Applications of Mesenchymal Stem Cells in Skin Regeneration and Rejuvenation
Abstract
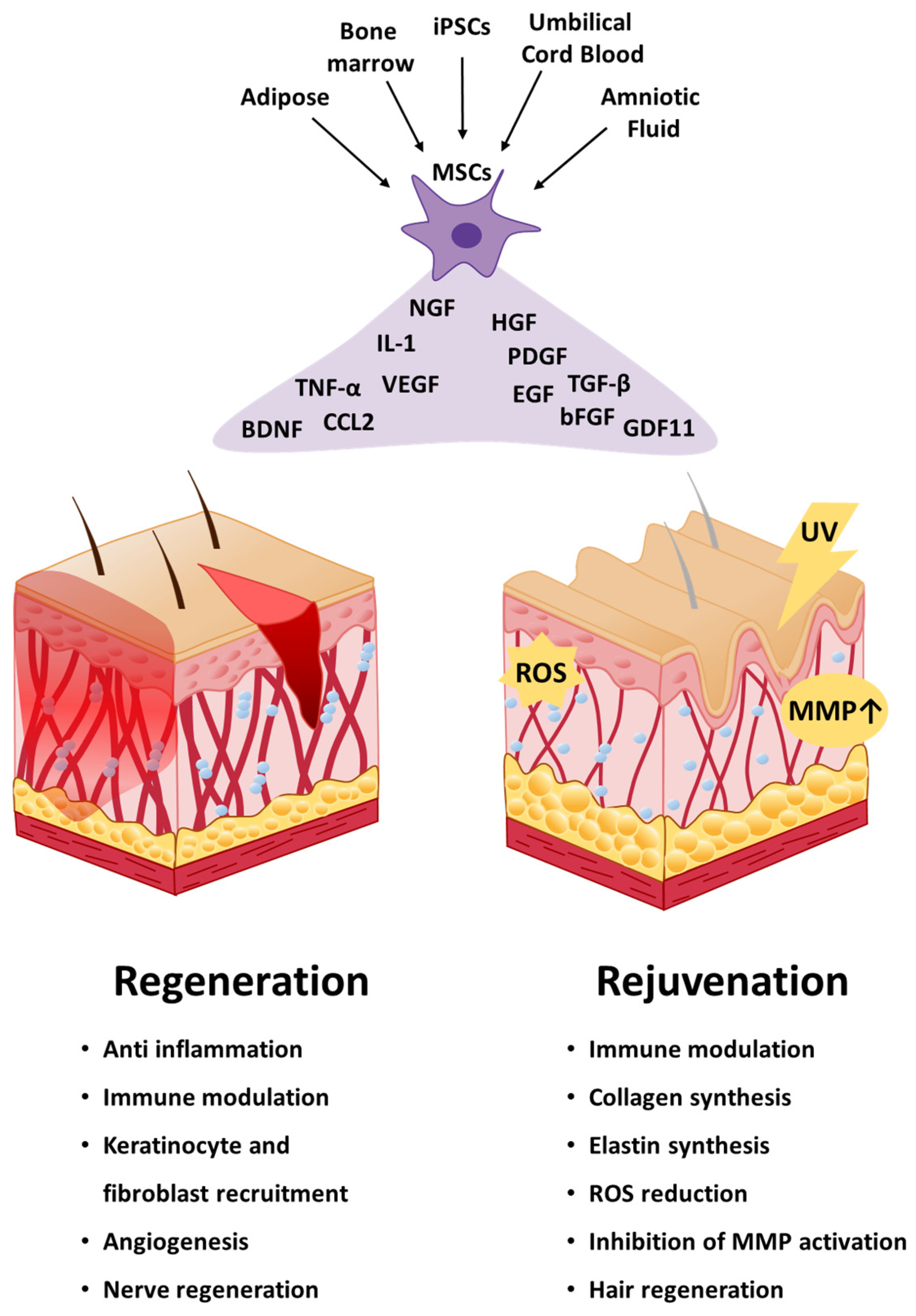
1. Mesenchymal Stem Cells
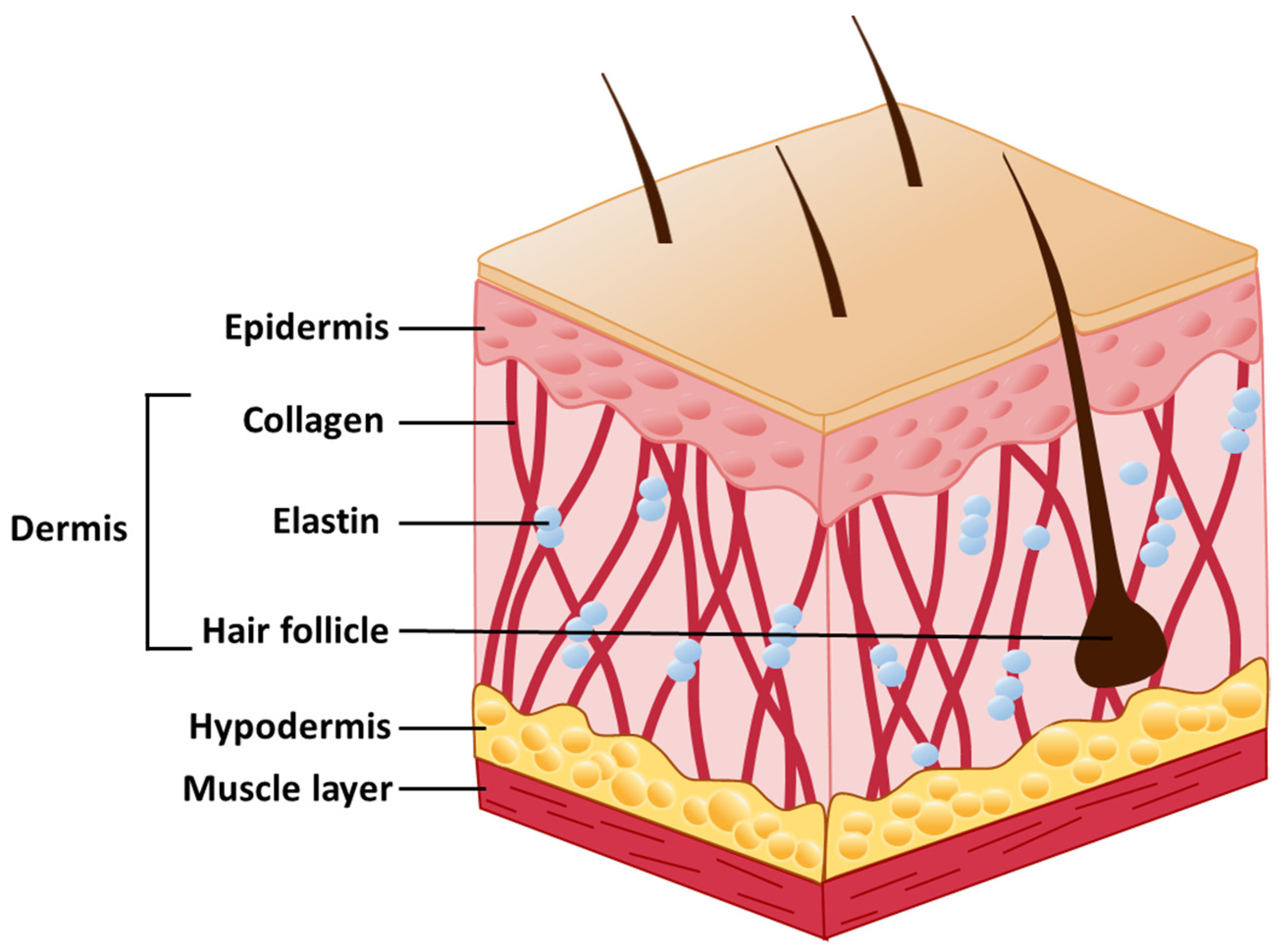
2. Skin Structure
3. Applications of Mesenchymal Stem Cells in Skin Regeneration
3.1. Wound Healing
3.2. Burn Injury
4. Applications of Mesenchymal Stem Cells in Skin Rejuvenation
4.1. Antiaging
4.2. Hair Loss
5. Induced Pluripotent Stem Cell-derived Mesenchymal Stem Cells
6. Embryonic Stem Cells-Derived Mesenchymal Stem Cells
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mizukami, A.; Swiech, K. Mesenchymal stromal cells: From discovery to manufacturing and commercialization. Stem. Cells Int. 2018, 2018, 4083921. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) mesenchymal stromal cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar]
- Prianishnikov, V.A. On the concept of stem cell and a model of functional-morphological structure of the endometrium. Contraception 1978, 18, 213–223. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Nat. Acad. Sci. USA 2000, 97, 13625. [Google Scholar] [CrossRef]
- Qu-Petersen, Z.; Deasy, B.; Jankowski, R.; Ikezawa, M.; Cummins, J.; Pruchnic, R.; Mytinger, J.; Cao, B.; Gates, C.; Wernig, A.; et al. Identification of a novel population of muscle stem cells in mice: Potential for muscle regeneration. J. Cell Biol. 2002, 157, 851–864. [Google Scholar] [CrossRef] [PubMed]
- In’t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; de Groot-Swings, G.M.J.S.; Claas, F.H.J.; Fibbe, W.E.; Kanhai, H.H.H. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Doi, H.; Kitajima, Y.; Luo, L.; Yan, C.; Tateishi, S.; Ono, Y.; Urata, Y.; Goto, S.; Mori, R.; Masuzaki, H.; et al. Potency of umbilical cord blood- and Wharton’s jelly-derived mesenchymal stem cells for scarless wound healing. Sci. Rep. 2016, 6, 18844. [Google Scholar] [CrossRef] [PubMed]
- McElreavey, K.D.; Irvine, A.I.; Ennis, K.T.; McLean, W.H.I. Isolation, culture and characterisation of fibroblast-like cells derived from the Wharton’s jelly portion of human umbilical cord. Biochem. Soc. Trans. 1991, 19, 29S. [Google Scholar] [CrossRef]
- Steens, J.; Klein, D. Current strategies to generate human mesenchymal stem cells in vitro. Stem Cells Int. 2018, 2018, 6726185. [Google Scholar] [CrossRef]
- Beeravolu, N.; McKee, C.; Alamri, A.; Mikhael, S.; Brown, C.; Perez-Cruet, M.; Chaudhry, G.R. Isolation and characterization of mesenchymal stromal cells from human umbilical cord and fetal placenta. J. Vis. Exp. 2017, 122, 55224. [Google Scholar] [CrossRef] [PubMed]
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.R.; Soria, B.; Capilla-Gonzalez, V. Therapeutic potential of mesenchymal stem cells for cancer therapy. Front. Bioeng. Biotechnol. 2020, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-Z.; Su, W.-R.; Shi, S.-H.; Wilder-Smith, P.; Xiang, A.P.; Wong, A.; Nguyen, A.L.; Kwon, C.W.; Le, A.D. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells 2010, 28, 1856–1868. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Abe, R.; Fujita, Y.; Ando, S.; Inokuma, D.; Shimizu, H. Mesenchymal Stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J. Immunol. 2008, 180, 2581. [Google Scholar] [CrossRef] [PubMed]
- Roura, S.; Pujal, J.M.; Gálvez-Montón, C.; Bayes-Genis, A. Impact of umbilical cord blood-derived mesenchymal stem cells on cardiovascular research. BioMed Res. Int. 2015, 2015, 975302. [Google Scholar] [CrossRef] [PubMed]
- Perdisa, F.; Gostyńska, N.; Roffi, A.; Filardo, G.; Marcacci, M.; Kon, E. Adipose-derived mesenchymal stem cells for the treatment of articular cartilage: a systematic review on preclinical and clinical evidence. Stem Cells Int. 2015, 2015, 597652. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, L.; Hufnagel, D.; Taylor, H.S. The endometrium as a source of mesenchymal stem cells for regenerative medicine. Biol. Reprod. 2015, 92, 138. [Google Scholar] [CrossRef]
- Satoh, H.; Kishi, K.; Tanaka, T.; Kubota, Y.; Nakajima, T.; Akasaka, Y.; Ishii, T. Transplanted mesenchymal stem cells are effective for skin regeneration in acute cutaneous wounds. Cell Transplant. 2004, 13, 405–412. [Google Scholar] [CrossRef]
- Nourian Dehkordi, A.; Mirahmadi Babaheydari, F.; Chehelgerdi, M.; Raeisi Dehkordi, S. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Yoo, S.M.; Park, H.H.; Lim, H.J.; Kim, Y.-L.; Lee, S.; Seo, K.-W.; Kang, K.-S. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulates rejuvenation of human skin. Biochem. Biophys. Res. Commun. 2017, 493, 1102–1108. [Google Scholar] [CrossRef]
