Molecular Background, Clinical Features and Management of Pediatric Mastocytosis: Status 2021
Abstract
:1. Introduction
2. Pathogenesis
2.1. Genetic Background and Molecular Markers in Mastocytosis
2.2. The Impact of Mediators Released by Mast Cells
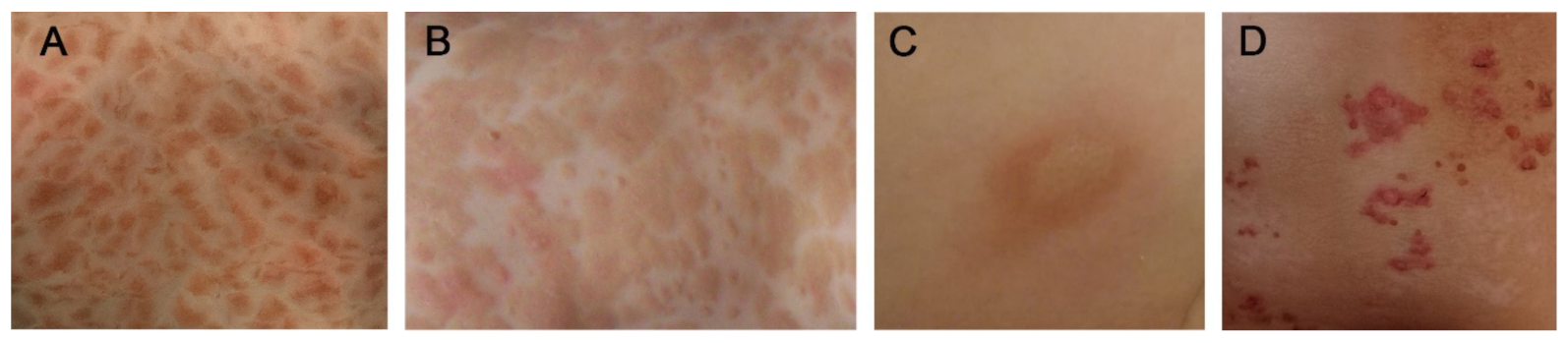
3. Cutaneous Mastocytosis
3.1. Maculopapular Cutaneous Mastocytosis (MPCM)
3.2. Mastocytoma
3.3. Diffuse Cutaneous Mastocytosis (DCM)
4. Mast Cell Mediator-Related Symptoms
4.1. Cutaneous Symptoms
4.2. Symptoms Resulting from Extracutaneous Involvement
4.3. Anaphylaxis
5. Systemic Mastocytosis
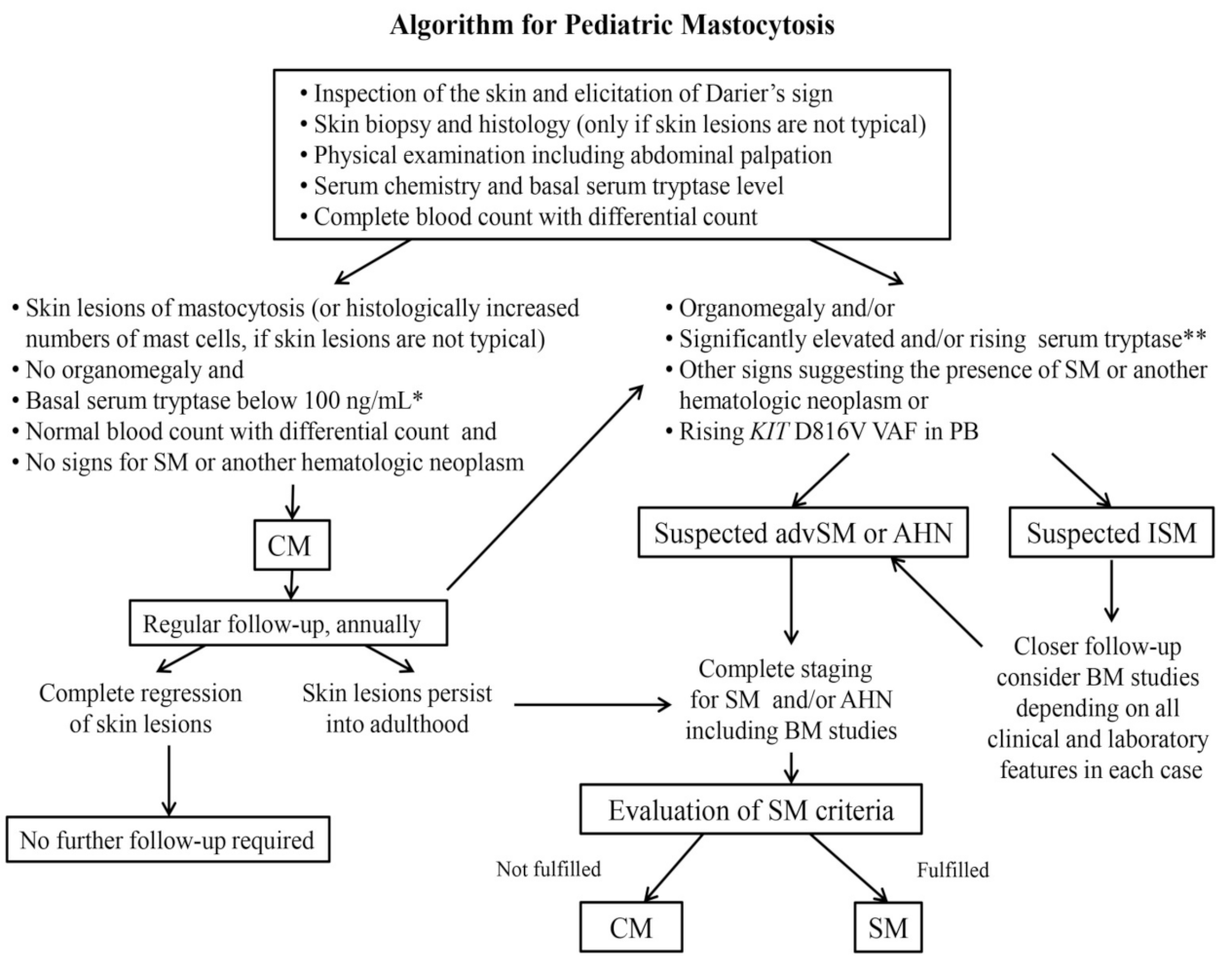
6. Diagnosis
7. Treatment of Pediatric Mastocytosis
7.1. Avoidance of Triggers
7.2. Emergency Medication
7.3. Anti-Mediator Therapy and Mast Cell-Targeted Treatment Options
7.4. Vaccination in Children with Mastocytosis
7.5. Topical Treatment Options and Phototherapy
8. Prognosis
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AML | acute myeloid leukemia |
| ASM | aggressive systemic mastocytosis |
| BM | bone marrow |
| CM | cutaneous mastocytosis |
| CMML | chronic myelomonocytic leukemia |
| DCM | diffuse cutaneous mastocytosis |
| HR | histamine receptor |
| IgE | immunoglobulin E |
| ISM | indolent systemic mastocytosis |
| MCL | mast cell leukemia |
| MCS | mast cell sarcoma |
| MPCM | maculopapular cutaneous mastocytosis |
| NB-UVB | narrow-band ultraviolet B |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| PB | peripheral blood |
| PUVA | psoralen plus ultraviolet A |
| SM | systemic mastocytosis |
| SM-AHN | systemic mastocytosis with associated hematologic neoplasm |
| TKI | tyrosine kinase inhibitors |
| WDSM | well-differentiated SM |
References
- Valent, P.; Akin, C.; Gleixner, K.V.; Sperr, W.R.; Reiter, A.; Arock, M.; Triggiani, M. Multidisciplinary challenges in mastocytosis and how to address with personalized medicine approaches. Int. J. Mol. Sci. 2019, 20, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Pardanani, A. Systemic mastocytosis in adults: 2019 update on diagnosis, risk stratification and management. Am. J. Hematol. 2018, 94, 363–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valent, P.; Akin, C.; Metcalfe, D.D. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood 2017, 129, 1420–1427. [Google Scholar] [CrossRef]
- Valent, P.; Horny, H.P.; Escribano, L.; Longley, B.J.; Li, C.Y.; Schwartz, L.B.; Marone, G.; Nuñez, R.; Akin, C.; Sotlar, K.; et al. Diagnostic criteria and classification of mastocytosis: A consensus proposal. Leuk. Res. 2001, 25, 603–625. [Google Scholar] [CrossRef]
- Hartmann, K.; Escribano, L.; Grattan, C.; Brockow, K.; Carter, M.C.; Alvarez-Twose, I.; Matito, A.; Broesby-Olsen, S.; Siebenhaar, F.; Lange, M.; et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J. Allergy Clin. Immunol. 2016, 137, 35–45. [Google Scholar] [PubMed]
- Horny, H.P.; Akin, C.; Arber, D.; Peterson, L.A.; Tefferi, A.; Metcalfe, D.D.; Bennett, J.M.; Bain, B.; Escribano, L.; Valent, P. Mastocytosis. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Arber, D.A., Hasserjian, R.P., Le Beau, M.M., et al., Eds.; IARC Press: Lyon, France, 2017; pp. 62–69. [Google Scholar]
- Alvarez-Twose, I.; Vañó-Galván, S.; Sánchez-Muñoz, L.; Morgado, J.M.; Matito, A.; Torrelo, A.; Jaén, P.; Schwartz, L.B.; Orfao, A.; Escribano, L. Increased serum baseline tryptase levels and extensive skin involvement are predictors for the severity of mast cell activation episodes in children with mastocytosis. Allergy 2012, 67, 813–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méni, C.; Bruneau, J.; Georgin-Lavialle, S.; Le Saché de Peufeilhoux, L.; Damaj, G.; Hadj-Rabia, S.; Fraitag, S.; Dubreuil, P.; Hermine, O.; Bodemer, C. Paediatric mastocytosis: A systematic review of 1747 cases. Br. J. Dermatol. 2015, 172, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.C.; Clayton, S.T.; Komarow, H.D.; Brittain, E.H.; Scott, L.M.; Cantave, D.; Gaskins, D.M.; Maric, I.; Metcalfe, D.D. Assessment of clinical findings, tryptase levels, and bone marrow histopathology in the management of pediatric mastocytosis. J. Allergy Clin. Immunol. 2015, 136, 1673–1679. [Google Scholar] [CrossRef] [Green Version]
- Carter, M.C.; Bai, Y.; Ruiz-Esteves, K.N.; Scott, L.M.; Cantave, D.; Bolan, H.; Eisch, R.; Sun, X.; Hahn, J.; Maric, I.; et al. Detection of KIT D816V in peripheral blood of children with manifestations of cutaneous mastocytosis suggests systemic disease. Br. J. Haematol. 2018, 183, 775–782. [Google Scholar] [CrossRef] [Green Version]
- Czarny, J.; Żuk, M.; Żawrocki, A.; Plata-Nazar, K.; Biernat, W.; Niedoszytko, M.; Ługowska-Umer, H.; Nedoszytko, B.; Wasąg, B.; Nowicki, R.J.; et al. New Approach to Paediatric Mastocytosis: Implications of KIT D816V Mutation Detection in Peripheral Blood. Acta Dermato-Venereol. 2020, 100, adv00149. [Google Scholar] [CrossRef]
- Lange, M.; Niedoszytko, M.; Renke, J.; Gleń, J.; Nedoszytko, B. Clinical aspects of paediatric mastocytosis: A review of 101 cases. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 97–102. [Google Scholar] [CrossRef]
- Hartmann, K.; Metcalfe, D.D. Pediatric mastocytosis. Hematol. Oncol. Clin. N. Am. 2000, 14, 625–640. [Google Scholar] [CrossRef]
- Cohen, S.S.; Skovbo, S.; Vestergaard, H.; Kristensen, T.; Møller, M.; Bindslev-Jensen, C.; Fryzek, J.P.; Broesby-Olsen, S. Epidemiology of systemic mastocytosis in Denmark. Br. J. Hematol. 2014, 166, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Van Doormaal, J.J.; Arends, S.; Brunekreeft, K.L.; van der Wal, V.B.; Sietsma, J.; van Voorst Vader, P.C.; Oude Elberink, J.N.; Kluin-Nelemans, J.C.; van der Veer, E.; de Monchy, J.G. Prevalence of indolent systemic mastocytosis in a Dutch region. J. Allergy Clin. Immunol. 2013, 131, 1429–1431.e1. [Google Scholar] [CrossRef]
- Méni, C.; Georgin-Lavialle, S.; Le Saché de Peufeilhoux, L.; Jais, J.P.; Hadj-Rabia, S.; Bruneau, J.; Fraitag, S.; Hanssens, K.; Dubreuil, P.; Hermine, O.; et al. Paediatric mastocytosis: Long-term follow-up of 53 patients with whole sequencing of KIT. A prospective study. Br. J. Dermatol. 2018, 179, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Wasąg, B.; Niedoszytko, M.; Piskorz, A.; Lange, M.; Renke, J.; Jassem, E.; Biernat, W.; Dębiec-Rychter, M.; Limon, J. Novel, activating KIT-N822I mutation in familial cutaneous mastocytosis. Exp. Hematol. 2011, 39, 859–865. [Google Scholar] [CrossRef]
