The Potential of Hydrogen Sulfide Donors in Treating Cardiovascular Diseases
Abstract
1. Introduction
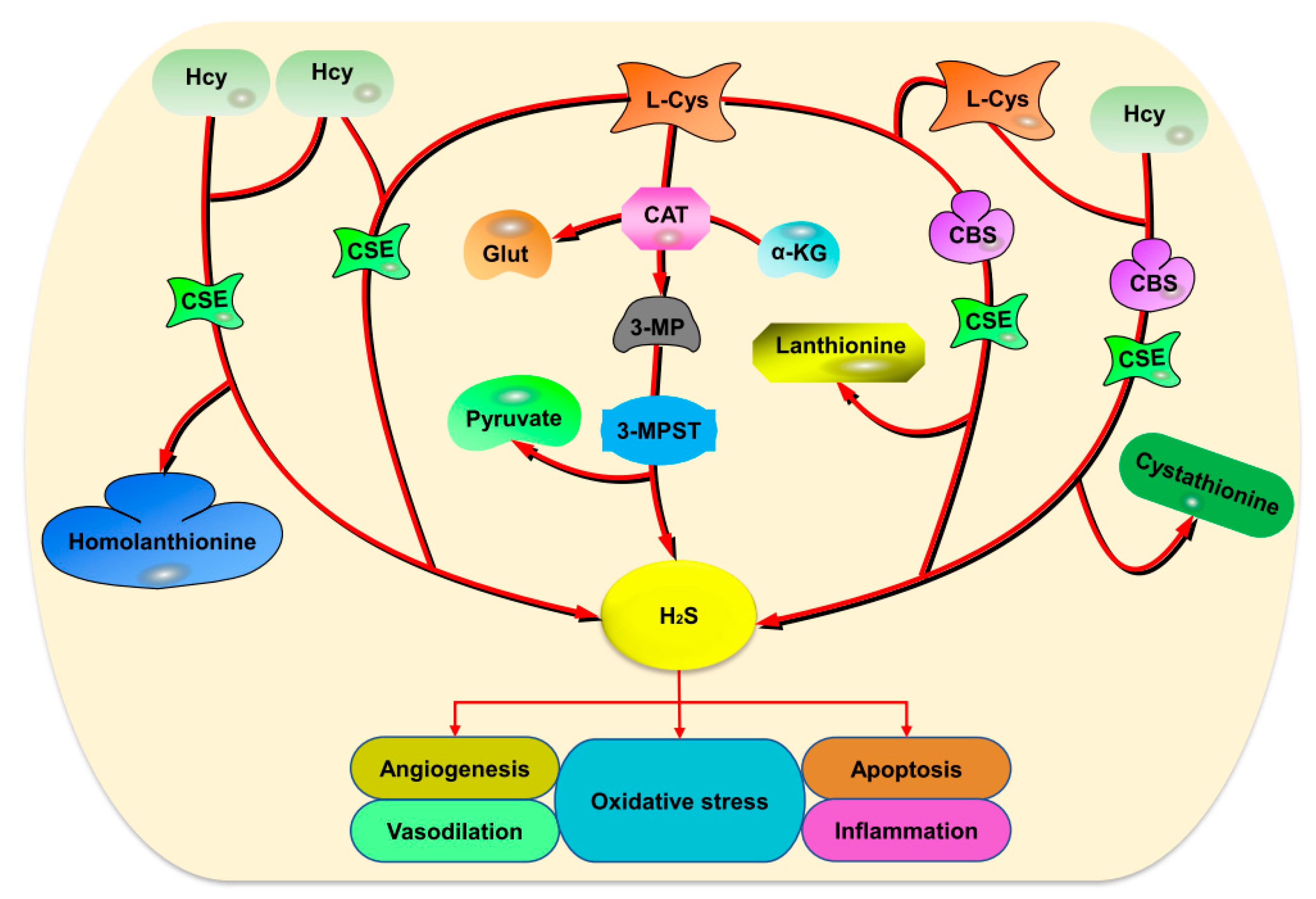
2. Endogenous H2S Production
2.1. Enzymatic Production
2.2. Non-Enzymatic Production
3. Exogenous H2S
3.1. Direct Inhalation of Exogenous H2S Gas
3.2. Inorganic Sulfide
3.3. Natural H2S Donors
3.4. Synthetic H2S Donors
4. Role of H2S in Vascular Function
4.1. Angiogenesis
4.2. Migration
4.3. Vasodilation
4.4. Apoptosis
4.5. Oxidative Stress
4.6. Autophagy
4.7. Inflammation
5. H2S and CVDs
5.1. Hypertension
5.2. Cardiac Fibrosis (CF)
5.3. Cardiac Hypertrophy (CH)
5.4. Cardiac Valve Calcification (CVC)
5.5. Takotsubo Cardiomyopathy (TCM)
5.6. Diabetic Cardiomyopathy (DCM)
5.7. Atherosclerosis
5.8. Myocardial Ischemia/Reperfusion (M-I/R) Injury
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-MP | 3-mercaptopyruvic acid |
| 3-MPST | 3-mercaptopyruvate sulfur transferase |
| ACE | Angiotensin-converting enzyme |
| Ang II | Angiotensin II |
| ATP | Adenosine triphosphate |
| Ca2+ | Calcium ion |
| CaS | Calcium sulfide |
| CBS | Cystathionine beta-synthase |
| CF | Cardiac fibrosis |
| cGMP | Cyclic guanosine 5′-monophosphate |
| CH | Cardiac hypertrophy |
| CO | Carbon monoxide |
| CSE | Cystathionine gamma-lyase |
| CVC | Cardiac valve calcification. |
| CVDs | Cardiovascular diseases |
| Cys | Cysteine |
| DADS | Diallyl disulfide |
| DAS | Diallyl sulfide |
| DATS | Diallyl trisulfide |
| PAG | D,L Propargylglycine |
| ECM | Extracellular matrix |
| FOXO-1 | Forkhead box protein O1 |
| H2S | Hydrogen sulfide |
| Hcy | Homocysteine |
| HUVECs | Human umbilical vein endothelial cells |
| IL | Interleukin |
| JAK | Janus kinase |
| K+ | Potassium |
| Keap-1 | Kelch-like ECH associated protein 1 |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| miR | microRNA |
| MMP | Matrix metalloproteinases |
| Na2S | Sodium sulfide |
| NaHS | Sodium hydrosulfide |
| NF-қB | Nuclear factor kappa B |
| NO | Nitric oxide |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| PI3K | Phosphatidylinositol-3-kinase |
| PLP | Pyridoxal 5′phosphate |
| PPAR | Peroxisome proliferator-activated receptor |
| ROS | Reactive oxygen species |
| STAT | Signal transducer and activator of transcription |
| TGF-β | Transforming growth factor beta |
| TIMP | Tissue inhibitor of metalloproteinase |
| VEGF | Vascular endothelial growth factor |
References
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.G.; Ohara, Y. Physiologic consequences of increased vascular oxidant stresses in hypercholesterolemia and atherosclerosis: Implications for impaired vasomotion. Am. J. Cardiol. 1995, 75, 75B–81B. [Google Scholar] [CrossRef]
- Bitar, M.S.; Nader, J.; Al-Ali, W.; Al Madhoun, A.; Arefanian, H.; Al-Mulla, F. Hydrogen Sulfide Donor NaHS Improves Metabolism and Reduces Muscle Atrophy in Type 2 Diabetes: Implication for Understanding Sarcopenic Pathophysiology. Oxid. Med. Cell Longev. 2018, 30, 5452. [Google Scholar] [CrossRef]
- Shirazi, M.K.; Azarnezhad, A.; Abazari, M.F.; Poorebrahim, M.; Ghoraeian, P.; Sanadgol, N.; Bokharaie, H.; Heydari, S.; Abbasi, A.; Kabiri, S.; et al. The role of nitric oxide signaling in renoprotective effects of hydrogen sulfide against chronic kidney disease in rats: Involvement of oxidative stress, autophagy and apoptosis. J. Cell Physiol. 2019, 234, 11411–11423. [Google Scholar] [CrossRef]
- Yue, L.M.; Gao, Y.M.; Han, B.H. Evaluation on the effect of hydrogen sulfide on the NLRP3 signaling pathway and its involvement in the pathogenesis of atherosclerosis. J. Cell Biochem. 2019, 120, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Peter, E.A.; Shen, X.; Shah, S.H.; Pardue, S.; Glawe, J.D.; Zhang, W.W.; Reddy, P.; Akkus, N.I.; Varma, J.; Kevil, C.G. Plasma free H2S levels are elevated in patients with cardiovascular disease. J. Am. Heart Assoc. 2013, 2, 387. [Google Scholar] [CrossRef]
- Polhemus, D.J.; Calvert, J.W.; Butler, J.; Lefer, D.J. The cardioprotective actions of hydrogen sulfide in acute myocardial infarction and heart failure. Scientifica 2014, 2014, 8607. [Google Scholar] [CrossRef] [PubMed]
- Whitcraft, D.D., 3rd; Bailey, T.D.; Hart, G.B. Hydrogen sulfide poisoning treated with hyperbaric oxygen. J. Emerg. Med. 1985, 3, 23–25. [Google Scholar] [CrossRef]
- Beauchamp, R.O., Jr.; Bus, J.S.; Popp, J.A.; Boreiko, C.J.; Andjelkovich, D.A. A critical review of the literature on hydrogen sulfide toxicity. Crit. Rev. Toxicol. 1984, 13, 25–97. [Google Scholar] [CrossRef]
- Guidotti, T.L. Hydrogen sulphide. Occup. Med. 1996, 46, 367–371. [Google Scholar] [CrossRef]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. Off. J. Soc. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef]
- Hosoki, R.; Matsuki, N.; Kimura, H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem. Biophys. Res. Commun. 1997, 237, 527–531. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef]
- Kimura, H. Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem. Biophys. Res. Commun. 2000, 267, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Ma, Y.; Xie, L.; Ferro, A.; Ji, Y. Emerging role of hydrogen sulfide in hypertension and related cardiovascular diseases. Br. J. Pharmacol. 2015, 172, 5501–5511. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Gui, D.D.; Yan, B.J.; Ren, Z.; Peng, L.J.; Wei, D.H.; Liu, L.S.; Zhang, D.W.; Jiang, Z.S. Hydrogen Sulfide Switch Phenomenon Regulating Autophagy in Cardiovascular Diseases. Cardiovasc. Drugs Ther. 2020, 34, 113–121. [Google Scholar] [CrossRef]
- Stipanuk, M.H.; Beck, P.W. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem. J. 1982, 206, 267–277. [Google Scholar] [CrossRef]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox Signal. 2009, 11, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 2009, 146, 623–626. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Dziegelewska, M.; Holtze, S.; Vole, C.; Wachter, U.; Menzel, U.; Morhart, M.; Groth, M.; Szafranski, K.; Sahm, A.; Sponholz, C.; et al. Low sulfide levels and a high degree of cystathionine β-synthase (CBS) activation by S-adenosylmethionine (SAM) in the long-lived naked mole-rat. Redox Biol. 2016, 8, 192–198. [Google Scholar] [CrossRef]
- Majtan, T.; Krijt, J.; Sokolová, J.; Křížková, M.; Ralat, M.A.; Kent, J.; Gregory, J.F., 3rd; Kožich, V.; Kraus, J.P. Biogenesis of Hydrogen Sulfide and Thioethers by Cystathionine Beta-Synthase. Antioxid. Redox Signal. 2018, 28, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Prudova, A.; Bauman, Z.; Braun, A.; Vitvitsky, V.; Lu, S.C.; Banerjee, R. S-adenosylmethionine stabilizes cystathionine beta-synthase and modulates redox capacity. Proc. Natl. Acad. Sci. USA 2006, 103, 6489–6494. [Google Scholar] [CrossRef]
- Finkelstein, J.D.; Kyle, W.E.; Martin, J.L.; Pick, A.M. Activation of cystathionine synthase by adenosylmethionine and adenosylethionine. Biochem. Biophys. Res. Commun. 1975, 66, 81–87. [Google Scholar] [CrossRef]
- Gregory, J.F.; DeRatt, B.N.; Avila, R.L.; Ralat, M.; Stacpoole, P.W. Vitamin B6 nutritional status and cellular availability of pyridoxal 5’-phosphate govern the function of the transsulfuration pathway’s canonical reactions and hydrogen sulfide production via side reactions. Biochimie 2016, 126, 21–26. [Google Scholar] [CrossRef]
- Chen, X.; Jhee, K.H.; Kruger, W.D. Production of the neuromodulator H2S by cystathionine beta-synthase via the condensation of cysteine and homocysteine. J. Biol. Chem. 2004, 279, 52082–52086. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Vlcek, C.; Paces, V.; Kraus, J.P. Identification and tissue distribution of human cystathionine beta-synthase mRNA isoforms. Arch. Biochem. Biophys. 1998, 350, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.; Naughten, E.R.; Wilcken, B.; Wilcken, D.E.; Boers, G.H. Vascular complications of severe hyperhomocysteinemia in patients with homocystinuria due to cystathionine beta-synthase deficiency: Effects of homocysteine-lowering therapy. Semin. Thromb. Hemost. 2000, 26, 335–340. [Google Scholar] [CrossRef]
