Role of Polymeric Immunoglobulin Receptor in IgA and IgM Transcytosis
Abstract
:1. Introduction
2. Polymeric Immunoglobulin Receptor (pIgR)
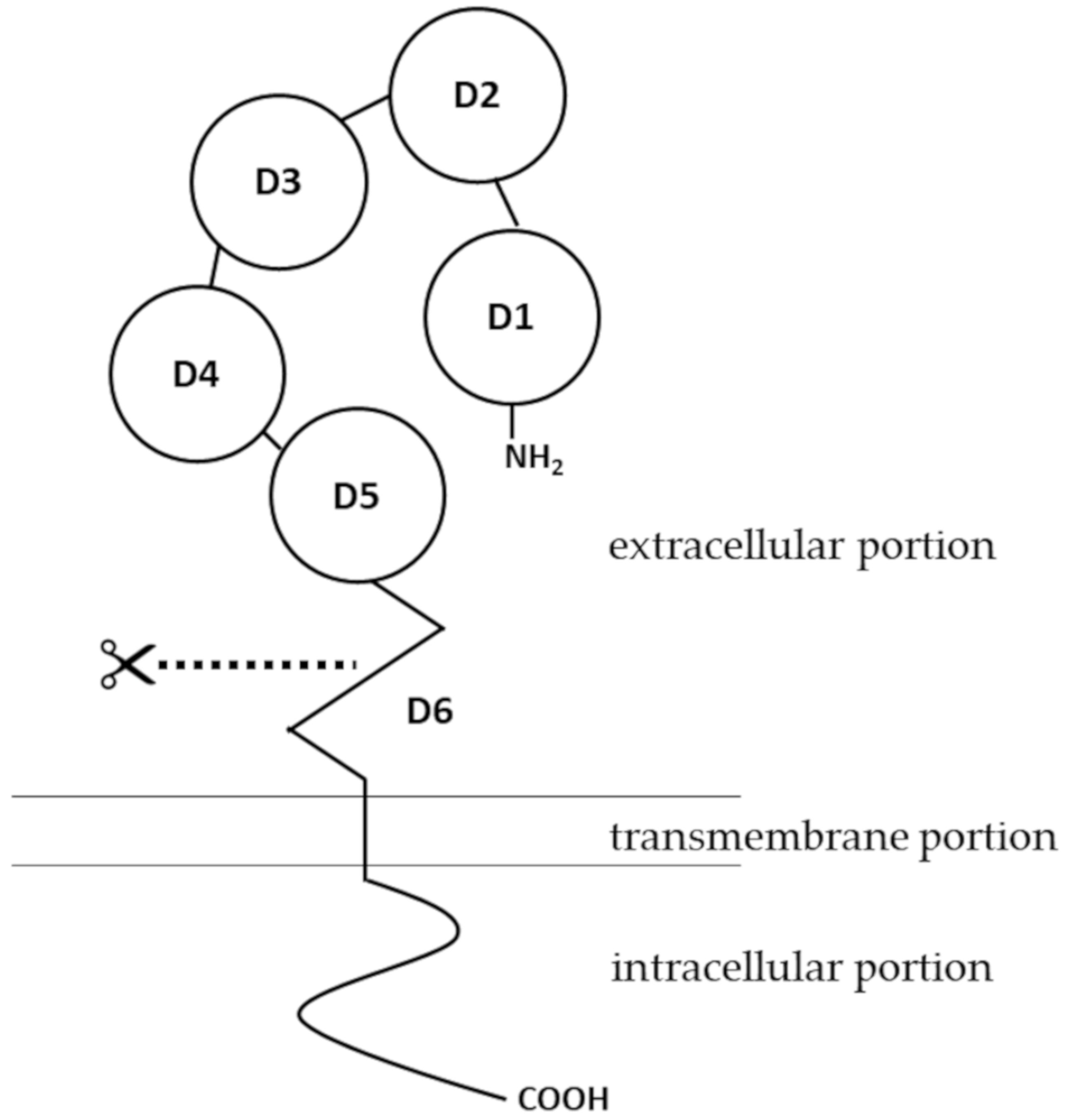
2.1. Structure and Expression of pIgR
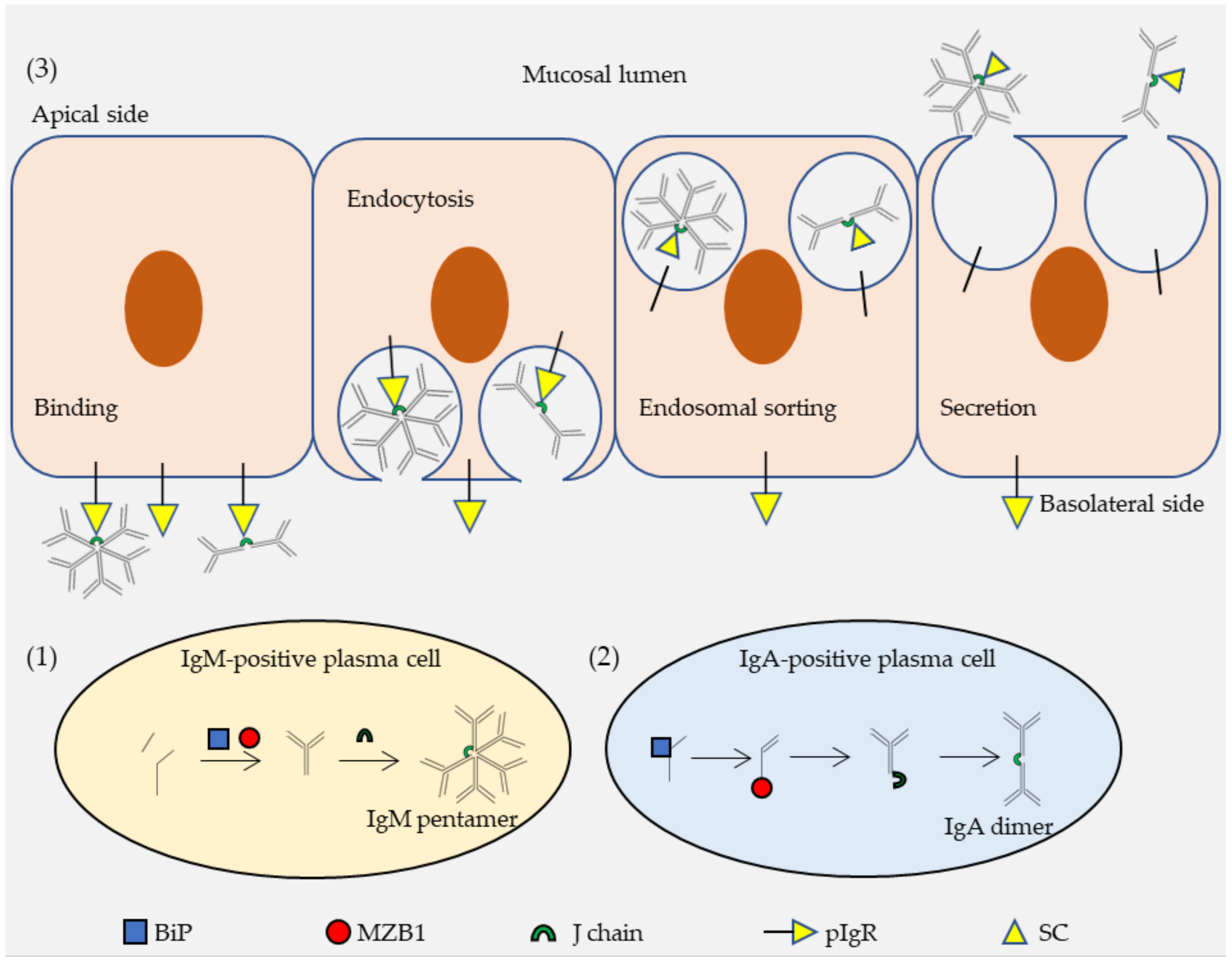
2.2. Functions of pIgR in Transcytosis of IgA and IgM
2.3. Consequences of pIgR Deficiency
3. Joining Chain (J Chain)
3.1. Structure and Expression of J Chain
3.2. J Chain in IgA Polymerization, Binding to pIgR and Function
3.3. J Chain in IgM Polymerization, Binding to pIgR and Function
4. Marginal Zone B and B-1 Cell-Specific Protein (MZB1)
4.1. Early Studies on MZB1 Revealed Its Role in Assembly of IgM
4.2. Functions of MZB1 in IgA Polymerization
4.3. Implications of MZB1 in Human Diseases
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Kaetzel, C.S. The Polymeric Immunoglobulin Receptor: Bridging Innate and Adaptive Immune Responses at Mucosal Surfaces. Immunol. Rev. 2005, 206, 83–99. [Google Scholar] [CrossRef]
- Perez-Lopez, A.; Behnsen, J.; Nuccio, S.-P.; Raffatellu, M. Mucosal Immunity to Pathogenic Intestinal Bacteria. Nat. Rev. Immunol. 2016, 16, 135–148. [Google Scholar] [CrossRef]
- Gutzeit, C.; Chen, K.; Cerutti, A. The Enigmatic Function of IgD: Some Answers at Last. Eur. J. Immunol. 2018, 48, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, B.; Tomela, K.; Olejnik-Schmidt, A.; Mackiewicz, A.; Schmidt, M. Secretory IgA in Intestinal Mucosal Secretions as an Adaptive Barrier against Microbial Cells. Int. J. Mol. Sci. 2020, 21, 9254. [Google Scholar] [CrossRef] [PubMed]
- Van Anken, E.; Pena, F.; Hafkemeijer, N.; Christis, C.; Romijn, E.P.; Grauschopf, U.; Oorschot, V.M.J.; Pertel, T.; Engels, S.; Ora, A.; et al. Efficient IgM Assembly and Secretion Require the Plasma Cell Induced Endoplasmic Reticulum Protein PERp1. Proc. Natl. Acad. Sci. USA 2009, 106, 17019–17024. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, Y.; Meunier, L.; Hendershot, L.M. PERp1 Is Significantly Up-Regulated during Plasma Cell Differentiation and Contributes to the Oxidative Folding of Immunoglobulin. Proc. Natl. Acad. Sci. USA 2009, 106, 17013–17018. [Google Scholar] [CrossRef] [Green Version]
- Flach, H.; Rosenbaum, M.; Duchniewicz, M.; Kim, S.; Zhang, S.L.; Cahalan, M.D.; Mittler, G.; Grosschedl, R. Mzb1 Protein Regulates Calcium Homeostasis, Antibody Secretion, and Integrin Activation in Innate-like B Cells. Immunity 2010, 33, 723–735. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Liu, C.; Cao, G.; Gao, H.; Zhang, Z. Expression of Polymeric Immunoglobulin Receptor and Its Biological Function in Endometrial Adenocarcinoma. J. Cancer Res. Ther. 2019, 15, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Mostov, K.E.; Friedlander, M.; Blobel, G. The Receptor for Transepithelial Transport of IgA and IgM Contains Multiple Immunoglobulin-like Domains. Nature 1984, 308, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Stadtmueller, B.M.; Huey-Tubman, K.E.; López, C.J.; Yang, Z.; Hubbell, W.L.; Bjorkman, P.J. The Structure and Dynamics of Secretory Component and Its Interactions with Polymeric Immunoglobulins. eLife 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Piskurich, J.F.; Blanchard, M.H.; Youngman, K.R.; France, J.A.; Kaetzel, C.S. Molecular Cloning of the Mouse Polymeric Ig Receptor. Functional Regions of the Molecule Are Conserved among Five Mammalian Species. J. Immunol. Baltim. Md 1950 1995, 154, 1735–1747. [Google Scholar]
- He, T.; Siwy, J.; Metzger, J.; Mullen, W.; Mischak, H.; Schanstra, J.P.; Zürbig, P.; Jankowski, V. Associations of Urinary Polymeric Immunoglobulin Receptor Peptides in the Context of Cardio-Renal Syndrome. Sci. Rep. 2020, 10, 8291. [Google Scholar] [CrossRef]
- Turula, H.; Wobus, C.E. The Role of the Polymeric Immunoglobulin Receptor and Secretory Immunoglobulins during Mucosal Infection and Immunity. Viruses 2018, 10, 237. [Google Scholar] [CrossRef] [Green Version]
- Braathen, R.; Hohman, V.S.; Brandtzaeg, P.; Johansen, F.-E. Secretory Antibody Formation: Conserved Binding Interactions between J Chain and Polymeric Ig Receptor from Humans and Amphibians. J. Immunol. 2007, 178, 1589–1597. [Google Scholar] [CrossRef] [Green Version]
- Wcisel, D.J.; Yoder, J.A. The Confounding Complexity of Innate Immune Receptors within and between Teleost Species. Fish Shellfish Immunol. 2016, 53, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Kong, X.; Pei, C.; Zhao, X.; Li, L. Molecular Characterization of Polymeric Immunoglobulin Receptor and Expression Response to Aeromonas Hydrophila Challenge in Carassius Auratus. Fish Shellfish Immunol. 2017, 70, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wang, L.; Pei, C.; Zhang, J.; Zhao, X.; Li, L. Comparison of Polymeric Immunoglobulin Receptor between Fish and Mammals. Vet. Immunol. Immunopathol. 2018, 202, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Parra, D.; Korytář, T.; Takizawa, F.; Sunyer, J.O. B Cells and Their Role in the Teleost Gut. Dev. Comp. Immunol. 2016, 64, 150–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stadtmueller, B.M.; Yang, Z.; Huey-Tubman, K.E.; Roberts-Mataric, H.; Hubbell, W.L.; Bjorkman, P.J. Biophysical and Biochemical Characterization of Avian Secretory Component Provides Structural Insights into the Evolution of the Polymeric Ig Receptor. J. Immunol. Baltim. 2016, 197, 1408–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akula, S.; Mohammadamin, S.; Hellman, L. Fc Receptors for Immunoglobulins and Their Appearance during Vertebrate Evolution. PLoS ONE 2014, 9, e96903. [Google Scholar] [CrossRef] [Green Version]
