Resveratrol Affects Insulin Signaling in Type 2 Diabetic Goto-Kakizaki Rats
Abstract
1. Introduction
2. Results
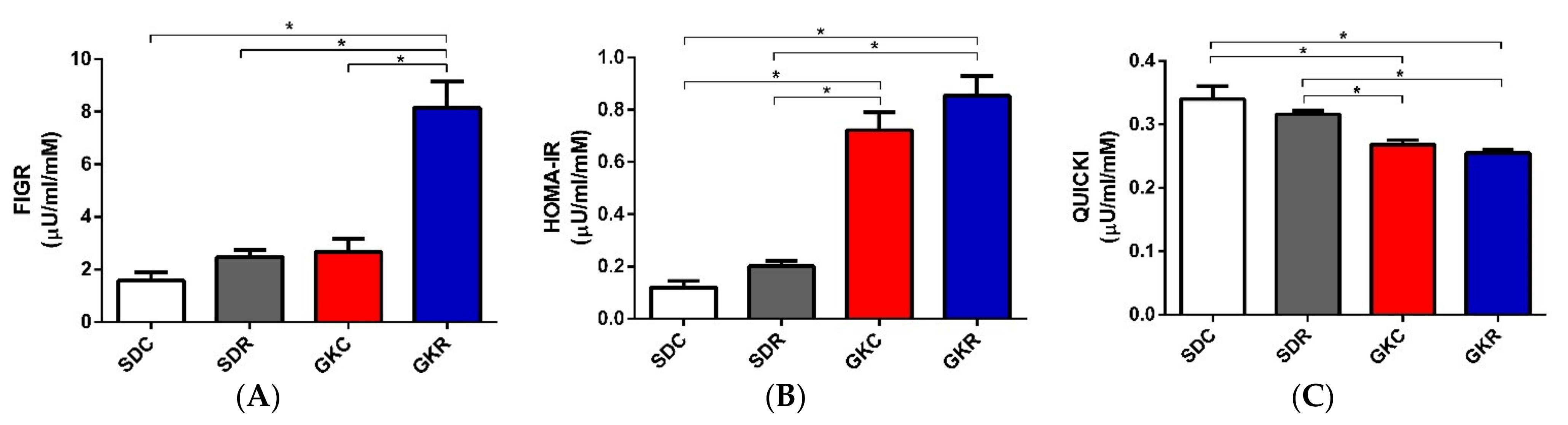
2.1. Effects of Resveratrol on FIGR, HOMA-IR, QUICKI, and Hormone Levels
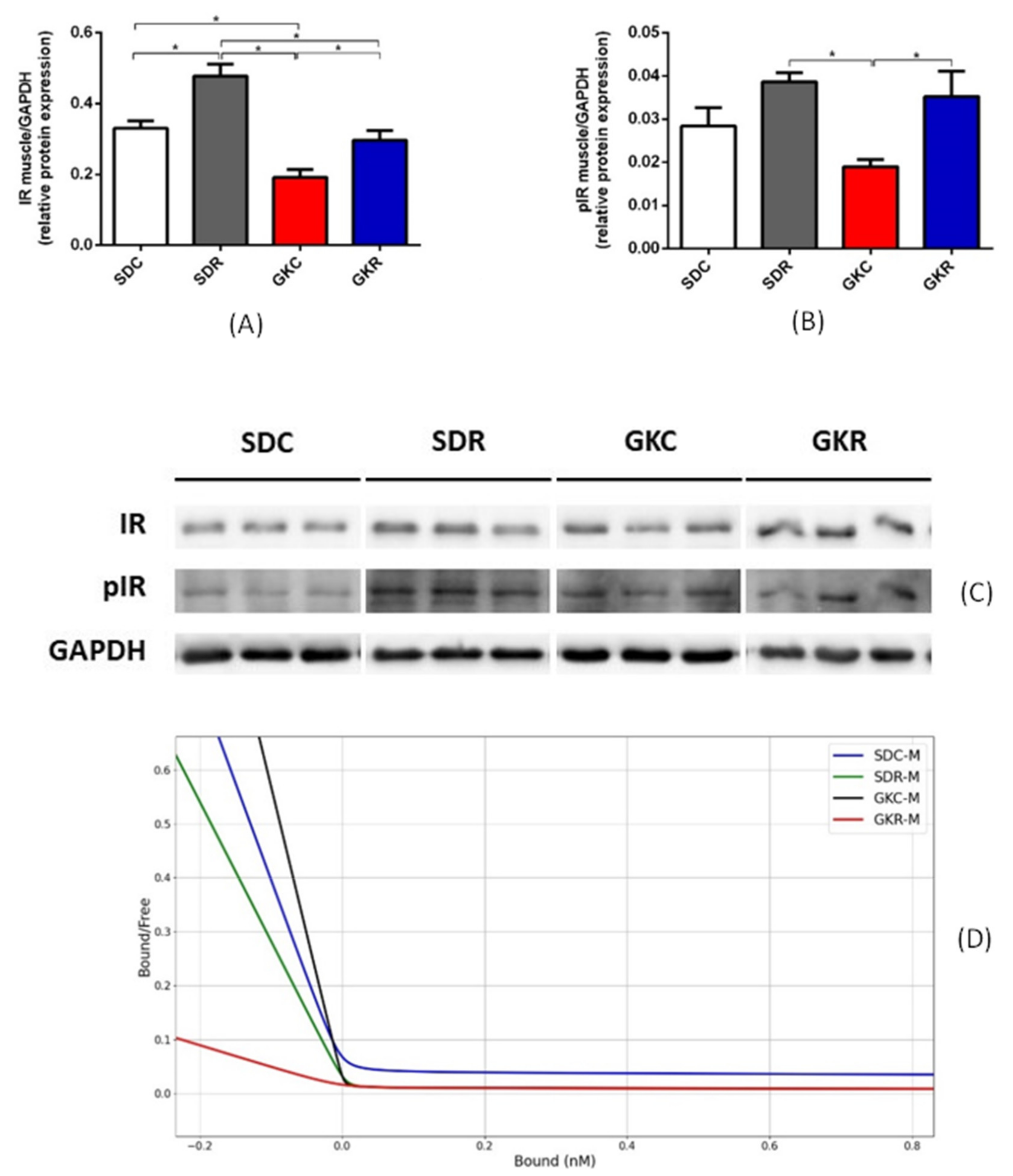
2.2. Effects of Resveratrol on Expression of Insulin Receptor and on Insulin Binding
2.3. Effects of Resveratrol on Expression of GLUT4 and TUG
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment
4.2. Determination of FIGR, HOMA-IR, QUICKI, and Tissue Sampling
4.3. Blood Hormone Levels
4.4. Western Blot Immunodetection
4.5. Insulin Receptor Kinetics
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Acetyl-CoA carboxylase |
| Akt | Protein kinase B |
| FIGR | Fasting insulin/glucose ratio |
| GIP | Glucose-dependent insulinotropic peptide |
| GKC | Goto-Kakizaki control rats |
| GKR | Goto-Kakizaki rats treated with resveratrol |
| GLP-1 | Glucagon-like peptide-1 |
| GLUT4 | Glucose transporter 4 |
| HAIRs | High-affinity insulin receptors |
| HOMA-IR | Homeostatic model assessment—insulin resistance |
| IDE | Insulin-degrading enzyme |
| IRS-1 | Insulin receptor substrate-1 |
| Kd1 | Dissociation constant for HAIRs |
| Kd2 | Dissociation constant for LAIRs |
| LAIR | Low-affinity insulin receptors |
| QUICKI | Quantitative insulin sensitivity check index |
| R1 | Maximum binding capacity of HAIRs |
| R2 | Maximum binding capacity of LAIRs |
| SDC | Sprague-Dawley control rats |
| SDR | Sprague-Dawley rats treated with resveratrol |
| TUG | Tether containing a UBX domain for GLUT4 |
References
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of red wine consumption to human health protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef] [PubMed]
- Szkudelski, T.; Szkudelska, K. Resveratrol and diabetes: From animal to human studies. Biochim. Biophys. Acta 2015, 1852, 1145–1154. [Google Scholar] [CrossRef]
- Öztürk, E.; Arslan, A.K.K.; Yerer, M.B.; Bishayee, A. Resveratrol and diabetes: A critical review of clinical studies. Biomed. Pharmacother. 2017, 95, 230–234. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37, s81–s90. [Google Scholar] [CrossRef]
- Hirsch, G.E.; Heck, T.G. Inflammation, oxidative stress and altered heat shock response in type 2 diabetes: The basis for new pharmacological and non-pharmacological interventions. Arch. Physiol. Biochem. 2019, 20, 1–15. [Google Scholar] [CrossRef]
