Dispirooxindoles Based on 2-Selenoxo-Imidazolidin-4-Ones: Synthesis, Cytotoxicity and ROS Generation Ability
Abstract
:1. Introduction
2. Results and Discussion
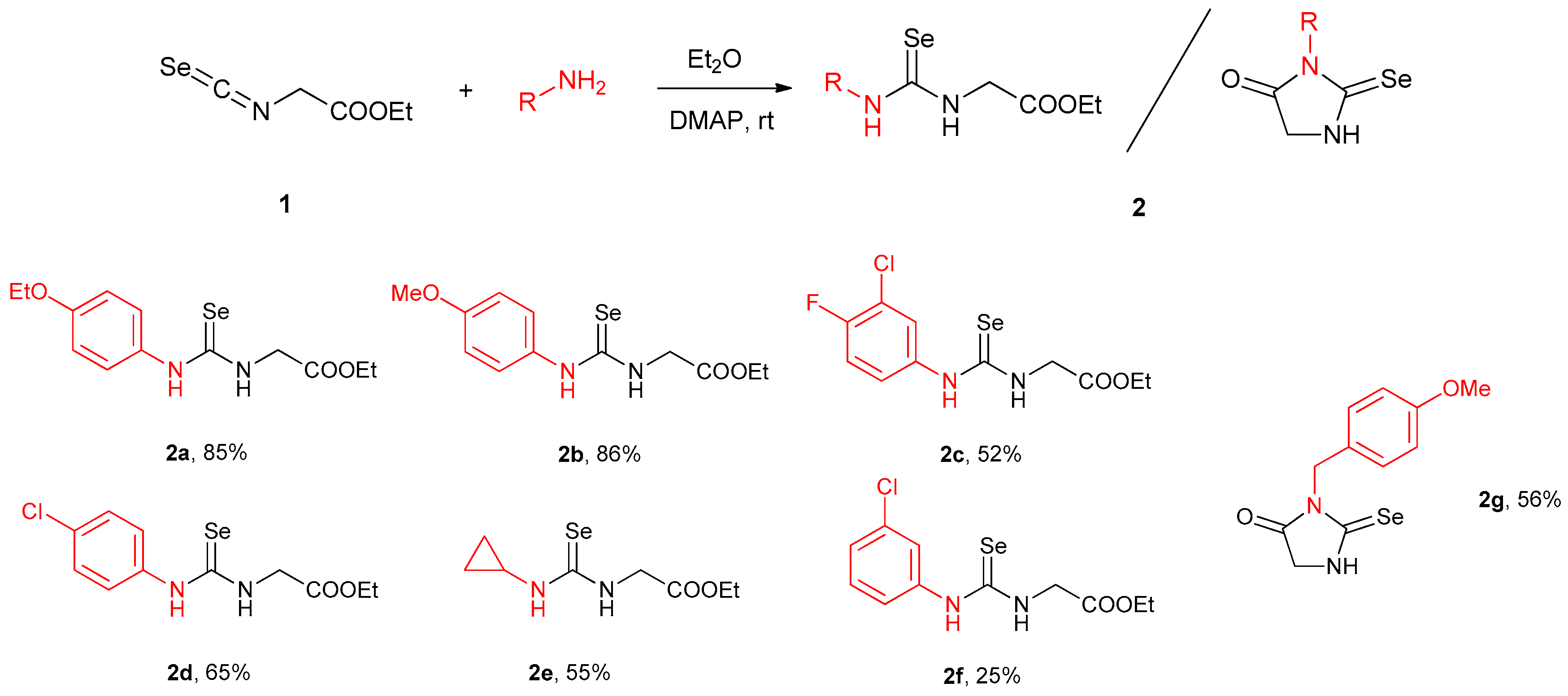
2.1. Synthesis
2.2. Cytotoxicity and p53 Activation
2.3. ROS Generation Ability
3. Materials and Methods
3.1. General
3.1.1. Cell Lines
3.1.2. In Vitro Survival Assay (MTT Assay)
3.1.3. Transcriptional Assay for P53 Activation (ONPG-Test)
3.1.4. Intracellular ROS Detection by Pt Nanoelectrodes
3.2. Synthesis
3.2.1. Glycine Ethyl Ester Hydrochloride
3.2.2. N-formylglycine Ethyl Ester
3.2.3. Ethyl 2-Isocyanoacetate
3.2.4. Ethyl Isoselenocyanatoacetate (1)
3.2.5. Selenoureas and 2-Selenoxohydantoins 2a-g (General Procedure)
3.2.6. 5-Arylidene-2-Selenoxohydantoins 3a-g (General Procedure)
3.2.7. 5-Indolinyliden-2-Selenoxohydantoins 4a-m (General Procedure)
3.2.8. Synthesis of Dispiroindolinones of Type I (Compounds 5a-h; General Procedure)
3.2.9. Synthesis of Dispiroindolinones of Type I (Compounds 6a-m; General Procedure)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ivanenkov, Y.A.; Vasilevski, S.V.; Beloglazkina, E.K.; Kukushkin, M.E.; Machulkin, A.E.; Veselov, M.S.; Chufarova, N.V.; Chernyagin, E.S.; Vanzcool, A.S.; Zyk, N.V.; et al. Design, synthesis and biological evaluation of novel potent MDM2/p53 small-molecule inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 404–409. [Google Scholar] [CrossRef]
- Beloglazkina, A.A.; Karpov, N.A.; Mefedova, S.R.; Polyakov, V.S.; Skvortsov, D.A.; Kalinina, M.A.; Tafeenko, V.A.; Majouga, A.G.; Zyk, N.V.; Beloglazkina, E.K. Synthesis of dispirooxindoles containing N-unsubstituted heterocyclic moieties and study of their anticancer activity. Russ. Chem. Bull. 2019, 68, 1006–1013. [Google Scholar] [CrossRef]
- Beloglazkina, A.A.; Skvortsov, D.A.; Tafeenko, V.A.; Majouga, A.G.; Zyk, N.V.; Beloglazkina, E.K. Synthesis and cytotoxicity of novel dispiro derivatives of 5-arylidenoxazolones, potential inhibitors of p53—MDM2 protein-protein interaction. Russ. Chem. Bull. 2018, 7, 562–569. [Google Scholar] [CrossRef]
- Kukushkin, M.E.; Skvortsov, D.A.; Kalinina, M.A.; Tafeenko, V.A.; Burmistrov, V.V.; Butov, G.M.; Zyk, N.V.; Majouga, A.G.; Beloglazkina, E.K. Synthesis and cytotoxicity of oxindoles dispiro derivatives with thiohydantoin and adamantane fragments. Phosph. Sulfur Relat. Elem. 2020, 195, 544–555. [Google Scholar] [CrossRef]
- Ding, K.; Lu, Y.; Nikolovska-Coleska, Z.; Qiu, S.; Ding, Y.; Gao, W.; Stuckey, J.; Krajewski, K.; Roller, P.P.; Tomita, Y.; et al. Structure-Based Design of Potent Non-Peptide MDM2 Inhibitors. J. Am. Chem. Soc. 2005, 127, 10130–10131. [Google Scholar] [CrossRef]