- Zarei, F.; Abbaszadeh, A. Application of cell therapy for anti-aging facial skin. Curr. Stem Cell Res. Ther. 2019, 14, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Gaur, M.; Dobke, M.; Lunyak, V.V. Mesenchymal stem cells from adipose tissue in clinical applications for dermatological indications and skin aging. Int. J. Mol. Sci. 2017, 18, 208. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Li, L.; Fuchs, E. Emerging interactions between skin stem cells and their niches. Nat. Med. 2014, 20, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Soulika, A.M. The dynamics of the skin’s immune system. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef]
- Wikramanayake, T.C.; Stojadinovic, O.; Tomic-Canic, M. Epidermal differentiation in barrier maintenance and wound healing. Adv. Wound Care 2014, 3, 272–280. [Google Scholar] [CrossRef]
- Bragulla, H.H.; Homberger, D.G. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J. Anat. 2009, 214, 516–559. [Google Scholar] [CrossRef]
- Yousef, H.; Alhajj, M.; Sharma, S. Anatomy, Skin (Integument), Epidermis; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Roger, M.; Fullard, N.; Costello, L.; Bradbury, S.; Markiewicz, E.; O’Reilly, S.; Darling, N.; Ritchie, P.; Määttä, A.; Karakesisoglou, I.; et al. Bioengineering the microanatomy of human skin. J. Anat. 2019, 234, 438–455. [Google Scholar] [CrossRef]
- Pincelli, C.; Marconi, A. Keratinocyte stem cells: Friends and foes. J. Cell. Physiol. 2010, 225, 310–315. [Google Scholar] [CrossRef]
- Clayton, K.; Vallejo, A.F.; Davies, J.; Sirvent, S.; Polak, M.E. Langerhans cells—Programmed by the epidermis. Front. Immunol. 2017, 8, 1676. [Google Scholar] [CrossRef]
- Haeberle, H.; Lumpkin, E.A. Merkel cells in somatosensation. Chemosens. Percept. 2008, 1, 110–118. [Google Scholar] [CrossRef]
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tymińska, A. Skin melanocytes: Biology and development. Postepy Dermatol. Alergol. 2013, 30, 30–41. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Sorrell, J.M.; Caplan, A.I. Fibroblast heterogeneity: More than skin deep. J. Cell Sci. 2004, 117, 667. [Google Scholar] [CrossRef]
- Roig-Rosello, E.; Rousselle, P. The human epidermal basement membrane: A shaped and cell instructive platform that aging slowly alters. Biomolecules 2020, 10, 1607. [Google Scholar] [CrossRef]
- Prost-Squarcioni, C.; Fraitag, S.; Heller, M.; Boehm, N. Functional histology of dermis. Ann. Dermatol. Venereol. 2008, 135, 1s5–20. [Google Scholar] [CrossRef]
- Grinnell, F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003, 13, 264–269. [Google Scholar] [CrossRef]
- Muiznieks, L.D.; Keeley, F.W. Molecular assembly and mechanical properties of the extracellular matrix: A fibrous protein perspective. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2013, 1832, 866–875. [Google Scholar] [CrossRef]
- Moon, T.C.; Befus, A.D.; Kulka, M. Mast cell mediators: Their differential release and the secretory pathways involved. Front. Immunol. 2014, 5, 569. [Google Scholar] [CrossRef]
- Driskell, R.R.; Jahoda, C.A.B.; Chuong, C.-M.; Watt, F.M.; Horsley, V. Defining dermal adipose tissue. Exp. Dermatol. 2014, 23, 629–631. [Google Scholar] [CrossRef]
- Stecco, C.; Hammer, W.; Vleeming, A.; De Caro, R. Functional Atlas of the Human Fascial System.; Stecco, C., Hammer, W., Vleeming, A., De Caro, R., Eds.; Churchill Livingstone: London, UK, 2015; pp. 21–49. [Google Scholar]
- Menon, G.K. New insights into skin structure: Scratching the surface. Adv. Drug Deliv. Rev. 2002, 54, S3–S17. [Google Scholar] [CrossRef]
- Gerber, P.A.; Buhren, B.A.; Schrumpf, H.; Homey, B.; Zlotnik, A.; Hevezi, P. The top skin-associated genes: A comparative analysis of human and mouse skin transcriptomes. Biol. Chem. 2014, 395, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Doeing, D.C.; Borowicz, J.L.; Crockett, E.T. Gender dimorphism in differential peripheral blood leukocyte counts in mice using cardiac, tail, foot, and saphenous vein puncture methods. BMC Clin. Pathol. 2003, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Gallo, R.L. Antimicrobial peptides in human skin disease. Eur. J. Dermatol. EJD 2008, 18, 11–21. [Google Scholar]
- Schittek, B.; Hipfel, R.; Sauer, B.; Bauer, J.; Kalbacher, H.; Stevanovic, S.; Schirle, M.; Schroeder, K.; Blin, N.; Meier, F.; et al. Dermcidin: A novel human antibiotic peptide secreted by sweat glands. Nat. Immunol. 2001, 2, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Cibelli, J.; Emborg, M.E.; Prockop, D.J.; Roberts, M.; Schatten, G.; Rao, M.; Harding, J.; Mirochnitchenko, O. Strategies for improving animal models for regenerative medicine. Cell Stem Cell 2013, 12, 271–274. [Google Scholar] [CrossRef]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin tissue regeneration for burn injury. Stem Cell Res. Ther. 2019, 10, 94. [Google Scholar] [CrossRef]
- Hu, M.S.; Borrelli, M.R.; Lorenz, H.P.; Longaker, M.T.; Wan, D.C. Mesenchymal stromal cells and cutaneous wound healing: A comprehensive review of the background, role, and therapeutic potential. Stem Cells Int. 2018, 2018, 6901983. [Google Scholar] [CrossRef]
