Pharmacological Conditioning of the Heart: An Update on Experimental Developments and Clinical Implications
Abstract
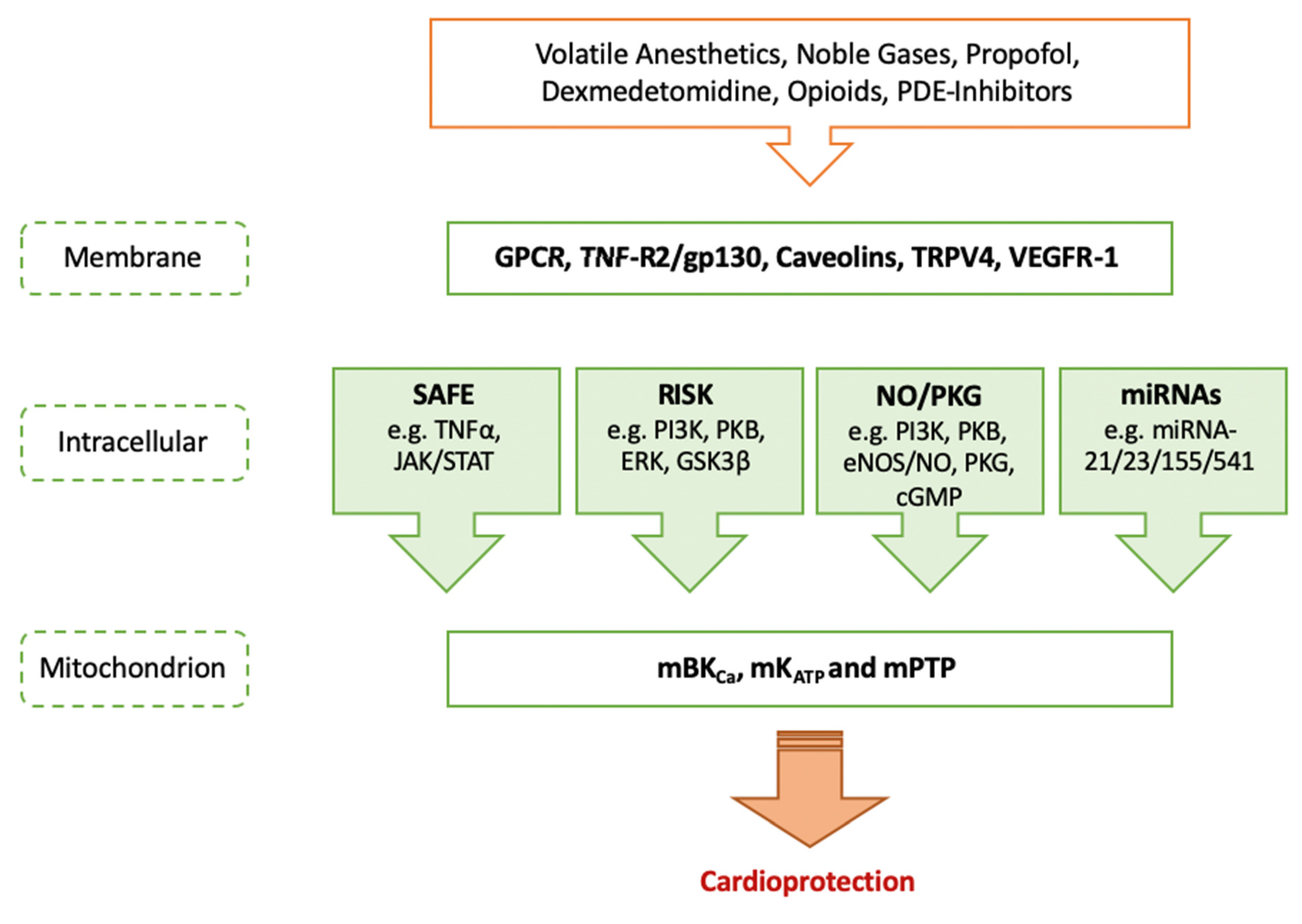
:1. Introduction
2. Volatile Anesthetics
2.1. Experimental Developments
2.2. Clinical Implications
3. Helium—A Noble Gas
3.1. Experimental Developments
3.2. Clinical Implications
4. Propofol
4.1. Experimental Developments
4.2. Clinical Implications
5. Opioids
5.1. Experimental Developments
5.2. Clinical Implications
6. Alpha-2 Agonists
6.1. Experimental Developments
6.2. Clinical Implications
7. Local Anesthetics
7.1. Experimental Developments
7.2. Clinical Implications
8. Phosphodiesterase Inhibitors
8.1. Experimental Developments
8.2. Clinical Implications
9. Future Perspectives
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torregroza, C.; Raupach, A.; Feige, K.; Hollmann, M.W.; Huhn, R. Perioperative Cardioprotection: General Mechanisms and Pharmacological Approaches. Anesth. Analg. 2020. [Google Scholar] [CrossRef] [PubMed]
- Torregroza, C.; Roth, S.; Feige, K.; Lurati Buse, G.; Hollmann, M.W.; Huhn, R. Perioperative cardioprotection—From bench to bedside: Current experimental evidence and possible reasons for the limited translation into the clinical setting. Anaesthesist 2021. [Google Scholar] [CrossRef]
- Roth, S.; Torregroza, C.; Huhn, R.; Hollmann, M.W.; Preckel, B. Perioperative Cardioprotection: Clinical Implications. Anesth. Analg. 2020, 131, 1751–1764. [Google Scholar] [CrossRef]
- Lemoine, S.; Tritapepe, L.; Hanouz, J.L.; Puddu, P.E. The mechanisms of cardio-protective effects of desflurane and sevoflurane at the time of reperfusion: Anaesthetic post-conditioning potentially translatable to humans? Br. J. Anaesth 2016, 116, 456–475. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Lotz, C.; Roewer, N.; Broscheit, J.A. Comparison of volatile anesthetic-induced preconditioning in cardiac and cerebral system: Molecular mechanisms and clinical aspects. Eur J. Med. Res. 2018, 23, 10. [Google Scholar] [CrossRef] [Green Version]
- Van Allen, N.R.; Krafft, P.R.; Leitzke, A.S.; Applegate, R.L., 2nd; Tang, J.; Zhang, J.H. The role of Volatile Anesthetics in Cardioprotection: A systematic review. Med Gas Res. 2012, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Yu, J.; Xie, P.; Maimaitili, Y.; Wang, J.; Yang, L.; Ma, H.; Zhang, X.; Yang, Y.; Zheng, H. Sevoflurane postconditioning protects the myocardium against ischemia/reperfusion injury via activation of the JAK2-STAT3 pathway. PeerJ 2017, 5, e3196. [Google Scholar] [CrossRef]
- Lu, Y.; Bu, M.; Yun, H. Sevoflurane prevents hypoxia/reoxygenation-induced cardiomyocyte apoptosis by inhibiting PI3KC3-mediated autophagy. Hum. Cell 2019, 32, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.G.; Sun, Y.; Sun, B.; Wang, A.; Qiu, J.; Hong, L.; An, J.Z.; Wang, C.; Zhang, H.L. Sevoflurane postconditioning protects against myocardial ischemia/reperfusion injury by restoring autophagic flux via an NO-dependent mechanism. Acta. Pharmacol. Sin. 2019, 40, 35–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, B.; Yang, Y.; Yao, Y.; Liao, Y.; Lin, Y. Upregulation of vascular endothelial growth factor receptor-1 contributes to sevoflurane preconditioning-mediated cardioprotection. Drug Des. Devel. Ther. 2018, 12, 769–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.B.; Liu, T.J.; Pu, G.H.; Li, B.Y.; Gao, X.Z.; Han, X.L. Suppression of Long Non-Coding RNA LINC00652 Restores Sevoflurane-Induced Cardioprotection Against Myocardial Ischemia-Reperfusion Injury by Targeting GLP-1R Through the cAMP/PKA Pathway in Mice. Cell Physiol. Biochem. 2018, 49, 1476–1491. [Google Scholar] [CrossRef]
- Huang, G.; Hao, F.; Hu, X. Downregulation of microRNA-155 stimulates sevoflurane-mediated cardioprotection against myocardial ischemia/reperfusion injury by binding to SIRT1 in mice. J. Cell Biochem. 2019, 120, 15494–15505. [Google Scholar] [CrossRef]
- Xie, D.; Zhao, J.; Guo, R.; Jiao, L.; Zhang, Y.; Lau, W.B.; Lopez, B.; Christopher, T.; Gao, E.; Cao, J.; et al. Sevoflurane Pre-conditioning Ameliorates Diabetic Myocardial Ischemia/Reperfusion Injury Via Differential Regulation of p38 and ERK. Sci. Rep. 2020, 10, 23. [Google Scholar] [CrossRef]
- Zhong, C.Y.; Qiu, H.; Chen, J.; Liu, H. Effects of volatile anesthetic preconditioning on expression of NFkB-regulated genes in aged rat myocardium. J. Biomed. Res. 2017. [Google Scholar] [CrossRef]
- Warltier, D.C.; al-Wathiqui, M.H.; Kampine, J.P.; Schmeling, W.T. Recovery of contractile function of stunned myocardium in chronically instrumented dogs is enhanced by halothane or isoflurane. Anesthesiology 1988, 69, 552–565. [Google Scholar] [CrossRef]
