A Smart Hyperthermia Nanofiber-Platform-Enabled Sustained Release of Doxorubicin and 17AAG for Synergistic Cancer Therapy
Abstract
:1. Introduction
2. Results and Discussion
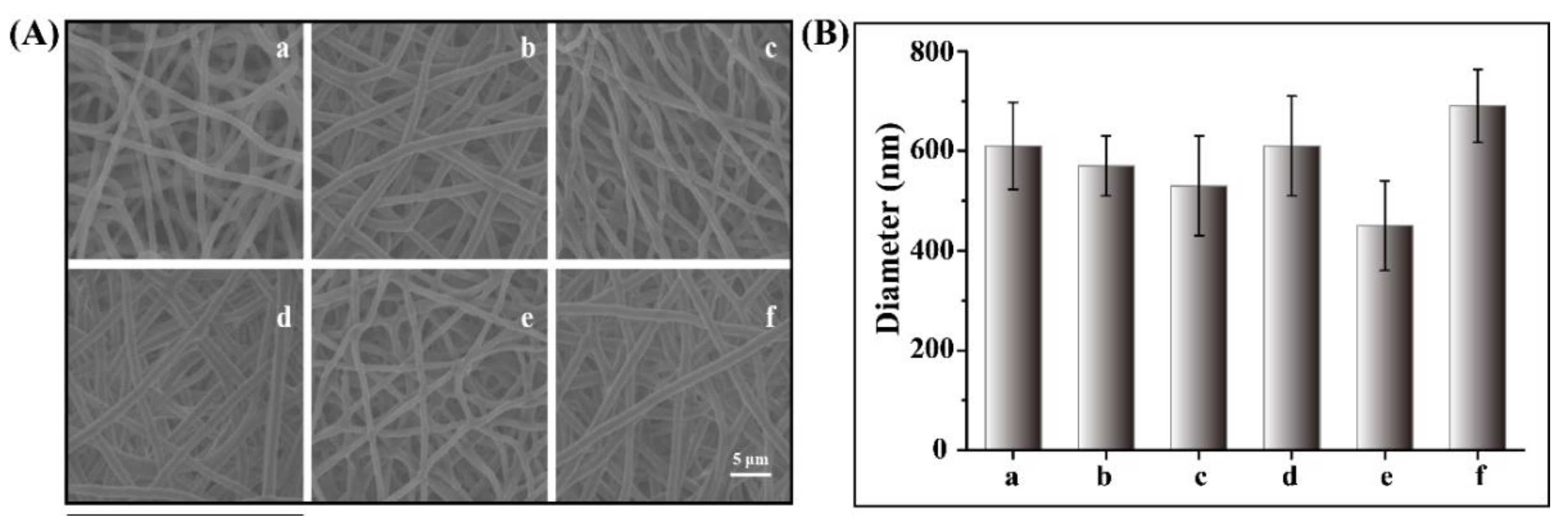
2.1. Fabrication of Nanofiber Meshes
2.2. Heating of MNPs within Nanofiber Meshes
2.3. DOX and 17AAG Release Behavior In Vitro
2.4. Effect of Heat on the Efficiency of DOX and 17AAG
2.5. Synergistic Anticancer Effects
3. Materials and Methods
3.1. Materials
3.2. Fabrication and Characterization of Nanofiber Mesh
3.3. Heating Profiles for Nanofiber Mesh
3.4. In Vitro Drug Release
3.5. Evaluation of DOX and 17AAG Efficacy after Heating
3.6. Antitumor Efficacy
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vargo-Gogola, T.; Rosen, J.M. Modelling breast cancer: One size does not fit all. Nat. Rev. Cancer 2007, 7, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Fernández, L.M. Use of Statistics to Assess the Global Burden of Breast Cancer. Glob. Epidemiol. Methods 2006, 12, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Rahul, J.; Cody, S.; Prakash, R. Nanoparticles for Effective Combination Therapy of Cancer. Int. J. Nanotechnol. Nanomed 2016, 1, 1–15. [Google Scholar]
- Brero, F.; Albino, M.; Antoccia, A.; Arosio, P.; Avolio, M.; Berardinelli, F.; Bettega, D.; Calzolari, P.; Ciocca, M.; Corti, M.; et al. Hadron Therapy, Magnetic Nanoparticles and Hyperthermia: A Promising Combined Tool for Pancreatic Cancer Treatment. Nanomaterials 2020, 10, 1919. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Sarkar, S.; Hashemi, M.; Saber, R. Treatment of Breast Cancer-Bearing BALB/c Mice with Magnetic Hyperthermia using Dendrimer Functionalized Iron-Oxide Nanoparticles. Nanomaterials 2020, 10, 2310. [Google Scholar] [CrossRef]
- Nemec, S.; Kralj, S.; Wilhelm, C.; Abou-Hassan, A.; Rols, M.-P.; Kolosnjaj-Tabi, J. Comparison of Iron Oxide Nanoparticles in Photothermia and Magnetic Hyperthermia: Effects of Clustering and Silica Encapsulation on Nanoparticles’ Heating Yield. Appl. Sci. 2020, 10, 7322. [Google Scholar] [CrossRef]
- Nashiry, M. Combination of Metformin and severe hyperthermia induces DNA damage and apoptosis in osteosarcoma cells in vitro. In The 2015 Annual Scientific Meeting of the College of Pathologists; Berjaya Time Square Hotel: Kuala Lumpur, Malaysia, 2018. [Google Scholar]
- Norouzi, M.; Abdali, Z.; Liu, S.; Miller, D.W. Salinomycin-loaded Nanofibers for Glioblastoma Therapy. Sci. Rep. 2018, 8, 9377–9386. [Google Scholar] [CrossRef] [Green Version]
- Xing, H.; Wang, Z.; Shao, D.; Chang, Z.; Ge, M.; Li, L.; Wu, M.; Yan, Z.; Dong, W. Janus nanocarriers for magnetically targeted and hyperthermia-enhanced curcumin therapy of liver cancer. RSC Adv. 2018, 8, 30448–30454. [Google Scholar] [CrossRef] [Green Version]
- Niiyama, E.; Uto, K.; Lee, C.M.; Sakura, K.; Ebara, M. Hyperthermia Nanofiber Platform Synergized by Sustained Release of Paclitaxel to Improve Antitumor Efficiency. Adv. Healthc. Mater. 2019, 8, 1900102–1900111. [Google Scholar] [CrossRef]
