Extracellular Vesicles in Allergic Rhinitis and Asthma and Laboratory Possibilities for Their Assessment
Abstract
1. Introduction
2. Asthma, Allergic Rhinitis, and EVs
2.1. Asthma and Allergic Rhinitis
2.2. EVs in General
2.3. EVs from Epithelial Cells
2.4. EVs from Immune Cells
2.5. Potential Diagnostic and Therapeutic Use of EVs
3. Laboratory Techniques
4. Collection, Preparation, and Storage of Material for EVs Assessment in Asthma and Allergic Rhinitis
5. Methods for the Detection of EVs from Specific Tissues or Cells
- Clinical flow cytometers, used primarily to perform tests on white and red blood cells, but with proper preparation of the analyzer and setting the reading parameters to enable the evaluation of EVs.
- Nanoparticles flow cytometers—specially designed for the detection and identification of small particles, EVs below 100 nm, but not widely used in laboratories
- Imaging flow cytometers.
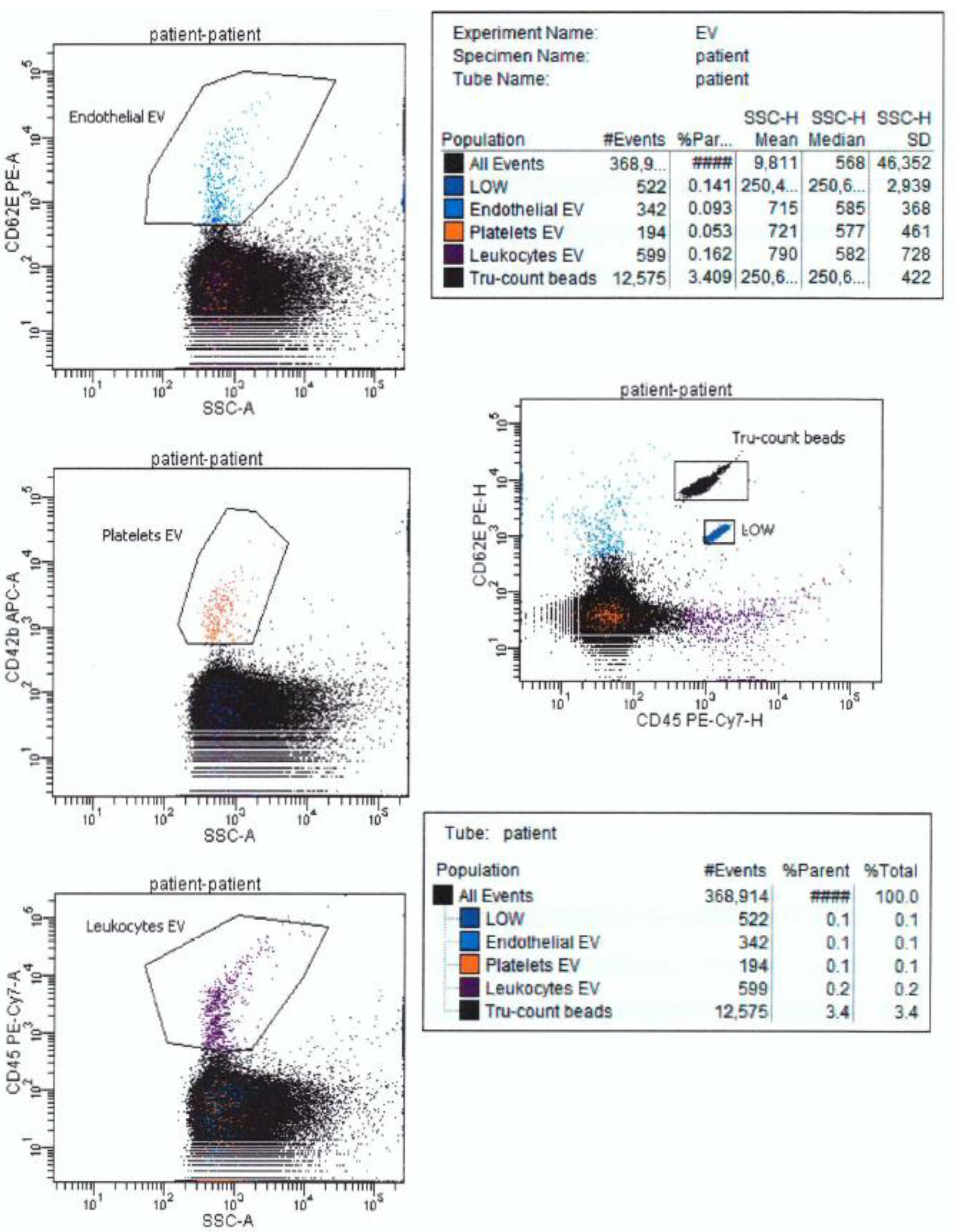
5.1. Clinical Flow Cytometers
5.2. Nanoparticles and Imaging Flow Cytometers
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Witwer, K.W.; Théry, C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J. Extracell. Vesicles 2019, 8, 1648167. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Seeger, R.C.; Fabbri, M.; Wang, L.; Wayne, A.S.; Jong, A.Y. Biological roles and potential applications of immune cell-derived extracellular vesicles. J. Extracell. Vesicles 2017, 6, 1400370. [Google Scholar] [CrossRef]
- Mohan, A.; Agarwal, S.; Clauss, M.; Britt, N.S.; Dhillon, N. Extracellular vesicles: Novel communicators in lung diseases. Respir. Res. 2020, 21, 175. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yin, Z.; Yang, L.; Fan, J.; Xu, J.; Jin, Y.; Yu, J.; Zhang, D.; Yang, G. Smoking Induced Extracellular Vesicles Release and Their Distinct Properties in Non-Small Cell Lung Cancer. J. Cancer 2019, 10, 3435–3443. [Google Scholar] [CrossRef]
- Sangaphunchai, P.; Todd, I.; Fairclough, L.C. Extracellular vesicles and asthma: A review of the literature. Clin. Exp. Allergy 2020, 50, 291–307. [Google Scholar] [CrossRef]
- Desrochers, L.M.; Antonyak, M.A.; Cerione, R.A. Extracellular Vesicles: Satellites of Information Transfer in Cancer and Stem Cell Biology. Dev. Cell 2016, 37, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Mandlik, D.S.; Mandlik, S.K. New perspectives in bronchial asthma: Pathological, immunological alterations, biological targets, and pharmacotherapy. Immunopharmacol. Immunotoxicol. 2020, 42, 521–544. [Google Scholar] [CrossRef] [PubMed]
- Calvén, J.; Ax, E.; Rådinger, M. The Airway Epithelium-A Central Player in Asthma Pathogenesis. Int. J. Mol. Sci. 2020, 21, 8907. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Jensen, S.S.; Lim, J.W. Proteomic profiling of exosomes: Current perspectives. Proteomics 2008, 8, 4083–4099. [Google Scholar] [CrossRef]
- Bartel, S.; Deshane, J.; Wilkinson, T.; Gabrielsson, S. Extracellular Vesicles as Mediators of Cellular Cross Talk in the Lung Microenvironment. Front. Med. 2020, 7, 326. [Google Scholar] [CrossRef]
- Gelfand, E.W.; Joetham, A.; Wang, M.; Takeda, K.; Schedel, M. Spectrum of T-lymphocyte activities regulating allergic lung inflammation. Immunol. Rev. 2017, 278, 63–86. [Google Scholar] [CrossRef]
