Mediator-Related Symptoms and Anaphylaxis in Children with Mastocytosis
Abstract
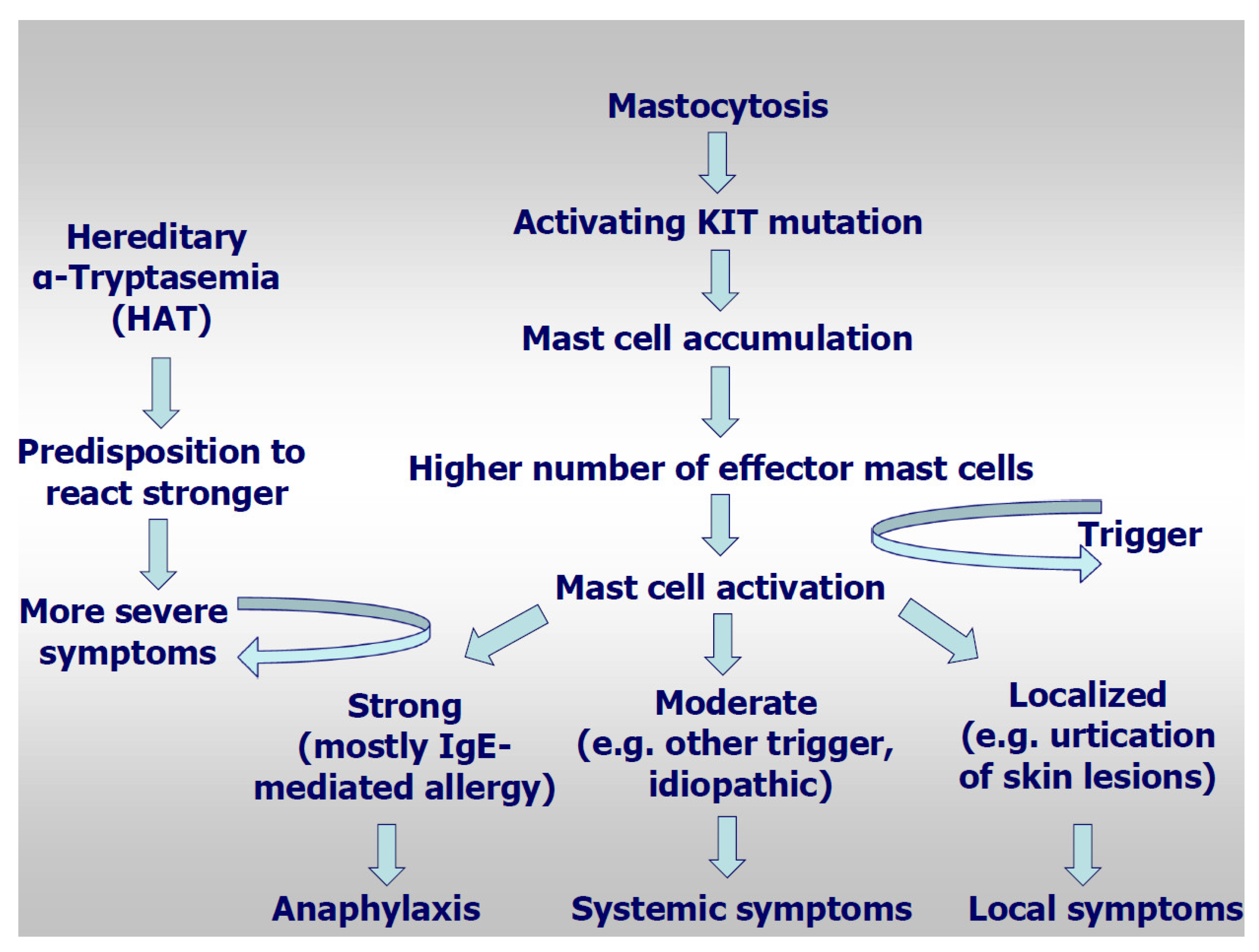
:1. Introduction
2. Mediators Released from MCs
3. Mediator-Related Symptoms in Children with Mastocytosis
3.1. Cutaneous Symptoms
3.2. Extracutaneous Systemic Symptoms
3.3. Anaphylaxis
3.4. Triggering Factors of Mediator-Related Symptoms and Anaphylaxis
3.5. Risk Factors for Anaphylaxis in Children with Mastocytosis
3.6. Hereditary Alpha-Tryptasemia as Risk Factor for Mediator-Related Symptoms and Anaphylaxis
4. Diagnostic Testing
5. Treatment
5.1. Topical Therapy of Skin Symptoms
5.2. Systemic Therapy of MC Mediator-Induced Symptoms
5.3. Management of Anaphylaxis
Author Contributions
Funding
Conflicts of Interest
References
- Castells, M.; Metcalfe, D.D.; Escribano, L. Diagnosis and treatment of cutaneous mastocytosis in children: Practical recommendations. Am. J. Clin. Dermatol. 2011, 12, 259–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broesby-Olsen, S.; Dybedal, I.; Gülen, T.; Kristensen, T.K.; Møller, M.B.; Ackermann, L.; Sääf, M.; Karlsson, M.A.; Agertoft, L.; Brixen, K.; et al. Multidisciplinary Management of Mastocytosis: Nordic Expert Group Consensus. Acta Derm. Venereol. 2016, 96, 602–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reite, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Advances in the Classification and Treatment of Mastocytosis: Current Status and Outlook toward the Future. Cancer Res. 2017, 77, 1261–1270. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.H. Pediatric mastocytosis. Curr. Opin. Pediatr. 2020, 32, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Méni, C.; Bruneau, J.; Georgin-Lavialle, S.; Le Saché de Peufeilhoux, L.; Damaj, G.; Hadj-Rabia, S.; Fraitag, S.; Dubreuil, P.; Hermine, O.; Bodemer, C. Paediatric mastocytosis: A systematic review of 1747 cases. Br. J. Dermatol. 2015, 172, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, T.I.; et al. Mast cells as a unique hematopoietic lineage and cell system: From Paul Ehrlich’s visions to precision medicine concepts. Theranostics 2020, 10, 10743–10768. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Gleixner, K.V.; Sperr, W.R.; Reiter, A.; Arock, M.; Triggiani, M. Multidisciplinary Challenges in Mastocytosis and How to Address with Personalized Medicine Approaches. Int. J. Mol. Sci. 2019, 20, 2976. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, K.; Escribano, L.; Grattan, C.; Brockow, K.; Carter, M.C.; Alvarez-Twose, I.; Matito, A.; Broesby-Olsen, S.; Siebenhaar, F.; Lange, M.; et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma &; Immunology; and the European Academy of Allergology and Clinical Immunology. J. Allergy Clin. Immunol. 2016, 137, 35–45. [Google Scholar]
- Valent, P.; Horny, H.P.; Escribano, L.; Longley, B.J.; Li, C.Y.; Schwartz, L.B.; Marone, G.; Nuñez, R.; Akin, C.; Sotlar, K.; et al. Diagnostic criteria and classification of mastocytosis: A consensus proposal. Leuk Res. 2001, 25, 603–625. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Metcalfe, D.D. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood 2017, 129, 1420–1427. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.C.; Clayton, S.T.; Komarow, H.D.; Brittain, E.H.; Scott, L.M.; Cantave, D.; Gaskins, D.M.; Maric, I.; Metcalfe, D.D. Assessment of clinical findings, tryptase levels, and bone marrow histopathology in the management of pediatric mastocytosis. J. Allergy Clin. Immunol. 2015, 136, 1673–1679. [Google Scholar] [CrossRef] [Green Version]