- De la Sotta, P.; Romero, W.A.; Kramer, D.; Cárdenas, C.; González, S. Cutaneous mastocytosis in twins: Multiple mastocytomas and urticarial pigmentosa in two pairs of monozygotic twins. Pediatr. Dermatol. 2011, 28, 585–587. [Google Scholar] [CrossRef]
- Bodemer, C.; Hermine, O.; Palmérini, F.; Yang, Y.; Grandpeix-Guyodo, C.; Leventhal, P.S.; Hadj-Rabia, S.; Nasca, L.; Georgin-Lavialle, S.; Cohen-Akenine, A.; et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J. Investig. Dermatol. 2010, 130, 804–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longley, B.J.; Metcalfe, D.D.; Tharp, M.; Wang, X.; Tyrrell, L.; Lu, S.; Heitjan, D.; Ma, Y. Activating and dominant inactivating c-KIT catalytic domain mutations in distinct clinical forms of human mastocytosis. Proc. Natl. Acad. Sci. USA 1999, 96, 1609–1614. [Google Scholar] [CrossRef] [Green Version]
- Verzijl, A.; Heide, R.; Oranje, A.P.; van Schaik, R.H.N. C-kit Asp-816-Val mutation analysis in patients with mastocytosis. Dermatology 2007, 214, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Martelli, M.; Monaldi, C.; De Santis, S.; Bruno, S.; Mancini, M.; Cavo, M.; Soverini, S. Recent Advances in the Molecular Biology of Systemic Mastocytosis: Implications for Diagnosis, Prognosis, and Therapy. Int. J. Mol. Sci. 2020, 21, 3987. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Ghosh, J.; Kapur, R. Mastocytosis: A mutated KIT receptor induced myeloproliferative disorder. Oncotarget 2015, 6, 18250–18264. [Google Scholar] [CrossRef] [PubMed]
- Komi, D.E.A.; Rambasek, T.; Wöhrl, S. Mastocytosis: From a Molecular Point of View. Clin. Rev. Allergy Immunol. 2018, 54, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Arase, N.; Wataya-Kaneda, M.; Murota, H.; Nakagawa, Y.; Yamaoka, T.; Itoi-Ochi, S.; Hirayasu, K.; Arase, H.; Fujimoto, M.; Katayama, I. Genotype and phenotype analysis of patients with pediatric cutaneous mastocytosis, especially wild-type KIT patients. J. Dermatol. 2020, 47, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Chan, I.J.; Tharp, M.D. Comparison of lesional skin c-KIT mutations with clinical phenotype in patients with mastocytosis. Clin. Exp. Dermatol. 2018, 43, 416–422. [Google Scholar] [CrossRef] [Green Version]
- Bibi, S.; Langenfeld, F.; Jeanningros, S.; Brenet, F.; Soucie, E.; Hermine, O.; Damaj, G.; Dubreuil, P.; Arock, M. Molecular defects in mastocytosis: KIT and beyond KIT. Immunol. Allergy Clin. N. Am. 2014, 34, 239–262. [Google Scholar] [CrossRef]
- Arock, M.; Sotlar, K.; Akin, C.; Broesby-Olsen, S.; Hoermann, G.; Escribano, L.; Kristensen, T.K.; Kluin-Nelemans, H.C.; Hermine, O.; Dubreuil, P.; et al. KIT mutation analysis in Mast Cell Neoplasms: Recommendations of European Competence Network on Mastocytosis. Leukemia 2015, 29, 1223–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akin, C.; Fumo, G.; Yavuz, A.S.; Lipsky, P.E.; Neckers, L.; Metcalfe, D.D. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood 2004, 103, 3222–3225. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.Y.; Smith, M.L.; Schultheis, B.; Fitzgibbon, J.; Lister, T.A.; Melo, J.V.; Cross, N.C.; Cavenagh, J.D. A novel K509I mutations of KIT identified in familial mastocytosis—In utero and in vivo responsiveness to imatinib therapy. Leuk. Res. 2006, 30, 373–378. [Google Scholar] [CrossRef]
- Wang, H.; Lin, Z.; Zhang, J.; Yin, J.; Yang, Y. A new germline mutation in KIT associated with diffuse cutaneous mastocytosis in Chinese family. Clin. Exp. Dermatol. 2013, 39, 146–149. [Google Scholar] [CrossRef]
- Pollard, W.L.; Beachkofsky, T.M.; Kobayashi, T.T. Novel R634W c-kit mutation identified in familial mastocytosis. Pediatr. Dermatol. 2015, 32, 267–270. [Google Scholar] [CrossRef]
- De Melo Campos, P.; Machado-Neto, J.A.; Scopim-Ribeiro, R.; Visconte, V.; Tabarroki, A.; Duarte, A.S.; Barra, F.F.; Vassalo, J.; Rogers, H.J.; Lorand-Metze, I.; et al. Familial systemic mastocytosis with germline KIT K509I mutation is sensitive to treatment with imatinib, dasatinib and PKC412. Leuk. Res. 2014, 38, 1245–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, E.C.; Bai, Y.; Kirshenbaum, A.S.; Fischer, E.R.; Simakova, O.; Bandara, G.; Scott, L.M.; Wisch, L.B.; Cantave, D.; Carter, M.C.; et al. Mastocytosis associated with a rare germline KIT K509I mutation displays a well-differentiated mast cell phenotype. J. Allergy Clin. Immunol. 2014, 134, 178–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otani, I.M.; Carroll, R.W.; Yager, P.; Kroshinsky, D.; Murphy, S.; Hornick, J.L.; Akin, C.; Castells, M.; Walter, J.E. Diffuse cutaneous mastocytosis with novel somatic KIT mutation K509I and association with tuberous sclerosis. Clin. Case Rep. 2018, 6, 1834–1840. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, K.; Wardelmann, E.; Ma, Y.; Merkelbach-Bruse, S.; Preussner, L.M.; Woolery, C.; Baldus, S.E.; Heinicke, T.; Thiele, J.; Buettner, R.; et al. Novel Germline Mutation of KIT Associated With Familial Gastrointestinal Stromal Tumors and Mastocytosis. Gastroenterology 2005, 129, 1042–1046. [Google Scholar] [CrossRef]
- Tang, X.; Boxer, M.; Drummond, A.; Ogston, P.; Hodgins, M.; Burden, A.D. A germline mutation in KIT in familial diffuse cutaneous mastocytosis. J. Med. Genet. 2004, 41, e88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Létard, S.; Borge, L.; Chaix, A.; Hanssens, K.; Lopez, S.; Vita, M.; Finetti, P.; Birnbaum, D.; Bertucci, F.; et al. Pediatric mastocytosis-associated KIT extracellular domain mutations exhibit different functional and signaling properties compared with KIT-phosphotransferase domain mutations. Blood 2010, 116, 1114–1123. [Google Scholar] [CrossRef] [Green Version]
- Peters, F.; Fiebig, B.; Lundberg, P.; Jaspers, N.I.; Holzapfel, B.; Ghadimi, M.P.; Drebber, U.; Tuchscherer, A.; Ullrich, R.; Hartmann, K.; et al. Detection of the Germline KIT S476I Mutation in a Kindred with Familial Mastocytosis associated with Gastrointestinal Stromal Tumors. J. Allergy Clin. Immunol. Pract. 2021, S2213-21989(21)00004-0. [Google Scholar]
- Li, Y.; Li, X.; Liu, X.; Kang, L.; Liu, X. Genotypic and phenotypic characteristics of Chinese neonates with cutaneous mastocytosis: A case report and literature review. J. Int. Med. Res. 2020, 48, 300060520952621. [Google Scholar] [CrossRef]