- Niiyama, E.; Uto, K.; Lee, C.; Sakura, K.; Ebara, M. Alternating Magnetic Field-Triggered Switchable Nanofiber Mesh for Cancer Thermo-Chemotherapy. Polymers 2018, 10, 1018. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, F.; Yusof, N.A. Doxorubicin-loaded magnetic gold nanoshells for a combination therapy of hyperthermia and drug delivery. J. Colloid Interface Sci. 2014, 4, 89–97. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Ebara, M.; Aoyagi, T. A Smart Hyperthermia Nanofiber with Switchable Drug Release for Inducing Cancer Apoptosis. Adv. Funct. Mater. 2013, 23, 5753–5761. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Y.; Ren, Z.; Li, X.; Mao, C.; Han, G. Enhanced cell uptake of fluorescent drug-loaded nanoparticles via an implantable photothermal fibrous patch for more effective cancer cell killing. J. Mater. Chem. B 2017, 5, 7504–7511. [Google Scholar] [CrossRef]
- Dev Kumar Chatterjee1, P.D.; Krishnan, S. Nanoparticle-mediated hyperthermia in cancer therapy. Ther. Deliv. 2011, 2, 1001–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, J.W.; Sarkar, S.; Buchanan, C.F.; Szot, C.S.; Whitney, J.; Hatcher, H.C.; Torti, S.V.; Rylander, C.G.; Rylander, M.N. Photothermal Response of Human and Murine Cancer Cells to Multiwalled Carbon Nanotubes after Laser Irradiation. Cancer Res. 2010, 70, 9855–9864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, S.; Fujita, N.; Tsuruo, T. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl. Acad. Sci. USA 2000, 97, 10832–10837. [Google Scholar] [CrossRef] [Green Version]
- Basso, A.D.; Solit, D.B.; Munster, P.N. Ansamycin antibiotics inhibit Akt activation and cyclin D expression in breast cancer cells that overexpress HER2. Oncogene 2002, 21, 1159–1166. [Google Scholar] [CrossRef] [Green Version]
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 2005, 5, 761–772. [Google Scholar] [CrossRef]
- Yoo, D.; Jeong, H.; Noh, S.-H.; Lee, J.-H.; Cheon, J. Magnetically Triggered Dual Functional Nanoparticles for Resistance-Free Apoptotic Hyperthermia. Angew. Chem. Int. Ed. 2013, 52, 13047–13051. [Google Scholar] [CrossRef]
- Jhaveri, K.; Taldone, T.; Modi, S.; Chiosis, G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2012, 1823, 742–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frazier, N.; Payne, A.; Dillon, C.; Subrahmanyam, N.; Ghandehari, H. Enhanced efficacy of combination heat shock targeted polymer therapeutics with high intensity focused ultrasound. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1235–1243. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Youn, P.; Pysher, T.J.; Scaife, C.L.; Furgeson, D.Y. Tumour eradication using synchronous thermal ablation and Hsp90 chemotherapy with protein engineered triblock biopolymer-geldanamycin conjugates. Int. J. Hyperth. 2014, 30, 550–564. [Google Scholar] [CrossRef]
