Synthesis of New Steroidal Carbamates with Plant-Growth-Promoting Activity: Theoretical and Experimental Evidence
Abstract
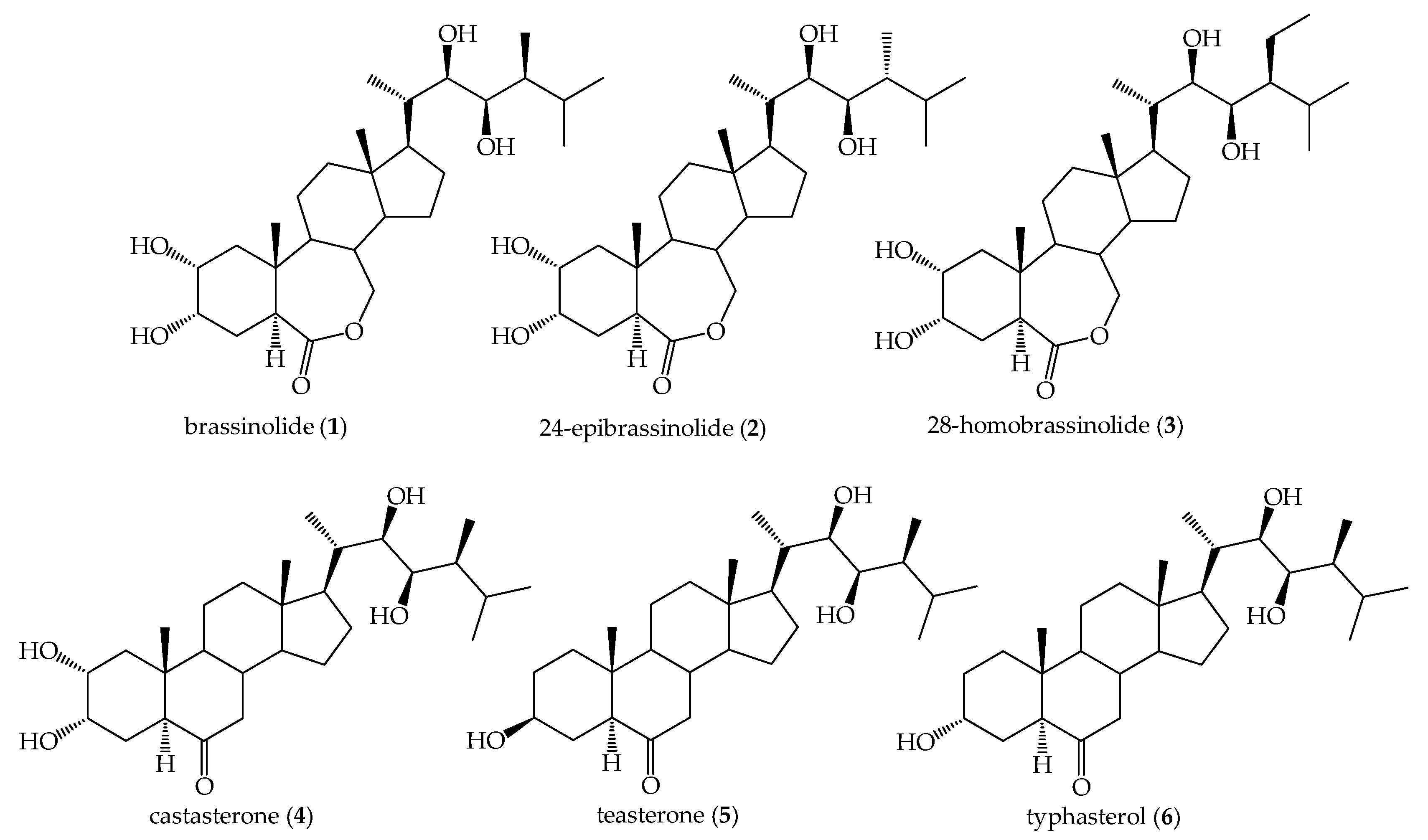
1. Introduction
2. Results and Discussion
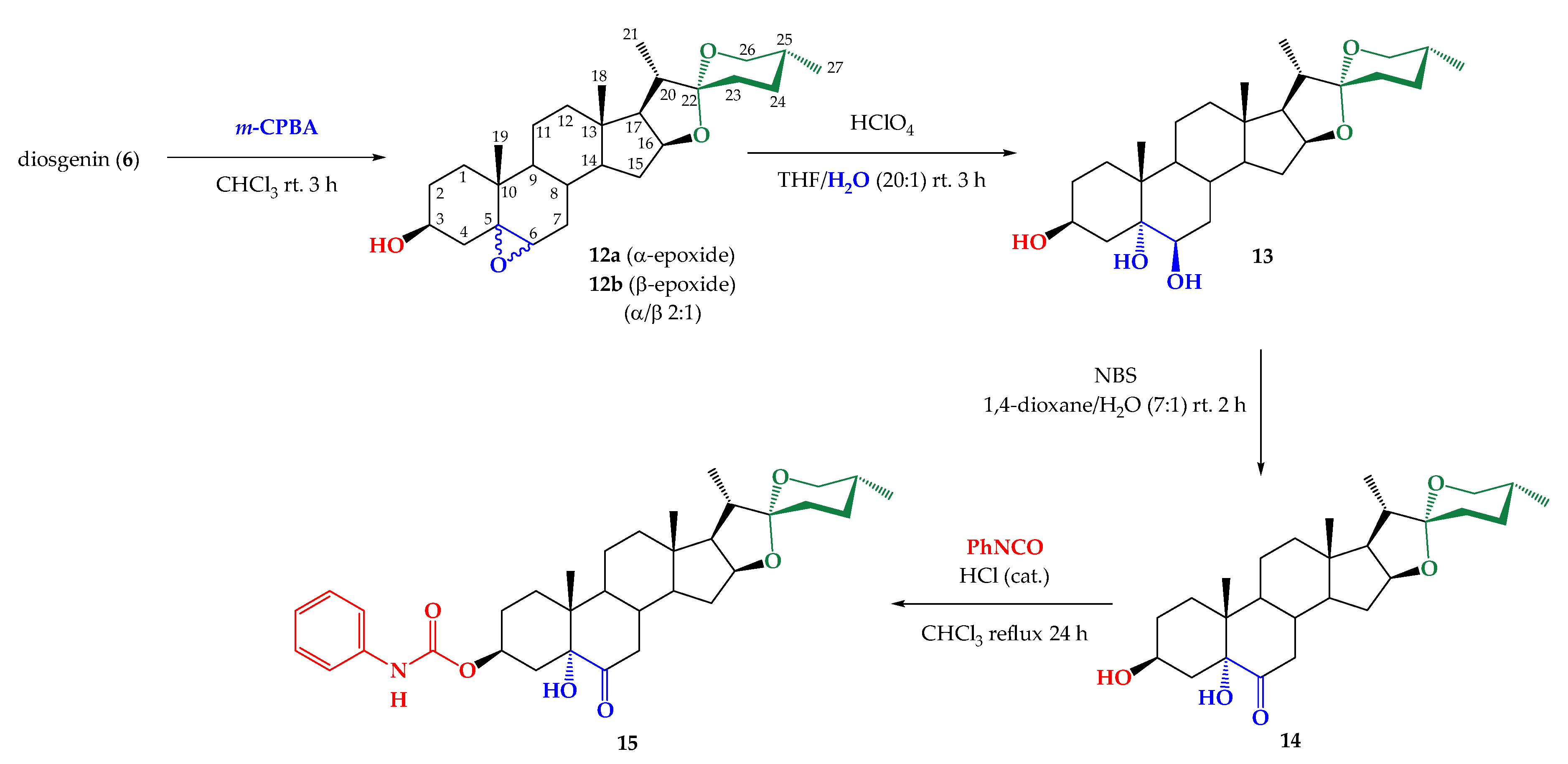
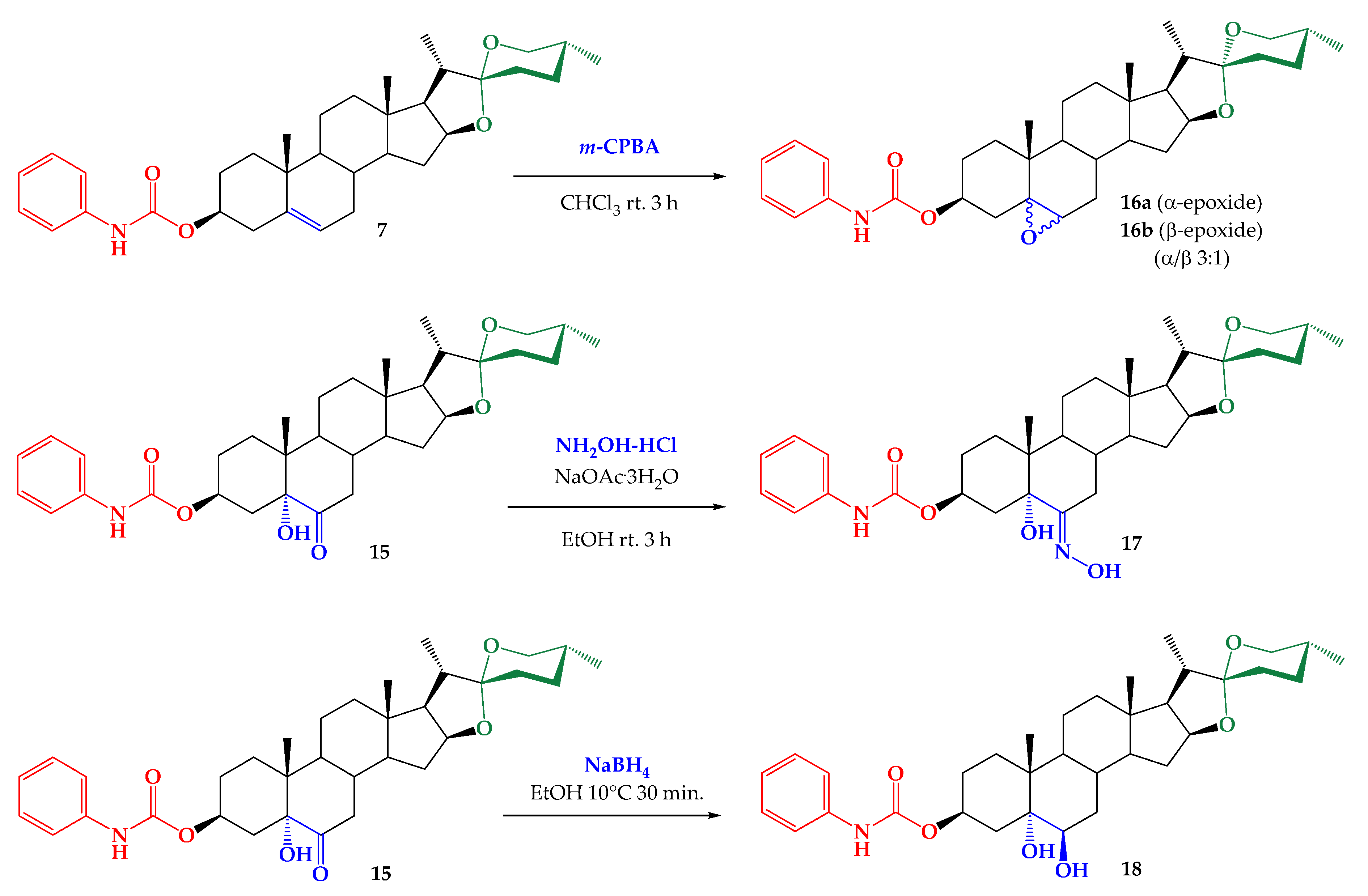
2.1. Synthesis
2.2. Rice-Lamina Inclination Test
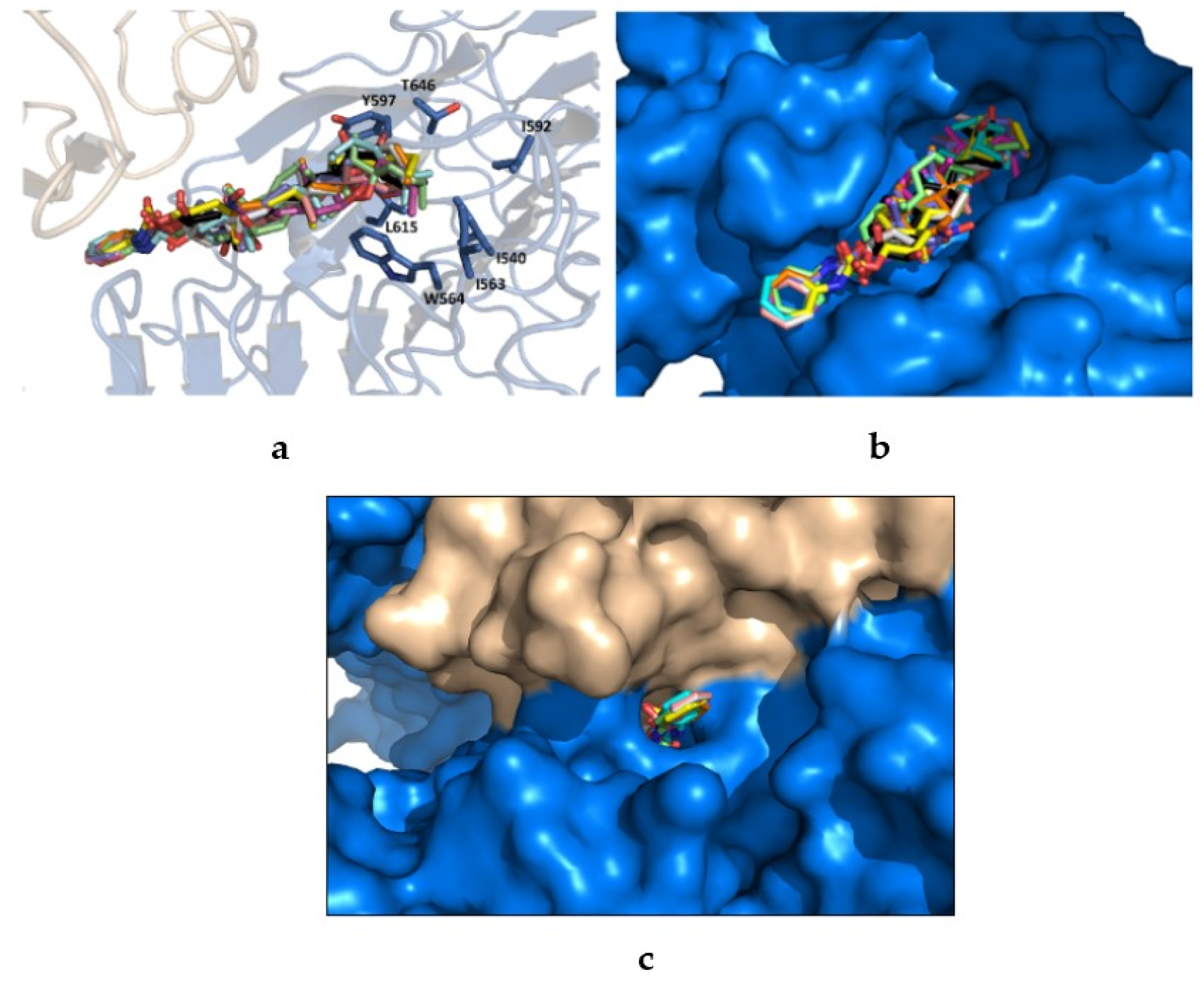
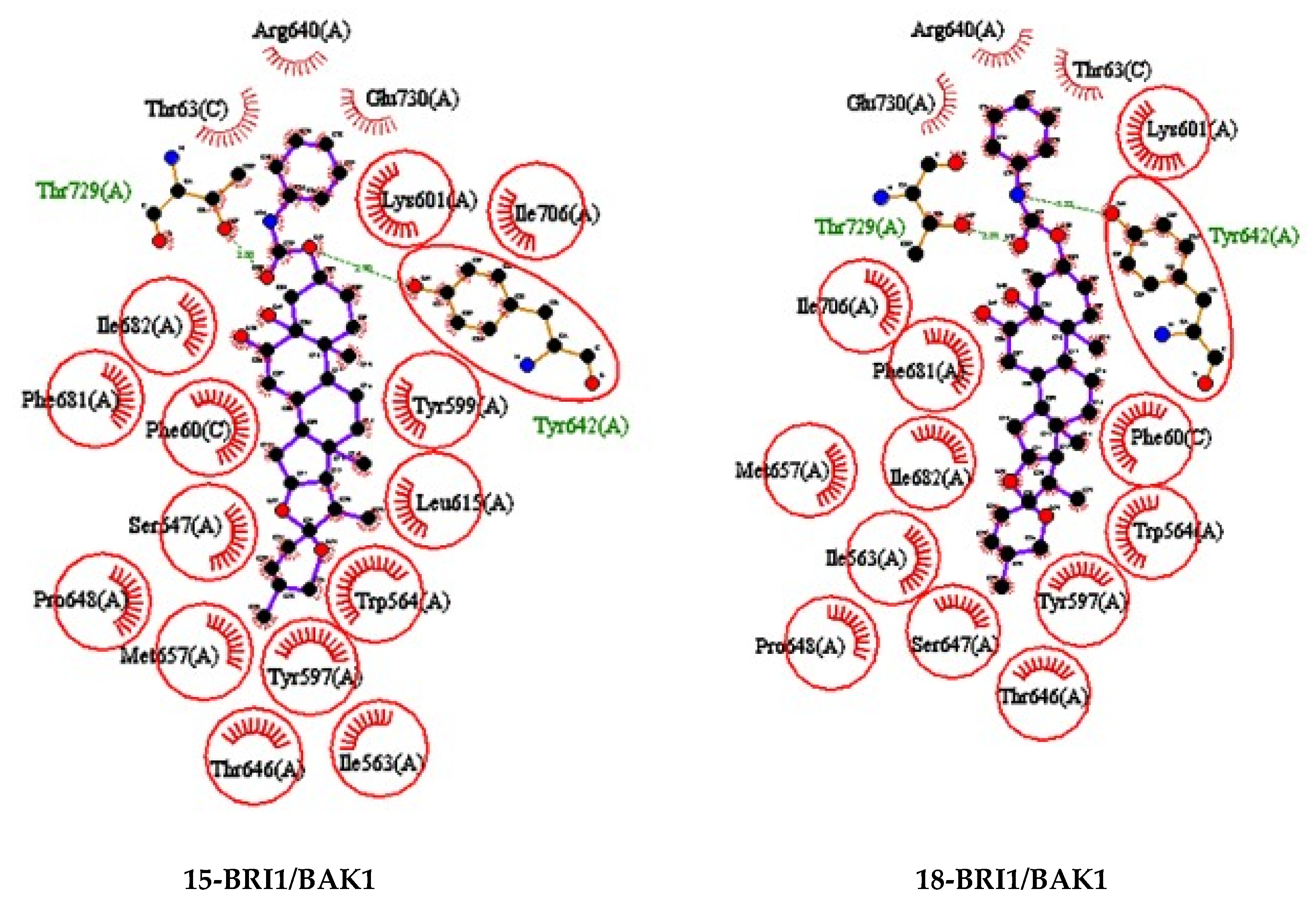
2.3. Molecular Docking Study
3. Materials and Methods.
3.1. Chemical
General
3.2. Methods of Synthesis
3.2.1. Synthesis of Carbamates
3.2.2. Synthesis of (25R)-5-en-Spirost-3β-yl Phenylcarbamate (7)
3.2.3. Synthesis of 5-en-Stigmast-3β-yl Phenylcarbamate (9)
3.2.4. Synthesis of 5,22-dien-Stigmast-3β-yl Phenylcarbamate (11)
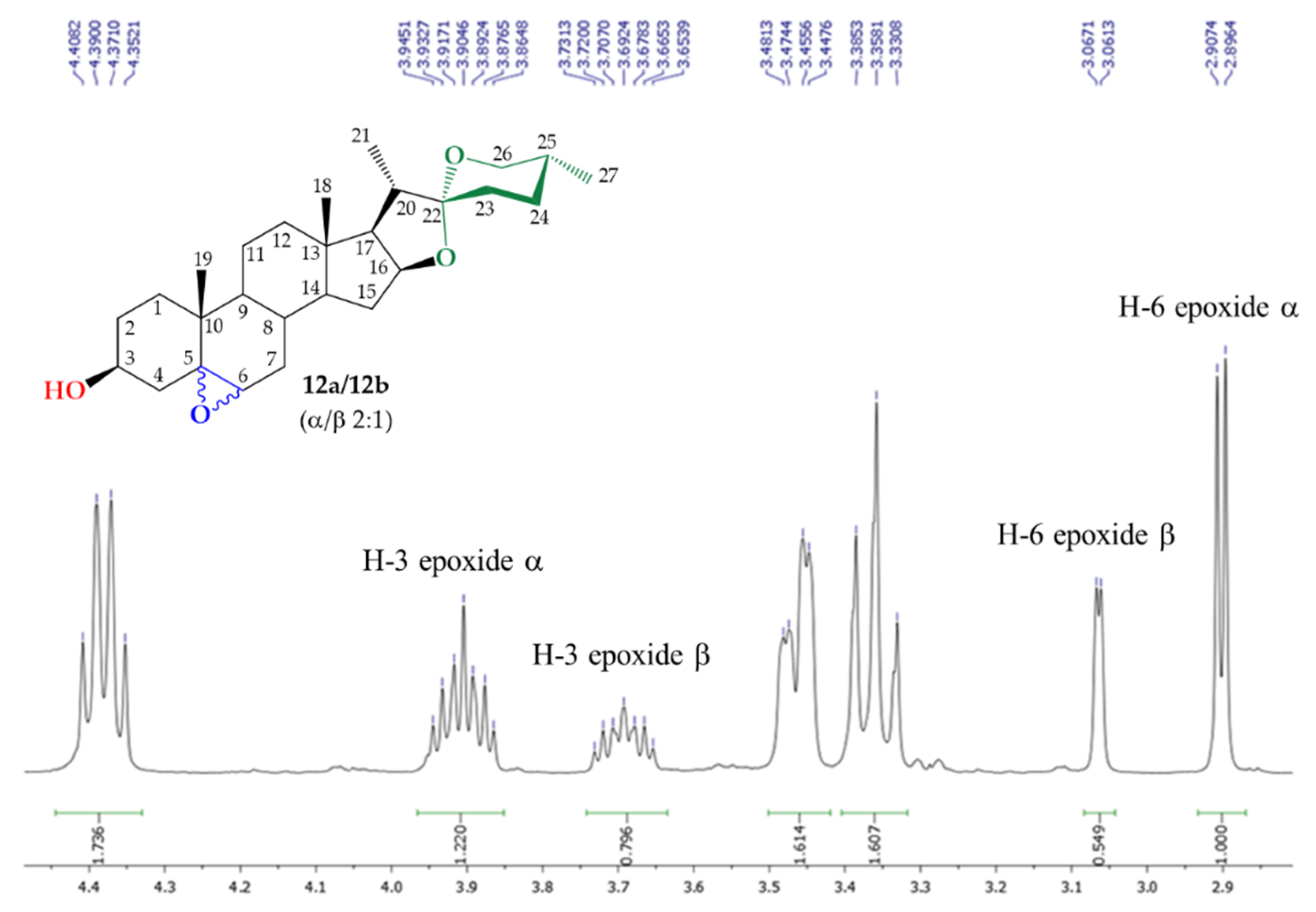
3.2.5. Synthesis of Mixture (25R)- 5α,6α-Epoxy-Spirostan-3β-ol (12a) and (25R)-5β,6β-Epoxy-Espirostan-3β-ol (12b)
3.2.6. Synthesis of (25R)-Spirostan-3β,5α,6β-Triol (13)
3.2.7. Synthesis of (25R)-3β,5α-Dihydroxy-Espirostan-6-ona (14)
3.2.8. Synthesis of (25R)- 5α- Hydroxy-6-oxo-Spirostan-3β-yl Phenylcarbamate (15)
3.2.9. Synthesis of Mixture (25R)-5α,6α-epoxy-Spirostan-3β-yl Phenylcarbamate (16a) and (25R)- 5β,6β-Epoxy-Spirostan-3β-yl Phenylcarbamate (16b)
3.2.10. Synthesis of ((25R)-5α-Hydroxy-6-Hydroxymino-3β-yl Phenylcarbamate (17)
3.2.11. Synthesis of (25R)-5α,6β-Dihydroxy-Spirostan-3β-yl Phenylcarbamate (18)
3.3. Rice-Lamina Inclination Assay
3.4. Molecular Docking
3.4.1. Ligand/Molecular Target Selection and Preparation
3.4.2. Docking Procedure and Analysis of Protein-Ligand Complexes.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BRI1 | Brassinosteroid Insensitive 1 |
| BAK1 | BRI-Associated Kinase 1 |
| BRs | Brassinosteroids |
| CC | Column Chromatography |
| TLC | Thin-Layer Chromatography |
| m-CPBA | meta-Chloroperoxybenzoic Acid |
| NBS | N-Bromosuccinimide |
| LRR-RLKs | Leucine-Rich Repeat Receptor-Like Kinases |
| SERK | Somatic Embryogenesis Receptor Kinase |
| NMR | Nuclear Magnetic Resonance |
| ppm | parts per millions |
| s | singlet |
| d | doublet |
| t | triplet |
| q | quartet |
| dd | double doublets |
| dt | double triplets |
| td | tripe doublets |
| ddd | doublet of double doublets |
| tt | triplet of triplets |
| m | multiplet |