- Marfia, G.; Navone, S.E.; Di Vito, C.; Ughi, N.; Tabano, S.; Miozzo, M.; Tremolada, C.; Bolla, G.; Crotti, C.; Ingegnoli, F.; et al. Mesenchymal stem cells: Potential for therapy and treatment of chronic non-healing skin wounds. Organogenesis 2015, 11, 183–206. [Google Scholar] [CrossRef]
- Chiossone, L.; Conte, R.; Spaggiari, G.M.; Serra, M.; Romei, C.; Bellora, F.; Becchetti, F.; Andaloro, A.; Moretta, L.; Bottino, C. Mesenchymal stromal cells induce peculiar alternatively activated macrophages capable of dampening both innate and adaptive immune responses. Stem Cells 2016, 34, 1909–1921. [Google Scholar] [CrossRef]
- He, X.; Dong, Z.; Cao, Y.; Wang, H.; Liu, S.; Liao, L.; Jin, Y.; Yuan, L.; Li, B. MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Int. 2019, 2019, 7132708. [Google Scholar] [CrossRef]
- Di, G.; Du, X.; Qi, X.; Zhao, X.; Duan, H.; Li, S.; Xie, L.; Zhou, Q. Mesenchymal stem cells promote diabetic corneal epithelial wound healing through tsg-6-dependent stem cell activation and macrophage switch. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4344–4354. [Google Scholar] [CrossRef]
- Qi, Y.; Jiang, D.; Sindrilaru, A.; Stegemann, A.; Schatz, S.; Treiber, N.; Rojewski, M.; Schrezenmeier, H.; Vander Beken, S.; Wlaschek, M.; et al. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. J. Investig. Dermatol. 2014, 134, 526–537. [Google Scholar] [CrossRef]
- Song, W.J.; Li, Q.; Ryu, M.O.; Ahn, J.O.; Bhang, D.H.; Jung, Y.C.; Youn, H.Y. TSG-6 released from intraperitoneally injected canine adipose tissue-derived mesenchymal stem cells ameliorate inflammatory bowel disease by inducing M2 macrophage switch in mice. Stem Cell Res. Ther. 2018, 9, 91. [Google Scholar] [CrossRef]
- Hu, C.; Yong, X.; Li, C.; Lü, M.; Liu, D.; Chen, L.; Hu, J.; Teng, M.; Zhang, D.; Fan, Y.; et al. CXCL12/CXCR4 axis promotes mesenchymal stem cell mobilization to burn wounds and contributes to wound repair. J. Surg. Res. 2013, 183, 427–434. [Google Scholar] [CrossRef]
- Roubelakis, M.G.; Trohatou, O.; Roubelakis, A.; Mili, E.; Kalaitzopoulos, I.; Papazoglou, G.; Pappa, K.I.; Anagnou, N.P. Platelet-rich plasma (PRP) promotes fetal mesenchymal stem/stromal cell migration and wound healing process. Stem Cell Rev. Rep. 2014, 10, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Stessuk, T.; Puzzi, M.B.; Chaim, E.A.; Alves, P.C.; de Paula, E.V.; Forte, A.; Izumizawa, J.M.; Oliveira, C.C.; Frei, F.; Ribeiro-Paes, J.T. Platelet-rich plasma (PRP) and adipose-derived mesenchymal stem cells: Stimulatory effects on proliferation and migration of fibroblasts and keratinocytes in vitro. Arch. Dermatol. Res. 2016, 308, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.X.; Xu, P.C.; Zhang, L.; Pang, M.R.; Tian, J.; Cheng, B. Effects of human adipose-derived mesenchymal stem cells and platelet-rich plasma on healing of wounds with full-thickness skin defects in mice. Zhonghua Shao Shang Za Zhi Zhonghua Shaoshang Zazhi Chin. J. Burns 2018, 34, 887–894. [Google Scholar]
- Holmes, H.L.; Wilson, B.; Goerger, J.P.; Silverberg, J.L.; Cohen, I.; Zipfel, W.R.; Fortier, L.A. Facilitated recruitment of mesenchymal stromal cells by bone marrow concentrate and platelet rich plasma. PLoS ONE 2018, 13, e0194567. [Google Scholar] [CrossRef]
- Furumoto, T.; Ozawa, N.; Inami, Y.; Toyoshima, M.; Fujita, K.; Zaiki, K.; Sahara, S.; Akita, M.; Kitamura, K.; Nakaoji, K.; et al. Mallotus philippinensis bark extracts promote preferential migration of mesenchymal stem cells and improve wound healing in mice. Phytomed. Int. J. F Phytother. Phytopharmacol. 2014, 21, 247–253. [Google Scholar] [CrossRef]
- Fujita, K.; Kuge, K.; Ozawa, N.; Sahara, S.; Zaiki, K.; Nakaoji, K.; Hamada, K.; Takenaka, Y.; Tanahashi, T.; Tamai, K.; et al. Cinnamtannin B-1 promotes migration of mesenchymal stem cells and accelerates wound healing in mice. PLoS ONE 2015, 10, e0144166. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, K.; Kweon, O.K.; Kim, W.H. Enhanced wound healing effect of canine adipose-derived mesenchymal stem cells with low-level laser therapy in athymic mice. J. Dermatol. Sci. 2012, 68, 149–156. [Google Scholar] [CrossRef]
- Whelan, D.S.; Caplice, N.M.; Clover, A.J.P. Mesenchymal stromal cell derived CCL2 is required for accelerated wound healing. Sci. Rep. 2020, 10, 2642. [Google Scholar] [CrossRef]
- Shou, K.; Niu, Y.; Zheng, X.; Ma, Z.; Jian, C.; Qi, B.; Hu, X.; Yu, A. Enhancement of bone-marrow-derived mesenchymal stem cell angiogenic capacity by NPWT for a combinatorial therapy to promote wound healing with large defect. BioMed Res. Int. 2017, 2017, 7920265. [Google Scholar] [CrossRef] [PubMed]
- Rustad, K.C.; Wong, V.W.; Sorkin, M.; Glotzbach, J.P.; Major, M.R.; Rajadas, J.; Longaker, M.T.; Gurtner, G.C. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials 2012, 33, 80–90. [Google Scholar] [CrossRef]