- Weber, N.C.; Goletz, C.; Huhn, R.; Grueber, Y.; Preckel, B.; Schlack, W.; Ebel, D. Blockade of anaesthetic-induced preconditioning in the hyperglycaemic myocardium: The regulation of different mitogen-activated protein kinases. Eur. J. Pharmacol. 2008, 592, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.D.; Pravdic, D.; Bienengraeber, M.; Pratt, P.F., Jr.; Auchampach, J.A.; Gross, G.J.; Kersten, J.R.; Warltier, D.C. Isoflurane postconditioning protects against reperfusion injury by preventing mitochondrial permeability transition by an endothelial nitric oxide synthase-dependent mechanism. Anesthesiology 2010, 112, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Lotz, C.; Kehl, F. Volatile anesthetic-induced cardiac protection: Molecular mechanisms, clinical aspects, and interactions with nonvolatile agents. J. Cardiothorac. Vasc. Anesth. 2015, 29, 749–760. [Google Scholar] [CrossRef]
- Ma, L.; Zhu, J.; Gao, Q.; Rebecchi, M.J.; Wang, Q.; Liu, L. Restoring Pharmacologic Preconditioning in the Aging Heart: Role of Mitophagy/Autophagy. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 489–498. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Zhang, K.; Hu, P. The Role of Autophagy in Acute Myocardial Infarction. Front. Pharmacol. 2019, 10, 551. [Google Scholar] [CrossRef] [Green Version]
- Hamacher-Brady, A.; Brady, N.R.; Logue, S.E.; Sayen, M.R.; Jinno, M.; Kirshenbaum, L.A.; Gottlieb, R.A.; Gustafsson, A.B. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007, 14, 146–157. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Jiao, Y.; Yan, N.; Wu, B.; Ren, Y.; Li, H.; Sun, J.; Gao, J. NOD2 mediates isoflurane preconditioning-induced protection of myocardial injury. Neurosci. Lett. 2017, 637, 154–160. [Google Scholar] [CrossRef]
- Xu, F.; Qiao, S.; Li, H.; Deng, Y.; Wang, C.; An, J. The Effect of Mitochondrial Complex I-Linked Respiration by Isoflurane Is Independent of Mitochondrial Nitric Oxide Production. Cardiorenal. Med. 2018, 8, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Harisseh, R.; Chiari, P.; Villedieu, C.; Sueur, P.; Abrial, M.; Fellahi, J.L.; Ovize, M.; Gharib, A. Cyclophilin D Modulates the Cardiac Mitochondrial Target of Isoflurane, Sevoflurane, and Desflurane. J. Cardiovasc. Pharmacol. 2017, 69, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Javadov, S.; Jang, S.; Parodi-Rullán, R.; Khuchua, Z.; Kuznetsov, A.V. Mitochondrial permeability transition in cardiac ischemia-reperfusion: Whether cyclophilin D is a viable target for cardioprotection? Cell. Mol. Life Sci. CMLS 2017, 74, 2795–2813. [Google Scholar] [CrossRef]
- Olson, J.M.; Yan, Y.; Bai, X.; Ge, Z.D.; Liang, M.; Kriegel, A.J.; Twaroski, D.M.; Bosnjak, Z.J. Up-regulation of microRNA-21 mediates isoflurane-induced protection of cardiomyocytes. Anesthesiology 2015, 122, 795–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, S.; Olson, J.M.; Paterson, M.; Yan, Y.; Zaja, I.; Liu, Y.; Riess, M.L.; Kersten, J.R.; Liang, M.; Warltier, D.C.; et al. MicroRNA-21 Mediates Isoflurane-induced Cardioprotection against Ischemia-Reperfusion Injury via Akt/Nitric Oxide Synthase/Mitochondrial Permeability Transition Pore Pathway. Anesthesiology 2015, 123, 786–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.J.; Liu, B. Inhibition of MicroRNA-23 Contributes to the Isoflurane-Mediated Cardioprotection Against Oxidative Stress. Cardiovasc. Toxicol. 2018, 18, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Heiberg, J.; Royse, C.F.; Royse, A.G.; Andrews, D.T. Propofol Attenuates the Myocardial Protection Properties of Desflurane by Modulating Mitochondrial Permeability Transition. Anesth. Analg. 2018, 127, 387–397. [Google Scholar] [CrossRef]
- Uhlig, C.; Bluth, T.; Schwarz, K.; Deckert, S.; Heinrich, L.; De Hert, S.; Landoni, G.; Serpa Neto, A.; Schultz, M.J.; Pelosi, P.; et al. Effects of Volatile Anesthetics on Mortality and Postoperative Pulmonary and Other Complications in Patients Undergoing Surgery: A Systematic Review and Meta-analysis. Anesthesiology 2016, 124, 1230–1245. [Google Scholar] [CrossRef]
- Likhvantsev, V.V.; Landoni, G.; Levikov, D.I.; Grebenchikov, O.A.; Skripkin, Y.V.; Cherpakov, R.A. Sevoflurane Versus Total Intravenous Anesthesia for Isolated Coronary Artery Bypass Surgery With Cardiopulmonary Bypass: A Randomized Trial. J. Cardiothorac. Vasc. Anesth. 2016, 30, 1221–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landoni, G.; Guarracino, F.; Cariello, C.; Franco, A.; Baldassarri, R.; Borghi, G.; Covello, R.D.; Gerli, C.; Crivellari, M.; Zangrillo, A. Volatile compared with total intravenous anaesthesia in patients undergoing high-risk cardiac surgery: A randomized multicentre study. Br. J. Anaesth. 2014, 113, 955–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landoni, G.; Lomivorotov, V.V.; Nigro Neto, C.; Monaco, F.; Pasyuga, V.V.; Bradic, N.; Lembo, R.; Gazivoda, G.; Likhvantsev, V.V.; Lei, C.; et al. Volatile Anesthetics versus Total Intravenous Anesthesia for Cardiac Surgery. N. Engl. J. Med. 2019, 380, 1214–1225. [Google Scholar] [CrossRef]
- Lurati Buse, G.A.; Schumacher, P.; Seeberger, E.; Studer, W.; Schuman, R.M.; Fassl, J.; Kasper, J.; Filipovic, M.; Bolliger, D.; Seeberger, M.D. Randomized comparison of sevoflurane versus propofol to reduce perioperative myocardial ischemia in patients undergoing noncardiac surgery. Circulation 2012, 126, 2696–2704. [Google Scholar] [CrossRef] [Green Version]