| eq | equatorial H disposition |
| ax | axial H disposition |
| ESI-HRMS | High Resolution Electrospray Ionization Mass Spectrometry |
| n-hex | n-hexane |
| EtOAc | Ethyl acetate |
| THF | Tetrahydrofuran |
| DMSO | Dimethyl sulfoxide |
| PhNCO | phenyl isocyante |
| rt. | room temperature |
References
- Mitchell, J.W.; Mandava, N.; Worley, J.F.; Plimmer, J.R.; Smith, M.V. Brassins—A New Family of Plant Hormones from Rape Pollen. Nature 1970, 225, 1065–1066. [Google Scholar] [CrossRef]
- Grove, M.D.; Spencer, G.F.; Rohwedder, W.K.; Mandava, N.; Worley, J.F.; Warthen, J.D.; Steffens, G.L.; Flippenanderson, J.L.; Cook, J.C. Brassinolide, A Plant Growth-Promoting Steroid Isolated from Brassica-Napus Pollen. Nature 1979, 281, 216–217. [Google Scholar] [CrossRef]
- Clouse, S.D.; Sasse, J.M. Brassinosteroids: Essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 427–451. [Google Scholar] [CrossRef]
- Fujioka, S. Natural Occurrence of Brassinosteroids in the Plant Kingdom. In Brassinosteroids: Steroidal Plant Hormones; Sakurai, A., Yokota, T., Clouse, S.D., Eds.; Springer: Tokyo, Japan, 1999; pp. 21–45. [Google Scholar]
- Oklestkova, J.; Rarova, L.; Kvasnica, M.; Strnad, M. Brassinosteroids: Synthesis and biological activities. Phytochem. Rev. 2015, 14, 1053–1072. [Google Scholar] [CrossRef]
- Clouse, S.D. A History of Brassinosteroid Research from 1970 through 2005: Thirty-Five Years of Phytochemistry, Physiology, Genes, and Mutants. J. Plant Growth Regul. 2015, 34, 828–844. [Google Scholar] [CrossRef]
- Oh, M.-H.; Honey, S.H.; Tax, F.E. The Control of Cell Expansion, Cell Division, and Vascular Development by Brassinosteroids: A Historical Perspective. Int. J. Mol. Sci. 2020, 21, 1743. [Google Scholar] [CrossRef]
- Sasse, J.M. Physiological actions of brassinosteroids: An update. J. Plant Growth Regul. 2003, 22, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Bajguz, A. Brassinosteroids—Occurence and chemical structures in plants. In Brassinosteroids: A Class of Plant Hormone; Hayat, S., Ahmad, A., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 1–27. [Google Scholar]
- Peres, A.L.G.L.; Soares, J.S.; Tavares, R.G.; Righetto, G.; Zullo, M.A.T.; Mandava, N.B.; Menossi, M. Brassinosteroids, the Sixth Class of Phytohormones: A Molecular View from the Discovery to Hormonal Interactions in Plant Development and Stress Adaptation. Int. J. Mol. Sci. 2019, 20, 331. [Google Scholar] [CrossRef]