- Carter, M.C.; Bai, Y.; Ruiz-Esteves, K.N.; Scott, L.M.; Cantave, D.; Bolan, H.; Eisch, R.; Sun, X.; Hahn, J.; Maric, I.; et al. Detection of KIT D816V in peripheral blood of children with manifestations of cutaneous mastocytosis suggests systemic disease. Br. J. Haematol. 2018, 183, 775–782. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Twose, I.; Vañó-Galván, S.; Sánchez-Muñoz, L.; Morgado, J.M.; Matito, A.; Torrelo, A.; Jaén, P.; Schwartz, L.B.; Orfao, A.; Escribano, L. Increased serum baseline tryptase levels and extensive skin involvement are predictors for the severity of mast cell activation episodes in children with mastocytosis. Allergy 2012, 67, 813–818. [Google Scholar] [CrossRef] [Green Version]
- Barnes, M.; Van, L.; DeLong, L.; Lawley, L.P. Severity of cutaneous findings predict the presence of systemic symptoms in pediatric maculopapular cutaneous mastocytosis. Pediatr. Dermatol. 2014, 31, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K.; Ring, J.; Alvarez-Twose, I.; Orfao, A.; Escribano, L. Extensive blistering is a predictor for severe complications in children with mastocytosis. Allergy 2012, 67, 1323–1324. [Google Scholar] [CrossRef]
- Valent, P. Mast cells, masters, and mastocytosis: Development of research since the times of Paul Ehrlich. Wiener Klinische Wochenschrift. 2004, 116, 645–646. [Google Scholar] [CrossRef] [PubMed]
- Bezzerri, V.; Borgatti, M.; Finotti, A.; Tamanini, A.; Gambari, R.; Cabrini, G. Mapping the transcriptional machinery of the IL-8 gene in human bronchial epithelial cells. J. Immunol. 2011, 187, 6069–6081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dispenza, M.C. Classification of hypersensitivity reactions. Allergy Asthma Proc. 2019, 40, 470–473. [Google Scholar] [CrossRef]
- Simons, F.E.R.; Ebisawa, M.; Sanchez-Borges, M.; Thong, B.Y.; Worm, M.; Tanno, L.K.; Lockey, R.F.; El-Gamal, Y.M.; Brown, S.G.; Park, H.-S.; et al. 2015 update of the evidence base: World Allergy Organization anaphylaxis guidelines. World Allergy Organ. J. 2015, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Greenhawt, M.; Akin, C. Mastocytosis and allergy. Curr. Opin. Allergy Clin. Immunol. 2007, 7, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Baghestanian, M.; Hofbauer, R.; Kiener, H.P.; Bankl, H.C.; Wimazal, F.; Willheim, M.; Scheiner, O.; Füreder, W.; Müller, M.R.; Bevec, D.; et al. The c-kit ligand stem cell factor and anti-IgE promote expression of monocyte chemoattractant protein-1 in human lung mast cells. Blood 1997, 90, 4438–4449. [Google Scholar] [CrossRef] [Green Version]
- Wimazal, F.; Jordan, J.H.; Sperr, W.R.; Chott, A.; Dabbass, S.; Lechner, K.; Horny, H.P.; Valent, P. Increased angiogenesis in the bone marrow of patients with systemic mastocytosis. Am. J. Pathol. 2002, 160, 1639–1645. [Google Scholar] [CrossRef] [Green Version]
- Hoermann, G.; Cerny-Reiterer, S.; Perné, A.; Klauser, M.; Hoetzenecker, K.; Klein, K.; Müllauer, L.; Gröger, M.; Nijman, S.M.; Klepetko, W.; et al. Identification of oncostatin M as a STAT5-dependent mediator of bone marrow remodeling in KIT D816V-positive systemic mastocytosis. Am. J. Pathol. 2011, 178, 2344–2356. [Google Scholar] [CrossRef] [Green Version]
- Greiner, G.; Witzeneder, N.; Berger, A.; Schmetterer, K.; Eisenwort, G.; Schiefer, A.I.; Roos, S.; Popow-Kraupp, T.; Müllauer, L.; Zuber, J.; et al. CCL2 is a KIT D816V-dependent modulator of the bone marrow microenvironment in systemic mastocytosis. Blood 2017, 129, 371–382. [Google Scholar] [CrossRef]
- Collington, S.J.; Williams, T.J.; Weller, C.L. Mechanisms underlying the localisation of mast cells in tissues. Trends Immunol. 2011, 32, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Ravanbakhsh, N.; Kesavan, A. The role of mast cells in pediatric gastrointestinal disease. Ann. Gastroenterol. 2019, 32, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.J.; Misseldine, S. Anesthetic considerations in pediatric mastocytosis: A review. J. Anesth. 2013, 27, 588–598. [Google Scholar] [CrossRef] [Green Version]