- Devereaux, P.J.; Sessler, D.I.; Leslie, K.; Kurz, A.; Mrkobrada, M.; Alonso-Coello, P.; Villar, J.C.; Sigamani, A.; Biccard, B.M.; Meyhoff, C.S.; et al. Clonidine in patients undergoing noncardiac surgery. N. Engl. J. Med. 2014, 370, 1504–1513. [Google Scholar] [CrossRef] [Green Version]
- Hofland, J.; Ouattara, A.; Fellahi, J.L.; Gruenewald, M.; Hazebroucq, J.; Ecoffey, C.; Joseph, P.; Heringlake, M.; Steib, A.; Coburn, M.; et al. Effect of Xenon Anesthesia Compared to Sevoflurane and Total Intravenous Anesthesia for Coronary Artery Bypass Graft Surgery on Postoperative Cardiac Troponin Release: An International, Multicenter, Phase 3, Single-blinded, Randomized Noninferiority Trial. Anesthesiology 2017, 127, 918–933. [Google Scholar] [CrossRef]
- Mehta, R.H.; Leimberger, J.D.; van Diepen, S.; Meza, J.; Wang, A.; Jankowich, R.; Harrison, R.W.; Hay, D.; Fremes, S.; Duncan, A.; et al. Levosimendan in Patients with Left Ventricular Dysfunction Undergoing Cardiac Surgery. N. Engl. J. Med. 2017, 376, 2032–2042. [Google Scholar] [CrossRef] [PubMed]
- Smit, K.F.; Weber, N.C.; Hollmann, M.W.; Preckel, B. Noble gases as cardioprotectants—Translatability and mechanism. Br. J. Pharmacol. 2015, 172, 2062–2073. [Google Scholar] [CrossRef] [Green Version]
- Pagel, P.S.; Krolikowski, J.G.; Shim, Y.H.; Venkatapuram, S.; Kersten, J.R.; Weihrauch, D.; Warltier, D.C.; Pratt, P.F., Jr. Noble gases without anesthetic properties protect myocardium against infarction by activating prosurvival signaling kinases and inhibiting mitochondrial permeability transition in vivo. Anesth. Analg. 2007, 105, 562–569. [Google Scholar] [CrossRef]
- Weber, N.C.; Smit, K.F.; Hollmann, M.W.; Preckel, B. Targets Involved in Cardioprotection by the Non-Anesthetic Noble Gas Helium. Curr. Drug Targets 2015, 16, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.P.; Zhang, J.Y.; Feng, D.X.; Kong, Y.; Xu, Z.; Chen, G. Advances in molecular mechanism of cardioprotection induced by helium. Med. Gas. Res. 2017, 7, 124–132. [Google Scholar] [CrossRef]
- Weber, N.C.; Preckel, B. Gaseous mediators: An updated review on the effects of helium beyond blowing up balloons. Intensive Care Med. Exp. 2019, 7, 73. [Google Scholar] [CrossRef]
- Feron, O.; Balligand, J.L. Caveolins and the regulation of endothelial nitric oxide synthase in the heart. Cardiovasc. Res. 2006, 69, 788–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pike, L.J. Lipid rafts: Bringing order to chaos. J. Lipid Res. 2003, 44, 655–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parton, R.G.; Way, M.; Zorzi, N.; Stang, E. Caveolin-3 associates with developing T-tubules during muscle differentiation. J. Cell Biol. 1997, 136, 137–154. [Google Scholar] [CrossRef]
- Chun, M.; Liyanage, U.K.; Lisanti, M.P.; Lodish, H.F. Signal transduction of a G protein-coupled receptor in caveolae: Colocalization of endothelin and its receptor with caveolin. Proc. Natl. Acad. Sci USA 1994, 91, 11728–11732. [Google Scholar] [CrossRef] [Green Version]
- Ballard-Croft, C.; Locklar, A.C.; Kristo, G.; Lasley, R.D. Regional myocardial ischemia-induced activation of MAPKs is associated with subcellular redistribution of caveolin and cholesterol. Am. J. Physiol. Heart Circ. Physiol 2006, 291, H658–H667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krajewska, W.M.; Masłowska, I. Caveolins: Structure and function in signal transduction. Cell Mol. Biol. Lett. 2004, 9, 195–220. [Google Scholar] [PubMed]
- Sargiacomo, M.; Scherer, P.E.; Tang, Z.; Kübler, E.; Song, K.S.; Sanders, M.C.; Lisanti, M.P. Oligomeric structure of caveolin: Implications for caveolae membrane organization. Proc. Natl. Acad. Sci. USA 1995, 92, 9407–9411. [Google Scholar] [CrossRef] [Green Version]
- Schilling, J.M.; Head, B.P.; Patel, H.H. Caveolins as Regulators of Stress Adaptation. Mol. Pharmacol. 2018, 93, 277–285. [Google Scholar] [CrossRef]
- Schilling, J.M.; Roth, D.M.; Patel, H.H. Caveolins in cardioprotection—Translatability and mechanisms. Br. J. Pharmacol. 2015, 172, 2114–2125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, K.S.; Scherer, P.E.; Tang, Z.; Okamoto, T.; Li, S.; Chafel, M.; Chu, C.; Kohtz, D.S.; Lisanti, M.P. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J. Biol. Chem. 1996, 271, 15160–15165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, H.H.; Tsutsumi, Y.M.; Head, B.P.; Niesman, I.R.; Jennings, M.; Horikawa, Y.; Huang, D.; Moreno, A.L.; Patel, P.M.; Insel, P.A.; et al. Mechanisms of cardiac protection from ischemia/reperfusion injury: A role for caveolae and caveolin-1. FASEB J. 2007, 21, 1565–1574. [Google Scholar] [CrossRef]
- Flick, M.; Albrecht, M.; Oei, G.; Steenstra, R.; Kerindongo, R.P.; Zuurbier, C.J.; Patel, H.H.; Hollmann, M.W.; Preckel, B.; Weber, N.C. Helium postconditioning regulates expression of caveolin-1 and -3 and induces RISK pathway activation after ischaemia/reperfusion in cardiac tissue of rats. Eur. J. Pharmacol. 2016, 791, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Aehling, C.; Weber, N.C.; Zuurbier, C.J.; Preckel, B.; Galmbacher, R.; Stefan, K.; Hollmann, M.W.; Popp, E.; Knapp, J. Effects of combined helium pre/post-conditioning on the brain and heart in a rat resuscitation model. Acta. Anaesthesiol. Scand. 2018, 62, 63–74. [Google Scholar] [CrossRef]