- Akula, S.; Hellman, L. The Appearance and Diversification of Receptors for IgM During Vertebrate Evolution. Curr. Top. Microbiol. Immunol. 2017, 408, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Krajci, P.; Kvale, D.; Taskén, K.; Brandtzaeg, P. Molecular Cloning and Exon-Intron Mapping of the Gene Encoding Human Transmembrane Secretory Component (the Poly-Ig Receptor). Eur. J. Immunol. 1992, 22, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Kaetzel, C.S. Cooperativity among Secretory IgA, the Polymeric Immunoglobulin Receptor, and the Gut Microbiota Promotes Host-Microbial Mutualism. Immunol. Lett. 2014, 162, 10–21. [Google Scholar] [CrossRef] [Green Version]
- Hamuro, K.; Suetake, H.; Saha, N.R.; Kikuchi, K.; Suzuki, Y. A Teleost Polymeric Ig Receptor Exhibiting Two Ig-like Domains Transports Tetrameric IgM into the Skin. J. Immunol. 2007, 178, 5682–5689. [Google Scholar] [CrossRef] [Green Version]
- Johansen, F.-E.; Kaetzel, C.S. Regulation of the Polymeric Immunoglobulin Receptor and IgA Transport: New Advances in Environmental Factors That Stimulate PIgR Expression and Its Role in Mucosal Immunity. Mucosal Immunol. 2011, 4, 598–602. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, M.; Takenouchi, N.; Asano, M.; Kato, M.; Tsurumachi, T.; Saito, T.; Moro, I. The Polymeric Immunoglobulin Receptor (Secretory Component) in a Human Intestinal Epithelial Cell Line Is up-Regulated by Interleukin-1. Immunology 1997, 92, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Monin, L.; Castillo, P.; Elsegeiny, W.; Horne, W.; Eddens, T.; Vikram, A.; Good, M.; Schoenborn, A.A.; Bibby, K.; et al. Intestinal Interleukin-17 Receptor Signaling Mediates Reciprocal Control of the Gut Microbiota and Autoimmune Inflammation. Immunity 2016, 44, 659–671. [Google Scholar] [CrossRef] [Green Version]
- Moon, C.; VanDussen, K.L.; Miyoshi, H.; Stappenbeck, T.S. Development of a Primary Mouse Intestinal Epithelial Cell Monolayer Culture System to Evaluate Factors That Modulate IgA Transcytosis. Mucosal Immunol. 2014, 7, 818–828. [Google Scholar] [CrossRef] [Green Version]
- Piskurich, J.F.; France, J.A.; Tamer, C.M.; Willmer, C.A.; Kaetzel, C.S.; Kaetzel, D.M. Interferon-Gamma Induces Polymeric Immunoglobulin Receptor MRNA in Human Intestinal Epithelial Cells by a Protein Synthesis Dependent Mechanism. Mol. Immunol. 1993, 30, 413–421. [Google Scholar] [CrossRef]
- Sarkar, J.; Gangopadhyay, N.N.; Moldoveanu, Z.; Mestecky, J.; Stephensen, C.B. Vitamin A Is Required for Regulation of Polymeric Immunoglobulin Receptor (PIgR) Expression by Interleukin-4 and Interferon-Gamma in a Human Intestinal Epithelial Cell Line. J. Nutr. 1998, 128, 1063–1069. [Google Scholar] [CrossRef] [Green Version]
- Ackermann, L.W.; Wollenweber, L.A.; Denning, G.M. IL-4 and IFN-Gamma Increase Steady State Levels of Polymeric Ig Receptor MRNA in Human Airway and Intestinal Epithelial Cells. J. Immunol. Baltim. Md 1950 1999, 162, 5112–5118. [Google Scholar]
- Blanch, V.J.; Piskurich, J.F.; Kaetzel, C.S. Cutting Edge: Coordinate Regulation of IFN Regulatory Factor-1 and the Polymeric Ig Receptor by Proinflammatory Cytokines. J. Immunol. Baltim. Md 1950 1999, 162, 1232–1235. [Google Scholar]
- Bruno, M.E.C.; Frantz, A.L.; Rogier, E.W.; Johansen, F.-E.; Kaetzel, C.S. Regulation of the Polymeric Immunoglobulin Receptor by the Classical and Alternative NF-ΚB Pathways in Intestinal Epithelial Cells. Mucosal Immunol. 2011, 4, 468–478. [Google Scholar] [CrossRef] [Green Version]
- Kushiro, A.; Sato, T. Polymeric Immunoglobulin Receptor Gene of Mouse: Sequence, Structure and Chromosomal Location. Gene 1997, 204, 277–282. [Google Scholar] [CrossRef]
- Pal, K.; Kaetzel, C.S.; Brundage, K.; Cunningham, C.A.; Cuff, C.F. Regulation of Polymeric Immunoglobulin Receptor Expression by Reovirus. J. Gen. Virol. 2005, 86, 2347–2357. [Google Scholar] [CrossRef]
- Deng, L.; Xu, H.; Liu, P.; Wu, S.; Shi, Y.; Lv, Y.; Chen, X. Prolonged Exposure to High Humidity and High Temperature Environment Can Aggravate Influenza Virus Infection through Intestinal Flora and Nod/RIP2/NF-ΚB Signaling Pathway. Vet. Microbiol. 2020, 251, 108896. [Google Scholar] [CrossRef] [PubMed]
- Armitage, C.W.; O’Meara, C.P.; Beagley, K.W. Chlamydial Infection Enhances Expression of the Polymeric Immunoglobulin Receptor (PIgR) and Transcytosis of IgA. Am. J. Reprod. Immunol. 2017, 77. [Google Scholar] [CrossRef] [PubMed]
- Godínez-Victoria, M.; Cruz-Hernández, T.R.; Reyna-Garfias, H.; Barbosa-Cabrera, R.E.; Drago-Serrano, M.E.; Sánchez-Gómez, M.C.; Campos-Rodríguez, R. Modulation by Bovine Lactoferrin of Parameters Associated with the IgA Response in the Proximal and Distal Small Intestine of BALB/c Mice. Immunopharmacol. Immunotoxicol. 2017, 39, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Godínez-Victoria, M.; Campos-Rodriguez, R.; Rivera-Aguilar, V.; Lara-Padilla, E.; Pacheco-Yepez, J.; Jarillo-Luna, R.A.; Drago-Serrano, M.E. Intermittent Fasting Promotes Bacterial Clearance and Intestinal IgA Production in Salmonella Typhimurium-Infected Mice. Scand. J. Immunol. 2014, 79, 315–324. [Google Scholar] [CrossRef]
- Zhang, J.R.; Mostov, K.E.; Lamm, M.E.; Nanno, M.; Shimida, S.; Ohwaki, M.; Tuomanen, E. The Polymeric Immunoglobulin Receptor Translocates Pneumococci across Human Nasopharyngeal Epithelial Cells. Cell 2000, 102, 827–837. [Google Scholar] [CrossRef] [Green Version]
- Van der Wielen, P.A.; Holmes, A.R.; Cannon, R.D. Secretory Component Mediates Candida Albicans Binding to Epithelial Cells. Oral Dis. 2016, 22, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Sixbey, J.W.; Yao, Q.Y. Immunoglobulin A-Induced Shift of Epstein-Barr Virus Tissue Tropism. Science 1992, 255, 1578–1580. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ren, W.; Fang, J.; Hu, C.-A.A.; Guan, G.; Al-Dhabi, N.A.; Yin, J.; Duraipandiyan, V.; Chen, S.; Peng, Y.; et al. L-Glutamine and L-Arginine Protect against Enterotoxigenic Escherichia Coli Infection via Intestinal Innate Immunity in Mice. Amino Acids 2017, 49, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, F.-J.; Yu, L.; Yao, W.-R.; Cui, Y.-F.; Yang, G.-B. Expression of PIgR in the Tracheal Mucosa of SHIV/SIV-Infected Rhesus Macaques. Zool. Res. 2017, 38, 44–48. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, G.B. Alteration of Polymeric Immunoglobulin Receptor and Neonatal Fc Receptor Expression in the Gut Mucosa of Immunodeficiency Virus-Infected Rhesus Macaques. Scand. J. Immunol. 2016, 83, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Wong, M.H.; Thelin, A.; Hansson, L.; Falk, P.G.; Gordon, J.I. Molecular Analysis of Commensal Host-Microbial Relationships in the Intestine. Science 2001, 291, 881–884. [Google Scholar] [CrossRef] [Green Version]