- Bajguz, A.; Hayat, S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bergonci, T.; Ribeiro, B.; Ceciliato, P.H.; Guerrero-Abad, J.C.; Silva-Filho, M.C.; Moura, D.S. Arabidopsis thaliana RALF1 opposes brassinosteroid effects on root cell elongation and lateral root formation. J. Exp. Bot. 2014, 65, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, D.; Sun, X.; Ding, T.; Lei, B.; Zhang, C. Structure-activity relationship of brassinosteroids and their agricultural practical usages. Steroids 2017, 124, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Khripach, V.; Zhabinskii, V.; De Groot, A. Twenty years of brassinosteroids: Steroidal plant hormones warrant better crops for the XXI century. Ann. Bot. 2000, 86, 441–447. [Google Scholar] [CrossRef]
- Salgado, R.; Cortes, M.A.; Del Rio, M.E. Uso de brasinoesteroides y sus análogos en la agricultura. Biologicas 2008, 10, 149–157. [Google Scholar]
- Wang, Q.; Xu, J.; Liu, X.; Gong, W.; Zhang, C. Synthesis of brassinosteroids analogues from laxogenin and their plant growth promotion. Nat. Prod. Res. 2015, 29, 149–157. [Google Scholar] [CrossRef]
- Perez, C.S.; Leliebre-Lara, V.; Concepcion, O.; Coll, F.; Rivera, D.G.; Pando, O. Synthesis and Biological Evaluation of Spirostanes Including Butyrolactone Moieties. Lett. Org. Chem. 2006, 3, 519–522. [Google Scholar] [CrossRef]
- Moreno-Castillo, E.; Ramírez-Echemendía, D.P.; Hernández-Campoalegre, G.; Mesa-Tejeda, D.; Coll-Manchado, F.; Coll-García, Y. In silico identification of new potentially active brassinosteroid analogues. Steroids 2018, 138, 35–42. [Google Scholar] [CrossRef]
- Takatsuto, S.; Ikekawa, N.; Morishita, T.; Abe, H. Structure Activity Relationship of Brassinosteroids with Respect to the A/B-Ring Functional-Groups. Chem. Pharm. Bull. 1987, 35, 211–216. [Google Scholar] [CrossRef]
- Brosa, C.; Capdevila, J.M.; Zamora, I. Brassinosteroids: A new way to define the structural requirements. Tetrahedron 1996, 52, 2435–2448. [Google Scholar] [CrossRef]
- Izquierdo, H.; Núñez, M.; González, M.C.; Proenza, R. Efectos de la aplicación de un análogo espirostánico de brasinoesteroides en vitroplantas de banano (Musa spp.) durante la fase de aclimatización. Cultiv. Trop. 2012, 33, 71–76. [Google Scholar]
