An Overview of the Molecular Mechanisms Contributing to Musculoskeletal Disorders in Chronic Liver Disease: Osteoporosis, Sarcopenia, and Osteoporotic Sarcopenia
Abstract
1. Introduction
2. Osteoporosis in Chronic Liver Disease
2.1. Prevalence and Clinical Outcomes of Osteoporosis in Chronic Liver Disease
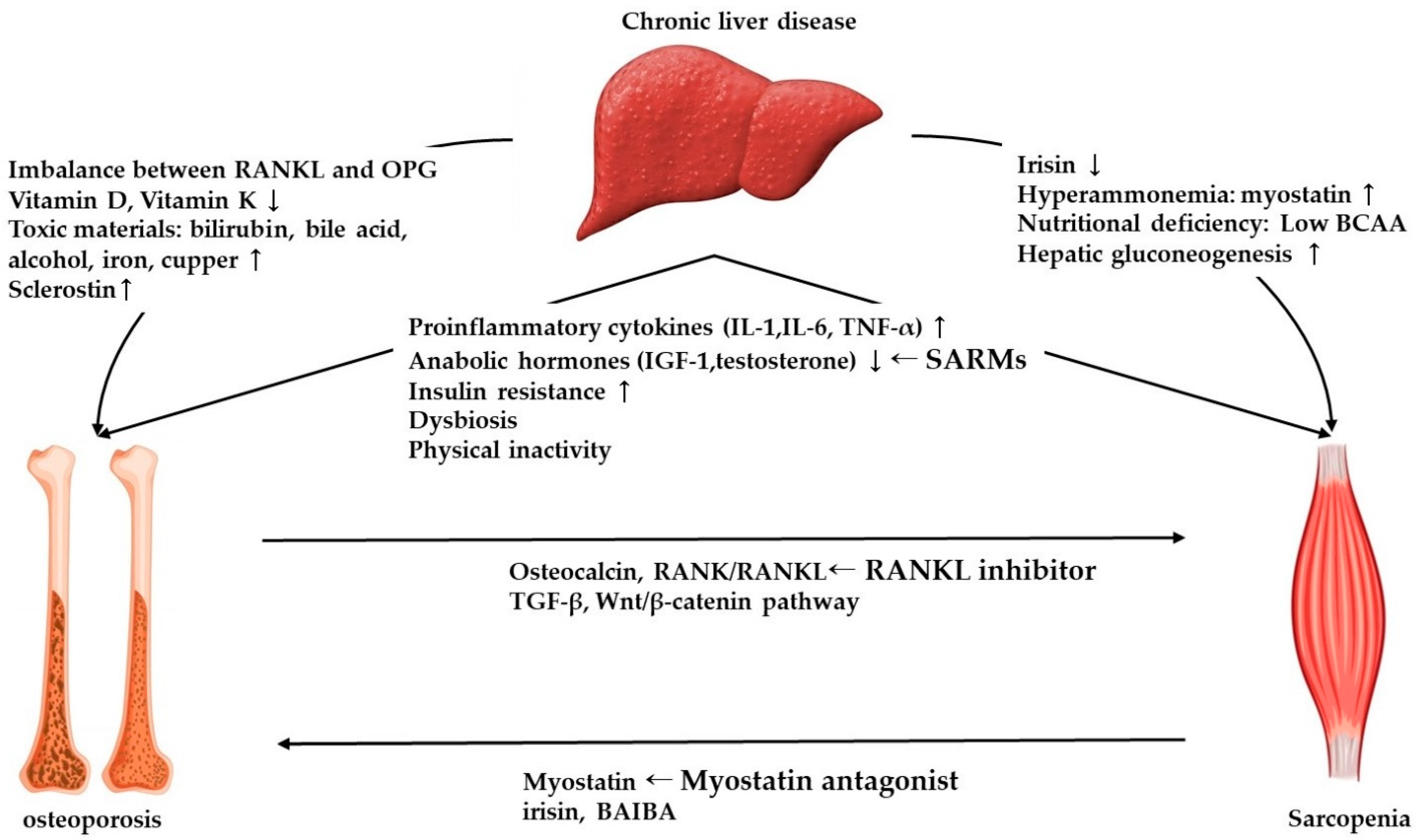
2.2. Molecular Mechanism of Osteoporosis in Chronic Liver Disease
3. Sarcopenia in Chronic Liver Disease
3.1. Definition of Sarcopenia
3.2. Prevalence and Clinical Outcomes of Sarcopenia in Chronic Liver Disease
3.3. Molecular Mechanism of Sarcopenia in Chronic Liver Disease
3.3.1. Sarcopenia in Non-Cirrhotic Liver Disease
3.3.2. Sarcopenia in Liver Cirrhosis
4. Osteosarcopenia
4.1. Prevalence of Osteosarcopenia
4.2. Crosstalk between Osteoporosis and Sarcopenia
4.3. Management
4.3.1. Exercise and Nutritional Support
4.3.2. Pharmacological Treatment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guañabens, N.; Parés, A. Osteoporosis in chronic liver disease. Liver Int. 2018, 38, 776–785. [Google Scholar] [CrossRef]
- Jeong, H.M.; Kim, D.J. Bone Diseases in Patients with Chronic Liver Disease. Int. J. Mol. Sci. 2019, 20, 4270. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Bhanji, R.A.; Mazurak, V.C.; Montano-Loza, A.J. Sarcopenia in cirrhosis: From pathogenesis to interventions. J. Gastroenterol. 2019, 54, 845–859. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Bannert, K.; Wiese, M.; Esau, S.; Sautter, L.F.; Ehlers, L.; Aghdassi, A.A.; Metges, C.C.; Garbe, L.-A.; Jaster, R.; et al. Molecular Mechanism Contributing to Malnutrition and Sarcopenia in Patients with Liver Cirrhosis. Int. J. Mol. Sci. 2020, 21, 5357. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Mincone, T.; Contreras-Briceño, F.; Espinosa-Ramírez, M.; García-Valdés, P.; López-Fuenzalida, A.; Riquelme, A.; Arab, J.P.; Cabrera, D.; Arrese, M.; Barrera, F. Nonalcoholic fatty liver disease and sarcopenia: Pathophysiological connections and therapeutic implications. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 1–17. [Google Scholar] [CrossRef]
- Jindal, A.; Jagdish, R.K. Sarcopenia: Ammonia metabolism and hepatic encephalopathy. Clin. Mol. Hepatol. 2019, 25, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Carey, E.J.; Lai, J.C.; Sonnenday, C.; Tapper, E.B.; Tandon, P.; Duarte-Rojo, A.; Dunn, M.A.; Tsien, C.; Kallwitz, E.R.; Ng, V.; et al. A North American Expert Opinion Statement on Sarcopenia in Liver Transplantation. Hepatology 2019, 70, 1816–1829. [Google Scholar] [CrossRef]
- Hsu, C.-S.; Kao, J.-H. Sarcopenia and chronic liver diseases. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 1229–1244. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S.; Merli, M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J. Hepatol. 2016, 65, 1232–1244. [Google Scholar] [CrossRef]
- Kirk, B.; Zanker, J.; Duque, G. Osteosarcopenia: Epidemiology, diagnosis, and treatment—Facts and numbers. J. Cachex-Sarcopenia Muscle 2020, 11, 609–618. [Google Scholar] [CrossRef]
- Kirk, B.; Al Saedi, A.; Duque, G. Osteosarcopenia: A case of geroscience. Aging Med. 2019, 2, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L. Use it or lose it to age: A review of bone and muscle communication. Bone 2019, 120, 212–218. [Google Scholar] [CrossRef]
- Picca, A.; Calvani, R.; Manes-Gravina, E.; Spaziani, L.; Landi, F.; Bernabei, R.; Marzetti, E. Bone-Muscle Crosstalk: Unraveling New Therapeutic Targets for Osteoporosis. Curr. Pharm. Des. 2018, 23, 6256–6263. [Google Scholar] [CrossRef]
- Hirschfeld, H.P.; Kinsella, R.; Duque, G. Osteosarcopenia: Where bone, muscle, and fat collide. Osteoporos. Int. 2017, 28, 2781–2790. [Google Scholar] [CrossRef]
- Kawao, N.; Kaji, H. Interactions Between Muscle Tissues and Bone Metabolism. J. Cell. Biochem. 2015, 116, 687–695. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Wang, Y.; Zhan, J.-K.; Tang, Z.-Y.; He, J.-Y.; Tan, P.; Deng, H.-Q.; Huang, W.; Liu, Y.-S. Sarco-Osteoporosis: Prevalence and Association with Frailty in Chinese Community-Dwelling Older Adults. Int. J. Endocrinol. 2015, 2015, 482940. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-I.; Kim, H.; Ha, Y.-C.; Kwon, H.-B.; Koo, K.-H. Osteosarcopenia in Patients with Hip Fracture Is Related with High Mortality. J. Korean Med. Sci. 2018, 33, e27. [Google Scholar] [CrossRef] [PubMed]
- Collier, J. Bone disorders in chronic liver disease. Hepatology 2007, 46, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Menon, K.; Angulo, P.; Weston, S.; Dickson, E.; Lindor, K.D. Bone disease in primary biliary cirrhosis: Independent indicators and rate of progression. J. Hepatol. 2001, 35, 316–323. [Google Scholar] [CrossRef]
- Guañabens, N.; Parés, A.; Ros, I.; Caballería, L.; Pons, F.; Vidal, S.; Monegal, A.; Peris, P.; Rodés, J. Severity of cholestasis and advanced histological stage but not menopausal status are the major risk factors for osteoporosis in primary biliary cirrhosis. J. Hepatol. 2005, 42, 573–577. [Google Scholar] [CrossRef]
- Angulo, P.; Grandison, G.A.; Fong, D.G.; Keach, J.C.; Lindor, K.D.; Bjornsson, E.; Koch, A. Bone Disease in Patients with Primary Sclerosing Cholangitis. Gastroenterology 2011, 140, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Solaymani—Dodaran, M.; Card, T.R.; Aithal, G.P.; West, J. Fracture Risk in People with Primary Biliary Cirrhosis: A Population-Based Cohort Study. Gastroenterology 2006, 131, 1752–1757. [Google Scholar] [CrossRef] [PubMed]
- Guañabens, N.; Cerdá, D.; Monegal, A.; Pons, F.; Caballería, L.; Peris, P.; Parés, A. Low Bone Mass and Severity of Cholestasis Affect Fracture Risk in Patients with Primary Biliary Cirrhosis. Gastroenterology 2010, 138, 2348–2356. [Google Scholar] [CrossRef] [PubMed]
- Orsini, L.G.S.; Pinheiro, M.M.; Castro, C.H.M.; Silva, A.E.B.; Szejnfeld, V.L. Bone Mineral Density Measurements, Bone Markers and Serum Vitamin D Concentrations in Men with Chronic Non-Cirrhotic Untreated Hepatitis C. PLoS ONE 2013, 8, e81652. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Shoback, D.M.; Zipperstein, J.; Lizaola, B.; Tseng, S.; Terrault, N.A. Bone Mineral Density, Bone Turnover, and Systemic Inflammation in Non-cirrhotics with Chronic Hepatitis C. Dig. Dis. Sci. 2015, 60, 1813–1819. [Google Scholar] [CrossRef]
- Huang, Z.; Wei, H.; Cheng, C.; Yang, S.; Wang, J.; Liu, X. Low bone mineral density in chronic hepatitis B virus infection: A case-control study. Pak. J. Med. Sci. 2017, 33, 457–461. [Google Scholar] [CrossRef]
- Wei, M.T.; Le, A.K.; Chang, M.S.; Hsu, H.; Nguyen, P.; Zhang, J.Q.; Wong, C.; Wong, C.; Cheung, R.; Nguyen, M.H. Antiviral therapy and the development of osteopenia/osteoporosis among Asians with chronic hepatitis B. J. Med. Virol. 2019, 91, 1288–1294. [Google Scholar] [CrossRef]
- Schiefke, I.; Fach, A.; Wiedmann, M.; Aretin, A.V.; Schenker, E.; Borte, G.; Wiese, M.; Moessner, J. Reduced bone mineral density and altered bone turnover markers in patients with non-cirrhotic chronic hepatitis B or C infection. World J. Gastroenterol. 2005, 11, 1843–1847. [Google Scholar] [CrossRef]
- Hansen, A.-B.E.; Omland, L.H.; Krarup, H.; Obel, N. Fracture risk in hepatitis C virus infected persons: Results from the DANVIR cohort study. J. Hepatol. 2014, 61, 15–21. [Google Scholar] [CrossRef]
- Goubraim, R.; Kabbaj, N.; Salihoun, M.; Chaoui, Z.; Nya, M.; Amrani, N. Metabolic Bone Disease in Viral Cirrhosis: A Prospective Study. ISRN Hepatol. 2013, 2013, 276563. [Google Scholar] [CrossRef]
- Sokhi, R.P.; Anantharaju, A.; Kondaveeti, R.; Creech, S.D.; Islam, K.K.; Van Thiel, D.H. Bone mineral density among cirrhotic patients awaiting liver transplantation. Liver Transplant. 2004, 10, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-P.; Miao, H.-X.; Zheng, S.-W.; Liu, W.-L.; Chen, C.-Q.; Zhong, H.-B.; Li, S.-F.; Fang, Y.-P.; Sun, C.-H. Risk factors for osteoporosis in liver cirrhosis patients measured by transient elastography. Medicine 2018, 97, e10645. [Google Scholar] [CrossRef] [PubMed]
- Monegal, A.; Navasa, M.; Guañabens, N.; Peris, P.; Pons, F.; De Osaba, M.J.M.; Rimola, A.; Rodés, J.; Muñoz-Gómez, J. Osteoporosis and Bone Mineral Metabolism Disorders in Cirrhotic Patients Referred for Orthotopic Liver Transplantation. Calcif. Tissue Int. 1997, 60, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Monegal, A.; Navasa, M.; Guañabens, N.; Peris, P.; Pons, F.; De Osaba, M.J.M.; Ordi, J.; Rimola, A.; Rodés, J.; Muñoz-Gómez, J. Bone Disease after Liver Transplantation: A Long-Term Prospective Study of Bone Mass Changes, Hormonal Status and Histomorphometric Characteristics. Osteoporos. Int. 2001, 12, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Guichelaar, M.M.J.; Kendall, R.; Malinchoc, M.; Hay, J.E. Bone mineral density before and after OLT: Long-term follow-up and predictive factors. Liver Transplant. 2006, 12, 1390–1402. [Google Scholar] [CrossRef]
- Li, M.; Xu, Y.; Xu, M.; Ma, L.; Wang, T.; Liu, Y.; Dai, M.; Chen, Y.; Lu, J.; Liu, J.; et al. Association between Nonalcoholic Fatty Liver Disease (NAFLD) and Osteoporotic Fracture in Middle-Aged and Elderly Chinese. J. Clin. Endocrinol. Metab. 2012, 97, 2033–2038. [Google Scholar] [CrossRef]
- Purnak, T.; Beyazit, Y.; Ozaslan, E.; Efe, C.; Hayretci, M.; Ozaslan, A.P.E. The evaluation of bone mineral density in patients with nonalcoholic fatty liver disease. Wien. Klin. Wochenschr. 2012, 124, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kim, K.J.; Rhee, Y.; Lim, S.-K. Significant liver fibrosis assessed using liver transient elastography is independently associated with low bone mineral density in patients with non-alcoholic fatty liver disease. PLoS ONE 2017, 12, e0182202. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Seo, D.H.; Kim, S.H.; Nam, M.-S.; Hong, S. The relationship between fatty liver index and bone mineral density in Koreans: KNHANES 2010–2011. Osteoporos. Int. 2017, 29, 181–190. [Google Scholar] [CrossRef]
- Malik, P.; Gasser, R.W.; Kemmler, G.; Moncayo, R.; Finkenstedt, G.; Kurz, M.; Fleischhacker, W.W. Low Bone Mineral Density and Impaired Bone Metabolism in Young Alcoholic Patients without Liver Cirrhosis: A Cross-Sectional Study. Alcohol. Clin. Exp. Res. 2009, 33, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Peris, P.; Guañabens, N.; Parés, A.; Pons, F.; Del Rio, L.; Monegal, A.; Surís, X.; Caballería, J.; Rodés, J.; Muñoz-Gómez, J. Vertebral fractures and osteopenia in chronic alcoholic patients. Calcif. Tissue Int. 1995, 57, 111–114. [Google Scholar] [CrossRef]