- Bruno, M.E.C.; Rogier, E.W.; Frantz, A.L.; Stefka, A.T.; Thompson, S.N.; Kaetzel, C.S. Regulation of the Polymeric Immunoglobulin Receptor in Intestinal Epithelial Cells by Enterobacteriaceae: Implications for Mucosal Homeostasis. Immunol. Invest. 2010, 39, 356–382. [Google Scholar] [CrossRef]
- Kurimoto, Y.; Saruta, J.; To, M.; Yamamoto, Y.; Kimura, K.; Tsukinoki, K. Voluntary Exercise Increases IgA Concentration and Polymeric Ig Receptor Expression in the Rat Submandibular Gland. Biosci. Biotechnol. Biochem. 2016, 80, 2490–2496. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, K.; Sugimoto, N.; Islam, R.; Hossain, M.E.; Sumiyoshi, E.; Katakura, M.; Shido, O. Salivary Immunoglobulin A Secretion and Polymeric Ig Receptor Expression in the Submandibular Glands Are Enhanced in Heat-Acclimated Rats. Int. J. Mol. Sci. 2020, 21, 815. [Google Scholar] [CrossRef] [Green Version]
- Ohkuma, R.; Yada, E.; Ishikawa, S.; Komura, D.; Kubota, Y.; Hamada, K.; Horiike, A.; Ishiguro, T.; Hirasawa, Y.; Ariizumi, H.; et al. High Expression Levels of Polymeric Immunoglobulin Receptor Are Correlated with Chemoresistance and Poor Prognosis in Pancreatic Cancer. Oncol. Rep. 2020, 44, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Ai, J.; Xu, Y.; Chen, Y.; Huang, M.; Yang, X.; Hu, B.; Zhang, H.; He, C.; Yang, X.; et al. Polymeric Immunoglobulin Receptor Promotes Tumor Growth in Hepatocellular Carcinoma. Hepatology 2017, 65, 1948–1962. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Tang, Q.; Wu, Y.; Xu, Y.; Feng, T.; Zhou, R.; Chen, Y.; Gao, X.; Zhu, Q.; Yue, X.; et al. The Role of Polymeric Immunoglobulin Receptor in Inflammation-Induced Tumor Metastasis of Human Hepatocellular Carcinoma. J. Natl. Cancer Inst. 2011, 103, 1696–1712. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Li, X.; Sun, X. Reduced Expression of Polymeric Immunoglobulin Receptor (PIgR) in Nasopharyngeal Carcinoma and Its Correlation with Prognosis. Tumour Biol. 2016, 37, 11099–11104. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, P.; Bhattacharya, S.; Chin-Aleong, J.; Capasso, M.; Kocher, H.M. Expression of Polymeric Immunoglobulin Receptor and Stromal Activity in Pancreatic Ductal Adenocarcinoma. Pancreatology 2017, 17, 295–302. [Google Scholar] [CrossRef]
- Liu, F.; Ye, P.; Bi, T.; Teng, L.; Xiang, C.; Wang, H.; Li, Y.; Jin, K.; Mou, X. COLORECTAL Polymeric Immunoglobulin Receptor Expression Is Correlated with Hepatic Metastasis and Poor Prognosis in Colon Carcinoma Patients with Hepatic Metastasis. Hepatogastroenterology 2014, 61, 652–659. [Google Scholar]
- Dewdney, B.; Hebbard, L. A Novel Role for Polymeric Immunoglobulin Receptor in Tumour Development: Beyond Mucosal Immunity and into Hepatic Cancer Cell Transformation. Hepatobiliary Surg. Nutr. 2018, 7, 52–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Castillo, M.D.; Chinnapen, D.J.-F.; Lencer, W.I. Membrane Transport across Polarized Epithelia. Cold Spring Harb. Perspect. Biol. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Jin, L.; Chen, T. The Effects of Secretory IgA in the Mucosal Immune System. BioMed Res. Int. 2020, 2020, 2032057. [Google Scholar] [CrossRef] [PubMed]
- Keyt, B.A.; Baliga, R.; Sinclair, A.M.; Carroll, S.F.; Peterson, M.S. Structure, Function, and Therapeutic Use of IgM Antibodies. Antibodies 2020, 9, 53. [Google Scholar] [CrossRef]
- Castro, C.D.; Flajnik, M.F. Putting J Chain Back on the Map: How Might Its Expression Define Plasma Cell Development? J. Immunol. 2014, 193, 3248–3255. [Google Scholar] [CrossRef] [Green Version]
- Mostov, K.E.; Deitcher, D.L. Polymeric Immunoglobulin Receptor Expressed in MDCK Cells Transcytoses IgA. Cell 1986, 46, 613–621. [Google Scholar] [CrossRef]
- Elkin, S.R.; Lakoduk, A.M.; Schmid, S.L. Endocytic Pathways and Endosomal Trafficking: A Primer. Wien. Med. Wochenschr. 1946 2016, 166, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Mostov, K.E. Transepithelial Transport of Immunoglobulins. Annu. Rev. Immunol. 1994, 12, 63–84. [Google Scholar] [CrossRef]
- Asano, M.; Komiyama, K. Polymeric Immunoglobulin Receptor. J. Oral Sci. 2011, 53, 147–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everett, M.L.; Palestrant, D.; Miller, S.E.; Bollinger, R.R.; Parker, W. Immune Exclusion and Immune Inclusion: A New Model of Host-Bacterial Interactions in the Gut. Clin. Appl. Immunol. Rev. 2004, 4, 321–332. [Google Scholar] [CrossRef]
- Wallace, A.L.; Schneider, M.I.; Toomey, J.R.; Schneider, R.M.; Klempner, M.S.; Wang, Y.; Cavacini, L.A. IgA as a Potential Candidate for Enteric Monoclonal Antibody Therapeutics with Improved Gastrointestinal Stability. Vaccine 2020, 38, 7490–7497. [Google Scholar] [CrossRef]
- Mathias, A.; Corthésy, B. N-Glycans on Secretory Component: Mediators of the Interaction between Secretory IgA and Gram-Positive Commensals Sustaining Intestinal Homeostasis. Gut Microbes 2011, 2, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Plomp, R.; de Haan, N.; Bondt, A.; Murli, J.; Dotz, V.; Wuhrer, M. Comparative Glycomics of Immunoglobulin A and G From Saliva and Plasma Reveals Biomarker Potential. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- Corthésy, B. Role of Secretory Immunoglobulin A and Secretory Component in the Protection of Mucosal Surfaces. Future Microbiol. 2010, 5, 817–829. [Google Scholar] [CrossRef]