- Huang, D.D.; Shi, G.; Jiang, Y.; Yao, C.; Zhu, C. A review on the potential of resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed. Pharmacother. 2020, 125, 109767. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors 2018, 44, 36–49. [Google Scholar] [CrossRef]
- Poulsen, M.M.; Fjeldborg, K.; Ornstrup, M.J.; Kjær, T.N.; Nøhr, M.K.; Pedersen, S.B. Resveratrol and inflammation: Challenges in translating pre-clinical findings to improved patient outcomes. Biochim. Biophys. Acta 2015, 1852, 1124–1136. [Google Scholar] [CrossRef]
- Brasnyó, P.; Molnár, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell. Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef]
- Movahed, A.; Nabipour, I.; Lieben Louis, X.; Thandapilly, S.J.; Yu, L.; Kalantarhormozi, M.; Rekabpour, S.J.; Netticadan, T. Antihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evid. Based Complement. Alternat. Med. 2013, e2013, 851267. [Google Scholar] [CrossRef] [PubMed]
- Bashmakov, Y.K.; Assaad-Khalil, S.H.; Abou Seif, M.; Udumyan, R.; Megallaa, M.; Rohoma, K.H.; Zeitoun, M.; Petyaev, I.M. Resveratrol promotes foot ulcer size reduction in type 2 diabetes patients. ISRN Endocrinol. 2014, 2014, e816307. [Google Scholar] [CrossRef] [PubMed]
- Zare Javid, A.; Hormoznejad, R.; Yousefimanesh, H.A.; Zakerkish, M.; Haghighi-Zadeh, M.H.; Dehghan, P.; Ravanbakhsh, M. The impact of resveratrol supplementation on blood glucose, insulin, insulin resistance, triglyceride, and periodontal markers in type 2 diabetic patients with chronic periodontitis. Phytother. Res. 2017, 31, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, A.; Namazi, G.; Farrokhian, A.; Reiner, Ž.; Aghadavod, E.; Bahmani, F.; Asemi, Z. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct. 2019, 10, 6042–6051. [Google Scholar] [CrossRef]
- Bo, S.; Ponzo, V.; Ciccone, G.; Evangelista, A.; Saba, F.; Goitre, I.; Procopio, M.; Pagano, G.F.; Cassader, M.; Gambino, R. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. A randomized, double blind, placebo-controlled trial. Pharmacol. Res. 2016, 111, 896–905. [Google Scholar] [CrossRef]
