Effector Profiles of Endophytic Fusarium Associated with Asymptomatic Banana (Musa sp.) Hosts
Abstract
:1. Introduction
2. Results
2.1. Universal Primers Successfully Detect SIX Genes in Pathogenic Formae Speciales
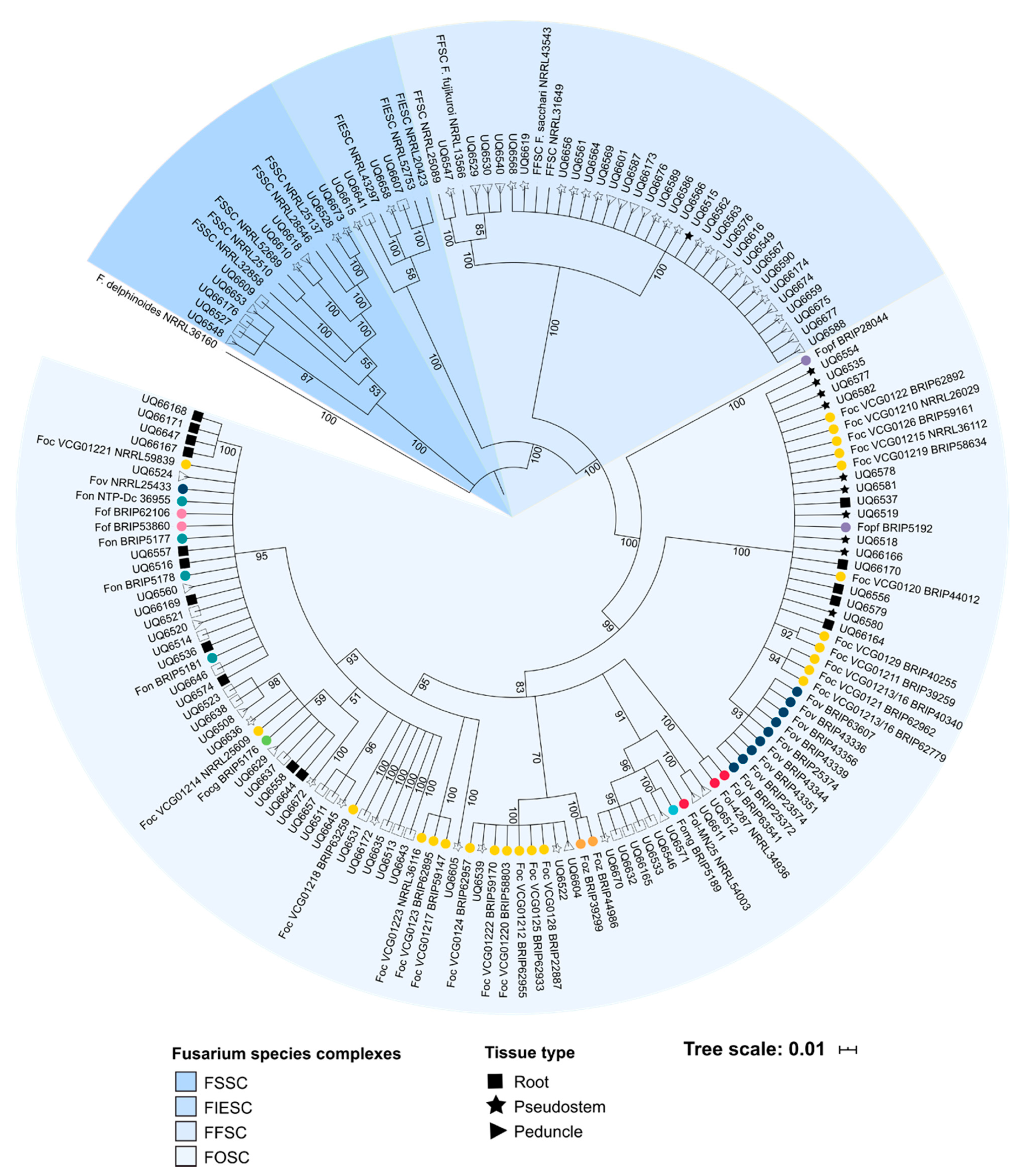
2.2. A Genetically Diverse Range of Fusarium Is Associated with Healthy Banana Tissue
2.3. A High Proportion of Endophytic Fusarium Carry SIX Gene Orthologues
2.4. Hierarchical Clustering Distinguishes Pathogenic and Non-Pathogenic Lineages of Fusarium
3. Discussion
4. Materials and Methods
4.1. Universal Primer Design for SIX1–SIX14
4.2. Culturing and Nucleic Acid Extraction
4.3. Optimisation and Screening of F. oxysporum Formae Speciales with Universal Primers for the SIX Genes
4.4. Isolation of Fusarium Species Associated with Asymptomatic Bananas
4.5. Phylogenetic Analysis
4.6. Screening of Fusarium Cultures for SIX Genes
4.7. Sequencing Analysis of the SIX Genes
4.8. Cluster Analysis of SIX Gene Profile
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| bp | base pair |
| BRIP | Brisbane Plant Pathology Herbarium |
| DOAJ | Directory of open access journals |
| EF1-α | translation elongation factor 1-α |
| f.sp. | forma specialis |
| ff.spp. | formae speciales |
| FFSC | Fusarium fujikuroi species complex |
| FIESC | Fusarium incarnatum-equiseti species complex |
| Foc | Fusarium oxysporum f.sp. cubense |
| Focg | Fusarium oxysporum f.sp. conglutinans |
| Fof | Fusarium oxsporum f.sp. fragariae |
| Fol | Fusarium oxysporum f.sp. lycopoersici |
| Fomg | Fusarium oxysporum f.sp. medicaginis |
| Fon | Fusarium oxysporum f.sp. niveum |
| Fopf | Fusarium oxysporum f.sp. passiflora |
| FOSC | Fusarium oxysporum species complex |
| Fov | Fusarium oxysporum f.sp. vasinfectum |
| Foz | Fusarium oxysporum f.sp. zingerberi |
| FSSC | Fusarium solani species complex |
| kb | kilobase |
| MCMC | Markov chain Monte Carlo |
| MDPI | Multidisciplinary Digital Publishing Institute |
| ML | Maximum likelihood |
| N/A | Not applicable |
| NCBI | National Centre Biotechnology Information |
| NRRL | Agricultural Research Service Culture Collection |
| NSW | New South Wales |
| PCR | polymerase chain reaction |
| PDA | Potato dextrose agar |
| QLD | Queensland |
| R4 | Race 4 |
| SIX | secreted in xylem |
| SR4 | Subtropical race 4 |
| TR4 | Tropical race 4 |
| VCG/s | Vegetative compatibility group/s |
References
- Snyder, W.C.; Hansen, H.N. The Species Concept in Fusarium. Am. J. Bot. 1940, 27, 64–67. [Google Scholar] [CrossRef]
- Gordon, T.R.; Martyn, R.D. The evolutionary biology of Fusarium oxysporum. Annu. Rev. Phytopathol. 1997, 35, 111–128. [Google Scholar] [CrossRef] [Green Version]
- Kistler, H.C.; Alabouvette, C.; Baayen, R.P.; Bentley, S.; Brayford, D.; Coddington, A.; Correll, J.; Daboussi, M.A.; Elias, K.; Fernandez, D.; et al. Systematic numbering of vegetative compatibility groups in the plant pathogenic fungus Fusarium oxysporum. Phytopathology 1998, 88, 30–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baayen, R.P.; O’Donnell, K.; Bonants, P.J.; Cigelnik, E.; Kroon, L.P.; Roebroeck, E.J.; Waalwijk, C. Gene genealogies and AFLP analyses in the Fusarium oxysporum complex identify monophyletic and nonmonophyletic formae speciales causing wilt and rot disease. Phytopathology 2000, 90, 891–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, K.; Gueidan, C.; Sink, S.; Johnston, P.R.; Crous, P.W.; Glenn, A.; Riley, R.; Zitomer, N.; Colyer, P.; Waalwijk, C.; et al. A two-locus DNA sequence database for typing plant and human pathogens within the Fusarium oxysporum species complex. Fungal Genet. Biol. 2009, 46, 936–948. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Correll, J.C. Vegetative Compatibility Among Races of Fusarium oxysporum f. sp. cubense. Plant Dis. 1988, 72, 325–328. [Google Scholar] [CrossRef]
- Katan, T.; Di Primo, P. Current status of vegetative compatibility groups in Fusarium oxysporum. Phytoparasitica 1999, 27, 51–64. [Google Scholar] [CrossRef]
- Stover, R.H. Fusarial wilt (Panama Disease) of Bananas and other Musa Species; Commonwealth Mycological Institute: Kew, UK, 1962. [Google Scholar]
- Ploetz, R.C. Panama disease: Return of the first banana menace. Int. J. Pest Manag. 1994, 40, 326–336. [Google Scholar] [CrossRef]