- Gollner, A.; Rudolph, D.; Arnhof, H.; Bauer, M.; Blake, S.M.; Boehmelt, G.; Cockroft, X.-L.; Dahmann, G.; Ettmayer, P.; Gerstberger, T.; et al. Discovery of Novel Spiro[3H-indole-3.2′-pyrrolidin]-2(1H)-one Compounds as Chemically Stable and Orally Active Inhibitors of the MDM2–p53 Interaction. J. Med. Chem. 2016, 59, 10147–10162. [Google Scholar] [CrossRef]
- Song, X.-J.; Ren, H.-X.; Xiang, M.; Li, C.-Y.; Zou, Y.; Li, X.; Huang, Z.-C.; Tian, F.; Wan, L.-X. Organocatalytic Enantioselective Michael Addition between 3-(3-hydroxy-1H-pyrazol-1-yl) Oxindole and β-Nitrostyrene for the Preparation of Chiral Disubstituted Oxindoles. J. Org. Chem. 2020, 85, 9290–9300. [Google Scholar] [CrossRef]
- Shu, L.; Li, Z.; Gu, C.; Fishlock, D. Synthesis of a SpiroindolinonePyrrolidinecarboxamide MDM2 Antagonist. Org. Process. Res. Dev. 2013, 17, 247–256. [Google Scholar] [CrossRef]
- Nakamaru, K.; Seki, T.; Tazaki, K.; Tse, A. Abstract B5: Preclinical characterization of a novel orally-available MDM2 inhibitor DS-3032b: Anti-tumor profile and predictive biomarkers for sensitivity. Mol. Cancer Ther. 2015, 14, B5. [Google Scholar] [CrossRef]
- Lane, D.P.; Crawford, L.V. T antigen is bound to a host protein in SY40-transformed cells. Nature 1979, 278, 261–263. [Google Scholar] [CrossRef]
- Chappell, W.H.; Lehmann, B.D.; Terrian, D.M.; Abrams, S.L.; Steelman, L.S.; McCubrey, J.A. p53 expression controls prostate cancer sensitivity to chemotherapy and the MDM2 inhibitor Nutlin-3. Cell Cycle 2012, 11, 4579–4588. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Liu, Z.; Yang, F.; Ding, M. Synthesis and Properties of Novel Imidazolone Derivatives Containing a Sulfur Atom. Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 939–950. [Google Scholar] [CrossRef]
- Jacob, C.; Giles, G.I.; Giles, N.M.; Sies, H. Sulfur and Selenium: The Role of Oxidation State in Protein Structure and Function. Angew. Chem. Int. Ed. 2003, 42, 4742–4758. [Google Scholar] [CrossRef]
- Ellis, K.O.; Castellion, A.W.; Honkomp, L.J.; Wessels, F.L.; Carpenter, J.F.; Halliday, R.P. Dantrolene, a direct acting skeletal muscle relaxant. J. Pharm. Sci. 1973, 62, 948–951. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, J.-P.; Bonne, C.; Bouton, M.-M.; Lagace, L.; Labrie, F. Action of a non-steroid anti-androgen, RU 23908, in peripheral and central tissues. J. Steroid Biochem. 1979, 11, 93–99. [Google Scholar] [CrossRef]
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From Selenium to Selenoproteins: Synthesis. Identity, and Their Role in Human Health. Antioxid. Redox Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Benedetti, R.; Handzlik, J.; Zwergel, C.; Battistelli, C. The innovative potential of selenium-containing agents for fighting cancer and viral infections. Drug Discov. Today 2020. [Google Scholar] [CrossRef]
