Gender-Specific Effects of Two Treatment Strategies in a Mouse Model of Niemann-Pick Disease Type C1
Abstract
:1. Introduction
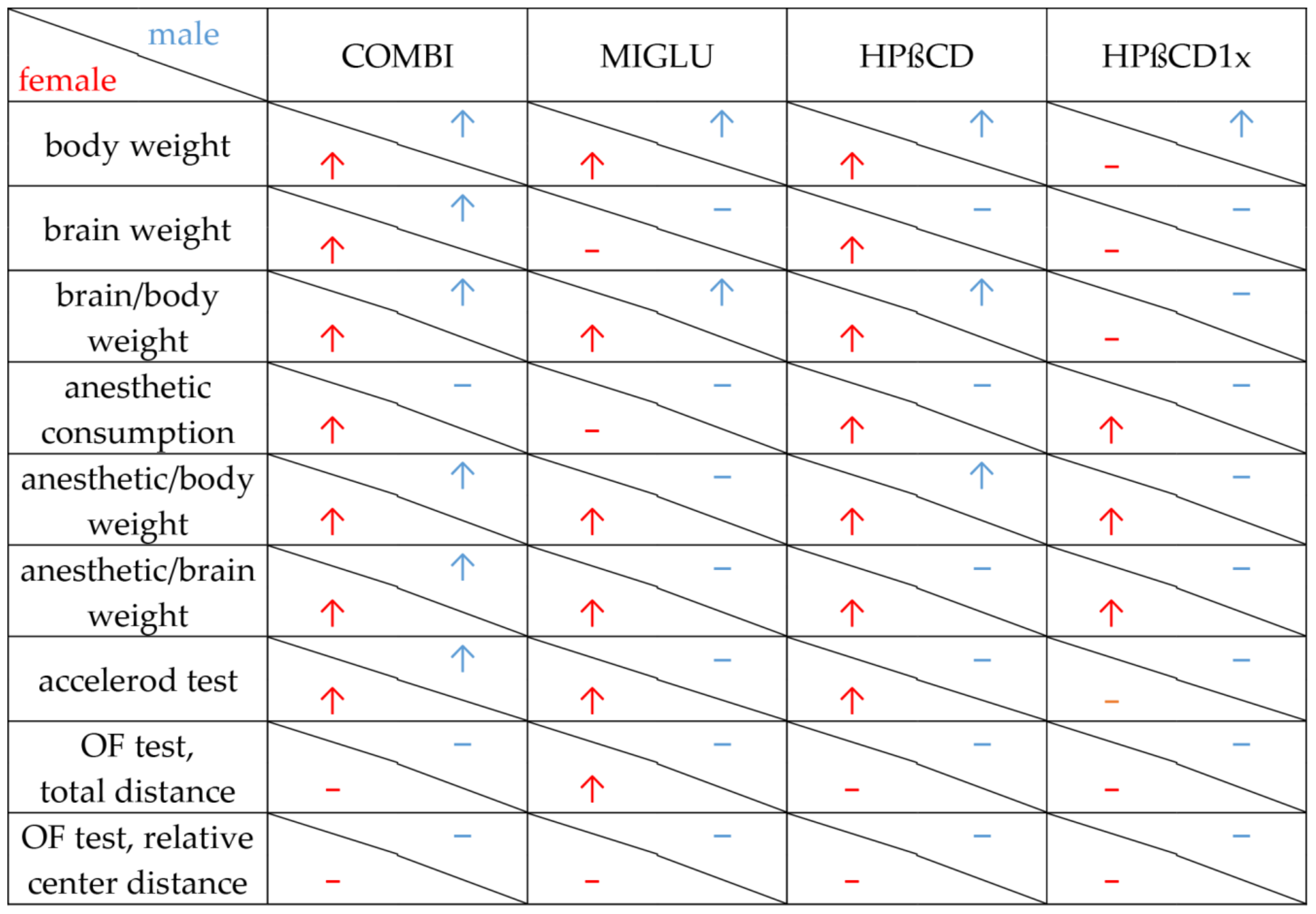
2. Results
2.1. Body Weight
2.2. Brain Weight
2.3. Brain Weight/Body Weight Ratio
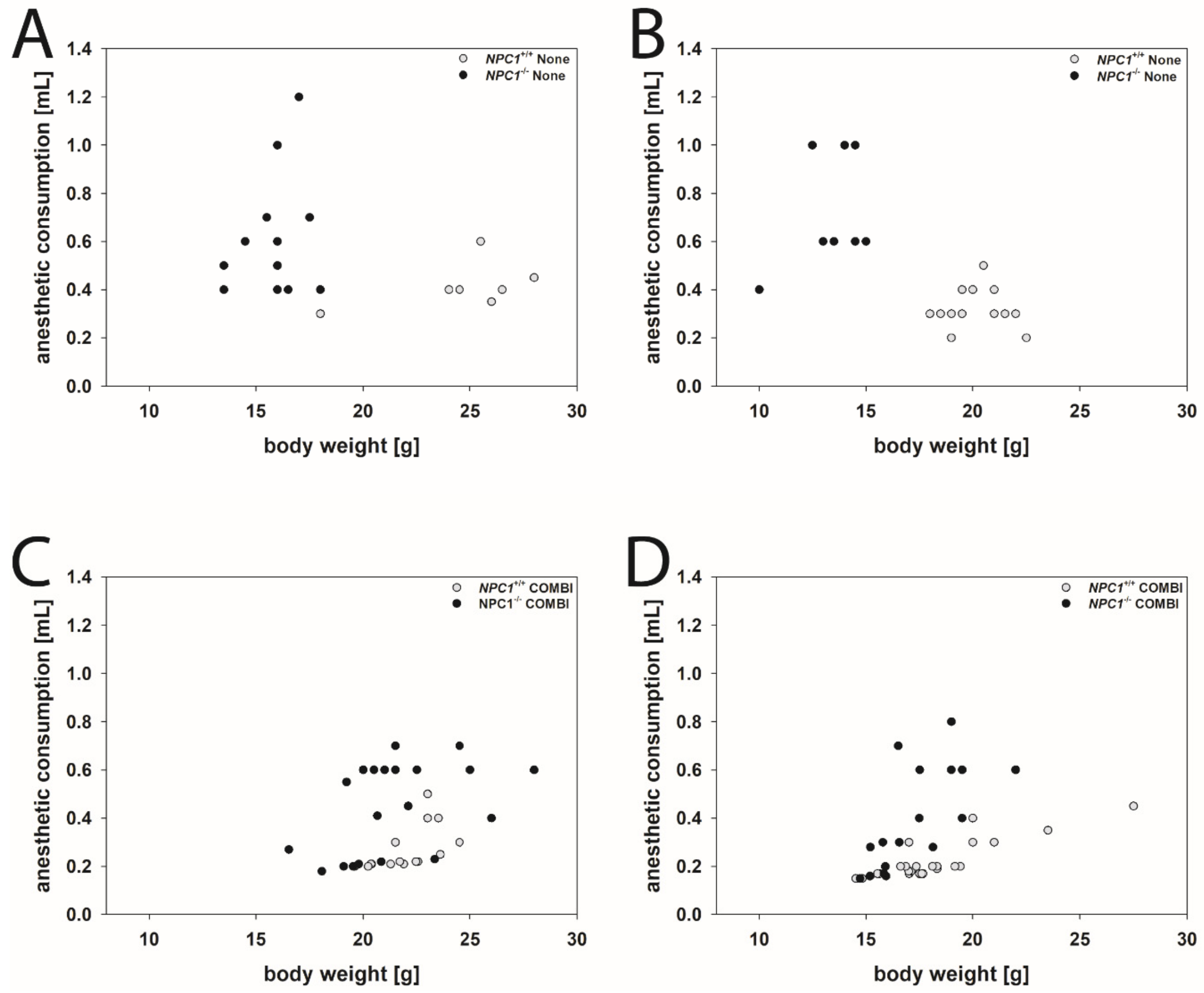
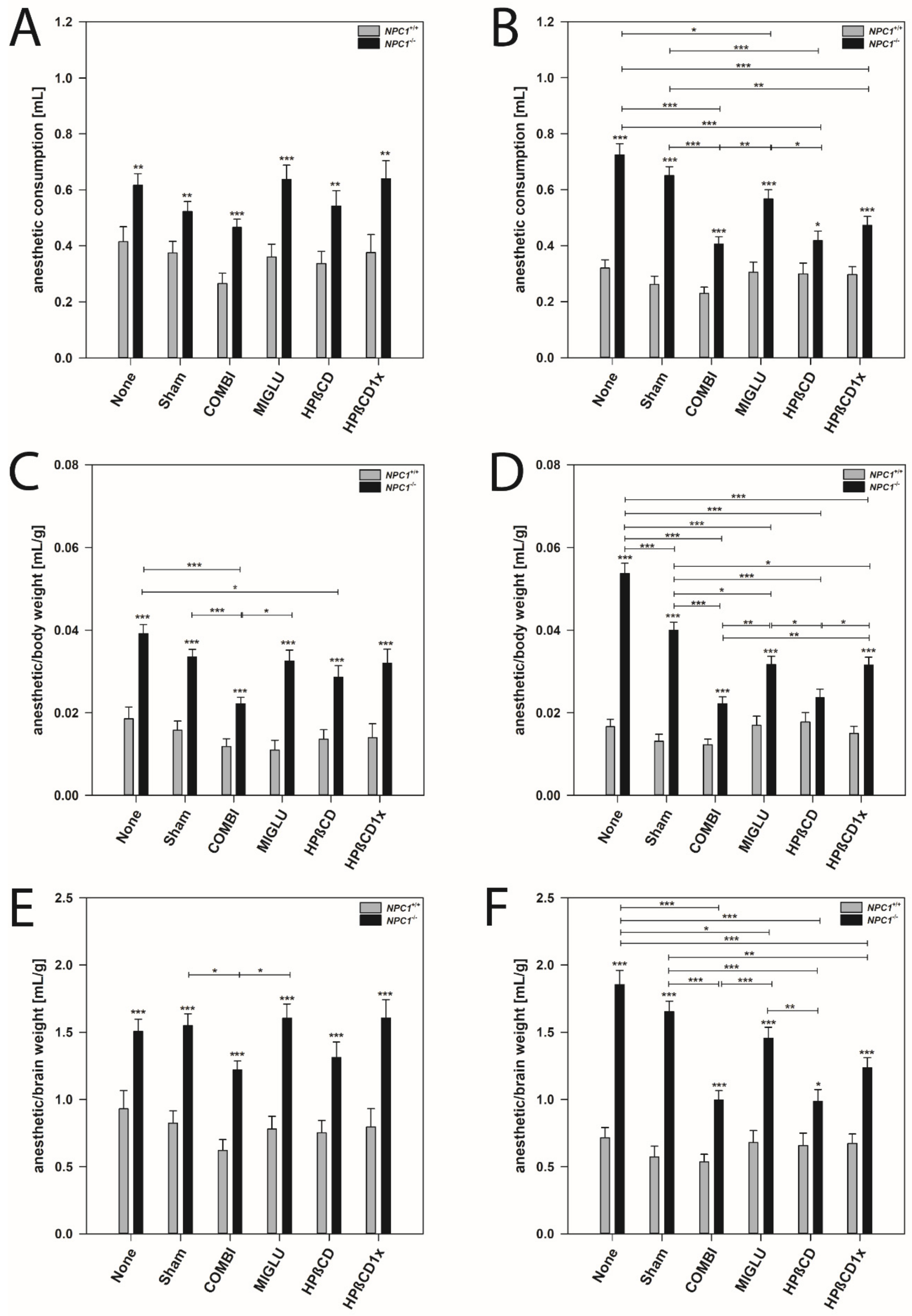
2.4. Anesthetic Consumption
2.5. Anesthetic Consumption Related to Body Weight
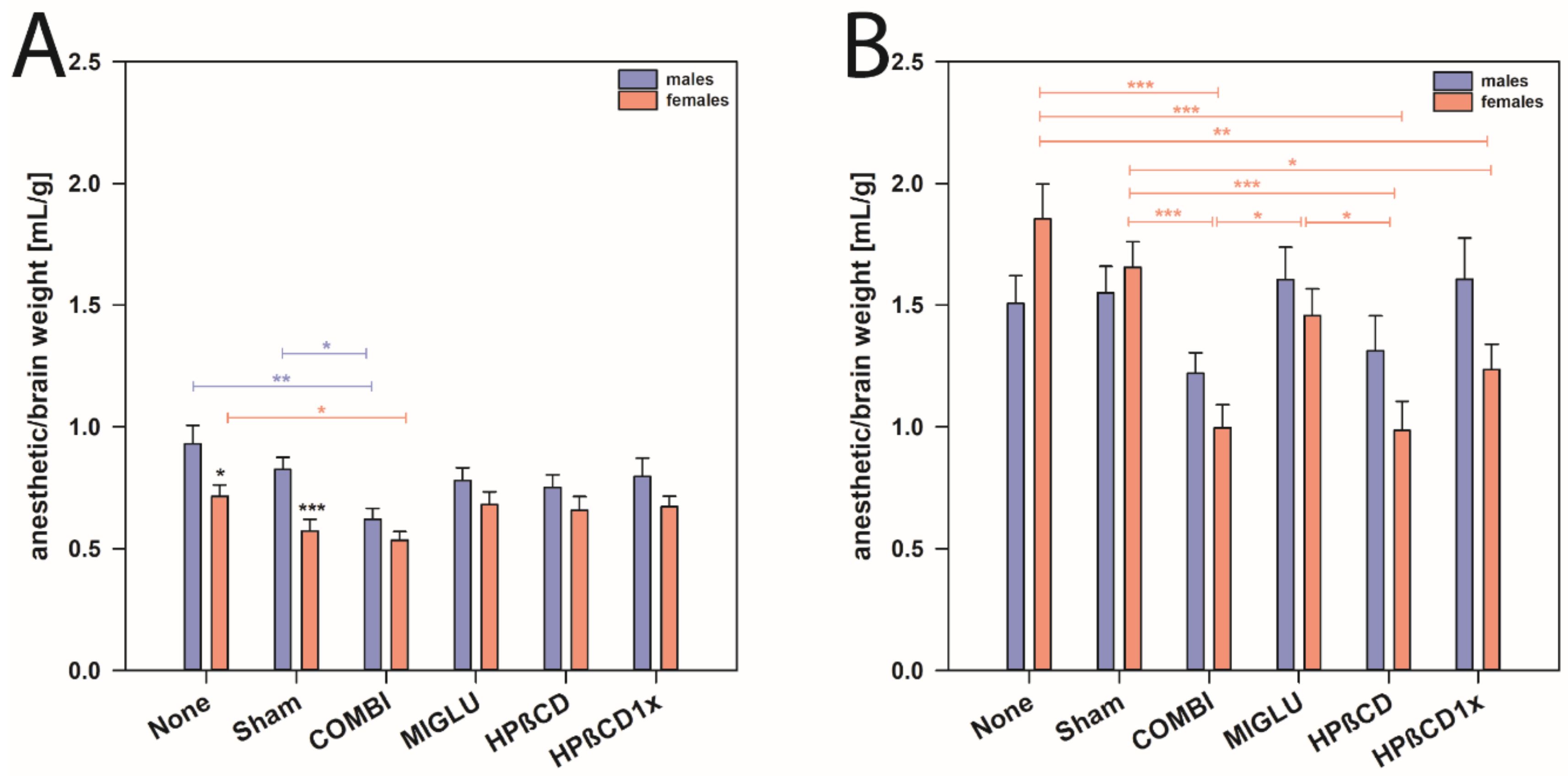
2.6. Anesthetic Consumption Related to Brain Weight
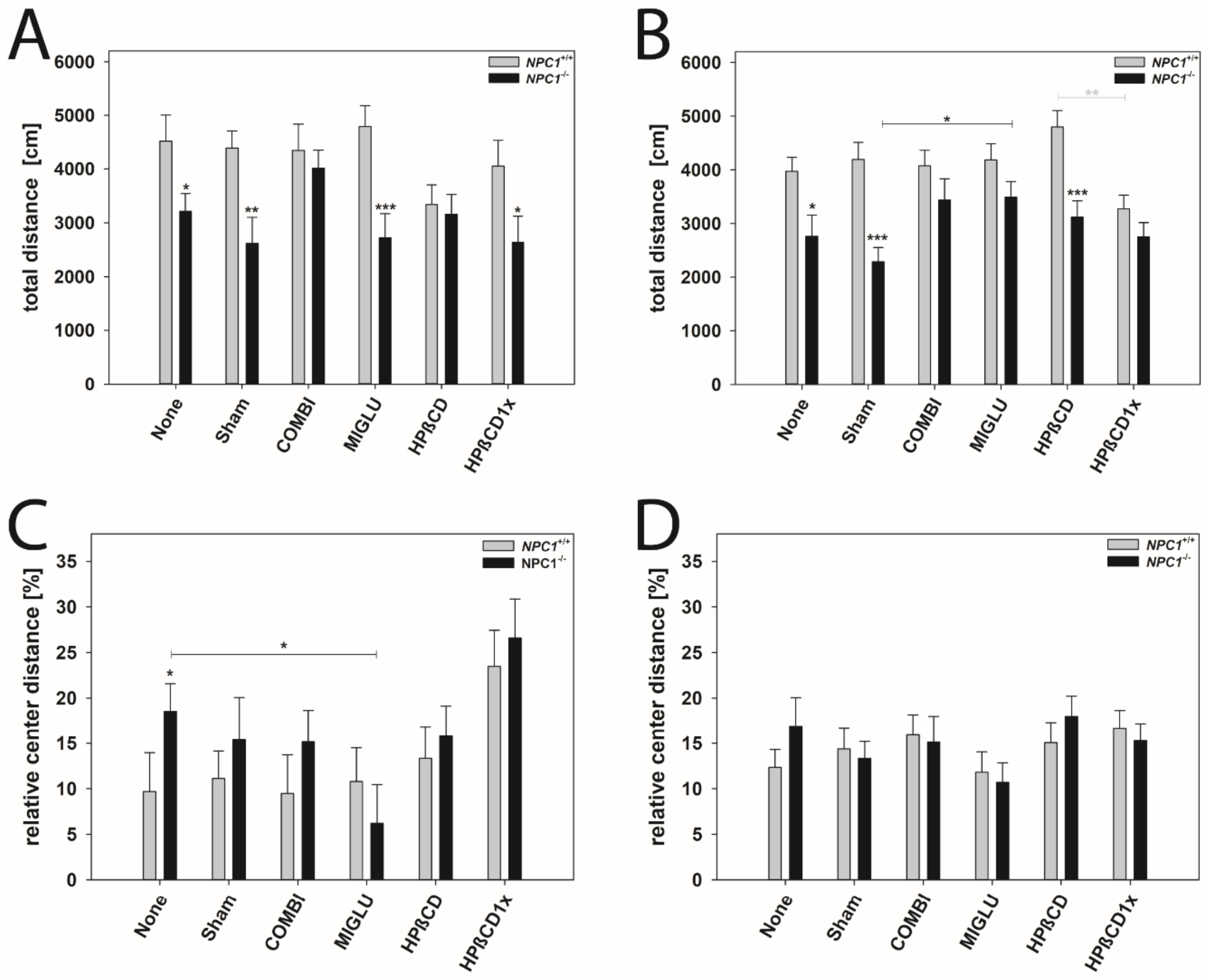
2.7. Accelerod Test
2.8. Open Field Test
3. Discussion
3.1. Both Medications Prevent Body Weight Loss, Preferably in Females
3.2. There Is No Drug-Related Effect on Brain Weights
3.3. Brain Weight/Body Weight Ratio
3.4. Female NPC1−/− Mice Need More Anesthetic Drugs Than Males
3.5. Accelerod Test—Motoric Coordination in NPC1−/− Mice Is Improved by All Drugs
3.6. Open Field Test—No Apparent Improvements upon Treatment
4. Materials and Methods
4.1. Animals
4.2. Treatment
4.3. Body Weight and Brain Weight
4.4. Anesthesia Consumption
4.5. Accelerod Test
4.6. Open Field Test
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALLO | Allopregnanolone |
| COMBI | Combination therapy |
| Cer | Ceramides |
| GABA | γ-aminobutyric acid |
| GCS | Glucosylceramide synthase |
| HPßCD | 2-hydroxypropyl-ß-cyclodextrin |
| LC | Lactosylceramides |
| MIGLU | Miglustat |
| MC | Monohexosyl ceramides |
| MDC | Monohexosyl dihydroceramides |
| NMDA | N-methyl-D-aspartate receptor |
| NPC1 | Niemann-Pick disease type C1 |
| Npc1 | NPC1 gene |
| NPC1 | NPC1 protein |
| OF | Open field test |
| OLs | Mature myelinatinggoligodendrocates |
| POMC | Pro-opiomelanocortin neurons |
| PSD95 | Postsynaptic density protein 95 |
| SREBP2 | Sterol regulatory element-binding protein-2 |
| S1P | Sphingosine-1-phosphate lipid |
References
- Cougnoux, A.; Cluzeau, C.; Mitra, S.; Li, R.; Williams, I.; Burkert, K.; Xu, X.; Wassif, C.A.; Zheng, W.; Porter, F.D.; et al. Necroptosis in niemann–pick disease, type C1: A potential therapeutic target. Cell Death Dis. 2016, 7, e2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garver, W.S.; Jelinek, D.; Meaney, F.J.; Flynn, J.; Pettit, K.M.; Shepherd, G.; Heidenreich, R.A.; Walsh Vockley, C.M.; Castro, G.; Francis, G.A.; et al. The national niemann-pick type c1 disease database: Correlation of lipid profi les, mutations, and biochemical phenotypes. J. Lipid Res. 2010, 51, 406–415. [Google Scholar] [CrossRef] [Green Version]
- Neufeld, E.B.; Wastney, M.; Patel, S.; Suresh, S.; Cooney, A.M.; Dwyer, N.K.; Roff, C.F.; Ohno, K.; Morris, J.A.; Carstea, E.D.; et al. The Niemann-Pick C1 protein resides in a vesicular compartment linked to retrograde transport of multiple lysosomal cargo. J. Biol. Chem. 1999, 274, 9627–9635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd-Evans, E.; Morgan, A.J.; He, X.; Smith, D.A.; Elliot-Smith, E.; Sillence, D.J.; Churchill, G.C.; Schuchman, E.H.; Galione, A.; Platt, F.M.; et al. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat. Med. 2008, 14, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Walkley, S.U.; Suzuki, K. Consequences of NPC1 and NPC2 loss of function in mammalian neurons. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2004, 1685, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Vanier, M.T. Niemann-Pick disease type C. Orphanet J. Rare Dis. 2010, 5, 16. [Google Scholar] [CrossRef] [Green Version]
- Maeda, Y.; Motoyama, K.; Nishiyama, R.; Higashi, T.; Onodera, R.; Nakamura, H.; Takeo, T.; Nakagata, N.; Yamada, Y.; Ishitsuka, Y.; et al. In vivo efficacy and safety evaluation of lactosyl-β-cyclodextrin as a therapeutic agent for hepatomegaly in Niemann-pick type C disease. Nanomaterials 2019, 9, 802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baguña Torres, J.; Yu, Z.; Bordoloi, J.; Sunassee, K.; Smith, D.; Smith, C.; Chen, O.; Purchase, R.; Tuschl, K.; Spencer, J.; et al. Imaging of changes in copper trafficking and redistribution in a mouse model of Niemann-Pick C disease using positron emission tomography. BioMetals 2019, 32, 293–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trilck, M.; Peter, F.; Zheng, C.; Frank, M.; Dobrenis, K.; Mascher, H.; Rolfs, A.; Frech, M.J. Diversity of glycosphingolipid GM2 and cholesterol accumulation in NPC1 patient-specific iPSC-derived neurons. Brain Res. 2017, 1657, 52–61. [Google Scholar] [CrossRef]
- Neßlauer, A.M.; Gläser, A.; Gräler, M.; Engelmann, R.; Müller-Hilke, B.; Frank, M.; Burstein, C.; Rolfs, A.; Neidhardt, J.; Wree, A.; et al. A therapy with miglustat, 2-hydroxypropyl-ß-cyclodextrin and allopregnanolone restores splenic cholesterol homeostasis in Niemann-pick disease type C1. Lipids Health Dis. 2019, 18. [Google Scholar] [CrossRef] [Green Version]
- Bonnot, O.; Klünemann, H.H.; Velten, C.; Torres Martin, J.V.; Walterfang, M. Systematic review of psychiatric signs in Niemann-Pick disease type C. World J. Biol. Psychiatry 2019, 20, 320–332. [Google Scholar] [CrossRef]
- Bonnot, O.; Gama, C.S.; Mengel, E.; Pineda, M.; Vanier, M.T.; Watson, L.; Watissée, M.; Schwierin, B.; Patterson, M.C. Psychiatric and neurological symptoms in patients with Niemann-Pick disease type C (NP-C): Findings from the International NPC Registry. World, J. Biol. Psychiatry 2019, 20, 310–319. [Google Scholar] [CrossRef]
- Patterson, M.C.; Clayton, P.; Gissen, P.; Anheim, M.; Bauer, P.; Bonnot, O.; Dardis, A.; Dionisi-Vici, C.; Klünemann, H.H.; Latour, P.; et al. Recommendations for the detection and diagnosis of Niemann-Pick disease type C: An update. Neurol. Clin. Pract. 2017, 7, 499–511. [Google Scholar] [CrossRef]