- Maryani, N.; Lombard, L.; Poerba, Y.S.; Subandiyah, S.; Crous, P.W.; Kema, G.H.J. Phylogeny and genetic diversity of the banana Fusarium wilt pathogen Fusarium oxysporum f. sp. cubense in the Indonesian centre of origin. Stud. Mycol. 2019, 92, 155–194. [Google Scholar] [CrossRef] [PubMed]
- Lombard, L.; Sandoval-Denis, M.; Lamprecht, S.C.; Crous, P.W. Epitypification of Fusarium oxysporum—Clearing the taxonomic chaos. Persoonia 2019, 43, 1–47. [Google Scholar] [CrossRef] [Green Version]
- Stover, R.H. Fusarium wilt of banana: Some history and current status of the disease. 1990. In Fusarium Wilt of Banana; Ploetz, R.C., Ed.; APS Press: St. Paul, MN, USA, 1990; pp. 1–7. [Google Scholar]
- Moore, N.Y.; Pegg, K.G.; Allen, R.N.; Irwin, J.A.G. Vegetative compatibility and distribution of Fusarium oxysporum f.sp. cubense in Australia. Aust. J. Exp. Agric. 1993, 33, 797–802. [Google Scholar] [CrossRef]
- García-Bastidas, F.A.; Ordóñez, N.; Konkol, J.; Al-Qasim, M.; Naser, Z.; Abdelwali, M.; Salem, N.; Waalwijk, C.; Ploetz, R.C.; Kema, G.H.J. First report of Fusarium oxysporum f. sp. cubense tropical race 4 associated with Panama disease of banana outside southeast Asia. Plant Dis. 2014, 98, 694. [Google Scholar]
- Ploetz, R.; Freeman, S.; Konkol, J.; Al-Abed, A.; Naser, Z.; Shalan, K.; Bakakat, R.; Israeli, Y. Tropical race 4 of Panama disease in the Middle East. Phytoparasitica 2015, 43, 283–293. [Google Scholar] [CrossRef]
- O’Neill, W.T.; Henderson, J.; Pattemore, J.A.; O’Dwyer, C.; Perry, S.; Beasley, D.R.; Tan, Y.P.; Smyth, A.L.; Goosem, C.H.; Thomson, K.M.; et al. Detection of Fusarium oxysporum f. sp. cubense tropical race 4 strain in northern Queensland. Australas. Plant Dis. Notes 2016, 11, 33. [Google Scholar] [CrossRef] [Green Version]
- Ordoñez, N.; García-Bastidas, F.; Laghari, H.B.; Akkary, M.Y.; Harfouche, E.N.; al Awar, B.N.; Kema, G.H.J. First Report of Fusarium oxysporum f. sp. cubense Tropical Race 4 Causing Panama disease in Cavendish bananas in Pakistan and Lebanon. Plant Dis. 2016, 100, 209. [Google Scholar]
- Zheng, S.-J.; García-Bastidas, F.A.; Li, X.; Zeng, L.; Bai, T.; Xu, S.; Yin, K.; Li, H.; Fu, G.; Yu, Y.; et al. New geographical insights of the latest expansion of Fusarium oxysporum f.sp. cubense Tropical Race 4 into the greater Mekong subregion. Front. Plant Sci. 2018, 9, 457. [Google Scholar]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. PNAS 1998, 95, 2044–2049. [Google Scholar] [CrossRef] [Green Version]
- Fourie, G.; Steenkamp, E.T.; Gordon, T.R.; Viljoen, A. Evolutionary relationships among the Fusarium oxysporum f. sp. cubense vegetative compatibility groups. Appl. Environ. Microbiol. 2009, 75, 4770–4781. [Google Scholar] [CrossRef] [Green Version]
- Ordonez, N.; Seidl, M.F.; Waalwijk, C.; Drenth, A.; Kilian, A.; Thomma, B.P.H.J.; Ploetz, R.C.; Kema, G.H.J. Worse comes to worst: Bananas and Panama Disease—When plant and pathogen clones meet. PLoS Pathog. 2015, 11, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czislowski, E.; Fraser-Smith, S.; Zander, M.; O’Neill, W.T.; Meldrum, R.A.; Tran-Nguyen, L.T.T.; Batley, J.; Aitken, E.A.B. Investigation of the diversity of effector genes in the banana pathogen, Fusarium oxysporum f. sp. cubense, reveals evidence of horizontal gene transfer. Mol. Plant Pathol. 2018, 19, 1155–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Rep, M.; van der Does, H.C.; Meijer, M.; van Wijk, R.; Houterman, P.M.; Dekker, H.L.; De Koster, C.G.; Cornelissen, B.J.C. A small, cysteine-rich protein secreted by Fusarium oxysporum during colonization of xylem vessels is required for I-3-mediated resistance in tomato. Mol. Microbiol. 2004, 53, 1373–1383. [Google Scholar] [CrossRef]
- Houterman, P.M.; Speijer, D.; Dekker, H.L.; de Koster, C.G.; Cornelissen, B.J.C.; Rep, M. The mixed xylem sap proteome of Fusarium oxysporum-infected tomato plants. Mol. Plant Pathol. 2007, 8, 215–221. [Google Scholar] [CrossRef]
- Lievens, B.; Houterman, P.M.; Rep, M. Effector gene screening allows unambiguous identification of Fusarium oxysporum f. sp. lycopersici races and discrimination from other formae speciales. FEMS Microbiol. Lett. 2009, 300, 201–215. [Google Scholar] [PubMed] [Green Version]
- Takken, F.; Rep, M. The arms race between tomato and Fusarium oxysporum. Mol. Plant Pathol. 2010, 11, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.M.; Houterman, P.M.; Schreiver, I.; Ma, L.; Amyotte, S.; Chellappan, B.; Boeren, S.; Takken, F.L.W.; Rep, M. MITEs in the promoters of effector genes allow prediction of novel virulence genes in Fusarium oxysporum. BMC Genomics 2013, 14, 119. [Google Scholar] [CrossRef] [Green Version]