- Bartolini, D.; Sancineto, L.; Fabro de Bem, A.; Tew, K.D.; Santi, C.; Radi, R.; Toquato, P.; Galli, F. Selenocompounds in Cancer Therapy: An Overview. Adv. Cancer Res. 2017, 136, 259–302. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2015, 1850, 1642–1660. [Google Scholar] [CrossRef]
- Hovens, C.; Costello, A.; Corcoran, N. Selenium fir Treatment of Cancer. Patent No. WO 2007109852, 4 October 2007. [Google Scholar]
- Cao, S.; Durrani, F.A.; Toth, K.; Rustum, Y.M. Se-methylselenocysteine offers selective protection against toxicity and potentiates the antitumour activity of anticancer drugs in preclinical animal models. Br. J. Cancer 2014, 110, 1733–1743. [Google Scholar] [CrossRef] [Green Version]
- Gowda, R.; Madhunapantula, S.V.; Desai, D.; Amin, S.; Robertson, G.P. Simultaneous Targeting of COX-2 and AKT Using Selenocoxib-1-GSH to Inhibit Melanoma. Mol. Cancer Ther. 2013, 12, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.K.; Jani, J.; Sharma, C.; Cleary, P.; Sharma, M.; Satish, S.; Gomez, E.; Prez, M.; Amezcua, N.; Navel, M.; et al. SC-4, a novel inhibitor of hedgehog-Gli signaling, inhibits growth of CSC and CTC of melanoma patients. In Proceedings of the 105th Annaul Meeting Amiraca Association Cancer Reserch (AACR), San-Diego, CA, USA, 5–9 April 2014. [Google Scholar]
- Allen, J.E.; Gallant, J.-N.; Dicker, D.T.; Amin, S.; Irby, R.B.; Sharma, A.K.; ElDeiry, W.S. The Akt Inhibitor ISC-4 Synergizes with Cetuximab in 5-FU-Resistant Colon Cancer. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Ibбcez, E.; Agliano, A.; Prior, C.; Nguewa, P.; Redrado, M.; Gonzбlez-Zubeldia, I.; Plano, D.; Palop, J.A.; Sanmartнn, C.; Calvo, A. The QuinolineImidoselenocarbamate EI201 Blocks the AKT/mTOR Pathway and Targets Cancer Stem Cells Leading to a Strong Antitumor Activity. Curr. Med. Chem. 2012, 19, 3031–3043. [Google Scholar] [CrossRef]
- Roy, S.S.; Chakraborty, P.; Bhattacharya, S. Intervention in cyclophosphamide induced oxidative stress and DNA damage by a flavonyl-thiazolidinedione based organoselenocyanate and evaluation of its efficacy during adjuvant therapy in tumor bearing mice. Eur. J. Med. Chem. 2014, 73, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Hwang, H.; Ham, J.; Ko, J. Organoselenium Containing Compounds and their Use. Patent No. WO 2006071103, 6 July 2006. [Google Scholar]
- Bijian, K.; Zhang, Z.W.; Xu, B.; Jie, S.; Chen, B.; Wan, S.B.; Wu, J.H.; Jiang, T.; Alaoui-Jamali, M.A. Synthesis and biological activity of novel organoselenium derivatives targeting multiple kinases and capable of inhibiting cancer progression to metastases. Eur. J. Med. Chem. 2012, 48, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Sheng, J.; Sun, Y.; Lu, C.; Yan, J.; Liu, A.; Luo, H.B.; Huang, L.; Li, X.J. Synthesis and Evaluation of Multi-Target-Directed Ligands against Alzheimer’s Disease Based on the Fusion of Donepezil and Ebselen. Med. Chem. 2013, 56, 9089–9099. [Google Scholar] [CrossRef]
