Effect of Different Wavelengths of Laser Irradiation on the Skin Cells
Abstract
1. Introduction
2. Lasers and LEDs
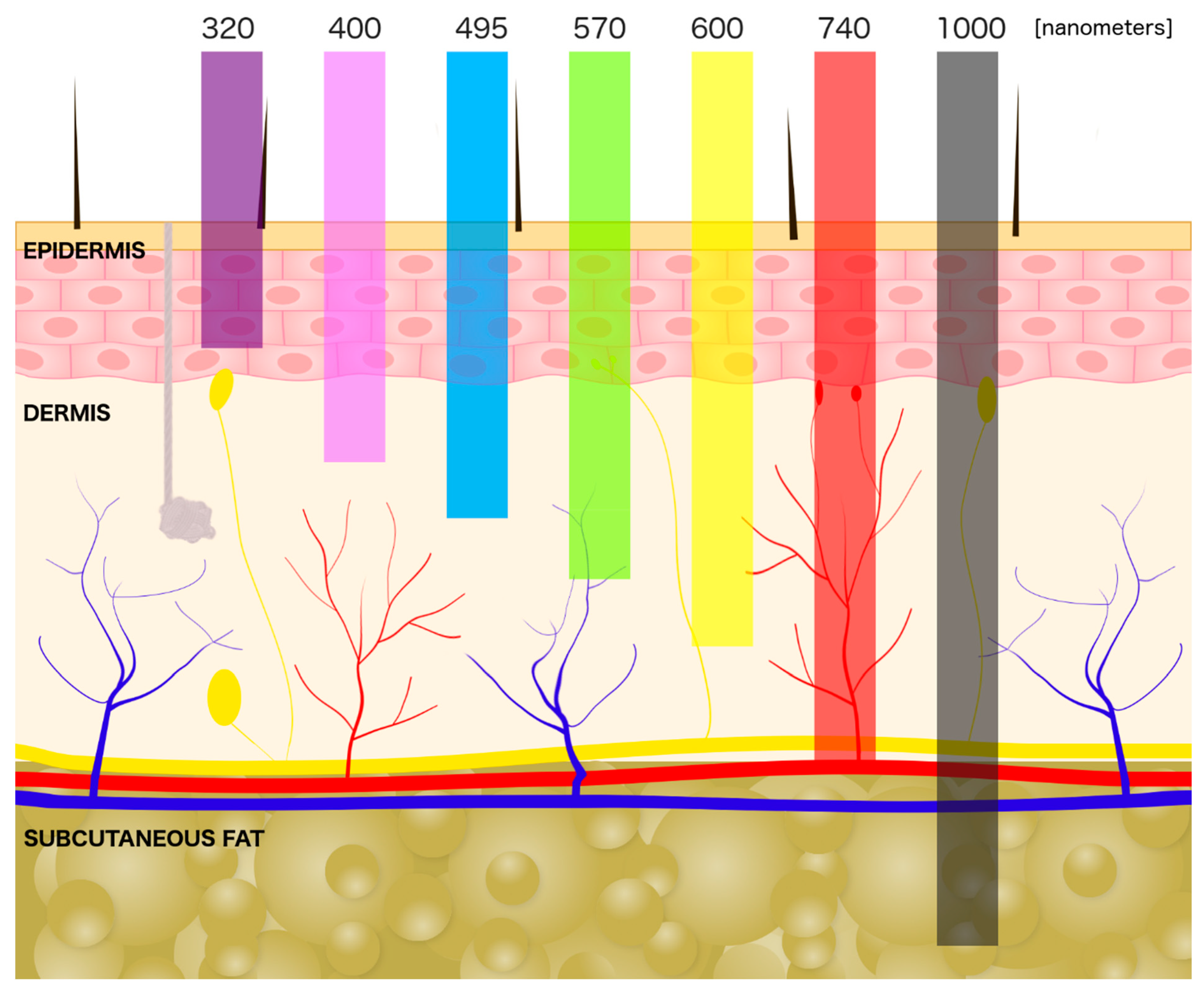
3. Laser Interaction with a Skin
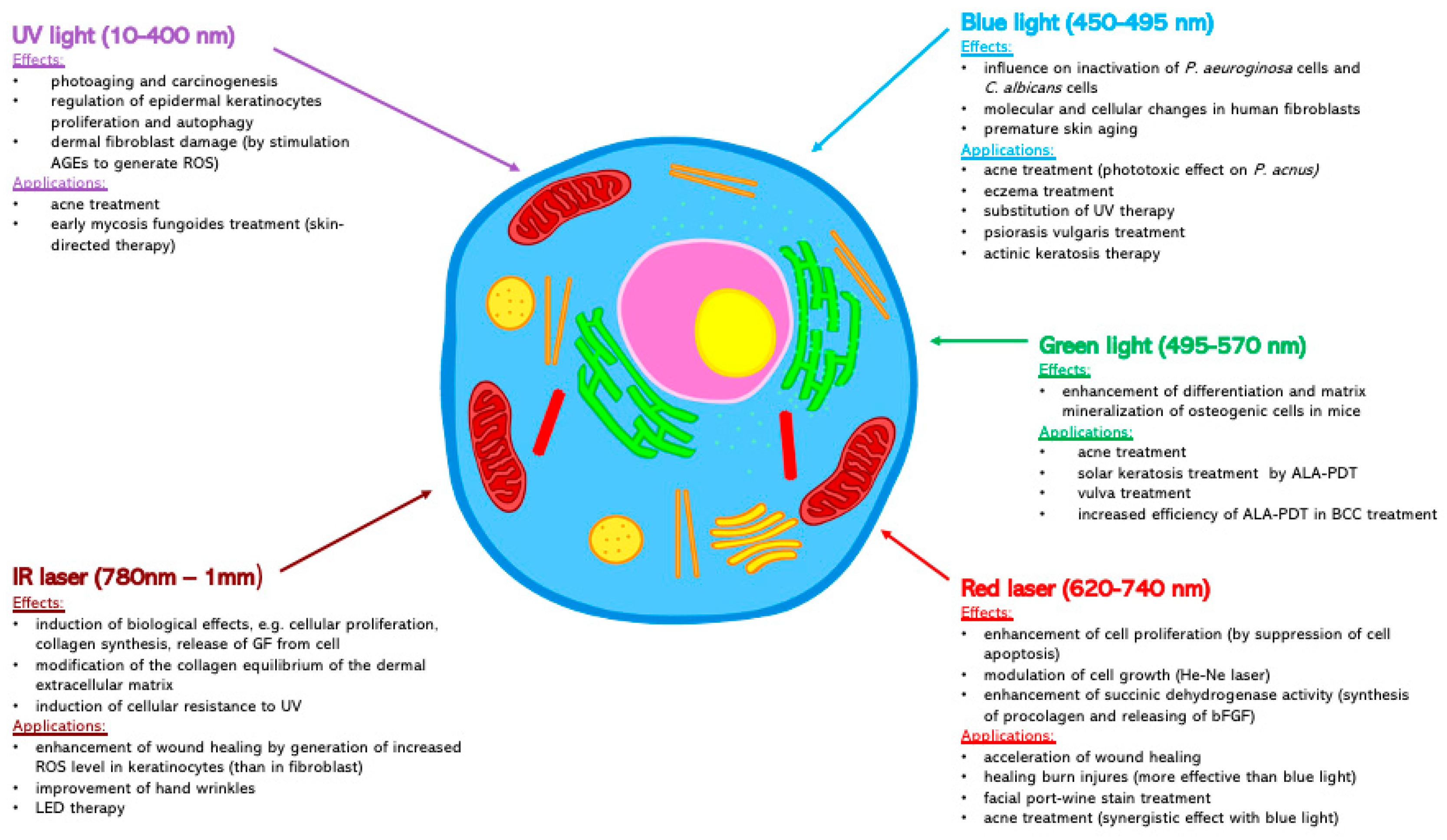
4. The UV Light (10–400 nm)
5. Blue Light (450–495 nm)
6. Green Light (495–570 nm)
7. Red Laser (620–740 nm)
8. IR Laser (780 nm–1 mm)
9. Conclusions
Funding
Conflicts of Interest
Abbreviations
| IR | infrared |
| UV | ultraviolet |
| ROS | reactive oxygen species |
| HDF | human dermal fibroblast |
| HaCaT | human normal epidermal keratinocytes cell lines |
| MMP | matrix metalloproteinase |
| IL | interleukin |
| NIR | near infrared |
References
- Hilton, P.A. Early Days of Laser Cutting. Lasers Mater. Process. 1997, 3097, 10–16. [Google Scholar]
- Riveiro, A.; Quintero, F.; Boutinguiza, M.; del Val, J.; Comesaña, R.; Lusquiños, F.; Pou, J. Laser cutting: A review on the influence of assist gas. Materials 2019, 12, 157. [Google Scholar] [CrossRef] [PubMed]
- Azadgoli, B.; Baker, R.Y. Laser applications in surgery. Ann. Transl. Med. 2016, 4. [Google Scholar] [CrossRef]
- Kaneko, S. Safety guidelines for diagnostic and therapeutic laser applications in the neurosurgical field. Laser Ther. 2012, 21, 129. [Google Scholar] [CrossRef]
- Cotler, H.B.; Chow, R.T.; Hamblin, M.R.; Carroll, J. The use of low level laser therapy (LLLT) for musculoskeletal pain. MOJ Orthop. Rheumatol. 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.M.A.; Barkokebas, A.; Pires, A.P.; Barros, L.F.; Carvalho, A.A.T.; Leão, J.C. Current use and future perspectives of diagnostic and therapeutic lasers in oral medicine. Minerva Stomatol. 2008, 57, 511–517. [Google Scholar]
- Km, B. Lasers in Urology. Available online: https://pubmed.ncbi.nlm.nih.gov/7651053/ (accessed on 16 September 2020).