- Mazorra, L.M.; Núñez, M. Estado Actual Sobre El Conocimiento De La Biosíntesis Y Los Mecanismos Moleculares De Acción De Los Brasinoesteroides En Las Plantas. Cultiv. Trop. 2008, 29, 91–105. [Google Scholar]
- Núñez, M.; Mazorra, L.M.; Reyes, Y.; Martínez, L. Los brasinoesteroides y las respuestas de las plantas a estrés abióticos. Una visión actualizada. Cultiv. Trop. 2010, 31, 56–65. [Google Scholar]
- Núñez Vázquez, M.; Reyes Guerrero, Y.; Rosabal Ayán, L.; Martínez González, L. Análogos espirostánicos de brasinoesteroides y sus potencialidades de uso en la agricultura. Cultiv. Trop. 2014, 35, 34–42. [Google Scholar]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine Function in Plants: Metabolism, Regulation on Development, and Roles in Abiotic Stress Responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Ledoux, S.; Witek, R.M.; Nair, S.K. 1-(Alkyl/arylthiocarbamoyl)benzotriazoles as stable isothiocyanate equivalents: Synthesis of Di- and trisubstituted thioureas. J. Org. Chem. 2004, 69, 2976–2982. [Google Scholar] [CrossRef]
- Wu, J.; Shi, Q.; Chen, Z.; He, M.; Jin, L.; Hu, D. Synthesis and bioactivity of pyrazole acyl thiourea derivatives. Molecules 2012, 17, 5139–5150. [Google Scholar] [CrossRef]
- Pivazyan, V.A.; Ghazaryan, E.A.; Shainova, R.S.; Tamazyan, R.A.; Ayvazyan, A.G.; Yengoyan, A.P. Synthesis and Growth Stimulant Properties of 2-Acetyl-3,7-dimethyl-5H-thiazolo[3,2-a]pyrimidin-5-one Derivatives. J. Chem. 2017, 2017, 8180913. [Google Scholar] [CrossRef]
- Korinkova, P.; Bazgier, V.; Oklestkova, J.; Rarova, L.; Strnad, M.; Kvasnica, M. Synthesis of novel aryl brassinosteroids through alkene cross-metathesis and preliminary biological study. Steroids 2017, 127, 46–55. [Google Scholar] [CrossRef]
- Duran, M.I.; Gonzalez, C.; Acosta, A.; Olea, A.F.; Diaz, K.; Espinoza, L. Synthesis of Five Known Brassinosteroid Analogs from Hyodeoxycholic Acid and Their Activities as Plant-Growth Regulators. Int. J. Mol. Sci. 2017, 18, 516. [Google Scholar] [CrossRef]
- Habibi, D.; Zolfigol, M.A.; Safaee, M. Synthesis of 1,4-Dihydropyridines Bearing a Carbamate Moiety on the 4-Position. J. Chem. 2013, 2013, 495982. [Google Scholar] [CrossRef]