- Peris, P.; Pares, A.; Guanabens, N.; Del Rio, L.; Pons, F.; De Osaba, M.J.M.; Monegal, A.; Caballería, J.; Rodés, J.; Muñoz-Gómez, J. Bone mass improves in alcoholics after 2 years of abstinence. J. Bone Miner. Res. 1994, 9, 1607–1612. [Google Scholar] [CrossRef]
- Sinigaglia, L.; Fargion, S.; Fracanzani, A.L.; Binelli, L.; Battafarano, N.; Varenna, M.; Piperno, A.; Fiorelli, G. Bone and joint involvement in genetic hemochromatosis: Role of cirrhosis and iron overload. J. Rheumatol. 1997, 24, 1809–1813. [Google Scholar]
- Guggenbuhl, P.; Deugnier, Y.; Boisdet, J.F.; Rolland, Y.; Perdriger, A.; Pawlotsky, Y.; Chalès, G. Bone mineral density in men with genetic hemochromatosis and HFE gene mutation. Osteoporos. Int. 2005, 16, 1809–1814. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Varenna, M.; Fracanzani, A.L.; Rossi, V.; Fargion, S.; Sinigaglia, L. Association between iron overload and osteoporosis in patients with hereditary hemochromatosis. Osteoporos. Int. 2009, 20, 549–555. [Google Scholar] [CrossRef]
- Weiss, K.H.; Van De Moortele, M.; Gotthardt, D.N.; Pfeiffenberger, J.; Seessle, J.; Ullrich, E.; Gielen, E.; Borghs, H.; Adriaens, E.; Stremmel, W.; et al. Bone demineralisation in a large cohort of Wilson disease patients. J. Inherit. Metab. Dis. 2015, 38, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Quemeneur, A.-S.; Trocello, J.-M.; Ea, H.-K.; Ostertag, A.; Leyendecker, A.; Duclos-Vallee, J.-C.; De Vernejoul, M.-C.; Woimant, F.; Lioté, F. Bone status and fractures in 85 adults with Wilson’s disease. Osteoporos. Int. 2014, 25, 2573–2580. [Google Scholar] [CrossRef]
- Danford, C.J.; Trivedi, H.D.; Bonder, A. Bone Health in Patients with Liver Diseases. J. Clin. Densitom. 2020, 23, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Danford, C.J.; Trivedi, H.D.; Papamichael, K.; Tapper, E.B.; Bonder, A. Osteoporosis in primary biliary cholangitis. World J. Gastroenterol. 2018, 24, 3513–3520. [Google Scholar] [CrossRef] [PubMed]
- Raggatt, L.J.; Partridge, N.C. Cellular and Molecular Mechanisms of Bone Remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef]
- Ono, T.; Hayashi, M.; Sasaki, F.; Nakashima, T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020, 40, 1–16. [Google Scholar] [CrossRef]
- Santos, L.A.A.; Romeiro, F.G. Diagnosis and Management of Cirrhosis-Related Osteoporosis. BioMed Res. Int. 2016, 2016, 1423462. [Google Scholar] [CrossRef]
- Moschen, A.R.; Kaser, A.; Stadlmann, S.; Millonig, G.; Kaser, S.; Mühllechner, P.; Habior, A.; Graziadei, I.; Vogel, W.; Tilg, H. The RANKL/OPG system and bone mineral density in patients with chronic liver disease. J. Hepatol. 2005, 43, 973–983. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gaspà, S.; Martinez-Ferrer, A.; Guañabens, N.; Dubreuil, M.; Peris, P.; Enjuanes, A.; De Osaba, M.J.M.; Alvarez, L.; Monegal, A.; Combalia, A.; et al. Effects of bilirubin and sera from jaundiced patients on osteoblasts: Contribution to the development of osteoporosis in liver diseases. Hepatology 2011, 54, 2104–2113. [Google Scholar] [CrossRef]
- Blaschke, M.; Koepp, R.; Cortis, J.; Komrakova, M.; Schieker, M.; Hempel, U.; Siggelkow, H. IL-6, IL-1β, and TNF-α only in combination influence the osteoporotic phenotype in Crohn’s patients via bone formation and bone resorption. Adv. Clin. Exp. Med. 2018, 27, 45–56. [Google Scholar] [CrossRef]
- Lorenzo, J.; Horowitz, M.; Choi, Y. Osteoimmunology: Interactions of the Bone and Immune System. Endocr. Rev. 2008, 29, 403–440. [Google Scholar] [CrossRef]
- Wu, Q.; Zhou, X.; Huang, D.; Ji, Y.; Kang, F. IL-6 Enhances Osteocyte-Mediated Osteoclastogenesis by Promoting JAK2 and RANKL Activity In Vitro. Cell. Physiol. Biochem. 2017, 41, 1360–1369. [Google Scholar] [CrossRef]
- Nakchbandi, I.A.; Mitnick, M.A.; Lang, R.; Gundberg, C.; Kinder, B.; Insogna, K. Circulating Levels of Interleukin-6 Soluble Receptor Predict Rates of Bone Loss in Patients with Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2002, 87, 4946–4951. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, X.; Zhou, Z.-Y.; Zhang, Y.-Y.; Yang, H.-L. IL-6 Contributes to the Defective Osteogenesis of Bone Marrow Stromal Cells from the Vertebral Body of the Glucocorticoid-Induced Osteoporotic Mouse. PLoS ONE 2016, 11, e0154677. [Google Scholar] [CrossRef] [PubMed]
- Axmann, R.; Böhm, C.; Krönke, G.; Zwerina, J.; Smolen, J.; Schett, G. Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis Rheum. 2009, 60, 2747–2756. [Google Scholar] [CrossRef]
- Norris, C.A.; He, M.; Kang, L.-I.; Ding, M.Q.; Radder, J.E.; Haynes, M.M.; Yang, Y.; Paranjpe, S.; Bowen, W.C.; Orr, A.; et al. Synthesis of IL-6 by Hepatocytes Is a Normal Response to Common Hepatic Stimuli. PLoS ONE 2014, 9, e96053. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Matsumata, T.; Taketomi, A.; Shirabe, K.; Yamamoto, K.; Takenaka, K.; Sugimachi, K. The role of interleukin-6, interleukin-16, tumor necrosis factor-alpha and endotoxin in hepatic resection. Hepatogastroenterology 1995, 42, 691–697. [Google Scholar] [PubMed]
- Guarino, M.; Loperto, I.; Camera, S.; Cossiga, V.; Di Somma, C.; Colao, A.; Caporaso, N.; Morisco, F. Osteoporosis across chronic liver disease. Osteoporos. Int. 2016, 27, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Handzlik-Orlik, G.; Holecki, M.; Wilczyński, K.; Duława, J. Osteoporosis in liver disease: Pathogenesis and management. Ther. Adv. Endocrinol. Metab. 2016, 7, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Grimes, S.N.; Li, S.; Hu, X.; Ivashkiv, L.B. TNF-induced osteoclastogenesis and inflammatory bone resorption are inhibited by transcription factor RBP-J. J. Exp. Med. 2012, 209, 319–334. [Google Scholar] [CrossRef]
- Boyce, B.E.; Li, P.; Yao, Z.; Zhang, Q.; Badell, I.R.; Schwarz, E.M.; O’Keefe, R.J.; Xing, L. TNF.ALPHA. and pathologic bone resorption. Keio J. Med. 2005, 54, 127–131. [Google Scholar] [CrossRef] [PubMed]
- González-Calvin, J.L.; Gallego-Rojo, F.; Fernández-Pérez, R.; Casado-Caballero, F.; Ruiz-Escolano, E.; Olivares, E.G. Osteoporosis, Mineral Metabolism, and Serum Soluble Tumor Necrosis Factor Receptor p55 in Viral Cirrhosis. J. Clin. Endocrinol. Metab. 2004, 89, 4325–4330. [Google Scholar] [CrossRef]
- Filip, R.; Radzki, R.P.; Bieńko, M. Novel insights into the relationship between nonalcoholic fatty liver disease and osteoporosis. Clin. Interv. Aging 2018, 13, 1879–1891. [Google Scholar] [CrossRef]
- Manco, M.; Marcellini, M.; Giannone, G.; Nobili, V. Correlation of Serum TNF-α Levels and Histologic Liver Injury Scores in Pediatric Nonalcoholic Fatty Liver Disease. Am. J. Clin. Pathol. 2007, 127, 954–960. [Google Scholar] [CrossRef]
- McCaughan, G.; Feller, R. Osteoporosis in Chronic Liver Disease: Pathogenesis, Risk Factors, and Management. Dig. Dis. 1994, 12, 223–231. [Google Scholar] [CrossRef]
- Koshihara, Y.; Hoshi, K.; Okawara, R.; Ishibashi, H.; Yamamoto, S. Vitamin K stimulates osteoblastogenesis and inhibits osteoclastogenesis in human bone marrow cell culture. J. Endocrinol. 2003, 176, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Hiruma, Y.; Nakahama, K.-I.; Fujita, H.; Morita, I. Vitamin K2 and geranylgeraniol, its side chain component, inhibited osteoclast formation in a different manner. Biochem. Biophys. Res. Commun. 2004, 314, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Cockayne, S.; Adamson, J.; Lanham-New, S.; Shearer, M.J.; Gilbody, S.; Torgerson, D.J. Vitamin K and the prevention of fractures: Systematic review and meta-analysis of randomized controlled trials. Arch. Intern. Med. 2006, 166, 1256–1261. [Google Scholar] [CrossRef]
- Kowdley, K.V.; Emond, M.J.; Sadowski, J.A.; Kaplan, M.M. Plasma vitamin K1 level is decreased in primary biliary cirrhosis. Am. J. Gastroenterol. 1997, 92, 2059–2061. [Google Scholar] [PubMed]
- Nishiguchi, S.; Shimoi, S.; Kurooka, H.; Tamori, A.; Habu, D.; Takeda, T.; Ochi, H. Randomized pilot trial of vitamin K2 for bone loss in patients with primary biliary cirrhosis. J. Hepatol. 2001, 35, 543–545. [Google Scholar] [CrossRef]
- Ruiz-Gaspà, S.; Dubreuil, M.; Guañabens, N.; Combalia, A.; Peris, P.; Monegal, A.; Parés, A. Ursodeoxycholic acid decreases bilirubin-induced osteoblast apoptosis. Eur. J. Clin. Investig. 2014, 44, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Ukon, Y.; Makino, T.; Kodama, J.; Tsukazaki, H.; Tateiwa, D.; Yoshikawa, H.; Kaito, T. Molecular-Based Treatment Strategies for Osteoporosis: A Literature Review. Int. J. Mol. Sci. 2019, 20, 2557. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Sato, A.Y.; Bellido, T. Role and mechanism of action of sclerostin in bone. Bone 2017, 96, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R. Targeting Sclerostin in Postmenopausal Osteoporosis: Focus on Romosozumab and Blosozumab. BioDrugs 2017, 31, 289–297. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Kang, H.; Liu, W.; Liu, P.; Zhang, J.; Harris, S.E.; Wu, D. Sclerostin Binds to LRP5/6 and Antagonizes Canonical Wnt Signaling. J. Biol. Chem. 2005, 280, 19883–19887. [Google Scholar] [CrossRef] [PubMed]
- Guañabens, N.; Gifre, L.; Miquel, R.; Peris, P.; Monegal, A.; Dubrueil, M.; Arias, A.; Parés, A.; Ruiz-Gaspà, S. Sclerostin Expression in Bile Ducts of Patients with Chronic Cholestasis May Influence the Bone Disease in Primary Biliary Cirrhosis. J. Bone Miner. Res. 2016, 31, 1725–1733. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.; Kim, W.J.; Han, K.J.; Kil Lim, S.; Kim, S.H. Effect of liver dysfunction on circulating sclerostin. J. Bone Miner. Metab. 2013, 32, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, V.; Bianchi, V.E. Effect of GH/IGF-1 on Bone Metabolism and Osteoporsosis. Int. J. Endocrinol. 2014, 2014, 235060. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Uehara, S.; Udagawa, N.; Takahashi, N. Regulation of bone metabolism by Wnt signals. J. Biochem. 2016, 159, 387–392. [Google Scholar] [CrossRef]
- Guerra-Menéndez, L.; Sádaba, M.C.; Puche, J.E.; Lavandera, J.L.; de Castro, L.F.; de Gortázar, A.R.; Castilla-Cortázar, I. IGF-I increases markers of osteoblastic activity and reduces bone resorption via osteoprotegerin and RANK-ligand. J. Trans. Med. 2013, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- De La Garza, R.G.; Morales-Garza, L.A.; Martin-Estal, I.; Castilla-Cortazar, I. Insulin-Like Growth Factor-1 Deficiency and Cirrhosis Establishment. J. Clin. Med. Res. 2017, 9, 233–247. [Google Scholar] [CrossRef]
- Thrailkill, K.M.; Lumpkin, C.K.; Bunn, R.C.; Kemp, S.F.; Fowlkes, J.L. Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am. J. Physiol. Metab. 2005, 289, E735–E745. [Google Scholar] [CrossRef] [PubMed]