- Timmers, S.; de Ligt, M.; Phielix, E.; van de Weijer, T.; Hansen, J.; Moonen-Kornips, E.; Schaart, G.; Kunz, I.; Hesselink, M.K.; Schrauwen-Hinderling, V.B.; et al. Resveratrol as add-on therapy in subjects with well-controlled type 2 diabetes: A randomized controlled trial. Diabetes Care 2016, 39, 2211–2217. [Google Scholar] [CrossRef]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human study and clinical trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef]
- Szkudelska, K.; Deniziak, M.; Hertig, I.; Wojciechowicz, T.; Tyczewska, M.; Jaroszewska, M.; Szkudelski, T. Effects of resveratrol in Goto-Kakizaki rat, a model of type 2 diabetes. Nutrients 2019, 11, 2488. [Google Scholar] [CrossRef]
- Szkudelska, K.; Okulicz, M.; Hertig, I.; Szkudelski, T. Resveratrol ameliorates inflammatory and oxidative stress in type 2 diabetic Goto-Kakizaki rats. Biomed. Pharmacother. 2020, 125, 110026. [Google Scholar] [CrossRef]
- Szkudelska, K.; Okulicz, M.; Szkudelski, T. Resveratrol reduces excessive cholesterol accumulation in Goto-Kakizaki rat, a model with congenital type 2 diabetes. J. Physiol. Pharmacol. 2020, 71, 581–587. [Google Scholar]
- Portha, B.; Lacraz, G.; Kergoat, M.; Homo-Delarche, F.; Giroix, M.H.; Bailbé, D.; Gangnerau, M.N.; Dolz, M.; Tourrel-Cuzin, C.; Movassat, J. The GK rat beta-cell: A prototype for the diseased human beta-cell in type 2 diabetes? Mol. Cell. Endocrinol. 2009, 297, 73–85. [Google Scholar] [CrossRef]
- Li, J.; Xu, S.; Liu, Y.; Yan, Z.; Zhang, F.; Lv, Q.; Tong, N. Activated PPARβ/δ protects pancreatic β cells in type 2 diabetic Goto-Kakizaki rats from lipoapoptosis via GPR40. Lipids 2019, 54, 603–616. [Google Scholar] [CrossRef]
- Cahová, M.; Habart, D.; Olejár, T.; Berková, Z.; Papáčková, Z.; Daňková, H.; Lodererova, A.; Heczková, M.; Saudek, F. Lipasin/betatrophin is differentially expressed in liver and white adipose tissue without association with insulin resistance in Wistar and Goto-Kakizaki rats. Physiol. Res. 2017, 66, 273–281. [Google Scholar] [CrossRef]
- Chia, C.W.; Egan, J.M. Incretins in obesity and diabetes. Ann. N. Y. Acad. Sci. 2020, 1461, 104–126. [Google Scholar] [CrossRef]
- Kuwabara, W.M.T.; Panveloski-Costa, A.C.; Yokota, C.N.F.; Pereira, J.N.B.; Filho, J.M.; Torres, R.P.; Hirabara, S.M.; Curi, R.; Alba-Loureiro, T.C. Comparison of Goto-Kakizaki rats and high fat diet-induced obese rats: Are they reliable models to study type 2 diabetes mellitus? PLoS ONE 2017, 12, e0189622. [Google Scholar] [CrossRef]