- Madhunapantula, S.V.; Desai, D.; Sharma, A.; Huh, S.J.; Amin, S.; Robertson, G.P. PBISe, a novel selenium-containing drug for the treatment of malignant melanoma. Mol. Cancer Ther. 2008, 7, 1297–1308. [Google Scholar] [CrossRef] [Green Version]
- Cao, S.; Durrani, F.A.; Rustum, Y.M. Selective Modulation of the Therapeutic Efficacy of Anticancer Drugs by Selenium Containing Compounds against Human Tumor Xenografts. Clin. Cancer Res. 2004, 10, 2561–2569. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Fu, J.N.; Wang, J.Y.; Jin, C.J.; Ren, X.Y.; Tan, Q.; Li, J.; Yin, H.W.; Xiong, K.; Wang, T.Y.; et al. Selenium-containing thioredoxin reductase inhibitor ethaselen sensitizes non-small cell lung cancer to radiotherapy. Anti-Cancer Drugs 2011, 22, 732–740. [Google Scholar] [CrossRef]
- Jiang, C.; Jiang, W.; Ip, C.; Ganther, H.; Lu, J. Selenium-induced inhibition of angiogenesis in mammary cancer at chemopreventive levels of intake. Mol. Carcinog. 1999, 26, 213–225. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Seshadri, M.; Oven, S.D.; Toth, K.; Vaughan, M.M.; Rustum, Y.M. Tumor Vascular Maturation and Improved Drug Delivery Induced by Methylselenocysteine Leads to Therapeutic Synergy with Anticancer Drugs. Clin. Cancer Res. 2008, 14, 3926–3932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.; Ganther, H.; Lu, J. Monomethyl selenium–specific inhibition of MMP-2 and VEGF expression: Implications for angiogenic switch regulation. Mol. Carcinog. 2000, 29, 236–250. [Google Scholar] [CrossRef]
- Novotortsev, V.K.; Kukushkin, M.E.; Tafeenko, V.A.; Zyk, N.V.; Beloglazkina, E.K. New spiro-linked indolinone pyrrolidine selenoxoimidazolones. Mendeleev Commun. 2020, 30, 320–321. [Google Scholar] [CrossRef]
- Ivanenkov, Y.A.; Veselov, M.S.; Rezekin, I.G.; Skvortsov, D.A.; Sandulenko, Y.B.; Polyakova, M.V.; Bezrukov, D.S.; Vasilevsky, S.V.; Kukushkin, M.E.; Moiseeva, A.A.; et al. Synthesis, isomerization and biological activity of novel 2-selenohydantoin derivatives. Bioorg. Med. Chem. 2016, 24, 802–811. [Google Scholar] [CrossRef]
- Vyhivskyi, O.; Dlin, E.A.; Finko, A.V.; Stepanova, S.P.; Ivanenkov, Y.A.; Skvortsov, D.A.; Mironov, A.V.; Zyk, N.V.; Majouga, A.G.; Beloglazkina, E.K. Copper-Promoted C–Se Cross-Coupling of 2-Selenohydantoins with Arylboronic Acids in an Open Flask. ACS Comb. Sci. 2019, 21, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, O.Y.; Antipin, R.L.; Udina, A.V.; Krasnovskaya, O.O.; Beloglazkina, E.K.; Terenin, V.I.; Koteliansky, V.E.; Zyk, N.V.; Majouga, A.G. An Improved Protocol for Synthesis of 3-Substituted 5-Arylidene-2-thiohydantoins: Two-step Procedure Alternative to Classical Methods. J. Heterocycl. Chem. 2016, 53, 1570–1577. [Google Scholar] [CrossRef]