- Houterman, P.M.; Ma, L.; van Ooijen, G.; de Vroomen, M.J.; Cornelissen, B.J.C.; Takken, F.L.W.; Rep, M. The effector protein Avr2 of the xylem-colonizing fungus Fusarium oxysporum activates the tomato resistance protein I-2 intracellularly. Plant J. 2009, 58, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, L.F.; Gardiner, D.M.; Kazan, K.; Manners, J.M. A Highly Conserved Effector in Fusarium oxysporum is required for full virulence on Arabidopsis. Mol. Plant Microbe Interact. 2012, 25, 180–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gawehns, F.; Houterman, P.M.; Ichou, F.A.; Michielse, C.B.; Hijdra, M.; Cornelissen, B.J.C.; Rep, M.; Takken, F.L.W. The Fusarium oxysporum effector Six6 contributes to virulence and suppresses I-2-mediated cell death. Mol. Plant Microbe Interact. 2014, 27, 336–348. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Houterman, P.M.; Gawehns, F.; Cao, L.; Sillo, F.; Richter, H.; Clavijo-Ortiz, M.J.; Schmidt, S.M.; Boeren, S.; Vervoort, J.; et al. The AVR2-SIX5 gene pair is required to activate I-mediated immunity in tomato. New Phytol. 2015, 5, 507–518. [Google Scholar] [CrossRef]
- van Dam, P.; Fokkens, L.; Ayukawa, Y.; Van Der Gragt, M.; Ter Horst, A.; Brankovics, B.; Houterman, D.M.; Arie, T.; Rep, M. A mobile pathogenicity chromosome in Fusarium oxysporum for infection of multiple cucurbit species. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Ma, L.J.; van der Does, H.C.; Borkovich, K.A.; Coleman, J.J.; Daboussi, M.J.; Di Pietro, A.; Dufresne, M.; Freitag, M.; Grabherr, M.; Henrissat, B.; et al. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 2010, 464, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Fraser-Smith, S.; Czislowski, E.; Meldrum, R.A.; Zander, M.; O’Neill, W.; Balali, G.R.R.; Aitken, E.A.B. Sequence variation in the putative effector gene SIX8 facilitates molecular differentiation of Fusarium oxysporum f. sp. cubense. Plant Pathol. 2014, 63, 1044–1052. [Google Scholar] [CrossRef]
- Laurence, M.H.; Summerell, B.A.; Liew, E.C.Y. Fusarium oxysporum f. sp. canariensis: Evidence for horizontal gene transfer of putative pathogenicity genes. Plant Pathol. 2015, 64, 1068–1075. [Google Scholar]
- Taylor, A.; Vagany, V.; Jackson, A.; Harrison, R.; Rainoni, A.; Clarkson, J.P. Identification of pathogenicity-related genes in Fusarium oxysporum f. sp. cepae. Mol. Plant Pathol. 2016, 17, 1032–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, A.H.; Sharma, M.; Thatcher, L.F.; Azam, S.; Hane, J.K.; Sperschneider, J.; Kidd, B.N.; Anderson, J.P.; Gosh, R.; Garg, G.; et al. Comparative genomics and prediction of conditionally dispensable sequences in legume-infecting Fusarium oxysporum formae speciales facilitates identification of candidate effectors. BMC Genom. 2016, 17, 191. [Google Scholar] [CrossRef] [Green Version]
- van Dam, P.; Fokkens, L.; Schmidt, S.M.; Linmans, J.H.J.; Corby Kistler, H.; Ma, L.J.; Rep, M. Effector profiles distinguish formae speciales of Fusarium oxysporum. Environ. Microbiol. 2016, 18, 4087–4102. [Google Scholar] [CrossRef]
- van der Does, H.C.; Lievens, B.; Claes, L.; Houterman, P.M.; Cornelissen, B.J.C.; Rep, M. The presence of a virulence locus discriminates Fusarium oxysporum isolates causing tomato wilt from other isolates. Environ. Microbiol. 2008, 10, 1475–1485. [Google Scholar] [CrossRef]
- van der Does, H.C.; Rep, M. Virulence genes and the evolution of host specificity in plant-pathogenic fungi. Mol. Plant Microbe Interact. 2007, 20, 1175–1182. [Google Scholar] [CrossRef] [Green Version]
- van Dam, P.; Rep, M. The distribution of miniature impala elements and SIX genes in the Fusarium genus is suggestive of horizontal gene transfer. J. Mol. Evol. 2017, 85, 14–25. [Google Scholar] [CrossRef]
- van de Wouw, A.P.; Cozijnsen, A.J.; Hane, J.K.; Brunner, P.C.; McDonald, B.A.; Oliver, R.P.; Howlett, B.J. Evolution of linked avirulence effectors in Leptosphaeria maculans is affected by genomic environment and exposure to resistance genes in host plants. PLoS Pathog. 2010, 6, e1001180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleemann, J.; Rincon-Rivera, L.J.; Takahara, H.; Neumann, U.; van Themaat, E.V.L.; van der Does, H.C.; Hacquard, S.; Stuber, K.; Will, I.; Schmalenbach, W.; et al. Sequential delivery of host-induced virulence effectors by appressoria and intracellular hyphae of the phytopathogen Colletotrichum higginsianum. PLoS Pathog. 2012, 8, e1002643. [Google Scholar] [CrossRef]