- Weber, N.C.; Schilling, J.M.; Warmbrunn, M.V.; Dhanani, M.; Kerindongo, R.; Siamwala, J.; Song, Y.; Zemljic-Harpf, A.E.; Fannon, M.J.; Hollmann, M.W.; et al. Helium-Induced Changes in Circulating Caveolin in Mice Suggest a Novel Mechanism of Cardiac Protection. Int. J. Mol. Sci. 2019, 20, 2640. [Google Scholar] [CrossRef] [Green Version]
- Smit, K.F.; Brevoord, D.; De Hert, S.; de Mol, B.A.; Kerindongo, R.P.; van Dieren, S.; Schlack, W.S.; Hollmann, M.W.; Weber, N.C.; Preckel, B. Effect of helium pre- or postconditioning on signal transduction kinases in patients undergoing coronary artery bypass graft surgery. J. Transl. Med. 2016, 14, 294. [Google Scholar] [CrossRef] [Green Version]
- Smit, K.F.; Oei, G.T.; Brevoord, D.; Stroes, E.S.; Nieuwland, R.; Schlack, W.S.; Hollmann, M.W.; Weber, N.C.; Preckel, B. Helium induces preconditioning in human endothelium in vivo. Anesthesiology 2013, 118, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Lucchinetti, E.; Wacker, J.; Maurer, C.; Keel, M.; Härter, L.; Zaugg, K.; Zaugg, M. Helium breathing provides modest antiinflammatory, but no endothelial protection against ischemia-reperfusion injury in humans in vivo. Anesth. Analg. 2009, 109, 101–108. [Google Scholar] [CrossRef]
- Zaugg, M.; Lucchinetti, E.; Behmanesh, S.; Clanachan, A.S. Anesthetic cardioprotection in clinical practice from proof-of-concept to clinical applications. Curr. Pharm. Des. 2014, 20, 5706–5726. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Sun, J.G.; Hu, L.H.; Ma, X.C.; Zhou, G.; Huang, X.Z. Propofol-mediated cardioprotection dependent of microRNA-451/HMGB1 against myocardial ischemia-reperfusion injury. J. Cell Physiol. 2019, 234, 23289–23301. [Google Scholar] [CrossRef] [PubMed]
- Engels, W.; Reiters, P.H.; Daemen, M.J.; Smits, J.F.; van der Vusse, G.J. Transmural changes in mast cell density in rat heart after infarct induction in vivo. J. Pathol. 1995, 177, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Sun, X.; Zhao, M.; Hou, Y.; Li, J.; Yu, J.; Hou, Y. Propofol attenuates myocardial ischemia reperfusion injury partly through inhibition of resident cardiac mast cell activation. Int. Immunopharmacol. 2018, 54, 267–274. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Q.; Liao, J.; Zhang, S.; Liu, H.; Yang, C.; Dong, Q.; Zhao, N.; Huang, Z.; Guo, K.; et al. Propofol Induces Cardioprotection Against Ischemia-Reperfusion Injury via Suppression of Transient Receptor Potential Vanilloid 4 Channel. Front. Pharmacol. 2019, 10, 1150. [Google Scholar] [CrossRef]
- Bunte, S.; Behmenburg, F.; Eckelskemper, F.; Mohr, F.; Stroethoff, M.; Raupach, A.; Heinen, A.; Hollmann, M.W.; Huhn, R. Cardioprotection by Humoral Factors Released After Remote Ischemic Preconditioning Depends on Anesthetic Regimen. Crit. Care Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Behmenburg, F.; van Caster, P.; Bunte, S.; Brandenburger, T.; Heinen, A.; Hollmann, M.W.; Huhn, R. Impact of Anesthetic Regimen on Remote Ischemic Preconditioning in the Rat Heart In Vivo. Anesth. Analg. 2018, 126, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Nam, K.; Kim, T.K.; Choi, S.W.; Kim, S.J.; Hausenloy, D.J.; Jeon, Y. Sevoflurane, Propofol and Carvedilol Block Myocardial Protection by Limb Remote Ischemic Preconditioning. Int. J. Mol. Sci. 2019, 20, 269. [Google Scholar] [CrossRef] [Green Version]
- Bunte, S.; Lill, T.; Falk, M.; Stroethoff, M.; Raupach, A.; Mathes, A.; Heinen, A.; Hollmann, M.W.; Huhn, R. Impact of Anesthetics on Cardioprotection Induced by Pharmacological Preconditioning. J. Clin. Med. 2019, 8, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucchinetti, E.; Lou, P.H.; Gandhi, M.; Clanachan, A.S.; Zaugg, M. Differential Effects of Anesthetics and Opioid Receptor Activation on Cardioprotection Elicited by Reactive Oxygen Species-Mediated Postconditioning in Sprague-Dawley Rat Hearts. Anesth. Analg. 2018, 126, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Mwamure, P.K.; Venugopal, V.; Harris, J.; Barnard, M.; Grundy, E.; Ashley, E.; Vichare, S.; Di Salvo, C.; Kolvekar, S.; et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: A randomised controlled trial. Lancet 2007, 370, 575–579. [Google Scholar] [CrossRef]
- Meybohm, P.; Bein, B.; Brosteanu, O.; Cremer, J.; Gruenewald, M.; Stoppe, C.; Coburn, M.; Schaelte, G.; Boning, A.; Niemann, B.; et al. A Multicenter Trial of Remote Ischemic Preconditioning for Heart Surgery. N. Engl. J. Med. 2015, 373, 1397–1407. [Google Scholar] [CrossRef]
- Ney, J.; Hoffmann, K.; Meybohm, P.; Goetzenich, A.; Kraemer, S.; Benstöm, C.; Weber, N.C.; Bickenbach, J.; Rossaint, R.; Marx, G.; et al. Remote Ischemic Preconditioning Does Not Affect the Release of Humoral Factors in Propofol-Anesthetized Cardiac Surgery Patients: A Secondary Analysis of the RIPHeart Study. Int. J. Mol. Sci. 2018, 19, 1094. [Google Scholar] [CrossRef] [Green Version]