- Han, K.S.; Ko, K.W.; Nam, S.J.; Park, S.H.; Kim, S.K. Optimization of a rice lamina inclination assay for detection of brassinosteroids: I. effect of phytohormones on the inclination activity. J. Plant Biol. 1997, 40, 240–244. [Google Scholar] [CrossRef]
- Iglesias-Arteaga, M.; Gil, R.; Leliebre-Lara, V.; Manchado, F.; Pérez, C.S.; Rosado, A. Synthesis of (25R)-5α-Spirostan-2α,3α,6β-triol Triacetate. Synth. Commun. 1998, 28, 75–81. [Google Scholar] [CrossRef]
- Iglesias-Arteaga, M.; Gil, R.P.; Leliebre-Lara, V.; Martinez, C.S.P.; Manchado, F.; Perez, A.R.; Rios, L.P. Synthesis of (22R,25R)-3 beta,26-dihydroxy-5 alpha-furostan-6-one. Synth. Commun. 1998, 28, 1381–1386. [Google Scholar] [CrossRef]
- Iglesias-Arteaga, M.A.; Símuta-Lopez, E.M.; Xochihua-Moreno, S.; Viñas-Bravo, O.; Montiel Smith, S.; Meza Reyes, S.; Sandoval-Ramírez, J. A Convenient Procedure for the Synthesis of 3β-Hydroxy-6-oxo-5α-steroids: Application to the Synthesis of Laxogenin. J. Mex. Chem. Soc. 2005, 49, 134–142. [Google Scholar]
- Rincón, S.; Del Río, R.E.; Sandoval-Ramírez, J.; Meza-Reyes, S.; Montiel-Smith, S.; Fernández, M.A.; Farfán, N.; Santillan, R. A new route for the preparation of the 22,23-dioxocholestane side chain from diosgenin and its application to the stereocontrolled construction of the 22R,23S-diol function. Tetrahedron 2006, 62, 2594–2602. [Google Scholar] [CrossRef]
- Romero-Avila, M.; De Dios-Bravo, G.; Mendez-Stivalet, J.M.; Rodriguez-Sotres, R.; Iglesias-Arteaga, M.A. Synthesis and biological activity of furostanic analogues of brassinosteroids bearing the 5 alpha-hydroxy-6-oxo moiety. Steroids 2007, 72, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Lawless, L.J.; Blackburn, A.G.; Ayling, A.J.; Pérez-Payán, M.N.; Davis, A.P. Steroidal guanidines as enantioselective receptors for N-acyl α-amino acids. Part 1. 3α-Guanylated carbamates derived from cholic acid. J. Chem. Soc. Perkin Trans. 1 2001, 1329–1341. [Google Scholar] [CrossRef]