- Gan, P.; Ikeda, K.; Irieda, H.; Narusaka, M.; O’Connell, R.J.; Narusaka, Y.; Takano, Y.; Kubo, Y.; Shirasu, K. Comparative genomic and transcriptomic analyses reveal the hemibiotrophic stage shift of Colletotrichum fungi. New Phytol. 2013, 197, 1236–1249. [Google Scholar] [CrossRef]
- Guo, L.; Han, L.; Yang, L.; Zeng, H.; Fan, D.; Zhu, Y.; Feng, Y.; Wang, G.; Peng, C.; Jiang, X.; et al. Genome and transcriptome analysis of the fungal pathogen Fusarium oxysporum f. sp. cubense causing banana vascular wilt disease. PLoS ONE 2014, 9, e95543. [Google Scholar]
- Photita, W.; Lumyong, S.; Lumyong, P.; Hyde, K.D. Endophytic fungi of wild banana (Musa acuminata) at Doi Suthep Pui National Park, Thailand. Mycol. Res. 2001, 105, 1508–1513. [Google Scholar] [CrossRef]
- Athman, S.Y.; Dubois, T.; Coyne, D.; Gold, C.S.; Labuschagne, N.; Viljoen, A. Effect of endophytic Fusarium oxysporum on host preference of Radopholus similis to tissue culture banana plants. J. Nematology 2006, 38, 455–460. [Google Scholar]
- Inami, K.; Kashiwa, T.; Kawabe, M.; Onokubo-Okabe, A.; Ishikawa, N.; Pérez, E.R.; Hozumi, T.; Caballero, L.A.; de Baldarrago, F.C.; Roco, M.J.; et al. The tomato wilt fungus Fusarium oxysporum f. sp. lycopersici shares common ancestors with nonpathogenic F. oxysporum isolated from wild tomatoes in the Peruvian Andes. Microbes Environ. 2014, 29, 200–210. [Google Scholar] [PubMed] [Green Version]
- Demers, J.E.; Gugino, B.K.; Jiménez-Gasco, M. Highly diverse endophytic and soil Fusarium oxysporum populations associated with field-grown tomato plants. Appl. Environ. Microbiol. 2015, 81, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, L.; Jamil, M.I.M.; Anuar, I.S.M. Molecular characterisation of endophytic fungi from roots of wild banana (Musa acuminata). Trop. Life Sci. Res. 2016, 27, 153–162. [Google Scholar] [PubMed]
- Rocha, L.O.; Laurence, M.H.; Ludowici, V.A.; Puno, V.I.; Lim, C.C.; Tesoriero, L.A.; Summerell, B.A.; Liew, E.C.Y. Putative effector genes detected in Fusarium oxysporum from natural ecosystems of Australia. Plant Pathol. 2016, 65, 914–929. [Google Scholar] [CrossRef] [Green Version]
- Jelinski, N.A.; Broz, K.; Jonkers, W.; Ma, L.J.; Kistler, H.C. Effector gene suites in some soil isolates of Fusarium oxysporum are not sufficient predictors of vascular wilt in tomato. Phytopathology 2017, 107, 842–851. [Google Scholar] [CrossRef]
- Wang, B.; Brubaker, C.L.; Tate, W.; Woods, M.J.; Matheson, B.A.; Burdon, J.J. Genetic variation and population structure of Fusarium oxysporum f.sp. vasinfectum in Australia. Plant Path. 2006, 55, 746–755. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Rep, M.; Wang, B.; Ashton, A.; Dodds, P.; Ellis, J. Variation in potential effector genes distinguishing Australian and non-Australian isolates of the cotton wilt pathogen Fusarium oxysporum f.sp. vasinfectum. Plant Path. 2011, 60, 232–243. [Google Scholar] [CrossRef]
- Rafiqi, M.; Ellis, J.G.; Ludowici, V.A.; Hardham, A.R.; Dodds, P.N. Challenges and progress towards understanding the role of effectors in plant–fungal interactions. Curr. Opin. Plant Biol. 2012, 15, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Plett, J.M.; Martin, F. Reconsidering mutualistic plant-fungal interactions through the lens of effector biology. Curr. Opin. Plant Biol. 2015, 26, 45–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camehl, I.; Sherameti, I.; Venus, Y.; Bethke, G.; Varma, A.; Lee, J.; Oelmüller, R. Ethylene signalling and ethylene-targeted transcription factors are required to balance beneficial and nonbeneficial traits in the symbiosis between the endophytic fungus Piriformospora indica and Arabidopsis thaliana. New Phytol. 2010, 185, 1062–1073. [Google Scholar] [CrossRef]
- Kloppholz, S.; Kuhn, H.; Requena, N. A secreted fungal effector of Glomus intraradices promotes symbiotic biotrophy. Curr. Biol. 2011, 21, 1204–1209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plett, J.M.; Kemppainen, M.; Kale, S.D.; Kohler, A.; Rie Legué, V.; Brun, A.; Tyler, B.M.; Pardo, A.G.; Martin, F. A secreted effector protein of Laccaria bicolor is required for symbiosis development. Curr. Biol. 2011, 21, 1197–1203. [Google Scholar] [CrossRef] [Green Version]