- Song, X.M.; Kawano, Y.; Krook, A.; Ryder, J.W.; Efendic, S.; Roth, R.A.; Wallberg-Henriksson, H.; Zierath, J.R. Muscle fiber type-specific defects in insulin signal transduction to glucose transport in diabetic GK rats. Diabetes 1999, 48, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Bitar, M.S.; Al-Saleh, E.; Al-Mulla, F. Oxidative stress-mediated alterations in glucose dynamics in a genetic animal model of type II diabetes. Life Sci. 2005, 77, 2552–2573. [Google Scholar] [CrossRef]
- Dadke, S.S.; Li, H.C.; Kusari, A.B.; Begum, N.; Kusari, J. Elevated expression and activity of protein-tyrosine phosphatase 1B in skeletal muscle of insulin-resistant type II diabetic Goto-Kakizaki rats. Biochem. Biophys. Res. Commun. 2000, 274, 583–589. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, K.J. Regulation of signaling molecules associated with insulin action, insulin secretion and pancreatic β-cell mass in the hypoglycemic effects of Korean red ginseng in Goto-Kakizaki rats. Ethnopharmacology 2012, 142, 53–58. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, Á.; Santamaría, B.; Mas-Gutierrez, J.A.; Rada, P.; Fernández-Millán, E.; Pardo, V.; Álvarez, C.; Cuadrado, A.; Ros, M.; Serrano, M.; et al. Resveratrol treatment restores peripheral insulin sensitivity in diabetic mice in a sirt1-independent manner. Mol. Nutr. Food. Res. 2015, 59, 1431–1442. [Google Scholar] [CrossRef]
- Gammeltoft, S. Insulin receptors: Binding kinetics and structure-function relationship of insulin. Physiol. Rev. 1984, 64, 1321–1378. [Google Scholar] [CrossRef]
- Bogan, J.S. Regulation of glucose transporter translocation in health and diabetes. Annu. Rev. Biochem. 2012, 81, 507–532. [Google Scholar] [CrossRef]
- Klip, A.; McGraw, T.E.; James, D.E. Thirty sweet years of GLUT4. J. Biol. Chem. 2019, 294, 11369–11381. [Google Scholar] [CrossRef] [PubMed]
- Belman, J.P.; Bian, R.R.; Habtemichael, E.N.; Li, D.T.; Jurczak, M.J.; Alcázar-Román, A.; McNally, L.J.; Shulman, G.I.; Bogan, J.S. Acetylation of TUG protein promotes the accumulation of GLUT4 glucose transporters in an insulin-responsive intracellular compartment. J. Biol. Chem. 2015, 290, 4447–4463. [Google Scholar] [CrossRef]
- Papazoglou, I.; Berthou, F.; Vicaire, N.; Rouch, C.; Markaki, E.M.; Bailbe, D.; Portha, B.; Taouis, M.; Gerozissis, K. Hypothalamic serotonin-insulin signaling cross-talk and alterations in a type 2 diabetic model. Mol. Cell. Endocrinol. 2012, 350, 136–144. [Google Scholar] [CrossRef]