- Headrick, J.P.; See Hoe, L.E.; Du Toit, E.F.; Peart, J.N. Opioid receptors and cardioprotection—‘Opioidergic conditioning’ of the heart. Br. J. Pharmacol. 2015, 172, 2026–2050. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Kersten, J.R.; Riess, M.L. Opioid-induced cardioprotection. Curr. Pharm. Des. 2014, 20, 5696–5705. [Google Scholar] [CrossRef] [Green Version]
- Irwin, M.G.; Wong, G.T. Remifentanil and opioid-induced cardioprotection. J. Cardiothorac. Vasc. Anesth. 2015, 29, S23–S26. [Google Scholar] [CrossRef] [PubMed]
- Melo, Z.; Ishida, C.; Goldaraz, M.P.; Rojo, R.; Echavarria, R. Novel Roles of Non-Coding RNAs in Opioid Signaling and Cardioprotection. Noncoding RNA 2018, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.Y.; Huang, J.; Zhu, H.J.; Wu, H.; Xu, S.J.; Irwin, M.G.; He, S.F.; Zhang, Y. Remifentanil preconditioning confers cardioprotection via c-Jun NH2-terminal kinases and extracellular signal regulated kinases pathways in ex-vivo failing rat heart. Eur. J. Pharmacol. 2018, 828, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, Q.; Chen, L.; Zhang, L.; Cheng, X.; Gu, E. HDAC3 Mediates Cardioprotection of Remifentanil Postconditioning by Targeting GSK-3β in H9c2 Cardiomyocytes in Hypoxia/Reoxygenation Injury. Shock 2018, 50, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, H.P.; Li, Y.; Shao, W.; Zhang, J.Z.; Wang, L.M. Influences of remifentanil on myocardial ischemia-reperfusion injury and the expressions of Bax and Bcl-2 in rats. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8951–8960. [Google Scholar] [CrossRef]
- Lei, S.; Su, W.; Xia, Z.Y.; Wang, Y.; Zhou, L.; Qiao, S.; Zhao, B.; Xia, Z.; Irwin, M.G. Hyperglycemia-Induced Oxidative Stress Abrogates Remifentanil Preconditioning-Mediated Cardioprotection in Diabetic Rats by Impairing Caveolin-3-Modulated PI3K/Akt and JAK2/STAT3 Signaling. Oxid. Med. Cell Longev. 2019, 2019, 9836302. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Cheng, X.; Gu, E.; Liu, X.; Zhang, L.; Cao, Y. Effect of aortic root infusion of sufentanil on ischemia-reperfusion injury in patients undergoing mitral valve replacement. J. Cardiothorac. Vasc. Anesth. 2014, 28, 1474–1478. [Google Scholar] [CrossRef]
- Wong, G.T.; Huang, Z.; Ji, S.; Irwin, M.G. Remifentanil reduces the release of biochemical markers of myocardial damage after coronary artery bypass surgery: A randomized trial. J. Cardiothorac. Vasc. Anesth. 2010, 24, 790–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, M.; Landoni, G.; Biondi-Zoccai, G.; Cabrini, L.; Ruggeri, L.; Pasculli, N.; Giacchi, V.; Sayeg, J.; Greco, T.; Zangrillo, A. Remifentanil in cardiac surgery: A meta-analysis of randomized controlled trials. J. Cardiothorac. Vasc. Anesth. 2012, 26, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Kwanten, L.E.; O’Brien, B.; Anwar, S. Opioid-Based Anesthesia and Analgesia for Adult Cardiac Surgery: History and Narrative Review of the Literature. J. Cardiothorac. Vasc. Anesth. 2019, 33, 808–816. [Google Scholar] [CrossRef]
- Nishina, K.; Mikawa, K.; Uesugi, T.; Obara, H.; Maekawa, M.; Kamae, I.; Nishi, N. Efficacy of clonidine for prevention of perioperative myocardial ischemia: A critical appraisal and meta-analysis of the literature. Anesthesiology 2002, 96, 323–329. [Google Scholar] [CrossRef]
- Castillo, R.L.; Ibacache, M.; Cortinez, I.; Carrasco-Pozo, C.; Farias, J.G.; Carrasco, R.A.; Vargas-Errazuriz, P.; Ramos, D.; Benavente, R.; Torres, D.H.; et al. Dexmedetomidine Improves Cardiovascular and Ventilatory Outcomes in Critically Ill Patients: Basic and Clinical Approaches. Front. Pharmacol. 2019, 10, 1641. [Google Scholar] [CrossRef] [PubMed]
- Bunte, S.; Behmenburg, F.; Majewski, N.; Stroethoff, M.; Raupach, A.; Mathes, A.; Heinen, A.; Hollmann, M.W.; Huhn, R. Characteristics of Dexmedetomidine Postconditioning in the Field of Myocardial Ischemia-Reperfusion Injury. Anesth. Analg. 2019. [Google Scholar] [CrossRef]
- Cheng, X.; Hu, J.; Wang, Y.; Ye, H.; Li, X.; Gao, Q.; Li, Z. Effects of Dexmedetomidine Postconditioning on Myocardial Ischemia/Reperfusion Injury in Diabetic Rats: Role of the PI3K/Akt-Dependent Signaling Pathway. J. Diabetes Res. 2018, 2018, 3071959. [Google Scholar] [CrossRef]
- Torregroza, C.; Feige, K.; Schneider, L.; Bunte, S.; Stroethoff, M.; Heinen, A.; Hollmann, M.W.; Huhn, R.; Raupach, A. Influence of Hyperglycemia on Dexmedetomidine-Induced Cardioprotection in the Isolated Perfused Rat Heart. J. Clin. Med. 2020, 9, 1445. [Google Scholar] [CrossRef]
- He, L.; Hao, S.; Wang, Y.; Yang, W.; Liu, L.; Chen, H.; Qian, J. Dexmedetomidine preconditioning attenuates ischemia/reperfusion injury in isolated rat hearts with endothelial dysfunction. Biomed. Pharmacother. 2019, 114, 108837. [Google Scholar] [CrossRef]