- Levänen, B.; Bhakta, N.R.; Torregrosa Paredes, P.; Barbeau, R.; Hiltbrunner, S.; Pollack, J.L.; Skold, M.; Svartengren, M.; Grunewald, J.; Gabrielsson, S.; et al. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J. Allergy Clin. Immunol. 2013, 131, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Bartel, S.; La Grutta, S.; Cilluffo, G.; Perconti, G.; Bongiovanni, A.; Giallongo, A.; Behrends, J.; Kruppa, J.; Hermann, S.; Chiang, D.; et al. Human airway epithelial extracellular vesicle miRNA signature is altered upon asthma development. Allergy 2020, 75, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Stumpfova, Z.; Hezova, R.; Meli, A.C.; Slaby, O.; Michalek, J. MicroRNA profiling of activated and tolerogenic human dendritic cells. Mediat. Inflamm. 2014, 2014, 259689. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Ahmad, T.; Sharma, A.; Mabalirajan, U.; Kulshreshtha, A.; Agrawal, A.; Ghosh, B. Let-7 microRNA-mediated regulation of IL-13 and allergic airway inflammation. J. Allergy Clin. Immunol. 2011, 128, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.X.; Munitz, A.; Rothenberg, M.E. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J. Immunol. 2009, 182, 4994–5002. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Tao, Y.-W.; Gao, S.; Li, P.; Zheng, J.-M.; Zhang, S.-E.; Liang, J.; Zhang, Y. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome mediated paracrine miR-34a-5p. EBioMedicine 2018, 36, 209–220. [Google Scholar] [CrossRef]
- Fujita, Y.; Kosaka, N.; Araya, J.; Kuwano, K.; Ochiya, T. Extracellular vesicles in lung microenvironment and pathogenesis. Trends Mol. Med. 2015, 21, 533–542. [Google Scholar] [CrossRef]
- Hashimi, S.T.; Fulcher, J.A.; Chang, M.H.; Gov, L.; Wang, S.; Lee, B. MicroRNA profiling identifies miR-34a and miR-21 and their target genes JAG1 and WNT1 in the coordinate regulation of dendritic cell differentiation. Blood 2009, 114, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Vallhov, H.; Gutzeit, C.; Hultenby, K.; valenta, R.; Groenlund, H.; Scheynius, A. Dendritic cell-derived exosomes carry the major cat allergen Fel d 1 and induce an allergic immune response. Allergy 2015, 70, 1651–1655. [Google Scholar] [CrossRef]
- Huang, A.; Yang, Y.I.; Chen, S.I.; Xia, F.; Sun, D.; Fang, D.; Xiong, S.; Jin, L.; Zhang, J. MiR-34a promotes DCs d evelopment and inhibits their function on T cell activation by targeting WNT1. Oncotarget 2017, 8, 17191–17201. [Google Scholar] [CrossRef]
- Skokos, D.; Le Panse, S.; Villa, I.; Pousselle, J.S.; Peronet, R.; Namane, A.; David, B.; Mecheri, S. Nonspecific B and T cell-stimulatory activity mediated by mast cells is associated with exosomes. Int. Arch. Allergy Immunol. 2001, 124, 133–136. [Google Scholar] [CrossRef]
- Xie, G.; Yang, H.; Peng, X.; Lin, L.; Wang, J.; Lin, K.; Cui, Z.; Li, J.; Xiao, H.; Linag, Y.; et al. Mast cell exosomes can suppress allergic reactions by binding to IgE. J. Allergy Clin. Immunol. 2018, 141, 788–791. [Google Scholar] [CrossRef]