- Bratoeff, E.; Sainz, T.; Cabeza, M.; Heuze, I.; Recillas, S.; Pérez, V.; Rodríguez, C.; Segura, T.; Gonzáles, J.; Ramírez, E. Steroids with a carbamate function at C-17, a novel class of inhibitors for human and hamster steroid 5alpha-reductase. J. Steroid Biochem. Mol. Biol. 2007, 107, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Mor, M.; Lodola, A.; Rivara, S.; Vacondio, F.; Duranti, A.; Tontini, A.; Sanchini, S.; Piersanti, G.; Clapper, J.R.; King, A.R.; et al. Synthesis and quantitative structure-activity relationship of fatty acid amide hydrolase inhibitors: Modulation at the N-portion of biphenyl-3-yl alkylcarbamates. J. Med. Chem. 2008, 51, 3487–3498. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, E.; Kim, J. Epoxidation of Diosgenin, 25(R)-1,4,6-Spirostatrien-3-one and 25(R)-4,6-Spirostadien-3β-ol. Molecules 2003, 8, 886–893. [Google Scholar] [CrossRef]
- Diggle, J.M.; Halliday, M.D.; Meakins, G.D.; Saltmarsh, M.S. The acid-catalysed ring-opening of epoxides in a largely nonaqueous medium. J. Chem. Soc. D Chem. Commun. 1969, 819–820. [Google Scholar] [CrossRef]
- Khurana, J.M.; Kandpal, B.M. A novel method of synthesis of 1,2-diketones from 1,2-diols using N-bromosuccinimide. Tetrahedron Lett. 2003, 44, 4909–4912. [Google Scholar] [CrossRef]
- Rodríguez, J.; Nuñez, L.; Peixinho, S.; Jiménez, C. Isolation and synthesis of the first natural 6-hydroximino 4-en-3-one- steroids from the sponges Cinachyrella spp. Tetrahedron Lett. 1997, 38, 1833–1836. [Google Scholar] [CrossRef]
- Cui, J.; Fan, L.; Huang, Y.; Xin, Y.; Zhou, A. Synthesis and evaluation of some steroidal oximes as cytotoxic agents: Structure/activity studies (II). Steroids 2009, 74, 989–995. [Google Scholar] [CrossRef]
- Clouse, S.D. Brassinosteroid signal transduction: From receptor kinase activation to transcriptional networks regulating plant development. Plant Cell 2011, 23, 1219–1230. [Google Scholar] [CrossRef]
- Gampala, S.S.; Kim, T.W.; He, J.X.; Tang, W.; Deng, Z.; Bai, M.Y.; Guan, S.; Lalonde, S.; Sun, Y.; Gendron, J.M.; et al. An essential role for 14-3-3 proteins in brassinosteroid signal transduction in Arabidopsis. Dev. Cell 2007, 13, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, M.; Khan, T.A.; Fariduddin, Q. Brassinosteroids: Physiological Roles and its Signalling in Plants. In Stress Signaling in Plants: Genomics and Proteomics Perspective; Sarwat, M., Ahmad, A., Abdin, M.Z., Ibrahim, M.M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 2, pp. 241–260. [Google Scholar]