- Chaudhary, N.; Shapiro, N.; Bhutada, A.; Rastogi, S. c-KIT-Positive Fatal Diffuse Cutaneous Mastocytosis With Systemic Manifestations in a Neonate. J. Pediatr. Hematol. Oncol. 2019, 41, e338–e340. [Google Scholar] [CrossRef]
- Jenkinson, H.A.; Lundgren, A.D.; Carter, M.C.; Diaz, L.Z.; Levy, M.L. Management of neonate with diffuse cutaneous mastocytosis: Case report and literature review. Pediatr. Dermatol. 2019, 36, 486–489. [Google Scholar] [CrossRef]
- Morren, M.A.; Hoppé, A.; Renard, M.; Debiec Rychter, M.; Uyttebroeck, A.; Dubreuil, P.; Martin, L. Imatinib mesylate in the treatment of diffuse cutaneous mastocytosis. J. Pediatr. 2013, 162, 205–207. [Google Scholar] [CrossRef]
- Ma, D.; Stence, A.A.; Bossler, A.B.; Hackman, J.R.; Bellizzi, A.M. Identification of KIT activating mutations in paediatric solitary mastocytoma. Histopathology 2014, 64, 218–225. [Google Scholar] [CrossRef]
- Intzes, S.; Wiersma, S.; Meyerson, H. Myelomastocytic leukemia with t(8;21) in a 3-year-old child. J. Pediatr. Hematol. Oncol. 2011, 33, 372–375. [Google Scholar] [CrossRef]
- Homan, M.; Avčin, T. Nodularity of the small intestine in a child with systemic mastocytosis associated with hyperimmunoglobulin M syndrome. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 245. [Google Scholar] [CrossRef]
- Sharma, S.; Harbhajanka, A.; Jain, A.; Seth, A. Systemic mastocytosis with an associated non mast cell lineage clonal hematological disease in a child. Indian J. Pathol. Microbiol. 2011, 54, 854–856. [Google Scholar]
- Gadage, V.S.; Kadam Amare, P.S.; Galani, K.S.; Mittal, N. Systemic mastocytosis with associated acute myeloid leukemia with t(8;21) (q22;q22). Indian J. Pathol. Microbiol. 2012, 55, 409–412. [Google Scholar] [CrossRef]
- Turner, P.J.; Kemp, A.S.; Rogers, M.; Mehr, S. Refractory Symptoms Successfully Treated with Leukotriene Inhibition in a Child with Systemic Mastocytosis. Pediatr. Dermatol. 2012, 29, 222–223. [Google Scholar] [CrossRef] [PubMed]
- Torun, Y.A.; Ergul, A.B.; Kazancı, E.G.; Serbetci, M.C.; Sarıguzel, F.M. Indolent systemic mastocytosis in a child: A rare and difficult diagnosis. Indian J. Paediatr. Dermatol. 2016, 17, 306–308. [Google Scholar] [CrossRef]
- Mahadeo, K.M.; Wolgast, L.; McMahon, C.; Cole, P.D. Systemic mastocytosis in a child with t(8;21) acute myeloid leukemia. Pediatr. Blood Cancer 2011, 57, 684–687. [Google Scholar] [CrossRef] [PubMed]
- Synakiewicz, A.; Stachowicz-Stencel, T.; Renke, J.; Lange, M.; Adamkiewicz-Drożyńska, E.; Balcerska, A. Systemic mastocytosis in children—Therapeutic problems. Dev. Period. Med. 2013, 17, 126–129. [Google Scholar]
- Gogia, A.; Sharawat, S.K.; Kumar, R.; Sarkar, C.; Bakhshi, S. Systemic mastocytosis associated with childhood acute myeloid leukemia. J. Pediatr. Hematol. Oncol. 2013, 35, 163–164. [Google Scholar] [CrossRef]
- Rabade, N.; Tembhare, P.; Patkar, N.; Amare, P.; Arora, B.; Subramanian, P.G.; Gujral, S. Childhood systemic mastocytosis associated with t (8; 21) (q22; q22) acute myeloid leukemia. Indian J. Pathol. Microbiol. 2016, 59, 407–409. [Google Scholar] [PubMed]
- Zheng, Y.; Nong, L.; Liang, L.; Wang, W.; Li, T. De novo mast cell leukemia without CD25 expression and KIT mutations: A rare case report in a 13-year-old child. Diagn. Pathol. 2018, 13, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yabe, M.; Masukawa, A.; Kato, S.; Yabe, H.; Nakamura, N.; Matsushita, H. Systemic mastocytosis associated with t(8;21) Acute Myeloid Leukemia in a child: Detection of the D816A mutation of KIT. Pediatr. Blood Cancer 2012, 59, 1313–1316. [Google Scholar] [CrossRef]
- Mitchell, S.G.; Bunting, S.T.; Saxe, D.; Olson, T.; Keller, F.G. A variant c-KIT mutation, D816H, fundamental to the sequential development of an ovarian mixed germ cell tumor and systemic mastocytosis with chronic myelomonocytic leukemia. Pediatr. Blood Cancer 2017, 64. [Google Scholar] [CrossRef]
- Huang, A.; Fiadorchanka, N.; Brar, K.; Balderacchi, J.; Glick, S. In utero presentation of aggressive systemic mastocytosis in a neonate. Br. J. Dermatol. 2017, 177, 1439–1441. [Google Scholar] [CrossRef]
- Liu, M.M.; Kohn, L.A.; Roach, G.D.; Sun, G.; Garcia-Lloret, M.I.; Butte, M.J. Treatment of systemic mastocytosis in an infant with midostaurin. J. Allergy Clin. Immunol. Pract. 2019, 7, 2929–2931. [Google Scholar] [CrossRef]
- Chantorn, R.; Shwayder, T. Death from mast cell leukemia: A young patient with longstanding cutaneous mastocytosis evolving into fatal mast cell leukemia. Pediatr. Dermatol. 2012, 29, 605–609. [Google Scholar] [CrossRef]
- Mital, A.; Piskorz, A.; Lewandowski, K.; Wasąg, B.; Limon, J.; Hellmann, A. A case of mast cell leukaemia with exon 9 KIT mutation and good response to imatinib. Eur. J. Haematol. 2011, 86, 531–535. [Google Scholar] [CrossRef]
- Georgin-Lavialle, S.; Aguilar, C.; Guieze, R.; Lhermitte, L.; Bruneau, J.; Fraitag, S.; Canioni, D.; Chandesris, M.O.; Suarez, F.; Grandpeix-Guyodo, C.; et al. Mast cell sarcoma: A rare and aggressive entity—Report of two cases and review of the literature. J. Clin. Oncol. 2013, 31, e90–e97. [Google Scholar] [CrossRef]
- Ryan, R.J.; Akin, C.; Castells, M.; Wills, M.; Selig, M.K.; Nielsen, G.P.; Ferry, J.A.; Hornick, J.L. Mast cell sarcoma: A rare and potentially under-recognized diagnostic entity with specific therapeutic implications. Mod. Pathol. 2013, 26, 533–543. [Google Scholar] [CrossRef]
- Boutista-Quach, M.; Booth, C.; Kheradpour, A.; Zuppan, C.; Rowsell, E.; Weiss, L.; Wang, J. Mast cell sarcoma in an infant: A case report and review of the literature. J. Pediatr. Hematol. Oncol. 2012, 35, 315–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S.; Wu, H.; Pawlowska, A.B.; Bautista-Quach, M.A.; Huang, Q.; Gaal, K.; Chang, K.L. Pediatric mast cell sarcoma of temporal bone with novel L799F (2395 C>T) KIT mutation, mimicking histiocytic neoplasm. Am. J. Surg. Pathol. 2013, 37, 453–458. [Google Scholar] [CrossRef]
- Chott, A.; Guenther, P.; Huebner, A.; Selzer, E.; Parwaresch, R.M.; Horny, H.-P.; Valent, P. Morphologic and immunophenotypic properties of neoplastic cells in a case of mast cell sarcoma. Am. J. Surg. Pathol. 2003, 27, 1013–1019. [Google Scholar] [CrossRef]