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.-Y.; Carroll, J.D.; Hamblin, M.R. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef]
- Ash, C.; Dubec, M.; Donne, K.; Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 2017, 32, 1909–1918. [Google Scholar] [CrossRef] [PubMed]
- Basso, F.G.; Pansani, T.N.; Cardoso, L.M.; Citta, M.; Soares, D.G.; Scheffel, D.S.; Hebling, J.; de Souza Costa, C.A. Epithelial cell-enhanced metabolism by low-level laser therapy and epidermal growth factor. Lasers Med. Sci. 2018, 33, 445–449. [Google Scholar] [CrossRef] [PubMed]
- De Chaves, M.E.A.; de Araújo, A.R.; Piancastelli, A.C.C.; Pinotti, M.; de Chaves, M.E.A.; de Araújo, A.R.; Piancastelli, A.C.C.; Pinotti, M. Effects of low-power light therapy on wound healing: LASER x LED. An. Bras. Dermatol. 2014, 89, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Watanabe, R.; Hanaoka, H.; Nakajima, T.; Choyke, P.L.; Kobayashi, H. Comparative effectiveness of light emitting diodes (LEDs) and lasers in near infrared photoimmunotherapy. Oncotarget 2016, 7, 14324–14335. [Google Scholar] [CrossRef]
- Lister, T.; Wright, P.A.; Chappell, P.H. Optical properties of human skin. J. Biomed. Opt. 2012, 17, 090901. [Google Scholar] [CrossRef]
- Cvetković, M.; Peratta, A.; Poljak, D. FETD computation of the temperature distribution induced into a human eye by a pulsed laser. Prog. Electromagn. Res. 2011, 120, 403–421. [Google Scholar] [CrossRef]
- Cvetkovic, M.; Peratta, A.; Poljak, D. Thermal modelling of the human eye exposed to infrared radiation of 1064 Nm Nd:YAG And 2090 Nm Ho:YAG lasers. Environ. Health Risk 2009, 14, 221–231. [Google Scholar] [CrossRef]
- Mirnezami, S.A.; Rajaei Jafarabadi, M.; Abrishami, M. Temperature distribution simulation of the human eye exposed to laser radiation. J. Lasers Med. Sci. 2013, 4, 175–181. [Google Scholar] [PubMed]
- Husain, Z.; Alster, T.S. The role of lasers and intense pulsed light technology in dermatology. Clin. Cosmet. Investig. Dermatol. 2016, 9, 29–40. [Google Scholar] [CrossRef]
- Carroll, L.; Humphreys, T.R. LASER-tissue interactions. Clin. Dermatol. 2006, 24, 2–7. [Google Scholar] [CrossRef]
- Joukar, A.; Nammakie, E.; Niroomand-Oscuii, H. A comparative study of thermal effects of 3 types of laser in eye: 3D simulation with bioheat equation. J. Therm. Biol. 2015, 49, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Pacholczyk, M.; Czernicki, J.; Ferenc, T. The effect of solar ultraviolet radiation (UVR) on induction of skin cancers. Medycyna Pracy 2016, 67, 255–266. [Google Scholar] [CrossRef]
- Micka-Michalak, K.; Biedermann, T.; Reichmann, E.; Meuli, M.; Klar, A.S. Induction of angiogenic and inflammation-associated dermal biomarkers following acute UVB exposure on bio-engineered pigmented dermo-epidermal skin substitutes In Vivo. Pediatric Surg. Int. 2019, 35, 129–136. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Xu, W.-W.; Liu, X.-M.; Zhang, R.-Z.; Xu, C.-X.; Xu, B.; Cheng, S.; Liu, Q. Self-control study of combination treatment of 308 nm excimer laser and calcipotriene ointment on stable Psoriasis vulgaris. Int. J. Clin. Exp. Med. 2014, 7, 2844–2850. [Google Scholar]
- Tacastacas, J.D.; Oyetakin-White, P.; Soler, D.C.; Young, A.; Groft, S.; Honda, K.; Cooper, K.D.; McCormick, T.S. Does imiquimod pretreatment optimize 308-nm excimer laser (UVB) therapy in psoriasis patients? Photodermatol. Photoimmunol. Photomed. 2017, 33, 193–202. [Google Scholar] [CrossRef]
- Zoghi, M.; Parvin, P.; Behrouzinia, S.; Salehinia, D.; Khorasani, K.; Mehravaran, H. Acoustic effects of metal vapor lasers. Appl. Opt. 2009, 48, 3460–3467. [Google Scholar] [CrossRef] [PubMed]
- Bradford, A.; Barlow, A.; Chazot, P.L. Probing the differential effects of infrared light sources IR1072 and IR880 on human lymphocytes: Evidence of selective cytoprotection by IR1072. J. Photochem. Photobiol. B Biol. 2005, 81, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Jobe, N.P.; Živicová, V.; Mifková, A.; Rösel, D.; Dvořánková, B.; Kodet, O.; Strnad, H.; Kolář, M.; Šedo, A.; Smetana, K.; et al. Fibroblasts potentiate melanoma cells In Vitro invasiveness induced by UV-irradiated keratinocytes. Histochem. Cell Biol. 2018, 149, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Michniak-Kohn, B.; Leonardi, G.R. An overview about oxidation in clinical practice of skin aging. Anais Vrasileiros Dermatologia 2017, 92, 367–374. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Goldstein, N.B.; Koster, M.I.; Jones, K.L.; Gao, B.; Hoaglin, L.G.; Robinson, S.E.; Wright, M.J.; Birlea, S.I.; Luman, A.; Lambert, K.A.; et al. Repigmentation of human vitiligo skin by NBUVB is controlled by transcription of GLI1 and activation of the β-catenin pathway in the hair follicle bulge stem cells. J. Investig. Derm. 2018, 138, 657–668. [Google Scholar] [CrossRef]