- Fanapour, P.C.; Yug, B.; Kochar, M.S. Hyperhomocysteinemia: An additional cardiovascular risk factor. WMJ Off. Publ. State Med. Soc. Wis. 1999, 98, 51–54. [Google Scholar]
- Kabil, O.; Vitvitsky, V.; Xie, P.; Banerjee, R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid. Redox Signal. 2011, 15, 363–372. [Google Scholar] [CrossRef]
- Zhao, W.; Ndisang, J.F.; Wang, R. Modulation of endogenous production of H2S in rat tissues. Can. J. Physiol. Pharmacol. 2003, 81, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhang, W.; Yang, G.; Wang, R. Is cystathionine gamma-lyase protein expressed in the heart? Biochem. Biophys. Res. Commun. 2012, 428, 469–474. [Google Scholar] [CrossRef]
- Kuo, M.M.; Kim, D.H.; Jandu, S.; Bergman, Y.; Tan, S.; Wang, H.; Pandey, D.R.; Abraham, T.P.; Shoukas, A.A.; Berkowitz, D.E.; et al. MPST but not CSE is the primary regulator of hydrogen sulfide production and function in the coronary artery. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H71–H79. [Google Scholar] [CrossRef]
- Leucker, T.M.; Nomura, Y.; Kim, J.H.; Bhatta, A.; Wang, V.; Wecker, A.; Jandu, S.; Santhanam, L.; Berkowitz, D.; Romer, L.; et al. Cystathionine γ-lyase protects vascular endothelium: A role for inhibition of histone deacetylase 6. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H711–H720. [Google Scholar] [CrossRef] [PubMed]
- Mikami, Y.; Shibuya, N.; Ogasawara, Y.; Kimura, H. Hydrogen sulfide is produced by cystathionine γ-lyase at the steady-state low intracellular Ca(2+) concentrations. Biochem. Biophys. Res. Commun. 2013, 431, 131–135. [Google Scholar] [CrossRef]
- Nagahara, N.; Yoshii, T.; Abe, Y.; Matsumura, T. Thioredoxin-dependent enzymatic activation of mercaptopyruvate sulfurtransferase. An intersubunit disulfide bond serves as a redox switch for activation. J. Biol. Chem. 2007, 282, 1561–1569. [Google Scholar] [CrossRef]
- Peleli, M.; Bibli, S.I.; Li, Z.; Chatzianastasiou, A.; Varela, A.; Katsouda, A.; Zukunft, S.; Bucci, M.; Vellecco, V.; Davos, C.H. Cardiovascular phenotype of mice lacking 3-mercaptopyruvate sulfurtransferase. Biochem. Pharmacol. 2020, 176, 3833. [Google Scholar] [CrossRef]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A novel pathway for the production of hydrogen sulfide from D-cysteine in mammalian cells. Nat. Commun. 2013, 4, 1366. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Minkler, P.; Grove, D.; Wang, R.; Willard, B.; Dweik, R.; Hine, C. Non-enzymatic hydrogen sulfide production from cysteine in blood is catalyzed by iron and vitamin B6. Commun. Biol. 2019, 2, 194. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Deleon, E.R.; Gao, Y.; Hurley, K.; Sadauskas, V.; Batz, C.; Stoy, G.F. Thiosulfate: A readily accessible source of hydrogen sulfide in oxygen sensing. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Searcy, D.G.; Lee, S.H. Sulfur reduction by human erythrocytes. J. Exp. Zool. 1998, 282, 310–322. [Google Scholar] [CrossRef]
- Volpato, G.P.; Searles, R.; Yu, B.; Scherrer-Crosbie, M.; Bloch, K.D.; Ichinose, F.; Zapol, W.M. Inhaled hydrogen sulfide: A rapidly reversible inhibitor of cardiac and metabolic function in the mouse. Anesthesiology 2008, 108, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Faller, S.; Zimmermann, K.K.; Strosing, K.M.; Engelstaedter, H.; Buerkle, H.; Schmidt, R.; Spassov, S.G.; Hoetzel, A. Inhaled hydrogen sulfide protects against lipopolysaccharide-induced acute lung injury in mice. Med. Gas Res. 2012, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhang, B.; Zhang, Y.; Li, H.; Cheng, L.; Zhao, X.; Yin, J.; Wang, G. Hydrogen Sulfide Inhalation Improves Neurological Outcome via NF-κB-Mediated Inflammatory Pathway in a Rat Model of Cardiac Arrest and Resuscitation. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 36, 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Derwall, M.; Francis, R.C.; Kida, K.; Bougaki, M.; Crimi, E.; Adrie, C.; Zapol, W.M.; Ichinose, F. Administration of hydrogen sulfide via extracorporeal membrane lung ventilation in sheep with partial cardiopulmonary bypass perfusion: A proof of concept study on metabolic and vasomotor effects. Crit. Care 2011, 15, R51. [Google Scholar] [CrossRef] [PubMed]
- Giggenbach, W. Optical spectra of highly alkaline sulfide solutions and the second dissociation constant of hydrogen sulfide. Inorg. Chem. 1971, 10, 1333–1338. [Google Scholar] [CrossRef]
- Li, Y.F.; Xiao, C.S.; Hui, R.T. Calcium sulfide (CaS), a donor of hydrogen sulfide (H(2)S): A new antihypertensive drug? Med. Hypotheses 2009, 73, 445–447. [Google Scholar] [CrossRef]
- Benavides, G.A.; Squadrito, G.L.; Mills, R.W.; Patel, H.D.; Isbell, T.S.; Patel, R.P.; Darley-Usmar, V.M.; Doeller, J.E.; Kraus, D.W. Hydrogen sulfide mediates the vasoactivity of garlic. Proc. Natl. Acad. Sci. USA 2007, 104, 17977–17982. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H. Clarifying the real bioactive constituents of garlic. J. Nutr. 2006, 136, 716s–725s. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Liu, C.J.; Kuo, C.H.; Chen, H.; Lin, W.Y.; Teng, K.Y.; Chang, S.W.; Tsai, C.H.; Tsai, F.J.; Huang, C.Y.; et al. Garlic Oil Alleviates MAPKs- and IL-6-mediated Diabetes-related Cardiac Hypertrophy in STZ-induced DM Rats. Evid. Based Complement. Altern. Med. eCAM 2011, 2011, 150. [Google Scholar] [CrossRef] [PubMed]
- Harauma, A.; Moriguchi, T. Aged garlic extract improves blood pressure in spontaneously hypertensive rats more safely than raw garlic. J. Nutr. 2006, 136, 769S–773S. [Google Scholar] [CrossRef] [PubMed]
- Powell, C.R.; Dillon, K.M.; Matson, J.B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.H.; Moore, P.K. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): New insights into the biology of hydrogen sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef]
- Zhang, Q.; Tian, P.; Zhai, M.; Lei, X.; Yang, Z.; Liu, Y.; Liu, M.; Huang, H.; Zhang, X.; Yang, X.; et al. Formaldehyde regulates vascular tensions through nitric oxide-cGMP signaling pathway and ion channels. Chemosphere 2018, 193, 60–73. [Google Scholar] [CrossRef]
- True, A.L.; Olive, M.; Boehm, M.; San, H.; Westrick, R.J.; Raghavachari, N.; Xu, X.; Lynn, E.G.; Sack, M.N.; Munson, P.J.; et al. Heme oxygenase-1 deficiency accelerates formation of arterial thrombosis through oxidative damage to the endothelium, which is rescued by inhaled carbon monoxide. Circ. Res. 2007, 101, 893–901. [Google Scholar] [CrossRef]
- Park, C.M.; Zhao, Y.; Zhu, Z.; Pacheco, A.; Peng, B.; Devarie-Baez, N.O.; Bagdon, P.; Zhang, H.; Xian, M. Synthesis and evaluation of phosphorodithioate-based hydrogen sulfide donors. Mol. Biosyst. 2013, 9, 2430–2434. [Google Scholar] [CrossRef]
- Kang, J.; Li, Z.; Organ, C.L.; Park, C.M.; Yang, C.T.; Pacheco, A.; Wang, D.; Lefer, D.J.; Xian, M. pH-Controlled Hydrogen Sulfide Release for Myocardial Ischemia-Reperfusion Injury. J. Am. Chem. Soc. 2016, 138, 6336–6339. [Google Scholar] [CrossRef]
- Polhemus, D.J.; Li, Z.; Pattillo, C.B. A novel hydrogen sulfide prodrug, SG1002, promotes hydrogen sulfide and nitric oxide bioavailability in heart failure patients. Cardiovasc. Ther. 2015, 33, 216–226. [Google Scholar] [CrossRef]
- Li, L.; Rossoni, G.; Sparatore, A.; Lee, L.C.; Del Soldato, P.; Moore, P.K. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic. Biol. Med. 2007, 42, 706–719. [Google Scholar] [CrossRef]
- Sidhapuriwala, J.; Li, L.; Sparatore, A.; Bhatia, M.; Moore, P.K. Effect of S-diclofenac, a novel hydrogen sulfide releasing derivative, on carrageenan-induced hindpaw oedema formation in the rat. Eur. J. Pharmacol. 2007, 569, 149–154. [Google Scholar] [CrossRef]