- Hothorn, M.; Belkhadir, Y.; Dreux, M.; Dabi, T.; Noel, J.P.; Wilson, I.A.; Chory, J. Structural basis of steroid hormone perception by the receptor kinase BRI1. Nature 2011, 474, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Kvasnica, M.; Oklestkova, J.; Bazgier, V.; Rárová, L.; Korinkova, P.; Mikulík, J.; Budesinsky, M.; Béres, T.; Berka, K.; Lu, Q.; et al. Design, synthesis and biological activities of new brassinosteroid analogues with a phenyl group in the side chain. Org. Biomol. Chem. 2016, 14, 8691–8701. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.; Henzler, C.; Hothorn, M. Molecular Mechanism for Plant Steroid Receptor Activation by Somatic Embryogenesis Co-Receptor Kinases. Science 2013, 341, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- She, J.; Han, Z.; Zhou, B.; Chai, J. Structural basis for differential recognition of brassinolide by its receptors. Protein Cell 2013, 4, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand–Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.C.; Laskowski, R.A.; Thornton, J.M. LIGPLOT: A program to generate schematic diagrams of protein-ligand interactions. Protein Eng. Des. Sel. 1995, 8, 127–134. [Google Scholar] [CrossRef]
- Lei, B.; Liu, J.; Yao, X. Unveiling the molecular mechanism of brassinosteroids: Insights from structure-based molecular modeling studies. Steroids 2015, 104, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lu, Y.; Wang, S. Comparative evaluation of 11 scoring functions for molecular docking. J. Med. Chem. 2003, 46, 2287–2303. [Google Scholar] [CrossRef] [PubMed]
- Deive, N.; Rodríguez, J.; Jiménez, C. Synthesis of Cytotoxic 6E-Hydroximino-4-ene Steroids: Structure/Activity Studies. J. Med. Chem. 2001, 44, 2612–2618. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, Y.; Alonso, E.; Coll, F.; Coll-García, D.; Pérez, C.; Agüero, G. Synthesis of (22R,23R)-22,23-epoxy-3β,5α-dihydroxystigmastan-6-one from Stigmasterol. J. Chem. Res. 2005, 2005, 475–477. [Google Scholar] [CrossRef]
- Bernardo-Otero, Y.; Alonso-Becerra, E.; Guerra-Martínez, F.; Martínez-Massanet, G.; Pérez-Martínez, C.; Coll-Manchado, F. Synthesis and Biological Activity of Epoxy Analogues of 3-dehydroteasterone. J. Chem. Res. 2007, 2007, 268–271. [Google Scholar] [CrossRef]