- Evdokimov, N.M.; Magedov, I.V.; McBrayer, D.; Kornienko, A. Isatin derivatives with activity against apoptosis-resistant cancer cells. Bioorg. Med. Chem. Lett. 2016, 26, 1558–1560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majouga, A.G.; Beloglazkina, E.K.; Vatsadze, S.Z.; Frolova, N.A.; Zyk, N.V. Synthesis of isomeric 3-phenyl-5-(pyridylmethylene)-2-thiohydantoins and their S-methylated derivatives. Molecular and crystal structures of (5Z)-3-phenyl-5-(pyridin-2-ylmethylene)-2-thiohydantoin and (5Z)-2-methylthio-3-phenyl-5-(pyridin-2-ylmethylene)-3. 5-dihydro-4H-imidazol-4-one. Russ. Chem. Bull. 2004, 53, 2850–2855. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Gazieva, G.A.; Kolotyrkina, N.G.; Kravchenko, A.N.; Makhova, N.N. Synthesis of novel spiro[indole-3.3′-pyrrolidin]-2(1H)-ones. Russ. Chem. Bull. 2014, 63, 431–434. [Google Scholar] [CrossRef]
- Sosnovskikh, V.Y.; Kornev, M.Y.; Moshkin, V.S.; Buev, E.M. Substituted chromones in [3+2] cycloadditions with nonstabilizedazomethineylides: Synthesis of 1-benzopyrano[2.3-c]pyrrolidines and 1-benzopyrano[2.3-c:3.4-c′]dipyrrolidines. Tetrahedron 2014, 70, 9253–9261. [Google Scholar] [CrossRef]
- Graves, B.; Thompson, T.; Xia, M.; Janson, C.; Lukacs, C.; Deo, D.; Di Lello, P.; Fry, D.; Garvie, C.; Huang, K.-S.; et al. Activation of the p53 pathway by small-molecule-induced MDM2 and MDMX dimerization. Proc. Natl. Acad. Sci. USA 2012, 109, 11788–11797. [Google Scholar] [CrossRef] [Green Version]
- Kravchenko, J.E.; Ilyinskaya, G.V.; Komarov, P.G.; Agapova, L.S.; Kochetkov, D.V.; Strom, E.; Frolova, E.I.; Kovriga, I.; Gudkov, A.V.; Feinstein, E.; et al. Small-molecule RETRA suppresses mutant p53-bearing cancer cells through a p73-dependent salvage pathway. Proc. Natl. Acad. Sci. USA 2008, 105, 6302–6307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaels, H.; Benesperi, I.; Edvinsson, T.; Mucoz-Garcia, A.B.; Pavone, M.; Boschloo, G.; Freitag, M. Copper complexes with tetradentate ligands for enhanced charge transport in dye-sensitized solar cells. Inorganics 2018, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, A.; Falahati-Pour, S.K.; Mohammadizadeh, F.; Hajizadeh, M.R.; Mirzaei, M.R.; Khoshdel, A.; Fahmidehkar, M.A.; Mahmoodi, M. Effect of a Copper (II) Complex on The Induction of Apoptosis in Human Hepatocellular Carcinoma Cells. Asian Pac. J. Cancer Prev. 2018, 19, 2877–2884. [Google Scholar] [CrossRef]