- Wang, L.; Tang, S.; Wang, Z.; Chen, H.; Rajcha, S.S.; Qian, J. The administration of dexmedetomidine changes microRNA expression profiling of rat hearts. Biomed. Pharmacother. 2019, 120, 109463. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yang, W.; Wang, W.; Wang, Z.; Pu, Y.; Chen, H.; Wang, F.; Qian, J. Involvement of miR-665 in protection effect of dexmedetomidine against Oxidative Stress Injury in myocardial cells via CB2 and CK1. Biomed. Pharmacother. 2019, 115, 108894. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Hirata, N.; Terada, H.; Sawashita, Y.; Yamakage, M. Identification of Candidate Genes and Pathways in Dexmedetomidine-Induced Cardioprotection in the Rat Heart by Bioinformatics Analysis. Int. J. Mol. Sci. 2019, 20, 1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Chen, H.; Chen, Y.; Birnbaum, Y.; Liang, R.; Ye, Y.; Qian, J. Circulating miRNA Expression Profiling and Target Prediction in Patients Receiving Dexmedetomidine. Cell Physiol. Biochem. 2018, 50, 552–568. [Google Scholar] [CrossRef]
- Peng, K.; Chen, W.R.; Xia, F.; Liu, H.; Meng, X.W.; Zhang, J.; Liu, H.Y.; Xia, Z.Y.; Ji, F.H. Dexmedetomidine post-treatment attenuates cardiac ischaemia/reperfusion injury by inhibiting apoptosis through HIF-1alpha signalling. J. Cell Mol. Med. 2020, 24, 850–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.R.; Li, T.; Cao, L.; Yu, Y.Y.; Chen, L.L.; Fan, X.H.; Yang, B.B.; Tan, X.Q. Dexmedetomidine attenuates H2O2-induced neonatal rat cardiomyocytes apoptosis through mitochondria- and ER-medicated oxidative stress pathways. Mol. Med. Rep. 2018, 17, 7258–7264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, H.; Liu, D.H.; Wang, G.N. Effects of dexmedetomidine on myocardial ischemia-reperfusion injury through PI3K-Akt-mTOR signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6736–6743. [Google Scholar] [CrossRef] [PubMed]
- Intachai, K.; C Chattipakorn, S.; Chattipakorn, N.; Shinlapawittayatorn, K. Revisiting the Cardioprotective Effects of Acetylcholine Receptor Activation against Myocardial Ischemia/Reperfusion Injury. Int. J. Mol. Sci. 2018, 19, 2466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Z.; Ma, L.; Zhong, Y.L.; Li, J.; Lv, J.; Xie, Y.B. Myocardial protective effects of dexmedetomidine in patients undergoing cardiac surgery: A meta-analysis and systematic review. Exp. Ther. Med. 2017, 13, 2355–2361. [Google Scholar] [CrossRef] [Green Version]
- Tosun, Z.; Baktir, M.; Kahraman, H.C.; Baskol, G.; Guler, G.; Boyaci, A. Does dexmedetomidine provide cardioprotection in coronary artery bypass grafting with cardiopulmonary bypass? A pilot study. J. Cardiothorac. Vasc. Anesth. 2013, 27, 710–715. [Google Scholar] [CrossRef]
- Zhou, H.M.; Ling, X.Y.; Ni, Y.J.; Wu, C.; Zhu, Z.P. Pre-cardiopulmonary bypass administration of dexmedetomidine decreases cardiac troponin I level following cardiac surgery with sevoflurane postconditioning. J. Int. Med. Res. 2019, 47, 3623–3635. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhou, D.; Lu, J.; Wu, C.; Zhu, Z. Effects of Pre-Cardiopulmonary Bypass Administration of Dexmedetomidine on Cardiac Injuries and the Inflammatory Response in Valve Replacement Surgery with a Sevoflurane Postconditioning Protocol: A Pilot Study. J. Cardiovasc. Pharmacol. 2019, 74, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elgebaly, A.S.; Fathy, S.M.; Sallam, A.A.; Elbarbary, Y. Cardioprotective effects of propofol-dexmedetomidine in open-heart surgery: A prospective double-blind study. Ann. Card. Anaesth. 2020, 23, 134–141. [Google Scholar] [CrossRef]
- Ebel, D.; Lipfert, P.; Fräßdorf, J.; Preckel, B.; Müllenheim, J.; Thämer, V.; Schlack, W. Lidocaine reduces ischaemic but not reperfusion injury in isolated rat heart. BJA: Br. J. Anaesth. 2001, 86, 846–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaczmarek, D.J.; Herzog, C.; Larmann, J.; Gillmann, H.-J.; Hildebrand, R.; Schmitz, M.; Westermann, A.; Harendza, T.; Werdehausen, R.; Osthaus, A.W.; et al. Lidocaine Protects from Myocardial Damage due to Ischemia and Reperfusion in Mice by Its Antiapoptotic Effects. Anesthesiology 2009, 110, 1041–1049. [Google Scholar] [CrossRef] [Green Version]
- Cassutto, B.H.; Gfeller, R.W. Use of intravenous lidocaine to prevent reperfusion injury and subsequent multiple organ dysfunction syndrome. J. Vet. Emerg. Crit. Care 2003, 13, 137–148. [Google Scholar] [CrossRef]
- Eroglu, A. The effect of intravenous anesthetics on ischemia-reperfusion injury. Biomed. Res. Int. 2014, 2014, 821513. [Google Scholar] [CrossRef]
- Granfeldt, A.; Letson, H.L.; Dobson, G.P.; Shi, W.; Vinten-Johansen, J.; Tønnesen, E. Adenosine, lidocaine and Mg2+ improves cardiac and pulmonary function, induces reversible hypotension and exerts anti-inflammatory effects in an endotoxemic porcine model. Crit. Care 2014, 18, 682. [Google Scholar] [CrossRef] [Green Version]
- Barthel, H.; Ebel, D.; Müllenheim, J.; Obal, D.; Preckel, B.; Schlack, W. Effect of lidocaine on ischaemic preconditioning in isolated rat heart. Br. J. Anaesth. 2004, 93, 698–704. [Google Scholar] [CrossRef] [Green Version]