- Kumar, N.; Arthur, C.P.; Ciferri, C.; Matsumoto, M.L. Structure of the Secretory Immunoglobulin A Core. Science 2020, 367, 1008–1014. [Google Scholar] [CrossRef]
- Rogier, E.W.; Frantz, A.L.; Bruno, M.E.; Wedlund, L.; Cohen, D.A.; Stromberg, A.J.; Kaetzel, C.S. Lessons from Mother: Long-Term Impact of Antibodies in Breast Milk on the Gut Microbiota and Intestinal Immune System of Breastfed Offspring. Gut Microbes 2014, 5, 663–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demers-Mathieu, V.; Underwood, M.A.; Beverly, R.L.; Nielsen, S.D.; Dallas, D.C. Comparison of Human Milk Immunoglobulin Survival during Gastric Digestion between Preterm and Term Infants. Nutrients 2018, 10, 631. [Google Scholar] [CrossRef] [Green Version]
- Goldman, A.S.; Garza, C.; Nichols, B.L.; Goldblum, R.M. Immunologic Factors in Human Milk during the First Year of Lactation. J. Pediatr. 1982, 100, 563–567. [Google Scholar] [CrossRef]
- Shimada, S.; Kawaguchi-Miyashita, M.; Kushiro, A.; Sato, T.; Nanno, M.; Sako, T.; Matsuoka, Y.; Sudo, K.; Tagawa, Y.; Iwakura, Y.; et al. Generation of Polymeric Immunoglobulin Receptor-Deficient Mouse with Marked Reduction of Secretory IgA. J. Immunol. 1999, 163, 5367–5373. [Google Scholar]
- Johansen, F.E.; Pekna, M.; Norderhaug, I.N.; Haneberg, B.; Hietala, M.A.; Krajci, P.; Betsholtz, C.; Brandtzaeg, P. Absence of Epithelial Immunoglobulin A Transport, with Increased Mucosal Leakiness, in Polymeric Immunoglobulin Receptor/Secretory Component-Deficient Mice. J. Exp. Med. 1999, 190, 915–922. [Google Scholar] [CrossRef] [Green Version]
- Uren, T.K.; Johansen, F.-E.; Wijburg, O.L.C.; Koentgen, F.; Brandtzaeg, P.; Strugnell, R.A. Role of the Polymeric Ig Receptor in Mucosal B Cell Homeostasis. J. Immunol. 2003, 170, 2531–2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turula, H.; Bragazzi Cunha, J.; Mainou, B.A.; Ramakrishnan, S.K.; Wilke, C.A.; Gonzalez-Hernandez, M.B.; Pry, A.; Fava, J.; Bassis, C.M.; Edelman, J.; et al. Natural Secretory Immunoglobulins Promote Enteric Viral Infections. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [Green Version]
- Gohy, S.T.; Detry, B.R.; Lecocq, M.; Bouzin, C.; Weynand, B.A.; Amatngalim, G.D.; Sibille, Y.M.; Pilette, C. Polymeric Immunoglobulin Receptor Down-Regulation in Chronic Obstructive Pulmonary Disease. Persistence in the Cultured Epithelium and Role of Transforming Growth Factor-β. Am. J. Respir. Crit. Care Med. 2014, 190, 509–521. [Google Scholar] [CrossRef]
- Richmond, B.W.; Brucker, R.M.; Han, W.; Du, R.-H.; Zhang, Y.; Cheng, D.-S.; Gleaves, L.; Abdolrasulnia, R.; Polosukhina, D.; Clark, P.E.; et al. Airway Bacteria Drive a Progressive COPD-like Phenotype in Mice with Polymeric Immunoglobulin Receptor Deficiency. Nat. Commun. 2016, 7, 11240. [Google Scholar] [CrossRef] [PubMed]
- Richmond, B.W.; Du, R.-H.; Han, W.; Benjamin, J.T.; van der Meer, R.; Gleaves, L.; Guo, M.; McKissack, A.; Zhang, Y.; Cheng, D.-S.; et al. Bacterial-Derived Neutrophilic Inflammation Drives Lung Remodeling in a Mouse Model of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2018, 58, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Reikvam, D.H.; Derrien, M.; Islam, R.; Erofeev, A.; Grcic, V.; Sandvik, A.; Gaustad, P.; Meza-Zepeda, L.A.; Jahnsen, F.L.; Smidt, H.; et al. Epithelial-Microbial Crosstalk in Polymeric Ig Receptor Deficient Mice. Eur. J. Immunol. 2012, 42, 2959–2970. [Google Scholar] [CrossRef] [PubMed]
- Kato-Nagaoka, N.; Shimada, S.-I.; Yamakawa, Y.; Tsujibe, S.; Naito, T.; Setoyama, H.; Watanabe, Y.; Shida, K.; Matsumoto, S.; Nanno, M. Enhanced Differentiation of Intraepithelial Lymphocytes in the Intestine of Polymeric Immunoglobulin Receptor-Deficient Mice. Immunology 2015, 146, 59–69. [Google Scholar] [CrossRef]
- Betz, K.J.; Maier, E.A.; Amarachintha, S.; Wu, D.; Karmele, E.P.; Kinder, J.M.; Steinbrecher, K.A.; McNeal, M.M.; Luzader, D.H.; Hogan, S.P.; et al. Enhanced Survival Following Oral and Systemic Salmonella Enterica Serovar Typhimurium Infection in Polymeric Immunoglobulin Receptor Knockout Mice. PLoS ONE 2018, 13, e0198434. [Google Scholar] [CrossRef] [Green Version]
- Wijburg, O.L.C.; Uren, T.K.; Simpfendorfer, K.; Johansen, F.-E.; Brandtzaeg, P.; Strugnell, R.A. Innate Secretory Antibodies Protect against Natural Salmonella Typhimurium Infection. J. Exp. Med. 2006, 203, 21–26. [Google Scholar] [CrossRef]
- Suzuki, K. Diversified IgA-Bacteria Interaction in Gut Homeostasis. Adv. Exp. Med. Biol. 2020, 1254, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Okai, S.; Usui, F.; Ohta, M.; Mori, H.; Kurokawa, K.; Matsumoto, S.; Kato, T.; Miyauchi, E.; Ohno, H.; Shinkura, R. Intestinal IgA as a Modulator of the Gut Microbiota. Gut Microbes 2017, 8, 486–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.T.; Yao, S.; Gong, B.; Elson, C.O.; Cong, Y. Th17 Cells Upregulate Polymeric Ig Receptor and Intestinal IgA and Contribute to Intestinal Homeostasis. J. Immunol. 2012, 189, 4666–4673. [Google Scholar] [CrossRef] [Green Version]
- Bruno, M.E.C.; Rogier, E.W.; Arsenescu, R.I.; Flomenhoft, D.R.; Kurkjian, C.J.; Ellis, G.I.; Kaetzel, C.S. Correlation of Biomarker Expression in Colonic Mucosa with Disease Phenotype in Crohn’s Disease and Ulcerative Colitis. Dig. Dis. Sci. 2015, 60, 2976–2984. [Google Scholar] [CrossRef] [Green Version]
- Kakiuchi, N.; Yoshida, K.; Uchino, M.; Kihara, T.; Akaki, K.; Inoue, Y.; Kawada, K.; Nagayama, S.; Yokoyama, A.; Yamamoto, S.; et al. Frequent Mutations That Converge on the NFKBIZ Pathway in Ulcerative Colitis. Nature 2020, 577, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Olafsson, S.; McIntyre, R.E.; Coorens, T.; Butler, T.; Jung, H.; Robinson, P.S.; Lee-Six, H.; Sanders, M.A.; Arestang, K.; Dawson, C.; et al. Somatic Evolution in Non-Neoplastic IBD-Affected Colon. Cell 2020, 182, 672–684.e11. [Google Scholar] [CrossRef] [PubMed]