- Yi, R.; Zhang, J.; Sun, P.; Qian, Y.; Zhao, X. Protective effects of Kuding tea (ilex kudingcha, C.J. Tseng) polyphenols on UVB-induced skin aging in SKH1 hairless mice. Molecules 2019, 24, 1016. [Google Scholar] [CrossRef]
- Gruber, J.V.; Holtz, R.; Yang, S.I. In Vitro examination of an oleosome-based sun protection product on the influence of UVB-induced inflammation markers in human epidermal skin equivalent tissue model. J. Photochem. Photobiol. B Biol. 2018, 179, 39–45. [Google Scholar] [CrossRef]
- Penna, I.; Albanesi, E.; Bertorelli, R.; Bandiera, T.; Russo, D. Cytoprotective, anti-inflammatory, and antioxidant properties of high-molecular-weight hyaluronan enriched with red orange extract in human fibroblasts exposed to ultra violet light b irradiation: Cytoprotective, anti-inflammatory, and antioxidant properties. Biotechnol. Appl. Biochem. 2019. [Google Scholar] [CrossRef]
- Szymański, Ł.; Jęderka, K.; Cios, A.; Ciepelak, M.; Lewicka, A.; Stankiewicz, W.; Lewicki, S. A simple method for the production of human skin equivalent in 3D, multi-cell culture. Int. J. Mol. Sci. 2020, 21, 4644. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Xu, S.; Bu, W.; Chen, K.; Li, M.; Gu, H. Ultraviolet B radiation down-regulates ULK1 and ATG7 expression and impairs the autophagy response in human keratinocytes. J. Photochem. Photobiol. B Biol. 2018, 178, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Brand, R.M.; Wipf, P.; Durham, A.; Epperly, M.W.; Greenberger, J.S.; Falo, L.D. Targeting mitochondrial oxidative stress to mitigate UV-induced skin damage. Front. Pharm. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.-R.; Kim, J.H.; Hong, J.-Y.; Seok, J.; Kim, J.M.; Bak, D.-H.; Choi, M.-J.; Mun, S.K.; Kim, C.W.; Kim, B.J.; et al. Irradiation with 310 Nm and 340 Nm ultraviolet light-emitting-diodes can improve atopic dermatitis-like skin lesions in NC/Nga mice. Photochem. Photobiol. Sci. 2018, 17, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Rascalou, A.; Lamartine, J.; Poydenot, P.; Demarne, F.; Bechetoille, N. Mitochondrial damage and cytoskeleton reorganization in human dermal fibroblasts exposed to artificial visible light similar to screen-emitted light. J. Dermatol. Sci. 2018, 91, 195–205. [Google Scholar] [CrossRef]
- Nakashima, Y.; Ohta, S.; Wolf, A.M. Blue light-induced oxidative stress in live skin. Free Radic. Biol. Med. 2017, 108, 300–310. [Google Scholar] [CrossRef]
- Lee, H.S.; Jung, S.-E.; Kim, S.K.; Kim, Y.-S.; Sohn, S.; Kim, Y.C. Low-level light therapy with 410 Nm light emitting diode suppresses collagen synthesis in human keloid fibroblasts: An In Vitro study. Ann. Derm. 2017, 29, 149–155. [Google Scholar] [CrossRef]
- Mignon, C.; Uzunbajakava, N.E.; Castellano-Pellicena, I.; Botchkareva, N.V.; Tobin, D.J. Differential response of human dermal fibroblast subpopulations to visible and near-infrared light: Potential of photobiomodulation for addressing Cutaneous conditions. Lasers Surg. Med. 2018. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Chen, J.; Wang, Y.; Sherwood, M.E.; Murray, C.K.; Vrahas, M.S.; Hooper, D.C.; Hamblin, M.R.; Dai, T.; et al. Antimicrobial blue light inactivation of Candida albicans: In Vitro and in Vivo studies. Virulence 2016, 7, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Y.; Gupta, A.; Huang, Y.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Baer, D.G.; Hamblin, M.R.; Dai, T.; et al. Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: Implications for prophylaxis and treatment of combat-related wound infections. J. Infect. Dis. 2014, 209, 1963–1971. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Z.; Peng, Y.; Guo, Y.; Yao, M.; Dong, J. Application of 460 Nm visible light for the elimination of Candida albicans In Vitro and in Vivo. Mol. Med. Rep. 2018, 18, 2017–2026. [Google Scholar] [CrossRef]
- Makdoumi, K.; Hedin, M.; Bäckman, A. Different photodynamic effects of blue light with and without riboflavin on methicillin-resistant Staphylococcus aureus (MRSA) and human keratinocytes In Vitro. Lasers Med. Sci. 2019. [Google Scholar] [CrossRef]