- Szczesny, B.; Módis, K.; Yanagi, K.; Coletta, C.; Le Trionnaire, S.; Perry, A.; Wood, M.E.; Whiteman, M.; Szabo, C. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide Biol. Chem. 2014, 41, 120–130. [Google Scholar] [CrossRef]
- Zhu, C.; Su, Y.; Juriasingani, S.; Zheng, H. Supplementing preservation solution with mitochondria-targeted H2 S donor AP39 protects cardiac grafts from prolonged cold ischemia-reperfusion injury in heart transplantation. Am. Soc. Transplant. Surg. 2019, 19, 3139–3148. [Google Scholar] [CrossRef]
- Ji, J.; Xiang, P.; Li, T.; Lan, L.; Xu, X.; Lu, G.; Ji, H.; Zhang, Y.; Li, Y. NOSH-NBP, a Novel Nitric Oxide and Hydrogen Sulfide- Releasing Hybrid, Attenuates Ischemic Stroke-Induced Neuroinflammatory Injury by Modulating Microglia Polarization. Front. Cell. Neurosci. 2017, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Longchamp, A.; Mirabella, T.; Arduini, A.; MacArthur, M.R.; Das, A.; Treviño-Villarreal, J.H.; Hine, C.; Ben-Sahra, I.; Knudsen, N.H.; Brace, L.E.; et al. Amino Acid Restriction Triggers Angiogenesis via GCN2/ATF4 Regulation of VEGF and H2S Production. Cell 2018, 173, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Adamis, A.P. Ten years of anti-vascular endothelial growth factor therapy. Nature reviews. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef]
- Li, J.; Zhou, W.; Chen, W.; Wang, H.; Zhang, Y.; Yu, T. Mechanism of the hypoxia inducible factor 1/hypoxic response element pathway in rat myocardial ischemia/diazoxide post-conditioning. Mol. Med. Rep. 2020, 21, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Xu, M.; Zhang, W.; Che, X. Effects of Sevoflurane Pretreatment on Myocardial Ischemia-Reperfusion Injury Through the Akt/Hypoxia-Inducible Factor 1-alpha (HIF-1α)/Vascular Endothelial Growth Factor (VEGF) Signaling Pathway. Med Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 3100–3107. [Google Scholar] [CrossRef] [PubMed]
- Arany, Z.; Foo, S.Y.; Ma, Y.; Ruas, J.L.; Bommi-Reddy, A.; Girnun, G.; Cooper, M.; Laznik, D.; Chinsomboon, J.; Rangwala, S.M.; et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 2008, 451, 1008–1012. [Google Scholar] [CrossRef]
- Papapetropoulos, A.; Pyriochou, A.; Altaany, Z.; Yang, G.; Marazioti, A.; Zhou, Z.; Jeschke, M.G.; Branski, L.K.; Herndon, D.N.; Wang, R.; et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 21972–21977. [Google Scholar] [CrossRef] [PubMed]
- Qipshidze, N.; Metreveli, N.; Mishra, P.K.; Lominadze, D.; Tyagi, S.C. Hydrogen sulfide mitigates cardiac remodeling during myocardial infarction via improvement of angiogenesis. Int. J. Biol. Sci. 2012, 8, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Aranguren, S.L.C.; Ahmad, S.; Dias, I.; Alzahrani, F.A.; Rezai, H.; Wang, K.; Ahmed, A. Bioenergetic effects of hydrogen sulfide suppress soluble Flt-1 and soluble endoglin in cystathionine gamma-lyase compromised endothelial cells. Sci. Rep. 2020, 10, 15810. [Google Scholar] [CrossRef]
- Xiong, Y.; Chang, L.L.; Tran, B.; Dai, T.; Zhong, R.; Mao, Y.C.; Zhu, Y.Z. ZYZ-803, A novel hydrogen sulfide-nitric oxide conjugated donor, promotes angiogenesis via cross-talk between STAT3 and CaMKII. Acta Pharmacol. Sin. 2020, 41, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Li, H.; Xue, H.; Jin, S.; Xiao, L.; Guo, Q.; Wu, Y. GABAA receptor, KATP channel and L-type Ca2+ channel is associated with facilitation effect of H2S on the baroreceptor reflex in spontaneous hypertensive rats. Pharmacol. Rep. 2019, 71, 968–975. [Google Scholar] [CrossRef]
- Dai, L.; Qian, Y.; Zhou, J.; Zhu, C.; Jin, L.; Li, S. Hydrogen sulfide inhibited L-type calcium channels (CaV1.2) via up-regulation of the channel sulfhydration in vascular smooth muscle cells. Eur. J. Pharmacol. 2019, 858, 2455. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; van Der Vlies, A.J.; Hasegawa, U. Hydrogen sulfide-releasing micelles for promoting angiogenesis. Polym. Chem. 2020, 11, 4454–4463. [Google Scholar] [CrossRef]
- Terzuoli, E.; Monti, M.; Vellecco, V.; Bucci, M.; Cirino, G.; Ziche, M.; Morbidelli, L. Characterization of zofenoprilat as an inducer of functional angiogenesis through increased H2S availability. Br. J. Pharmacol. 2015, 172, 2961–2973. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, X.H.; Zhang, C.C.; Wang, M.J.; Xue, W.L.; Wu, D.D.; Ma, F.F.; Li, W.W.; Tao, B.B.; Zhu, Y. Hydrogen sulfide promotes angiogenesis by downregulating miR-640 via the VEGFR2/mTOR pathway. American journal of physiology. Cell Physiol. 2016, 310, C305–C317. [Google Scholar] [CrossRef] [PubMed]
- van den Born, J.C.; Mencke, R.; Conroy, S.; Zeebregts, C.J.; van Goor, H.; Hillebrands, J.L. Cystathionine γ-lyase is expressed in human atherosclerotic plaque microvessels and is involved in micro-angiogenesis. Sci. Rep. 2016, 6, 4608. [Google Scholar] [CrossRef] [PubMed]
- Mokretar, K.; Velinov, H.; Postadzhiyan, A.; Apostolova, M. Association of Polymorphisms in Endothelial Nitric Oxide Synthesis and Renin-Angiotensin-Aldosterone System with Developing of Coronary Artery Disease in Bulgarian Patients. Genet. Test. Mol. Biomark. 2016, 20, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Bir, S.C.; Kolluru, G.K.; McCarthy, P.; Shen, X.; Pardue, S.; Pattillo, C.B.; Kevil, C.G. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1α and vascular endothelial growth factor-dependent angiogenesis. J. Am. Heart Assoc. 2012, 1, 4093. [Google Scholar] [CrossRef]
- Coletta, C.; Papapetropoulos, A.; Erdelyi, K.; Olah, G.; Módis, K.; Panopoulos, P.; Asimakopoulou, A.; Gerö, D.; Sharina, I.; Martin, E.; et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. USA 2012, 109, 9161–9166. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, C.; Deng, M.; Wang, F.; Ren, X.; Fan, Y.; Du, J.; Wang, Y. H2S promotes developmental brain angiogenesis via the NOS/NO pathway in zebrafish. Stroke and vascular neurology, svn-2020-000584. Adv. Online Publ. 2020. [Google Scholar] [CrossRef]
- Tao, B.; Wang, R.; Sun, C.; Zhu, Y. 3-Mercaptopyruvate Sulfurtransferase, Not Cystathionine β-Synthase Nor Cystathionine γ-Lyase, Mediates Hypoxia-Induced Migration of Vascular Endothelial Cells. Front. Pharmacol. 2017, 8, 657. [Google Scholar] [CrossRef] [PubMed]
- Govar, A.A.; Törő, G.; Szaniszlo, P.; Pavlidou, A.; Bibli, S.I.; Thanki, K.; Resto, V.A.; Chao, C.; Hellmich, M.R.; Szabo, C.; et al. 3-Mercaptopyruvate sulfurtransferase supports endothelial cell angiogenesis and bioenergetics. Br. J. Pharmacol. 2020, 177, 866–883. [Google Scholar] [CrossRef]
- Xue, W.L.; Chen, R.Q.; Zhang, Q.Q.; Li, X.H.; Cao, L.; Li, M.Y.; Li, Y.; Lin, G.; Chen, Y.; Wang, M.J.; et al. Hydrogen sulfide rescues high glucose-induced migration dysfunction in HUVECs by upregulating miR-126-3p. American journal of physiology. Cell Physiol. 2020, 318, C857–C869. [Google Scholar] [CrossRef] [PubMed]
- Grambow, E.; Klee, G.; Xie, W.; Schafmayer, C.; Vollmar, B. Hydrogen sulfide reduces the activity of human endothelial cells. Clin. Hemorheol. Microcirc. 2020, 76, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.L.; Zhao, F.R.; Zhu, T.T.; Wang, Q.Q.; Wu, Z.Q.; Song, P.; Xu, J.; Wan, G.R.; Yin, Y.L.; Li, P. The antihypertension effect of hydrogen sulfide (H2S) is induced by activating VEGFR2 signaling pathway. Life Sci. 2020, 267, 8831. [Google Scholar] [CrossRef]
- Wu, J.; Agbor, L.N.; Fang, S.; Mukohda, M.; Nair, A.R.; Nakagawa, P.; Sigmund, C.D. Failure to vasodilate in response to salt loading blunts renal blood flow and causes salt-sensitive hypertension. Cardiovasc. Res. 2021, 117, 308–319. [Google Scholar] [CrossRef]
- Marques, A.; da Silva, C.; de Souza, P.; de Almeida, C.; Cechinel-Filho, V.; Lourenço, E.; Gasparotto, A., Jr. Nitric oxide and Ca2+-activated high-conductance K+ channels mediate nothofagin-induced endothelium-dependent vasodilation in the perfused rat kidney. Chem. Biol. Interact. 2020, 327, 9182. [Google Scholar] [CrossRef] [PubMed]