- Plett, J.M.; Daguerre, Y.; Wittulsky, S.; Vayssières, A.; Deveau, A.; Melton, S.J.; Kohler, A.; Morrell-Falvey, J.L.; Brun, A.; Veneault-Fourrey, C.; et al. Effector MiSSP7 of the mutualistic fungus Laccaria bicolor stabilizes the Populus JAZ6 protein and represses jasmonic acid (JA) responsive genes. PNAS 2014, 111, 8299–8304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plett, J.M.; Khachane, A.; Ouassou, M.; Sundberg, B.; Kohler, A.; Martin, F. Ethylene and jasmonic acid act as negative modulators during mutualistic symbiosis between Laccaria bicolor and Populus roots. New Phytol. 2014, 202, 270–286. [Google Scholar] [CrossRef]
- Martin, F.; Aerts, A.; Ahrén, D.; Brun, A.; Danchin, E.G.J.; Duchaussoy, F.; Gibon, J.; Kohler, A.; Lindquist, E.; Pereda, V.; et al. The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature 2008, 452, 88–92. [Google Scholar] [CrossRef]
- Zuccaro, A.; Lahrmann, U.; Gü Ldener, U.; Langen, G.; Pfiffi, S.; Biedenkopf, D.; Wong, P.; Samans, B.; Grimm, C.; Basiewicz, M.; et al. Endophytic life strategies decoded by genome and transcriptome analyses of the mutualistic root symbiont Piriformospora indica. PLOS Pathog. 2011, 7, e1002290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohler, A.; Kuo, A.; Nagy, L.G.; Morin, E.; Barry, K.W.; Buscot, F.; Canback, B.; Choi, C.; Cichocki, N.; Clum, A.; et al. Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nat. Genet. 2015, 47, 410–415. [Google Scholar] [CrossRef]
- Carvalhais, L.; Henderson, J.; Rincon-Florez, V.A.; O’Dwyer, C.; Czislowski, E.; Aitken, E.A.B.; Drenth, A. Molecular diagnostics of banana Fusarium Wilt targeting secreted-in-xylem genes. Front. Plant Sci. 2019, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. J. Bioinform. 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publisher: Oxford, UK, 2007. [Google Scholar]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. J. Bioinform. 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamatakis, A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. J. Bioinform. 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, J.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. 2018, R Package Version 2.5-2. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 23 June 2018).
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. cluster: Cluster Analysis Basics and Extensions. 2018, R Package Version 2.0.7-1. Available online: https://cran.r-project.org/web/packages/cluster/index.html (accessed on 23 June 2018).



| Race | VCG | Species Name within FOSC |
|---|---|---|
| 1 | 0123 | Fusarium phialophorum |
| 1 | 01210 | Fusarium purpurascens |
| 1, 2 | 0124 | Fusarium tardichlamydosporum |
| 1, 2 | 0125 | Fusarium tardichlamydosporum |
| 1, 2 | 0128 | Fusarium tardichlamydosporum |
| 1, SR4 | 01220 | sp. (not determined) |
| 2 | 01214 | Fusarium tardicrescens |
| R4 | 0121 | Fusarium odoratissimum |
| R4 | 0122 | Fusarium phialophorum |
| SR4 | 0120 | Fusarium phialophorum |
| SR4 | 0129 | Fusarium phialophorum |
| SR4 | 01211 | Fusarium phialophorum |
| SR4 | 0121 | Fusarium phialophorum |
| SR4 1 | 0126 | Fusarium purpurascens |
| TR4 | 01213 | Fusarium odoratissimum |
| TR4 2 | 01216 | Fusarium odoratissimum |
| Undetermined | 01212 | Fusarium tardichlamydosporum |
| Undetermined | 01217 | Fusarium duoseptum |
| Undetermined | 01218 | sp. (not determined) |
| Undetermined | 01219 | Fusarium phialophorum |
| Undetermined | 01221 | Fusarium grosmichelli |
| Undetermined | 01222 | Fusarium tardichlamydosporum |
| Undetermined | 01223 | Fusarium duoseptum |
| Undetermined | 01224 | Fusarium duoseptum |
| N/A 3 | 0127 | N/A 3 |
| Forma Specialis (Host Name) 1 | Isolate 2 | SIX1 | SIX2 | SIX3 | SIX4 | SIX5 | SIX6 | SIX7 | SIX8 | SIX9-G1 3 | SIX9-G2 3 | SIX10 | SIX11 | SIX12 | SIX13 | SIX14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fol (tomato) | 4287 NRRL349364 | + | + | + | - | + | + | + | + | - | + | + | + | + | + | + |
| Fol (tomato) | MN25 NRRL540034 | + | + | + | - | + | + | + | + | - | + | + | + | + | + | + |
| Fol (tomato) | BRIP63541 | + | + | + | - | + | + | + | + | - | + | + | + | + | + | + |
| Fopf (passionfruit) | BRIP280445 | - | - | - | - | - | + | - | + | - | + | - | + | - | - | - |