- Castells, M.; Butterfield, J. Mast cell activation syndrome and mastocytosis: Initial treatment options and long-term management. J. Allergy Clin. Immunol Pract. 2019, 7, 1097–1106. [Google Scholar] [CrossRef]
- Wodnar-Filipowicz, A.; Heusser, C.H.; Moroni, C. Production of the haemopoietic growth factors GM-CSF and interleukin-3 by mast cells in response to IgE receptor-mediated activation. Nature 1989, 339, 150–152. [Google Scholar] [CrossRef]
- Echtenacher, B.; Männel, D.N.; Hültner, L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature 1996, 381, 75–77. [Google Scholar] [CrossRef]
- Malaviya, R.; Ikeda, T.; Ross, E.; Abraham, S.N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 1996, 381, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.R.; Galli, S.J. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature 1990, 346, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Matito, A.; Carter, M. Cutaneous and Systemic Mastocytosis in Children: A Risk Factor for Anaphylaxis? Curr. Allergy Asthma Rep. 2015, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.; Staubach, P. Mastocytosis—Pathogenesis, clinical manifestation and treatment. JDDG 2018, 16, 42–57. [Google Scholar] [CrossRef] [Green Version]
- Lange, M.; Niedoszytko, M.; Renke, J.; Glen, J.; Nedoszytko, B. Clinical aspects of paediatric mastocytosis: A review of 101 cases. J. Eur. Acad Dermatol. Venereol. 2013, 27, 97–102. [Google Scholar] [CrossRef]
- Klaiber, N.; Kumar, S.; Irani, A.M. Mastocytosis in Children. Curr. Allergy Asthma Rep. 2017, 17, 80. [Google Scholar] [CrossRef]
- Matito, A.; Azaña, J.M.; Torrelo, A.; Alvarez-Twose, I. Cutaneous Mastocytosis in Adults and Children: New Classification and Prognostic Factors. Immunol. Allergy Clin. N. Am. 2018, 38, 351–363. [Google Scholar] [CrossRef]
- Lange, M.; Zawadzka, A.; Schrors, S.; Slomka, J.; Lugowska-Umer, H.; Nedoszytko, B.; Nowicki, R. The role of serum tryptase in the diagnosis and monitoring of pediatric mastocytosis: A single-center experience. Postepy Dermatol. Alergol. 2017, 34, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Heide, R.; Beishuizen, A.; De Groot, H.; Den Hollander, J.C.; Van Doormaal, J.J.; De Monchy, J.G.R.; Pasmans, S.; Van Gysel, D.; Oranje, A. Dutch National Mastocytosis Work Group Mastocytosis in children: A protocol for management. Pediatr. Dermatol. 2008, 25, 493–500. [Google Scholar] [CrossRef]
- Theoharides, T.C. Autism spectrum disorders and mastocytosis. Int. J. Immunopathol. Pharmacol. 2009, 22, 859–865. [Google Scholar] [CrossRef] [Green Version]
- Lange, M.; Niedoszytko, M.; Nedoszytko, B.; Łata, J.; Trzeciak, M.; Biernat, W. Diffuse cutaneus mastocytosis: Analysis of 10 cases and a brief review of the literature. J. Eur. Acad Dermatol. Venereol. 2012, 26, 1565–1571. [Google Scholar] [PubMed]
- Brockow, K.; Jofer, C.; Behrendt, H.; Ring, J. Anaphylaxis in patients with mastocytosis: A study on history, clinical features and risk factors in 120 patients. Allergy 2008, 63, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Heinze, A.; Kuemmet, T.J.; Chiu, Y.E.; Galbraith, S.S. Longitudinal Study of Pediatric Urticaria Pigmentosa. Pediatr. Dermatol. 2017, 34, 144–149. [Google Scholar] [CrossRef]
- Lieberman, P.; Camargo, C.A., Jr.; Bohlke, K.; Jick, H.; Miller, R.L.; Sheikh, A.; Simons, F.E. Epidemiology of anaphylaxis: Findings of the American College of Allergy, Asthma and Immunology Epidemiology of Anaphylaxis Working Group. Ann. Allergy Asthma Immunol. 2006, 97, 596–602. [Google Scholar] [CrossRef]
