Alteration of 3D Matrix Stiffness Regulates Viscoelasticity of Human Mesenchymal Stem Cells
Abstract
1. Introduction
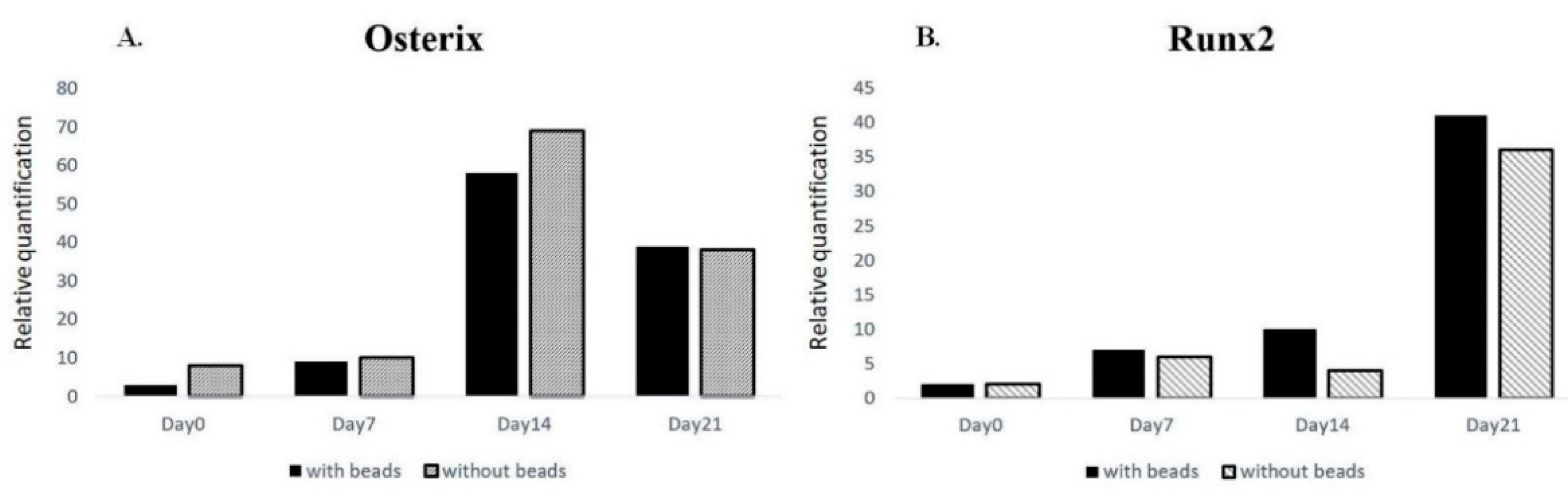
2. Results
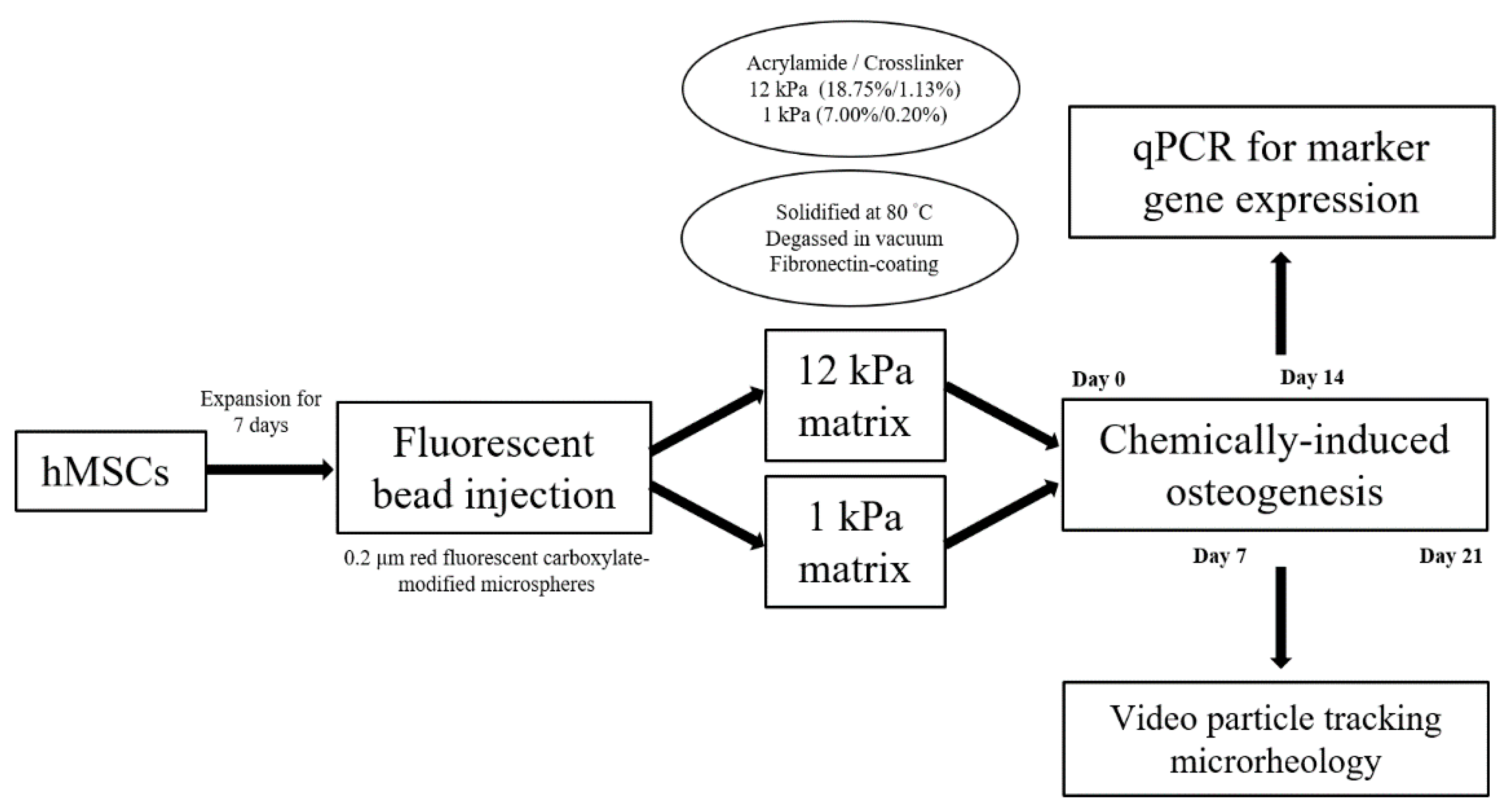
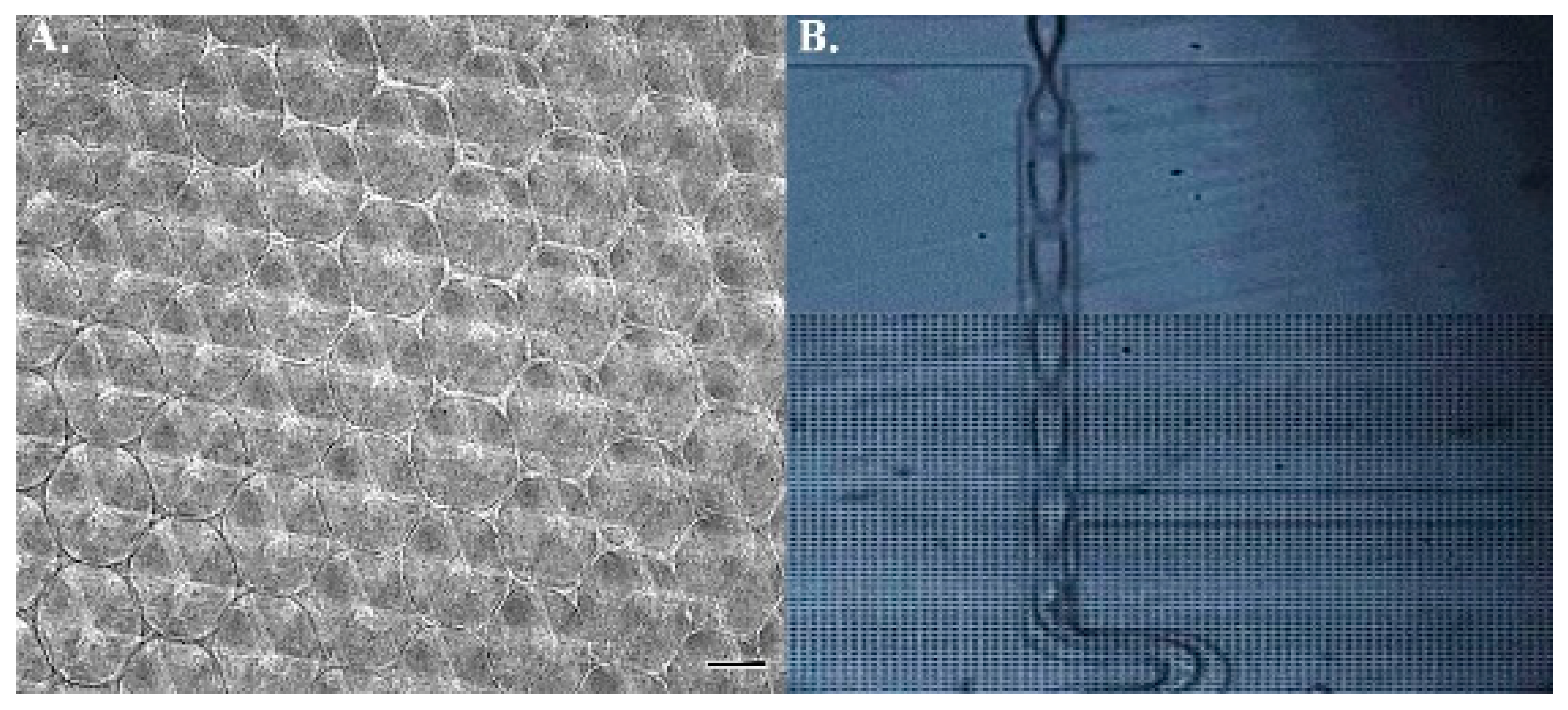
2.1. Scaffold Profiling and Bead Concentration
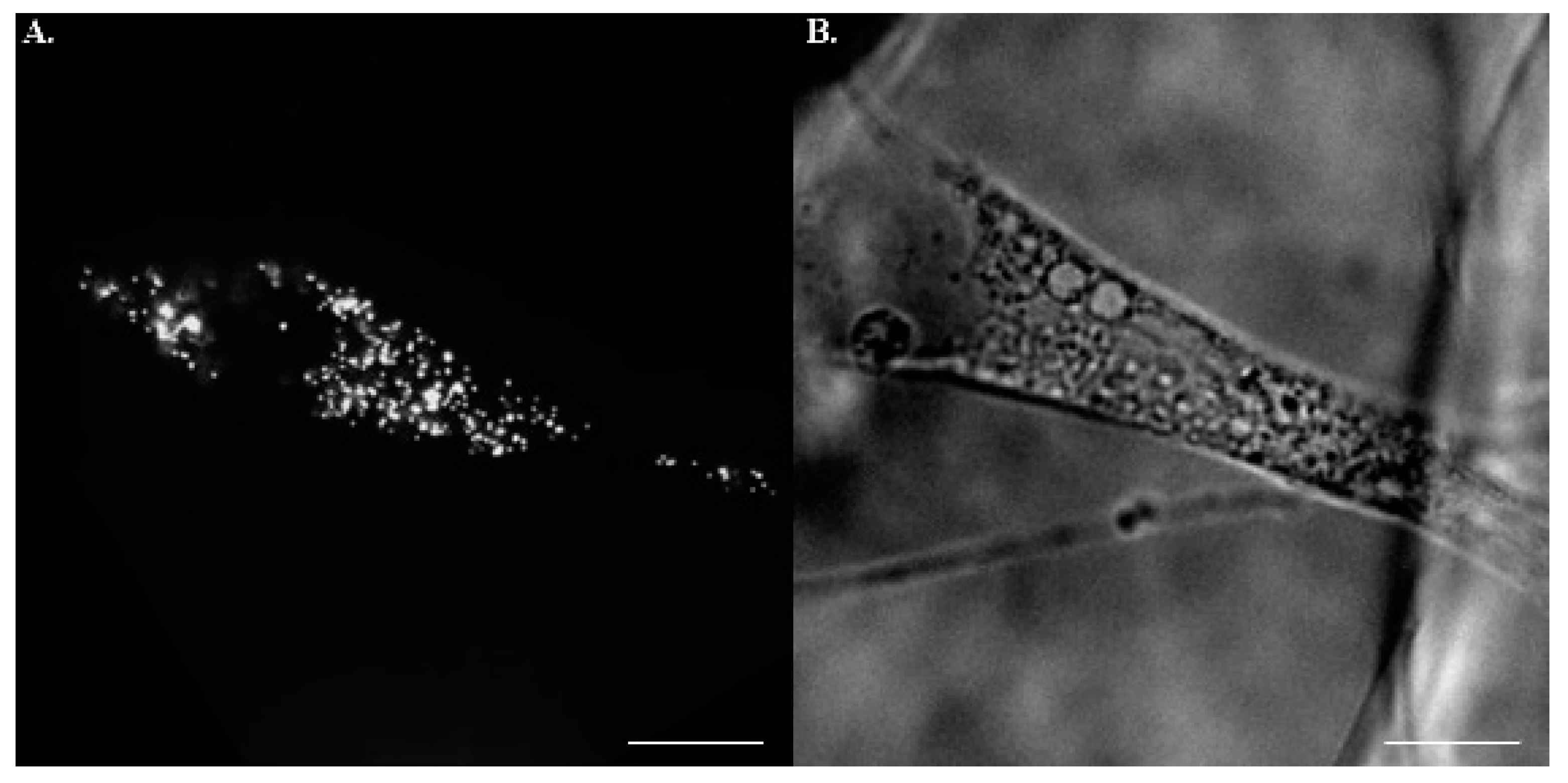
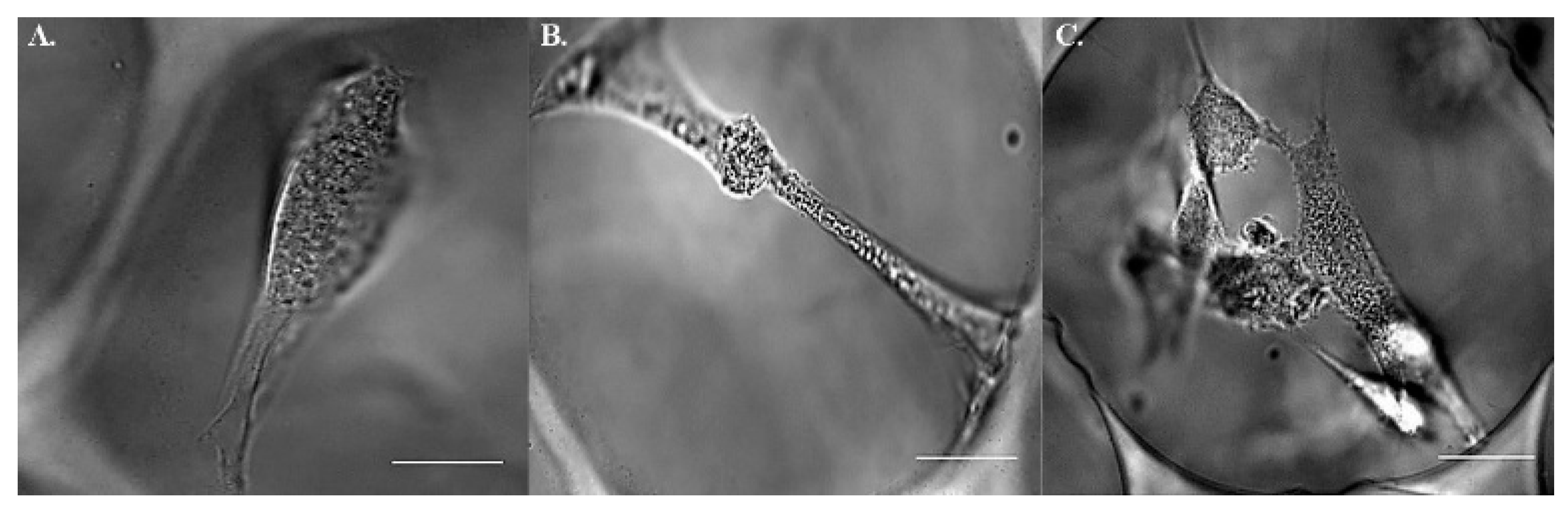
2.2. Postures of hMSCs in Porous Scaffolds
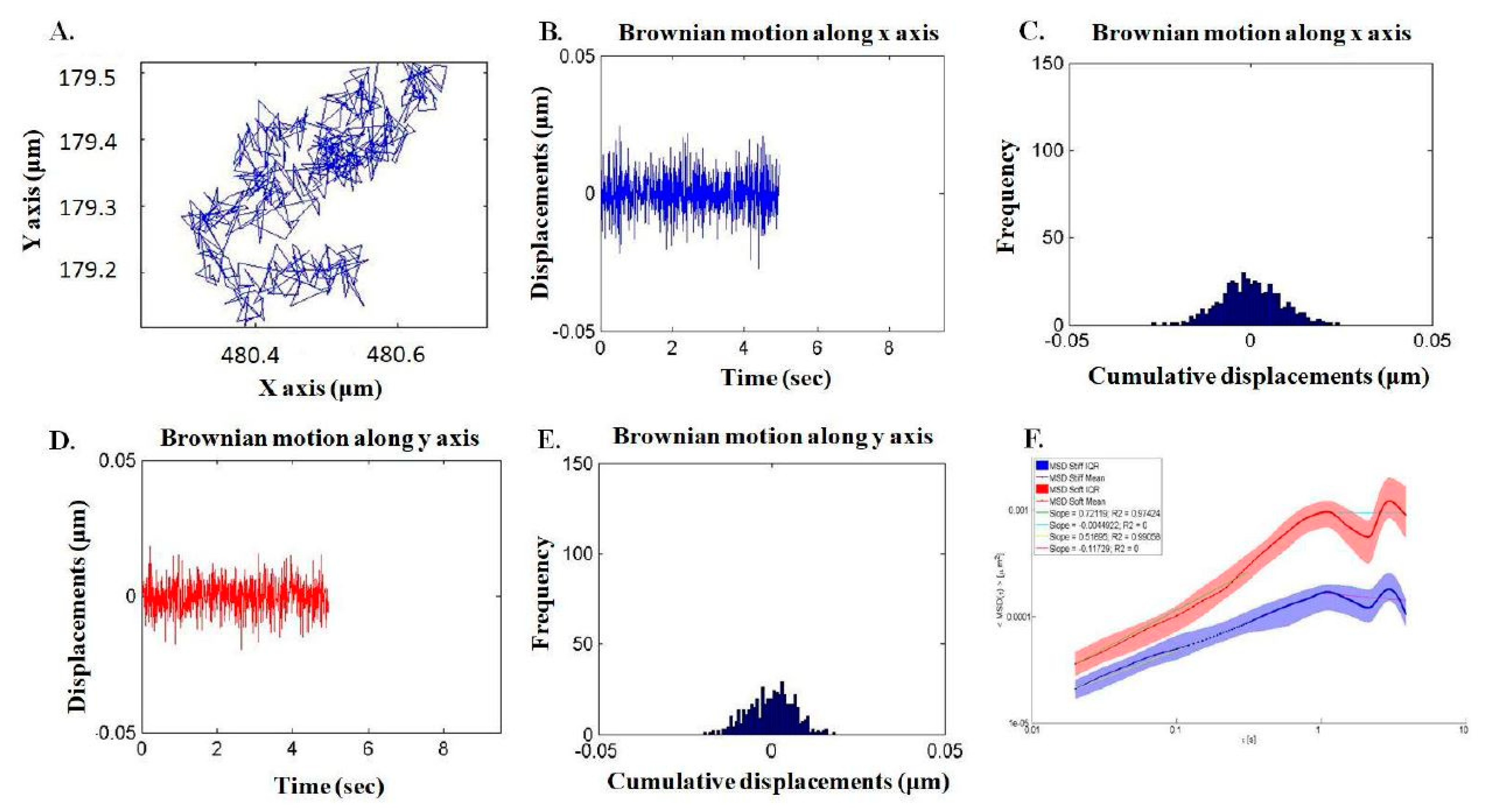
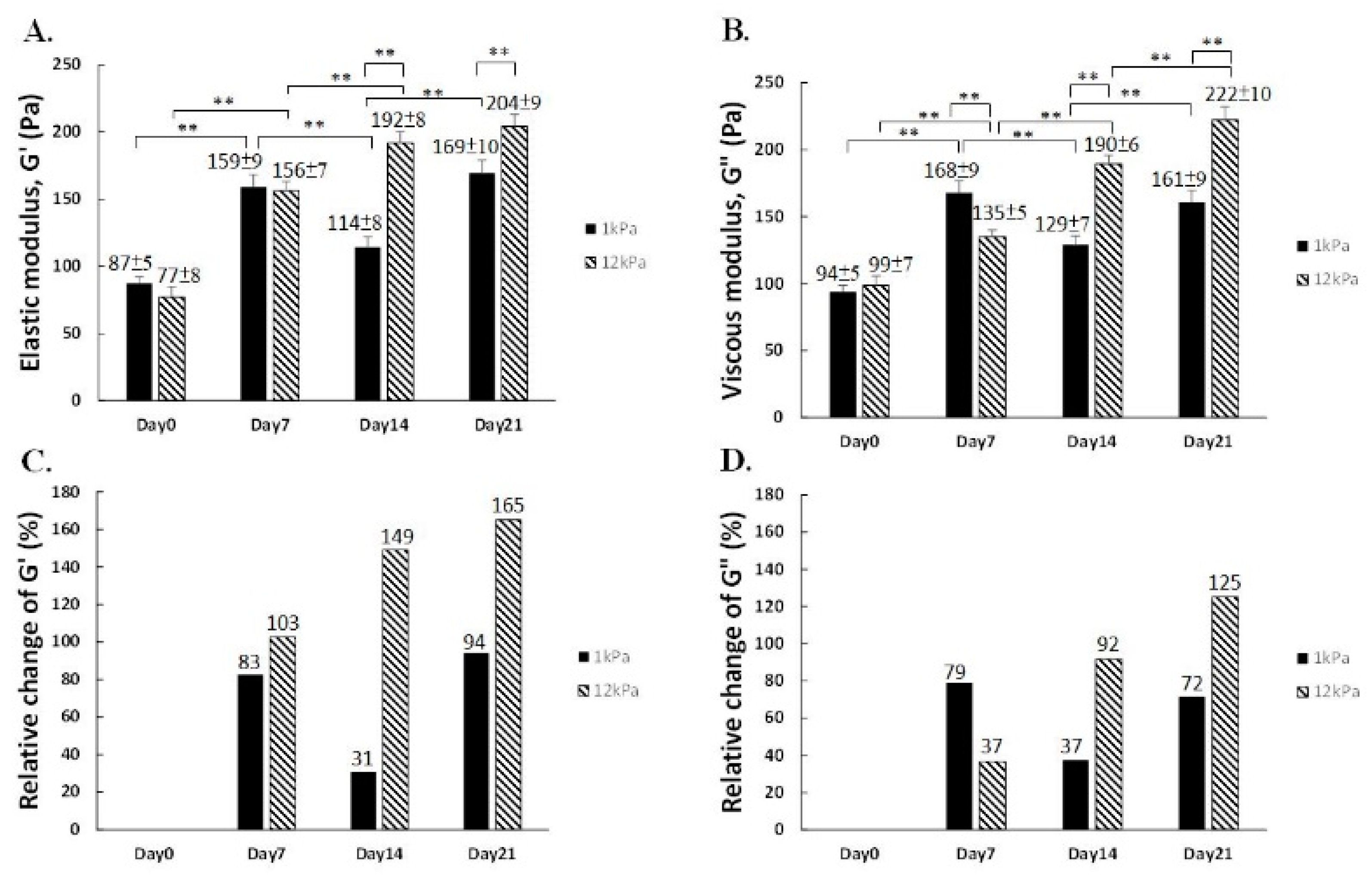
2.3. Assessment of Intracellular Viscoelasticity by VPTM
3. Discussion
4. Materials and Methods
4.1. Fabrication of Polyacrylamide 3D Scaffolds
4.2. Injection and Tracking of Fluorescent Particles
4.3. hMSCs Culture, Maintenance and Chemical Induction
4.4. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The epidemiology of osteoporosis. Br. Med. Bull. 2020, 16, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Cummings, S.R.; San Martin, J.; McClung, M.R.; Siris, E.S.; Eastell, R.; Reid, I.R.; Pierre Delmas, M.D.; Matt Austin, M.S.; Andrea Wang, M.A.; Stepan Kutilek, M.D.; et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N. Engl. J. Med. 2009, 361, 756–765. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Khatiwala, C.B.; Kim, P.D.; Peyton, S.R.; Putnam, A.J. ECM compliance regulates osteogenesis by influencing MAPK signaling downstream of RhoA and ROCK. J. Bone Miner. Res. 2009, 24, 886–898. [Google Scholar] [CrossRef]
- Wrighton, K.H. Mechanotransduction: YAP and TAZ feel the force. Nat. Rev. Mol. Cell Biol. 2011, 12, 404. [Google Scholar] [CrossRef]
- Mullen, C.A.; Vaughan, T.J.; Billiar, K.L.; McNamara, L.M. The effect of substrate stiffness, thickness, and cross-linking density on osteogenic cell behavior. Biophys. J. 2015, 108, 1604–1612. [Google Scholar] [CrossRef]
- Caiazzo, M.; Okawa, Y.; Ranga, A.; Piersigilli, A.; Tabata, Y.; Lutolf, M.P. Defined three-dimensional microenvironments boost induction of pluripotency. Nat. Mater. 2016, 15, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension: How 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef]
- Hsieh, W.T.; Liu, Y.S.; Lee, Y.H.; Rimando, M.G.; Lin, K.H.; Lee, O.K. Matrix dimensionality and stiffness cooperatively regulate osteogenesis of mesenchymal stromal cells. Acta Biomater. 2016, 32, 210–222. [Google Scholar] [CrossRef]