- Lo Castro, A.; Murdocca, M.; Pucci, S.; Zaratti, A.; Greggi, C.; Sangiuolo, F.; Tancredi, V.; Frank, C.; D’Arcangelo, G. Early hippocampal i-LTP and LOX-1 overexpression induced by anoxia: A potential role in neurodegeneration in NPC mouse model. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [Green Version]
- Shulman, L.M.; David, N.J.; Weiner, W.J. Psychosis as the initial manifestation of adult-onset niemann-pick disease type c. Neurology 1995, 45, 1739–1743. [Google Scholar] [CrossRef] [PubMed]
- Garver, W.S.; Francis, G.A.; Jelinek, D.; Shepherd, G.; Flynn, J.; Castro, G.; Vockley, C.W.; Coppock, D.L.; Pettit, K.M.; Heidenreich, R.A.; et al. The National Niemann-Pick C1 disease database: Report of clinical features and health problems. Am. J. Med. Genet. Part A 2007, 143, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Cluzeau, C.V.M.; Watkins-Chow, D.E.; Fu, R.; Borate, B.; Yanjanin, N.; Dail, M.K.; Davidson, C.D.; Walkley, S.U.; Ory, D.S.; Wassif, C.A.; et al. Microarray expression analysis and identification of serum biomarkers for niemann-pick disease, type c1. Hum. Mol. Genet. 2012, 21, 3632–3646. [Google Scholar] [CrossRef] [Green Version]
- Bonnot, O.; Klünemann, H.H.; Sedel, F.; Tordjman, S.; Cohen, D.; Walterfang, M. Diagnostic and treatment implications of psychosis secondary to treatable metabolic disorders in adults: A systematic review. Orphanet J. Rare Dis. 2014, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiegel, R.; Raas-Rothschild, A.; Reish, O.; Regev, M.; Meiner, V.; Bargal, R.; Sury, V.; Meir, K.; Nadjari, M.; Hermann, G.; et al. The clinical spectrum of fetal Niemann-Pick type C. Am. J. Med. Genet. Part A 2009, 149, 446–450. [Google Scholar] [CrossRef]
- Morris, M.D.; Bhuvaneswaran, C.; Shio, H.; Fowler, S. Lysosome lipid storage disorder in NCTR-BALB/c mice. I. Description of the disease and genetics. Am. J. Pathol. 1982, 108, 140–149. [Google Scholar] [PubMed]
- Loftus, S.K.; Erickson, R.P.; Walkley, S.U.; Bryant, M.A.; Incao, A.; Heidenreich, R.A.; Pavan, W.J. Rescue of neurodegeneration in Niemann-Pick C mice by a prion-promoter-driven Npc1 cDNA transgene. Hum. Mol. Genet. 2002, 11, 3107–3114. [Google Scholar] [CrossRef] [Green Version]
- Sarna, J.R.; Larouche, M.; Marzban, H.; Sillitoe, R.V.; Rancourt, D.E.; Hawkes, R. Patterned purkinje cell degeneration in mouse models of niemann-pick type C disease. J. Comp. Neurol. 2003, 456, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Ohara, S.; Ukita, Y.; Ninomiya, H.; Ohno, K. Degeneration of cholecystokinin-immunoreactive afferents to the VPL thalamus in a mouse model of Niemann-Pick disease type C. Brain Res. 2004, 1022, 244–246. [Google Scholar] [CrossRef]
- Luan, Z.; Saito, Y.; Miyata, H.; Ohama, E.; Ninomiya, H.; Ohno, K. Brainstem neuropathology in a mouse model of Niemann-Pick disease type C. J. Neurol. Sci. 2008, 268, 108–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Repa, J.J.; Valasek, M.A.; Beltroy, E.P.; Turley, S.D.; German, D.C.; Dietschy, J.M. Molecular, anatomical, and biochemical events associated with neurodegeneration in mice with Niemann-Pick type C disease. J. Neuropathol. Exp. Neurol. 2005, 64, 323–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Võikar, V.; Rauvala, H.; Ikonen, E. Cognitive deficit and development of motor impairment in a mouse model of Niemann-Pick type C disease. Behav. Brain Res. 2002, 132, 1–10. [Google Scholar] [CrossRef]
- Hovakimyan, M.; Meyer, A.; Lukas, J.; Luo, J.; Gudziol, V.; Hummel, T.; Rolfs, A.; Wree, A.; Witt, M. Olfactory deficits in Niemann-Pick type C1 (NPC1) disease. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- King, K.A.; Gordon-Salant, S.; Pawlowski, K.S.; Taylor, A.M.; Griffith, A.J.; Houser, A.; Kurima, K.; Wassif, C.A.; Wright, C.G.; Porter, F.D.; et al. Hearing loss is an early consequence of Npc1 gene deletion in the mouse model of Niemann-Pick disease, type C. JARO J. Assoc. Res. Otolaryngol. 2014, 15, 529–541. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Ma, L.; Hovakimyan, M.; Lukas, J.; Wree, A.; Frank, M.; Guthoff, R.; Rolfs, A.; Witt, M.; Luo, J. Defects in the retina of Niemann-pick type C 1 mutant mice. BMC Neurosci. 2014, 15. [Google Scholar] [CrossRef] [Green Version]
- Santos-Lozano, A.; Villamandos García, D.; Sanchis-Gomar, F.; Fiuza-Luces, C.; Pareja-Galeano, H.; Garatachea, N.; Nogales Gadea, G.; Lucia, A. Niemann-Pick disease treatment: A systematic review of clinical trials. Ann. Transl. Med. 2015, 3, 360. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Cai, X.; Wang, G.; Ouyang, G.; Cao, H. Model construction of Niemann-Pick type C disease in zebrafish. Biol. Chem. 2018, 399, 903–910. [Google Scholar] [CrossRef]
- Schlegel, V.; Thieme, M.; Holzmann, C.; Witt, M.; Grittner, U.; Rolfs, A.; Wree, A. Pharmacologic treatment assigned for Niemann Pick Type C1 disease partly changes behavioral traits in wild-type mice. Int. J. Mol. Sci. 2016, 17, 1866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, M.C.; Vecchio, D.; Prady, H.; Abel, L.; Wraith, J.E. Miglustat for treatment of Niemann-Pick C disease: A randomised controlled study. Lancet. Neurol. 2007, 6, 765–772. [Google Scholar] [CrossRef]