- Neong, S.F.; Billington, E.O.; Congly, S.E. Sexual Dysfunction and Sex Hormone Abnormalities in Patients with Cirrhosis: Review of Pathogenesis and Management. Hepatology 2018, 69, 2683–2695. [Google Scholar] [CrossRef] [PubMed]
- Golds, G.; Houdek, D.; Arnason, T. Male Hypogonadism and Osteoporosis: The Effects, Clinical Consequences, and Treatment of Testosterone Deficiency in Bone Health. Int. J. Endocrinol. 2017, 2017, 4602129. [Google Scholar] [CrossRef]
- Sarkar, M.; Lai, J.C.; Sawinski, D.; Zeigler, T.E.; Cedars, M.; Forde, K.A. Sex hormone levels by presence and severity of cirrhosis in women with chronic hepatitis C virus infection. J. Viral Hepat. 2018, 26, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Nakchbandi, I.A. Osteoporosis and fractures in liver disease: Relevance, pathogenesis and therapeutic implications. World J. Gastroenterol. 2014, 20, 9427–9438. [Google Scholar]
- Zumoff, B.; Fishman, J.; Gallagher, T.F.; Hellman, L. Estradiol metabolism in cirrhosis. J. Clin. Investig. 1968, 47, 20–25. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C.; Manini, T.M. Sarcopenia =/= dynapenia. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Jung, K.S.; Kim, S.U.; Yoon, H.-J.; Yun, Y.J.; Lee, B.-W.; Kang, E.S.; Han, K.-H.; Lee, H.C.; Cha, B.-S. Sarcopaenia is associated with NAFLD independently of obesity and insulin resistance: Nationwide surveys (KNHANES 2008–2011). J. Hepatol. 2015, 63, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.C.; Hwang, S.Y.; Choi, H.Y.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; Choi, D.S.; Choi, K.M. Relationship between sarcopenia and nonalcoholic fatty liver disease: The Korean Sarcopenic Obesity Study. Hepatology 2014, 59, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Carias, S.; Castellanos, A.L.; Vilchez, V.; Nair, R.; Cruz, A.C.D.; Watkins, J.; Barrett, T.; Trushar, P.; Esser, K.; Gedaly, R. Nonalcoholic steatohepatitis is strongly associated with sarcopenic obesity in patients with cirrhosis undergoing liver transplant evaluation. J. Gastroenterol. Hepatol. 2016, 31, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.K.; Kim, D.; Joo, S.K.; Kim, J.H.; Chang, M.S.; Kim, B.G.; Lee, K.L.; Kim, W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J. Hepatol. 2017, 66, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Gan, D.; Wang, L.; Jia, M.; Ru, Y.; Ma, Y.; Zheng, W.; Zhao, X.; Yang, F.; Wang, T.; Mu, Y.; et al. Low muscle mass and low muscle strength associate with nonalcoholic fatty liver disease. Clin. Nutr. 2020, 39, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Lee, S.; Lee, Y.; Jun, J.E.; Ahn, J.; Bae, J.C.; Jin, S.; Hur, K.Y.; Jee, J.H.; Lee, M.; et al. Relationship Between Relative Skeletal Muscle Mass and Nonalcoholic Fatty Liver Disease: A 7-Year Longitudinal Study. Hepatology 2018, 68, 1755–1768. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Ney, M.; Irwin, I.; Ma, M.M.; Gramlich, L.; Bain, V.G.; Esfandiari, N.; Baracos, V.; Montano-Loza, A.J.; Myers, R.P. Severe muscle depletion in patients on the liver transplant wait list: Its prevalence and independent prognostic value. Liver Transplant. 2012, 18, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J.; Meza-Junco, J.; Prado, C.M.; Lieffers, J.R.; Baracos, V.E.; Bain, V.G.; Sawyer, M.B. Muscle Wasting Is Associated with Mortality in Patients with Cirrhosis. Clin. Gastroenterol. Hepatol. 2012, 10, 166–173.e1. [Google Scholar] [CrossRef]
- Merli, M.; Giusto, M.; Lucidi, C.; Giannelli, V.; Pentassuglio, I.; Di Gregorio, V.; Lattanzi, B.; Riggio, O. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: Results of a prospective study. Metab. Brain Dis. 2013, 28, 281–284. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kim, M.Y.; Sohn, J.H.; Kim, S.M.; Ryu, J.A.; Lim, S.; Kim, Y. Sarcopenia as a Useful Predictor for Long-Term Mortality in Cirrhotic Patients with Ascites. J. Korean Med. Sci. 2014, 29, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Durand, F.; Buyse, S.; Francoz, C.; Laouénan, C.; Bruno, O.; Belghiti, J.; Moreau, R.; Vilgrain, V.; Valla, D. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J. Hepatol. 2014, 60, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Hanai, T.; Shiraki, M.; Nishimura, K.; Ohnishi, S.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M.; Moriwaki, H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrient 2015, 31, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J.; Duarte-Rojo, A.; Meza-Junco, J.; Baracos, V.E.; Sawyer, M.B.; Pang, J.X.Q.; Beaumont, C.; Esfandiari, N.; Myers, R.P. Inclusion of Sarcopenia Within MELD (MELD-Sarcopenia) and the Prediction of Mortality in Patients with Cirrhosis. Clin. Transl. Gastroenterol. 2015, 6, e102. [Google Scholar] [CrossRef]
- Hanai, T.; Shiraki, M.; Ohnishi, S.; Miyazaki, T.; Ideta, T.; Kochi, T.; Imai, K.; Suetsugu, A.; Takai, K.; Moriwaki, H.; et al. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol. Res. 2016, 46, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, S.; Lattanzi, B.; Torrisi, S.; Greco, F.; Farcomeni, A.; Gioia, S.; Merli, M.; Riggio, O. Sarcopenia Is Risk Factor for Development of Hepatic Encephalopathy after Transjugular Intrahepatic Portosystemic Shunt Placement. Clin. Gastroenterol. Hepatol. 2017, 15, 934–936. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Jeong, W.K.; Baik, S.K.; Cha, S.H.; Kim, M.Y. Impact of sarcopenia on prognostic value of cirrhosis: Going beyond the hepatic venous pressure gradient and MELD score. J. Cachex-Sarcopenia Muscle 2018, 9, 860–870. [Google Scholar] [CrossRef]
- Englesbe, M.J.; Patel, S.P.; He, K.; Lynch, R.J.; Schaubel, D.E.; Harbaugh, C.; Holcombe, S.A.; Wang, S.C.; Segev, D.L.; Sonnenday, C.J. Sarcopenia and Mortality after Liver Transplantation. J. Am. Coll. Surg. 2010, 211, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Kaido, T.; Ogawa, K.; Fujimoto, Y.; Ogura, Y.; Hata, K.; Ito, T.; Tomiyama, K.; Yagi, S.; Mori, A.; Uemoto, S. Impact of Sarcopenia on Survival in Patients Undergoing Living Donor Liver Transplantation. Arab. Archaeol. Epigr. 2013, 13, 1549–1556. [Google Scholar] [CrossRef]
- Krell, R.W.; Kaul, D.R.; Martin, A.R.; Englesbe, M.J.; Sonnenday, C.J.; Cai, S.; Malani, P.N. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transplant. 2013, 19, 1396–1402. [Google Scholar] [CrossRef]
- Tsien, C.; Garber, A.; Narayanan, A.; Shah, S.N.; Barnes, D.; Eghtesad, B.; Fung, J.; McCullough, A.J.; Dasarathy, S. Post-liver transplantation sarcopenia in cirrhosis: A prospective evaluation. J. Gastroenterol. Hepatol. 2014, 29, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J.; Meza-Junco, J.; Baracos, V.E.; Prado, C.M.M.; Ma, M.; Meeberg, G.; Beaumont, C.; Tandon, P.; Esfandiari, N.; Sawyer, M.B.; et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transplant. 2014, 20, 640–648. [Google Scholar] [CrossRef]
- Masuda, T.; Shirabe, K.; Ikegami, T.; Harimoto, N.; Yoshizumi, T.; Soejima, Y.; Uchiyama, H.; Ikeda, T.; Baba, H.; Maehara, Y. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transplant. 2014, 20, 401–407. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Kaido, T.; Okumura, S.; Fujimoto, Y.; Ogawa, K.; Mori, A.; Hammad, A.; Tamai, Y.; Inagaki, N.; Uemoto, S. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transplant. 2014, 20, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Kalafateli, M.; Mantzoukis, K.; Yau, Y.C.; Mohammad, A.O.; Arora, S.; Rodrigues, S.; De Vos, M.; Papadimitriou, K.; Thorburn, D.; O’Beirne, J.; et al. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. J. Cachex-Sarcopenia Muscle 2017, 8, 113–121. [Google Scholar] [CrossRef]
- Meza-Junco, J.; Montano-Loza, A.J.; Baracos, V.E.; Prado, C.M.; Bain, V.G.; Beaumont, C.; Esfandiari, N.; Lieffers, J.R.; Sawyer, M.B. Sarcopenia as a Prognostic Index of Nutritional Status in Concurrent Cirrhosis and Hepatocellular Carcinoma. J. Clin. Gastroenterol. 2013, 47, 861–870. [Google Scholar] [CrossRef]
- Harimoto, N.; Shirabe, K.; Yamashita, Y.-I.; Ikegami, T.; Yoshizumi, T.; Soejima, Y.; Ikeda, T.; Maehara, Y.; Nishie, A.; Yamanaka, T. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. BJS 2013, 100, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Nakagawa, H.; Kudo, Y.; Tateishi, R.; Taguri, M.; Watadani, T.; Nakagomi, R.; Kondo, M.; Nakatsuka, T.; Minami, T.; et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J. Hepatol. 2015, 63, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.J.; Dreyer, H.C.; Fry, C.S.; Glynn, E.L.; Rasmussen, B.B. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J. Appl. Physiol. 2009, 106, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Enomoto, H.; Ishii, A.; Iwata, Y.; Miyamoto, Y.; Ishii, N.; Yuri, Y.; Hasegawa, K.; Nakano, C.; Nishimura, T.; et al. Elevated serum myostatin level is associated with worse survival in patients with liver cirrhosis. J. Cachex-Sarcopenia Muscle 2017, 8, 915–925. [Google Scholar] [CrossRef]
- Han, H.; Zhou, X.; Mitch, W.E.; Goldberg, A.L. Myostatin/activin pathway antagonism: Molecular basis and therapeutic potential. Int. J. Biochem. Cell Biol. 2013, 45, 2333–2347. [Google Scholar] [CrossRef]
- Garikipati, D.K.; Rodgers, B.D. Myostatin inhibits myosatellite cell proliferation and consequently activates differentiation: Evidence for endocrine-regulated transcript processing. J. Endocrinol. 2012, 215, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Margioris, A.N. Sarcopenic obesity. Hormones 2018, 17, 321–331. [Google Scholar] [CrossRef]
- Kalyani, R.R.; Corriere, M.; Ferrucci, L. Age-related and disease-related muscle loss: The effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014, 2, 819–829. [Google Scholar] [CrossRef]
- Vitale, G.; Cesari, M.; Mari, D. Aging of the endocrine system and its potential impact on sarcopenia. Eur. J. Intern. Med. 2016, 35, 10–15. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Z.; Hu, J.; Du, J.; Mitch, W.E. Insulin Resistance Accelerates Muscle Protein Degradation: Activation of the Ubiquitin-Proteasome Pathway by Defects in Muscle Cell Signaling. Endocrinology 2006, 147, 4160–4168. [Google Scholar] [CrossRef]
- Schiaffino, S.; Mammucari, C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: Insights from genetic models. Skelet. Muscle 2011, 1, 4. [Google Scholar] [CrossRef]
