plasma | [18] |
| Aging-associated T-cell population decrease | ↑ | Serum | [19] |
| Aging (athletes) | ↓ | Serum | [22] |
| Glaucoma | ↓ | Serum. Decreased ratio of sFasL to membrane FasL (mFasL) leads to increased susceptibility to fibrosis | [5] |
| Oxidative Stress | |||
| Condition | Direction of Change | Notes | References |
| Disruption of spermatogenesis | ↑ | Testicular tissue | [24] |
| Renal degeneration | ↑ | Plasma, urine | [25] |
| Condition | Direction of Change | Notes | References |
|---|---|---|---|
| Idiopathic pulmonary fibrosis (IPF) | ↑ | Serum and BAL | [67,84] |
| Hypersensitivity pneumonitis | ↑ | Serum and BAL | [66] |
| Interstitial pneumonia | ↑ | Serum and BAL | [67] |
| Chronic obstructive pulmonary disease (COPD) | ↮ | Serum | [78] |
| Asthma | ↮ | Serum | [83] |
| Asthma (uncontrolled allergic patients) | ↓ | Serum | [83] |
| Asthma (omalizumab treatment) | ↑ | Serum | [81] |
| Asthma (allergic children) | ↑ | Serum, during symptomatic period | [82] |
| Acute lung injury | ↑ | [73] | |
| Acute respiratory distress syndrome | ↑ | BAL of patients at risk of death | [85] |
| Lung cancer chemotherapy | ↑ | Serum | [77] |
| Lung cancer | ↮ | BAL | [69] |
| Small cell lung cancer | ↑ | Serum | [70] |
| Non-small cell lung cancer | ↑ | Serum | [71] |
| Pulmonary sarcoidosis | ↑ | BAL | [72] |
| Condition | Direction of Change | Notes | References |
|---|---|---|---|
| Inflammation | ↑ | Serum | [2] |
| Autoimmune lymphoproliferative syndrome | ↑ | Serum | [96,98] |
| Sjögren’s syndrome | ↓ | Serum | [99] |
| Chronic inflammation (pemphigus) | ↑ | Serum | [95] |
| Graft-versus-host disease | ↑ | Serum | [100] |
| Tuberculosis pleurisy | ↑ | BAL | [75] |
| Asymptomatic carriers of human T-lymphotropic virus type-1 (HTLV-1) | ↑ | Serum and BAL | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wallach-Dayan, S.B.; Petukhov, D.; Ahdut-HaCohen, R.; Richter-Dayan, M.; Breuer, R. sFasL—The Key to a Riddle: Immune Responses in Aging Lung and Disease. Int. J. Mol. Sci. 2021, 22, 2177. https://doi.org/10.3390/ijms22042177
Wallach-Dayan SB, Petukhov D, Ahdut-HaCohen R, Richter-Dayan M, Breuer R. sFasL—The Key to a Riddle: Immune Responses in Aging Lung and Disease. International Journal of Molecular Sciences. 2021; 22(4):2177. https://doi.org/10.3390/ijms22042177
Chicago/Turabian StyleWallach-Dayan, Shulamit B., Dmytro Petukhov, Ronit Ahdut-HaCohen, Mark Richter-Dayan, and Raphael Breuer. 2021. "sFasL—The Key to a Riddle: Immune Responses in Aging Lung and Disease" International Journal of Molecular Sciences 22, no. 4: 2177. https://doi.org/10.3390/ijms22042177
APA StyleWallach-Dayan, S. B., Petukhov, D., Ahdut-HaCohen, R., Richter-Dayan, M., & Breuer, R. (2021). sFasL—The Key to a Riddle: Immune Responses in Aging Lung and Disease. International Journal of Molecular Sciences, 22(4), 2177. https://doi.org/10.3390/ijms22042177