- Davidson, C.D.; Ali, N.F.; Micsenyi, M.C.; Stephney, G.; Renault, S.; Dobrenis, K.; Ory, D.S.; Vanier, M.T.; Walkley, S.U. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS ONE 2009, 4, e6951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pineda, M.; Walterfang, M.; Patterson, M.C. Miglustat in Niemann-Pick disease type C patients: A review. Orphanet J. Rare Dis. 2018, 13, 140. [Google Scholar] [CrossRef]
- Treiber, A.; Morand, O.; Clozel, M. The pharmacokinetics and tissue distribution of the glucosylceramide synthase inhibitor miglustat in the rat. Xenobiotica 2007, 37, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Platt, F.M.; Jeyakumar, M. Substrate reduction therapy. Acta Paediatr. 2008, 97, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Platt, F.M.; Neises, G.R.; Dwek, R.A.; Butters, T.D. N-butyldeoxynojirimycin is a novel inhibitor of glycolipid biosynthesis. J. Biol. Chem. 1994, 269, 8362–8365. [Google Scholar] [CrossRef]
- Zervas, M.; Somers, K.L.; Thrall, M.A.; Walkley, S.U. Critical role for glycosphingolipids in Niemann-Pick disease type C. Curr. Biol. 2001, 11, 1283–1287. [Google Scholar] [CrossRef] [Green Version]
- Jeyakumar, M.; Dwek, R.A.; Butters, T.D.; Platt, F.M. Storage solutions: Treating lysosomal disorders of the brain. Nat. Rev. Neurosci. 2005, 6, 713. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, R.H.; te Vruchte, D.; Lloyd-Evans, E.; Reinkensmeier, G.; Sillence, D.J.; Fernandez-Guillen, L.; Dwek, R.A.; Butters, T.D.; Cox, T.M.; Platt, F.M.; et al. Treatment with miglustat reverses the lipid-trafficking defect in Niemann–Pick disease type C. Neurobiol. Dis. 2004, 16, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Pineda, M.; Wraith, J.E.E.; Mengel, E.; Sedel, F.; Hwu, W.-L.L.; Rohrbach, M.; Bembi, B.; Walterfang, M.; Korenke, G.C.C.; Marquardt, T.; et al. Miglustat in patients with Niemann-Pick disease Type C (NP-C): A multicenter observational retrospective cohort study. Mol. Genet. Metab. 2009, 98, 243–249. [Google Scholar] [CrossRef]
- Cologna, S.M.; Jiang, X.S.; Backlund, P.S.; Cluzeau, C.V.M.; Dail, M.K.; Yanjanin, N.M.; Siebel, S.; Toth, C.L.; Jun, H.S.; Wassif, C.A.; et al. Quantitative proteomic analysis of niemann-pick disease, type C1 cerebellum identifies protein biomarkers and provides pathological insight. PLoS ONE 2012, 7, e47845. [Google Scholar] [CrossRef]
- Ebner, L.; Gläser, A.; Bräuer, A.; Witt, M.; Wree, A.; Rolfs, A.; Frank, M.; Vollmar, B.; Kuhla, A. Evaluation of two liver treatment strategies in a mouse model of niemann–pick-disease type C1. Int. J. Mol. Sci. 2018, 19, 972. [Google Scholar] [CrossRef] [Green Version]
- Elstein, D.; Hollak, C.; Aerts, J.M.F.G.; van Weely, S.; Maas, M.; Cox, T.M.; Lachmann, R.H.; Hrebicek, M.; Platt, F.M.; Butters, T.D.; et al. Sustained therapeutic effects of oral miglustat (Zavesca, N-butyldeoxynojirimycin, OGT 918) in type I Gaucher disease. J. Inherit. Metab. Dis. 2004, 27, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Pastores, G.M.; Barnett, N.L.; Kolodny, E.H. An open-label, noncomparative study of miglustat in type I Gaucher disease: Efficacy and tolerability over 24 months of treatment. Clin. Ther. 2005, 27, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Wraith, J.E.; Vecchio, D.; Jacklin, E.; Abel, L.; Chadha-Boreham, H.; Luzy, C.; Giorgino, R.; Patterson, M.C. Miglustat in adult and juvenile patients with Niemann–Pick disease type C: Long-term data from a clinical trial. Mol. Genet. Metab. 2010, 99, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Griffin, L.D.; Gong, W.; Verot, L.; Mellon, S.H. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat. Med. 2004, 10, 704–711. [Google Scholar] [CrossRef]
- Ahmad, I.; Lope-Piedrafita, S.; Bi, X.; Hicks, C.; Yao, Y.; Yu, C.; Chaitkin, E.; Howison, C.M.; Weberg, L.; Trouard, T.P.; et al. Allopregnanolone treatment, both as a single injection or repetitively, delays demyelination and enhances survival of Niemann-Pick C mice. J. Neurosci. Res. 2005, 82, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, H.; Repa, J.J.; Turley, S.D.; Dietschy, J.M. Genetic variations and treatments that affect the lifespan of the NPC1 mouse. J. Lipid Res. 2008, 49, 663–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Turley, S.D.; Burns, D.K.; Miller, A.M.; Repa, J.J.; Dietschy, J.M. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1-/- mouse. Proc. Natl. Acad. Sci. 2009, 106, 2377–2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Ramirez, C.M.; Miller, A.M.; Repa, J.J.; Turley, S.D.; Dietschy, J.M. Cyclodextrin overcomes the transport defect in nearly every organ of NPC1 mice leading to excretion of sequestered cholesterol as bile acid. J. Lipid Res. 2010, 51, 933–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Yamada, Y.; Ishitsuka, Y.; Matsuo, M.; Shiraishi, K.; Wada, K.; Uchio, Y.; Kondo, Y.; Takeo, T.; Nakagata, N.; et al. Efficacy of 2-hydroxypropyl-β-cyclodextrin in Niemann-Pick disease type C model mice and its pharmacokinetic analysis in a patient with the disease. Biol. Pharm. Bull. 2015, 38, 844–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, C.M.; Liu, B.; Taylor, A.M.; Repa, J.J.; Burns, D.K.; Weinberg, A.G.; Turley, S.D.; Dietschy, J.M. Weekly cyclodextrin administration normalizes cholesterol metabolism in nearly every organ of the Niemann-Pick type C1 mouse and markedly prolongs life. Pediatr. Res. 2010, 68, 309–315. [Google Scholar] [CrossRef] [Green Version]