- Kim, H.; Bae, C.; Kook, Y.M.; Koh, W.G.; Lee, K.; Park, M.H. Mesenchymal stem cell 3D encapsulation technologies for biomimetic microenvironment in tissue regeneration. Stem Cell Res. Ther. 2019, 10, 51. [Google Scholar] [CrossRef]
- Yen, M.H.; Chen, Y.H.; Liu, Y.S.; Lee, O.K. Alteration of Young’s modulus in mesenchymal stromal cells during osteogenesis measured by atomic force microscopy. Biochem. Biophys. Res. Commun. 2020, 526, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.G. Estimating the viscoelastic moduli of complex fluids using the generalized Stokes–Einstein equation. Rheol. Acta 2000, 39, 371–378. [Google Scholar] [CrossRef]
- Banks, J.M.; Mozdzen, L.C.; Harley, B.A.; Bailey, R.C. The combined effects of matrix stiffness and growth factor immobilization on the bioactivity and differentiation capabilities of adipose-derived stem cells. Biomaterials 2014, 35, 8951–8959. [Google Scholar] [CrossRef] [PubMed]
- Keogh, M.B.; O’Brien, F.J.; Daly, J.S. Substrate stiffness and contractile behaviour modulate the functional maturation of osteoblasts on a collagen-GAG scaffold. Acta Biomater. 2010, 6, 4305–4313. [Google Scholar] [CrossRef]