- Thieme, M.; Sivritas, S.H.; Mergia, E.; Potthoff, S.A.; Yang, G.; Hering, L.; Grave, K.; Hoch, H.; Rump, L.C.; Stegbauer, J. Phosphodiesterase 5 inhibition ameliorates angiotensin II-dependent hypertension and renal vascular dysfunction. American journal of physiology. Ren. Physiol. 2017, 312, F474–F481. [Google Scholar] [CrossRef] [PubMed]
- Greaney, J.L.; Kutz, J.L.; Shank, S.W.; Jandu, S.; Santhanam, L.; Alexander, L.M. Impaired Hydrogen Sulfide-Mediated Vasodilation Contributes to Microvascular Endothelial Dysfunction in Hypertensive Adults. Hypertension 2017, 69, 902–909. [Google Scholar] [CrossRef]
- Te Winkel, J.; John, Q.E.; Hosfield, B.D.; Drucker, N.A.; Das, A.; Olson, K.R.; Markel, T.A. Mesenchymal stem cells promote mesenteric vasodilation through hydrogen sulfide and endothelial nitric oxide. American journal of physiology. Gastrointest. Liver Physiol. 2019, 317, G441–G446. [Google Scholar] [CrossRef]
- Barrera, A.; Loredo, M.H.; Garcia, J.M.; Fregoso, G.; Pace, C.E.; Mendiola, P.J.; Naik, J.S.; Gonzalez Bosc, L.V.; Kanagy, N. Simulated sleep apnea alters hydrogen sulfide regulation of blood flow and pressure. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H511–H519. [Google Scholar] [CrossRef]
- Mys, L.A.; Strutynska, N.A.; Goshovska, Y.V.; Sagach, V.F. Stimulation of the endogenous hydrogen sulfide synthesis suppresses oxidative-nitrosative stress and restores endothelial-dependent vasorelaxation in old rats. Can. J. Physiol. Pharmacol. 2020, 98, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bai, J.; Yang, Y.H.; Hoshi, N.; Chen, D.B. Hydrogen Sulfide Relaxes Human Uterine Artery via Activating Smooth Muscle BKCa Channels. Antioxidants 2020, 9, 1127. [Google Scholar] [CrossRef] [PubMed]
- Parfenova, H.; Liu, J.; Hoover, D.T.; Fedinec, A.L. Vasodilator effects of sulforaphane in cerebral circulation: A critical role of endogenously produced hydrogen sulfide and arteriolar smooth muscle KATP and BK channels in the brain. J. Cereb. Blood Flow Metab. 2020, 40, 1987–1996. [Google Scholar] [CrossRef]
- Lobov, G.I.; Sokolova, I.B.; Gorshkova, O.P.; Shvetsova, M.E.; Dvoretskii, D.P. Contribution of Hydrogen Sulfide to Dilation of Rat Cerebral Arteries after Ischemia/Reperfusion Injury. Bull. Exp. Biol. Med. 2020, 168, 597–601. [Google Scholar] [CrossRef]
- Naik, J.S.; Osmond, J.M.; Walker, B.R.; Kanagy, N.L. Hydrogen sulfide-induced vasodilation mediated by endothelial TRPV4 channels. Heart Circ. Physiol. 2016, 311, H1437–H1444. [Google Scholar] [CrossRef] [PubMed]
- Sélley, E.; Kun, S.; Szijártó, I.A.; Laczy, B.; Kovács, T.; Fülöp, F.; Wittmann, I.; Molnár, G.A. Exenatide induces aortic vasodilation increasing hydrogen sulphide, carbon monoxide and nitric oxide production. Cardiovasc. Diabetol. 2014, 13, 69. [Google Scholar] [CrossRef]
- Abbate, A.; Zoccai, B.G.G.; Bussani, R.; Dobrina, A.; Camilot, D.; Feroce, F.; Rossiello, R.; Baldi, F.; Silvestri, F.; Biasucci, L.M.; et al. Increased myocardial apoptosis in patients with unfavorable left ventricular remodeling and early symptomatic post-infarction heart failure. J. Am. Coll. Cardiol. 2003, 41, 753–760. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, C. The cystathionine γ-lyase/hydrogen sulfide pathway mediates the trimetazidine-induced protection of H9c2 cells against hypoxia/reoxygenation-induced apoptosis and oxidative stress. Anatol. J. Cardiol. 2019, 22, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.E.; Huang, G.Q.; Wu, B.; Lin, X.D.; Yang, W.Z.; Ke, Z.Y.; Liu, J. Hydrogen sulfide protects H9c2 cardiomyoblasts against H2O2-induced apoptosis. Braz. J. Med. Biol. Res. 2019, 52, 7626. [Google Scholar] [CrossRef]
- Zhou, X.; An, G.; Chen, J. Hydrogen sulfide improves left ventricular function in smoking rats via regulation of apoptosis and autophagy. Apoptosis Int. J. Program. Cell Death 2014, 19, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yuan, Y.Q.; Zhang, L.; Zhang, H.; Zhang, S.W.; Zhang, Y.; Xuan, X.X.; Wang, M.J.; Zhang, J.Y. Exogenous hydrogen sulfide protects against high glucose-induced apoptosis and oxidative stress by inhibiting the STAT3/HIF-1α pathway in H9c2 cardiomyocytes. Exp. Ther. Med. 2019, 18, 3948–3958. [Google Scholar] [CrossRef]
- Ehara, S.; Ueda, M.; Naruko, T.; Haze, K.; Itoh, A.; Otsuka, M.; Komatsu, R.; Matsuo, T.; Itabe, H.; Takano, T.; et al. Elevated levels of oxidized low-density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation 2001, 103, 1955–1960. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, D.; Xu, R.X.; Guo, Y.L.; Zhu, C.G.; Wu, N.Q.; Zhang, Y.; Li, S.; Gao, Y.; Liu, G.; et al. Low-density lipoprotein-associated variables and the severity of coronary artery disease: An untreated Chinese cohort study. Biomark. Biochem. Indic. Expo. Response Susceptibility Chem. 2018, 23, 647–653. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, H.W.; Xu, R.X.; Guo, Y.L.; Zhu, C.G.; Wu, N.Q.; Gao, Y.; Li, J.J. Oxidized-LDL is a useful marker for predicting the very early coronary artery disease and cardiovascular outcomes. Pers. Med. 2018, 15, 521–529. [Google Scholar] [CrossRef]
- Jain, S.K.; Micinski, D.; Lieblong, B.J.; Stapleton, T. Relationship between hydrogen sulfide levels and HDL-cholesterol, adiponectin, and potassium levels in the blood of healthy subjects. Atherosclerosis 2012, 225, 242–245. [Google Scholar] [CrossRef]
- Jeney, V.; Komódi, E.; Nagy, E.; Zarjou, A.; Vercellotti, G.M.; Eaton, J.W.; Balla, G.; Balla, J. Suppression of hemin-mediated oxidation of low-density lipoprotein and subsequent endothelial reactions by hydrogen sulfide (H(2)S). Free Radic. Biol. Med. 2009, 46, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.J.; Li, B.; Yang, M.; Liu, Y.; Liu, Y.L.; Su, J.W. Sirtuin 1 Mediates Hydrogen Sulfide-induced Cytoprotection Effects in Neonatal Mouse Cardiomyocytes. Chin. Med. J. 2017, 130, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Mao, Y.; Tan, B.; Luo, S.; Zhu, Y. The protective effects of endogenous hydrogen sulfide modulator, S-propargyl-cysteine, on high glucose-induced apoptosis in cardiomyocytes: A novel mechanism mediated by the activation of Nrf2. Eur. J. Pharmacol. 2015, 761, 135–143. [Google Scholar] [CrossRef]
- Corsetti, G.; Scarabelli, C.C.; Romano, C.; Pasini, E.; Dioguardi, F.S.; Onorati, F.; Knight, R.; Patel, H.; Saravolatz, L.; Faggian, G.; et al. Autophagy and Oncosis/Necroptosis Are Enhanced in Cardiomyocytes from Heart Failure Patients. Med. Sci. Monit. Basic Res. 2019, 25, 33–44. [Google Scholar] [CrossRef]
- Zhou, J.; Xi, C.; Wang, W.; Yang, Y.; Qiu, Y.; Huang, Z. Autophagy plays an important role in triptolide-induced apoptosis in cardiomyocytes. Toxicol. Lett. 2015, 236, 168–183. [Google Scholar] [CrossRef]
- Feng, A.; Ling, C.; Duo, X.L.; Bing, W.; Wu, S.W.; Yu, Z.; Lan, Y.H.; En, Y.Z. Hydrogen Sulfide Protects Human Cardiac Fibroblasts Against H2O2-induced Injury Through Regulating Autophagy-Related Proteins. Cell Transplant. 2018, 27, 1222–1234. [Google Scholar] [CrossRef]
- Jiang, H.; Xiao, J.; Kang, B.; Zhu, X.; Xin, N.; Wang, Z. PI3K/SGK1/GSK3β signaling pathway is involved in inhibition of autophagy in neonatal rat cardiomyocytes exposed to hypoxia/reoxygenation by hydrogen sulfide. Exp. Cell Res. 2016, 345, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Duan, W.; Wu, G.; Zhang, D.; Wang, L.; Chen, D.; Chen, Z.; Yang, B. Protective effect of hydrogen sulfide on endothelial cells through Sirt1-FoxO1-mediated autophagy. Ann. Transl. Med. 2020, 8, 1586. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, V.; Lobasso, A.; Barbieri, L.; Parrella, P.; Ciervo, D.; Liccardo, B.; Bonaduce, D.; Tocchetti, C.G.; De Paulis, A.; Rossi, F.W. Inflammatory, Serological and Vascular Determinants of Cardiovascular Disease in Systemic Lupus Erythematosus Patients. Int. J. Mol. Sci. 2019, 20, 2154. [Google Scholar] [CrossRef]