- Monnier, J.; Georgin-Lavialle, S.; Canioni, D.; Lhermitte, L.; Soussan, M.; Arock, M.; Bruneau, J.; Dubreuil, P.; Bodemer, C.; Chandesris, M.O.; et al. Mast cell sarcoma: New cases and literature review. Oncotarget 2016, 7, 66299–66309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brcić, L.; Vuletić, L.B.; Stepan, J.; Bonevski, A.; Jakovljević, G.; Gasparov, S.; Marjanović, K.; Seiwerth, S. Mast-cell sarcoma of the tibia. J. Clin. Pathol. 2007, 60, 424–425. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Mast cells as a unique hematopoietic lineage and cell system: From Paul Ehrlich’s visions to precision medicine concepts. Theranostics 2020, 10, 10743–10768. [Google Scholar] [CrossRef]
- Metcalfe, D.D. Mast cells and mastocytosis. Blood 2008, 112, 946–956. [Google Scholar] [CrossRef] [Green Version]
- Theoharides, T.C.; Valent, P.; Akin, C. Mast Cells, Mastocytosis, and Related Disorders. N. Engl. J. Med. 2015, 373, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Valent, P. KIT D816V and the cytokine storm in mastocytosis: Production and role of interleukin-6. Haematologica 2020, 105, 5–6. [Google Scholar] [CrossRef]
- Tobío, A.; Bandara, G.; Morris, D.A.; Kim, D.K.; O’Connell, M.P.; Komarow, H.D.; Carter, M.C.; Smrz, D.; Metcalfe, D.D.; Olivera, A. Oncogenic D816V-KIT signaling in mast cells causes persistent IL-6 production. Haematologica 2020, 105, 124–135. [Google Scholar] [CrossRef]
- Hoermann, G.; Cerny-Reiterer, S.; Perné, A.; Klauser, M.; Hoetzenecker, K.; Klein, K.; Müllauer, L.; Gröger, M.; Nijman, S.M.; Klepetko, W.; et al. Identification of oncostatin M as a STAT5-dependent mediator of bone marrow remodeling in KIT D816V-positive systemic mastocytosis. Am. J. Pathol. 2011, 178, 2344–2356. [Google Scholar] [CrossRef] [Green Version]
- Greiner, G.; Witzeneder, N.; Berger, A.; Schmetterer, K.; Eisenwort, G.; Schiefer, A.I.; Roos, S.; Popow-Kraupp, T.; Müllauer, L.; Zuber, J.; et al. CCL2 is a KIT D816V-dependent modulator of the bone marrow microenvironment in systemic mastocytosis. Blood 2017, 129, 371–382. [Google Scholar] [CrossRef]
- Brockow, K.; Akin, C.; Huber, M.; Metcalfe, D.D. IL-6 levels predict disease variant and extent of organ involvement in patients with mastocytosis. Clin. Immunol. 2005, 115, 216–223. [Google Scholar] [CrossRef]
- Renke, J.; Kędzierska-Mieszkowska, S.; Lange, M.; Nedoszytko, B.; Wasilewska, E.; Liberek, A.; Renke, M.; Niedoszytko, M.; Witkowski, J.; Skórko-Glonek, J.; et al. Mast cells in mastocytosis and allergy—Important player in metabolic and immunological homeostasis. Adv. Med. Sci. 2019, 64, 124–130. [Google Scholar] [CrossRef]
- Da Silva, E.Z.M.; Jamur, M.C.; Oliver, C. Mast Cell Function. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef]
- Butterfield, J.H.; Weiler, C.R. Prevention of mast cell activation disorder-associated clinical sequelae of excessive prostaglandin D2 production. Int. Arch. Allergy Immunol. 2008, 147, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Kleewein, K.; Lang, R.; Diem, A.; Vogel, T.; Pohla-Gubo, G.; Bauer, J.W.; Hintner, H.; Laimer, M. Diffuse Cutaneous Mastocytosis masquering as epidermolysis bullosa. Pediatr. Dermatol. 2011, 28, 720–725. [Google Scholar] [CrossRef]
- Rabenhorst, A.; Christopeit, B.; Leja, S.; Gerbaulet, A.; Kleiner, S.; Förster, A.; Raap, U.; Wickenhauser, C.; Hartmann, K. Serum levels of bone cytokines are increased in indolent systemic mastocytosis associated with osteopenia or osteoporosis. J. Allergy Clin. Immunol. 2013, 132, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Teodosio, C.; García-Montero, A.C.; Jara-Acevedo, M.; Sánchez-Muñoz, L.; Pedreira, C.E.; Álvarez-Twose, I.; Matarraz, S.; Morgado, J.M.; Bárcena, P.; Matito, A.; et al. Gene expression profile of highly purified bone marrow mast cells in systemic mastocytosis. J. Allergy Clin. Immunol. 2013, 131, 1213–1224. [Google Scholar] [CrossRef]
- Ahn, Y.M.; Hong, G.U.; Kim, S.H.; Lee, H.J.; Baek, H.S.; Kim, M.N.; Park, K.Y.; Ro, J.Y. Transglutaminase 2 expressed in mast cells recruited into skin or bone marrow induces the development of pediatric mastocytosis. Pediatr. Allergy Immunol. 2015, 26, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Akin, C.; Escribano, L.; Födinger, M.; Hartmann, K.; Brockow, K.; Castells, M.; Sperr, W.R.; Kluin-Nelemans, H.C.; Hamdy, N.A.; et al. Standards and standardization in mastocytosis: Consensus statements on diagnostics, treatment recommendations and response criteria. Eur. J. Clin. Investig. 2007, 37, 435–453. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Escribano, L.; Broesby-Olsen, S.; Hartmann, K.; Grattan, C.; Brockow, K.; Niedoszytko, M.; Nedoszytko, B.; Oude Elberink, J.N.; Kristensen, T.; et al. Proposed diagnostic algorithm for patients with suspected mastocytosis: A proposal of the European Competence Network on Mastocytosis. Allergy 2014, 69, 1267–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, K.; Valent, P.; Horny, H.P. Cutaneous mastocytosis. In World Health Organization (WHO) Classification of Skin Tumours, 4th ed.; Elder, D.E., Massi, D., Scolyer, R., Willemze, R., Eds.; International Agency for Research on Cancer: Lyon, France, 2018; pp. 275–278. [Google Scholar]
- Castells, M.; Metcalfe, D.D.; Escribano, L. Diagnosis and treatment of cutaneous mastocytosis in children: Practical recommendations. Am. J. Clin. Dermatol. 2011, 12, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Matito, A.; Azaña, J.M.; Torrelo, A.; Alvarez-Twose, I. Cutaneous Mastocytosis in Adults and Children: New Classification and Prognostic Factors. Immunol. Allergy Clin. N. Am. 2018, 38, 351–363. [Google Scholar] [CrossRef]
- Torrello, A.; Alvarez-Twose, I.; Escribano, L. Childhood mastocytosis. Curr. Opin. Pediatr. 2012, 24, 480–486. [Google Scholar] [CrossRef]
- Barnes, M.; Van, L.; De Long, L.; Lawley, L.P. Severity of cutaneous findings predict the presence of systemic symptoms in pediatric maculopapular cutaneous mastocytosis. Pediatr. Dermatol. 2014, 31, 271–275. [Google Scholar] [CrossRef]
- Brockow, K.; Akin, C.; Huber, M.; Metcalfe, D.D. Assessment of the extent of cutaneous involvement in children and adults with mastocytosis: Relationship to symptomatology, tryptase levels, and bone marrow pathology. J. Am. Acad. Dermatol. 2003, 48, 508–516. [Google Scholar] [CrossRef]