- Eldh, M.; Ekström, K.; Valadi, H.; Sjostrand, M.; Olsson, B.; Jernas, M.; Lotvall, J. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS ONE 2010, 5, e15353. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Konno, S.; Makita, H.; Shimizu, K.; Kimura, H.; Kimura, H.; Nishimura, M. Altered circulating exosomal RNA profiles detected by next-generation sequencing in patients with s evere asthma. Eur. Respiratory Soc. 2016, 48, PA3410. [Google Scholar]
- Bahmer, T.; Krauss-Etschmann, S.; Buschmann, D.; Behrends, J.; Watz, H.; Kirsten, A.-M.; Pedersen, F.; Waschki, B.; Fuchs, O.; Pfaffl, M.W.; et al. RNA-seq-based profiling of extracellular vesicles in plasma reveals a potential role of miR-122-5p in asthma. Allergy 2020, 76, 366–371. [Google Scholar] [CrossRef]
- Worthington, E.N.; Hagood, J.S. Therapeutic Use of Extracellular Vesicles for Acute and Chronic Lung Disease. Int. J. Mol. Sci. 2020, 21, 2318. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.F.; Borg, Z.D.; Goodwin, M.; Sokocevic, D.; Wagner, D.E.; Coffey, A.; Antunes, M.; Robinson, K.L.; Mitsialis, S.A.; Kourembanas, S.; et al. Systemic Administration of Human Bone Marrow-Derived Mesenchymal Stromal Cell Extracellular Vesicles Ameliorates Aspergillus Hyphal Extract-Induced Allergic Airway Inflammation in Immunocompetent Mice. Stem. Cells Transl. Med. 2015, 4, 1302–1316. [Google Scholar] [CrossRef] [PubMed]
- de Castro, L.L.; Xisto, D.G.; Kitoko, J.Z.; Cruz, F.F.; Olsen, P.C.; Redondo, P.A.G.; Ferreira, T.P.T.; Weiss, D.J.; Martins, M.A.; Morales, M.M.; et al. Human adipose tissue mesenchymal stromal cells and their extracellular vesicles act differentially on lung mechanics and inflammation in experimental allergic asthma. Stem. Cell Res. Ther. 2017, 8, 151. [Google Scholar] [CrossRef]
- Yuana, Y.; Bertina, R.M.; Osanto, S. Pre-analytical and analytical issues in the analysis of blood microparticles. Thromb. Haemost. 2011, 105, 396–408. [Google Scholar] [CrossRef]
- Panagopoulou, M.S.; Wark, A.W.; Birch, D.J.S.; Gregory, C.D. Phenotypic analysis of extracellular vesicles: A review on the applications of fluorescence. J. Extracell. Vesicles 2020, 9, 1710020. [Google Scholar] [CrossRef] [PubMed]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.D.D.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F. Methodological guidelines to study extracellular vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef] [PubMed]
- Yuana, Y.; Böing, A.N.; Grootemaat, A.E.; van der Pol, E.; Hau, C.M.; Cizmar, P.; Buhr, E.; Sturk, A.; Nieuwland, R. Handling and storage of human body fluids for analysis of extracellular vesicles. Extracell. Vesicles 2015, 4, 29260. [Google Scholar] [CrossRef]
- Lotvall, J.; Hill, A.F.; Hochberg, F.; Buzas, E.L.; Di Vizio, D.; Gardiner, C.; Gho, Y.S.; Kurochkin, I.V.; Mathivanan, S.; Quesenberry, P.; et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the international society for extracellular vesicles. J. Extracell. Vesicles 2014, 3, 26913. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Andreson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 [MISEV2018]: A position statement of the International Society for Extracellular Vesicles and update of the MIS ev2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Lässer, C.; O’Neil, S.E.; Shelke, G.V.; Sihlbom, C.; Hansson, S.F.; Gho, Y.S.; Lundback, B.; Lotvall, J. Exosomes in the nose induce immune cell trafficking and harbour an altered protein cargo in chronic airway inflammation. J. Transl. Med. 2016, 14, 181. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Yadav, A.K.; Chakraborty, S.; Kabra, S.K.; Lodha, R.; Kumar, M.; Kulshreshtha, A.; Sethi, T.; Pandey, R.; Malik, G.; et al. Exosome-enclosed microRNAs in exhaled breath hold potential for biomarker discovery in patients with pulmonary diseases. J. Allergy Clin. Immunol. 2013, 132, 219–222. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biologicalfluids. Curr. Protoc. Cell Biol. 2006, 3, 3–22. [Google Scholar]
- Héliot, A.; Landkocz, Y.; Roy Saint-Georges, F.; Gosset, P.; Billet, S.; Shirali, P.; Courcot, D.; Martin, P.J. Smoker extracellular vesicles influence status of human bronchial epithelial cells. Int. J. Hyg. Environ. Heal. 2017, 220, 445–454. [Google Scholar] [CrossRef]
- Torregrosa Paredes, P.; Esser, J.; Admyre, C.; Nord, M.; Rahman, Q.K.; Lukic, A.; Radmark, O.; Groenneberg, R.; Grunewald, J.; Eklund, A.; et al. Bronchoalveolar lavage fluid exosomes contribute to cytokine and leukotriene production in allergic asthma. Allergy 2012, 67, 911–919. [Google Scholar] [CrossRef]
- Qazi, K.R.; Torregrosa Paredes, P.; Dahlberg, B.; Grunewald, J.; Eklund, A.; Gabrielsson, S. Proinflammatory exosomes in bronchoalveolar lavage fluid of patients with sarcoidosis. Thorax 2010, 65, 1016–1024. [Google Scholar] [CrossRef]
- Hough, K.P.; Wilson, L.S.; Trevor, J.L.; Strenkowski, J.G.; Maina, N.; Kim, Y.-I.; Spell, M.L.; Wang, Y.; Chanda, D.; Rodriguez Dager, J.; et al. Unique Lipid Signatures of Extracellular Vesicles from the Airways of Asthmatics. Sci. Rep. 2018, 8, 10340. [Google Scholar] [CrossRef]
- Kuiper, M.; van de Nes, A.; Nieuwland, R.; Varga, Z.; van der Pol, E. Reliable measurements of extracellular vesicles by clinical flow cytometry. Am. J. Reprod. Immunol. 2021, 85, e13350. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P. Flow cytometry of extracellular vesicles: Potential, pitfalls, and prospects. Curr. Protoc. Cytom. 2015, 73, 13–14. [Google Scholar] [CrossRef]
- Cossarizza, A.; Chang, H.-D.; Radbruch, A.; Acs, A.; Adam, D.; Adam-Klages, S.; Agace, W.W.; Aghaeepour, N.; Akdis, M.; Allez, M.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies (second edition). Eur. J. Immunol. 2019, 49, 1457–1973. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.M. Practical Flow Cytometry, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Adan, A.; Alizada, G.; Kiraz, Y.; Baran, Y.; Nalbant, A. Flow cytometry: Basic principles and applications. Crit. Rev. Biotechnol. 2017, 37, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Van der Pol, E.; Pálmai, M.; Garcia-Diez, R.; Gollwitzer, C.; Krumrey, M.; Frajkin, J.-L.; Gasecka, A.; Hajji, N.; van Leeuwen, T.G.; et al. Hollow organosilica beads as reference particles for optical detection of extracellular vesicles. J. Thromb. Haemost. 2018, 16, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Horak, P.; Wilkinson, J.S.; Ford, V.J.; Jones, J.C.; Smith, D.; Holloway, J.A.; Englyst, N.A. FCMPASS software aids extracellular vesicle light scatter standardization. Cytom. Part A 2019, 97, 569–581. [Google Scholar] [CrossRef]