- Pai, J.K.; Pischon, T.; Ma, J.; Manson, J.E.; Hankinson, S.E.; Joshipura, K.; Curhan, G.C.; Rifai, N.; Cannuscio, C.C.; Stampfer, M.J.; et al. Inflammatory markers and the risk of coronary heart disease in men and women. N. Engl. J. Med. 2004, 351, 2599–2610. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Stampfer, M.J.; Hennekens, C.H. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000, 101, 1767–1772. [Google Scholar] [CrossRef]
- Schieffer, B.; Schieffer, E.; Kleiner, H.D.; Hilfiker, A.; Kovanen, P.T.; Kaartinen, M.; Nussberger, J.; Harringer, W.; Drexler, H. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: Potential implications for inflammation and plaque instability. Circulation 2000, 101, 1372–1378. [Google Scholar] [CrossRef]
- Machida, Y.; Kubota, T.; Kawamura, N.; Funakoshi, H.; Ide, T.; Utsumi, H.; Li, Y.Y.; Feldman, A.M.; Tsutsui, H.; Shimokawa, H.; et al. Overexpression of tumor necrosis factor-alpha increases production of hydroxyl radical in murine myocardium. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H449–H455. [Google Scholar] [CrossRef] [PubMed]
- Obama, T.; Ohinata, H.; Takaki, T.; Iwamoto, S.; Sawada, N.; Aiuchi, T.; Kato, R.; Itabe, H. Cooperative Action of Oxidized Low-Density Lipoproteins and Neutrophils on Endothelial Inflammatory Responses Through Neutrophil Extracellular Trap Formation. Front. Immunol. 2019, 10, 1899. [Google Scholar] [CrossRef]
- Lin, Z.; Altaf, N.; Li, C.; Chen, M.; Pan, L.; Wang, D.; Xie, L.; Zheng, Y.; Fu, H.; Han, Y.; et al. Hydrogen sulfide attenuates oxidative stress-induced NLRP3 inflammasome activation via S-sulfhydrating c-Jun at Cys269 in macrophages. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2890–2900. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Wang, J.; Li, D.; Li, Z.; Liu, H.; Ding, M.; Lu, W. Hydrogen sulfide inhibits cigarette smoke-induced inflammation and injury in alveolar epithelial cells by suppressing PHD2/HIF-1α/MAPK signaling pathway. Int. Immunopharmacol. 2020, 81, 5979. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wu, K.; Chen, J.; Mo, L.; Hua, X.; Zheng, D.; Chen, P.; Chen, G.; Xu, W.; Feng, J. Exogenous hydrogen sulfide protects against doxorubicin-induced inflammation and cytotoxicity by inhibiting p38MAPK/NFκB pathway in H9c2 cardiac cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2013, 32, 1668–1680. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Peng, H.; Du, Q.; Lin, W.; Liu, Y. GYY4137, A hydrogen sulfide-releasing molecule, inhibits the inflammatory response by suppressing the activation of nuclear factor-kappa B and mitogen-activated protein kinases in Coxsackie virus B3-infected rat cardiomyocytes. Mol. Med. Rep. 2015, 11, 1837–1844. [Google Scholar] [CrossRef]
- Pan, L.L.; Liu, X.H.; Gong, Q.H.; Zhu, Y.Z. S-Propargyl-cysteine (SPRC) attenuated lipopolysaccharide-induced inflammatory response in H9c2 cells involved in a hydrogen sulfide-dependent mechanism. Amino Acids 2011, 41, 205–215. [Google Scholar] [CrossRef]
- Kitt, J.; Fox, R.; Tucker, K.L.; McManus, R.J. New Approaches in Hypertension Management: A Review of Current and Developing Technologies and Their Potential Impact on Hypertension Care. Curr. Hypertens. Rep. 2019, 21, 44. [Google Scholar] [CrossRef]
- Chen, L.; Ingrid, S.; Ding, Y.G.; Liu, Y.; Qi, J.G.; Tang, C.S.; DU, J.B. Imbalance of endogenous homocysteine and hydrogen sulfide metabolic pathway in essential hypertensive children. Chin. Med. J. 2007, 120, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.L.; Xi, Y.; Yang, S.N.; Ma, Z.; Tang, C.S. Plasma hydrogen sulfide and homocysteine levels in hypertensive patients with different blood pressure levels and complications. Zhonghua Xin Xue Guan Bing Za Zhi 2007, 35, 1145–1148. [Google Scholar]
- Lu, M.; Liu, Y.H.; Goh, H.S.; Wang, J.J.; Yong, Q.C.; Wang, R.; Bian, J.S. Hydrogen sulfide inhibits plasma renin activity. J. Am. Soc. Nephrol. 2010, 21, 993–1002. [Google Scholar] [CrossRef]
- Huang, P.; Chen, S.; Wang, Y.; Liu, J.; Yao, Q.; Huang, Y.; Li, H.; Zhu, M.; Wang, S.; Li, L.; et al. Down-regulated CBS/H2S pathway is involved in high-salt-induced hypertension in Dahl rats. Nitric Oxide Biol. Chem. 2015, 46, 192–203. [Google Scholar] [CrossRef]
- Magableh, A.M.R.; Harper, K.B.K.; Hart, J.L. Hydrogen sulfide treatment reduces blood pressure and oxidative stress in angiotensin II-induced hypertensive mice. Hypertension Res. Off. J. Jpn. Soc. Hypertens. 2015, 38, 13–20. [Google Scholar] [CrossRef]
- Laggner, H.; Hermann, M.; Esterbauer, H.; Muellner, M.K.; Exner, M.; Gmeiner, B.M.; Kapiotis, S. The novel gaseous vasorelaxant hydrogen sulfide inhibits angiotensin-converting enzyme activity of endothelial cells. J. Hypertens. 2007, 25, 2100–2104. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Fan, J.; Zeng, Q.; Cai, J.; Chen, Y.; Chen, Z.; Wang, W.; Li, S.Y.; Cui, Q.; Yang, J.; et al. CD4+ T-Cell Endogenous Cystathionine γ Lyase-Hydrogen Sulfide Attenuates Hypertension by Sulfhydrating Liver Kinase B1 to Promote T Regulatory Cell Differentiation and Proliferation. Circulation 2020, 142, 1752–1769. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N.; Lu, P.C. Early short-term treatment with exogenous hydrogen sulfide postpones the transition from prehypertension to hypertension in spontaneously hypertensive rat. Clin. Exp. Hypertens. 2018, 40, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Dong, J.H.; Jin, S.; Xue, H.M.; Guo, Q.; Teng, X.; Wu, Y.M. Hydrogen Sulfide Improves Endothelial Dysfunction via Downregulating BMP4/COX-2 Pathway in Rats with Hypertension. Oxidative Med. Cell. Longev. 2016, 2016, 8957. [Google Scholar] [CrossRef]
- Xiao, L.; Dong, J.H.; Teng, X.; Jin, S.; Xue, H.M.; Liu, S.Y.; Guo, Q.; Shen, W.; Ni, X.C.; Wu, Y.M. Hydrogen sulfide improves endothelial dysfunction in hypertension by activating peroxisome proliferator-activated receptor delta/endothelial nitric oxide synthase signaling. J. Hypertens. 2018, 36, 651–665. [Google Scholar] [CrossRef]
- Morris, M.T.; Camboa, G.N.; Banerjee, I.; Zambon, A.C.; Kisseleva, T.; Velayoudon, A.; Stallcup, W.B.; Gu, Y.; Dalton, N.D.; Cedenilla, M. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J. Clin. Investig. 2014, 124, 2921–2934. [Google Scholar] [CrossRef] [PubMed]
- van Wamel, A.J.; Ruwhof, C.; van der Kokshoom, V.L.; Schrier, P.I.; van der Laarse, A. The role of angiotensin II, endothelin-1 and transforming growth factor-beta as autocrine/paracrine mediators of stretch-induced cardiomyocyte hypertrophy. Mol. Cell. Biochem. 2001, 218, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Borer, J.S.; Truter, S.; Herrold, E.M.; Falcone, D.J.; Pena, M.; Carter, J.N.; Dumlao, T.F.; Lee, J.A.; Supino, P.G. Myocardial fibrosis in chronic aortic regurgitation: Molecular and cellular responses to volume overload. Circulation 2002, 105, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Stuart, F.S.D.; De Jesus, N.M.; Lindsey, M.L.; Ripplinger, C.M. The crossroads of inflammation, fibrosis, and arrhythmia following myocardial infarction. J. Mol. Cell. Cardiol. 2016, 91, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Talman, V.; Ruskoaho, H. Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 2016, 365, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Shim, W.; Wei, H.; Lim, S.Y.; Liew, R.; Lim, T.S.; Ong, B.H.; Chua, Y.L.; Wong, P. Hydrogen sulphide suppresses human atrial fibroblast proliferation and transformation to myofibroblasts. J. Cell. Mol. Med. 2013, 17, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.L.; Liu, X.H.; Shen, Y.Q.; Wang, N.Z.; Xu, J.; Wu, D.; Xiong, Q.H.; Deng, H.Y.; Huang, G.Y.; Zhu, Y.Z. Inhibition of NADPH oxidase 4-related signaling by sodium hydrosulfide attenuates myocardial fibrotic response. Int. J. Cardiol. 2013, 168, 3770–3778. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Song, X.; Zheng, X.; Yi, J.; Liu, D.; Wang, S.; Chu, C.; Yang, J. Exogenous Hydrogen Sulfide Ameliorates Diabetic Myocardial Fibrosis by Inhibiting Cell Aging Through SIRT6/AMPK Autophagy. Front. Pharmacol. 2020, 11, 1150. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Ling, X.; Xi, W.; Wang, P.; Sun, J.; Yang, Q.; Xiao, J. Exogenous hydrogen sulfide reduces atrial remodeling and atrial fibrillation induced by diabetes mellitus via activation of the PI3K/Akt/eNOS pathway. Mol. Med. Rep. 2020, 22, 1759–1766. [Google Scholar] [CrossRef]