- Gonzalez de Olano, D.; de la Hoz Caballer, B.; Nunez Lopez, R.; Sanchez Munoz, L.; Cuevas Agustin, M.; Dieguez, M.C.; Alvarez Twose, I.; Castells, M.C.; Escribano Mora, L. Prevalence of allergy and anaphylactic symptoms in 210 adult and pediatric patients with mastocytosis in Spain: A study of the Spanish network on mastocytosis (REMA). Clin. Exp. Allergy 2007, 37, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Matito, A.; Morgado, J.M.; Sánchez-López, P.; Álvarez-Twose, I.; Sánchez-Muñoz, L.; Orfao, A.; Escribano, L. Management of Anesthesia in Adult and Pediatric Mastocytosis: A Study of the Spanish Network on Mastocytosis (REMA) Based on 726 Anesthetic Procedures. Int. Arch. Allergy Immunol. 2015, 167, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Bonadonna, P.; Perbellini, O.; Passalacqua, G.; Caruso, B.; Colarossi, S.; Dal Fior, D.; Castellani, L.; Bonetto, C.; Frattini, F.; Dama, A.; et al. Clonal mast cell disorders in patients with systemic reactions to Hymenoptera stings and increased serum tryptase levels. J. Allergy Clin. Immunol. 2009, 123, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Niedoszytko, M.; de Monchy, J.; van Doormaal, J.J.; Jassem, E.; Oude Elberink, J.N. Mastocytosis and insect venom allergy: Diagnosis, safety and efficacy of venom immunotherapy. Allergy 2009, 64, 1237–1245. [Google Scholar] [CrossRef]
- Brockow, K.; Akin, C. Hymenoptera-induced anaphylaxis: Is it a mast cell driven hematological disorder? Curr. Opin. Allergy Clin. Immunol. 2017, 17, 356–362. [Google Scholar] [CrossRef]
- Oropeza, A.R.; Bindslev-Jensen, C.; Broesby-Olsen, S.; Kristensen, T.; Moller, M.B.; Vestergaard, H.; Kjaer, H.F.; Halken, S.; Lassen, A.; Mortz, C.G. Patterns of anaphylaxis after diagnostic work-up: A follow-up study of 226 patients with suspected anaphylaxis. Allergy 2017, 72, 1944–1952. [Google Scholar] [CrossRef]
- Jarkvist, J.; Brockow, K.; Gulen, T. Low Frequency of IgE-Mediated Food Hypersensitivity in Mastocytosis. J. Allergy Clin. Immunol. Pract. 2020, 8, 3093–3101. [Google Scholar] [CrossRef]
- Brockow, K.; Bonadonna, P. Drug allergy in mast cell disease. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.C.; Metcalfe, D.D.; Matito, A.; Escribano, L.; Butterfield, J.H.; Schwartz, L.B.; Bonadonna, P.; Zanotti, R.; Triggiani, M.; Castells, M.; et al. Adverse reactions to drugs and biologics in patients with clonal mast cell disorders: A group report of the Mast Cells Disorder Committee, American Academy of Allergy and Immunology. J. Allergy Clin. Immunol. 2019, 143, 880–893. [Google Scholar] [CrossRef] [PubMed]
- Parente, R.; Pucino, V.; Magliacane, D.; Petraroli, A.; Loffredo, S.; Marone, G.; Triggiani, M. Evaluation of vaccination safety in children with mastocytosis. Pediatr. Allergy Immunol. 2017, 28, 93–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanoni, G.; Zanotti, R.; Schena, D.; Sabbadini, C.; Opri, R.; Bonadonna, P. Vaccination management in children and adults with mastocytosis. Clin. Exp. Allergy 2017, 47, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.C.; Uzzaman, A.; Scott, L.M.; Metcalfe, D.D.; Quezado, Z. Pediatric mastocytosis: Routine anesthetic management for a complex disease. Anesth Analg 2008, 107, 422–427. [Google Scholar] [CrossRef] [Green Version]
- Akin, C.; Scott, L.M.; Kocabas, C.N.; Kushnir-Sukhov, N.; Brittain, E.; Noel, P.; Metcalfe, D.D. Demonstration of an aberrant mast-cell population with clonal markers in a subset of patients with “idiopathic” anaphylaxis. Blood 2007, 110, 2331–2333. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, H.C.; Parsons, D.J.; Peden, D.B.; Morrell, D. Recurrent syncope and anaphylaxis as presentation of systemic mastocytosis in a pediatric patient: Case report and literature review. J. Am. Acad Dermatol. 2006, 54, 210–213. [Google Scholar] [CrossRef]