- Wang, L.; Gao, C.; Liu, K.; Liu, Y.; Ma, L.; Liu, L.; Du, X.; Zhou, J. Cypate-Conjugated Porous Upconversion Nanocomposites for Programmed Delivery of Heat Shock Protein 70 Small Interfering RNA for Gene Silencing and Photothermal Ablation. Adv. Funct. Mate. 2016, 26, 3480–3489. [Google Scholar] [CrossRef]
- Kamal, A.; Thao, L.; Sensintaffar, J. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 2003, 425, 407–410. [Google Scholar] [CrossRef]
- Ding, Q.; Li, Z.; Yang, Y.; Guo, G.; Luo, F.; Chen, Z.; Yang, Y.; Qian, Z.; Shi, S. Preparation and therapeutic application of docetaxel-loaded poly(d,l-lactide) nanofibers in preventing breast cancer recurrence. Drug Deliv. 2015, 23, 2677–2685. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.; Mun, G.-H.; Choi, B.; Aseh, A.; Mildred, L.; Patel, A.; Zhang, Q.; Price, J.E.; Chang, D.; Robb, G.; et al. Repair and Reconstruction of a Resected Tumor Defect Using a Composite of Tissue Flap–Nanotherapeutic–Silk Fibroin and Chitosan Scaffold. Ann. Biomed. Eng. 2011, 39, 2374–2387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yohe, S.T.; Herrera, V.L.M.; Colson, Y.L.; Grinstaff, M.W. 3D superhydrophobic electrospun meshes as reinforcement materials for sustained local drug delivery against colorectal cancer cells. J. Controll. Release 2012, 162, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, D.; Chen, P.; Yang, Y.; Li, Q.; Wan, W.; Fang, X.; Zhang, J.; Han, Z.; Tian, J.; Ouyang, J. Fabrication of Gelatin/PCL Electrospun Fiber Mat with Bone Powder and the Study of Its Biocompatibility. J. Funct. Biomater. 2016, 7, 6. [Google Scholar] [CrossRef]
- Ban, Q.; Bai, T.; Duan, X.; Kong, J. Noninvasive photothermal cancer therapy nanoplatforms via integrating nanomaterials and functional polymers. Biomater. Sci. 2017, 5, 190–210. [Google Scholar] [CrossRef] [PubMed]
- Azfarniam, L.; Norouzi, M. Multifunctional polyester fabric using a multicomponent treatment. Fibers Polym. 2016, 17, 298–304. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Ebara, M.; Aoyagi, T. Temperature-responsive electrospun nanofibers for ‘on–off’ switchable release of dextran. Sci. Technol. Adv. Mater. 2016, 13, 64203–64213. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-J.; Ebara, M.; Aoyagi, T. A Smart Nanofiber Web That Captures and Releases Cells. Angew. Chem. Int. Ed. 2012, 51, 10537–10541. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Niiyama, E.; Uto, K.; Aoyagi, T.; Ebara, M. Inactivated Sendai Virus (HVJ-E) Immobilized Electrospun Nanofiber for Cancer Therapy. Materials 2015, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrett, R.; Niiyama, E.; Kotsuchibashi, Y.; Uto, K.; Ebara, M. Biodegradable Nanofiber for Delivery of Immunomodulating Agent in the Treatment of Basal Cell Carcinoma. Fibers 2015, 3, 478. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K.; Tanaka, H.; Ebara, M.; Uto, K.; Matsuoka, H.; Nishimoto, S.; Okada, K.; Murase, T.; Yoshikawa, H. Electrospun nanofiber sheets incorporating methylcobalamin promote nerve regeneration and functional recovery in a rat sciatic nerve crush injury model. Acta Biomater. 2017, 5, 250–259. [Google Scholar] [CrossRef]