- Mamalis, A.; Garcha, M.; Jagdeo, J. Light emitting diode-generated blue light modulates fibrosis characteristics: Fibroblast proliferation, migration speed, and reactive oxygen species generation. Lasers Surg. Med. 2015, 47, 210–215. [Google Scholar] [CrossRef]
- Teuschl, A.; Balmayor, E.R.; Redl, H.; van Griensven, M.; Dungel, P. Phototherapy with LED light modulates healing processes in an In Vitro scratch-wound model using 3 different cell types. Derm. Surg. 2015, 41, 261–268. [Google Scholar] [CrossRef] [PubMed]
- De Alencar, F.N.J.; Nonaka, C.F.W.; de Vasconcelos Catão, M.H.C. Effect of blue LED on the healing process of third-degree skin burns: Clinical and histological evaluation. Lasers Med. Sci. 2019, 34, 721–728. [Google Scholar] [CrossRef]
- AlGhamdi, K.M.; Kumar, A.; Ashour, A.E.; AlGhamdi, A.A. A comparative study of the effects of different low-level lasers on the proliferation, viability, and migration of human melanocytes in Vitro. Lasers Med. Sci. 2015, 30, 1541–1551. [Google Scholar] [CrossRef]
- AlGhamdi, K.M.; Kumar, A.; Al-Ghamdi, A.; AL-Rikabi, A.C.; Mubarek, M.; Ashour, A.E. Ultra-structural effects of different low-level lasers on normal cultured human melanocytes: An in Vitro comparative study. Lasers Med. Sci. 2016, 31, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Castellano-Pellicena, I.; Uzunbajakava, N.E.; Mignon, C.; Raafs, B.; Botchkarev, V.A.; Thornton, M.J. Does blue light restore human epidermal barrier function via activation of opsin during cutaneous wound healing? Lasers Surg. Med. 2018. [Google Scholar] [CrossRef]
- Marra, K.; LaRochelle, E.P.; Chapman, M.S.; Hoopes, P.J.; Lukovits, K.; Maytin, E.V.; Hasan, T.; Pogue, B.W. Comparison of blue and white lamp light with sunlight for daylight-mediated, 5-ALA photodynamic therapy, In Vivo. Photochem. Photobiol. 2018, 94, 1049–1057. [Google Scholar] [CrossRef]
- Campiche, R.; Curpen, S.J.; Lutchmanen-Kolanthan, V.; Gougeon, S.; Cherel, M.; Laurent, G.; Gempeler, M.; Schuetz, R. Pigmentation effects of blue light irradiation on skin and how to protect against them. Int. J. Cosmet. Sci. 2020, 42, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Osiecka, B.J.; Jurczyszyn, K.; Nockowski, P.; Murawski, M.; Ziółkowski, P. Photodynamic therapy with green light for the treatment of vulvar lichen sclerosus-preliminary results. Photodiagnosis Photodyn. Ther. 2016. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, C.; Stege, H.; Saalmann, G.; Goerz, G.; Ruzicka, T.; Krutmann, J. Green light is effective and less painful than red light in photodynamic therapy of facial solar keratoses. Photodermatol. Photoimmunol. Photomed. 1997, 13, 181–185. [Google Scholar] [CrossRef]
- Li-Qiang, G.; Hua, W.; Si-li, N.; Chun-hua, T. A Clinical study of HMME-PDT therapy in Chinese pediatric patients with port-wine stain. Photodiagnosis Photodyn. Ther. 2018, 23, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zou, X.; Chen, H.; Yang, Y.; Lin, H.; Guo, X. Clinical study on clinical operation and post-treatment reactions of HMME-PDT in treatment of PWS. Photodiagnosis Photodyn. Ther. 2017, 20, 253–256. [Google Scholar] [CrossRef]
- JalalKamali, M.; Nematollahi-Mahani, S.N.; Shojaei, M.; Shamsoddini, A.; Arabpour, N. Effect of light polarization on the efficiency of photodynamic therapy of basal cell carcinomas: An In Vitro cellular study. Lasers Med. Sci. 2018, 33, 305–313. [Google Scholar] [CrossRef]
- Fushimi, T.; Inui, S.; Nakajima, T.; Ogasawara, M.; Hosokawa, K.; Itami, S. Green light emitting diodes accelerate wound healing: Characterization of the effect and its molecular basis In Vitro and In Vivo. Wound Repair. Regen. 2012, 20, 226–235. [Google Scholar] [CrossRef]
- Vinck, E.M.; Cagnie, B.J.; Cornelissen, M.J.; Declercq, H.A.; Cambier, D.C. Increased fibroblast proliferation induced by light emitting diode and low power laser irradiation. Lasers Med. Sci. 2003, 18, 95–99. [Google Scholar] [CrossRef]
- Sperandio, F.F.; Simões, A.; Corrêa, L.; Aranha, A.C.C.; Giudice, F.S.; Hamblin, M.R.; Sousa, S.C.O.M. Low-level laser irradiation promotes the proliferation and maturation of keratinocytes during epithelial wound repair. J. Biophotonics 2015, 8, 795–803. [Google Scholar] [CrossRef]
- Osiecka, B.; Nockowski, P.; Szepietowski, J. Treatment of actinic keratosis with photodynamic therapy using red or green light: A Comparative Study. Acta Derm. Venerol. 2018, 98, 689–693. [Google Scholar] [CrossRef]