- Muralidhar, A.; Borbon, I.A.; Esharif, D.M.; Ke, W.; Manacheril, R.; Daines, M.; Erickson, R.P. Pulmonary function and pathology in hydroxypropyl-beta-cyclodextin-treated and untreated Npc1-/- mice. Mol. Genet. Metab. 2011, 103, 142–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramirez, C.M.; Lopez, A.M.; Le, L.Q.; Posey, K.S.; Weinberg, A.G.; Turley, S.D. Ontogenic changes in lung cholesterol metabolism, lipid content, and histology in mice with Niemann-Pick type C disease. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2014, 1841, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Yancey, P.G.; Rodrigueza, W.V.; Kilsdonk, E.P.C.; Stoudt, G.W.; Johnson, W.J.; Phillips, M.C.; Rothblat, G.H. Cellular cholesterol efflux mediated by cyclodextrins: Demonstration of kinetic pools and mechanism of efflux. J. Biol. Chem. 1996, 271, 16026–16034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christian, A.E.; Byun, H.S.; Zhong, N.; Wanunu, M.; Marti, T.; Fürer, A.; Diederich, F.; Bittman, R.; Rothblat, G.H. Comparison of the capacity of β-cyclodextrin derivatives and cyclophanes to shuttle cholesterol between cells and serum lipoproteins. J. Lipid Res. 1999, 40, 1475–1482. [Google Scholar] [CrossRef]
- Atger, V.M.; De La Llera Moya, M.; Stoudt, G.W.; Rodrigueza, W.V.; Phillips, M.C.; Rothblat, G.H. Cyclodextrins as catalysts for the removal of cholesterol from macrophage foam cells. J. Clin. Invest. 1997, 99, 773–780. [Google Scholar] [CrossRef] [Green Version]
- Camargo, F.; Erickson, R.P.; Garver, W.S.; Hossain, G.S.; Carbone, P.N.; Heidenreich, R.A.; Blanchard, J. Cyclodextrins in the treatment of a mouse model of Niemann-Pick C disease. Life Sci. 2001, 70, 131–142. [Google Scholar] [CrossRef]
- Maass, F.; Petersen, J.; Hovakimyan, M.; Schmitt, O.; Witt, M.; Hawlitschka, A.; Lukas, J.; Rolfs, A.; Wree, A. Reduced cerebellar neurodegeneration after combined therapy with cyclodextrin/allopregnanolone and miglustat in NPC1: A mouse model of Niemann-Pick type C1 disease. J. Neurosci. Res. 2015, 93, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Hovakimyan, M.; Maass, F.; Petersen, J.; Holzmann, C.; Witt, M.; Lukas, J.; Frech, M.J.J.; Hübner, R.; Rolfs, A.; Wree, A.; et al. Combined therapy with cyclodextrin/allopregnanolone and miglustat improves motor but not cognitive functions in Niemann-Pick Type C1 mice. Neuroscience 2013, 252, 201–211. [Google Scholar] [CrossRef]
- Bräuer, A.U.A.U.; Kuhla, A.; Holzmann, C.; Wree, A.; Witt, M. Current challenges in understanding the cellular and molecular mechanisms in niemann-pick disease type C1. Int. J. Mol. Sci. 2019, 20, 4392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, A.; Wree, A.; Günther, R.; Holzmann, C.; Schmitt, O.; Rolfs, A.; Witt, M.; Günther, R.; Holzmann, C.; Schmitt, O.; et al. Increased regenerative capacity of the olfactory epithelium in Niemann-Pick disease type C1. Int. J. Mol. Sci. 2017, 18, 777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, C.D.; Fishman, Y.I.; Puskás, I.; Szemán, J.; Sohajda, T.; McCauliff, L.A.; Sikora, J.; Storch, J.; Vanier, M.T.; Szente, L.; et al. Efficacy and ototoxicity of different cyclodextrins in Niemann-Pick C disease. Ann. Clin. Transl. Neurol. 2016, 3, 366–380. [Google Scholar] [CrossRef]
- Xie, C.; Turley, S.D.; Dietschy, J.M. Centripetal cholesterol flow from the extrahepatic organs through the liver is normal in mice with mutated Niemann-Pick type C protein (NPC1). J. Lipid Res. 2000, 41, 1278–1289. [Google Scholar] [CrossRef]
- Witt, M.; Thiemer, R.; Meyer, A.; Schmitt, O.; Wree, A. Main olfactory and vomeronasal epithelium are differently affected in niemann-pick disease type C1. Int. J. Mol. Sci. 2018, 19, 3563. [Google Scholar] [CrossRef] [Green Version]
- Meyer, A.; Gläser, A.; Bräuer, A.U.; Wree, A.; Strotmann, J.; Rolfs, A.; Witt, M. Olfactory performance as an indicator for protective treatment effects in an animal model of neurodegeneration. Front. Integr. Neurosci. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Scantlebery, A.M.L.; Ochodnicky, P.; Kors, L.; Rampanelli, E.; Butter, L.M.; El Boumashouli, C.; Claessen, N.; Teske, G.J.; van den Bergh Weerman, M.A.; Leemans, J.C.; et al. β-Cyclodextrin counteracts obesity in Western diet-fed mice but elicits a nephrotoxic effect. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.R.; Klein, A.; Castro, J.; Cancino, G.I.; Amigo, J.; Mosqueira, M.; Vargas, L.M.; Yévenes, L.F.; Bronfman, F.C.; Zanlungo, S. Imatinib therapy blocks cerebellar apoptosis and improves neurological symptoms in a mouse model of Niemann-Pick type C disease. FASEB J. 2008, 22, 3617–3627. [Google Scholar] [CrossRef]
- Beltroy, E.P.; Richardson, J.A.; Horton, J.D.; Turley, S.D.; Dietschy, J.M. Cholesterol accumulation and liver cell death in mice with Niemann-Pick type C disease. Hepatology 2005, 42, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Vanier, M.; Millat, G. Niemann-Pick disease type C. Clin. Genet. 2003, 64, 269–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wraith, J.E.; Sedel, F.; Pineda, M.; Wijburg, F.A.; Hendriksz, C.J.; Fahey, M.; Walterfang, M.; Patterson, M.C.; Chadha-Boreham, H.; Kolb, S.A.; et al. Niemann-Pick type C Suspicion Index tool: Analyses by age and association of manifestations. J. Inherit. Metab. Dis. 2014, 37, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Stampfer, M.; Theiss, S.; Amraoui, Y.; Jiang, X.; Keller, S.; Ory, D.S.; Mengel, E.; Fischer, C.; Runz, H. Niemann-Pick disease type C clinical database: Cognitive and coordination deficits are early disease indicators. Orphanet J. Rare Dis. 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mengel, E.; Pineda, M.; Hendriksz, C.J.; Walterfang, M.; Torres, J.V.; Kolb, S.A. Differences in Niemann-Pick disease Type C symptomatology observed in patients of different ages. Mol. Genet. Metab. 2017, 120, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, S.E.; Hammond, D.I.; Farhat, N.Y.; Dang Do, A.; Jenkins, K.; Cougnoux, A.; Martin, K.; Porter, F.D. Evaluation of age of death in Niemann-Pick disease, type C: Utility of disease support group websites to understand natural history. Mol. Genet. Metab. 2019, 126, 466–469. [Google Scholar] [CrossRef]