- Peng, L.-H.; Xu, X.-H.; Huang, Y.-F.; Zhao, X.-L.; Zhao, B.; Cai, S.-Y.; Xie, M.-J.; Wang, M.-Z.; Yuan, T.-J.; He, Y.; et al. Self-adaptive all-in-one delivery chip for rapid skin nerves regeneration by endogenous mesenchymal stem cells. Adv. Funct. Mater. 2020, 30, 2001751. [Google Scholar] [CrossRef]
- Shou, K.; Huang, Y.; Qi, B.; Hu, X.; Ma, Z.; Lu, A.; Jian, C.; Zhang, L.; Yu, A. Induction of mesenchymal stem cell differentiation in the absence of soluble inducer for cutaneous wound regeneration by a chitin nanofiber-based hydrogel. J. Tissue Eng. Regen. Med. 2018, 12, e867–e880. [Google Scholar] [CrossRef]
- Nakayama, C.; Fujita, Y.; Matsumura, W.; Ujiie, I.; Takashima, S.; Shinkuma, S.; Nomura, T.; Abe, R.; Shimizu, H. The development of induced pluripotent stem cell-derived mesenchymal stem/stromal cells from normal human and RDEB epidermal keratinocytes. J. Dermatol. Sci. 2018, 91, 301–310. [Google Scholar] [CrossRef]
- Yoon, D.; Yoon, D.; Sim, H.; Hwang, I.; Lee, J.S.; Chun, W. Accelerated wound healing by fibroblasts differentiated from human embryonic stem cell-derived mesenchymal stem cells in a pressure ulcer animal model. Stem Cells Int. 2018, 2018, 4789568. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Zhao, L.; Yang, W.; Wan, X.; Yue, C.; Mo, Z. Mesenchymal stem cells coated by the extracellular matrix promote wound healing in diabetic rats. Stem Cells Int. 2019, 2019, 9564869. [Google Scholar]
- Luz-Crawford, P.; Djouad, F.; Toupet, K.; Bony, C.; Franquesa, M.; Hoogduijn, M.; Jorgensen, C.; Noël, D. Mesenchymal stem CelŒ derived interleukin 1 receptor antagonist promotes macrophage polarization and inhibits B cell differentiation. Stem Cells 2016, 34, 483–492. [Google Scholar] [CrossRef]
- Ikeda, S.; Saijo, S.; Murayama, M.A.; Shimizu, K.; Akitsu, A.; Iwakura, Y. Excess IL-1 signaling enhances the development of Th17 cells by downregulating TGF-β-induced Foxp3 expression. J. Immunol. 2014, 192, 1449–1458. [Google Scholar] [CrossRef]
- Zhao, M.M.; Cui, J.Z.; Cui, Y.; Li, R.; Tian, Y.X.; Song, S.X.; Zhang, J.; Gao, J.L. Therapeutic effect of exogenous bone marrow-derived mesenchymal stem cell transplantation on silicosis via paracrine mechanisms in rats. Mol. Med. Rep. 2013, 8, 741–746. [Google Scholar] [CrossRef]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of wound healing. Curr Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Y.; Hao, H.; Dai, H.; Han, Q.; Tong, C.; Liu, J.; Han, W.; Fu, X. Mesenchymal stem cell–conditioned medium improves the proliferation and migration of keratinocytes in a diabetes-like microenvironment. Int. J. Lower Extrem. Wounds 2015, 14, 73–86. [Google Scholar] [CrossRef]
- Smith, A.N.; Willis, E.; Chan, V.T.; Muffley, L.A.; Isik, F.F.; Gibran, N.S.; Hocking, A.M. Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp. Cell Res. 2010, 316, 48–54. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, J.; Zheng, C.; Su, Y.; Bao, L.; Zhu, B.; Liu, S.; Wang, L.; Wang, X.; Wang, Y.; et al. Exosomes released from educated mesenchymal stem cells accelerate cutaneous wound healing via promoting angiogenesis. Cell Prolif. 2020, 53, e12830. [Google Scholar] [CrossRef]
- Martin-Piedra, M.Á.; Alfonso-Rodríguez, C.A.; Zapater Latorre, A.; Durand-Herrera, D.; Chato-Astrain, J.; Campos, F.; Sánchez Quevedo, M.d.C.; Alaminos Mingorance, M.; Garzón Bello, I.J. Effective use of mesenchymal stem cells in human skin substitutes generated by tissue engineering. Eur. Cell Mater. 2019, 37, 233–249. [Google Scholar] [CrossRef]
- Cooney, D.S.; Wimmers, E.G.; Ibrahim, Z.; Grahammer, J.; Christensen, J.M.; Brat, G.A.; Wu, L.W.; Sarhane, K.A.; Lopez, J.; Wallner, C.; et al. Mesenchymal stem cells enhance nerve regeneration in a rat sciatic nerve repair and hindlimb transplant model. Sci. Rep. 2016, 6, 31306. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte–Fibroblast Interactions in Wound Healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef]
- Zhou, Z.Q.; Chen, Y.; Chai, M.; Tao, R.; Lei, Y.H.; Jia, Y.Q.; Shu, J.; Ren, J.; Li, G.; Wei, W.X.; et al. Adipose extracellular matrix promotes skin wound healing by inducing the differentiation of adipose-derived stem cells into fibroblasts. Int. J. Mol. Med. 2019, 43, 890–900. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudian-Sani, M.-R.; Rafeei, F.; Amini, R.; Saidijam, M. The effect of mesenchymal stem cells combined with platelet-rich plasma on skin wound healing. J. Cosmet. Dermatol. 2018, 17, 650–659. [Google Scholar] [CrossRef]
- Hersant, B.; Sid-Ahmed, M.; Braud, L.; Jourdan, M.; Baba-Amer, Y.; Meningaud, J.P.; Rodriguez, A.M. Platelet-rich plasma improves the wound healing potential of mesenchymal stem cells through paracrine and metabolism alterations. Stem Cells Int. 2019, 2019, 1234263. [Google Scholar] [CrossRef]