- Nanki, K.; Fujii, M.; Shimokawa, M.; Matano, M.; Nishikori, S.; Date, S.; Takano, A.; Toshimitsu, K.; Ohta, Y.; Takahashi, S.; et al. Somatic Inflammatory Gene Mutations in Human Ulcerative Colitis Epithelium. Nature 2020, 577, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Brandtzaeg, P.; Prydz, H. Direct Evidence for an Integrated Function of J Chain and Secretory Component in Epithelial Transport of Immunoglobulins. Nature 1984, 311, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Suzuki, Y.; Novak, J.; Tomino, Y. Development of Animal Models of Human IgA Nephropathy. Drug Discov. Today Dis. Models 2014, 11, 5–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steffen, U.; Koeleman, C.A.; Sokolova, M.V.; Bang, H.; Kleyer, A.; Rech, J.; Unterweger, H.; Schicht, M.; Garreis, F.; Hahn, J.; et al. IgA Subclasses Have Different Effector Functions Associated with Distinct Glycosylation Profiles. Nat. Commun. 2020, 11, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roos Ljungberg, K.; Börjesson, E.; Martinsson, K.; Wetterö, J.; Kastbom, A.; Svärd, A. Presence of Salivary IgA Anti-Citrullinated Protein Antibodies Associate with Higher Disease Activity in Patients with Rheumatoid Arthritis. Arthritis Res. Ther. 2020, 22, 274. [Google Scholar] [CrossRef]
- Guo, J.; Han, X.; Huang, W.; You, Y.; Jicheng, Z. Interaction between IgA and Gut Microbiota and Its Role in Controlling Metabolic Syndrome. Obes. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lombana, T.N.; Rajan, S.; Zorn, J.A.; Mandikian, D.; Chen, E.C.; Estevez, A.; Yip, V.; Bravo, D.D.; Phung, W.; Farahi, F.; et al. Production, Characterization, and in Vivo Half-Life Extension of Polymeric IgA Molecules in Mice. mAbs 2019, 11, 1122–1138. [Google Scholar] [CrossRef] [Green Version]
- Cattaneo, A.; Neuberger, M.S. Polymeric Immunoglobulin M Is Secreted by Transfectants of Non-Lymphoid Cells in the Absence of Immunoglobulin J Chain. EMBO J. 1987, 6, 2753–2758. [Google Scholar] [CrossRef]
- Randall, T.D.; Brewer, J.W.; Corley, R.B. Direct Evidence That J Chain Regulates the Polymeric Structure of IgM in Antibody-Secreting B Cells. J. Biol. Chem. 1992, 267, 18002–18007. [Google Scholar] [CrossRef]
- Jones, K.; Savulescu, A.F.; Brombacher, F.; Hadebe, S. Immunoglobulin M in Health and Diseases: How Far Have We Come and What Next? Front. Immunol. 2020, 11, 595535. [Google Scholar] [CrossRef]
- Johansen, F.E.; Braathen, R.; Brandtzaeg, P. Role of J Chain in Secretory Immunoglobulin Formation. Scand. J. Immunol. 2000, 52, 240–248. [Google Scholar] [CrossRef]
- Frutiger, S.; Hughes, G.J.; Paquet, N.; Lüthy, R.; Jaton, J.C. Disulfide Bond Assignment in Human J Chain and Its Covalent Pairing with Immunoglobulin, M. Biochemistry 1992, 31, 12643–12647. [Google Scholar] [CrossRef] [PubMed]
- Bastian, A.; Kratzin, H.; Fallgren-Gebauer, E.; Eckart, K.; Hilschmann, N. Intra- and Inter-Chain Disulfide Bridges of J Chain in Human S-IgA. Adv. Exp. Med. Biol. 1995, 371A, 581–583. [Google Scholar] [CrossRef]
- De Sousa-Pereira, P.; Woof, J.M. IgA: Structure, Function, and Developability. Antibodies 2019, 8, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klimovich, V.B.; Samoĭlovich, M.P.; Klimovich, B.V. Problem of J-chain of immunoglobulins. Zh. Evol. Biokhim. Fiziol. 2008, 44, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Johansen, F.E.; Braathen, R.; Brandtzaeg, P. The J Chain Is Essential for Polymeric Ig Receptor-Mediated Epithelial Transport of IgA. J. Immunol. 2001, 167, 5185–5192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, N.C.; Rise, M.L.; Christian, S.L. A Comparison of the Innate and Adaptive Immune Systems in Cartilaginous Fish, Ray-Finned Fish, and Lobe-Finned Fish. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Johansen, F.E.; Natvig Norderhaug, I.; Røe, M.; Sandlie, I.; Brandtzaeg, P. Recombinant Expression of Polymeric IgA: Incorporation of J Chain and Secretory Component of Human Origin. Eur. J. Immunol. 1999, 29, 1701–1708. [Google Scholar] [CrossRef]
- Max, E.E.; McBride, O.W.; Morton, C.C.; Robinson, M.A. Human J Chain Gene: Chromosomal Localization and Associated Restriction Fragment Length Polymorphisms. Proc. Natl. Acad. Sci. USA 1986, 83, 5592–5596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.; Karray, S.; Gackstetter, E.R.; Koshland, M.E. Myocyte Enhancer Factor-Related B-MEF2 Is Developmentally Expressed in B Cells and Regulates the Immunoglobulin J Chain Promoter. J. Biol. Chem. 1998, 273, 26123–26129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinkenberger, J.L.; Wallin, J.J.; Johnson, K.W.; Koshland, M.E. An Interleukin-2 Signal Relieves BSAP (Pax5)-Mediated Repression of the Immunoglobulin J Chain Gene. Immunity 1996, 5, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Shapiro-Shelef, M.; Lin, K.-I.; McHeyzer-Williams, L.J.; Liao, J.; McHeyzer-Williams, M.G.; Calame, K. Blimp-1 Is Required for the Formation of Immunoglobulin Secreting Plasma Cells and Pre-Plasma Memory B Cells. Immunity 2003, 19, 607–620. [Google Scholar] [CrossRef] [Green Version]
- Brandtzaeg, P.; Johansen, F.-E. Mucosal B Cells: Phenotypic Characteristics, Transcriptional Regulation, and Homing Properties. Immunol. Rev. 2005, 206, 32–63. [Google Scholar] [CrossRef]
- Hendrickson, B.A.; Conner, D.A.; Ladd, D.J.; Kendall, D.; Casanova, J.E.; Corthesy, B.; Max, E.E.; Neutra, M.R.; Seidman, C.E.; Seidman, J.G. Altered Hepatic Transport of Immunoglobulin A in Mice Lacking the J Chain. J. Exp. Med. 1995, 182, 1905–1911. [Google Scholar] [CrossRef]