- Furlani, E.J.; Furlani, E.P. A model for predicting magnetic targeting of multifunctional particles in the microvasculature. J. Magn. Magn. Mater. 2007, 312, 187–193. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.S. Monodisperse Magnetic Nanoparticles for Theranostic Applications. Acc. Chem. Res. 2011, 44, 875–882. [Google Scholar]
- Barry, S.E. Challenges in the development of magnetic particles for therapeutic applications. Int. J. Hyperth. 2009, 24, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Karathanasis, E.; Cervadoro, A.; Giverso, C.; Pande, R.; Sarangi, S.; Preziosi, L.; Wosik, J.; Brazdeikis, A.; Decuzzi, P. Design Maps for the Hyperthermic Treatment of Tumors with Superparamagnetic Nanoparticles. PLoS ONE 2013, 8, 57332–57346. [Google Scholar]
- Hayashi, K.; Sakamoto, W.; Yogo, T. Smart Ferrofluid with Quick Gel Transformation in Tumors for MRI-Guided Local Magnetic Thermochemotherapy. Adv. Funct. Mater. 2016, 26, 1708–1718. [Google Scholar] [CrossRef]
- Wu, K.; Wang, J.P. Magnetic hyperthermia performance of magnetite nanoparticle assemblies under different driving fields. AIP Adv. 2017, 7, 56327–56336. [Google Scholar] [CrossRef] [Green Version]
- Mamiya, H.; Takeda, Y.; Naka, T.; Kawazoe, N.; Chen, G.; Jeyadevan, B. Practical Solution for Effective Whole-Body Magnetic Fluid Hyperthermia Treatment. J. Nanomater. 2017, 20, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Xia, Q.; Li, L.; Zhao, L. Silica nanoparticle-based dual-responsive nanoprodrug system for liver cancer therapy. Exp. Ther. Med. 2017, 14, 2071–2077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, K.; Chen, S.; Chen, W.-H.; Lei, Q.; Liu, Y.; Zhuo, R.-X.; Zhang, X.-Z. Synergistic gene and drug tumor therapy using a chimeric peptide. Biomaterials 2013, 34, 4680–4689. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Wang, Q.; Deng, Y.; Yang, T.; Ke, H.; Yang, H.; He, H.; Guo, Z.; Yu, D.; Wu, H.; et al. Mutually Synergistic Nanoparticles for Effective Thermo-Molecularly Targeted Therapy. Adv. Funct. Mater. 2017, 27, 1702834–1702844. [Google Scholar] [CrossRef]
- Li, M.; Bu, W.; Ren, J.; Li, J.; Deng, L.; Gao, M.; Gao, X.; Wang, P. Enhanced Synergism of Thermo-chemotherapy For Liver Cancer with Magnetothermally Responsive Nanocarriers. Theranostics 2018, 8, 693–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, X.P.; Tmai, T.T.; Ha, P.T.; Pham, H.N.; Luu, H.N.; Do, H.M.; Tran, D.L.; Nguyen, H.N.; Nguyen, L.T.; Ho, A.S.; et al. Multifunctional Drug Nanosystems: A Summary of Recent Researches at IMS/VAST. Biomed. Eng. 2015, 4, 55–57. [Google Scholar]
- Lin, T.; Guo, W.; Long, Q.; Ma, A.; Liu, Q.; Zhang, H.; Huang, Y.; Chandrasekaran, S.; Pan, C.; Lam, K.S.; et al. HSP90 Inhibitor Encapsulated Photo-Theranostic Nanoparticles for Synergistic Combination Cancer Therapy. Theranostics 2016, 6, 1324–1335. [Google Scholar] [CrossRef]