- Wirtz, D. Particle-tracking microrheology of living cells: Principles and applications. Annu. Rev. Biophys. 2009, 38, 301–326. [Google Scholar] [CrossRef]
- McGlynn, J.A.; Wu, N.; Schultz, K.M. Multiple particle tracking microrheological characterization: Fundamentals, emerging techniques and applications. J. Appl. Phys. 2020, 127, 201101. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Chou, P.L.; Cheng, C.Y.; Chiang, C.C.; Wei, M.T.; Chuang, C.T.; Chen, Y.L.S.; Chiou, A. Microrheology of human synovial fluid of arthritis patients studied by diffusing wave spectroscopy. J. Biophoton. 2012, 5, 777–784. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Kuo, C.Y.; Wei, M.T.; Wu, K.; Su, P.T.; Huang, C.S.; Chiou, A.E. Intracellular viscoelasticity of HeLa cells during cell division studied by video particle-tracking microrheology. J. Biomed. Opt. 2014, 19, 011008. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Huang, J.R.; Wang, Y.K.; Lin, K.H. Three-dimensional fibroblast morphology on compliant substrates of controlled negative curvature. Integr. Biol. Camb. 2013, 5, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.P.; Liu, Y.S.; Rimando, M.G.; Lin, K.H. Three-dimensional spherical spatial boundary conditions differentially regulate osteogenic differentiation of mesenchymal stromal cells. Sci. Rep. 2016, 6, 21253. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Arany, P.R.; Mao, A.S.; Shvartsman, D.; Ali, O.A.; Bencherif, S.A.; Rivera-Feliciano, J.; Mooney, D.J. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010, 9, 518–526. [Google Scholar] [CrossRef]
- Haugh, M.G.; Vaughan, T.J.; Madl, C.M.; Raftery, R.M.; McNamara, L.M.; O’Brien, F.J.; Heilshorn, S.C. Investigating the interplay between substrate stiffness and ligand chemistry in directing mesenchymal stem cell differentiation within 3D macro-porous substrates. Biomaterials 2018, 171, 23–33. [Google Scholar] [CrossRef]
- Li, Z.; Gong, Y.; Sun, S.; Du, Y.; Lü, D.; Liu, X.; Long, M. Differential regulation of stiffness, topography, and dimension of substrates in rat mesenchymal stem cells. Biomaterials 2013, 34, 7616–7625. [Google Scholar] [CrossRef]