- Paganelli, A.; Benassi, L.; Pastar, I.; Pellegrini, M.; Azzoni, P.; Vaschieri, C.; Pisciotta, A.; Carnevale, G.; Pellacani, G.; Magnoni, C. In vitro engineering of a skin substitute based on adipose-derived stem cells. Cells Tissues Organs 2019, 207, 46–57. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, X.; Zhao, B.; Li, Y.; Zhang, Y.; Li, Z.; Wang, X.; Luo, L.; Han, F.; Zhang, J.; et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp. Cell Res. 2018, 370, 333–342. [Google Scholar] [CrossRef]
- Hettiaratchy, S.; Papini, R. Initial management of a major burn: II—Assessment and resuscitation. BMJ 2004, 329, 101–103. [Google Scholar] [CrossRef]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef]
- Temnov, A.A.T.; Rogov, K.; Moroz, B.; Lebedev, V.; Nasonova, T.; Lyrshchikova, A.; Dobrynina, O.; Deshevoy, Y.; Melerzanov, A.; Bader, A.; et al. Use of paracrine factors from stem cells to treat local radiation burns in rats. Stem Cells Cloning 2018, 11, 69–76. [Google Scholar] [CrossRef]
- Alapure, B.V.; Lu, Y.; He, M.; Chu, C.C.; Peng, H.; Muhale, F.; Brewerton, Y.L.; Bunnell, B.; Hong, S. Accelerate healing of severe burn wounds by mouse bone marrow mesenchymal stem cell-seeded biodegradable hydrogel scaffold synthesized from arginine-based poly(ester amide) and chitosan. Stem Cells Develop. 2018, 27, 1605–1620. [Google Scholar] [CrossRef]
- Liu, P.; Deng, Z.; Han, S.; Liu, T.; Wen, N.; Lu, W.; Geng, X.; Huang, S.; Jin, Y. Tissue-engineered skin containing mesenchymal stem cells improves burn wounds. Artif. Organs 2008, 32, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, X.; Zhang, B.; Shi, Q.; Li, D.; Ju, X. A human umbilical cord mesenchymal stem cell-conditioned medium/chitosan/collagen/β-glycerophosphate thermosensitive hydrogel promotes burn injury healing in mice. Biomed. Res. Int. 2019, 2019, 5768285. [Google Scholar] [CrossRef]
- Revilla, G.; Darwin, E.; Yanwirasti; Rantam, F.A. Effect of allogeneic bone marrow-mesenchymal stem cells (BM-MSCs) to accelerate burn healing of rat on the expression of collagen type i and integrin α2β1. Pak. J. Biol. Sci. PJBS 2016, 19, 345–351. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, J.; La, X.; Fan, L.; Li, P.; Yu, Y.; Huang, Y.; Ding, J.; Xing, Y. Immunosuppressive effects of mesenchymal stem cell transplantation in rat burn models. Int. J. Clin. Exp. Pathol. 2015, 8, 5129–5136. [Google Scholar] [PubMed]
- Iso, Y.; Spees, J.L.; Serrano, C.; Bakondi, B.; Pochampally, R.; Song, Y.H.; Sobel, B.E.; Delafontaine, P.; Prockop, D.J. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem. Biophys. Res. Commun. 2007, 354, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Burst, V.R.; Gillis, M.; Pütsch, F.; Herzog, R.; Fischer, J.H.; Heid, P.; Müller-Ehmsen, J.; Schenk, K.; Fries, J.W.; Baldamus, C.A.; et al. Poor cell survival limits the beneficial impact of mesenchymal stem cell transplantation on acute kidney injury. Nephron. Exp. Nephrol. 2010, 114, e107–e116. [Google Scholar] [CrossRef]
- Di Bonzo, L.V.; Ferrero, I.; Cravanzola, C.; Mareschi, K.; Rustichell, D.; Novo, E.; Sanavio, F.; Cannito, S.; Zamara, E.; Bertero, M.; et al. Human mesenchymal stem cells as a two-edged sword in hepatic regenerative medicine: Engraftment and hepatocyte differentiation versus profibrogenic potential. Gut 2008, 57, 223–231. [Google Scholar] [CrossRef]
- Lee, R.H.; Seo, M.J.; Reger, R.L.; Spees, J.L.; Pulin, A.A.; Olson, S.D.; Prockop, D.J. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc. Nat. Acad. Sci. USA 2006, 103, 17438–17443. [Google Scholar] [CrossRef] [PubMed]
- Arthur, A.; Zannettino, A.; Gronthos, S. The therapeutic applications of multipotential mesenchymal/stromal stem cells in skeletal tissue repair. J. Cell. Physiol. 2009, 218, 237–245. [Google Scholar] [CrossRef]
- Kean, T.J.; Lin, P.; Caplan, A.I.; Dennis, J.E. MSCs: Delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. 2013, 2013, 732742. [Google Scholar] [CrossRef]
- Wang, Z.; Man, M.-Q.; Li, T.; Elias, P.M.; Mauro, T.M. Aging-associated alterations in epidermal function and their clinical significance. Aging 2020, 12, 5551–5565. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Berardesca, E.; Maibach, H.I. Clinical implications of aging skin: Cutaneous disorders in the elderly. Am. J. Clin. Dermatol. 2009, 10, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Uitto, J. The role of elastin and collagen in cutaneous aging: Intrinsic aging versus photoexposure. J. Drugs Dermatol. JDD 2008, 7, s12–s16. [Google Scholar]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Panich, U.; Sittithumcharee, G.; Rathviboon, N.; Jirawatnotai, S. Ultraviolet radiation-induced skin aging: The role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int. 2016, 2016, 7370642. [Google Scholar] [CrossRef] [PubMed]