- Cabrera, D.; Ruiz, A.; Cabello-Verrugio, C.; Brandan, E.; Estrada, L.; Pizarro, M.; Solis, N.; Torres, J.; Barrera, F.; Arrese, M. Diet-Induced Nonalcoholic Fatty Liver Disease Is Associated with Sarcopenia and Decreased Serum Insulin-Like Growth Factor-1. Dig. Dis. Sci. 2016, 61, 3190–3198. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, D.; Cabello-Verrugio, C.; Solís, N.; Martín, D.S.; Cofré, C.; Pizarro, M.; Arab, J.P.; Abrigo, J.; Campos, F.; Irigoyen, B.; et al. Somatotropic Axis Dysfunction in Non-Alcoholic Fatty Liver Disease: Beneficial Hepatic and Systemic Effects of Hormone Supplementation. Int. J. Mol. Sci. 2018, 19, 1339. [Google Scholar] [CrossRef]
- Serrano, A.L.; Baeza-Raja, B.; Perdiguero, E.; Jardí, M.; Muñoz-Cánoves, P. Interleukin-6 Is an Essential Regulator of Satellite Cell-Mediated Skeletal Muscle Hypertrophy. Cell Metab. 2008, 7, 33–44. [Google Scholar] [CrossRef]
- Haddad, F.; Zaldivar, F.; Cooper, D.M.; Adams, G.R. IL-6-induced skeletal muscle atrophy. J. Appl. Physiol. 2005, 98, 911–917. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Michell, B.J.; Van Denderen, B.J.; Watt, M.J.; Carey, A.L.; Fam, B.C.; Andrikopoulos, S.; Proietto, J.; Görgün, C.Z.; Carling, D.; et al. Tumor necrosis factor α-induced skeletal muscle insulin resistance involves suppression of AMP-kinase signaling. Cell Metab. 2006, 4, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef] [PubMed]
- Saxena, N.K.; Anania, F.A. Adipocytokines and hepatic fibrosis. Trends Endocrinol. Metab. 2015, 26, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.B.; McAinch, A.J.; Macaulay, S.L.; Castelli, L.A.; O’Brien, P.E.; Dixon, J.B.; Cameron-Smith, D.; Kemp, B.E.; Steinberg, G.R. Impaired activation of AMP-kinase and fatty acid oxidation by globular adiponectin in cultured human skeletal muscle of obese type 2 diabetics. J. Clin. Endocrinol. Metab. 2005, 90, 3665–3672. [Google Scholar] [CrossRef]
- Hamrick, M.; Dukes, A.; Arounleut, P.; Davis, C.; Periyasamy-Thandavan, S.; Mörk, S.; Herberg, S.; Johnson, M.; Isales, C.; Hill, W.; et al. The adipokine leptin mediates muscle- and liver-derived IGF-1 in aged mice. Exp. Gerontol. 2015, 70, 92–96. [Google Scholar] [CrossRef]
- Yvan-Charvet, L.; Quignard-Boulangé, A. Role of adipose tissue renin–angiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney Int. 2011, 79, 162–168. [Google Scholar] [CrossRef]
- Sowers, J.R.; Whaley-Connell, A.; Epstein, M. Narrative review: The emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann. Intern. Med. 2009, 150, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Clark, S.E.; Morris, E.M.; Thyfault, J.P.; Uptergrove, G.M.; Whaley-Connell, A.T.; Ferrario, C.M.; Sowers, J.R.; Ibdah, J.A. Angiotensin II-induced non-alcoholic fatty liver disease is mediated by oxidative stress in transgenic TG(mRen2)27(Ren2) rats. J. Hepatol. 2008, 49, 417–428. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of Cachexia in Chronic Disease States. Am. J. Med. Sci. 2015, 350, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Bhanji, R.A.; Narayanan, P.; Allen, A.M.; Malhi, H.; Watt, K.D. Sarcopenia in hiding: The risk and consequence of underestimating muscle dysfunction in nonalcoholic steatohepatitis. Hepatology 2017, 66, 2055–2065. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.T.; Zhao, B.; Yang, J. Enhanced muscle by myostatin propeptide increases adipose tissue adiponectin, PPAR-α, and PPAR-γ expressions. Biochem. Biophys. Res. Commun. 2008, 369, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S. Is the adiponectin-AMPK-mitochondrial axis involved in progression of nonalcoholic fatty liver disease? Hepatology 2014, 60, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; McFarlane, C.; Lokireddy, S.; Bonala, S.; Ge, X.; Masuda, S.; Gluckman, P.D.; Sharma, M.; Kambadur, R. Myostatin-deficient mice exhibit reduced insulin resistance through activating the AMP-activated protein kinase signalling pathway. Diabetology 2011, 54, 1491–1501. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.O.; Kim, N.; Kim, J.K.; Kim, H.I.; Lee, Y.W.; Kim, S.J.; Choi, J.-I.; Oh, Y.; Kim, J.H.; et al. Irisin, a Novel Myokine, Regulates Glucose Uptake in Skeletal Muscle Cells via AMPK. Mol. Endocrinol. 2015, 29, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Bostroem, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Bostroem, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nat. Cell Biol. 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Metwally, M.; Bayoumi, A.; Romero-Gomez, M.; Thabet, K.; John, M.; Adams, L.A.; Huo, X.; Aller, R.; García-Monzón, C.; Arias-Loste, M.T.; et al. A polymorphism in the Irisin-encoding gene (FNDC5) associates with hepatic steatosis by differential miRNA binding to the 3′UTR. J. Hepatol. 2019, 70, 494–500. [Google Scholar] [CrossRef]
- Tajiri, K.; Shimizu, Y. Branched-chain amino acids in liver diseases. World J. Gastroenterol. 2013, 19, 7620–7629. [Google Scholar] [CrossRef] [PubMed]
- Tsien, C.; Davuluri, G.; Singh, D.; Allawy, A.; Have, G.A.T.; Thapaliya, S.; Schulze, J.M.; Barnes, D.; McCullough, A.J.; Engelen, M.P.; et al. Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology 2015, 61, 2018–2029. [Google Scholar] [CrossRef]
- Shangraw, R.E.; Jahoor, F. Effect of liver disease and transplantation on urea synthesis in humans: Relationship to acid-base status. Am. J. Physiol. Liver Physiol. 1999, 276, G1145–G1152. [Google Scholar] [CrossRef] [PubMed]
- Dam, G.; Ott, P.; Aagaard, N.K.; Vilstrup, H. Branched-chain amino acids and muscle ammonia detoxification in cirrhosis. Metab. Brain Dis. 2013, 28, 217–220. [Google Scholar] [CrossRef]
- Holecek, M. Evidence of a vicious cycle in glutamine synthesis and breakdown in pathogenesis of hepatic encephalopathy–therapeutic perspectives. Metab. Brain Dis. 2013, 29, 9–17. [Google Scholar] [CrossRef]
- Lockwood, A.H.; McDonald, J.M.; Reiman, R.E.; Gelbard, A.S.; Laughlin, J.S.; Duffy, T.E.; Plum, F. The dynamics of ammonia metabolism in man. Effects of liver disease and hyperammonemia. J. Clin. Investig. 1979, 63, 449–460. [Google Scholar] [CrossRef]
- Dasarathy, S. Myostatin and beyond in cirrhosis: All roads lead to sarcopenia. J. Cachex-Sarcopenia Muscle 2017, 8, 864–869. [Google Scholar] [CrossRef]
- Dasarathy, S.; Muc, S.; Hisamuddin, K.; Edmison, J.M.; Dodig, M.; McCullough, A.J.; Kalhan, S.C. Altered expression of genes regulating skeletal muscle mass in the portacaval anastamosis rat. Am. J. Physiol. Liver Physiol. 2007, 292, G1105–G1113. [Google Scholar] [CrossRef][Green Version]
- Dasarathy, S.; McCullough, A.J.; Muc, S.; Schneyer, A.; Bennett, C.D.; Dodig, M.; Kalhan, S.C. Sarcopenia associated with portosystemic shunting is reversed by follistatin. J. Hepatol. 2011, 54, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Tsien, C.; Thapalaya, S.; Narayanan, A.; Weihl, C.C.; Ching, J.K.; Eghtesad, B.; Singh, K.; Fu, X.; Dubyak, G.; et al. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am. J. Physiol. Metab. 2012, 303, E983–E993. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Thapaliya, S.; Runkana, A.; Yang, Y.; Tsien, C.; Mohan, M.L.; Narayanan, A.; Eghtesad, B.; Mozdziak, P.E.; McDonald, C.; et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 18162–18167. [Google Scholar] [CrossRef]
- Kosenko, E.; Venediktova, N.; Kaminsky, Y.; Montoliu, C.; Felipo, V. Sources of oxygen radicals in brain in acute ammonia intoxication in vivo. Brain Res. 2003, 981, 193–200. [Google Scholar] [CrossRef]
- Holeček, M. Branched-chain amino acid supplementation in treatment of liver cirrhosis: Updated views on how to attenuate their harmful effects on cataplerosis and ammonia formation. Nutrient 2017, 41, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Owen, O.E.; Kalhan, S.C.; Hanson, R.W. The Key Role of Anaplerosis and Cataplerosis for Citric Acid Cycle Function. J. Biol. Chem. 2002, 277, 30409–30412. [Google Scholar] [CrossRef]
- Davuluri, G.; Allawy, A.; Thapaliya, S.; Rennison, J.H.; Singh, D.; Kumar, A.; Sandlers, Y.; Van Wagoner, D.R.; Flask, C.A.; Hoppel, C.; et al. Hyperammonaemia-induced skeletal muscle mitochondrial dysfunction results in cataplerosis and oxidative stress. J. Physiol. 2016, 594, 7341–7360. [Google Scholar] [CrossRef]
- McDaniel, J.G.; Davuluri, G.; Hill, E.A.; Moyer, M.; Runkana, A.; Prayson, R.; Van Lunteren, E.; Dasarathy, S. Hyperammonemia results in reduced muscle function independent of muscle mass. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G163–G170. [Google Scholar] [CrossRef]
- Davuluri, G.; Krokowski, D.; Guan, B.-J.; Kumar, A.; Thapaliya, S.; Singh, D.; Hatzoglou, M.; Dasarathy, S. Metabolic adaptation of skeletal muscle to hyperammonemia drives the beneficial effects of l-leucine in cirrhosis. J. Hepatol. 2016, 65, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Harding, H.P.; Novoa, I.; Zhang, Y.; Zeng, H.; Wek, R.; Schapira, M.; Ron, D. Regulated Translation Initiation Controls Stress-Induced Gene Expression in Mammalian Cells. Mol. Cell 2000, 6, 1099–1108. [Google Scholar] [CrossRef]
- Plauth, M.; Egberts, E.H.; Abele, R.; Müller, P.H.; Fürst, P. Characteristic pattern of free amino acids in plasma and skeletal muscle in stable hepatic cirrhosis. Hepatogastroenterology 1990, 37, 135–139. [Google Scholar] [PubMed]
- Montanari, A.; Simoni, I.; Vallisa, D.; Trifirò, A.; Colla, R.; Abbiati, R.; Borghi, L.; Novarini, A. Free amino acids in plasma and skeletal muscle of patients with liver cirrhosis. Hepatology 1988, 8, 1034–1039. [Google Scholar] [CrossRef]
- Grossmann, M.; Hoermann, R.; Gani, L.; Chan, I.; Cheung, A.; Gow, P.J.; Li, A.; Zajac, J.D.; Angus, P. Low testosterone levels as an independent predictor of mortality in men with chronic liver disease. Clin. Endocrinol. 2012, 77, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Orr, R.; Singh, M.F. The anabolic androgenic steroid oxandrolone in the treatment of wasting and catabolic disorders: Review of efficacy and safety. Drugs 2004, 64, 725–750. [Google Scholar] [CrossRef]
- Liu, W.; Thomas, S.G.; Asa, S.L.; Gonzalez-Cadavid, N.; Bhasin, S.; Ezzat, S. Myostatin Is a Skeletal Muscle Target of Growth Hormone Anabolic Action. J. Clin. Endocrinol. Metab. 2003, 88, 5490–5496. [Google Scholar] [CrossRef]