- Bisbis, S.; Bailbe, D.; Tormo, M.A.; Picarel-Blanchot, F.; Derouet, M.; Simon, J.; Portha, B. Insulin resistance in the GK rat: Decreased receptor number but normal kinase activity in liver. Am. J. Physiol. 1993, 265, E807–E813. [Google Scholar] [CrossRef]
- Torlinska, T.; Perz, M.; Madry, E.; Hryniewiecki, T.; Nowak, K.W.; Mackowiak, P. Effect of hypothermia on insulin-receptor interaction in different rat tissues. Physiol. Res. 2002, 51, 261–266. [Google Scholar]
- Barchetta, I.; Cimini, F.A.; Ciccarelli, G.; Baroni, M.G.; Cavallo, M.G. Sick fat: The good and the bad of old and new circulating markers of adipose tissue inflammation. J. Endocrinol. Investig. 2019, 42, 1257–1272. [Google Scholar] [CrossRef]
- Xue, B.; Nie, J.; Wang, X.; DuBois, D.C.; Jusko, W.J.; Almon, R.R. Effects of high fat feeding on adipose tissue gene expression in diabetic Goto-Kakizaki rats. Gene Regul. Syst. Biol. 2015, 9, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Hong, H.J.; Guan, J.; Kim, D.G.; Yang, E.J.; Koh, G.; Park, D.; Han, C.H.; Lee, Y.J.; Lee, D.H. Resveratrol improves insulin signaling in a tissue-specific manner under insulin-resistant conditions only: In vitro and in vivo experiments in rodents. Metabolism 2012, 61, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Politis, T.; Darvesh, A.S. Resveratrol in the chemoprevention and treatment of hepatocellular carcinoma. Cancer Treat. Rev. 2010, 36, 43–53. [Google Scholar] [CrossRef]
- Edwards, J.A.; Beck, M.; Riegger, C.; Bausch, J. Safety of resveratrol with examples for high purity, trans-resveratrol, resVida(). Ann. N. Y. Acad. Sci. 2011, 1215, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Havrankova, J.; Roth, J.; Brownstein, M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature 1978, 272, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Scatchard, G. The attractions of proteins for small molecules and ions. Ann. N. Y. Acad. Sci. 1949, 51, 660–672. [Google Scholar] [CrossRef]
- Feldman, H.A. Mathematical theory of complex ligand-binding systems of equilibrium: Some methods for parameter fitting. Annal. Biochem. 1972, 48, 317–338. [Google Scholar] [CrossRef]


| Parameter | SDC | SDR | GKC | GKR |
|---|---|---|---|---|