- Brockow, K. Epidemiology, prognosis, and risk factors in mastocytosis. Immunol. Allergy Clin. North. Am. 2014, 34, 283–295. [Google Scholar] [CrossRef]
- Schuch, A.; Brockow, K. Mastocytosis and Anaphylaxis. Immunol. Allergy Clin. North. Am. 2017, 37, 153–164. [Google Scholar] [CrossRef]
- O’Connell, M.P.; Lyons, J.J. Hymenoptera venom-induced anaphylaxis and hereditary alpha-tryptasemia. Curr. Opin. Allergy Clin. Immunol. 2020, 20, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.C.; Wilcock, A.; Bonin, H.; Beaman, G.; Myers, B.; Grattan, C.; Briggs, T.A.; Arkwright, P.D. Hereditary Alpha-Tryptasemia: UK Prevalence and Variability in Disease Expression. J. Allergy Clin. Immunol. Pract. 2020, 8, 3549–3556. [Google Scholar] [CrossRef]
- Lyons, J.J. Hereditary Alpha Tryptasemia: Genotyping and Associated Clinical features. Immunol. Allergy Clin. North. Am. 2018, 63, 483–495. [Google Scholar] [CrossRef]
- Greiner, G.; Sprinzl, B.; Górska, A.; Ratzinger, F.; Gurbisz, M.; Witzeneder, N.; Schmetterer, K.G.; Gisslinger, B.; Uyanik, G.; Hadzijusufovic, E.; et al. Hereditary alpha tryptasemia is a valid genetic biomarker for severe mediator-related symptoms in mastocytosis. Blood 2020, 10, 2020006157. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.T.; Lyons, J.J.; Naranjo, A.N.; Olivera, A.; Lazarus, R.A.; Metcalfe, D.D.; Milner, J.D.; Schwartz, L.B. Impact of naturally forming human α/β-tryptase heterotetramers in the pathogenesis of hereditary α-tryptasemia. J. Exp. Med. 2019, 216, 2348–2361. [Google Scholar] [CrossRef] [PubMed]
- Lyons, J.J.; Chovanec, J.; O’Connell, M.P.; Liu, Y.; Šelb, J.; Zanotti, R.; Bai, Y.; Kim, J.; Le, Q.T.; DiMaggio, T.; et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase-encoding germline copy number at TPSAB. J. Allergy Clin. Immunol. 2021, 147, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Sabato, V.; Van De Vijver, E.; Hagendorens, M.; Vrelust, I.; Reyniers, E.; Fransen, E.; Bridts, C.; De Clerck, L.; Mortier, G.; Valent, P.; et al. Familial hypertryptasemia with associated mast cell activation syndrome. J. Allergy Clin. Immunol. 2014, 134, 1448–1450.e3. [Google Scholar] [CrossRef] [PubMed]
- Sznurkowska, K.; Plata- Nazar, K.; Sikorska-Wiśniewska, G.; Gruszczyńska, I.; Renke, J.; Niedoszytko, M.; Gleń, J.; Kamińska, B. Serum Concentrations of Tryptase in Children. Pediatric. Allergy Immunol. Pulmonol. 2014, 27, 70–74. [Google Scholar] [CrossRef]
- Valent, P.; Escribano, L.; Broesby-Olsen, S.; Hartmann, K.; Grattan, C.; Brockow, K.; Niedoszytko, M.; Nedoszytko, B.; Oude Elberink, J.N.; Kristensen, T.; et al. Proposed diagnostic algorithm for patients with suspected mastocytosis: A proposal of the European Competence Network on Mastocytosis. Allergy 2014, 69, 1267–1274. [Google Scholar] [CrossRef] [Green Version]
- Horny, H.P.; Akin, C.; Arber, D.; Peterson, L.A.; Tefferi, A.; Metcalfe, D.D.; Bennett, J.M.; Bain, B.; Escribano, L.; Valent, P. Mastocytosis. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Arber, D.A., Hasserjian, R.P., Le Beau, M.M., et al., Eds.; IARC Press: Lyon, France, 2017; pp. 62–69. [Google Scholar]