- Lakshman, K.M.; Bhasin, S.; Corcoran, C.; Collins-Racie, L.A.; Tchistiakova, L.; Forlow, S.B.; Ledger, K.S.; Burczynski, M.E.; Dorner, A.J.; LaVallie, E.R. Measurement of myostatin concentrations in human serum: Circulating concentrations in young and older men and effects of testosterone administration. Mol. Cell. Endocrinol. 2009, 302, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S.; Mullen, K.D.; Dodig, M.; Donofrio, B.; McCullough, A.J. Inhibition of aromatase improves nutritional status following portacaval anastomosis in male rats. J. Hepatol. 2006, 45, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, J.G.; Damink, S.W.O.; Venema, K.; Buurman, W.A.; Jalan, R.; Dejong, C.H. Short chain fatty acids exchange: Is the cirrhotic, dysfunctional liver still able to clear them? Clin. Nutr. 2010, 29, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Rainer, F.; Horvath, A.; Sandahl, T.D.; Leber, B.; Schmerboeck, B.; Blesl, A.; Groselj-Strele, A.; Stauber, R.E.; Fickert, P.; Stiegler, P.; et al. Soluble CD163 and soluble mannose receptor predict survival and decompensation in patients with liver cirrhosis, and correlate with gut permeability and bacterial translocation. Aliment. Pharmacol. Ther. 2018, 47, 657–664. [Google Scholar] [CrossRef]
- Elswefy, S.E.-S.; Abdallah, F.R.; Atteia, H.H.; Wahba, A.S.; Hasan, R.A. Inflammation, oxidative stress and apoptosis cascade implications in bisphenol A-induced liver fibrosis in male rats. Int. J. Exp. Pathol. 2016, 97, 369–379. [Google Scholar] [CrossRef]
- Nishikawa, H.; Enomoto, H.; Nishiguchi, S.; Iijima, H. Liver Cirrhosis and Sarcopenia from the Viewpoint of Dysbiosis. Int. J. Mol. Sci. 2020, 21, 5254. [Google Scholar] [CrossRef]
- Huo, Y.R.; Suriyaarachchi, P.; Gomez, F.; Curcio, C.L.; Boersma, D.; Muir, S.W.; Montero-Odasso, M.; Gunawardene, P.; Demontiero, O.; Duque, G. Phenotype of Osteosarcopenia in Older Individuals with a History of Falling. J. Am. Med. Dir. Assoc. 2015, 16, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Abe, K.; Fujita, M.; Okai, K.; Takahashi, A.; Ohira, H. Association between sarcopenia and osteoporosis in chronic liver disease. Hepatol. Res. 2018, 48, 893–904. [Google Scholar] [CrossRef]
- Hayashi, F.; Kaibori, M.; Sakaguchi, T.; Matsui, K.; Ishizaki, M.; Kwon, A.-H.; Iwasaka, J.; Kimura, Y.; Habu, D. Loss of skeletal muscle mass in patients with chronic liver disease is related to decrease in bone mineral density and exercise tolerance. Hepatol. Res. 2018, 48, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Bering, T.; Diniz, K.G.; Coelho, M.P.P.; Vieira, D.A.; Soares, M.M.S.; Kakehasi, A.M.; Correia, M.I.T.; Teixeira, R.; Queiroz, D.M.; Rocha, G.A.; et al. Association between pre-sarcopenia, sarcopenia, and bone mineral density in patients with chronic hepatitis C. J. Cachex-Sarcopenia Muscle 2018, 9, 255–268. [Google Scholar] [CrossRef]
- Santos, L.A.A.; Lima, T.B.; Augusti, L.; Franzoni, L.D.C.; Yamashiro, F.D.S.; Bolfi, F.; Nunes, V.D.S.; Dorna, M.D.S.; De Oliveira, C.V.; Caramori, C.A.; et al. Handgrip strength as a predictor of bone mineral density in outpatients with cirrhosis. J. Gastroenterol. Hepatol. 2015, 31, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Saeki, C.; Kanai, T.; Nakano, M.; Oikawa, T.; Torisu, Y.; Abo, M.; Saruta, M.; Tsubota, A. Relationship between Osteosarcopenia and Frailty in Patients with Chronic Liver Disease. J. Clin. Med. 2020, 9, 2381. [Google Scholar] [CrossRef] [PubMed]
- Saeki, C.; Takano, K.; Oikawa, T.; Aoki, Y.; Kanai, T.; Takakura, K.; Nakano, M.; Torisu, Y.; Sasaki, N.; Abo, M.; et al. Comparative assessment of sarcopenia using the JSH, AWGS, and EWGSOP2 criteria and the relationship between sarcopenia, osteoporosis, and osteosarcopenia in patients with liver cirrhosis. BMC Musculoskelet. Disord. 2019, 20, 1–12. [Google Scholar] [CrossRef]
- Karasik, D.; Cohen-Zinder, M. The genetic pleiotropy of musculoskeletal aging. Front. Physiol. 2012, 3, 303. [Google Scholar] [CrossRef]
- Guo, Y.-F.; Zhang, L.-S.; Liu, Y.-J.; Hu, H.-G.; Li, J.; Tian, Q.; Yu, P.; Zhang, F.; Yang, T.-L.; Peng, X.-L.; et al. Suggestion of GLYAT gene underlying variation of bone size and body lean mass as revealed by a bivariate genome-wide association study. Qual. Life Res. 2012, 132, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hsu, Y.-H.; Mo, C.; Abreu, E.; Kiel, D.P.; Bonewald, L.F.; Brotto, M.; Karasik, D. METTL21CIs a Potential Pleiotropic Gene for Osteoporosis and Sarcopenia Acting Through the Modulation of the NF-κB Signaling Pathway. J. Bone Miner. Res. 2014, 29, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, D.G.; Lyons, G.E.; Martin, J.F.; Olson, E.N. Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesis. Development 1994, 120, 1251–1263. [Google Scholar] [PubMed]
- Kramer, I.; Baertschi, S.; Halleux, C.; Keller, H.; Kneissel, M. Mef2cdeletion in osteocytes results in increased bone mass. J. Bone Miner. Res. 2012, 27, 360–373. [Google Scholar] [CrossRef]
- Hamrick, M.W. The skeletal muscle secretome: An emerging player in muscle–bone crosstalk. BoneKEy Rep. 2012, 1, 60. [Google Scholar] [CrossRef]
- Dankbar, B.; Fennen, M.; Brunert, D.; Hayer, S.; Frank, S.; Wehmeyer, C.; Beckmann, D.; Paruzel, P.; Bertrand, J.; Redlich, K.; et al. Myostatin is a direct regulator of osteoclast differentiation and its inhibition reduces inflammatory joint destruction in mice. Nat. Med. 2015, 21, 1085–1090. [Google Scholar] [CrossRef]
- Puolakkainen, T.; Ma, H.; Kainulainen, H.; Pasternack, A.; Rantalainen, T.; Ritvos, O.; Heikinheimo, K.; Hulmi, J.J.; Kiviranta, R. Treatment with soluble activin type IIB-receptor improves bone mass and strength in a mouse model of Duchenne muscular dystrophy. BMC Musculoskelet. Disord. 2017, 18, 20. [Google Scholar] [CrossRef]
- DiGirolamo, D.J.; Singhal, V.; Chang, X.; Lee, S.-J.; Germain-Lee, E.L. Administration of soluble activin receptor 2B increases bone and muscle mass in a mouse model of osteogenesis imperfecta. Bone Res. 2015, 3, 14042. [Google Scholar] [CrossRef] [PubMed]
- Hamrick, M.; Shi, X.; Zhang, W.; Pennington, C.; Thakore, H.; Haque, M.; Kang, B.; Isales, C.; Fulzele, S.; Wenger, K. Loss of myostatin (GDF8) function increases osteogenic differentiation of bone marrow-derived mesenchymal stem cells but the osteogenic effect is ablated with unloading. Bone 2007, 40, 1544–1553. [Google Scholar] [CrossRef]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Oranger, A.; Mori, G.; Brunetti, G.; Colucci, S.; Cinti, S.; Grano, M. Irisin Enhances Osteoblast Differentiation In Vitro. Int. J. Endocrinol. 2014, 2014, 902186. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G.; et al. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef]
- Kitase, Y.; Vallejo, J.A.; Gutheil, W.; Vemula, H.; Jähn, K.; Yi, J.; Zhou, J.; Brotto, M.; Bonewald, L.F. β-aminoisobutyric Acid, l-BAIBA, Is a Muscle-Derived Osteocyte Survival Factor. Cell Rep. 2018, 22, 1531–1544. [Google Scholar] [CrossRef]
- Karsenty, G.; Ferron, M. The contribution of bone to whole-organism physiology. Nat. Cell Biol. 2012, 481, 314–320. [Google Scholar] [CrossRef]
- Mera, P.; Laue, K.; Ferron, M.; Confavreux, C.; Wei, J.; Galán-Díez, M.; Lacampagne, A.; Mitchell, S.J.; Mattison, J.A.; Chen, Y.; et al. Osteocalcin Signaling in Myofibers Is Necessary and Sufficient for Optimum Adaptation to Exercise. Cell Metab. 2016, 23, 1078–1092. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, S.S.; Dumont, N.A.; Boulanger-Piette, A.; Fajardo, V.A.; Gamu, D.; Kake-Guena, S.A.; David, R.O.; Bouchard, P.; Lavergne, É.; Penninger, J.M.; et al. Muscle RANK is a key regulator of Ca2+ storage, SERCA activity, and function of fast-twitch skeletal muscles. Am. J. Physiol. Physiol. 2016, 310, C663–C672. [Google Scholar] [CrossRef]
- Bonnet, N.; Bourgoin, L.; Biver, E.; Douni, E.; Ferrari, S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J. Clin. Investig. 2019, 129, 3214–3223. [Google Scholar] [CrossRef]
- Dole, N.S.; Mazur, C.M.; Acevedo, C.; Lopez, J.P.; Monteiro, D.A.; Fowler, T.W.; Gludovatz, B.; Walsh, F.; Regan, J.N.; Messina, S.; et al. Osteocyte-Intrinsic TGF-β Signaling Regulates Bone Quality through Perilacunar/Canalicular Remodeling. Cell Rep. 2017, 21, 2585–2596. [Google Scholar] [CrossRef]
- Waning, D.L.; Mohammad, K.S.; Reiken, S.; Xie, W.; Andersson, D.C.; John, S.K.; Chiechi, A.; Wright, L.E.; Umanskaya, A.; Niewolna, M.; et al. Excess TGF-β mediates muscle weakness associated with bone metastases in mice. Nat. Med. 2015, 21, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F.; Johnson, M.L. Osteocytes, mechanosensing and Wnt signaling. Bone 2008, 42, 606–615. [Google Scholar] [CrossRef]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/β-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Von Maltzahn, J.; Chang, N.C.; Bentzinger, C.F.; Rudnicki, M.A. Wnt signaling in myogenesis. Trends Cell Biol. 2012, 22, 602–609. [Google Scholar] [CrossRef]
- Huang, J.; Romero-Suarez, S.; Lara, N.; Mo, C.; Kaja, S.; Brotto, L.; Dallas, S.L.; Johnson, M.L.; Jähn, K.; Bonewald, L.F.; et al. Crosstalk Between MLO-Y4 Osteocytes and C2C12 Muscle Cells Is Mediated by the Wnt/β-Catenin Pathway. JBMR Plus 2017, 1, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Strope, M.A.; Nigh, P.; Carter, M.I.; Lin, N.; Jiang, J.; Hinton, P.S. Physical Activity–Associated Bone Loading During Adolescence and Young Adulthood Is Positively Associated with Adult Bone Mineral Density in Men. Am. J. Men’s Health 2014, 9, 442–450. [Google Scholar] [CrossRef]
- De Kam, D.; Smulders, E.; Weerdesteyn, V.; Smits-Engelsman, B.C.M. Exercise interventions to reduce fall-related fractures and their risk factors in individuals with low bone density: A systematic review of randomized controlled trials. Osteoporos. Int. 2009, 20, 2111–2125. [Google Scholar] [CrossRef]
- Kirk, B.; Mooney, K.; Amirabdollahian, F.; Khaiyat, O. Exercise and Dietary-Protein as a Countermeasure to Skeletal Muscle Weakness: Liverpool Hope University—Sarcopenia Aging Trial (LHU-SAT). Front. Physiol. 2019, 10, 445. [Google Scholar] [CrossRef]
- Kirk, B.; Mooney, K.; Cousins, R.; Angell, P.; Jackson, M.; Pugh, J.N.; Coyles, G.; Amirabdollahian, F.; Khaiyat, O. Effects of exercise and whey protein on muscle mass, fat mass, myoelectrical muscle fatigue and health-related quality of life in older adults: A secondary analysis of the Liverpool Hope University—Sarcopenia Ageing Trial (LHU-SAT). Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 120, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Román, E.; Torrades, M.T.; Nadal, M.J.; Cárdenas, G.; Nieto, J.C.; Vidal, S.; Bascuñana, H.; Juárez, C.; Guarner, C.; Córdoba, J.; et al. Randomized Pilot Study: Effects of an Exercise Programme and Leucine Supplementation in Patients with Cirrhosis. Dig. Dis. Sci. 2014, 59, 1966–1975. [Google Scholar] [CrossRef]