- Canyon, S.J.; Dobson, G.P. Pretreatment with an adenosine A1 receptor agonist and lidocaine: A possible alternative to myocardial ischemic preconditioning. J. Thorac. Cardiovasc. Surg. 2005, 130, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Weibel, S.; Jokinen, J.; Pace, N.L.; Schnabel, A.; Hollmann, M.W.; Hahnenkamp, K.; Eberhart, L.H.; Poepping, D.M.; Afshari, A.; Kranke, P. Efficacy and safety of intravenous lidocaine for postoperative analgesia and recovery after surgery: A systematic review with trial sequential analysis. Br. J. Anaesth. 2016, 116, 770–783. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.H.; Lee, H.M.; Chung, C.H.; Chin, J.H.; Choi, D.K.; Chung, H.J.; Sim, J.Y.; Choi, I.C. Impact of intravenous lidocaine on myocardial injury after off-pump coronary artery surgery. Br. J. Anaesth. 2011, 106, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wu, X.; Li, J.; Xiao, F.; Liu, X.; Meng, M. The effect of lidocaine on early postoperative cognitive dysfunction after coronary artery bypass surgery. Anesth. Analg. 2002, 95, 1134–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, J.P.; Mackensen, G.B.; Phillips-Bute, B.; Grocott, H.P.; Glower, D.D.; Laskowitz, D.T.; Blumenthal, J.A.; Newman, M.F. Randomized, double-blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery. Stroke 2009, 40, 880–887. [Google Scholar] [CrossRef] [Green Version]
- Behmenburg, F.; Trefz, L.; Dorsch, M.; Strothoff, M.; Mathes, A.; Raupach, A.; Heinen, A.; Hollmann, M.W.; Berger, M.M.; Huhn, R. Milrinone-Induced Postconditioning Requires Activation of Mitochondrial Ca(2+)-sensitive Potassium (mBKCa) Channels. J. Cardiothorac. Vasc. Anesth. 2018, 32, 2142–2148. [Google Scholar] [CrossRef] [PubMed]
- Raupach, A.; Reinle, J.; Stroethoff, M.; Mathes, A.; Heinen, A.; Hollmann, M.W.; Huhn, R.; Bunte, S. Milrinone-Induced Pharmacological Preconditioning in Cardioprotection: Hints for a Role of Mitochondrial Mechanisms. J. Clin. Med. 2019, 8, 507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanada, S.; Kitakaze, M.; Papst, P.J.; Asanuma, H.; Node, K.; Takashima, S.; Asakura, M.; Ogita, H.; Liao, Y.; Sakata, Y.; et al. Cardioprotective effect afforded by transient exposure to phosphodiesterase III inhibitors: The role of protein kinase A and p38 mitogen-activated protein kinase. Circulation 2001, 104, 705–710. [Google Scholar] [CrossRef] [Green Version]
- Reffelmann, T.; Kloner, R.A. Phosphodiesterase 5 inhibitors: Are they cardioprotective? Cardiovasc. Res. 2009, 83, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Kukreja, R.C.; Ockaili, R.; Salloum, F.; Yin, C.; Hawkins, J.; Das, A.; Xi, L. Cardioprotection with phosphodiesterase-5 inhibition—A novel preconditioning strategy. J. Mol. Cell. Cardiol. 2004, 36, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, D.C.; Anderson, S.G.; Caldwell, J.L.; Trafford, A.W. Phosphodiesterase-5 inhibitors and the heart: Compound cardioprotection? Heart 2018, 104, 1244–1250. [Google Scholar] [CrossRef]
- Orstavik, O.; Manfra, O.; Andressen, K.W.; Andersen, G.O.; Skomedal, T.; Osnes, J.B.; Levy, F.O.; Krobert, K.A. The inotropic effect of the active metabolite of levosimendan, OR-1896, is mediated through inhibition of PDE3 in rat ventricular myocardium. PLoS ONE 2015, 10, e0115547. [Google Scholar] [CrossRef] [Green Version]
- Efentakis, P.; Varela, A.; Chavdoula, E.; Sigala, F.; Sanoudou, D.; Tenta, R.; Gioti, K.; Kostomitsopoulos, N.; Papapetropoulos, A.; Tasouli, A.; et al. Levosimendan prevents doxorubicin-induced cardiotoxicity in time- and dose-dependent manner: Implications for inotropy. Cardiovasc. Res. 2020, 116, 576–591. [Google Scholar] [CrossRef]
- Bunte, S.; Behmenburg, F.; Bongartz, A.; Stroethoff, M.; Raupach, A.; Heinen, A.; Minol, J.P.; Hollmann, M.W.; Huhn, R.; Sixt, S.U. Preconditioning by Levosimendan is Mediated by Activation of Mitochondrial Ca(2+)-Sensitive Potassium (mBKCa) Channels. Cardiovasc. Drugs Ther. 2018, 32, 427–434. [Google Scholar] [CrossRef]
- Stroethoff, M.; Bunte, S.; Raupach, A.; van de Snepscheut, M.; Torregroza, C.; Heinen, A.; Mathes, A.; Hollmann, M.W.; Huhn, R.; Sixt, S.U. Impact of Ca(2+)-Sensitive Potassium Channels in Levosimendan-Induced Postconditioning. Cardiovasc. Drugs Ther. 2019, 33, 581–588. [Google Scholar] [CrossRef]
- Uhlig, K.; Efremov, L.; Tongers, J.; Frantz, S.; Mikolajczyk, R.; Sedding, D.; Schumann, J. Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst. Rev. 2020, 11, Cd009669. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Bøtker, H.E.; Ovize, M.; Hausenloy, D.J.; Heusch, G. Co-morbidities and co-medications as confounders of cardioprotection-Does it matter in the clinical setting? Br. J. Pharmacol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Hausenloy, D.J.; Garcia-Dorado, D.; Bøtker, H.E.; Davidson, S.M.; Downey, J.; Engel, F.B.; Jennings, R.; Lecour, S.; Leor, J.; Madonna, R.; et al. Novel targets and future strategies for acute cardioprotection: Position Paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc. Res. 2017, 113, 564–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, S.M.; Ferdinandy, P.; Andreadou, I.; Bøtker, H.E.; Heusch, G.; Ibáñez, B.; Ovize, M.; Schulz, R.; Yellon, D.M.; Hausenloy, D.J.; et al. Multitarget Strategies to Reduce Myocardial Ischemia/Reperfusion Injury: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Rossello, X.; Yellon, D.M. The RISK pathway and beyond. Basic Res. Cardiol. 2017, 113, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwiebert, C.; Huhn, R.; Heinen, A.; Weber, N.C.; Hollmann, M.W.; Schlack, W.; Preckel, B. Postconditioning by xenon and hypothermia in the rat heart in vivo. Eur J. Anaesthesiol. 2010, 27, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yang, S.; Zhang, X.; Cao, Y.; Huang, Y. Comparison of cardioprotective efficacy resulting from a combination of atorvastatin and ischaemic post-conditioning in diabetic and non-diabetic rats. Clin. Exp. Pharmacol. Physiol. 2012, 39, 938–943. [Google Scholar] [CrossRef]