- Wiechers, T.; Rabenhorst, A.; Schick, T.; Preussner, L.M.; Förster, A.; Valent, P.; Horny, H.P.; Sotlar, K.; Hartmann, K. Large maculopapular cutaneous lesions are associated with favorable outcome in childhood-onset mastocytosis. J. Allergy Clin. Immunol. 2015, 136, 1581–1590. [Google Scholar] [CrossRef] [Green Version]
- Leung, A.K.C.; Lam, J.M.; Leong, K.F. Childhood Solitary Cutaneous Mastocytoma: Clinical Manifestations, Diagnosis, Evaluation, and Management. Curr. Pediatr. Rev. 2019, 15, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Niedoszytko, M.; Nedoszytko, B.; Łata, J.; Trzeciak, M.; Biernat, W. Diffuse cutaneus mastocytosis: Analysis of 10 cases and a brief review of the literature. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 1565–1571. [Google Scholar]
- Heide, R.; Zuidema, E.; Beishuizen, A.; Den Hollander, J.C.; Van Gysel, D.; Seyger, M.M.; Pasmans, S.G.; Kakourou, T.; Oranje, A.P. Clinical aspects of Diffuse Cutaneous Mastocytosis in children: Two variants. Dermatology 2009, 219, 309–315. [Google Scholar] [CrossRef]
- Hannaford, R.; Rogers, M. Presentation of cutaneous mastocytosis in 173 children. Australas. J. Dermatol. 2001, 42, 15–21. [Google Scholar] [CrossRef]
- Álvarez-Twose, I.; Jara-Acevedo, M.; Morgado, J.M.; García-Montero, A.; Sánchez-Muñoz, L.; Teodósio, C.; Matito, A.; Mayado, A.; Caldas, C.; Mollejo, M.; et al. Clinical, immunophenotypic, and molecular characteristics of well-differentiated systemic mastocytosis. J. Allergy Clin. Immunol. 2016, 137, 168–178.e1. [Google Scholar] [CrossRef]
- Willemze, R.; Ruiter, D.J.; Scheffer, E.; Van Vloten, W.A. Diffuse cutaneous mastocytosis with multiple cutaneous mastocytomas. Br. J. Dermatol. 1980, 102, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.; von Komorowski, G.; Scheurlen, W.; Dorn-Beineke, A.; Back, W.; Bayerl, C. Neonatal mastocytosis with pachydermic bullous skin without c-kit 816 mutation. Dermatology 2006, 212, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Wawrzycki, B.; Pietrzak, A.; Chodorowska, G.; Kanitakis, J. Diffuse cutaneous bullous mastocytosis in a newborn. Dermatol. Ther. 2013, 26, 176–179. [Google Scholar] [CrossRef]
- Hosking, A.M.; Makdisi, J.; Ortenzio, F.; de Feraudy, S.; Smith, J.; Linden, K. Diffuse cutaneous mastocytosis: Case report and literature review. Pediatr. Dermatol. 2018, 35, e348–e352. [Google Scholar] [CrossRef]
- Waxtein, L.M.; Vega-Memije, M.E.; Cortes-Franco, R.; Domingues-Soto, L. Diffuse cutaneous mastocytosis with bone marrow infiltration in a child: A case report. Pediatr. Dermatol. 2000, 3, 198–201. [Google Scholar] [CrossRef]
- Angus, J.; Leach, I.H.; Grant, J.; Ravenscroft, J.C. Systemic mastocytosis with diffuse cutaneous involvement and haematological disease presenting in utero treated unsuccessfully with vincristine. Clin. Exp. Dermatol. 2007, 33, 36–39. [Google Scholar] [CrossRef]
- Olgun, N.; Oren, H.; Oren, B.; Irken, G.; Polat, M.; Cevik, N. Diffuse erythrodermic cutaneous mastocytosis with bone marrow infiltration. Dermatology 1993, 2, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.C.; Metcalfe, D.D.; Komarow, H.D. Mastocytosis. Immunol. Allergy Clin. N. Am. 2014, 34, 181–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González de Olano, D.; de la Hoz Caballer, B.; Núñez López, R.; Sánchez Muñoz, L.; Cuevas Agustín, M.; Diéguez, M.C.; Alvarez Twose, I.; Castells, M.C.; Escribano Mora, L. Prevalence of allergy and anaphylactic symptoms in 210 adult and pediatric patients with mastocytosis in Spain: A study of the Spanish network on mastocytosis (REMA). Clin. Exp. Allergy 2007, 37, 1547–1555. [Google Scholar] [CrossRef]
- Brockow, K.; Jofer, C.; Behrendt, H.; Ring, J. Anaphylaxis in patients with mastocytosis: A study on history, clinical features and risk factors in 120 patients. Allergy 2008, 63, 226–232. [Google Scholar] [CrossRef]
- Matito, A.; Carter, M. Cutaneous and systemic mastocytosis in children: A risk factor for anaphylaxis? Curr Allergy Asthma Rep. 2015, 15, 22. [Google Scholar] [CrossRef]
- Lange, M.; Zawadzka, A.; Schrörs, S.; Słomka, J.; Ługowska-Umer, H.; Nedoszytko, B.; Nowicki, R. The role of serum tryptase in the diagnosis and monitoring of pediatric mastocytosis: A single-center experience. Adv. Dermatol. Alergol. 2017, 34, 306–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broesby-Olsen, S.; Carter, M.; Kjaer, H.F.; Mortz, C.G.; Møller, M.B.; Kristensen, T.K.; Bindslev-Jensen, C.; Agertoft, L. Pediatric Expression of Mast Cell Activation Disorders. Immunol. Allergy Clin. N. Am. 2018, 38, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K.; Ring, J.; Alvarez-Twose, I.; Orfao, A.; Escribano, L. Extensive blistering is a predictor for severe complications in children with mastocytosis. Allergy 2012, 67, 1323–1324. [Google Scholar] [CrossRef]
- Dosanjh, A. Infant anaphylaxis: The importance of early recognition. J. Asthma Allergy 2013, 6, 103–107. [Google Scholar] [CrossRef] [Green Version]
- Macpherson, J.L.; Kemp, A.; Rogers, M.; Mallet, A.I.; Toia, R.F.; Spur, B.; Earl, J.W.; Chesterman, C.N.; Krilis, S.A. Occurrence of platelet-activating factor (PAF) and an endogenous inhibitor of platelet aggregation in diffuse cutaneous mastocytosis. Clin. Exp. Immunol. 1989, 77, 391–396. [Google Scholar] [PubMed]
- Lange, M.; Ługowska-Umer, H.; Niedoszytko, M.; Wasąg, B.; Limon, J.; Żawrocki, A.; Nedoszytko, B.; Sobjanek, M.; Plata-Nazar, K.; Nowicki, R. Diagnosis of Mastocytosis in Children and Adults in Daily Clinical Practice. Acta Dermato-Venereol. 2016, 96, 292–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theoharides, T.C. Autism spectrum disorders and mastocytosis. Int. J. Immunopathol. Pharmacol. 2009, 22, 859–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gülen, T.; Hägglund, H.; Dahlén, B.; Nilsson, G. High prevalence of anaphylaxis in patients with systemic mastocytosis—A single-centre experience. Clin. Exp. Allergy 2014, 44, 121–129. [Google Scholar] [CrossRef]
- Alvarez-Twose, I.; González de Olano, D.; Sánchez-Muñoz, L.; Matito, A.; Esteban-López, M.I.; Vega, A.; Mateo, M.B.; Alonso Díaz de Durana, M.D.; de la Hoz, B.; Del Pozo Gil, M.D.; et al. Clinical, biological, and molecular characteristics of clonal mast cell disorders presenting with systemic mast cell activation symptoms. J. Allergy Clin. Immunol. 2010, 125, 1269–1278. [Google Scholar] [CrossRef]