- Kitajima, Y.; Takahashi, H.; Akiyama, T.; Murayama, K.; Iwane, S.; Kuwashiro, T.; Tanaka, K.; Kawazoe, S.; Ono, N.; Eguchi, T.; et al. Supplementation with branched-chain amino acids ameliorates hypoalbuminemia, prevents sarcopenia, and reduces fat accumulation in the skeletal muscles of patients with liver cirrhosis. J. Gastroenterol. 2017, 53, 427–437. [Google Scholar] [CrossRef]
- Glass, D.J. Molecular mechanisms modulating muscle mass. Trends Mol. Med. 2003, 9, 344–350. [Google Scholar] [CrossRef]
- Dukes, A.; Davis, C.; El Refaey, M.; Upadhyay, S.; Mork, S.; Arounleut, P.; Johnson, M.H.; Hill, W.D.; Isales, C.M.; Hamrick, M.W. The aromatic amino acid tryptophan stimulates skeletal muscle IGF1/p70s6k/mTor signaling in vivo and the expression of myogenic genes in vitro. Nutrient 2015, 31, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.D.; Smith, T.K.; Bayley, H.S. A Role for Tryptophan in Regulation of Protein Synthesis in Porcine Muscle. J. Nutr. 1988, 118, 445–449. [Google Scholar] [CrossRef]
- Cruzat, V.; Rogero, M.M.; Keane, K.N.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Mignon, M.; Beaufrère, A.-M.; Combaret, L.; Meynial-Denis, D. Does Long-Term Intermittent Treatment with Glutamine Improve the Well-being of Fed and Fasted Very Old Rats? J. Parenter. Enter. Nutr. 2007, 31, 456–462. [Google Scholar] [CrossRef]
- Meynial-Denis, D. Glutamine metabolism in advanced age. Nutr. Rev. 2016, 74, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Zanker, J.; Duque, G. Osteoporosis in Older Persons: Old and New Players. J. Am. Geriatr. Soc. 2018, 67, 831–840. [Google Scholar] [CrossRef]
- Kumar, A.; Davuluri, G.; DeSilva, R.N.; Engelen, M.P.; Have, G.A.T.; Prayson, R.; Deutz, N.E.; Dasarathy, S. Ammonia lowering reverses sarcopenia of cirrhosis by restoring skeletal muscle proteostasis. Hepatology 2017, 65, 2045–2058. [Google Scholar] [CrossRef]
- Rose, C.F. Ammonia-Lowering Strategies for the Treatment of Hepatic Encephalopathy. Clin. Pharmacol. Ther. 2012, 92, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Phu, S.; Hassan, E.B.; Vogrin, S.; Kirk, B.; Duque, G. Effect of Denosumab on Falls, Muscle Strength, and Function in Community-Dwelling Older Adults. J. Am. Geriatr. Soc. 2019, 67, 2660–2661. [Google Scholar] [CrossRef]
- Narayanan, R.; Coss, C.C.; Dalton, J.T. Development of selective androgen receptor modulators (SARMs). Mol. Cell. Endocrinol. 2018, 465, 134–142. [Google Scholar] [CrossRef]
- Attie, K.M.; Borgstein, N.G.; Yang, Y.; Condon, C.H.; Wilson, D.M.; Pearsall, A.E.; Kumar, R.; Willins, D.A.; Seehra, J.S.; Sherman, M.L. A single ascending-dose study of muscle regulator ace-031 in healthy volunteers. Muscle Nerve 2013, 47, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Lord, S.R.; Studenski, S.A.; Warden, S.J.; Fielding, R.A.; Recknor, C.P.; Hochberg, M.C.; Ferrari, S.L.; Blain, H.; Binder, E.F.; et al. Myostatin antibody (LY2495655) in older weak fallers: A proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015, 3, 948–957. [Google Scholar] [CrossRef]
- Parise, G.; Snijders, T. Myostatin inhibition for treatment of sarcopenia. Lancet Diabetes Endocrinol. 2015, 3, 917–918. [Google Scholar] [CrossRef]
| Studies on Patients with Primary Biliary Cholangitis (PBC) or Primary Sclerosing Cholangitis (PSC) | |||||
|---|---|---|---|---|---|
| Author, Year | Study Aim | Study Design | Study Population | Method to Diagnosis Osteoporosis | Outcome |
| Menon et al., 2001 [19] | To evaluate the prevalence and risk factor of bone disease in patients with PBC and to determine the rate of bone loss over time. | Retrospective study | 176 patients with PBC | DEXA | The prevalence of osteoporosis is 20% in patients with PBC. Age (OR 1.2 (1.1–1.2)), BMI (OR 0.8 (0.7–0.9)), advanced stage (3 or 4) (OR 6.3 (1.8–21.6)), and history of fractures (OR 4.1 (1.0–16.8)) were independent indicators of osteoporosis. Serum bilirubin level was independently associated with the rate of bone loss over time. |
| Guanabens et al., 2005 [20] | To find out the prevalence and risk factors for osteoporosis in women with PBC | Cross-sectional study | 142 women with PBC and 1305 age-matched control subjects | DEXA | Prevalence of osteoporosis was higher in PBC (32.4%) than in normal women (11.1%). Older age, higher Mayo risk score, lower BMI and advanced histological stage were independent risk factors for osteoporosis. |
| Solaymani-Dodaran et al., 2006 [22] | To quantify the excess fracture risk in people with PBC | Retrospective cohort study | 930 patients with PBC and 9202 age- and sex-matched control subjects. | NA | There were approximately 2-fold relative increases in the risk of any fracture (HR 2.03 (1.70–2.44)), hip fracture (HR 2.14 (1.40–3.28)), and ulna/radius fracture (HR 1.96 (1.42–2.71)) for the PBC cohort compared with the general population. |
| Guanabens et al., 2010 [23] | To assess the prevalence and risk factors for fractures and the fracture threshold in women with PBC | Prospective study | 185 women with PBC | DEXA | The prevalences of vertebral, non-vertebral, and overall fractures were 11.2%, 12.2%, and 20.8%, respectively. Vertebral fractures, are associated with osteoporosis (OR 8.48 (2.67–26.95)). Osteoporosis and osteopenia are associated with the severity of liver damage. |
| Angulo et al., 2011 [21] | To identify prevalence and rate of progression of bone disease in patients with PSC and to identify predictors of bone disease and progression. | Retrospective longitudinal cohort study | 237 patients with PSC | DEXA | Osteoporosis was found in 15% of patients (RR 23.8 (4.6–122.8)). Old age (OR 7.8 (3.3–18.3), BMI (OR 4.9 (1.9–12.6), and long duration of inflammatory bowel disease (OR 3.6 (1.5–8.4)) correlated with the presence of osteoporosis. |
| Studies on Patients with Viral Hepatitis | |||||
| Author, Year | Study Aim | Study Design | Study Population | Method to Diagnosis Osteoporosis | Outcome |
| Schiefke et al., 2005 [28] | To evaluate BMD and bone turnover markers in patients with non-cirrhotic CHB or CHC | Cross-sectional study | 43 patients with HCV (n = 30) or HBV (n = 13) infection without histological evidence for liver cirrhosis. | DEXA | Osteoporosis is observed in 32% of non-cirrhotic CHB or CHC patients. Altered bone metabolism with increased bone-specific ALP and iPTH already occurred in advanced liver fibrosis without cirrhosis. |
| Orsini et al., 2013 [24] | To identify the prevalence of osteoporotic vertebral fractures and low BMD measurements in men with non-cirrhotic CHC. | Cross-sectional study | 60 non-cirrhotic CHC patients and 59 healthy controls | DEXA | Non-cirrhotic untreated CHC patients have lower BMD at the femur as compared to healthy men in spite of the absence of significant bone and mineral abnormalities. |
| Hansen et al., 2014 [29] | Comparison of fracture risk between HCV-seropositive (HCV-exposed) patients and the general population, and between patients with cleared and CHC infection. | Retrospective cohort study | 12,013 HCV-exposed patients from the Danish HCV cohort, and 60,065 general population | NA | HCV-exposed patients had increased risk of all fracture types (adjusted incidence rate ratio (aIRR) 2.13–2.18) whereas overall risk of fracture did not differ between patients with chronic vs. cleared HCV-infection. |
| Lai et al., 2015 [25] | Association between BMD, systemic inflammation, and markers of bone turnover in CHC without cirrhosis | Cross-sectional study | 60 non-cirrhotic CHC patients | DEXA | Low BMD was observed in 42% (30% had osteopenia, 12% had osteoporosis) of non-cirrhotic CHC patients, but not associated with systemic inflammatory markers. Patients with low BMD had higher serum phosphorus and pro-peptide of type 1 collagen. |
| Huang et al., 2017 [26] | To assess BMD and prevalence of osteoporosis in CHB patients | Case-control study | 148 CHB patients and 148 age- and gender-matched healthy controls | DEXA | The prevalence of osteoporosis in either of lumbar spine, total hip or the femur neck was significantly higher in the CHB patients group (12.8%, 11.5%, 12.2%) compared with the healthy control (4.7%, 4.1%, 4.7%). CHB infection was associated with low BMD and increased the risk of osteoporosis. |
| Wei et al., 2019 [27] | To identify the effect of ETV and TDF on the development of osteopenia/osteoporosis | Retrospective cohort study | 1224 Asian CHB patients | DEXA | There is no significant increase in the incidence of osteopenia/osteoporosis for patients with CHB treated with TDF (HR 0.74 (0.34–1.59)) or ETV (HR 0.98 (0.51–1.90)) during a median follow-up of about 4 to 5 years. |
| Studies on Patients with Liver Cirrhosis (LC) | |||||
| Author, Year | Study Aim | Study Design | Study Population | Method to Diagnosis Osteoporosis | Outcome |
| Monegal et al. 1997 [33] | To find out the prevalence and risk factor of bone disease in patients with end-stage liver disease waiting for OLT. | Prospective study | 58 cirrhotic patients | DEXA | 43% patients had osteoporosis and vitamin D deficiency, reduced PTH levels, and hypogonadism are observed in cirrhotic patients. Alcoholic and Child-Pugh C patients showed the lowest femoral BMD. |
| Sokhi et al., 2004 [31] | To assess the BMD in different subgroups among pretransplant cirrhotic patients. | Retrospective study | 104 cirrhotic patients | DPA | The overall prevalence of osteopenia and osteoporosis were 34.6% and 11.5%, respectively, being significantly higher in females than in males. BMD is significantly lower in those with CTP class C than those with CTB class B in both males and females. |
| Goubraim et al., 2013 [30] | To evaluate prevalence and risk factors for metabolic bone disease in patients with viral cirrhosis | Prospective study | 46 cirrhotic patients | DEXA. | Osteopenia and osteoporosis is observed in 52.2% and 28.2% patients, respectively. There was no independent factor associated with bone disorders although bone disorders were significantly more frequent in old patients with low BMI, long duration of liver disease, and low vitamin D level. |
| Zheng et al., 2018 [32] | To evaluate osteoporosis or osteopenia in patients with cirrhosis | Retrospective study | 217 LC patients and 229 subjects without liver diseases | DEXA | Osteoporosis was found in 20.3% and older age (OR 1.78), lower BMI (OR 0.63), greater fibroscan score (OR 1.15), and alcoholic liver cirrhosis (OR 3.42) were independently associated with osteoporosis in cirrhotic patients. |
| Studies on Patients Who Underwent Liver Transplantation | |||||
| Author, Year | Study Aim | Study Design | Study Population | Method to Diagnosis Osteoporosis | Outcome |