| Fopf (passionfruit) | BRIP28044 | - | - | - | - | - | + | - | + | - | + | - | + | - | - | - |
| Fopf (passionfruit) | BRIP5192 | - | - | - | - | - | + | - | + | - | + | - | + | - | - | - |
| Fon (watermelon) | NT-DPI39655 4 | - | - | - | + | - | + | - | + | - | + | - | + | - | + | - |
| Fon (watermelon) | BRIP5178 | - | - | - | - | - | + | - | + | - | + | - | + | - | + | - |
| Fon (watermelon) | BRIP5177 | - | - | - | - | - | + | - | + | - | - | - | + | - | - | - |
| Fon (watermelon) | BRIP5181 | - | - | - | + | - | + | - | + | - | + | - | + | - | + | - |
| Fon (watermelon) | NT-DPI39655 | - | - | - | + | - | + | - | + | + | + | - | + | - | + | - |
| Foz (ginger) | BRIP39299 4 | - | - | - | - | - | - | + | - | + | - | + | - | + | - | - |
| Foz (ginger) | BRIP39299 | - | - | - | - | - | - | + | - | + | - | + | - | + | - | - |
| Foz (ginger) | BRIP44986 | - | - | - | - | - | - | + | - | + | - | + | - | + | - | - |
| Fof (strawberry) | BRIP538604 | + | - | - | - | - | - | - | - | - | - | - | - | - | + | - |
| Fof (strawberry) | BRIP53860 | + | - | - | - | - | - | - | - | - | - | - | - | - | + | - |
| Fof (strawberry) | BRIP62106 | + | - | - | - | - | - | - | - | - | - | - | - | - | + | - |
| Fov (cotton) | NRRL25433 4 | - | - | - | - | - | - | - | - | - | + | - | - | - | + | - |
| Fov (cotton) | BRIP63607 | - | - | - | - | - | + | - | - | - | - | - | + | - | + | + |
| Fov (cotton) | BRIP43351 | - | - | - | - | - | + | - | - | - | - | - | + | - | + | + |
| Fov (cotton) | BRIP25374 | - | - | - | - | - | + | - | - | - | - | - | + | - | + | + |
| Fov (cotton) | BRIP43344 | - | - | - | - | - | + | - | - | - | - | - | + | - | + | + |
| Fov (cotton) | BRIP43336 | - | - | - | - | - | + | - | - | - | - | - | + | - | + | + |
| Fov (cotton) | BRIP43339 | - | - | - | - | - | + | - | - | - | - | - | + | - | + | + |
| Fov (cotton) | BRIP43356 | - | - | - | - | - | + | - | - | - | - | - | + | - | + | + |
| Focg (Brassicae family) | BRIP51764 | + | - | - | + | - | - | - | + | + | - | - | - | - | - | - |
| Focg (Brassicae family) | BRIP5176 | + | - | - | + | - | - | - | + | + | - | - | - | - | - | - |
| Fomg (alfalfa) | BRIP5189 4 | + | - | - | - | - | - | - | + | - | - | - | - | - | + | - |
| Fomg (alfalfa) | BRIP5189 | + | - | - | - | - | - | - | + | - | - | - | - | - | + | - |
| Foc (banana) | BRIP62933 4 (VCG0124) | + | - | - | + | - | + | - | - | + | - | - | - | - | + | - |
| Foc (banana) | BRIP62933 (VCG0124) | + | - | - | + | - | + | - | - | + | - | - | - | - | + | - |
| Foc (banana) | BRIP62895 4 (VCG0123) | + | - | - | + | - | + | - | - | + | - | - | - | - | + | - |
| Foc (banana) | BRIP62895 (VCG0123) | + | - | - | + | - | + | - | - | + | - | - | - | - | + | - |
| Foc (banana) | BRIP58698 4 (VCG01217) | + | - | - | + | - | + | - | - | + | - | - | - | - | + | - |
| Foc (banana) | BRIP58698 (VCG01217) | + | - | - | + | - | + | - | + | + | - | - | - | - | + | - |
| Foc (banana) | NRRL36116 4 (VCG01223) | + | - | - | + | - | + | - | - | + | - | - | - | - | + | - |
| Foc (banana) | NRRL36116 (VCG01223) | + | - | - | + | - | + | - | + | + | - | - | - | - | + | - |
| Foc (banana) | BRIP40340 4 (VCG01213/16) | + | + | - | + | - | + | - | + | + | - | - | - | - | + | - |
| Foc (banana) | BRIP40340 (VCG01213/16) | + | + | - | + | - | + | - | + | + | - | - | - | - | + | - |
| Foc (banana) | BRIP62962 4 (VCG0121) | + | + | - | + | - | + | + | + | + | - | + | - | - | + | - |
| Foc (banana) | BRIP62962 (VCG0121) | + | + | - | + | - | + | + | + | + | - | + | - | - | + | - |
| Foc (banana) | BRIP62892 4 (VCG0122) | + | - | - | - | - | - | - | + | + | - | - | - | - | + | - |
| Foc (banana) | BRIP62892 (VCG0122) | + | - | - | - | - | - | - | + | + | - | - | - | - | + | - |
| Foc (banana) | NRRL36118 4 (VCG01221) | + | - | - | - | - | - | - | - | + | - | - | - | - | + | - |
| Foc (banana) | NRRL36118 (VCG01221) | + | - | - | - | - | - | - | + | + | - | - | - | - | + | - |
| Foc (banana) | BRIP44012 4 (VCG0120) | + | + | - | + | - | - | + | + | + | - | - | - | - | - | - |
| Foc (banana) | BRIP44012 (VCG0120) | + | + | - | + | - | - | + | + | + | - | - | - | - | - | - |
| Foc (banana) | BRIP63259 4 (VCG01218) | + | - | - | + | - | + | - | - | + | - | - | - | - | + | - |
| Foc (banana) | BRIP63259 (VCG01218) | + | - | - | + | - | + | - | - | + | - | - | - | - | + | - |