- Zanotti, R.; Bonadonna, P.; Bonifacio, M.; Artuso, A.; Schena, D.; Rossini, M.; Perbellini, O.; Colarossi, S.; Chilosi, M.; Pizzolo, G. Isolated bone marrow mastocytosis: An underestimated subvariant of indolent systemic mastocytosis. Haematologica 2011, 96, 482–484. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Twose, I.; González-de-Olano, D.; Sánchez-Muñoz, L.; Matito, A.; Jara-Acevedo, M.; Teodosio, C.; García-Montero, A.; Morgado, J.M.; Orfao, A.; Escribano, L. Validation of the REMA score for predicting mast cell clonality and systemic mastocytosis in patients with systemic mast cell activation symptoms. Int. Arch. Allergy Immunol. 2012, 157, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Bussmann, C.; Hagemann, T.; Hanfland, J.; Haidl, G.; Bieber, T.; Novak, N. Flushing and increase of serum tryptase after mechanical irritation of a solitary mastocytoma. Eur. J. Dermatol. 2007, 17, 332–334. [Google Scholar]
- Schuch, A.; Brockow, K. Mastocytosis and Anaphylaxis. Immunol. Allergy Clin. N. Am. 2017, 37, 153–164. [Google Scholar] [CrossRef]
- Carter, M.C.; Uzzaman, A.; Scott, L.M.; Metcalfe, D.D.; Quezado, Z. Pediatric mastocytosis: Routine anesthetic management for a complex disease. Anesth. Analg. 2008, 107, 422–427. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Matas, I.; Matito, A.; González-de-Olano, D.; Alvarez-Twose, I.; Sánchez-Muñoz, L.; de la Hoz Caballer, B.; Escribano, L. Prevalence of hypersensitivity reactions to nonsteroidal anti-inflammatory drugs in 212 patients with mastocytosis in Spain. Allergy 2009, 64, 574–575. [Google Scholar]
- Ahmad, N.; Evans, P.; Lloyd-Thomas, A.R. Anesthesia in children with mastocytosis—A case based review. Paediatr. Anaesth. 2009, 19, 97–107. [Google Scholar] [CrossRef]
- Matito, A.; Morgado, J.M.; Sánchez-López, P.; Álvarez-Twose, I.; Sánchez-Muñoz, L.; Orfao, A.; Escribano, L. Management of Anesthesia in Adult and Pediatric Mastocytosis: A Study of the Spanish Network on Mastocytosis (REMA) Based on 726 Anesthetic Procedures. Int. Arch. Allergy Immunol. 2015, 167, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinze, A.; Kuemmet, T.J.; Chiu, Y.E.; Galbraith, S.S. Longitudinal Study of Pediatric Urticaria Pigmentosa. Pediatr. Dermatol. 2017, 34, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.C.; Metcalfe, D.D.; Clark, A.S.; Wayne, A.S.; Maric, I. Abnormal bone marrow histopathology in paediatric mastocytosis. Br. J. Haematol. 2015, 168, 865–873. [Google Scholar] [CrossRef]
- Kristensen, T.; Vestergaard, H.; Møller, M.B. Improved detection of the KIT D816V mutation in patients with systemic mastocytosis using a quantitative and highly sensitive real-time qPCR assay. J. Mol. Diagn. 2011, 13, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, T.; Vestergaard, H.; Bindslev-Jensen, C.; Mortz, C.G.; Kjaer, H.F.; Ollert, M.; Møller, M.B.; Broesby-Olsen, S.; Mastocytosis Centre Odense University Hospital (MastOUH). Prospective evaluation of the diagnostic value of sensitive KIT D816V mutation analysis of blood in adults with suspected systemic mastocytosis. Allergy 2017, 72, 1737–1743. [Google Scholar] [CrossRef]
- Uzzaman, A.; Maric, I.; Noel, P.; Kettelhut, B.V.; Metcalfe, D.; Carter, M. Pediatric-onset Mastocytosis: A Long term Clinical follow-up and correlation with Bone Marrow Histopathology. Pediatr. Blood Cancer 2009, 53, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.H. Pediatric mastocytosis. Curr. Opin. Pediatr. 2020, 32, 531–538. [Google Scholar] [CrossRef]
- Broesby Broesby-Olsen, S.; Dybedal, I.; Gülen, T.; Kristensen, T.K.; Møller, M.B.; Ackermann, L.; Sääf, M.; Karlsson, M.A.; Agertoft, L.; Brixen, K.; et al. Multidisciplinary Management of Mastocytosis: Nordic Expert Group Consensus. Acta Dermato-Venereol. 2016, 96, 602–612. [Google Scholar] [CrossRef] [Green Version]
- Seth, N.; Chinen, J.; Buheis, M.G.; Chan, A.J.; Hunt, R.D.; Tuano, K.T.S. Serum tryptase levels in 114 pediatric patients with cutaneous mastocytosis. J. Allergy Clin. Immunol. 2018, 141, AB89. [Google Scholar] [CrossRef]
- Lyons, J.J. Hereditary Alpha Tryptasemia: Genotyping and Associated Clinical features. Immunol. Allergy Clin. N. Am. 2018, 38, 483–495. [Google Scholar] [CrossRef]
- Siebenhaar, F.; Akin, C.; Bindslev-Jensen, C.; Maurer, M.; Broesby-Olsen, S. Treatment strategies in mastocytosis. Immunol. Allergy Clin. N. Am. 2014, 34, 433–447. [Google Scholar] [CrossRef]
- Azana, J.M.; Torrelo, A.; Matito, A. Update on mastocytosis (part 2): Categories, Prognosis, and Treatment. Actas Dermo-Sifiliogr. 2016, 107, 15–22. [Google Scholar]
- Castells, M.; Butterfield, J. Mast cell activation syndrome and mastocytosis: Initial treatment options and long-term management. J. Allergy Clin. Immunol. Pract. 2019, 7, 1097–1106. [Google Scholar] [CrossRef]
- Gülen, T.; Akin, C. Pharmacotherapy of mast cell disorders. Curr. Opin. Allergy Clin. Immunol. 2017, 17, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Czarny, J.; Lange, M.; Ługowska-Umer, H.; Nowicki, R.J. Cutaneous mastocytosis treatment: Strategies, limitations, and perspectives. Adv. Dermatol. Alergol. 2018, 6, 541–545. [Google Scholar] [CrossRef] [Green Version]
- Carter, M.C.; Metcalfe, D.D.; Matito, A.; Escribano, L.; Butterfield, J.H.; Schwartz, L.B.; Bonadonna, P.; Zanotti, R.; Triggiani, M.; Castells, M.; et al. Adverse reactions to drugs and biologics in patients with clonal mast cell disorders: A group report of the Mast Cells Disorder Committee, American Academy of Allergy and Immunology. J. Allergy Clin. Immunol. 2019, 143, 880–893. [Google Scholar] [CrossRef]
- Muraro, A.; Roberts, G.; Worm, M.; Bilò, M.B.; Brockow, K.; Fernández Rivas, M.; Santos, A.F.; Zolkipli, Z.Q.; Bellou, A.; Beyer, K.; et al. EAACI Food Allergy and Anaphylaxis Guidelines Group. Anaphylaxis: Guidelines from the European Academy of Allergy and Clinical Immunology. Allergy 2014, 69, 1026–1045. [Google Scholar] [CrossRef]
- Bilò, M.B.; Cichocka-Jarosz, E.; Pumphrey, R.; Oude-Elberink, J.N.; Lange, J.; Jakob, T.; Bonadonna, P.; Fernandez, J.; Kosnik, M.; Helbling, A.; et al. Self-medication of anaphylactic reactions due to Hymenoptera stings-an EAACI Task Force Consensus Statement. Allergy 2016, 71, 931–943. [Google Scholar] [CrossRef] [Green Version]