| Monegal et al., 2001 [34] | To determine the incidence and risk factors of skeletal fractures and to analyze the long-term evolution of bone mass, bone turnover and hormonal status after LT | Prospective study | 45 patients following LT | DEXA | Fifteen patients (33%) developed fractures after liver transplantation, and pre- transplant risk factors for fractures were age and low bone mass (OR 5.69 (1.32–24.53)). Bone mass decreased during the first 6 months and after then bone formation parameters is increased. |
| Guichelaar et al., 2006 [35] | To identify the prevalence and predictive factors for low bone mass before OLT, Posttransplant bone loss, and bone gain at the lumbar spine with long-term follow-up after OLT | Prospective cohort study | 360 patients with end-stage PBC and PSC | DPA & DEXA | Most patients (77%) with advanced PBC and PSC have osteopenic bone disease, and risk factors for hepatic osteopenia are low BMI, older age, postmenopausal status, the presence of muscle wasting, high ALP, and low serum albumin. After OLT, aggressive bone loss occurs during the first 4 months, with risk factor of younger age, PSC, higher pretransplant BMD, no IBD, shorter duration of disease, current smoking and ongoing cholestasis at 4 months. After the first 4 postoperative months, bone gain occurs during the first 2 years with favoring factors for improvement of lower baseline and/or 4-month BMD, premenopausal status for females, lesser glucocorticoids, no ongoing cholestasis, and higher levels of vitamin D and parathyroid function. |
| Studies on Patients with Non-Alcoholic Fatty Liver Disease (NAFLD) | |||||
| Author, Year | Study Aim | Study Design | Study Population | Method to Diagnosis Osteoporosis | Outcome |
| Li et al., 2012 [36] | Association between NAFLD and osteoporotic fracture | Cross-sectional study | 7797 Chinese adults (including 2352 patients with NAFLD) | NA | The prevalence of osteoporotic fractures was significantly higher in men with NAFLD (3.6 vs. 1.7%), and the presence of NAFLD was significantly associated with osteoporotic fracture among men. (OR 2.53 (1.26–5.07)) |
| Purnak et al., 2012 [37] | Association between BMD and liver function in patients with NASH. | Cross-sectional study | 102 patients with NAFLD and 54 healthy controls | DEXA | The presence of elevated serum ALT and hs-CRP levels, which are suggestive of NASH, was associated with lower BMD although simple steatosis of the liver does not affect BMD. |
| Kim et al., 2017 [38] | Association between liver fibrosis and BMD in patients with NAFLD | Retrospective cross-sectional study | 231 subjects (including 129 patients with NAFLD) | DEXA | Significant liver fibrosis was independently associated with overall osteopenia and osteoporosis in subjects with NAFLD. (OR 4.10 (1.02–16.45)). |
| Ahn et al., 2018 [39] | Association between fatty liver index (scoring model for NAFLD) and BMD | Population-based, cross-sectional study | 4264 adults | DEXA | Fatty liver index was negatively correlated with total hip (p = 0.004), femoral neck (p < 0.001), and whole body BMD (p = 0.01) in men independent of insulin resistance. |
| Studies on Alcoholics | |||||
| Author, Year | Study Aim | Study Design | Study Population | Method to Diagnosis Osteoporosis | Outcome |
| Peris et al., 1994 [42] | To evaluate the effect of abstinence on bone mass and bone mineral metabolism in chronic alcoholics. | 2 year longitudinal follow-up study | 30 chronic alcoholic males | DPA | After 2 years of abstinence, Lumbar and femoral neck BMD increased in alcoholics and Baseline low osteocalcin increased after 1 year and 2 years of abstinence. |
| Peris et al., 1995 [41] | Association between vertebral facture and osteopenia in chronic alcoholics patients. | Cross-sectional study | 76 chronic alcoholics and 62 age matched healthy males. | DPA | Chronic alcoholics frequently have traumas (68%) and vertebral fractures (36%) in spite of having a lumbar BMD above the fracture threshold. |
| Malik et al., 2009 [40] | To evaluate BMD according to alcohol consumption and sex. | Cross-sectional study | 57 noncirrhotic alcoholic patients | DEXA | 24.3% of men and 5% of women had low BMD and 75.7% of the men and 90% of the women had vitamin D insufficiency or deficiency. |
| Studies on Patients with Genetic Hemochromatosis (GH) or Wilson Disease (WD) | |||||
| Author, Year | Study Aim | Study Design | Study Population | Method to Diagnosis Osteoporosis | Outcome |
| Sinigaglia et al., 1997 [43] | To evaluate the prevalence and risk factor of osteoporosis in GH | Cross sectional study | 32 patients with histologically proven GH | DEXA | Osteoporosis is observed in 28% and osteoporosis is highly associated with degree of iron overload (OR 3.23 (1.09–9.58)) |
| Guggenbuhl et al., 2005 [44] | To assess BMD and bone remodeling in patients with GH | Retrospective study | 38 men with HFE-related GH | DEXA | Osteopenia was observed in 78.9% of patients and osteoporosis in 34.2% that cannot solely be explained by hypogonadism or cirrhosis |
| Valenti et al., 2009 [45] | To identify the prevalence, clinical characteristics and genetic background associated with osteoporosis in patients with HHC | Retrospective study | 87 patients with HHC | DEXA | Osteoporosis was identified in 25.3%, and osteopenia in 41.4% patients regardless of genetic background. Lumbar spine osteoporosis was independently associated with lower BMI (OR 0.73 (0.54–0.94)), total ALP (OR 1.17 (1–1.39)), and the amount of iron removed (OR 1.53 (1–2.5)). |
| Quemeneur et al., 2014 [47] | To assess the prevalent fractures, BMD and related risk factors in patients with WD. | Prospective cross-sectional study | 85 patients with WD | DEXA | Prevalent peripheral fractures were presented in 51%, and vertebral fracture in 8% patients. Patients with severe neurological involvement, low BMI, old age are at risk factors for vertebral fractures |
| Weiss et al., 2015 [46] | Comparison of BMD between adult WD and healthy control population | Cross sectional study | 148 adult WD patients and 345 age and gender matched control subjects | DEXA | Osteoporosis (8.8% vs. 4.1%) and osteopenia (50.0% vs. 41.2%) is significantly more prevalent in patient with WD than healthy population. There was no significant correlation between BMD and any of the WD disease parameters (e.g., the severity of liver disease), lab results, type of treatment or known osteoporosis risk factors. |
| Studies on Patients with Non-Alcoholic Fatty Liver Disease (NAFLD) | |||||
|---|---|---|---|---|---|
| Author, Year | Study Aim | Study Design | Study Population | Method to Diagnosis Sarcopenia | Outcome |
| Hong et al., 2014 [100] | Relationship between sarcopenia and NAFLD | Cross-sectional study | 452 subjects | DEXA | Lower muscle mass increased the risk of NAFLD (OR 5.16 (1.63–16.33)). |
| Lee et al., 2015 [99] | Association between sarcopenia and NAFLD or NASH | Cross-sectional study | 15,132 subjects | DEXA | There was independent association between sarcopenia and NAFLD after adjusting for confounding factors related to obesity or insulin resistance (ORs 1.18 to 1.22) |
| Carias et al., 2016 [101] | Association between sarcopenic obestiy and NASH in patients with LC | Retrospective study | 207 patients with LC | L3–L4 skeletal muscle mass on CT imaging, DEXA | NASH is independent predictor of sarcopenia obesity in patients with LC (OR 6.03 (1.44–25.26)) |
| Koo et al., 2017 [102] | Association between sarcopenia and histological severity of NAFLD | Prospective cross-sectional study | 309 patients (including 240 biopsy proven NAFLD patients) | BIA | Sarcopenia was significantly associated with NASH (OR 2.28 (1.12–4.30)) and significant fibrosis (OR 2.05 (1.01–4.16)). |
| Kim et al., 2018 [104] | Effect of skeletal muscle mass changes on NAFLD | 7-year longitudinal cohort study | 10,534 subjects without baseline NAFLD and 2631 subjects with baseline NAFLD | BIA | Increases in relative skeletal muscle mass over time may lead to benefits either in the development of NAFLD (aHR 0.44 (0.38–0.51)) or the resolution of existing NAFLD (aHR 2.09 (1.02–4.28)). |
| Gan et al., 2020 [103] | Associations of NAFLD with low muscle mass, low muscle strength, sarcopenia, and sarcopenic obesity | Cross-sectional study | 5132 participants (including 1088 patients with NAFLD) | DEXA | Low muscle mass (OR 2.57 (2.03–3.25)), low muscle strength (OR 1.47 (1.21–1.80)), sarcopenia (OR 3.91 (2.90–5.28)), sarcopenic obesity (OR 1.42 (7.14–15.22)) were positively and associated with NAFLD. |
| Studies on Patients with Liver Cirrhosis (LC) | |||||
| Author, Year | Study Aim | Study Design | Study Population | Method to Diagnosis Sarcopenia | Outcome |
| Tandon et al., 2012 [105] | Prevalence of sarcopenia and clinical significance of sarcopenia in patients with cirrhosis listed for LT | Retrospective study | 142 LC patients waiting for LT | L3 skeletal muscle on CT and MRI images. DEXA | The prevalence of sarcopenia was 41%, and sarcopenia is independent predictor of mortality (HR 2.36 (1.23–4.53)) |
| Montano-Loza et al., 2012 [106] | Incidence of sarcopenia, association between sarcopenia and mortality and prognosis in LC patients | Prospective study | 112 patients with LC | L3 skeletal muscle on CT images | The incidence of sarcopenia is 40%. Sarcopenia is associated with mortality in patients with cirrhosis. (HR 2.21) |
| Merli et al., 2013 [107] | The relationship between muscle depletion and hepatic encephalopathy (HE) | Prospective study | 300 patients with LC | Mid-Arm-Muscle-Circumference, Triceps Skinfold-Thickness, Handgrip strength | HE were significantly higher in cirrhotic patients with muscle depletion or decreased muscle strength. (30% vs. 15%, and 29% vs. 16%, respectively) |
| Kim et al., 2014 [108] | The association between sarcopenia and long term mortality in LC patients with ascites | Retrospective study | 65 patients with LC | psoas muscle thickness on CT images | Sarcopenia is an independent useful predictor for long-term mortality in cirrhotic patients with ascites. (HR 0.812 (0.684–0.965)). |
| Durand et al., 2014 [109] | Prognostic value of muscle atrophy in cirrhosis | Retrospective study | 562 patients with LC | TPMT on CT image | TPMT/height on CT predicted mortality in cirrhotic patients, independent of the MELD and MELD-Na scores. (HR 0.86 (0.78–0.94) and HR 0.87 (0.79–0.95)). |