- Actis, P.; Tokar, S.; Clausmeyer, J.; Babakinejad, B.; Mikhaleva, S.; Cornut, R.; Takahashi, Y.; Lopez Cordoba, A.; Novak, P.; Shevchuck, A.I.; et al. Electrochemical Nanoprobes for Single-Cell Analysis. ACS Nano 2014, 8, 875–884. [Google Scholar] [CrossRef] [Green Version]
- Erofeev, A.; Gorelkin, P.; Garanina, A.; Alova, A.; Efremova, M.; Vorobyeva, N.; Edwards, C.; Korchev, Y.; Majouga, A. Novel method for rapid toxicity screening of magnetic nanoparticles. Sci. Rep. 2018, 8, 7462. [Google Scholar] [CrossRef] [Green Version]
- Krasnovskaya, O.; Guk, D.A.; Naumov, A.; Nikitina, V.N.; Semkina, A.; Vlasova, K.Y.; Pokrovsky, V.; Ryabaya, O.O.; Karshieva, S.; Skvortsov, D.A.; et al. Novel Copper-Containing Cytotoxic Agents Based on 2-Thioxoimidazolones. J. Med. Chem. 2020, 63. [Google Scholar] [CrossRef] [PubMed]
- Tietze, L.F.; Eicher, T. Preparative Organic Chemistry; Mir: Moscow, Russia, 1999. (In Russian) [Google Scholar]
- GraphPad Software, Inc. GraphPad Prism 8.0.0; GraphPad Software, Inc.: San Diego, CA, USA, 2018. [Google Scholar]
- Bříza, T.; Králová, J.; Rimpelová, S.; Havlík, M.; Kaplánek, R.; Kejík, Z.; Martásek, P.; Mikula, I.; Džubák, P.; Hajdúch, M.; et al. Pentamethinium salts as ligands for cancer: Sulfated polysaccharide co-receptors as possible therapeutic target. Bioorg. Chem. 2019, 82, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, X.; Phillips, D.L.; Zhao, C. Mechanistic Insights into the Factors That Influence the DNA Nuclease Activity of Mononuclear Facial Copper Complexes Containing Hetero-Substituted Cyclens. ACS Catal. 2016, 6, 248–257. [Google Scholar] [CrossRef]
- OriginLab Corporation. Origin 2018; OriginLab Corporation: Northampton, MA, USA, 2018. [Google Scholar]









| Compound | Cell Line | Selectivity Indexes | ||||
|---|---|---|---|---|---|---|
| VA13 | HEK293T | A549 | MCF7 | VA13/A549 | HEK293T/A549 | |
| 5a | 79.9 ± 34.7 | 25.8 ± 3.6 | 15.6 ± 2.1 | 82.5 ± 18.3 | 5.1 | 1.7 |
| 5b | 45.2 ± 23.3 | 19.8 ± 6 | 8.7 ± 2.5 | 20.9 ± 3.6 | 5.2 | 2.3 |
| 5c | 57.6 ± 24.7 | 14.4 ± 5.4 | 8.4 ± 1.5 | 29.5 ± 9.1 | 6.9 | 1.7 |
| 5d | 36.1 ± 4.5 | 33 ± 10.7 | 11.5 ± 2.5 | 54.3 ± 10.2 | 3.1 | 2.9 |
| 5e | 31.3 ± 12.4 | 17.3 ± 7.4 | 10.1 ± 2.3 | 24.8 ± 5.1 | 3.1 | 1.7 |
| 5f | 49 ± 8.4 | 16.2 ± 3.3 | 7.9 ± 0.9 | 33.1 ± 6.9 | 6.2 | 2.1 |
| 6a | 20.4 ± 2.2 | 23.6 ± 3.3 | 20 ± 3.3 | 31.6 ± 7.1 | 1 | 1.2 |
| 6b | 12.5 ± 1.5 | 14 ± 1.5 | 15.8 ± 1.5 | 20.4 ± 3.6 | 0.8 | 0.9 |
| 6c | 19.3 ± 5.9 | 10.3 ± 1.3 | 11.9 ± 1.5 | 12.8 ± 2.3 | 1.6 | 0.9 |
| 6d | 21.1 ± 2.4 | 27.07 ± 4.1 | 20.4 ± 1.8 | 35.1 ± 3 | 1 | 1.3 |
| 6e | 11.8 ± 4.3 | 9.7 ± 5.2 | 7.6 ± 1.6 | 20.6 ± 1.4 | 1.6 | 1.3 |
| 6g | 11.1 ± 0.6 | 15.3 ± 12.8 | 12 ± 6.7 | 16.9 ± 11.1 | 0.9 | 1.3 |
| 6h | 8.5 ± 3.2 | 16.6 ± 2.7 | 11.3 ± 1.2 | 22.1 ± 2.7 | 0.8 | 1.5 |