| Foc (banana) | BRIP62779 4 (VCG01213/16) | + | + | - | + | - | + | - | + | + | - | - | - | - | + | - |
| Foc (banana) | BRIP62779 (VCG01213/16) | + | + | - | + | - | + | - | + | + | - | - | - | - | + | - |
| Foc (banana) | BRIP39259 4 (VCG01211) | + | + | - | + | - | - | + | + | + | - | - | - | - | - | - |
| Foc (banana) | BRIP39259 (VCG01211) | + | + | - | + | - | - | + | + | + | - | - | - | - | - | - |
| Foc (banana) | NRRL25609 4 (VCG01214) | + | - | - | - | - | - | - | - | + | - | - | - | - | + | - |
| Foc (banana) | NRRL25609 (VCG01214) | + | - | - | - | - | - | - | - | + | - | - | - | - | + | - |
| Foc (banana) | NRRL26029 4 (VCG01210) | + | + | - | + | - | + | - | - | + | - | - | - | - | + | - |
| Foc (banana) | NRRL26029 (VCG01210) | + | + | - | + | - | + | - | - | + | - | - | - | - | + | - |
| Foc (banana) | BRIP62933 4 (VCG0125) | + | - | - | + | - | + | - | - | + | - | - | - | - | + | - |
| Foc (banana) | BRIP62933 (VCG0125) | + | - | - | + | - | + | - | - | + | - | - | - | - | + | - |
| Foc (banana) | BRIP59161 4 (VCG0126) | + | + | - | + | - | - | + | + | + | - | - | - | - | - | - |
| Foc (banana) | BRIP59161 (VCG0126) | + | + | - | + | - | - | + | + | + | - | - | - | - | - | - |
| Foc (banana) | BRIP22887 4 (VCG0128) | + | - | - | + | - | + | - | - | + | - | - | - | - | - | - |
| Foc (banana) | BRIP22887 (VCG0128) | + | - | - | + | - | + | - | - | + | - | - | - | - | - | - |
| Foc (banana) | BRIP40255 4 (VCG0129) | + | + | - | + | - | - | + | + | + | - | - | - | - | - | - |
| Foc (banana) | BRIP40255 (VCG0129) | + | + | - | + | - | - | + | + | + | - | - | - | - | - | - |
| Foc (banana) | NRRL36112 4 (VCG01215) | + | + | - | + | - | - | + | + | + | - | - | - | - | - | - |
| Foc (banana) | NRRL36112 (VCG01215) | + | + | - | + | - | - | + | + | + | - | - | - | - | - | - |
| Foc (banana) | BRIP59147 4 (VCG01217) | + | - | - | + | - | + | - | - | + | - | - | - | - | + | - |
| Foc (banana) | BRIP59147 (VCG01217) | + | - | - | + | - | + | - | - | + | - | - | - | - | + | - |
| Foc (banana) | BRIP58634 4 (VCG01219) | + | + | - | + | - | - | + | + | + | - | - | - | - | - | - |
| Foc (banana) | BRIP58634 (VCG01219) | + | + | - | + | - | - | + | + | + | - | - | - | - | - | - |
| Foc (banana) | BRIP58803 4 (VCG01220) | + | - | - | + | - | + | - | - | + | - | - | - | - | + | - |
| Foc (banana) | BRIP58803 (VCG01220) | + | - | - | + | - | + | - | - | + | - | - | - | - | + | - |
| Foc (banana) | BRIP59170 4 (VCG01222) | + | - | - | - | - | - | - | - | + | - | - | - | - | + | - |
| Foc (banana) | BRIP59170 (VCG01222) | + | - | - | - | - | - | - | - | + | - | - | - | - | + | - |
| Foc (banana) | BRIP62955 4 (VCG01212) | + | - | - | + | - | + | - | - | + | - | - | - | - | + | - |
| Foc (banana) | BRIP62955 (VCG01212) | + | - | - | + | - | + | - | - | + | - | - | - | - | + | - |
| Site | No. of Plants Sampled | Species Complex 2 | Tissue Type | Total | ||
|---|---|---|---|---|---|---|
| Root | Pseudostem | Peduncle | ||||
| Mullumbimby (NSW 1) | 20 | FFSC | 0 | 16 | 14 | 30 |
| FIESC | 2 | 2 | 0 | 4 | ||
| FOSC | 17 | 8 | 9 | 34 | ||
| FSSC | 3 | 3 | 3 | 9 | ||
| TOTAL | 22 | 29 | 26 | 77 | ||
| Redlands (QLD 1) | 8 | FFSC | 0 | 1 | N/A | 1 |
| FIESC | 0 | 0 | N/A | 0 | ||
| FOSC | 17 | 10 | N/A | 27 | ||
| FSSC | 0 | 0 | N/A | 0 | ||
| TOTAL | 17 | 11 | N/A | 28 | ||
| Gene Target | Primers | Sequence (5′–3′) | Expected Amplicon Size (Base Pairs) | Annealing Temperature (°C) |
|---|---|---|---|---|
| EF1-α | EF1-F | ATGGGTAAGGARGACAAGAC | ~650 | 55 °C |
| EF2-R | GGARGTACCAGTSATCATGTT | |||
| SIX1 | SIX1f | TCT CCA TTA CTT TGT CTC ACG | 694–733 | 58 °C |
| SIX1r | CGA TTT AGG CGA TTC GGG G | |||
| SIX2 | SIX2f | GGT TCC CAT CGT TGA AGC | 327–330 | 57 °C |
| SIX2r | TTG GTT TAA ATC TGC GTG TC | |||
| SIX3 | SIX3f | TTA CTA CGA GCT TCA GCA CC | 223 | 60 °C |
| SIX3r | GCA TTA GGT GTT GCA ACA GG | |||
| SIX4 | SIX4f | CAG CTC AGA CAG TCA GCC | ~491 | 58 °C |
| SIX4r | GGC CTT GAG TCG AAT GAG C | |||
| SIX5 | SIX5f | TCA TCA GTA CTG TGC TTG CC | 347–354 | 59 °C |
| SIX5r | CAT GTT GAG TCT GCT CCT CC | |||
| SIX6 | SIX6f | CTC TCG AGA CAC SCT TCC | 396–399 | 58 °C |
| SIX6r | GAT CCA CCA ATA CCT TCA T | |||
| SIX7 | SIX7f | GAG GTG ACA TTT GAC ATC ACC | 113 | 60 °C |
| SIX7r | TAG TAT GCG CGC CAT TGG | |||
| SIX8 | SIX8f | CCC TAG CCG TCT CTG TGG C | 163–165 | 64 °C |
| SIX8r | CGT TCG ACA AGG GCT CTC TCG | |||
| SIX9 Group 1 | SIX9f-G1 | TTC AAG TCG GTT GCT ACG C | 118 | 58 °C |