- Charles-de-Sá, L.; Gontijo-de-Amorim, N.F.; Maeda Takiya, C.; Borojevic, R.; Benati, D.; Bernardi, P.; Sbarbati, A.; Rigotti, G. Antiaging treatment of the facial skin by fat graft and adipose-derived stem cells. Plast. Reconstr. Surg. 2015, 135, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Charles-de-Sá, L.; Gontijo-de-Amorim, N.F.; Rigotti, G.; Sbarbati, A.; Bernardi, P.; Benati, D.; Bizon Vieira Carias, R.; Maeda Takiya, C.; Borojevic, R. Photoaged skin therapy with adipose-derived stem cells. Plast. Reconstr. Surg. 2020, 145, 1037e–1049e. [Google Scholar] [CrossRef]
- Xu, P.; Xin, Y.; Zhang, Z.; Zou, X.; Xue, K.; Zhang, H.; Zhang, W.; Liu, K. Extracellular vesicles from adipose-derived stem cells ameliorate ultraviolet B-induced skin photoaging by attenuating reactive oxygen species production and inflammation. Stem Cell Res. Ther. 2020, 11, 264. [Google Scholar] [CrossRef]
- Prakoeswa, C.R.S.; Pratiwi, F.D.; Herwanto, N.; Citrashanty, I.; Indramaya, D.M.; Murtiastutik, D.; Sukanto, H.; Rantam, F.A. The effects of amniotic membrane stem cell-conditioned medium on photoaging. J. Dermatol. Treat. 2019, 30, 478–482. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, G.D.; Luo, X.B.; Yin, B.; Shu, B.; Guan, J.Z.; Jia, C.Y. Potential of bone marrow mesenchymal stem cells in rejuvenation of the aged skin of rats. Biomed. Rep. 2017, 6, 279–284. [Google Scholar] [CrossRef]
- Kim, Y.J.; Seo, D.H.; Lee, S.H.; Lee, S.H.; An, G.H.; Ahn, H.J.; Kwon, D.; Seo, K.W.; Kang, K.S. Conditioned media from human umbilical cord blood-derived mesenchymal stem cells stimulate rejuvenation function in human skin. Biochem. Biophys. Rep. 2018, 16, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Abhange, K.K.; Wen, Y.; Chen, Y.; Xue, F.; Wang, G.; Tong, J.; Zhu, C.; He, X.; Wan, Y. Preparation of engineered extracellular vesicles derived from human umbilical cord mesenchymal stem cells with ultrasonication for skin rejuvenation. ACS Omega 2019, 4, 22638–22645. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.; Manchanda, K.; Pandey, S.S. Role of caffeine in the management of androgenetic alopecia. Int. J. Trichol. 2012, 4, 185–186. [Google Scholar] [CrossRef] [PubMed]
- Bassino, E.; Gasparri, F.; Munaron, L. Protective role of nutritional plants containing flavonoids in hair follicle disruption: A review. Int. J. Mol. Sci. 2020, 21, 523. [Google Scholar] [CrossRef]
- Choi, B.Y. Targeting Wnt/β-catenin pathway for developing therapies for hair loss. Int. J. Mol. Sci. 2020, 21, 4915. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, M.; Villareal, M.O.; Isoda, H. β-catenin-mediated hair growth induction effect of 3,4,5-tri-O-caffeoylquinic acid. Aging 2019, 11, 4216–4237. [Google Scholar] [CrossRef]
- Winiarska, A.; Mandt, N.; Kamp, H.; Hossini, A.; Seltmann, H.; Zouboulis, C.C.; Blume-Peytavi, U. Effect of 5alpha-dihydrotestosterone and testosterone on apoptosis in human dermal papilla cells. Skin Pharmacol. Physiol. 2006, 19, 311–321. [Google Scholar] [CrossRef]
- Huang, C.-F.; Chang, Y.-J.; Hsueh, Y.-Y.; Huang, C.-W.; Wang, D.-H.; Huang, T.-C.; Wu, Y.-T.; Su, F.-C.; Hughes, M.; Chuong, C.-M.; et al. Assembling composite dermal papilla spheres with adipose-derived stem cells to enhance hair follicle induction. Sci. Rep. 2016, 6, 26436. [Google Scholar] [CrossRef]
- Dong, L.; Hao, H.; Xia, L.; Liu, J.; Ti, D.; Tong, C.; Hou, Q.; Han, Q.; Zhao, Y.; Liu, H.; et al. Treatment of MSCs with Wnt1a-conditioned medium activates DP cells and promotes hair follicle regrowth. Sci. Rep. 2014, 4, 5432. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.S.; Moon, J.H.; Jun, E.K.; Kim, J.; Maeng, I.; Kim, J.S.; Lee, J.H.; Baik, C.S.; Kim, A.; Cho, K.S.; et al. Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells. Stem Cells Dev. 2010, 19, 887–902. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jun, E.K.; Son, D.; Hong, W.; Jang, J.; Yun, W.; Yoon, B.S.; Song, G.; Kim, I.Y.; You, S. Overexpression of Nanog in amniotic fluid-derived mesenchymal stem cells accelerates dermal papilla cell activity and promotes hair follicle regeneration. Exp. Mol. Med. 2019, 51, 72. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.L.; Gangadaran, P.; Bak, S.S.; Oh, J.M.; Kalimuthu, S.; Lee, H.W.; Baek, S.H.; Zhu, L.; Sung, Y.K.; Jeong, S.Y.; et al. Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci. Rep. 2017, 7, 15560. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Bieback, K.; Kern, S.; Kocaömer, A.; Ferlik, K.; Bugert, P. Comparing mesenchymal stromal cells from different human tissues: Bone marrow, adipose tissue and umbilical cord blood. Bio-Med. Mater. Eng. 2008, 18, S71–S76. [Google Scholar]
- Herrmann, R.; Sturm, M.; Shaw, K.; Purtill, D.; Cooney, J.; Wright, M.; Phillips, M.; Cannell, P. Mesenchymal stromal cell therapy for steroid-refractory acute and chronic graft versus host disease: A phase 1 study. Int. J. Hematol. 2012, 95, 182–188. [Google Scholar] [CrossRef]
- Connick, P.; Kolappan, M.; Crawley, C.; Webber, D.J.; Patani, R.; Michell, A.W.; Du, M.Q.; Luan, S.L.; Altmann, D.R.; Thompson, A.J.; et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: An open-label phase 2a proof-of-concept study. Lancet Neurol. 2012, 11, 150–156. [Google Scholar] [CrossRef]