| Insulin (ng/mL) | 2.01 ± 0.27 | 2.51 ± 0.39 | 5.48 ± 0.53 *,# | 6.2 ± 0.63 *,# |
| Proinsulin (pmol/mL) | 42.37 ± 1.6 | 43.20 ± 0.9 | 22.51 ± 0.8 *,# | 23.35 ± 6.7 *,# |
| Glucagon (pg/mL) | 115 ± 12.3 | 103 ± 8.7 | 122 ± 16.4 | 113 ± 13.4 |
| GIP (ng/mL) | 14.26 ± 0.62 | 13.87 ± 0.45 | 8.35 ± 0.49 *,# | 7.99 ± 0.35 *,# |
| GLP-1 (ng/mL) | 0.58 ± 0.02 | 0.53 ± 0.03 | 0.74 ± 0.06 # | 0.71 ± 0.05 # |
| Parameter | SDC | SDR | GKC | GKR |
|---|---|---|---|---|
| Kd1 Liver (pM) | 1.028 ± 0.211 * | 2.206 ± 0.560 | 2.570 ± 0.373 | 4.658 ± 0.561 * |
| Kd2 Liver (pM) | 513.858 ± 44.447 * | 518.362 ± 79.162 | 295.119± 75.735 | 798.021 ± 175.886 * |
| R1 Liver (pM) | 48.006 ± 11.211 | 23.154 ± 7.192 | 30.897 ± 2.499 | 9.240 ± 1.378 * |
| R2 Liver (pM) | 0.190 ± 0.017 | 0.287 ± 0.054 | 0.335 ± 0.109 | 0.114 ± 0.032 * |
| Kd1 Skeletal muscle (fM) | 4.247 ± 1.431 | 1.238 ± 0.008 | 2.791 ± 0.567 | 0.647 ± 0.074 * |
| Kd2 Skeletal muscle (fM) | 3636.828 ± 61.378 | 2592.685 ± 64.992 | 5487.313 ± 114.611 | 406.922 ± 44.274 * |
| R1 Skeletal muscle (fM) | 9038.727 ±3511.087 | 7789.672 ± 89.998 | 3963.148 ± 425.352 | 14137.999 ± 427.379 * |
| R2 Skeletal muscle (fM) | 8.175 ± 0.075 * | 8.807 ± 0.045 | 3.953 ± 1.046 | 17.973 ± 2.517 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szkudelska, K.; Deniziak, M.; Sassek, M.; Szkudelski, I.; Noskowiak, W.; Szkudelski, T. Resveratrol Affects Insulin Signaling in Type 2 Diabetic Goto-Kakizaki Rats. Int. J. Mol. Sci. 2021, 22, 2469. https://doi.org/10.3390/ijms22052469
Szkudelska K, Deniziak M, Sassek M, Szkudelski I, Noskowiak W, Szkudelski T. Resveratrol Affects Insulin Signaling in Type 2 Diabetic Goto-Kakizaki Rats. International Journal of Molecular Sciences. 2021; 22(5):2469. https://doi.org/10.3390/ijms22052469
Chicago/Turabian StyleSzkudelska, Katarzyna, Marzanna Deniziak, Maciej Sassek, Ignacy Szkudelski, Wojciech Noskowiak, and Tomasz Szkudelski. 2021. "Resveratrol Affects Insulin Signaling in Type 2 Diabetic Goto-Kakizaki Rats" International Journal of Molecular Sciences 22, no. 5: 2469. https://doi.org/10.3390/ijms22052469
APA StyleSzkudelska, K., Deniziak, M., Sassek, M., Szkudelski, I., Noskowiak, W., & Szkudelski, T. (2021). Resveratrol Affects Insulin Signaling in Type 2 Diabetic Goto-Kakizaki Rats. International Journal of Molecular Sciences, 22(5), 2469. https://doi.org/10.3390/ijms22052469