- Walterfang, M.; Fietz, M.; Abel, L.; Bowman, E.; Mocellin, R.; Velakoulis, D. Gender dimorphism in siblings with schizophrenia-like psychosis due to Niemann-Pick disease type C. J. Inherit. Metab. Dis. 2009, 32. [Google Scholar] [CrossRef]
- Comerford, K.B.; Artiss, J.D.; Jen, K.-L.C.; Karakas, S.E. The Beneficial Effects α-Cyclodextrin on Blood Lipids and Weight Loss in Healthy Humans. Obesity 2011, 19, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Walterfang, M.; Chien, Y.H.; Imrie, J.; Rushton, D.; Schubiger, D.; Patterson, M.C. Dysphagia as a risk factor for mortality in Niemann-Pick disease type C: Systematic literature review and evidence from studies with miglustat. Orphanet J. Rare Dis. 2012, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flanagan, E.P.; Hunderfund, A.N.L.; Kumar, N.; Murray, J.A.; Krecke, K.N.; Katz, B.S.; Pittock, S.J. Clinical reasoning: A 55-year-old man with weight loss, ataxia, and foot drop. Neurology 2014, 82, e214–e219. [Google Scholar] [CrossRef] [Green Version]
- Patterson, M.C.; Mengel, E.; Wijburg, F.A.; Muller, A.; Schwierin, B.; Drevon, H.; Vanier, M.T.; Pineda, M. Disease and patient characteristics in NP-C patients: Findings from an international disease registry. Orphanet J. Rare Dis. 2013, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.E.; Karten, B. Niemann-Pick C disease and mobilization of lysosomal cholesterol by cyclodextrin. J. Lipid Res. 2014, 55, 1609–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diallo, A.; Jacobi, H.; Schmitz-Hübsch, T.; Cook, A.; Labrum, R.; Durr, A.; Brice, A.; Charles, P.; Marelli, C.; Mariotti, C.; et al. Body mass index decline is related to spinocerebellar ataxia disease progression. Mov. Disord. Clin. Pract. 2017, 4, 689–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rönnefarth, M.; Hanisch, N.; Brandt, A.U.; Mähler, A.; Endres, M.; Paul, F.; Doss, S. Dysphagia affecting quality of life in cerebellar ataxia—A large survey. Cerebellum 2020, 19, 437–445. [Google Scholar] [CrossRef] [Green Version]
- Kapur, R.; Donohue, C.; Jelinek, D.; Erickson, R.P. Amelioration of enteric neuropathology in a mouse model of Niemann-Pick C by Npc1 expression in enteric glia. J. Neurosci. Res. 2009, 87, 2994–3001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Arcangelo, G.; Grossi, D.; Racaniello, M.; Cardinale, A.; Zaratti, A.; Rufini, S.; Cutarelli, A.; Tancredi, V.; Merlo, D.; Frank, C.; et al. Miglustat reverts the impairment of synaptic plasticity in a mouse model of NPC disease. Neural Plast. 2016, 2016, 3830424. [Google Scholar] [CrossRef] [Green Version]
- Zervas, M.; Dobrenis, K.; Walkley, S.U. Neurons in Niemann-Pick disease type C accumulate gangliosides as well as unesterified cholesterol and undergo dendritic and axonal alterations. J. Neuropathol. Exp. Neurol. 2001, 60, 49–64. [Google Scholar] [CrossRef] [Green Version]
- Butters, T.D.; Van Den Broek, L.A.G.M.; Fleet, G.W.J.; Krulle, T.M.; Wormald, M.R.; Dwek, R.A.; Platt, F.M. Molecular requirements of imino sugars for the selective control of N-linked glycosylation and glycosphingolipid biosynthesis. Tetrahedron Asymmetry 2000, 11, 113–124. [Google Scholar] [CrossRef]
- Lloyd-Evans, E.; Pelled, D.; Riebeling, C.; Bodennec, J.; De-Morgan, A.; Waller, H.; Schiffmann, R.; Futerman, A.H. Glucosylceramide and glucosylsphingosine modulate calcium mobilization from brain microsomes via different mechanisms. J. Biol. Chem. 2003, 278, 23594–23599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd-Evans, E.; Platt, F.M. Lysosomal Ca2+ homeostasis: Role in pathogenesis of lysosomal storage diseases. Cell Calcium 2011, 50, 200–205. [Google Scholar] [CrossRef]
- Asarian, L.; Geary, N. Sex differences in the physiology of eating. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305. [Google Scholar] [CrossRef] [Green Version]
- Palmer, B.F.; Clegg, D.J. The sexual dimorphism of obesity. Mol. Cell. Endocrinol. 2015, 402, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; McClusky, R.; Chen, J.; Beaven, S.W.; Tontonoz, P.; Arnold, A.P.; Reue, K. The number of X chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiyama, M.G.; Agellon, L.B. Sex differences in lipid metabolism and metabolic disease risk. Biochem. Cell Biol. 2012, 90, 124–141. [Google Scholar] [CrossRef]
- Shi, H.; Seeley, R.J.; Clegg, D.J. Sexual differences in the control of energy homeostasis. Front. Neuroendocrinol. 2009, 30, 396–404. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; He, Y.; Xu, P.; Yang, Y.; Saito, K.; Xia, Y.; Yan, X.; Hinton, A.; Yan, C.; Ding, H.; et al. TAp63 contributes to sexual dimorphism in POMC neuron functions and energy homeostasis. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Akpovi, C.D.; Murphy, B.D.; Erickson, R.P.; Pelletier, R.M. Dysregulation of testicular cholesterol metabolism following spontaneous mutation of the niemann-pick C1 gene in mice. Biol. Reprod. 2014, 91, 42. [Google Scholar] [CrossRef]