- Liu, M.; Li, Z.; Liang, B.; Li, L.; Liu, S.; Tan, W.; Long, J.; Tang, F.; Chu, C.; Yang, J. Hydrogen sulfide ameliorates rat myocardial fibrosis induced by thyroxine through PI3K/AKT signaling pathway. Endocr. J. 2018, 65, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Ying, R.; Wang, X.Q.; Yang, Y.; Gu, Z.J.; Mai, J.T.; Qiu, Q.; Chen, Y.X.; Wang, J.F. Hydrogen sulfide suppresses endoplasmic reticulum stress-induced endothelial-to-mesenchymal transition through Src pathway. Life Sci. 2016, 144, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Zhu, J.; Xiao, Y.; Huang, Z.; Zhang, Y.; Tang, X.; Xie, L.; Chen, Y.; Shao, Y.; Ferro, A.; et al. Hydrogen Sulfide Donor GYY4137 Protects against Myocardial Fibrosis. Oxid. Med. Cell. Longev. 2015, 2015, 1070. [Google Scholar] [CrossRef]
- Liu, M.; Li, Y.; Liang, B.; Li, Z.; Jiang, Z.; Chu, C.; Yang, J. Hydrogen sulfide attenuates myocardial fibrosis in diabetic rats through the JAK/STAT signaling pathway. Int. J. Mol. Med. 2018, 41, 1867–1876. [Google Scholar] [CrossRef]
- Yang, R.; Jia, Q.; Ma, S.F.; Wang, Y.; Mehmood, S.; Chen, Y. Exogenous H2S mitigates myocardial fibrosis in diabetic rats through suppression of the canonical Wnt pathway. Int. J. Mol. Med. 2019, 44, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Gu, Y.; Zhu, Y.R.; Chao, Y.L.; Kong, X.Q.; Luo, J.; Ren, X.M.; Zuo, G.F.; Zhang, D.M.; Chen, S.L. Exogenous hydrogen sulfide attenuates the development of diabetic cardiomyopathy via the FoxO1 pathway. J. Cell. Physiol. 2018, 233, 9786–9798. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, E.; Sutherland, L.B.; Thatcher, J.E.; DiMaio, J.M.; Naseem, R.H.; Marshall, W.S.; Hill, J.A.; Olson, E.N. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13027–13032. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shen, Z.; Miao, L.; Xin, X.; Lin, S.; Zhu, Y.; Guo, W.; Zhu, Y.Z. miRNA-30 family inhibition protects against cardiac ischemic injury by regulating cystathionine-γ-lyase expression. Antioxid. Redox Signal. 2015, 22, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Xiao, T.; Long, J.; Liu, M.; Li, Z.; Liu, S.; Yang, J. Hydrogen sulfide alleviates myocardial fibrosis in mice with alcoholic cardiomyopathy by downregulating autophagy. Int. J. Mol. Med. 2017, 40, 1781–1791. [Google Scholar] [CrossRef]
- Iismaa, S.E.; Graham, R.M. Dissecting cardiac hypertrophy and signaling pathways: Evidence for an interaction between multifunctional g proteins and prostanoids. Circ. Res. 2003, 92, 1059–1061. [Google Scholar] [CrossRef][Green Version]
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016, 97, 245–262. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Zhang, Y.; Kang, Y.; Wang, H.; Si, M.; Su, L.; Xin, X.; Xue, F.; Hao, F.; et al. Celecoxib prevents pressure overload-induced cardiac hypertrophy and dysfunction by inhibiting inflammation, apoptosis and oxidative stress. J. Cell. Mol. Med. 2016, 20, 116–127. [Google Scholar] [CrossRef]
- Liu, J.; Hao, D.D.; Zhang, J.S.; Zhu, Y.C. Hydrogen sulphide inhibits cardiomyocyte hypertrophy by up-regulating miR-133a. Biochem. Biophys. Res. Commun. 2011, 413, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, Y.Y.; Zhang, Y.Y.; Zhang, Y. Effects of hydrogen sulfide (H2S) on cardiac hypertrophy and miRNA-133a-mediated Ca2+/calcineurin/NFATc4 signal pathway in rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2018, 34, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; Monir, R. Hydrogen sulfide generation protects against renal ischemia/reperfusion-induced cardiac hypertrophy and arrhythmia via amelioration of connexin-43 expression and opening of KATP channels. Bull. Egypt. Soc. Physiol. Sci. 2020, 40, 46–68. [Google Scholar] [CrossRef]
- Kar, S.; Shahshahan, H.R.; Kambis, T.N.; Yadav, S.K.; Li, Z.; Lefer, D.J.; Mishra, P.K. Hydrogen Sulfide Ameliorates Homocysteine-Induced Cardiac Remodeling and Dysfunction. Front. Physiol. 2019, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.A.; Paramesha, B.; Kumar, Y.; Tariq, U.; Arava, S.K.; Banerjee, S.K. Allylmethylsulfide, a Sulfur Compound Derived from Garlic, Attenuates Isoproterenol-Induced Cardiac Hypertrophy in Rats. Oxid. Med. Cell. Longev. 2020, 2020, 6318. [Google Scholar] [CrossRef]
- Lu, F.; Xing, J.; Zhang, X.; Dong, S.; Zhao, Y.; Wang, L.; Li, H.; Yang, F.; Xu, C.; Zhang, W. Exogenous hydrogen sulfide prevents cardiomyocyte apoptosis from cardiac hypertrophy induced by isoproterenol. Mol. Cell. Biochem. 2013, 381, 41–50. [Google Scholar] [CrossRef]
- Snijder, P.M.; Frenay, A.R.; de Boer, R.A.; Pasch, A.; Hillebrands, J.L.; Leuvenink, H.G.; van Goor, H. Exogenous administration of thiosulfate, a donor of hydrogen sulfide, attenuates angiotensin II-induced hypertensive heart disease in rats. Br. J. Pharmacol. 2015, 172, 1494–1504. [Google Scholar] [CrossRef]
- Ahmad, A.; Sattar, M.A.; Rathore, H.A.; Abdulla, M.H.; Khan, S.A.; Azam, M.; Abdullah, N.A.; Johns, E.J. Up Regulation of cystathione γ lyase and Hydrogen Sulphide in the Myocardium Inhibits the Progression of Isoproterenol-Caffeine Induced Left Ventricular Hypertrophy in Wistar Kyoto Rats. PLoS ONE 2016, 11, 137. [Google Scholar] [CrossRef]
- Shimizu, Y.; Nicholson, C.K.; Lambert, J.P.; Barr, L.A.; Kuek, N.; Herszenhaut, D.; Tan, L.; Murohara, T.; Hansen, J.M.; Husain, A.; et al. Sodium Sulfide Attenuates Ischemic-Induced Heart Failure by Enhancing Proteasomal Function in an Nrf2-Dependent Manner. Circulation. Heart Fail. 2016, 9, 2368. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Xiao, Y.; Ma, Y.; Tang, X.; Xie, L.; Liu, J.; Gu, Y.; Yu, Y.; Park, C.M.; Xian, M.; et al. Hydrogen Sulfide Regulates Krüppel-Like Factor 5 Transcription Activity via Specificity Protein 1 S-Sulfhydration at Cys664 to Prevent Myocardial Hypertrophy. J. Am. Heart Assoc. 2016, 5, 4160. [Google Scholar] [CrossRef]
- Meng, G.; Liu, J.; Liu, S.; Song, Q.; Liu, L.; Xie, L.; Han, Y.; Ji, Y. Hydrogen sulfide pretreatment improves mitochondrial function in myocardial hypertrophy via a SIRT3-dependent manner. Br. J. Pharmacol. 2018, 175, 1126–1145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, J.; Chen, Y.; Liu, L.; Xu, M.; Sun, L.; Luo, H.; Wang, Y.; Meng, G. Exogenous Hydrogen Sulfide Supplement Attenuates Isoproterenol-Induced Myocardial Hypertrophy in a Sirtuin 3-Dependent Manner. Oxid. Med. Cell. Longev. 2018, 2018, 6089. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Sikura, K.É.; Potor, L.; Szerafin, T.; Oros, M.; Nagy, P.; Méhes, G.; Hendrik, Z.; Zarjou, A.; Agarwal, A.; Posta, N.; et al. Hydrogen sulfide inhibits calcification of heart valves; implications for calcific aortic valve disease. Br. J. Pharmacol. 2020, 177, 793–809. [Google Scholar] [CrossRef]
- Sikura, K.É.; Combi, Z.; Potor, L.; Szerafin, T.; Hendrik, Z.; Méhes, G.; Gergely, P.; Whiteman, M.; Beke, L.; Fürtös, I.; et al. Hydrogen sulfide inhibits aortic valve calcification in heart via regulating RUNX2 by NF-κB, a link between inflammation and mineralization. J. Adv. Res. 2020. [Google Scholar] [CrossRef]