- Vaerman, J.P.; Langendries, A.; Giffroy, D.; Brandtzaeg, P.; Kobayashi, K. Lack of SC/PIgR-Mediated Epithelial Transport of a Human Polymeric IgA Devoid of J Chain: In Vitro and in Vivo Studies. Immunology 1998, 95, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, B.A.; Rindisbacher, L.; Corthesy, B.; Kendall, D.; Waltz, D.A.; Neutra, M.R.; Seidman, J.G. Lack of Association of Secretory Component with IgA in J Chain-Deficient Mice. J. Immunol. 1996, 157, 750–754. [Google Scholar] [PubMed]
- Pabst, O.; Slack, E. IgA and the Intestinal Microbiota: The Importance of Being Specific. Mucosal Immunol. 2020, 13, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Magri, G.; Grasset, E.K.; Cerutti, A. Rethinking Mucosal Antibody Responses: IgM, IgG and IgD Join IgA. Nat. Rev. Immunol. 2020, 20, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Bollinger, R.R.; Everett, M.L.; Palestrant, D.; Love, S.D.; Lin, S.S.; Parker, W. Human Secretory Immunoglobulin A May Contribute to Biofilm Formation in the Gut. Immunology 2003, 109, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Van Gool, M.M.J.; van Egmond, M. IgA and FcαRI: Versatile Players in Homeostasis, Infection, and Autoimmunity. ImmunoTargets Ther. 2021, 9, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Pallav, K.; Xu, H.; Leffler, D.A.; Kabbani, T.; Kelly, C.P. Immunoglobulin A Deficiency in Celiac Disease in the United States. J. Gastroenterol. Hepatol. 2016, 31, 133–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berbers, R.-M.; Franken, I.A.; Leavis, H.L. Immunoglobulin A and Microbiota in Primary Immunodeficiency Diseases. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 563–570. [Google Scholar] [CrossRef]
- Matsumura, S.; Van De Water, J.; Leung, P.; Odin, J.A.; Yamamoto, K.; Gores, G.J.; Mostov, K.; Ansari, A.A.; Coppel, R.L.; Shiratori, Y.; et al. Caspase Induction by IgA Antimitochondrial Antibody: IgA-Mediated Biliary Injury in Primary Biliary Cirrhosis. Hepatology 2004, 39, 1415–1422. [Google Scholar] [CrossRef]
- Tanaka, A.; Nezu, S.; Uegaki, S.; Mikami, M.; Okuyama, S.; Kawamura, N.; Aiso, M.; Gershwin, M.E.; Takahashi, S.-I.; Selmi, C.; et al. The Clinical Significance of IgA Antimitochondrial Antibodies in Sera and Saliva in Primary Biliary Cirrhosis. Ann. NY Acad. Sci. 2007, 1107, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Martín-Nares, E.; Hernández-Molina, G. Novel Autoantibodies in Sjögren’s Syndrome: A Comprehensive Review. Autoimmun. Rev. 2019, 18, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, Z.; Frazer, G.; Ferro, A.; Clare, S.; Bouladoux, N.; Ferdinand, J.; Tuong, Z.K.; Negro-Demontel, M.L.; Kumar, N.; Suchanek, O.; et al. Gut-Educated IgA Plasma Cells Defend the Meningeal Venous Sinuses. Nature 2020, 587, 472–476. [Google Scholar] [CrossRef]
- Feinstein, A.; Munn, E.A. Conformation of the Free and Antigen-Bound IgM Antibody Molecules. Nature 1969, 224, 1307–1309. [Google Scholar] [CrossRef]
- Davis, A.C.; Roux, K.H.; Shulman, M.J. On the Structure of Polymeric IgM. Eur. J. Immunol. 1988, 18, 1001–1008. [Google Scholar] [CrossRef]
- Hiramoto, E.; Tsutsumi, A.; Suzuki, R.; Matsuoka, S.; Arai, S.; Kikkawa, M.; Miyazaki, T. The IgM Pentamer Is an Asymmetric Pentagon with an Open Groove That Binds the AIM Protein. Sci. Adv. 2018, 4, eaau1199. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, G.; Li, N.; Wang, Y.; Zhu, Q.; Chu, H.; Wu, W.; Tan, Y.; Yu, F.; Su, X.-D.; et al. Structural Insights into Immunoglobulin, M. Science 2020, 367, 1014–1017. [Google Scholar] [CrossRef]
- Sharp, T.H.; Boyle, A.L.; Diebolder, C.A.; Kros, A.; Koster, A.J.; Gros, P. Insights into IgM-Mediated Complement Activation Based on in Situ Structures of IgM-C1-C4b. Proc. Natl. Acad. Sci. USA 2019, 116, 11900–11905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erlandsson, L.; Andersson, K.; Sigvardsson, M.; Lycke, N.; Leanderson, T. Mice with an Inactivated Joining Chain Locus Have Perturbed IgM Secretion. Eur. J. Immunol. 1998, 28, 2355–2365. [Google Scholar] [CrossRef]
- Van Es, J.H.; Meyling, F.H.; Logtenberg, T. High Frequency of Somatically Mutated IgM Molecules in the Human Adult Blood B Cell Repertoire. Eur. J. Immunol. 1992, 22, 2761–2764. [Google Scholar] [CrossRef]
- Klein, U.; Küppers, R.; Rajewsky, K. Evidence for a Large Compartment of IgM-Expressing Memory B Cells in Humans. Blood 1997, 89, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Ruprecht, R.M. Immunoglobulin M: An Ancient Antiviral Weapon—Rediscovered. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Blandino, R.; Baumgarth, N. Secreted IgM: New Tricks for an Old Molecule. J. Leukoc. Biol. 2019, 106, 1021–1034. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Xiong, E.; Hong, R.; Lu, Q.; Ohno, H.; Wang, J.-Y. Role of the IgM Fc Receptor in Immunity and Tolerance. Front. Immunol. 2019, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Yamazaki, T.; Sugisawa, R.; Gershwin, M.E.; Arai, S. AIM Associated with the IgM Pentamer: Attackers on Stand-by at Aircraft Carrier. Cell. Mol. Immunol. 2018, 15, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, G.; Nonomura, T.; Sasaki, M.; Ishida, Y.; Arai, S.; Miyazaki, T. AIM-Deficient Mouse Fed a High-Trans Fat, High-Cholesterol Diet: A New Animal Model for Nonalcoholic Fatty Liver Disease. Exp. Anim. 2019, 68, 147–158. [Google Scholar] [CrossRef]
- Ozawa, T.; Maehara, N.; Kai, T.; Arai, S.; Miyazaki, T. Dietary Fructose-Induced Hepatocellular Carcinoma Development Manifested in Mice Lacking Apoptosis Inhibitor of Macrophage (AIM). Genes Cells 2016, 21, 1320–1332. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.M.E.; Peters, K.E.; Lipscombe, R. Apoptosis Inhibitor of Macrophage and Diabetic Kidney Disease. Cell. Mol. Immunol. 2019, 16, 521. [Google Scholar] [CrossRef]