- Valent, P.; Akin, C.; Escribano, L.; Födinger, M.; Hartmann, K.; Brockow, K.; Castells, M.; Sperr, W.R.; Kluin-Nelemans, H.C.; Hamdy, N.A.; et al. Standards and standardization in mastocytosis: Consensus statements on diagnostics, treatment recommendations and response criteria. Eur. J. Clin. Investig. 2007, 37, 435–553. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Arock, M.; Brockow, K.; Butterfield, J.H.; Carter, M.C.; Castells, M.; Escribano, L.; Hartmann, K.; Lieberman, P.; et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: A consensus proposal. Int. Arch. Allergy Immunol. 2012, 157, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Brockow, K.; Akin, C.; Huber, M.; Metcalfe, D. Assessment of the extent of cutaneous involvement in children and adults with mastocytosis: Relationship to symptomatology, tryptase levels, and bone marrow pathology. J. Am. Acad Dermatol. 2003, 48, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Czarny, J.; Żuk, M.; Żawrocki, A.; Plata-Nazar, K.; Biernat, W.; Niedoszytko, M.; Ługowska-Umer, H.; Nedoszytko, B.; Wasąg, B.; Nowicki, R.J.; et al. New Approach to Paediatric Mastocytosis: Implications of KIT D816V Mutation Detection in Peripheral Blood. Acta Derm Venereol. 2020, 100, adv00149. [Google Scholar] [CrossRef]
- Broesby-Olsen, S.; Carter, M.; Kjaer, H.F.; Mortz, C.G.; Møller, M.B.; Kristensen, T.K.; Bindslev-Jensen, C.; Agertoft, L. Pediatric Expression of Mast Cell Activation Disorders. Immunol. Allergy Clin. N. Am. 2018, 38, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Azana, J.M.; Torrelo, A.; Matito, A. Update on mastocytosis (part 2): Categories, Prognosis, and Treatment. Actas Derm. 2016, 107, 15–22. [Google Scholar]
- Edwards, A.M.; Hagberg, H. Oral and inhaled sodium cromoglicate in the management of systemic mastocytosis: A case report. J. Med. Case Rep. 2010, 4, 193. [Google Scholar] [CrossRef] [Green Version]
- Jenkinson, H.A.; Lundgren, A.D.; Carter, M.C.; Diaz, L.Z.; Levy, M.L. Management of neonate with diffuse cutaneous mastocytosis: Case report and literature review. Pediatric. Dermatol. 2019, 36, 486–489. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Lam, J.M.; Leong, K.F. Childhood Solitary Cutaneous Mastocytoma: Clinical Manifestations, Diagnosis, Evaluation, and Management. Curr. Pediatr. Rev. 2019, 15, 42–46. [Google Scholar] [CrossRef]
- Patrizi, A.; Tabanelli, M.; Neri, I.; Virdi, A. Topical corticosteroids versus “wait and see” in the management of solitary mastocytoma in pediatric patients: A long-term follow-up. Dermatol. Ther. 2015, 28, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Mashiah, J.; Harel, A.; Bodemer, C.; Hadj-Rabia, S.; Goldberg, I.; Sprecher, E.; Kutz, A. Topical pimecrolimus for pediatric cutaneous mastocytosis. Clin. Exp. Dermatol. 2018, 43, 559–565. [Google Scholar] [CrossRef]
- Correia, O.; Duarte, A.; Quirino, P.; Azevedo, R.; Delgado, L. Cutaneous mastocytosis: Two pediatric cases treated with topical pimecrolimus. Dermatol. Online J. 2010, 16, 8. [Google Scholar] [PubMed]
- Brazzelli, V.; Grassi, S.; Merante, S.; Grasso, V.; Ciccocioppo, R.; Bossi, G.; Borroni, G. Narrow-band UVB phototherapy and psoralen-ultraviolet A photochemotherapy in the treatment of cutaneous mastocytosis: A study in 20 pateints. Photodermatol. Photoimmunol. Photomed. 2016, 32, 238–246. [Google Scholar] [CrossRef]
- Kinsler, V.A.; Hawk, J.L.M.; Atherton, D.J. Diffuse cutaneous mastocytosis treated with psoralen photochemtherapy: Case report and review of literature. Br. J. Dermatol. 2005, 152, 179–180. [Google Scholar]
- Siebenhaar, F.; Akin, C.; Bindslev-Jensen, C.; Maurer, M.; Broesby-Olsen, S. Treatment strategies in mastocytosis. Immunol. Allergy Clin. North. Am. 2014, 34, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Eustace, K.; Dolman, S.; Alsharqi, A.; Sharpe, G.; Parslew, R. Use of Phototherapy in Children. Pediatric. Dermatol. 2017, 34, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Hosking, A.M.; Makdisi, J.; Ortenzio, F.; de Feraudy, S.; Smith, J.; Linden, K. Diffuse cutaneous mastocytosis: Case report and literature review. Pediatr. Dermatol. 2018, 35, e348–e352. [Google Scholar] [CrossRef]