- Sloth, A.D.; Schmidt, M.R.; Munk, K.; Schmidt, M.; Pedersen, L.; Sørensen, H.T.; Bøtker, H.E. Impact of cardiovascular risk factors and medication use on the efficacy of remote ischaemic conditioning: Post hoc subgroup analysis of a randomised controlled trial. BMJ Open 2015, 5, e006923. [Google Scholar] [CrossRef] [PubMed]
- Chiari, P.; Durand, M.; Desebbe, O.; Fischer, M.O.; Lena-Quintard, D.; Palao, J.C.; Mercier, C.; Samson, G.; Varillon, Y.; Pozzi, M.; et al. Multimodal cardioprotective strategy in cardiac surgery (the ProCCard trial): Study protocol for a multicenter randomized controlled trial. Trials 2019, 20, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Study Title | Population | Intervention | Endpoints | Results |
|---|---|---|---|---|
| Volatile anesthetics versus total intravenous anesthesia for cardiac surgery [34] | Elective CABG (RCT; n = 5400) | Volatile anesthetic vs. TIVA | Death from any cause at 1 year | No difference regarding deaths at 1 year between a volatile agent and total intravenous anesthesia |
| Volatile compared with total intravenous anaesthesia in patients undergoing high-risk cardiac surgery: a randomized multicentre study [33] | High-risk cardiac surgery patients with CPB (RCT; n = 200) | Sevoflurane versus TIVA | Composite of death, prolonged intensive care unit stay | No observed beneficial effect of sevoflurane on the composite endpoint |
| Sevoflurane Versus Total Intravenous Anesthesia for Isolated Coronary Artery Bypass Surgery with Cardiopulmonary Bypass: A Randomized Trial [32] | CABG with CPB (RCT; n = 868) | Sevoflurane versus TIVA | Hospital length of stay | Reduction of cardiac biomarker release and length of hospital stay after CABG by Sevoflurane |
| Randomized comparison of sevoflurane versus propofol to reduce perioperative myocardial ischemia in patients undergoing noncardiac surgery [35] | Noncardiac surgery patients at increased cardiovascular risk (RCT; n = 385) | Sevoflurane versus Propofol | Composite of myocardial ischemia detected by continuous ECG and/or troponin elevation | Sevoflurane did not reduce the incidence of myocardial ischemia |
| Clonidine in patients undergoing noncardiac surgery [36] | Patients at risk for atherosclerotic disease undergoing noncardiac surgery (RCT; n = 10,010) | Clonidine vs. Placebo | Composite endpoint of death or nonfatal myocardial infarction at 30 days | Clonidine did not reduce the rate of the composite outcome, but increased risk of hypotension and cardiac arrest |
| Effect of Xenon Anesthesia Compared to Sevoflurane and Total Intravenous Anesthesia for Coronary Artery Bypass Graft Surgery on Postoperative Cardiac Troponin Release [37] | Low-risk, on-pump CABG (RCT; n = 492) | Xenon vs. sevoflurane and TIVA | Cardiac troponin I concentration in the blood 24 h postsurgery | In postoperative troponin I release, xenon was noninferior to sevoflurane in CABG patients |
| Levosimendan in patients with left ventricular dysfunction undergoing cardiac surgery [38] | LVEF of 35% or less and cardiac surgery with CPB (RCT; n = 882) | Levosimendan vs. Placebo | Composite of death, RRT, MI and use of ECLS | Levosimendan did not reduce the incidence of the composite endpoint |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roth, S.; Torregroza, C.; Feige, K.; Preckel, B.; Hollmann, M.W.; Weber, N.C.; Huhn, R. Pharmacological Conditioning of the Heart: An Update on Experimental Developments and Clinical Implications. Int. J. Mol. Sci. 2021, 22, 2519. https://doi.org/10.3390/ijms22052519
Roth S, Torregroza C, Feige K, Preckel B, Hollmann MW, Weber NC, Huhn R. Pharmacological Conditioning of the Heart: An Update on Experimental Developments and Clinical Implications. International Journal of Molecular Sciences. 2021; 22(5):2519. https://doi.org/10.3390/ijms22052519
Chicago/Turabian StyleRoth, Sebastian, Carolin Torregroza, Katharina Feige, Benedikt Preckel, Markus W. Hollmann, Nina C. Weber, and Ragnar Huhn. 2021. "Pharmacological Conditioning of the Heart: An Update on Experimental Developments and Clinical Implications" International Journal of Molecular Sciences 22, no. 5: 2519. https://doi.org/10.3390/ijms22052519
APA StyleRoth, S., Torregroza, C., Feige, K., Preckel, B., Hollmann, M. W., Weber, N. C., & Huhn, R. (2021). Pharmacological Conditioning of the Heart: An Update on Experimental Developments and Clinical Implications. International Journal of Molecular Sciences, 22(5), 2519. https://doi.org/10.3390/ijms22052519