- Jendoubi, F.; Gaudenzio, N.; Gallini, A.; Negretto, M.; Paul, C.; Bulai Livideanu, C. Omalizumab in the treatment of adult patients with mastocytosis: A systematic review. Clin. Exp. Allergy 2020, 50, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Olynyc, T.; Chapdelaine, H.; Segal, L.; Miedzybrodzki, B.; Ben-Shoshan, M. Effective management of severe cutaneous mastocytosis in young children with omalizumab (Xolair(R)). Clin. Exp. Dermatol. 2018, 43, 573–576. [Google Scholar] [CrossRef]
- Distler, M.; Maul, J.T.; Steiner, U.C.; Jandus, P.; Kolios, A.G.A.; Murer, C.; Graf, N.; Seebach, J.D.; Pichler, W.J.; Navarini, A.A.; et al. Efficacy of omalizumab in mastocytosis: Allusive indication obtained from a prospective, double-blind, multicenter study (XOLIMA study). Dermatology 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Broesby-Olsen, S.; Vestergaard, H.; Mortz, C.G.; Jensen, B.; Havelund, T.; Hermann, A.P.; Siebenhaar, F.; Møller, M.B.; Kristensen, T.K.; Bindslev-Jensen, C.; et al. Omalizumab prevents anaphylaxis and improves symptoms in systemic mastocytosis: Efficacy and safety observations. Allergy 2018, 73, 230–238. [Google Scholar] [CrossRef]
- Constantine, G.M.; Bressler, P.B.; Petroni, D.; Metcalfe, D.D.; Carter, M.C. Twelve-year follow-up of omalizumab therapy for anaphylaxis in 2 patients with systemic mastocytosis. J. Allergy Clin. Immunol. Pract. 2019, 7, 1314–1316. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, K.M.; Moser, A.; Lohse, P.; Winkler, A.; Binder, B.; Sovinz, P.; Lackner, H.; Schwinger, W.; Benesch, M.; Urban, C. Successful treatment of progressive cutaneous mastocytosis with imatinib in a 2-year-old boy carring a somatic KIT mutation. Blood 2008, 112, 1655–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parente, R.; Pucino, V.; Magliacane, D.; Petraroli, A.; Loffredo, S.; Marone, G.; Triggiani, M. Evaluation of vaccination safety in children with mastocytosis. Pediatr. Allergy Immunol. 2017, 28, 93–95. [Google Scholar] [CrossRef] [Green Version]
- Abuhay, H.; Clark, A.S.; Carter, M.C. Occurrence of unexpected adverse reactions to vaccines in children with mastocytosis. J. Pediatr. Res. 2020, 7, 81–86. [Google Scholar] [CrossRef]
- Nilsson, L.; Brockow, K.; Alm, J.; Cardona, V.; Caubet, J.-C.; Gomes, E.; Jenmalm, M.C.; Lau, S.; Netterlid, E.; Schwarze, J.; et al. Vaccination and allergy: EAACI position paper, practical aspects. Pediatr. Allergy Immunol. 2017, 28, 628–640. [Google Scholar] [CrossRef]
- Edwards, A.M.; Capková, S. Oral and topical sodium cromoglicate in the treatment of diffuse cutaneous mastocytosis in an infant. BMJ Case Rep. 2011, 2011, bcr0220113910. [Google Scholar] [CrossRef] [Green Version]
- Vieira Dos Santos, R.; Magerl, M.; Martus, P.; Zuberbier, T.; Church, M.K.; Escribano, L.; Maurer, M. Topical sodium cromoglicate relieves allergen- and histamine-induced dermal pruritus. Br. J. Dermatol. 2010, 162, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Patrizi, A.; Tabanelli, M.; Neri, I.; Virdi, A. Topical corticosteroids versus “wait and see” in the management of solitary mastocytoma in pediatric patients: A long-term follow-up. Dermatol. Ther. 2015, 28, 57–61. [Google Scholar] [CrossRef]
- Mashiah, J.; Harel, A.; Bodemer, C.; Hadj-Rabia, S.; Goldberg, I.; Sprecher, E.; Kutz, A. Topical pimecrolimus for pediatric cutaneous mastocytosis. Clin. Exp. Dermatol. 2018, 43, 559–565. [Google Scholar] [CrossRef]
- Correia, O.; Duarte, A.; Quirino, P.; Azevedo, R.; Delgado, L. Cutaneous mastocytosis: Two pediatric cases treated with topical pimecrolimus. Dermatol. Online J. 2010, 16, 8. [Google Scholar]
- Stege, H.; Schopf, E.; Ruzicka, T.; Krutmann, J. High-dose UVA1 for urticaria pigmentosa. Lancet 1996, 347, 64. [Google Scholar] [CrossRef]
- Husain, Z.; Waterman, D.; Ellison, K.; DeSimone, J.A. Management of poorly controlled indolent systemic mastocytosis using narrowband UVB phototherapy. Cutis 2017, 99, 30–33. [Google Scholar]
- Brazzelli, V.; Grassi, S.; Merante, S.; Grasso, V.; Ciccocioppo, R.; Bossi, G.; Borroni, G. Narrow-band UVB phototherapy and psoralen-ultraviolet A photochemotherapy in the treatment of cutaneous mastocytosis: A study in 20 pateints. Photodermatol. Photoimmunol. Photomed. 2016, 32, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Eustace, K.; Dolman, S.; Alsharqi, A.; Sharpe, G.; Parslew, R. Use of Phototherapy in Children. Pediatr. Dermatol. 2017, 34, 150–155. [Google Scholar] [CrossRef]
- Siiskonen, H.; Smorodchenko, A.; Krause, K.; Maurer, M. Ultraviolet radiation and skin mast cells: Effects, mechanisms and relevance for skin diseases. Exp. Dermatol. 2018, 27, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Grimbaldeston, M.A.; Simpson, A.; Finlay-Jones, P.H.; Hart, P.H. The effect of ultraviolet radiation exposure on the prevalence of mast cells in human skin. Br. J. Dermatol. 2003, 148, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Gobello, T.; Mazzanti, C.; Sordi, D.; Annessi, G.; Abeni, D.; Chinni, L.; Girolomoni, G. Medium-versus high-dose ultraviolet A1 therapy for urticaria pigmentosa: A pilot study. J. Am. Acad. Dermatol. 2003, 4, 679–684. [Google Scholar] [CrossRef]
- Ghul, S.; Stefaniak, R.; Strathmann, M.; Babina, M.; Piazena, H.; Henz, B.M.; Zuberbier, T. Bivalent effect of UV light on human skin mast cells-low-level mediator release at baseline but potent suppression upon mast cell triggering. J. Investig. Dermatol. 2005, 2, 453–456. [Google Scholar] [CrossRef] [Green Version]
- Hart, P.H.; Grombaldeston, M.A.; Finlay-Jones, J.J. Mast cells in UV-B-induced immunosuppression. J. Photochem. Photobiol. 2000, 55, 81–87. [Google Scholar] [CrossRef]
- Smith, M.L.; Orton, P.W.; Chu, H.; Weston, W.L. Photochemotherapy of dominant, diffuse, cutaneous mastocytosis. Pediatr. Dermatol. 1990, 7, 251–255. [Google Scholar] [CrossRef]
- Kinsler, V.A.; Hawk, J.L.M.; Atherton, D.J. Diffuse cutaneous mastocytosis treated with psoralen photochemtherapy: Case report and review of literaturę. Br. J. Dermatol. 2005, 152, 179–180. [Google Scholar] [PubMed]
- Archier, E.; Devaux, S.; Castela, E.; Gallini, A.; Aubin, F.; Le Maitre, M.; Aractingi, S.; Bachelez, H.; Cribier, B.; Joly, P.; et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: A systematic literature review. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 22–31. [Google Scholar] [CrossRef]