- Frieri, M.; Quershi, M. Pediatric Mastocytosis: A Review of the Literature. Pediatr. Allergy Immunol. Pulmonol. 2013, 26, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Nurmatov, U.B.; Rhatigan, E.; Simons, F.E.R.; Sheikh, A. H1-antihistamines for primary mast cell activation syndromes: A systematic review. Allergy 2015, 70, 1052–1061. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.M.; Vagin, O.; Munson, K.; Kidd, M.; Modlin, I.M.; Sachs, G. Molecular mechanisms in therapy of acid-related diseases. Cell. Mol. Life Sci. 2008, 65, 264–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ring, J.; Beyer, K.; Biedermann, T.; Bircher, A.; Duda, D.; Fischer, J.; Friedrichs, F.; Fuchs, T.; Gieler, U.; Jakob, T.; et al. Guideline for acute therapy and management of anaphylaxis: S2 Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Association of German Allergologists (AeDA), the Society of Pediatric Allergy and Environmental Medicine (GPA), the German Academy of Allergology and Environmental Medicine (DAAU), the German Professional Association of Pediatricians (BVKJ), the Austrian Society for Allergology and Immunology (OGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Anaesthesiology and Intensive Care Medicine (DGAI), the German Society of Pharmacology (DGP), the German Society for Psychosomatic Medicine (DGPM), the German Working Group of Anaphylaxis Training and Education (AGATE) and the patient organization German Allergy and Asthma Association (DAAB). Allergo J. Int. 2014, 23, 96–112. [Google Scholar] [PubMed]
- Brockow, K.; Schallmayer, S.; Beyer, K.; Biedermann, T.; Fischer, J.; Gebert, N.; Grosber, M.; Jakob, T.; Klimek, L.; Kugler, C.; et al. Effects of a structured educational intervention on knowledge and emergency management in patients at risk for anaphylaxis. Allergy 2015, 70, 227–235. [Google Scholar] [CrossRef]
- Le, M.; Miedzybrodzki, B.; Olynych, T.; Chapdelaine, H.; Ben-Shoshan, M. Natural history and treatment of cutaneous and systemic mastocytosis. Postgrad Med. 2017, 129, 896–901. [Google Scholar] [CrossRef]
- Slapnicar, C.; Trinkaus, M.; Lisa Hicks, L.; Vadas, P. Efficacy of Omalizumab in Indolent Systemic Mastocytosis. Case Rep. Hematol. 2019, 16, 3787586. [Google Scholar] [CrossRef] [Green Version]
- Hinojosa, T.; Lewis, D.J.; Vangipuram, R.; Safeer, L.; Mui, U.N.; Haley, C.; Konoplev, S.; Tyring, S.K. The efficacy of omalizumab in Cutaneous Mastocytosis: A case series. Dermatol. Ther. 2019, 32, e12848. [Google Scholar] [CrossRef]
- Matito, A.; Blázquez-Goñi, C.; Morgado, J.M.; Álvarez-Twose, I.; Mollejo, M.; Sánchez-Muñoz, L.; Escribano, L. Short-term omalizumab treatment in an adolescent with cutaneous mastocytosis. Ann. Allergy Asthma Immunol. 2013, 111, 425–426. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.D.M.; Olynyc, T.; Chapdelaine, H.; Segal, L.; Miedzybrodzki, B.; Ben-Shoshan, M. Effective management of severe cutaneous mastocytosis in young children with omalizumab (Xolair®). Clin. Exp. Dermatol. 2018, 43, 573–576. [Google Scholar] [CrossRef]
- Broesby-Olsen, S.; Vestergaard, H.; Mortz, C.G.; Jensen, B.; Havelund, T.; Hermann, A.P.; Siebenhaar, F.; Møller, M.B.; Kristensen, T.K.; Bindslev-Jensen, C. Mastocytosis Centre Odense University Hospital (MastOUH). Omalizumab prevents anaphylaxis and improves symptoms in systemic mastocytosis: Efficacy and safety observations. Allergy 2018, 73, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Jendoubi, F.; Gaudenzio, N.; Gallini, A.; Negretto, M.; Paul, C.; Bulai Livideanu, C. Omalizumab in the treatment of adult patients with mastocytosis: A systematic review. Clin. Exp. Allergy 2020, 50, 654–661. [Google Scholar] [CrossRef]