- Iwamura, Y.; Mori, M.; Nakashima, K.; Mikami, T.; Murayama, K.; Arai, S.; Miyazaki, T. Apoptosis Inhibitor of Macrophage (AIM) Diminishes Lipid Droplet-Coating Proteins Leading to Lipolysis in Adipocytes. Biochem. Biophys. Res. Commun. 2012, 422, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-J.; Wang, X.; Zhang, M.-L.; Li, L.; Wang, R.-T. Association between Apoptosis Inhibitor of Macrophage and Microsatellite Instability Status in Colorectal Cancer. BMC Gastroenterol. 2020, 20, 373. [Google Scholar] [CrossRef]
- Sugisawa, R.; Komatsu, G.; Hiramoto, E.; Takeda, N.; Yamamura, K.-I.; Arai, S.; Miyazaki, T. Independent Modes of Disease Repair by AIM Protein Distinguished in AIM-Felinized Mice. Sci. Rep. 2018, 8, 13157. [Google Scholar] [CrossRef]
- Tomita, T.; Arai, S.; Kitada, K.; Mizuno, M.; Suzuki, Y.; Sakata, F.; Nakano, D.; Hiramoto, E.; Takei, Y.; Maruyama, S.; et al. Apoptosis Inhibitor of Macrophage Ameliorates Fungus-Induced Peritoneal Injury Model in Mice. Sci. Rep. 2017, 7, 6450. [Google Scholar] [CrossRef] [Green Version]
- Sowa, S.T.; Moilanen, A.; Biterova, E.; Saaranen, M.J.; Lehtiö, L.; Ruddock, L.W. High-Resolution Crystal Structure of Human PERp1, a Saposin-like Protein Involved in IgA, IgM and Integrin Maturation in the Endoplasmic Reticulum. J. Mol. Biol. 2021, 433, 166826. [Google Scholar] [CrossRef] [PubMed]
- Xiong, E.; Li, Y.; Min, Q.; Cui, C.; Liu, J.; Hong, R.; Lai, N.; Wang, Y.; Sun, J.; Matsumoto, R.; et al. MZB1 Promotes the Secretion of J-Chain-Containing Dimeric IgA and Is Critical for the Suppression of Gut Inflammation. Proc. Natl. Acad. Sci. USA 2019, 116, 13480–13489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minnich, M.; Tagoh, H.; Bönelt, P.; Axelsson, E.; Fischer, M.; Cebolla, B.; Tarakhovsky, A.; Nutt, S.L.; Jaritz, M.; Busslinger, M. Multifunctional Role of the Transcription Factor Blimp-1 in Coordinating Plasma Cell Differentiation. Nat. Immunol. 2016, 17, 331–343. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, T.; Corrado, M.; Pearce, E.L.; Pearce, E.J.; Grosschedl, R. MZB1 Enables Efficient Interferon α Secretion in Stimulated Plasmacytoid Dendritic Cells. Sci. Rep. 2020, 10, 21626. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, K.; Braakman, I. Protein Quality Control at the Endoplasmic Reticulum. Essays Biochem. 2016, 60, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Yadav, K.; Yadav, A.; Vashistha, P.; Pandey, V.P.; Dwivedi, U.N. Protein Misfolding Diseases and Therapeutic Approaches. Curr. Protein Pept. Sci. 2019, 20, 1226–1245. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Andreani, V.; Kapoor, T.; Herp, S.; Flach, H.; Duchniewicz, M.; Grosschedl, R. MZB1 Is a GRP94 Cochaperone That Enables Proper Immunoglobulin Heavy Chain Biosynthesis upon ER Stress. Genes Dev. 2014, 28, 1165–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreani, V.; Ramamoorthy, S.; Pandey, A.; Lupar, E.; Nutt, S.L.; Lämmermann, T.; Grosschedl, R. Cochaperone Mzb1 Is a Key Effector of Blimp1 in Plasma Cell Differentiation and Β1-Integrin Function. Proc. Natl. Acad. Sci. USA 2018, 115, E9630–E9639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Vogelzang, A.; Fagarasan, S. MZB1 Folding and Unfolding the Role of IgA. Proc. Natl. Acad. Sci. USA 2019, 116, 13163–13165. [Google Scholar] [CrossRef] [Green Version]
- Guzeldemir-Akcakanat, E.; Sunnetci-Akkoyunlu, D.; Orucguney, B.; Cine, N.; Kan, B.; Yılmaz, E.B.; Gümüşlü, E.; Savli, H. Gene-Expression Profiles in Generalized Aggressive Periodontitis: A Gene Network-Based Microarray Analysis. J. Periodontol. 2016, 87, 58–65. [Google Scholar] [CrossRef]
- Lundmark, A.; Gerasimcik, N.; Båge, T.; Jemt, A.; Mollbrink, A.; Salmén, F.; Lundeberg, J.; Yucel-Lindberg, T. Gene Expression Profiling of Periodontitis-Affected Gingival Tissue by Spatial Transcriptomics. Sci. Rep. 2018, 8, 9370. [Google Scholar] [CrossRef] [Green Version]
- Guzeldemir-Akcakanat, E.; Alkan, B.; Sunnetci-Akkoyunlu, D.; Gurel, B.; Balta, V.M.; Kan, B.; Akgun, E.; Yilmaz, E.B.; Baykal, A.T.; Cine, N.; et al. Molecular Signatures of Chronic Periodontitis in Gingiva: A Genomic and Proteomic Analysis. J. Periodontol. 2019, 90, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa-Hayashino, A.; Yoshifuji, H.; Kitagori, K.; Ito, S.; Oku, T.; Hirayama, Y.; Salah, A.; Nakajima, T.; Kiso, K.; Yamada, N.; et al. Increase of MZB1 in B Cells in Systemic Lupus Erythematosus: Proteomic Analysis of Biopsied Lymph Nodes. Arthritis Res. Ther. 2018, 20, 13. [Google Scholar] [CrossRef] [Green Version]
- Wildschütz, L.; Ackermann, D.; Witten, A.; Kasper, M.; Busch, M.; Glander, S.; Melkonyan, H.; Walscheid, K.; Tappeiner, C.; Thanos, S.; et al. Transcriptomic and Proteomic Analysis of Iris Tissue and Aqueous Humor in Juvenile Idiopathic Arthritis-Associated Uveitis. J. Autoimmun. 2019, 100, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.A.R.; Pascoal, L.B.; Dotti, I.; de Setsuko Ayrizono, L.M.; Aguilar, D.; Rodrigues, B.L.; Arroyes, M.; Ferrer-Picon, E.; Milanski, M.; Velloso, L.A.; et al. Whole Transcriptional Analysis Identifies Markers of B, T and Plasma Cell Signaling Pathways in the Mesenteric Adipose Tissue Associated with Crohn’s Disease. J. Transl. Med. 2020, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Christakoudi, S.; Runglall, M.; Mobillo, P.; Rebollo-Mesa, I.; Tsui, T.-L.; Nova-Lamperti, E.; Taube, C.; Norris, S.; Kamra, Y.; Hilton, R.; et al. Development and Validation of the First Consensus Gene-Expression Signature of Operational Tolerance in Kidney Transplantation, Incorporating Adjustment for Immunosuppressive Drug Therapy. EBioMedicine 2020, 58, 102899. [Google Scholar] [CrossRef] [PubMed]
- Herold, T.; Mulaw, M.A.; Jurinovic, V.; Seiler, T.; Metzeler, K.H.; Dufour, A.; Schneider, S.; Kakadia, P.M.; Spiekermann, K.; Mansmann, U.; et al. High Expression of MZB1 Predicts Adverse Prognosis in Chronic Lymphocytic Leukemia, Follicular Lymphoma and Diffuse Large B-Cell Lymphoma and Is Associated with a Unique Gene Expression Signature. Leuk. Lymphoma 2013, 54, 1652–1657. [Google Scholar] [CrossRef]