- Roff, C.F.; Strauss, J.F.; Goldin, E.; Jaffe, H.; Patterson, M.C.; Agritellis, G.C.; Hibbs, A.M.; Garfield, M.; Brady, R.O.; Pentchev, P.G.; et al. The murine Niemann-Pick type C lesion affects testosterone production. Endocrinology 1993, 133, 2913–2923. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Richardson, J.A.; Turley, S.D.; Dietschy, J.M. Cholesterol substrate pools and steroid hormone levels are normal in the face of mutational inactivation of NPC1 protein. J. Lipid Res. 2006, 47, 953–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, R.P. Current controversies in Niemann-Pick C1 disease: Steroids or gangliosides; neurons or neurons and glia. J. Appl. Genet. 2013, 54, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, H.M.; Chen, Y.R.; Gu, X.S.; Duan, S. Decreased estradiol release from astrocytes contributes to the neurodegeneration in a mouse model of Niemann-Pick disease type C. Glia 2007, 55, 1509–1518. [Google Scholar] [CrossRef]
- Gévry, N.Y.; Lopes, F.L.; Ledoux, S.; Murphy, B.D. Aberrant intracellular cholesterol transport disrupts pituitary and ovarian function. Mol. Endocrinol. 2004, 18, 1778–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gläser, A.; Hammerl, F.; Gräler, M.H.; Coldewey, S.M.; Völkner, C.; Frech, M.J.; Yang, F.; Luo, J.; Tönnies, E.; Halbach, O.; et al. Identification of brain-specific treatment effects in NPC1 disease by focusing on cellular and molecular changes of sphingosine-1-phosphate metabolism. Int. J. Mol. Sci. 2020, 21, 4502. [Google Scholar] [CrossRef]
- Dupuis, N.; Fafouri, A.; Bayot, A.; Kumar, M.; Lecharpentier, T.; Ball, G.; Edwards, D.; Bernard, V.; Dournaud, P.; Drunat, S.; et al. Dymeclin deficiency causes postnatal microcephaly, hypomyelination and reticulum-to-Golgi trafficking defects in mice and humans. Hum. Mol. Genet. 2015, 24, 2771–2783. [Google Scholar] [CrossRef] [PubMed]
- Thakurela, S.; Garding, A.; Jung, R.B.; Müller, C.; Goebbels, S.; White, R.; Werner, H.B.; Tiwari, V.K. The transcriptome of mouse central nervous system myelin. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Roderick, T.H.; Wimer, R.E.; Wimer, C.C.; Schwartzkroin, P.A. Genetic and phenotypic variation in weight of brain and spinal cord between inbred strains of mice. Brain Res. 1973, 64, 345–353. [Google Scholar] [CrossRef]
- Tanaka, J.; Nakamura, H.; Mlyawaki, S. Cerebellar involvement in murine sphingomyelinosis: A new model of niemann-pick disease. J. Neuropathol. Exp. Neurol. 1988, 47, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.Y.; Kumar, U.; Switzer, R.C.; Sidhu, A.; Suresh, G.; Hu, C.Y.; Patel, S.C. Neurodegeneration in Niemann-Pick type C disease mice. Exp. Brain Res. 2001, 141, 218–231. [Google Scholar] [CrossRef]
- German, D.C.; Matthew Quintero, E.; Liang, C.L.; Ng, B.; Punia, S.; Xie, C.; Dietschy, J.M. Selective neurodegeneration, without neurofibrillary tangles, in a mouse model of Niemann-Pick C disease. J. Comp. Neurol. 2001, 433, 415–425. [Google Scholar] [CrossRef] [Green Version]
- Boyle, B.R.; Melli, S.E.; Altreche, R.S.; Padron, Z.M.; Yousufzai, F.A.K.; Kim, S.; Vasquez, M.D.; Carone, D.M.; Carone, B.R.; Soto, I. NPC1 deficiency impairs cerebellar postnatal development of microglia and climbing fiber refinement in a mouse model of Niemann-Pick disease type C. Development 2020, 147. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Murayama, S.; Pentchev, P.G.; Suzuki, K. Cerebellar degeneration in the Niemann-Pick type C mouse. Acta Neuropathol. 1993, 85, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Pentchev, P.; Vanier, M.; Suzuki, K.; Patterson, M. Niemann-Pick disease, type C: A cellular cholesterol lipidosis. In The Metabolic and Molecular Bases of Inherited Disease; Scriver, C., Beaudet, A., Sly, W., Valle, D., Eds.; McGraw-Hill: New York, NY, USA, 1995; pp. 2625–2640. [Google Scholar]
- Patel, S.; Barton, N.; Argoff, C. Niemann-Pick disease types A, C and D, Gaucher’s disease types I, II and III and Wolman’s disease.e. In Handbook of Clinical Neurology; de Jong, J., Ed.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 147–164. [Google Scholar]
- Takikita, S.; Fukuda, T.; Mohri, I.; Yagi, T.; Suzuki, K. Perturbed myelination process of premyelinating oligodendrocyte in Niemann-Pick type C mouse. J. Neuropathol. Exp. Neurol. 2004, 63, 660–673. [Google Scholar] [CrossRef] [Green Version]
- Qiao, L.; Yang, E.; Luo, J.; Lin, J.; Yan, X. Altered myelination in the niemann-pick type c1 mutant mouse. Histol. Histopathol. 2019, 33, 1311–1321. [Google Scholar] [CrossRef]
- De Nuccio, C.; Bernardo, A.; Ferrante, A.; Pepponi, R.; Martire, A.; Falchi, M.; Visentin, S.; Popoli, P.; Minghetti, L. Adenosine A2A receptor stimulation restores cell functions and differentiation in Niemann-Pick type C-like oligodendrocytes. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Guan, Y.; Feng, X.; Rolfs, A.; Schlüter, H.; Luo, J. Proteomics of the corpus callosum to identify novel factors involved in hypomyelinated Niemann-Pick Type C disease mice. Mol. Brain 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Lukas, J.; Witt, M.; Wree, A.; Hübner, R.; Frech, M.; Köhling, R.; Rolfs, A.; Luo, J. Decreased expression of myelin gene regulatory factor in Niemann-Pick type C 1 mouse. Metab. Brain Dis. 2011, 26, 299–306. [Google Scholar] [CrossRef]
- Yang, F.; Feng, X.; Rolfs, A.; Luo, J. Lovastatin promotes myelin formation in NPC1 mutant oligodendrocytes. J. Neurol. Sci. 2018, 386, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yang, F.; Lukas, J.; Witt, M.; Wree, A.; Rolfs, A.; Luo, J. Hyperactive glial cells contribute to axonal pathologies in the spinal cord of Npc1 mutant mice. Glia 2014, 62, 1024–1040. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Lieberman, A.P. Npc1 Acting in Neurons and Glia Is Essential for the Formation and Maintenance of CNS Myelin. PLoS Genet. 2013, 9. [Google Scholar] [CrossRef] [Green Version]