| Hanai et al., 2015 [110] | The prevalence of sarcopenia in patients with LC, Association between sarcopenia and outcomes. | Retrospective study | 130 patients with LC | L3 skeletal muscle on CT images | The prevalence of sarcopenia was 68% and Sarcopenia is significantly associated with mortality in patients with LC. (HR 3.03) |
| Montano-Loza et al., 2015 [111] | The impact of sarcopenia in cirrhosis and mortality prediction of inclusion muscularity assessment within model for end-stage liver disease (MELD) | Retrospective study | 669 patients with LC | L3 skeletal muscle on CT images | Sarcopenia (HR 0.97 (0.96–0.99)) were associated with mortality. Modification of MELD to include sarcopenia is associated with improved prediction of mortality in patients with cirrhosis, primarily in patients with low MELD scores. (C-statistics 0.73 (0.70–0.77)). |
| Hanai et al., 2016 [112] | The relationship between time-course change in skeletal muscle and the prognosis of patients with LC | Retrospective study | 149 patients with LC | L3 skeletal muscle on CT images | The relative change in skeletal muscle area per year (>−3.1%) is useful for predicting mortality in patients with liver cirrhosis. (HR 2.73 (1.43–5.44)). |
| Nardelli et al., 2017 [113] | The association between sarcopenia and HE after TIPS | Prospective study | 46 patients with LC | L3 skeletal muscle on CT images | Sarcopenia is independently associated with the development of HE after TIPS (subdistribution HR, 31.3 (4.5–218.07)). |
| Kang et al., 2018 [114] | Impact of sarcopenia to the conventional prognostic factors (MELD, CTP, HVPG) | Retrospective study | 452 patients with LC | L3 skeletal muscle on CT images | The prevalence of sarcopenia was 42%. Sarcopenia is associated with mortality in compensated and early decompensated cirrhosis. Existing conventional prognostic factors had limited value in severe sarcopenia. (MELD, p = 0.182; CTP, p = 0.187; HVPG, p = 0.077). |
| Studies on Patients Who Underwent Liver Transplantation | |||||
| Author, Year | Study Aim | Study Design | Study Population | Method to Diagnosis Sarcopenia | Outcome |
| Englesbe et al., 2010 [115] | Association between sarcopenia and mortality after LT | Retrospective study | 163 patients undergoing LT | psoas muscle at L4 vertebra on CT images | Total psoas area strongly correlates with mortality after LT (HR 0.274 (0.141–0.531)). |
| Kaido et al., 2013 [116] | Impact of sarcopenia on survival after LT | Retrospective study | 124 undergoing LT | BIA | Low skeletal muscle mass was an independent risk factor for death after transplantation. (OR 4.846 (2.092–11.790)) |
| Krell et al., 2013 [117] | Association between sarcopenia and serious infection after LT | Retrospective study | 207 patients undergoing LT | TPA on CT images | Recipient age (HR 1.04), pre-transplant total psoas muscle area (HR 0.38) and pre-transplant total bilirubin level (HR 1.05) were independently associated with the risk of developing severe infections. |
| Tsien et al., 2014 [118] | The effect of changes in skeletal muscle mass on outcomes after LT | Prospective study | 53 patients undergoing LT | Psoas and paraspinal muscles on CT images | Loss of muscle mass post-OLT increased risk of diabetes mellitus (HR 3.1 (1.01–9.38)) and a trend toward higher mortality. |
| Montano-Loza et al., 2014 [119] | Impact of muscle depletion on morbidity or mortality after LT | Retrospective study | 248 patients undergoing LT | L3 skeletal muscle on CT images | Sarcopenia is predictive of longer hospital stays (40 ± 4 vs. 25 ± 3 days) and a higher risk of perioperative bacterial infection (26% vs. 15%) after LT. |
| Masuda et al., 2014 [120] | Impact of sarcopenia on mortality and sepsis after living donor LT | Retrospective study | 204 patients undergoing LT | Psoas muscle at L3 vertebra on CT images | Sarcopenia is an independent predictor of mortality (HR 2.06) and sepsis after LDLT (HR 5.31) |
| Hamaguchi et al., 2014 [121] | Impact of quality and quantity of skeletal muscle on preoperative CT on outcomes after LT | Retrospective study | 200 patients undergoing LT | IMAC and PMI on CT images | High IMAC (OR 3.898 (2.025–7.757)) and low PMI (OR 3.635 (1.896–7.174) were independent risk factors for death after LT. |
| Kalafateli et al., 2016 [122] | The impact of sarcopenia on post LT outcomes | Retrospective study | 232 patients undergoing LT | L3-PMI on CT images | Sarcopenia were independent predictors of Hospital stay >20 days (OR 0.996 (0.994–0.999)) and 12 month mortality (OR 0.996 (0.992–0.999)) |
| Studies on Patients with Hepatocellular Carcinoma (HCC) | |||||
| Author, Year | Study Aim | Study Design | Study Population | Method to Diagnosis Sarcopenia | Outcome |
| Meza-Junco et al., 2013 [123] | Frequency and prognostic significance of sarcopenia in patients with HCC. | Prospective study | 116 patients with HCC | L3 SMI on CT images | Sarcopenia is present in 30% of patients with HCC and independent risk factor for mortality. (HR, 2.04) with median survival of 16 ± 6 (vs. 28 ± 3 months in nonsarcopenic). |
| Harimoto et al., 2013 [124] | The effect of sarcopenia on outcomes after partial hepatectomy for HCC | Retrospective study | 186 patients with HCC | L3 skeletal muscle on CT images | 5-year overall survival rate and 5-year recurrence-free survival rate was 71% vs. 83.7% and 13% vs. 33.2% in patients with and without sarcopenia, respectively. Sarcopenia was predictive of an overall survival (HR 3.27 (1.39–7.69)) and recurrence free survival (HR 0.97 (0.95–1.00)). |
| Fujiwara et al., 2015 [125] | Impact of body composition on HCC | Retrospective study | 1257 patients with different stages of HCC | SMI, mean MA, visceral adipose tissue index, subcutaneous adipose tissue index, VSR via on CT images | Sarcopenia (HR 1.52 (1.18–1.96)), intramuscular fat deposition (HR 1.34 (1.05–1.71)), and visceral adiposity (HR 1.35 (1.09–1.66)) independently predict mortality in patients with HCC. |
| Author, Year [Reference] | Study Aim | Study Design | Study Population | Method to Diagnosis Osteosarcopenia | Outcome |
|---|---|---|---|---|---|
| Santos et al., 2016 [188] | To evaluate whether handgrip strength, bone, and liver tests may be useful as predictors of bone disease in outpatients with LC | Prospective study | 129 patients with LC | DEXA, dynamometer | For lumbar spine, only low handgrip strength and high PTH levels were clearly related to low T scores. |
| Hayashi et al., 2018 [185] | Association between sarcopenia and osteoporosis in patients with CLD | Retrospective study | 112 CLD patients including 40 cirrhotic patients | BIA and DEXA | The sarcopenia rate was 13%, and the osteoporosis and osteopenia rates were 17% and 65%, respectively. Sarcopenia was significantly associated with the BMD of the lumbar spine and the femur neck. Sarcopenia (OR 6.16) and cirrhosis (OR 15.8) were independent risk factors for osteoporosis. |
| Hayashi et al., 2018 [186] | Association between loss of skeletal muscle mass and clinical factors such as osteoporosis in patients with chronic liver disease. | Cross-sectional study | 112 HCC patients undergoing hepatectomy | DEXA | The T-score and PeakVO2 was significantly lower in the low skeletal mass index (SMI) group. T-score (OR 3.508 (1.074–11.456)) and PeakVO2 (OR 3.512 (1.114–11.066)) were significantly related to SMI, independent of age and sex. |
| Bering et al., 2018 [187] | To assess the prevalence of low BMD and its association with body composition, muscle strength, and nutritional status in patients with CHC. | Prospective cross-sectional study | 104 patients with CHC | DEXA | Low BMD, low muscle strength, pre-sarcopenia, sarcopenia, and sarcopenic obesity were presented in 34.6%, 27.9%, 14.4%, 8.7%, and 3.8% of the patients, respectively. Appendicular skeletal muscle mass is an independent predictor of BMD in CHC. Sarcopenia was independently associated with bone mineral content and malnutrition. |
| Saeki et al., 2020 [189] | Association between osteosarcopenia and frailty in patients with CLD | Cross-sectional study | 291 patients with CLD | DEXA | 49 (16.8%) and 81 (27.8%) had osteosarcopenia and frailty, respectively. Frailty was an independently associated with osteosarcopenia (OR 9.837), and vice versa (OR 10.069) and increased the risk of vertebral fracture in patients with CLD. |
| Disorders | Effect | Agents |
|---|---|---|
| Osteoporosis | Inhibit bone resorption | Calcium |
| Inhibit bone resorption | Vitamin D | |
| Inhibit bone resorption | Calcitonin | |
| Inhibit bone resorption | SERMs (ex, raloxifene) | |
| Inhibit bone resorption | Bisphosphates (ex, alendronate, zoledronic acid, ibandronate) | |
| Inhibit bone resorption | Anti-RANKL antibody (ex, denosumab) | |
| Activate bone formation | PTH (ex, teriparatide) | |
| Activate bone formation | Sclerostin inhibitors (ex, romosozumab) | |
| Sarcopenia | Reduce Ammonia | L-ornithine L-aspartate |
| Reduce Ammonia | Rifaximin | |
| Increase muscle protein synthesis | Testosterone | |
| Increase muscle protein synthesis | Myostatin antagonists (ex, follistatin) | |
| Increase muscle protein synthesis | IGF-1 antagonist | |
| Osteosarcopenia | Inhibit bone resorption Increase muscle protein synthesis (insulin sensitivity) | Anti-RANKL antibody (ex, denosumab) |
| Activate bone and muscle formation | SARMs (ex, VK5211) | |
| Activate bone and muscle formation | Myostatin antagonists (ex, ACE-031) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.J.; Kim, D.J. An Overview of the Molecular Mechanisms Contributing to Musculoskeletal Disorders in Chronic Liver Disease: Osteoporosis, Sarcopenia, and Osteoporotic Sarcopenia. Int. J. Mol. Sci. 2021, 22, 2604. https://doi.org/10.3390/ijms22052604
Yang YJ, Kim DJ. An Overview of the Molecular Mechanisms Contributing to Musculoskeletal Disorders in Chronic Liver Disease: Osteoporosis, Sarcopenia, and Osteoporotic Sarcopenia. International Journal of Molecular Sciences. 2021; 22(5):2604. https://doi.org/10.3390/ijms22052604
Chicago/Turabian StyleYang, Young Joo, and Dong Joon Kim. 2021. "An Overview of the Molecular Mechanisms Contributing to Musculoskeletal Disorders in Chronic Liver Disease: Osteoporosis, Sarcopenia, and Osteoporotic Sarcopenia" International Journal of Molecular Sciences 22, no. 5: 2604. https://doi.org/10.3390/ijms22052604
APA StyleYang, Y. J., & Kim, D. J. (2021). An Overview of the Molecular Mechanisms Contributing to Musculoskeletal Disorders in Chronic Liver Disease: Osteoporosis, Sarcopenia, and Osteoporotic Sarcopenia. International Journal of Molecular Sciences, 22(5), 2604. https://doi.org/10.3390/ijms22052604