- Le, M.; Miedzybrodzki, B.; Olynych, T.; Chapdelaine, H.; Ben-Shoshan, M. Natural history and treatment of cutaneous and systemic mastocytosis. Postgrad. Med. 2017, 129, 896–901. [Google Scholar] [CrossRef]
- Brockow, K. Epidemiology, prognosis, and risk factors in mastocytosis. Immunol. Allergy Clin. N. Am. 2014, 34, 283–295. [Google Scholar] [CrossRef]
- Morgado, J.M.; Sánchez-Muñoz, L.; Matito, A.; Mollejo, M.; Escribano, L.; Alvarez-Twose, I. Patterns of Expression of CD25 and CD30 on Skin Mast Cells in Pediatric Mastocytosis. J. Contemp. Immunol. 2014, 1, 46–56. [Google Scholar] [CrossRef]
- Greenberger, S.; Landov, H.; Confino, Y.; Vaknine, H.; Avivi, C.; Baum, S.; Barzilai, A. Immunophenotype of pediatric-onset mastocytosis does not correlate with clinical course. Pediatr. Dermatol. 2019, 36, 477–481. [Google Scholar] [CrossRef]
| Reference | Diagnosis, Category of SM | KIT Mutation in BM | Cytogenetics, Molecular Diagnostics | Age Sex | Cutaneous Involvement, Type of Lesions |
|---|---|---|---|---|---|
| Intzes S. et al. (2011) [45] | SM-AHN (AML) | D816V (−) | t(8;21)(q22;q22) | 5 years F | no |
| Mahadeo K.M. et al. (2011) [51] | SM-AHN (AML) | D816V (−) No mutation in exon 8, 9, 11, 13, 17 | t(8;21)(q22;q22) | 10 years F | no |
| Yabe M. et al. (2012) [56] | SM-AHN (AML) | D816A (+) | t(8;21)(q22;q22) | 5 years F | no |
| Gadage V.S. et al. (2012) [48] | SM-AHN (AML) | D816V: ND | t(8;21)(q22;q22) | 14 years F | no |
| Gogia A. et al. (2013) [53] | SM-AHN (AML) | Codon 816 (−) | 46,XX | 3 years F | maculopapular lesions |
| Rabade N. et al. (2016) [54] | SM-AHN (AML) | D816V (−) | t(8;21)(q22;q22) | 7 years F | no |
| Mitchell S.G. et al. (2017) [57] | SM-AHN (CMML) | D816H (+) | 49,XX,+8+8, der(8)t(1;8)(q21;p11.2), +12,i(12)(p10) | 13 years F | no |
| Huang A. et al. (2017) [58] | ASM | D816V (+) | ND | 1 month M | diffuse lesions |
| Zheng Y. et al. (2018) [55] | MCL | D816V (−) | 47,XY+5,t(1;9) | 13 years M | no |
| Reference | Number of Patients | Number of SM Patients (%) | Form of SM |
|---|---|---|---|
| Bodemer C. et al. (2010) [19] | 65 | 1 (1.5) | no data reported |
| Alvarez-Twose I. et al. (2012) [7] | 111 | 2 (1.8) | 1 ISM, 1 WDSM ** |
| Lange M. et al. (2013) [12] | 101 | 1 (1) | 1 ISM |
| Méni C. et al. (2015) [8] * | 1747 | 16 (0.9) | 4 ISM; 8 MCS; 4 MCL |
| Carter M. et al. (2015) [9] | 108 | 18 (16.6) | 18 ISM |
| Matito A. et al. (2015) [125] | 42 | 2 (4.8) | 2 WDSM ** |
| Méni C. et al. (2018) [16] | 53 | 1 (1.9) | 1 ISM |
| Carter M. et al. (2018) [10] | 65 | 23 (35.4) | 23 ISM |
| Czarny J. et al. (2020) [11] *** | 32 | 4 (12.5) | 1 SSM, 3 ISM |
| Environmental and General Factors: |
| Physical: friction, pressure, cold, heat, sudden temperature change |
| Nutrition: alcohol, caffeine, hot spices, rarely also fermented and matured foods (histamine-rich) * |
| Infectious diseases and fever (typically viral infections) |
| Teething |
| Emotional stress |
| Intensive exercise |
| Allergens: |
| Hymenoptera venoms and allergens that can be unique for the individual patient |
| (pollens, animal dander, molds, dust mite, food, among others) |
| Drugs: |
| Analgesics (i.e., aspirin, NSAIDs) |
| Opioids (i.e., morphine, codeine) |
| Muscle relaxants (i.e., atracurium, mivacurium, rocuronium) |
| Cough suppressants (i.e., dextromethorphan, codeine) |
| Contrast media (i.e., hyperosmolar and ionic contrast media) |
| Antibiotics (i.e., quinolones) |
| Vaccinations |
| Systems and Symptoms | First-Line Therapy | Other Therapeutic Options |
|---|---|---|
| Skin: pruritus flushing, blistering | HR1-antagonists | HR2-antagonists Oral corticosteroids (short course) Topical corticosteroids (class 1–3, short cycles ± occlusion) Leukotriene antagonist Pimecrolimus cream Topical sodium cromolyn Excision (for mastocytoma) NB-UVB * or PUVA ** Local care (for blistering) |
| Gastrointestinal: diarrhea, abdominal cramping/pain, reflux, ulceration | HR2-antagonists | Proton pump inhibitors Oral sodium cromolyn Oral corticosteroids |
| Neuro/psychiatric: headache, poor concentration, cognitive impairment | HR1 and HR2-antagonists | Neuro/psychiatric treatment specific for the individual patient according to symptoms |
| Cardiovascular: presyncope, syncope, hypotension | HR1 and HR2-antagonists | Oral corticosteroids Epinephrine |
| Osteopenia/osteoporosis | Calcium, vitamin D3 | Treatment specific for the individual patient according to the age and T-score |
| Anaphylaxis | Epinephrine | Acute anaphylaxis: HR1 and HR2-antagonists Oral corticosteroids Intravenous fluids Prevention of anaphylaxis: Epinephrine auto-injector HR1-antagonists Allergen specific immunotherapy (typically Hymenoptera venom) Omalizumab (for recurrent episodes) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lange, M.; Hartmann, K.; Carter, M.C.; Siebenhaar, F.; Alvarez-Twose, I.; Torrado, I.; Brockow, K.; Renke, J.; Irga-Jaworska, N.; Plata-Nazar, K.; et al. Molecular Background, Clinical Features and Management of Pediatric Mastocytosis: Status 2021. Int. J. Mol. Sci. 2021, 22, 2586. https://doi.org/10.3390/ijms22052586
Lange M, Hartmann K, Carter MC, Siebenhaar F, Alvarez-Twose I, Torrado I, Brockow K, Renke J, Irga-Jaworska N, Plata-Nazar K, et al. Molecular Background, Clinical Features and Management of Pediatric Mastocytosis: Status 2021. International Journal of Molecular Sciences. 2021; 22(5):2586. https://doi.org/10.3390/ijms22052586
Chicago/Turabian StyleLange, Magdalena, Karin Hartmann, Melody C. Carter, Frank Siebenhaar, Ivan Alvarez-Twose, Inés Torrado, Knut Brockow, Joanna Renke, Ninela Irga-Jaworska, Katarzyna Plata-Nazar, and et al. 2021. "Molecular Background, Clinical Features and Management of Pediatric Mastocytosis: Status 2021" International Journal of Molecular Sciences 22, no. 5: 2586. https://doi.org/10.3390/ijms22052586
APA StyleLange, M., Hartmann, K., Carter, M. C., Siebenhaar, F., Alvarez-Twose, I., Torrado, I., Brockow, K., Renke, J., Irga-Jaworska, N., Plata-Nazar, K., Ługowska-Umer, H., Czarny, J., Belloni Fortina, A., Caroppo, F., Nowicki, R. J., Nedoszytko, B., Niedoszytko, M., & Valent, P. (2021). Molecular Background, Clinical Features and Management of Pediatric Mastocytosis: Status 2021. International Journal of Molecular Sciences, 22(5), 2586. https://doi.org/10.3390/ijms22052586