- Karamouzian, S.; Nematollahi-Mahani, S.N.; Nakhaee, N.; Eskandary, H. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin. Neurol. Neurosurg. 2012, 114, 935–939. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, Q. The clinical application of mesenchymal stromal cells in hematopoietic stem cell transplantation. J. Hematol. Oncol. 2016, 9, 46. [Google Scholar] [CrossRef]
- Reinders, M.E.; de Fijter, J.W.; Roelofs, H.; Bajema, I.M.; de Vries, D.K.; Schaapherder, A.F.; Claas, F.H.; van Miert, P.P.; Roelen, D.L.; van Kooten, C.; et al. Autologous bone marrow-derived mesenchymal stromal cells for the treatment of allograft rejection after renal transplantation: Results of a phase I study. Stem Cells Trans. Med. 2013, 2, 107–111. [Google Scholar] [CrossRef]
- Cooper, K.; Viswanathan, C. Establishment of a mesenchymal stem cell bank. Stem Cells Int. 2011, 2011, 905621. [Google Scholar] [CrossRef]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and safety issues of stem cell-based therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Lian, Q.; Zhang, Y.; Zhang, J.; Zhang, H.K.; Wu, X.; Zhang, Y.; Lam, F.F.; Kang, S.; Xia, J.C.; Lai, W.H.; et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation 2010, 121, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Shaw, G.; Murphy, M.; Barry, F. Induced pluripotent stem cell-derived mesenchymal stromal cells are functionally and genetically different from bone marrow-derived mesenchymal stromal cells. Stem Cells 2019, 37, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Villa-Diaz, L.G.; Brown, S.E.; Liu, Y.; Ross, A.M.; Lahann, J.; Parent, J.M.; Krebsbach, P.H. Derivation of mesenchymal stem cells from human induced pluripotent stem cells cultured on synthetic substrates. Stem Cells 2012, 30, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Pelekanos, R.A.; Ellis, R.L.; Horne, R.; Wolvetang, E.J.; Fisk, N.M. Small molecule mesengenic induction of human induced pluripotent stem cells to generate mesenchymal stem/stromal cells. Stem Cells Trans. Med. 2012, 1, 83–95. [Google Scholar] [CrossRef]
- Soontararak, S.; Chow, L.; Johnson, V.; Coy, J.; Wheat, W.; Regan, D.; Dow, S. Mesenchymal Stem Cells (MSC) Derived from Induced Pluripotent Stem Cells (iPSC) equivalent to adipose-derived msc in promoting intestinal healing and microbiome normalization in mouse inflammatory bowel disease model. Stem Cells Trans. Med. 2018, 7, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.L.; Chow, Y.Y.; Sun, S.J.; Zeng, Q.X.; Li, H.B.; Shi, J.B.; Sun, Y.Q.; Wen, W.; Tse, H.F.; Lian, Q.; et al. Mesenchymal stem cells derived from human induced pluripotent stem cells modulate T-cell phenotypes in allergic rhinitis. Allergy 2012, 67, 1215–1222. [Google Scholar] [CrossRef]
- Gao, W.X.; Sun, Y.Q.; Shi, J.; Li, C.L.; Fang, S.B.; Wang, D.; Deng, X.Q.; Wen, W.; Fu, Q.L. Effects of mesenchymal stem cells from human induced pluripotent stem cells on differentiation, maturation, and function of dendritic cells. Stem Cell Res. Ther. 2017, 8, 48. [Google Scholar] [CrossRef]
- Chow, L.; Johnson, V.; Regan, D.; Wheat, W.; Webb, S.; Koch, P.; Dow, S. Safety and immune regulatory properties of canine induced pluripotent stem cell-derived mesenchymal stem cells. Stem Cell Res. 2017, 25, 221–232. [Google Scholar] [CrossRef]
- Wei, H.; Tan, G.; Manasi; Qiu, S.; Kong, G.; Yong, P.; Koh, C.; Ooi, T.H.; Lim, S.Y.; Wong, P.; et al. One-step derivation of cardiomyocytes and mesenchymal stem cells from human pluripotent stem cells. Stem Cell Res. 2012, 9, 87–100. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.K.; Kim, H.; Kim, T.M. Exosomes secreted from induced pluripotent stem cell-derived mesenchymal stem cells accelerate skin cell proliferation. Int. J. Mol. Sci. 2018, 19, 3119. [Google Scholar] [CrossRef]
- Veraitch, O.; Mabuchi, Y.; Matsuzaki, Y.; Sasaki, T.; Okuno, H.; Tsukashima, A.; Amagai, M.; Okano, H.; Ohyama, M. Induction of hair follicle dermal papilla cell properties in human induced pluripotent stem cell-derived multipotent LNGFR(+)THY-1(+) mesenchymal cells. Sci. Rep. 2017, 7, 42777. [Google Scholar] [CrossRef]
- Spitzhorn, L.-S.; Megges, M.; Wruck, W.; Rahman, M.S.; Otte, J.; Degistirici, Ö.; Meisel, R.; Sorg, R.V.; Oreffo, R.O.C.; Adjaye, J. Human iPSC-derived MSCs (iMSCs) from aged individuals acquire a rejuvenation signature. Stem Cell Res. Ther. 2019, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Barberi, T.; Willis, L.M.; Socci, N.D.; Studer, L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005, 2, e161. [Google Scholar] [CrossRef]