- Yu, L.; Wu, Y.; Liu, J.; Li, B.; Ma, B.; Li, Y.; Huang, Z.; He, J. 3D culture of bone marrow-derived mesenchymal stem cells could improve bone regeneration in 3D-printed porous Ti6Al4V scaffolds. Stem Cells Int. 2018, 2018, 2074021. [Google Scholar] [CrossRef]
- Yao, B.; Wang, R.; Wang, Y.; Zhang, Y.; Hu, T.; Song, W.; Li, Z.; Huang, S.; Fu, X. Biochemical and structural cues of 3D-printed matrix synergistically direct MSC differentiation for functional sweat gland regeneration. Sci. Adv. 2020, 6, 1094. [Google Scholar] [CrossRef] [PubMed]
- Egger, D.; Oliveira, A.C.; Mallinger, B.; Hemeda, H.; Charwat, V.; Kasper, C. From 3D to 3D: Isolation of mesenchymal stem/stromal cells into a three-dimensional human platelet lysate matrix. Stem Cell Res. Ther. 2019, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Liu, A.; Sun, J.; Chen, S.; Wu, C.; Zhu, H.; Chen, Y.; Luo, H.; Fan, H. Mechanics-controlled dynamic cell niches guided osteogenic differentiation of stem cells via preserved cellular mechanical memory. ACS Appl. Mater. Interfaces 2020, 12, 260–274. [Google Scholar] [CrossRef]