| SIX9r-G1 | GCA TCC CAA AAT CCA AAG CG | |||
| SIX9 Group 2 | SIX9f-G2 | CCG TCT TCT CTA CCG CCG | 288 | 58 °C |
| SIX9r-G2 | AGT TGA CGC AAG CAA AGT CG | |||
| SIX10 | SIX10f | TCA CGT TTC GAG TTG GTC C | 202 | 60 °C |
| SIX10r | ACA CCA AAT CGA GTC GAT GC | |||
| SIX11 | SIX11f | GTT GCT CCT CCT TTG CTG G | 163 | 62 °C |
| SIX11r | TAC CAC TCT GAC CAG TCA CC | |||
| SIX12 | SIX12f | CAG AAT GCT TGT GTG TGT GG | 171 | 61 °C |
| SIX12r | ATC ACC AGA GCA TGA ACC CC | |||
| SIX13 | SIX13f | TCT GAT CAG CCT CCT AGC GT | 840 | 60 °C |
| SIX13r | CCA CTG TAA CTC GGC ATC GA | |||
| SIX14 | SIX14f | TGT CTC AGC GTA TCC TCG GC | 147–197 | 61 °C |
| SIX14r | ATT CAG TGA CAA CGG GAC CG |
| Forma Specialis | Accession Code 1 | VCG 2 | Host |
|---|---|---|---|
| conglutinans (Focg) | BRIP5176 | NA | Brassica oleracea var. capitate |
| cubense (Foc) | BRIP62933 | 0124 | Musa sp. (unidentified) |
| BRIP62895 | 0123 | Musa sp. AAB ‘Latundan’ | |
| BRIP58698 | 01217 | Musa sp. (unidentified) | |
| BRIP40340 | 01213 | Musa sp. AAA ‘Cavendish’ | |
| BRIP62962 | 0121 | Musa sp. AA ‘Sucrier’ | |
| BRIP62892 | 0122 | Musa sp. AAA ‘Cavendish’ | |
| NRRL36118 | 01221 | Musa sp. ABB ‘Pisang Awak’ | |
| BRIP44012 | 0120 | Musa sp. AAA ‘Cavendish’ | |
| BRIP63259 | 01218 | Musa sp. (unidentified) | |
| BRIP62779 | 01216 | Musa sp. AAA ‘Cavendish’ | |
| BRIP39259 | 01211 | Musa sp. AAB ‘Lady Finger’ | |
| BRIP62911 | 0124 | Musa sp. (unidentified) | |
| NRRL36113 | 01214 | Musa sp. ABB ‘Bluggoe’ | |
| NRRL25609 | 01214 | Musa sp. ABB ‘Harare’ | |
| NRRL26029 | 01210 | Musa sp. AAB ‘Silk’ | |
| BRIP62957 | 0125 | Musa sp. (unidentified) | |
| BRIP59161 | 0126 | Musa sp. (unidentified) | |
| BRIP22887 | 0128 | Musa sp. ABB ‘Bluggoe’ | |
| BRIP40255 | 0129 | Musa sp. AAB ‘Lady Finger’ | |
| NRRL36112 | 01215 | Musa sp. AAA ‘Cavendish’ | |
| BRIP59147 | 01217 | Musa sp. (unidentified) | |
| BRIP58634 | 01219 | Musa sp. (unidentified) | |
| BRIP58803 | 01220 | Musa sp. (unidentified) | |
| BRIP59170 | 01222 | Musa sp. (unidentified) | |
| BRIP62955 | 01212 | Musa sp. (unidentified) | |
| fragariae (Fof) | BRIP53860 | NA | Fragaria × ananassa |
| BRIP62107 | NA | Fragaria × ananassa | |
| BRIP62106 | NA | Fragaria × ananassa | |
| lycopersici (Fol) | BRIP63541 | NA | Solanum lycopersicum |
| 4287 3 (NRRL34936) | 0030 | Solanum lycopersicum | |
| MN25 3 (NRRL54003) | 0033 | Solanum lycopersicum | |
| medicaginis (Fomg) | BRIP5189 | NA | Medicago sativa |
| niveum (Fon) | NT-DPI36955 | NA | Citrullus sp. |
| BRIP5178 | NA | Citrullus lanatus | |
| BRIP5177 | NA | Citrullus lanatus | |
| BRIP5181 | NA | Citrullus lanatus | |
| passiflorae (Fopf) | BRIP28044 | NA | Passiflora edulis |
| BRIP5192 | NA | Passiflora edulis | |
| vasinfectum (Fov) | NRRL25433 | 0114 | Gossypium hirsutum |
| BRIP25372 | 01111 | Gossypium hirsutum | |
| BRIP43365 | NA | Gossypium hirsutum | |
| BRIP63607 | NA | Gossypium hirsutum | |
| BRIP43351 | NA | Gossypium hirsutum | |
| BRIP25374 | 01112 | Gossypium hirsutum | |
| BRIP43344 | NA | Gossypium hirsutum | |
| BRIP43336 | NA | Gossypium hirsutum | |
| BRIP43339 | NA | Gossypium hirsutum | |
| BRIP43356 | NA | Gossypium hirsutum | |
| zingerberi (Foz) | BRIP39299 | NA | Zingiber officinale |
| BRIP44986 | NA | Zingiber officinale |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czislowski, E.; Zeil-Rolfe, I.; Aitken, E.A.B. Effector Profiles of Endophytic Fusarium Associated with Asymptomatic Banana (Musa sp.) Hosts. Int. J. Mol. Sci. 2021, 22, 2508. https://doi.org/10.3390/ijms22052508
Czislowski E, Zeil-Rolfe I, Aitken EAB. Effector Profiles of Endophytic Fusarium Associated with Asymptomatic Banana (Musa sp.) Hosts. International Journal of Molecular Sciences. 2021; 22(5):2508. https://doi.org/10.3390/ijms22052508
Chicago/Turabian StyleCzislowski, Elizabeth, Isabel Zeil-Rolfe, and Elizabeth A. B. Aitken. 2021. "Effector Profiles of Endophytic Fusarium Associated with Asymptomatic Banana (Musa sp.) Hosts" International Journal of Molecular Sciences 22, no. 5: 2508. https://doi.org/10.3390/ijms22052508
APA StyleCzislowski, E., Zeil-Rolfe, I., & Aitken, E. A. B. (2021). Effector Profiles of Endophytic Fusarium Associated with Asymptomatic Banana (Musa sp.) Hosts. International Journal of Molecular Sciences, 22(5), 2508. https://doi.org/10.3390/ijms22052508