- Espahbodi, E.; Yaghooti, A.A.; Ostadalipour, A.; Marashi, S. Anesthetic management in a child with niemann-pick disease. Iran. J. Pediatr. 2016, 26, e5479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, N.; Lu, X.; O’Grady, N.P.; Yanjanin, N.; Porter, F.D.; Quezado, Z.M.N. Niemann-pick disease type C: Implications for sedation and anesthesia for diagnostic procedures. J. Child Neurol. 2012, 27, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.M. Ambulatory anaesthesia in a patient with Niemann-Pick Disease Type, C.J. Anesth. Clin. Res. 2015, 06, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Schilling, T.; Kozian, A.; Pfau, G.; Friedl, A.; Hachenberg, T. Anesthetic management of a patient with Niemann-Pick Type B disease undergoing cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2007, 21, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.S.; Bakkal, M.; Yeşiltaş, S. Dental treatment of two children with niemann pick disease type B under general anesthesia: Case reports. Bezmialem Sci. 2019, 7, 74–76. [Google Scholar] [CrossRef]
- Tikkanen, A.U.; Buxton, K.; Ullrich, C.K.; Stone, S.S.D.; Nimec, D.L. The palliative use of intrathecal baclofen in Niemann-Pick disease Type, C. Pediatrics 2019, 144. [Google Scholar] [CrossRef] [PubMed]
- Bujok, L.S.; Bujok, G.; Knapik, P. Niemann-Pick disease: A rare problem in anaesthesiological practice. Paediatr. Anaesth. 2002, 12, 806–808. [Google Scholar] [CrossRef]
- Grafft, C.A.; Fervenza, F.C.; Semret, M.H.; Orloff, S.; Sethi, S. Renal involvement in Neimann-Pick Disease. NDT Plus 2009, 2, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Murrough, J.W.; Iosifescu, D.V.; Chang, L.C.; Al Jurdi, R.K.; Green, C.E.; Perez, A.M.; Iqbal, S.; Pillemer, S.; Foulkes, A.; Shah, A.; et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: A two-site randomized controlled trial. Am. J. Psychiatry 2013, 170, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S. Stahl’s Essential Psychopharmacology, 4th ed.; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Aleksandrova, L.R.; Phillips, A.G.; Wang, Y.T. Antidepressant effects of ketamine and the roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. J. Psychiatry Neurosci. 2017, 42, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Hohlbaum, K.; Bert, B.; Dietze, S.; Palme, R.; Fink, H.; Thöne-Reineke, C. Impact of repeated anesthesia with ketamine and xylazine on the well-being of C57BL/6JRj mice. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gigliucci, V.; O’Dowd, G.; Casey, S.; Egan, D.; Gibney, S.; Harkin, A. Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology 2013, 228, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, H.; Yokoyama, C.; Mizuma, H.; Kurai, S.; Finnema, S.J.; Halldin, C.; Doi, H.; Onoe, H. A possible mechanism of the nucleus accumbens and ventral pallidum 5-HT1B receptors underlying the antidepressant action of ketamine: A PET study with macaques. Transl. Psychiatry 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Kokras, N.; Antoniou, K.; Polissidis, A.; Papadopoulou-Daifoti, Z. Antidepressants induce regionally discrete, sex-dependent changes in brain’s glutamate content. Neurosci. Lett. 2009, 464, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Pitychoutis, P.M.; Pallis, E.G.; Mikail, H.G.; Papadopoulou-Daifoti, Z. Individual differences in novelty-seeking predict differential responses to chronic antidepressant treatment through sex- and phenotype-dependent neurochemical signatures. Behav. Brain Res. 2011, 223, 154–168. [Google Scholar] [CrossRef]
- Thelen, C.; Sens, J.; Mauch, J.; Pandit, R.; Pitychoutis, P.M. Repeated ketamine treatment induces sex-specific behavioral and neurochemical effects in mice. Behav. Brain Res. 2016, 312, 305–312. [Google Scholar] [CrossRef]
- Nishitani, N.; Nagayasu, K.; Asaoka, N.; Yamashiro, M.; Shirakawa, H.; Nakagawa, T.; Kaneko, S. Raphe AMPA receptors and nicotinic acetylcholine receptors mediate ketamine-induced serotonin release in the rat prefrontal cortex. Int. J. Neuropsychopharmacol. 2014, 17, 1321–1326. [Google Scholar] [CrossRef] [Green Version]
- Winters, W.D.; Hance, A.J.; Cadd, G.C.; Lakin, M.L. Seasonal and sex influences on ketamine-induced analgesia and catalepsy in the rat; a possible role for melatonin. Neuropharmacology 1986, 25, 1095–1101. [Google Scholar] [CrossRef]
- Sarkar, A.; Kabbaj, M. Sex Differences in effects of ketamine on behavior, spine density, and synaptic proteins in socially isolated rats. Biol. Psychiatry 2016, 80, 448–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrier, N.; Kabbaj, M. Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology 2013, 70, 27–34. [Google Scholar] [CrossRef]
- Franceschelli, A.; Sens, J.; Herchick, S.; Thelen, C.; Pitychoutis, P.M. Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naïve and “depressed” mice exposed to chronic mild stress. Neuroscience 2015, 290, 49–60. [Google Scholar] [CrossRef] [Green Version]
- Karl, T.; Pabst, R.; von Hörsten, S. Behavioral phenotyping of mice in pharmacological and toxicological research. Exp. Toxicol. Pathol. Off. J. Gesellschaft Toxikologische Pathol. 2003, 55, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.J.; Roberts, D.J. The quantitative measurement of motor inco-ordination in naive naïve using an accelerating rotarod. J. Pharm. Pharmacol. 1968, 20, 1968. [Google Scholar] [CrossRef]