- Scally, C.; Rudd, A.; Mezincescu, A.; Wilson, H.; Srivanasan, J.; Horgan, G.; Broadhurst, P.; Newby, D.E.; Henning, A.; Dawson, D.K. Persistent Long-Term Structural, Functional, and Metabolic Changes after Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2018, 137, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Khera, R.; McGroary, L.K.; Zahr, F.; Horwitz, P.A.; Girotra, S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am. Heart J. 2016, 172, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Scally, C.; Abbas, H.; Ahearn, T.; Srinivasan, J.; Mezincescu, A.; Rudd, A.; Spath, N.; Yucel-Finn, A.; Yuecel, R.; Oldroyd, K.; et al. Myocardial and Systemic Inflammation in Acute Stress-Induced (Takotsubo) Cardiomyopathy. Circulation 2019, 139, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, T.; Kawabe, T.; Hano, T.; Tsuruo, Y.; Ueda, K.; Ichinose, M.; Kimura, H.; Yoshida, K. Upregulation of heme oxygenase-1 in an animal model of Takotsubo cardiomyopathy. Circ. J. Off. J. Jpn. Circ. Soc. 2009, 73, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jin, S.; Teng, X.; Duan, X.; Chen, Y.; Wu, Y. Hydrogen sulfide attenuates cardiac injury in takotsubo cardiomyopathy by alleviating oxidative stress. Nitric Oxide Biol. Chem. 2017, 67, 10–25. [Google Scholar] [CrossRef]
- Borchert, T.; Hübscher, D.; Guessoum, C.I.; Lam, T.D.; Ghadri, J.R.; Schellinger, I.N.; Tiburcy, M.; Liaw, N.Y.; Li, Y.; Haas, J.; et al. Catecholamine-Dependent β-Adrenergic Signaling in a Pluripotent Stem Cell Model of Takotsubo Cardiomyopathy. J. Am. Coll. Cardiol. 2017, 70, 975–991. [Google Scholar] [CrossRef]
- Anderson, E.J.; Kypson, A.P.; Rodriguez, E.; Anderson, C.A.; Lehr, E.J.; Neufer, P.D. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J. Am. Coll. Cardiol. 2009, 54, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Montaigne, D.; Marechal, X.; Coisne, A.; Debry, N.; Modine, T.; Fayad, G.; Potelle, C.; El Arid, J.-M.; Mouton, S.; Sebti, Y.; et al. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation 2014, 130, 554–564. [Google Scholar] [CrossRef]
- Barr, L.A.; Shimizu, Y.; Lambert, J.P.; Nicholson, C.K.; Calvert, J.W. Hydrogen sulfide attenuates high fat diet-induced cardiac dysfunction via the suppression of endoplasmic reticulum stress. Nitric Oxide Biol. Chem. 2015, 46, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; An, G.; Lu, X. Hydrogen sulfide attenuates the development of diabetic cardiomyopathy. Clin. Sci. 2015, 128, 325–335. [Google Scholar] [CrossRef]
- Li, F.; Luo, J.; Wu, Z.; Xiao, T.; Zeng, O.; Li, L.; Li, Y.; Yang, J. Hydrogen sulfide exhibits cardioprotective effects by decreasing endoplasmic reticulum stress in a diabetic cardiomyopathy rat model. Mol. Med. Rep. 2016, 14, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tian, Z.; Sun, Y.; Lu, C.; Liu, N.; Gao, Z.; Zhang, L.; Dong, S.; Yang, F.; Zhong, X.; et al. Exogenous H2S facilitating ubiquitin aggregates clearance via autophagy attenuates type 2 diabetes-induced cardiomyopathy. Cell Death Dis. 2017, 8, e2992. [Google Scholar] [CrossRef]
- Kar, S.; Shahshahan, H.R.; Hackfort, B.T.; Yadav, S.K.; Yadav, R.; Kambis, T.N.; Lefer, D.J.; Mishra, P.K. Exercise Training Promotes Cardiac Hydrogen Sulfide Biosynthesis and Mitigates Pyroptosis to Prevent High-Fat Diet-Induced Diabetic Cardiomyopathy. Antioxidants. 2019, 8, 638. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, D.; Lu, F.; Peng, S.; Yu, M.; Liu, N.; Sun, Y.; Du, H.; Wang, B.; Chen, J.; et al. Hydrogen sulfide regulates muscle RING finger-1 protein S-sulfhydration at Cys44 to prevent cardiac structural damage in diabetic cardiomyopathy. Br. J. Pharmacol. 2020, 177, 836–856. [Google Scholar] [CrossRef]
- Zhang, M.; Ye, M. Hydrogen Sulfide Attenuates High Glucose-induced Myocardial Injury in Rat Cardiomyocytes by Suppressing Wnt/beta-catenin Pathway. Curr. Med. Sci. 2019, 39, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Sauty, A.; Iarossi, A.S.; Sukhova, G.K.; Neote, K.; Libby, P.; Luster, A.D. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J. Clin. Investig. 1999, 104, 1041–1050. [Google Scholar] [CrossRef]
- Gu, L.; Okada, Y.; Clinton, S.K.; Gerard, C.; Sukhova, G.K.; Libby, P.; Rollins, B.J. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol. Cell 1998, 2, 275–281. [Google Scholar] [CrossRef]
- Clarkson, T.B. Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause 2018, 25, 1262–1274. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mani, S.; Wu, L.; Fu, M.; Shuang, T.; Xu, C.; Wang, R. The interaction of estrogen and CSE/H2S pathway in the development of atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H406–H414. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zheng, F.; Li, S.; Cui, C.; Jiang, S.; Zhang, J.; Cai, J.; Cui, Q.; Yang, J.; Tang, X.; et al. Hydrogen sulfide lowers hyperhomocysteinemia dependent on cystathionine γ lyase S-sulfhydration in ApoE-knockout atherosclerotic mice. Br. J. Pharmacol. 2019, 176, 3180–3192. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Y.; Zhu, N.; Zhao, S.; Fan, J.; Liu, E. Hydrogen sulfide inhibits development of atherosclerosis through up-regulating protein S-nitrosylation. Biomed. Pharmacother. Biomed. Pharmacother. 2016, 83, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Gu, Y.; Wen, M.; Zhao, S.; Wang, W.; Ma, Y.; Meng, G.; Han, Y.; Wang, Y.; Liu, G.; et al. Hydrogen Sulfide Induces Keap1 S-sulfhydration and Suppresses Diabetes-Accelerated Atherosclerosis via Nrf2 Activation. Diabetes 2016, 65, 3171–3184. [Google Scholar] [CrossRef]
- Cheung, S.H.; Lau, J. Hydrogen sulfide mediates athero-protection against oxidative stress via S-sulfhydration. PLoS ONE 2018, 13, 4176. [Google Scholar] [CrossRef]
- Xiong, Q.; Wang, Z.; Yu, Y.; Wen, Y.; Suguro, R.; Mao, Y.; Zhu, Y.Z. Hydrogen sulfide stabilizes atherosclerotic plaques in apolipoprotein E knockout mice. Pharmacol. Res. 2019, 144, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Lin, X.; Xu, W.; Zheng, F.; Cai, J.; Yang, J.; Cui, Q.; Tang, C.; Cai, J.; Xu, G.; et al. Sulfhydrated Sirtuin-1 Increasing Its Deacetylation Activity Is an Essential Epigenetics Mechanism of Anti-Atherogenesis by Hydrogen Sulfide. Antioxid. Redox Signal. 2019, 30, 184–197. [Google Scholar] [CrossRef]
- Lin, F.; Yang, Y.; Wei, S.; Huang, X.; Peng, Z.; Ke, X.; Zeng, Z.; Song, Y. Hydrogen Sulfide Protects Against High Glucose-Induced Human Umbilical Vein Endothelial Cell Injury Through Activating PI3K/Akt/eNOS Pathway. Drug Des. Dev. Ther. 2020, 14, 621–633. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, K.; Xiao, L.; Jin, S.; Dong, J.; Teng, X.; Guo, Q.; Chen, Y.; Wu, Y. Alpha-lipoic acid regulates the autophagy of vascular smooth muscle cells in diabetes by elevating hydrogen sulfide level. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3723–3738. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, S.; Feng, S.; Li, H. CSE/H2S system alleviates uremic accelerated atherosclerosis by regulating TGF-β/Smad3 pathway in 5/6 nephrectomy ApoE-/- mice. BMC Nephrol. 2020, 21, 527. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Lu, X.; Song, J.; Li, H.; Wang, S. Molecular mechanisms of hydrogen sulfide against uremic accelerated atherosclerosis through cPKCβII/Akt signal pathway. BMC Nephrol. 2019, 20, 358. [Google Scholar] [CrossRef]
- Zheng, Q.; Pan, L.; Ji, Y. H 2S protects against diabetes-accelerated atherosclerosis by preventing the activation of NLRP3 inflammasome. J. Biomed. Res. 2019, 34, 94–102. [Google Scholar] [CrossRef]
- Zheng, Y.; Lv, P.; Huang, J.; Ke, J.; Yan, J. GYY4137 exhibits anti-atherosclerosis effect in apolipoprotein E (-/-) mice via PI3K/Akt and TLR4 signalling. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1231–1239. [Google Scholar] [CrossRef]