- van der Pol, E.; Sturk, A.; van Leeuwen, T.; Nieuwland, R.; Coumans, F. ISTH-SSC-VB Working Group Standardization of extracellular vesicle measurements by flow cytometry through vesicle diameter approximation. J. Thromb. Haemost. 2018, 16, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Van Der Pol, E.; Arkesteijn, G.J.A.; Bremer, M.; Brisson, A.; Coumans, F.; Dignat-George, F.; Doggan, E.; Ghiran, I.; Giebel, B.; et al. MIFlowCyt- ev: A framework for standardized reporting of extracellular vesicle flow cytometry experiments. J. Extracell. Vesicles 2020, 9, 1713526. [Google Scholar] [CrossRef]
- Welsh, J.A.; Tang, V.A.; van der Pol, E.; Gorgens, A. MIFlowCyt-EV: The Next Chapter in the Reporting and Reliability of Single Extracellular Vesicle Flow Cytometry Experiments. Cytom. Part A 2020. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Simeone, P.; Celia, C.; Bologna, G.; Ercolino, E.; Pierdomenico, L.; Cilurzo, F.; Grande, R.; Diomede, F.; Vespa, S.; Canonico, B.; et al. Diameters and Fluorescence Calibration for Extracellular Vesicle Analyses by Flow Cytometry. Int. J. Mol. Sci. 2020, 21, 7885. [Google Scholar] [CrossRef] [PubMed]
- Oesterreicher, J.; Pultar, M.; Schneider, J.; Muehleder, S.; Zipperle, J.; Grillari, J.; Holnthoner, W. Fluorescence-Based Nanoparticle Tracking Analysis and Flow Cytometry for Characterization of Endothelial Extracellular Vesicle Release. Int. J. Mol. Sci. 2020, 21, 9278. [Google Scholar] [CrossRef] [PubMed]
- Diehl, P.; Aleker, M.; Helbing, T.; Sossong, V.; Germann, M.; Sorichter, S.; Bode, C.; Mozer, M. Incerased platelet, leukocyte and endothelial microparticles predict enhanced cpagulation and vascular inflammation in pulmonary hypertension. J. Thromb. Thrombolysis 2011, 31, 173–179. [Google Scholar] [CrossRef]
- Nanou, A.; Zeune, L.L.; Terstappen, L.W.M.M. Leukocyte-Derived Extracellular Vesicles in Blood with and without EpCAM Enrichment. Cells 2019, 8, 937. [Google Scholar] [CrossRef]
- Italiano, J.E.; Mairuhu, A.T.A., Jr.; Flaumenhaft, R. Clinical Relevance of Microparticles from Platelets and Megacaryocytes. Curr. Opin. Hematol. 2010, 17, 578–584. [Google Scholar] [CrossRef]
- Yang, K.S.; Lin, H.Y.; Curley, C.; Welch, M.W.; Wolpin, B.M.; Lee, H.; Weissleder, R.; Im, H.; Castro, C.M. Bead-Based Extracellular Vesicle Analysis Using Flow Cytometry. Adv. Biosyst. 2020, 4, e2000203. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Bostancioglu, R.B.; Welsh, J.A.; Zickler, A.M.; Murke, F.; Corso, G.; Felldin, U.; Hagey, D.W.; Evertsson, B.; Liang, X.-M.; et al. Systematic methodological evaluation of a multiplex bead-based flow cytometry assay for detection of extracellular vesicle surface signatures. Front. Immunol. 2018, 9, 1326. [Google Scholar] [CrossRef]
- Stoner, S.A.; Duggan, E.; Condello, D.; Guerrero, A.; Turk, J.R.; Narayanan, P.K.; Nolan, J.P. High sensitivity flow cytometry of membrane vesicles. Cytom. A 2016, 89, 196–206. [Google Scholar] [CrossRef]