- Miyake, K.; Mori, R.; Homma, Y.; Matsuyama, R.; Okayama, A.; Murakami, T.; Hirano, H.; Endo, I. MZB1 in Borderline Resectable Pancreatic Cancer Resected after Neoadjuvant Chemoradiotherapy. J. Surg. Res. 2017, 220, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Chen, Y.; Li, Q.; Zhang, L. Exploration of the Hub Genes and MiRNAs in Lung Adenocarcinoma. Oncol. Lett. 2019, 18, 1713–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niemira, M.; Collin, F.; Szalkowska, A.; Bielska, A.; Chwialkowska, K.; Reszec, J.; Niklinski, J.; Kwasniewski, M.; Kretowski, A. Molecular Signature of Subtypes of Non-Small-Cell Lung Cancer by Large-Scale Transcriptional Profiling: Identification of Key Modules and Genes by Weighted Gene Co-Expression Network Analysis (WGCNA). Cancers 2019, 12, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Liu, D.; Wang, Y.; Dong, M. Salidroside Suppresses Nonsmall Cell Lung Cancer Cells Proliferation and Migration via MicroRNA-103-3p/Mzb1. Anticancer. Drugs 2020, 31, 663–671. [Google Scholar] [CrossRef]
- Zhong, R.; Zhang, Y.; Chen, D.; Cao, S.; Han, B.; Zhong, H. Single-Cell RNA Sequencing Reveals Cellular and Molecular Immune Profile in a Pembrolizumab-Responsive PD-L1-Negative Lung Cancer Patient. Cancer Immunol. Immunother. 2021. [Google Scholar] [CrossRef]
- Bauer, M.A.; Ashby, C.; Wardell, C.; Boyle, E.M.; Ortiz, M.; Flynt, E.; Thakurta, A.; Morgan, G.; Walker, B.A. Differential RNA Splicing as a Potentially Important Driver Mechanism in Multiple Myeloma. Haematologica 2020. [Google Scholar] [CrossRef] [Green Version]
- Chanukuppa, V.; Paul, D.; Taunk, K.; Chatterjee, T.; Sharma, S.; Shirolkar, A.; Islam, S.; Santra, M.K.; Rapole, S. Proteomics and Functional Study Reveal Marginal Zone B and B1 Cell Specific Protein as a Candidate Marker of Multiple Myeloma. Int. J. Oncol. 2020, 57, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Shibata, M.; Inaishi, T.; Ichikawa, T.; Soeda, I.; Miyajima, N.; Takano, Y.; Takeuchi, D.; Tsunoda, N.; Kanda, M.; et al. MZB1 Expression Indicates Poor Prognosis in Estrogen Receptor-Positive Breast Cancer. Oncol. Lett. 2020, 20, 198. [Google Scholar] [CrossRef]
- Ji, C.; Li, Y.; Yang, K.; Gao, Y.; Sha, Y.; Xiao, D.; Liang, X.; Cheng, Z. Identification of Four Genes Associated with Cutaneous Metastatic Melanoma. Open Med. Wars. Pol. 2020, 15, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Imoto, I.; Kozaki, K.; Matsui, T.; Muramatsu, T.; Furuta, M.; Tanaka, S.; Sakamoto, M.; Arii, S.; Inazawa, J. Integrative Array-Based Approach Identifies MZB1 as a Frequently Methylated Putative Tumor Suppressor in Hepatocellular Carcinoma. Clin. Cancer Res. 2012, 18, 3541–3551. [Google Scholar] [CrossRef] [Green Version]
- Kanda, M.; Tanaka, C.; Kobayashi, D.; Tanaka, H.; Shimizu, D.; Shibata, M.; Takami, H.; Hayashi, M.; Iwata, N.; Niwa, Y.; et al. Epigenetic Suppression of the Immunoregulator MZB1 Is Associated with the Malignant Phenotype of Gastric Cancer. Int. J. Cancer 2016, 139, 2290–2298. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Yang, Z.; Long, F.; Luo, L.; Deng, Q.; Wu, J.; Ouyang, S.; Tang, D. COL1A1 and MZB1 as the Hub Genes Influenced the Proliferation, Invasion, Migration and Apoptosis of Rectum Adenocarcinoma Cells by Weighted Correlation Network Analysis. Bioorganic Chem. 2020, 95, 103457. [Google Scholar] [CrossRef]
- Schiller, H.B.; Mayr, C.H.; Leuschner, G.; Strunz, M.; Staab-Weijnitz, C.; Preisendörfer, S.; Eckes, B.; Moinzadeh, P.; Krieg, T.; Schwartz, D.A.; et al. Deep Proteome Profiling Reveals Common Prevalence of MZB1-Positive Plasma B Cells in Human Lung and Skin Fibrosis. Am. J. Respir. Crit. Care Med. 2017, 196, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
- Maltby, V.E.; Lea, R.A.; Ribbons, K.A.; Sanders, K.A.; Kennedy, D.; Min, M.; Scott, R.J.; Lechner-Scott, J. DNA Methylation Changes in CD4+ T Cells Isolated from Multiple Sclerosis Patients on Dimethyl Fumarate. Mult. Scler. J. Exp. Transl. Clin. 2018, 4, 2055217318787826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| State of MZB1 Expression | Implicated Human Disease(s) | Reference |
|---|---|---|
| Elevated | Periodontitis | [156,157,158] |
| Elevated | Systemic lupus erythematosus, Rheumatoid arthritis | [159] |
| Elevated | Juvenile idiopathic arthritis-associated uveitis | [160] |
| Elevated | Crohn’s disease | [161] |
| Elevated | Rejection of kidney transplant | [162] |
| Elevated | Chronic lymphocytic leukemia, Follicular lymphoma, diffuse large B-cell lymphoma | [163] |
| Elevated | Borderline resectable pancreatic cancer | [164] |
| Elevated | Lung adenocarcinoma | [165,166,167,168] |
| Elevated | Multiple myeloma | [169,170] |
| Elevated | Estrogen receptor-positive breast cancer | [171] |
| Elevated | Cutaneous metastatic melanoma | [172] |
| Reduced | Hepatocellular carcinoma | [173] |
| Reduced | Malignant gastric cancer | [174] |
| Reduced | Colorectal adenocarcinoma | [175] |
| Elevated | Lung and skin fibrosis | [176] |
| Reduced | Multiple sclerosis | [177] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Wang, J.-Y. Role of Polymeric Immunoglobulin Receptor in IgA and IgM Transcytosis. Int. J. Mol. Sci. 2021, 22, 2284. https://doi.org/10.3390/ijms22052284
Wei H, Wang J-Y. Role of Polymeric Immunoglobulin Receptor in IgA and IgM Transcytosis. International Journal of Molecular Sciences. 2021; 22(5):2284. https://doi.org/10.3390/ijms22052284
Chicago/Turabian StyleWei, Hao, and Ji-Yang Wang. 2021. "Role of Polymeric Immunoglobulin Receptor in IgA and IgM Transcytosis" International Journal of Molecular Sciences 22, no. 5: 2284. https://doi.org/10.3390/ijms22052284
APA StyleWei, H., & Wang, J.-Y. (2021). Role of Polymeric Immunoglobulin Receptor in IgA and IgM Transcytosis. International Journal of Molecular Sciences, 22(5), 2284. https://doi.org/10.3390/ijms22052284