- Maiman, T.H. Stimulated optical radiation in Ruby. Nature 1960, 187, 493. [Google Scholar] [CrossRef]
- Souza-Barros, L.; Dhaidan, G.; Maunula, M.; Solomon, V.; Gabison, S.; Lilge, L.; Nussbaum, E.L. Skin color and tissue thickness effects on transmittance, reflectance, and skin temperature when using 635 and 808 Nm lasers in low intensity therapeutics. Lasers Surg. Med. 2018, 50, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Z.; Liu, H.; Chen, X.; Liu, Y.; Tan, H. Photodynamic inactivation of fibroblasts and inhibition of staphylococcus epidermidis adhesion and biofilm formation by toluidine blue, O. Mol. Med. Rep. 2017, 15, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, V.; Hamblin, M.R. Photobiomodulation: Lasers vs light emitting diodes? Photochem. Photobiol. Sci. 2018, 17, 1003–1017. [Google Scholar] [CrossRef]
- Evans, D.; Abrahamse, H.; Maskew, M. Laser irradiation with 648 Nm light stimulates autophagic human skin keratinocytes: Peer reviewed original article. Med. Technol. SA 2012, 26, 34–43. [Google Scholar]
- Niu, T.; Tian, Y.; Cai, Q.; Ren, Q.; Wei, L. Red light combined with blue light irradiation regulates proliferation and apoptosis in skin keratinocytes in combination with low concentrations of curcumin. PLoS ONE 2015, 10, e0138754. [Google Scholar] [CrossRef] [PubMed]
- Leong, C.; Bigliardi, P.L.; Sriram, G.; Au, V.B.; Connolly, J.; Bigliardi-Qi, M. Physiological doses of red light induce IL-4 release in cocultures between human keratinocytes and immune cells. Photochem. Photobiol. 2018, 94, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Hyun Soo, K.; Yeo Jin, K.; Su Ji, K.; Doo Seok, K.; Lee, T.R.; Hyoung-June, K.; Dong Wook, S.; Young Rok, S. Transcriptomic analysis of human dermal fibroblast cells reveals potential mechanisms underlying the protective effects of visible red light against damage from ultraviolet B light. J. Dermatol. Sci. 2019. [Google Scholar] [CrossRef]
- Song, S.; Zhang, Y.; Fong, C.-C.; Tsang, C.-H.; Yang, Z.; Yang, M. CDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red light. J. Investig. Dermatol. 2003, 120, 849–857. [Google Scholar] [CrossRef]
- George, S.; Hamblin, M.R.; Abrahamse, H. Effect of red light and near infrared laser on the generation of reactive oxygen species in primary dermal fibroblasts. J. Photochem. Photobiol. B Biol. 2018, 188, 60–68. [Google Scholar] [CrossRef]
- Ayuk, S.M.; Houreld, N.N.; Abrahamse, H. Collagen production in diabetic wounded fibroblasts in response to low-intensity laser irradiation at 660 Nm. Diabetes Technol. 2012, 14, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, T.; Gonzalez, A.C.; Sá, M.F.; de Andrade, Z.A.; Reis, S.R.A.; Medrado, A.R.A.P. Effect of 670 Nm laser photobiomodulation on vascular density and fibroplasia in late stages of tissue repair. Int. Wound, J. 2018, 15, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Epithelial Cell-Enhanced Metabolism by Low-Level Laser Therapy and Epidermal Growth Factor. Available online: https://pubmed.ncbi.nlm.nih.gov/28285410/ (accessed on 13 July 2020).
- Passarella, S.; Karu, T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J. Photochem. Photobiol. B 2014, 140, 344–358. [Google Scholar] [CrossRef]
- Yao, J.; Liu, B.; Qin, F. Rapid temperature jump by infrared diode laser irradiation for patch-clamp studies. Biophys. J. 2009, 96, 3611–3619. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Tao, D.; Dong, Z.; Chen, Q.; Chao, Y.; Liu, Z.; Chen, M. Near-Infrared light activation of quenched liposomal Ce6 for synergistic cancer phototherapy with effective skin protection. Biomaterials 2017, 127, 13–24. [Google Scholar] [CrossRef]
- Solmaz, H.; Ulgen, Y.; Gulsoy, M. Photobiomodulation of wound healing via visible and infrared laser irradiation. Lasers Med. Sci. 2017, 32, 903–910. [Google Scholar] [CrossRef]
- Engel, K.W.; Khan, I.; Arany, P.R. Cell lineage responses to photobiomodulation therapy. J. Biophotonics. 2016. [Google Scholar] [CrossRef]
- Schmitt, L.; Amann, P.M.; Marquardt, Y.; Heise, R.; Czaja, K.; Gerber, P.A.; Steiner, T.; Hölzle, F.; Baron, J.M. Molecular effects of fractional ablative erbium: YAG laser treatment with multiple stacked pulses on standardized human three-dimensional organotypic skin models. Lasers Med. Sci. 2017, 32, 805–814. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, A.; Perfetto, B.; Guerrera, L.P.; Oliviero, G.; Baroni, A. Q-switched 1064 Nm Nd-Yag nanosecond laser effects on skin barrier function and on molecular rejuvenation markers in keratinocyte-fibroblasts interaction. Lasers Med. Sci. 2018. [Google Scholar] [CrossRef]