- Allinger, N.L. Conformational analysis. 130. MM2. A hydrocarbon force field utilizing V1 and V2 torsional terms. J. Am. Chem. Soc. 1977, 99, 8127–8134. [Google Scholar] [CrossRef]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar]




| BRs Analogs | Inclination between Laminae and Sheaths (Degrees ± Standard Error) | ||
|---|---|---|---|
| Number | Concentration (μM) | ||
| 0.01 | 0.10 | 1.0 | |
| 7 | 21 ± 3 a | 16 ± 5 c | 21 ± 3 a |
| 9 | 35 ± 3 b | 30 ± 3 b | 11 ± 5 c |
| 11 | 31 ± 3 b | 31 ± 3 b | 14 ± 1 c |
| 15 | 29 ± 1 b | 33 ± 3 b | 40 ± 3 b |
| 16a/16b | 25 ± 3 a | 51 ± 1 d | 41 ± 1 b |
| 17 | 13 ± 5 c | 36 ± 5 b | 38 ± 2 b |
| 18 | 13 ± 5 c | 29 ± 1 b | 29 ± 1 b |
| 1 (C+) | 34 ± 6 b | 55 ± 8 d | 78 ± 9 e |
| C- (water) | 11 ± 6 c | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, D.F.; González Ceballos, L.; Castro, A.Z.; Conde González, M.R.; González de la Torre, L.; Rostgaard, L.P.; Espinoza, L.; Díaz, K.; Olea, A.F.; Coll García, Y. Synthesis of New Steroidal Carbamates with Plant-Growth-Promoting Activity: Theoretical and Experimental Evidence. Int. J. Mol. Sci. 2021, 22, 2330. https://doi.org/10.3390/ijms22052330
Pacheco DF, González Ceballos L, Castro AZ, Conde González MR, González de la Torre L, Rostgaard LP, Espinoza L, Díaz K, Olea AF, Coll García Y. Synthesis of New Steroidal Carbamates with Plant-Growth-Promoting Activity: Theoretical and Experimental Evidence. International Journal of Molecular Sciences. 2021; 22(5):2330. https://doi.org/10.3390/ijms22052330
Chicago/Turabian StylePacheco, Daylin Fernández, Leonardo González Ceballos, Armando Zaldo Castro, Marcos R. Conde González, Laura González de la Torre, Lia Pérez Rostgaard, Luis Espinoza, Katy Díaz, Andrés F. Olea, and Yamilet Coll García. 2021. "Synthesis of New Steroidal Carbamates with Plant-Growth-Promoting Activity: Theoretical and Experimental Evidence" International Journal of Molecular Sciences 22, no. 5: 2330. https://doi.org/10.3390/ijms22052330
APA StylePacheco, D. F., González Ceballos, L., Castro, A. Z., Conde González, M. R., González de la Torre, L., Rostgaard, L. P., Espinoza, L., Díaz, K., Olea, A. F., & Coll García, Y. (2021). Synthesis of New Steroidal Carbamates with Plant-Growth-Promoting Activity: Theoretical and Experimental Evidence. International Journal of Molecular Sciences, 22(5), 2330. https://doi.org/10.3390/ijms22052330