| 6i | 9.1 ± 0.4 | 13.1 ± 5.1 | 11 ± 0.4 | 17.2 ± 0.3 | 0.8 | 1.2 |
| 6k | 45.7 ± 15.7 | 71.9 ± 27.5 | 54.9 ± 15 | 79.4 ± 5.9 | 0.8 | 1.3 |
| 6l | 62.2 ± 21.4 | 43.2 ± 19 | 26.6 ± 0.9 | >100 | 2.3 | 1.6 |
| 6m | 16.1 ± 2.7 | 28.8 ± 5.1 | 14.7 ± 1.5 | 29 ± 5.7 | 1.1 | 2 |
| dox | 0.11 ± 0.04 | 0.020 ± 0.010 | 0.014 ± 0.005 | 0.037 ± 0.015 | 8.1 | 1.4 |
| Concentration, µM | 100 | 25 | 6.25 | 1.56 | 100 | 25 | 6.25 | 1.56 |
|---|---|---|---|---|---|---|---|---|
| Compound | ONPG/MTT | Survived Cells, % | ||||||
| Nutlin-3a | 8.62 | 6.83 | 3.82 | 1.97 | 77.34 | 103.42 | 103.41 | 106.33 |
| 5b | 1.03 | 0.96 | 0.95 | 0.97 | 72.94 | 81.74 | 99.16 | 100.89 |
| 5c | 0.96 | 0.86 | 0.97 | 0.78 | 72.54 | 66.33 | 68.71 | 89.37 |
| 6a | 1.57 | 1.02 | 1.08 | 1.12 | 73.66 | 91.27 | 101.07 | 105.44 |
| 6b | 1.22 | 1.31 | 1.11 | 1.09 | 45.20 | 52.40 | 83.34 | 80.78 |
| 6c | 1.21 | 1.03 | 1.16 | 0.97 | 23.27 | 84.28 | 90.43 | 98.25 |
| 6l | 2.08 | 0.92 | 0.94 | 1.15 | 50.91 | 79.87 | 94.15 | 93.47 |
| 6m | 1.03 | 1.15 | 1.06 | 1.04 | 41.91 | 79.19 | 91.11 | 91.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novotortsev, V.K.; Kukushkin, M.E.; Tafeenko, V.A.; Skvortsov, D.A.; Kalinina, M.A.; Timoshenko, R.V.; Chmelyuk, N.S.; Vasilyeva, L.A.; Tarasevich, B.N.; Gorelkin, P.V.; et al. Dispirooxindoles Based on 2-Selenoxo-Imidazolidin-4-Ones: Synthesis, Cytotoxicity and ROS Generation Ability. Int. J. Mol. Sci. 2021, 22, 2613. https://doi.org/10.3390/ijms22052613
Novotortsev VK, Kukushkin ME, Tafeenko VA, Skvortsov DA, Kalinina MA, Timoshenko RV, Chmelyuk NS, Vasilyeva LA, Tarasevich BN, Gorelkin PV, et al. Dispirooxindoles Based on 2-Selenoxo-Imidazolidin-4-Ones: Synthesis, Cytotoxicity and ROS Generation Ability. International Journal of Molecular Sciences. 2021; 22(5):2613. https://doi.org/10.3390/ijms22052613
Chicago/Turabian StyleNovotortsev, Vladimir K., Maxim E. Kukushkin, Viktor A. Tafeenko, Dmitry A. Skvortsov, Marina A. Kalinina, Roman V. Timoshenko, Nelly S. Chmelyuk, Liliya A. Vasilyeva, Boris N. Tarasevich, Petr V. Gorelkin, and et al. 2021. "Dispirooxindoles Based on 2-Selenoxo-Imidazolidin-4-Ones: Synthesis, Cytotoxicity and ROS Generation Ability" International Journal of Molecular Sciences 22, no. 5: 2613. https://doi.org/10.3390/ijms22052613
APA StyleNovotortsev, V. K., Kukushkin, M. E., Tafeenko, V. A., Skvortsov, D. A., Kalinina, M. A., Timoshenko, R. V., Chmelyuk, N. S., Vasilyeva, L. A., Tarasevich, B. N., Gorelkin, P. V., Erofeev, A. S., Majouga, A. G., Zyk, N. V., & Beloglazkina, E. K. (2021). Dispirooxindoles Based on 2-Selenoxo-Imidazolidin-4-Ones: Synthesis, Cytotoxicity and ROS Generation Ability. International Journal of Molecular Sciences, 22(5), 2613. https://doi.org/10.3390/ijms22052613