- Asghari, M.; Kanonisabet, A.; Safakhah, M.; Azimzadeh, Z.; Mostafavinia, A.; Taheri, S.; Amini, A.; Ghorishi, S.K.; JalaliFiroozkohi, R.; Bayat, S.; et al. The effect of combined photobiomodulation and metformin on open skin wound healing in a non-genetic model of type II diabetes. J. Photochem. Photobiol. B Biol. 2017, 169, 63–69. [Google Scholar] [CrossRef]
- Bitter, P. Acne treatment with 3-step broadband light protocol. J. Drugs Derm. 2016, 15, 1382–1388. [Google Scholar]
- Robati, R.M.; Asadi, E. Efficacy and safety of fractional CO2 Laser versus fractional Er:YAG laser in the treatment of facial skin wrinkles. Lasers Med. Sci. 2017, 32, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Robati, R.M.; Asadi, E.; Shafiee, A.; Namazi, N.; Talebi, A. Efficacy of long pulse Nd:YAG Laser versus fractional Er: YAG laser in the treatment of hand wrinkles. Lasers Med. Sci. 2018, 33, 461–467. [Google Scholar] [CrossRef] [PubMed]
| Author | Parameters | Material | Main Findings after Laser Treatment |
|---|---|---|---|
| Micka-Michalak et al. [21] | 308 nm; 250 mJ/cm2 | Pigmented human skin analog (fibroblasts, melanocytes, and keratinocytes isolated from healthy patients) engrafted to the skin of immuno-deficient female nu/nu rats |
|
| Tang et al. [22] | 308 nm; 0.05, 0.075, 0.10, 0.125, 0.150 and 0.175 J/cm2 | 36 patients with psoriasis, self-control study |
|
| Jobe et al. [26] | 365 nm; 10, 50 and 100 mJ/cm2 | Co-culture of dermal cells (keratinocytes and fibroblast) isolated from human skin (healthy and with melanoma) |
|
| Goldstein et al. [29] | 311–313 nm, starting dose - 0.2 J/cm2 | Skin biopsies of vitiligo patients |
|
| Yi et al. [30] | 313 nm, 90 mJ/cm2 | 50 SKH1 hairless mice |
|
| Gruber et al. [31] | 302 nm, 300 mJ/cm2 | MatTek® Human Epidermal Skin Equivalent |
|
| Penna et al. [32] | 302 nm, 500 and 1000 mJ/cm2 | Human Foreskin Fibroblasts |
|
| Chen et al. [34] | 290–315 nm, 1.5–50 mJ/cm2 | Human epidermal keratinocytes (HEK) Human skin tissues |
|
| Kwon et al. [36] | 310 nm, 50 mJ/cm2 combined with 340 nm, 5 mJ/cm2 | NC/Nga mice with induced atopic dermatitis |
|
| Author | Parameters | Material | Main Findings |
|---|---|---|---|
| Rascalou et al. [37] | 450, 525 and 625 nm combined, 99 J/cm2 | normal human fibroblasts |
|
| Nakashima et al. [38] | 460 nm, 0.133 J/cm2 | hairless mice expressing roGFP1 Human normal epidermal keratinocyte (HaCaT) cells expressing roGFP1 human skin in vivo |
|
| Lee et al. [39] | 410 nm, 10 J/cm2 | keloid fibroblasts isolated from keloid-revision surgery |
|
| Mignon et al. [40] | 450 nm, 0–250 J/cm2 | primary human reticular and papillary dermal fibroblasts |
|
| Zhang et al. [41] | 415 nm, 432 J/cm2 | C. albicans infected 16 adult mice: 8 untreated control, 8 study group |
|
| Zhang et al. [42] | 415 nm, 0, 28.0, 56.2, 84.2, 112.3, 140.4, and 168.5 J/cm2– for keratinocytes 415 nm, 55.8 J/cm2, 70.2 J/cm2, 195 J/cm2– for mice | Human normal epidermal keratinocyte (HaCaT), A. baumannii infected adult mice |
|
| Wang et al. [43] | 460 nm, 240 J/cm2 | C. albicans biofilm model, Human normal epidermal keratinocyte (HaCaT), Human normal foreskin fibroblast (Hs27), C. albicans infected mice |
|
| Makdoumi et al. [44] | 450 nm, 15 J/cm2, 30 J/cm2, 56 J/cm2, 84 J/cm2 | Human normal epidermal keratinocyte (HaCaT), MRSA HaCaT in vitro liquid layer model. |
|
| Mamalis et al. [45] | 415 nm, 0,5,10,15, 30, 80 J/cm2 | Primary human skin fibroblasts |
|
| Teuschl et al. [46] | 470 nm, 30 J/cm2 | NIH/3T3 fibroblasts, BICR10 keratinocytes |
|
| de Alencar Fernandes Neto et al. [47] | 470 nm, 12.5 J/cm2 | Wistar rats: control (n = 20) and blue LED (n = 20) |
|
| AlGhamdi et al. [48,49] | 457 nm, 0–5 J/cm2 | Human normal, foreskin melanocytes |
|
| Castellano-Pellicena et al. [50] | 453 nm, 2 J/cm2 | ex vivo human skin wound healing model primary human skin keratinocytes, primary human skin dermal fibroblasts |
|
| Marra et al. [51] | 415 nm, 20 J/cm2 | 30 normal nude mouse skin |
|
| Campiche et al. [52] | 450 nm, 4 x 60 J/cm2 | 33 human female (skin phototypes III and IV) |
|
| Author | Parameters | Material | Main Findings |
|---|---|---|---|