- Datzmann, T.; Hoffmann, A.; McCook, O.; Merz, T.; Wachter, U.; Preuss, J.; Vettorazzi, S.; Calzia, E.; Gröger, M.; Kohn, F.; et al. Effects of sodium thiosulfate (Na2S2O3) during resuscitation from hemorrhagic shock in swine with preexisting atherosclerosis. Pharmacol. Res. 2020, 151, 4536. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zeng, H.; Gao, L.; Gu, T.; Wang, C.; Zhang, H. Hydrogen Sulfide Attenuates Atherosclerosis in a Partially Ligated Carotid Artery Mouse model via Regulating Angiotensin Converting Enzyme 2 Expression. Front. Physiol. 2017, 8, 782. [Google Scholar] [CrossRef]
- Piper, H.M.; Dorado, G.D.; Ovize, M. A fresh look at reperfusion injury. Cardiovasc. Res. 1998, 38, 291–300. [Google Scholar] [CrossRef]
- Yellon, D.M.; Hausenloy, D.J. Myocardial reperfusion injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef]
- Murphy, E.; Steenbergen, C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 2008, 88, 581–609. [Google Scholar] [CrossRef] [PubMed]
- Sivarajah, A.; McDonald, M.C.; Thiemermann, C. The production of hydrogen sulfide limits myocardial ischemia and reperfusion injury and contributes to the cardioprotective effects of preconditioning with endotoxin, but not ischemia in the rat. Shock 2006, 26, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Li, Z.; Sharp, T.E., 3rd; Polhemus, D.J.; Carnal, J.; Moles, K.H.; Tao, Y.X.; Elrod, J.; Pfeilschifter, J.; Beck, K.F.; et al. Endothelial Cell Cystathionine γ-Lyase Expression Level Modulates Exercise Capacity, Vascular Function, and Myocardial Ischemia Reperfusion Injury. J. Am. Heart Assoc. 2020, 9, 7544. [Google Scholar] [CrossRef] [PubMed]
- Ellmers, L.J.; Templeton, E.M.; Pilbrow, A.P.; Frampton, C.; Ishii, I.; Moore, P.K.; Bhatia, M.; Richards, A.M.; Cameron, V.A. Hydrogen Sulfide Treatment Improves Post-Infarct Remodeling and Long-Term Cardiac Function in CSE Knockout and Wild-Type Mice. Int. J. Mol. Sci. 2020, 21, 4284. [Google Scholar] [CrossRef]
- Elrod, J.W.; Calvert, J.W.; Morrison, J.; Doeller, J.E.; Kraus, D.W.; Tao, L.; Jiao, X.; Scalia, R.; Kiss, L.; Szabo, C.; et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc. Natl. Acad. Sci. USA 2007, 104, 15560–15565. [Google Scholar] [CrossRef]
- Sun, X.; Wang, W.; Dai, J.; Jin, S.; Huang, J.; Guo, C.; Wang, C.; Pang, L.; Wang, Y. A Long-Term and Slow-Releasing Hydrogen Sulfide Donor Protects against Myocardial Ischemia/Reperfusion Injury. Sci. Rep. 2017, 7, 3541. [Google Scholar] [CrossRef]
- Li, S.; Xiao, F.Y.; Shan, P.R.; Su, L.; Chen, D.L.; Ding, J.Y.; Wang, Z.Q. Overexpression of microRNA-133a inhibits ischemia-reperfusion-induced cardiomyocyte apoptosis by targeting DAPK2. J. Hum. Genet. 2015, 60, 709–716. [Google Scholar] [CrossRef]
- Wang, W.; Liu, H.; Lu, Y.; Wang, X.; Zhang, B.; Cong, S.; Zhao, Y.; Ji, M.; Tao, H.; Wei, L. Controlled-releasing hydrogen sulfide donor based on dual-modal iron oxide nanoparticles protects myocardial tissue from ischemia-reperfusion injury. Int. J. Nanomed. 2019, 14, 875–888. [Google Scholar] [CrossRef]
- Lambert, J.P.; Nicholson, C.K.; Amin, H.; Amin, S.; Calvert, J.W. Hydrogen sulfide provides cardioprotection against myocardial/ischemia reperfusion injury in the diabetic state through the activation of the RISK pathway. Med. Gas Res. 2014, 4, 20. [Google Scholar] [CrossRef]
- Qiu, Y.; Wu, Y.; Meng, M.; Luo, M.; Zhao, H.; Sun, H.; Gao, S. GYY4137 protects against myocardial ischemia/reperfusion injury via activation of the PHLPP-1/Akt/Nrf2 signaling pathway in diabetic mice. J. Surg. Res. 2018, 225, 29–39. [Google Scholar] [CrossRef]
- Jeddi, S.; Gheibi, S.; Kashfi, K.; Carlström, M.; Ghasemi, A. Protective effect of intermediate doses of hydrogen sulfide against myocardial ischemia-reperfusion injury in obese type 2 diabetic rats. Life Sci. 2020, 256, 7855. [Google Scholar] [CrossRef]
- Yu, L.; Li, S.; Tang, X.; Li, Z.; Zhang, J.; Xue, X.; Han, J.; Liu, Y.; Zhang, Y.; Zhang, Y.; et al. Diallyl trisulfide ameliorates myocardial ischemia-reperfusion injury by reducing oxidative stress and endoplasmic reticulum stress-mediated apoptosis in type 1 diabetic rats: Role of SIRT1 activation. Apoptosis Int. J. Program. Cell Death 2017, 22, 942–954. [Google Scholar] [CrossRef]
- Xie, H.; Xu, Q.; Jia, J.; Ao, G.; Sun, Y.; Hu, L.; Alkayed, N.J.; Wang, C.; Cheng, J. Hydrogen sulfide protects against myocardial ischemia and reperfusion injury by activating AMP-activated protein kinase to restore autophagic flux. Biochem. Biophys. Res. Commun. 2015, 458, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.; Xiao, F.Y. Effect of hydrogen sulfide on cardiomyocyte apoptosis in rats with myocardial ischemia-reperfusion injury via the JNK signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2054–2061. [Google Scholar] [CrossRef]
- Hu, X.; Liu, B.; Wu, P.; Lang, Y.; Li, T. LncRNA Oprm1 overexpression attenuates myocardial ischemia/reperfusion injury by increasing endogenous hydrogen sulfide via Oprm1/miR-30b-5p/CSE axis. Life Sci. 2020, 254, 7699. [Google Scholar] [CrossRef] [PubMed]
- Ge, N.; Liu, C.; Li, G.; Xie, L.; Zhang, Q.; Li, L.; Hao, N.; Zhang, J. Hydrosulfide attenuates acute myocardial ischemic injury through the glycogen synthase kinase-3β/β-catenin signaling pathway. Int. J. Mol. Med. 2016, 37, 1281–1289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Ye, J.; Nassab, H.N.; Flores, A.; Kalashnikova, I.; Paluri, S.L.; Lotfi, M.; Leeper, N.J.; Smith, B.R. Macrophage-targeted single walled carbon nanotubes stimulate phagocytosis via pH-dependent drug release. Nano Res. 2021, 14, 762–769. [Google Scholar] [CrossRef]

| H2S Donors | Cysteine Residues | Resulting Effects | References |
|---|---|---|---|
| NaHS | Cys-269 of c-jun | Induces anti-inflammatory properties | [177] |
| GYY4137 | Cys-664 of specificity protein-1 | Reduces myocardial hypertrophy | [170] |
| NaHS | Cys-44 of muscle ring finger-1 | Improves cardiac function | [189] |
| NaHS and GYY4137 | Cys-252, -225, -307, and -310 of CSE | Induces anti-atherosclerotic properties | [195] |
| NaHS | S-nitrosylation of aortic VSMC proteins | Reduces the progression of atherosclerosis | [196] |
| GYY4137 | Cys-151 of Keap-1 | Promotes antioxidative properties | [197] |
| GYY4137 | S-sulfuration of multiple proteins including cathepsin B, fatty-acid binding protein, and glutathione peroxidase 1 | Reduces pro-oxidative events | [198] |
| NaHS and GYY4137 | S-sulfuration at the two Zn2+ domains of the SIRT-1 | Reduces atherosclerotic plaques | [200] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-Z.; Ngowi, E.E.; Wang, D.; Qi, H.-W.; Jing, M.-R.; Zhang, Y.-X.; Cai, C.-B.; He, Q.-L.; Khattak, S.; Khan, N.H.; et al. The Potential of Hydrogen Sulfide Donors in Treating Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 2194. https://doi.org/10.3390/ijms22042194
Wang Y-Z, Ngowi EE, Wang D, Qi H-W, Jing M-R, Zhang Y-X, Cai C-B, He Q-L, Khattak S, Khan NH, et al. The Potential of Hydrogen Sulfide Donors in Treating Cardiovascular Diseases. International Journal of Molecular Sciences. 2021; 22(4):2194. https://doi.org/10.3390/ijms22042194
Chicago/Turabian StyleWang, Yi-Zhen, Ebenezeri Erasto Ngowi, Di Wang, Hui-Wen Qi, Mi-Rong Jing, Yan-Xia Zhang, Chun-Bo Cai, Qing-Lin He, Saadullah Khattak, Nazeer Hussain Khan, and et al. 2021. "The Potential of Hydrogen Sulfide Donors in Treating Cardiovascular Diseases" International Journal of Molecular Sciences 22, no. 4: 2194. https://doi.org/10.3390/ijms22042194
APA StyleWang, Y.-Z., Ngowi, E. E., Wang, D., Qi, H.-W., Jing, M.-R., Zhang, Y.-X., Cai, C.-B., He, Q.-L., Khattak, S., Khan, N. H., Jiang, Q.-Y., Ji, X.-Y., & Wu, D.-D. (2021). The Potential of Hydrogen Sulfide Donors in Treating Cardiovascular Diseases. International Journal of Molecular Sciences, 22(4), 2194. https://doi.org/10.3390/ijms22042194