- Bell, M.C.; Jackson, D.J. Prevention of anaphylaxis related to mast cell activation syndrome with omalizumab. Ann. Allergy Asthma Immunol. 2012, 108, 383–384. [Google Scholar] [CrossRef] [Green Version]
- Constantine, G.M.; Bressler, P.B.; Petroni, D.; Metcalfe, D.D.; Carter, M.C. Twelve-year follow-up of omalizumab therapy for anaphylaxis in 2 patients with systemic mastocytosis. J. Allergy Clin. Immunol. Pract. 2019, 7, 1314–1316. [Google Scholar] [CrossRef] [PubMed]
- Distler, M.; Maul, J.T.; Steiner, U.C.; Jandus, P.; Kolios, A.G.A.; Murer, C.; Graf, N.; Seebach, J.D.; Pichler, W.J.; Navarini, A.A.; et al. Efficacy of omalizumab in mastocytosis: Allusive indication obtained from a prospective, double-blind, multicenter study (XOLIMA study). Dermatology 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lemal, R.; Fouquet, G.; Terriou, L.; Vaes, M.; Livideanu, C.B.; Frenzel, L.; Barete, S.; Canioni, D.; Lhermitte, L.; Rossignol, J.; et al. Omalizumab Therapy for Mast Cell-Mediator Symptoms in Patients with ISM, CM, MMAS, and MCAS. J. Allergy Clin. Immunol. Pract. 2019, 7, 2387–2395.e3. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.P.; Giménez-Arnau, A.M.; Saini, S.S. Mechanisms of action that contribute to efficacy of omalizumab in chronic spontaneous urticaria. Allergy 2017, 72, 519–533. [Google Scholar] [CrossRef] [Green Version]
| Study * | Year | No | Frequency | Elicitors |
|---|---|---|---|---|
| Gonzales [46] | 2007 | 47 | 3 (6.4%) | Food, idiopathic |
| Brockow [43] | 2008 | 46 | 4 (9%) | Food, vaccination, cold water, idiopathic |
| Alvarez-Twose [14] | 2012 | 111 | 4 (3.6%) | Heat, skin rubbing, fever |
| Lange [36] | 2013 | 101 | 7 (7%) | Clindamycin, ketamine, gadolinium contrast, stress, idiopathic |
| Barnes [15] | 2014 | 67 | 1 (1,5%) | NA |
| Matito [47] | 2015 | 48 | 1 (2.0%) | Perioperative |
| Lange [39] | 2017 | 102 | 2 (2%) | Ketamine, idiopathic |
| Heinze [44] | 2017 | 43 | 0 (0%) | NA |
| Studies combined | 565 | 22 (3.9%) |
| Criterium | Risk Factor Outcome | Study |
|---|---|---|
| Extent and density of skin lesions | Anaphylaxis in those with extent >45% and density >15% | Brockow et al. [43] |
| Serum tryptase | Significantly elevated in patients with anaphylaxis | Brockow et al. [43] |
| Correlation with severity | Alvarez-Twose et al. [14] | |
| Skin involvement | >90%: risk factor for hospitalization * | Alvarez-Twose et al. [14] |
| Blistering | Risk factor for hospitalization * | Brockow et al. [16] |
| Diffuse cutaneous mastocytosis | Risk factor for hospitalization * | Alvarez-Twose et al. [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brockow, K.; Plata-Nazar, K.; Lange, M.; Nedoszytko, B.; Niedoszytko, M.; Valent, P. Mediator-Related Symptoms and Anaphylaxis in Children with Mastocytosis. Int. J. Mol. Sci. 2021, 22, 2684. https://doi.org/10.3390/ijms22052684
Brockow K, Plata-Nazar K, Lange M, Nedoszytko B, Niedoszytko M, Valent P. Mediator-Related Symptoms and Anaphylaxis in Children with Mastocytosis. International Journal of Molecular Sciences. 2021; 22(5):2684. https://doi.org/10.3390/ijms22052684
Chicago/Turabian StyleBrockow, Knut, Katarzyna Plata-Nazar, Magdalena Lange, Bogusław Nedoszytko, Marek Niedoszytko, and Peter Valent. 2021. "Mediator-Related Symptoms and Anaphylaxis in Children with Mastocytosis" International Journal of Molecular Sciences 22, no. 5: 2684. https://doi.org/10.3390/ijms22052684
APA StyleBrockow, K., Plata-Nazar, K., Lange, M., Nedoszytko, B., Niedoszytko, M., & Valent, P. (2021). Mediator-Related Symptoms and Anaphylaxis in Children with Mastocytosis. International Journal of Molecular Sciences, 22(5), 2684. https://doi.org/10.3390/ijms22052684