| Osiecka et al. [53] | 540 nm, 62.5 J/cm2 | 11 patients with chronic lichen sclerosus |
|
| Fritsch et al. [54] | 543–548 nm, 30 J/cm2 | six patients with extended solar keratoses |
|
| Li-qiang et al. [55] | 532 nm, 96–115 J/cm2 | 82 patients with port wine stains (PWS) |
|
| Zhang et al. [56] | 532 nm, 9.6–15 J/cm2 | 16 patients with port wine stains (PWS) |
|
| JalalKamali et al. [57] | 532 nm, 1.2 J/cm2 | basal skin carcinoma cells (BCC) |
|
| Fushimi et al. [58] | 518 nm, 0.2 J/cm2 | human normal epidermal keratinocyte (HaCaT), primary human skin dermal fibroblasts |
|
| Vinck et al. [59] | 570 nm, 0.1 J/cm2 | fibroblasts from chicken embryos |
|
| Osiecka et al. [61] | 540 nm, 62.5 J/cm2 | 20 patients with actinic keratosis |
|
| Author | Parameters | Material | Main Findings |
|---|---|---|---|
| Souza-Barros et al. [63] | 635 nm, 10.75–57.6 mJ/cm2 | 40 patients |
|
| Gonçalves Basso et al. [74] | 780 nm, 0.5, 1.5 and 3 J/cm2 | human keratinocytes (HaCaT cell line) |
|
| Li et al. [64] | 635 nm, 10, 20, 30 J/cm2 | mouse fibroblasts (L929 cell line) |
|
| Sperandino et al. [60] | cells: 660nm, 3, 6 or 12 J/cm2; animals: 660 nm, 117.85 J/cm2 | human keratinocytes (HaCaT cell line) 40 Wistar rats |
|
| Evans et al. [66] | 648 nm, 1.5 J/cm2 | human keratinocytes (CCD 1102 KERTr cell line) |
|
| Niu et al. [67] | combined 405 nm (1.604 J/cm2) and 630 nm (3.409 J/cm2) /660 nm, (6.538 J/cm2) | human keratinocytes (HaCaT cell line) treated with curcumin |
|
| Leong et al. [68] | 380 to 660 nm, 1 J/cm2 | co culture model: human keratinocytes (N/TERT-1 cell line) with human monocytic cells (THP-1 cell line) |
|
| Hyun-Soo et al. [69] | 620–690 nm, 60 J/cm2 | normal human dermal fibroblasts (NHDF cell line) |
|
| Song et al. [70] | 628nm, 0, 0.44, 0.88, 2.00, 4.40, and 8.68 J/cm2 | normal human fibroblasts of the newborn foreskin (HS27 cell line) |
|
| Ayuk et al. [72] | 660 nm, 5 J/cm2 | isolated human skin fibroblast in in vitro model of diabetic wound |
|
| George et al. [71] | 636 nm, 5, 10, 15, 20, 25 J/cm2 | fibroblasts isolated from the skin of donor undergoing abdominoplasty |
|
| Fortuna et al. [73] | 670 nm, 4 J/cm2 | 40 rats with scalpel-made wound |
|
| Author | Parameters | Material | Main Findings |
|---|---|---|---|
| George et al. [71] | 825 nm, 5, 10, 15, 20, 25 J/cm2 | Fibroblasts isolated from the skin of donor undergoing abdominoplasty |
|
| Solmaz et al. [78] | 809 nm, 1 and 3 J/cm2 | Mouse fibroblasts (L929 cell line) |
|
| Engel et al. [79] | 808 nm, 11.3, 13.2 15.1, 17 J/cm2 | Human oral fibroblasts, human normal oral keratinocytes-spontaneously immortalized |
|
| Schmitt et al. [80] | 2940 nm, 60 J/cm2 | 3D standardized organotypic model of human skin (keratinocytes and fibroblasts isolated from patients) |
|
| De Filippis et al. [81] | 1064 nm, 2, 4, 6, and 8 J/cm2 | Human normal epidermal keratinocyte (HaCaT) Human Dermal Fibroblasts (HDF) |
|
| Asghari et.al. [82] | 890 nm, 0.324 J/cm2 | 20 Wistar rats |
|
| Robati and Asadi [84] | Er:YAG laser: 2940 nm, 3.12 J/cm2 CO2 laser (far-infrared): 10600 nm, 20–18 mJ/cm2 | 40 patients |
|
| Robati et al. [85] | Er:YAG laser: 2940 nm, 3.12 J/cm2 Nd:YAG laser: 1064nm, 10-20 J/cm2 | 27 patients |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cios, A.; Ciepielak, M.; Szymański, Ł.; Lewicka, A.; Cierniak, S.; Stankiewicz, W.; Mendrycka, M.; Lewicki, S. Effect of Different Wavelengths of Laser Irradiation on the Skin Cells. Int. J. Mol. Sci. 2021, 22, 2437. https://doi.org/10.3390/ijms22052437
Cios A, Ciepielak M, Szymański Ł, Lewicka A, Cierniak S, Stankiewicz W, Mendrycka M, Lewicki S. Effect of Different Wavelengths of Laser Irradiation on the Skin Cells. International Journal of Molecular Sciences. 2021; 22(5):2437. https://doi.org/10.3390/ijms22052437
Chicago/Turabian StyleCios, Aleksandra, Martyna Ciepielak, Łukasz Szymański, Aneta Lewicka, Szczepan Cierniak, Wanda Stankiewicz, Mariola Mendrycka, and Sławomir Lewicki. 2021. "Effect of Different Wavelengths of Laser Irradiation on the Skin Cells" International Journal of Molecular Sciences 22, no. 5: 2437. https://doi.org/10.3390/ijms22052437
APA StyleCios, A., Ciepielak, M., Szymański, Ł., Lewicka, A., Cierniak, S., Stankiewicz, W., Mendrycka, M., & Lewicki, S. (2021). Effect of Different Wavelengths of Laser Irradiation on the Skin Cells. International Journal of Molecular Sciences, 22(5), 2437. https://doi.org/10.3390/ijms22052437