- Doulazmi, M.; Frédéric, F.; Lemaigre-Dubreuil, Y.; Hadj-Sahraoui, N.; Delhaye-Bouchaud, N.; Mariani, J. Cerebellar Purkinje cell loss during life span of the heterozygous staggerer mouse (Rora+/Rora(sg)) is gender-related. J. Comp. Neurol. 1999, 411, 267–273. [Google Scholar] [CrossRef]
- Ogura, H.; Matsumoto, M.; Mikoshiba, K. Motor discoordination in mutant mice heterozygous for the type 1 inositol 1,4,5-trisphosphate receptor. Behav. Brain Res. 2001, 122, 215–219. [Google Scholar] [CrossRef]
- Hadj-Sahraoui, N.; Frédéric, F.; Delhaye-Bouchaud, N.; Mariani, J. Gender effect on Purkinje cell loss in the cerebellum of the heterozygous Reeler mouse. J. Neurogenet. 1996, 11, 45–58. [Google Scholar] [CrossRef]
- Hall, C.; Ballachey, E.L. A study of the rat’s behavior in a field. A contribution to method in comparative psychology. Univ. Calif. Publ. Psychol. 1932, 6, 1–12. [Google Scholar]
- Hall, C. Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality. J. Comp. Psychol. 1934, 18, 385–403. [Google Scholar] [CrossRef]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Yadid, G.; Sotnik-Barkai, I.; Tornatore, C.; Baker-Cairns, B.; Harvey-White, J.; Pentchev, P.G.; Goldin, E. Neurochemical alterations in the cerebellum of a murine model of Niemann-Pick type C disease. Brain Res. 1998, 799, 250–256. [Google Scholar] [CrossRef]
- Clement, Y.; Chapouthier, G. Biological bases of anxiety. Neurosci. Biobehav. Rev. 1998, 22, 623–633. [Google Scholar] [CrossRef]
- Barlow, C.; Hirotsune, S.; Paylor, R.; Liyanage, M.; Eckhaus, M.; Collins, F.; Shiloh, Y.; Crawley, J.N.; Ried, T.; Tagle, D.; et al. Atm-deficient mice: A paradigm of ataxia telangiectasia. Cell 1996, 86, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Andringa, G.; Van Oosten, R.V.; Unger, W.; Hafmans, T.G.M.; Veening, J.; Stoof, J.C.; Cools, A.R. Systemic administration of the propargylamine CGP 3466B prevents behavioural and morphological deficits in rats with 6-hydroxydopamine-induced lesions in the substantia nigra. Eur. J. Neurosci. 2000, 12, 3033–3043. [Google Scholar] [CrossRef] [PubMed]
- Denenberg, V.H. Open-field bheavior in the rat: What does it mean? Ann. N. Y. Acad. Sci. 1969, 159, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.A.; Thurm, A.; Farhat, N.; Bianconi, S.; Keener, L.A.; Porter, F.D. Long-term neuropsychological outcomes from an open-label phase I/IIa trial of 2-hydroxypropyl-β-cyclodextrins (VTS-270) in Niemann-Pick disease, type C1. CNS Drugs 2019, 33, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Berry-Kravis, E.; Chin, J.; Hoffmann, A.; Winston, A.; Stoner, R.; LaGorio, L.; Friedmann, K.; Hernandez, M.; Ory, D.S.; Porter, F.D.; et al. Long-Term treatment of Niemann-Pick type C1 disease with intrathecal 2-hydroxypropyl-β-cyclodextrin. Pediatr. Neurol. 2018, 80, 24–34. [Google Scholar] [CrossRef]
- Open-label Study of VTS-270 in Participants With Neurologic Manifestations of Niemann-Pick Type C1-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03879655?term=niemann-pick&draw=3&rank=2 (accessed on 15 February 2021).
- VTS-270 to Treat Niemann-Pick Type C1 (NPC1) Disease-Full Text View-ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02534844 (accessed on 19 February 2021).
- Puskás, I. Cyclodextrin News. Available online: https://cyclodextrinnews.com/2018/11/13/hpbcd-treatment-for-niemann-pick-type-c-performed-no-differently-than-placebo/ (accessed on 19 February 2021).




| Parameter | Male | Female | ||
|---|---|---|---|---|
| NPC1+/+ | NPC1−/− | NPC1+/+ | NPC1−/− | |
| body weight [g] | 24.643 | 15.833 AAA | 20.067 CCC | 13.375 BBB D |
| ±0.876 | ±0.669 | ±0.614 | ±0.840 | |
| brain weight [g] | 0.474 | 0.385 AAA | 0.463 | 0.370 BBB |
| ±0.009 | ±0.006 | ±0.006 | ±0.008 | |
| brain/body weight [%] | 1.812 | 2.434 AAA | 2.325 CCC | 2.816 BBB DD |
| ±0.117 | ±0.079 | ±0.065 | ±0.088 | |
| anesthetic consumption [mL] | 0.414 | 0.617 AA | 0.320 CC | 0.725 BBB |
| ±0.055 | ±0.042 | ±0.030 | ±0.040 | |
| anesthetic/body weight [mL/g] | 0.019 | 0.039 AAA | 0.017 | 0.054 BBB DDD |
| ±0.003 | ±0.002 | ±0.002 | ±0.002 | |
| anesthetic/brain weight [mL/g] | 0.930 | 1.506 AAA | 0.714 C | 1.855 BBB |
| ±0.136 | ±0.092 | ±0.077 | ±0.105 | |
| accelerod test [rpm] | 16.563 | 9.615 AA | 19.214 | 10.982 BBB |
| ±1.821 | ±1.345 | ±1.192 | ±1.821 | |
| open field test, | 4519.48 | 3214.20 A | 3974.49 | 2762.27 B |
| total distance [cm] | ±478.04 | ±328.36 | ±258.00 | ±394.11 |
| open field test, | 8.20 | 17.80 A | 12.80 | 18,70 |
| relative center distance [%] | ±3.21 | ±2.16 | ±2.23 | ±3.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holzmann, C.; Witt, M.; Rolfs, A.; Antipova, V.; Wree, A. Gender-Specific Effects of Two Treatment Strategies in a Mouse Model of Niemann-Pick Disease Type C1. Int. J. Mol. Sci. 2021, 22, 2539. https://doi.org/10.3390/ijms22052539
Holzmann C, Witt M, Rolfs A, Antipova V, Wree A. Gender-Specific Effects of Two Treatment Strategies in a Mouse Model of Niemann-Pick Disease Type C1. International Journal of Molecular Sciences. 2021; 22(5):2539. https://doi.org/10.3390/ijms22052539
Chicago/Turabian StyleHolzmann, Carsten, Martin Witt, Arndt Rolfs, Veronica Antipova, and Andreas Wree. 2021. "Gender-Specific Effects of Two Treatment Strategies in a Mouse Model of Niemann-Pick Disease Type C1" International Journal of Molecular Sciences 22, no. 5: 2539. https://doi.org/10.3390/ijms22052539