- Lannigan, J.; Erdbruegger, U. Imaging flow cytometry for the characterization of extracellular vesicles. Methods 2017, 112, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.M.; Banyard, A.; Smith, C.; Mironov, A.; McCabe, M.G. Large Extracellular Vesicles Can be Characterised by Multiplex Labelling Using Imaging Flow Cytometry. Int. J. Mol. Sci. 2020, 21, 8723. [Google Scholar] [CrossRef] [PubMed]
- Mykhailova, O.; Seghatchian, J.; Acker, J.P. Assessment of extracellular vesicles using IFC for application in transfusion medicine. Transfus. Apher. Sci. 2020, 59, 102942. [Google Scholar] [CrossRef]
- Erdbrügger, U.; Rudy, C.K.E.; Etter, M.; Dryden, K.A.; Yeager, M.; Klibanov, A.L.; Lanningan, L. Imaging flow cytometry elucidates limitations of microparticle analysis by conventional flow cytometry. Cytom. Part A 2014, 85, 756–770. [Google Scholar]


| Operation Steps | Thery et al., 2006 | Modifications | Purpose of Action |
|---|---|---|---|
| Step 1 | Viscous body fluids Dilute fluid with an equal volume of PBS | BALF Centrifugation: 300–500× g, 10 min, at 4 °C | To remove airway cells (BALF) |
| Step 2 | Centrifugation: 2000× g, 30 min, at 4 °C | Transfer supernatant, remove pellet centrifugation: 2000–3000× g, 10 min, at 4 °C | To remove dead cells and large cellular debris |
| Step 3 | Transfer supernatant, remove pellet centrifugation: 12,000× g, 45 min, at 4 °C | Transfer supernatant, remove pellet centrifugation: 10,000–16,500× g, 30 min, at 4 °C Filter supernatant (0.22 µm) | Again, to remove dead cells and cellular debris |
| Step 4 | Transfer supernatant, centrifugation: 110,000× g, 120 min, at 4 °C | Transfer supernatant centrifugation: 100,000–140,000× g, 70–130 min, at 4 °C | To have EVs in pellet |
| Step 5 | Discard supernatant, Resuspend pellet in 1 mL PBS Filter the suspension (0.22 µm), centrifugation of the filtrate: 110,000× g, 70 min, 4 °C. Discard supernatant, wash pellet in PBS, centrifugation: 110,000× g, 70 min, 4 °C. Pellet resuspended in PBS, stored at −80 °C | Discard supernatant, wash pellet in PBS, centrifugation: 100,000–110,000× g, 70 min, at 4 °C. Pellet resuspended in PBS, stored at −80 °C | To remove any contaminating proteins and to have EVs in the pellet |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demkow, U.; Stelmaszczyk-Emmel, A. Extracellular Vesicles in Allergic Rhinitis and Asthma and Laboratory Possibilities for Their Assessment. Int. J. Mol. Sci. 2021, 22, 2273. https://doi.org/10.3390/ijms22052273
Demkow U, Stelmaszczyk-Emmel A. Extracellular Vesicles in Allergic Rhinitis and Asthma and Laboratory Possibilities for Their Assessment. International Journal of Molecular Sciences. 2021; 22(5):2273. https://doi.org/10.3390/ijms22052273
Chicago/Turabian StyleDemkow, Urszula, and Anna Stelmaszczyk-Emmel. 2021. "Extracellular Vesicles in Allergic Rhinitis and Asthma and Laboratory Possibilities for Their Assessment" International Journal of Molecular Sciences 22, no. 5: 2273. https://doi.org/10.3390/ijms22052273
APA StyleDemkow, U., & Stelmaszczyk-Emmel, A. (2021). Extracellular Vesicles in Allergic Rhinitis and Asthma and Laboratory Possibilities for Their Assessment. International Journal of Molecular Sciences, 22(5), 2273. https://doi.org/10.3390/ijms22052273