- Rabel, K.; Kohal, R.J.; Steinberg, T.; Tomakidi, P.; Rolauffs, B.; Adolfsson, E.; Palmero, P.; Fürderer, T.; Altmann, B. Controlling osteoblast morphology and proliferation via surface micro-topographies of implant biomaterials. Sci. Rep. 2020, 10, 12810. [Google Scholar] [CrossRef] [PubMed]
- Becquart, P.; Cruel, M.; Hoc, T.; Sudre, L.; Pernelle, K.; Bizios, R.; Logeart-Avramoglou, D.; Petite, H.; Bensidhoum, M. Human mesenchymal stem cell responses to hydrostatic pressure and shear stress. Eur. Cell Mater. 2016, 31, 160–173. [Google Scholar] [CrossRef] [PubMed]
| Technique | Purpose | Application in this Study |
|---|---|---|
| Polydimethylsiloxane-based microfluidic device | To propel the movement of those ingredients introduced for scaffold manufacturing | The device was employed to facilitate mixing of input materials |
| Biolistic delivery system | To place particle into cell of interest | Fluorescent beads were injected intracellularly to hMSCs |
| Charge-coupled camera | To capture cellular image under designated resolution | TxRed and bright images of hMSCs were obtained. |
| Video particle tracking microrheology | To deduce Young’s modulus from Brownian motion of intracellular particles | The viscoelasticity of hMSCs were yielded by tracing the random walk of fluorescent particles |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, T.-W.; Chiou, A.; Lin, K.-H.; Liu, Y.-S.; Lee, O.K.-S. Alteration of 3D Matrix Stiffness Regulates Viscoelasticity of Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 2441. https://doi.org/10.3390/ijms22052441
Kao T-W, Chiou A, Lin K-H, Liu Y-S, Lee OK-S. Alteration of 3D Matrix Stiffness Regulates Viscoelasticity of Human Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2021; 22(5):2441. https://doi.org/10.3390/ijms22052441
Chicago/Turabian StyleKao, Ting-Wei, Arthur Chiou, Keng-Hui Lin, Yi-Shiuan Liu, and Oscar Kuang-Sheng Lee. 2021. "Alteration of 3D Matrix Stiffness Regulates Viscoelasticity of Human Mesenchymal Stem Cells" International Journal of Molecular Sciences 22, no. 5: 2441. https://doi.org/10.3390/ijms22052441
APA StyleKao, T.-W., Chiou, A., Lin, K.-H., Liu, Y.-S., & Lee, O. K.-S. (2021). Alteration of 3D Matrix Stiffness Regulates Viscoelasticity of Human Mesenchymal Stem Cells. International Journal of Molecular Sciences, 22(5), 2441. https://doi.org/10.3390/ijms22052441