- Olivier, E.N.; Rybicki, A.C.; Bouhassira, E.E. Differentiation of human embryonic stem cells into bipotent mesenchymal stem cells. Stem Cells 2006, 24, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Lian, Q.; Lye, E.; Suan Yeo, K.; Khia Way Tan, E.; Salto-Tellez, M.; Liu, T.M.; Palanisamy, N.; El Oakley, R.M.; Lee, E.H.; Lim, B.; et al. Derivation of clinically compliant MSCs from CD105+, CD24- differentiated human ESCs. Stem Cells 2007, 25, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Lee, H.N.; Kang, H.J.; Kim, K.H.; Hur, J.; Cho, H.J.; Lee, J.; Chung, H.M.; Cho, J.; Cho, M.Y.; et al. Novel embryoid body-based method to derive mesenchymal stem cells from human embryonic stem cells. Tissue Eng. Part A 2010, 16, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Hwang, N.S.; Varghese, S.; Lee, H.J.; Zhang, Z.; Ye, Z.; Bae, J.; Cheng, L.; Elisseeff, J. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc. Nat. Acad. Sci. USA 2008, 105, 20641–20646. [Google Scholar] [CrossRef] [PubMed]
- Laurila, J.P.; Laatikainen, L.; Castellone, M.D.; Trivedi, P.; Heikkila, J.; Hinkkanen, A.; Hematti, P.; Laukkanen, M.O. Human embryonic stem cell-derived mesenchymal stromal cell transplantation in a rat hind limb injury model. Cytotherapy 2009, 11, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Gadkari, R.; Zhao, L.; Teklemariam, T.; Hantash, B.M. Human embryonic stem cell derived-mesenchymal stem cells: An alternative mesenchymal stem cell source for regenerative medicine therapy. Regen. Med. 2014, 9, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, C.; Emanuelsson, K.; Wessberg, F.; Kajic, K.; Axell, M.Z.; Eriksson, P.S.; Lindahl, A.; Hyllner, J.; Strehl, R. Human embryonic stem cell-derived mesenchymal progenitors--potential in regenerative medicine. Stem Cell Res. 2009, 3, 39–50. [Google Scholar] [CrossRef]

| Wound Healing Process | Treatment to MSCs | Function of MSCs | Source of MSCs | Model | Reference |
|---|---|---|---|---|---|
| Anti-inflammation | - | Polarization of macrophages to an M2 phenotype | BM | MSCs co-culture with macrophage | [53] |
| MSC-derived exosome | Polarization of macrophages to an M2 phenotype | BM | MSCs co-culture with macrophage | [54] | |
| TNF-α, IL-6 | Polarization of macrophages to an M2 phenotype | Gingiva | MSCs co-culture with macrophage | [15] | |
| TSG-6 | Polarization of macrophages to an M2 phenotype | BM | Diabetic mice model | [55] | |
| TNF-α | Limiting macrophage activation | BM | Skin injury mice model | [56] | |
| siTSG-6 (negative effect) | Polarization of macrophages to an M2 phenotype | cAD | Inflammatory bowel disease mice model | [57] | |
| Proliferation | CXCR4 antagonist (negative effect) | Chemotaxis of MSCs | BM | Burn mice model | [58] |
| PRP | Chemotaxis of MSCs | AF | Transwell migration assay | [59] | |
| PRP | Fibroblast migration | AD | Wound healing assay in culture dish | [60] | |
| PRP | Re-epithelialization | AD | Skin injury mice model | [61] | |
| PRP | Chemotaxis of MSCs | BM | Chemotaxis device | [62] | |
| EMPB | Migration of MSCs | Endogenous MSCs in mice | Diabetic mice model | [63] | |
| Cinnamtannin B-1 | Migration of MSCs | Endogenous MSCs in mice | Diabetic mice model | [64] | |
| Angiogenesis | Low-level laser therapy | VEGF, bFGF secretion in the wound bed | cAD | Skin injury mice model | [65] |
| - | CCL2 | Primary MSCs in CCL2-KO mice | Skin injury mice model | [66] | |
| Negative pressure wound therapy | CD31, VEGF, α-SMA | BM | Skin injury mice model | [67] | |
| Biomimetic hydrogel scaffold | Wound vascularization | BM | Skin injury mice model | [68] | |
| Increase in wound closure | Self-adaptive all-in-one delivery chip | Skin nerve regeneration | BM | Skin injury mice model | [69] |
| Chitin nanofiber-based hydrogel | Granulation tissue formation | BM | Skin injury mice model | [70] | |
| - | Collagen type VII | iPSC | Skin injury mice model | [71] | |
| CTGF | Fibroblast differentiation | ESCs | Skin pressure ulcer mice model | [72] | |
| ECM | VEGF, PDGF, EGF | UCB | Diabetic rat model | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Brito, S.; Kwak, B.M.; Park, S.; Lee, M.-G.; Bin, B.-H. Applications of Mesenchymal Stem Cells in Skin Regeneration and Rejuvenation. Int. J. Mol. Sci. 2021, 22, 2410. https://doi.org/10.3390/ijms22052410
Jo H, Brito S, Kwak BM, Park S, Lee M-G, Bin B-H. Applications of Mesenchymal Stem Cells in Skin Regeneration and Rejuvenation. International Journal of Molecular Sciences. 2021; 22(5):2410. https://doi.org/10.3390/ijms22052410
Chicago/Turabian StyleJo, Hantae, Sofia Brito, Byeong Mun Kwak, Sangkyu Park, Mi-Gi Lee, and Bum-Ho Bin. 2021. "Applications of Mesenchymal Stem Cells in Skin Regeneration and Rejuvenation" International Journal of Molecular Sciences 22, no. 5: 2410. https://doi.org/10.3390/ijms22052410
APA StyleJo, H., Brito, S., Kwak, B. M., Park, S., Lee, M.-G., & Bin, B.-H. (2021). Applications of Mesenchymal Stem Cells in Skin Regeneration and Rejuvenation. International Journal of Molecular Sciences, 22(5), 2410. https://doi.org/10.3390/ijms22052410