- Partanen, A.; Yarmolenko, P.S.; Viitala, A. Mild hyperthermia with magnetic resonance-guided high-intensity focused ultrasound for applications in drug delivery. Int. J. Hyperth. 2012, 28, 320–336. [Google Scholar] [CrossRef]
- Jain, T.K.; Richey, J.; Strand, M.; Leslie-Pelecky, D.L.; Flask, C.A.; Labhasetwar, V. Magnetic nanoparticles with dual functional properties: Drug delivery and magnetic resonance imaging. Biomaterials 2008, 29, 4012–4021. [Google Scholar] [CrossRef] [Green Version]
- Dilnawaz, F.; Singh, A.; Mewar, S.; Sharma, U.; Jagannathan, N.R.; Sahoo, S.K. The transport of non-surfactant based paclitaxel loaded magnetic nanoparticles across the blood brain barrier in a rat model. Biomaterials 2012, 33, 2936–2951. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Li, S. Hydroxycamptothecin-loaded nanoparticles enhance target drug delivery and anticancer effect. BMC Biotechnol. 2008, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Blasiak, J.; Widera, K.; Pertyński, T. Hyperthermia can differentially modulate the repair of doxorubicin-damaged DNA in normal and cancer cells. Acta Biochim. Pol. 2003, 50, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Hui, G.; Yu, L.L.; Man, Z. Inactivated Sendai Virus Induces Apoptosis Mediated by Reactive Oxygen Species in Murine Melanoma Cells. Biomed. Environ. Sci. 2016, 12, 877–884. [Google Scholar]
- Barrott, J.J.; Haystead, T.A.J. Hsp90, an unlikely ally in the war on cancer. FEBS J. 2013, 280, 1381–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pick, E.; Kluger, Y.; Giltnane, J.M.; Moeder, C.; Camp, R.L.; Rimm, D.L.; Kluger, H.M. High HSP90 Expression Is Associated with Decreased Survival in Breast Cancer. Cancer Res. 2007, 67, 2932–2937. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Fujisawa, N.; Takanohashi, M.; Najmina, M.; Uto, K.; Ebara, M. A Smart Hyperthermia Nanofiber-Platform-Enabled Sustained Release of Doxorubicin and 17AAG for Synergistic Cancer Therapy. Int. J. Mol. Sci. 2021, 22, 2542. https://doi.org/10.3390/ijms22052542
Chen L, Fujisawa N, Takanohashi M, Najmina M, Uto K, Ebara M. A Smart Hyperthermia Nanofiber-Platform-Enabled Sustained Release of Doxorubicin and 17AAG for Synergistic Cancer Therapy. International Journal of Molecular Sciences. 2021; 22(5):2542. https://doi.org/10.3390/ijms22052542
Chicago/Turabian StyleChen, Lili, Nanami Fujisawa, Masato Takanohashi, Mazaya Najmina, Koichiro Uto, and Mitsuhiro Ebara. 2021. "A Smart Hyperthermia Nanofiber-Platform-Enabled Sustained Release of Doxorubicin and 17AAG for Synergistic Cancer Therapy" International Journal of Molecular Sciences 22, no. 5: 2542. https://doi.org/10.3390/ijms22052542
APA StyleChen, L., Fujisawa, N., Takanohashi, M., Najmina, M., Uto, K., & Ebara, M. (2021). A Smart Hyperthermia Nanofiber-Platform-Enabled Sustained Release of Doxorubicin and 17AAG for Synergistic Cancer Therapy. International Journal of Molecular Sciences, 22(5), 2542. https://doi.org/10.3390/ijms22052542






