A Narrative Review on Plasminogen Activator Inhibitor-1 and Its (Patho)Physiological Role: To Target or Not to Target?
Abstract
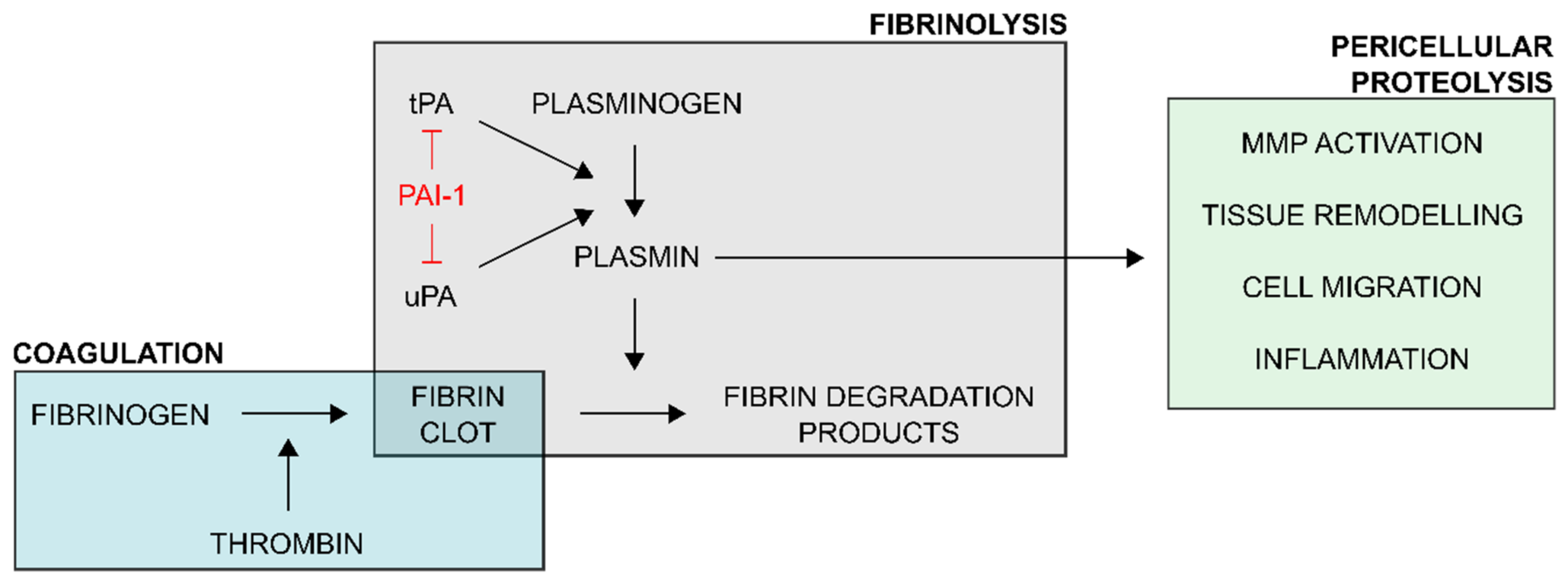
1. Introduction
2. PAI-1 Synthesis and Distribution
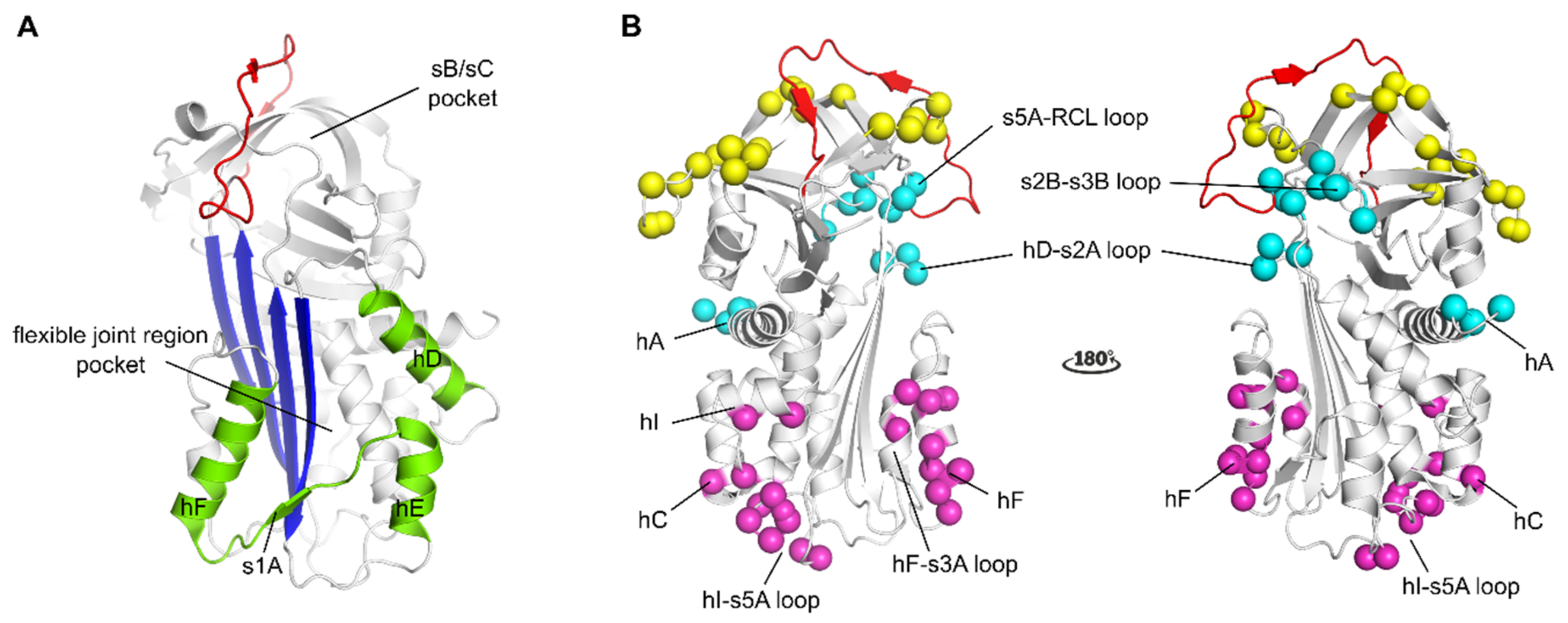
3. PAI-1 Structure and Function
3.1. PAI-1 Is an Inhibitory Serpin
3.2. PAI-1 Stability
3.3. Interactions with Non-Proteinase Ligands
4. Role of PAI-1 in Diverse Pathologies
4.1. PAI-1 in Cardiovascular Disease
4.2. PAI-1 in Metabolic Disturbances
4.3. PAI-1 in Inflammation and Infectious Disease
4.4. PAI-1 in Cancer
4.5. PAI-1 in Fibrosis
4.6. PAI-1 in the Central Nervous System
4.7. PAI-1 in Aging
5. Diverse Approaches to Inhibit PAI-1
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-β |
| Abs | Antibodies |
| ARDS | Acute respiratory distress syndrome |
| COVID-19 | Coronavirus disease 2019 |
| CVD | Cardiovascular disease |
| ECM | Extracellular matrix |
| IHD | Ischemic heart disease |
| IL-6 | Interleukin-6 |
| LDL | Low-density lipoprotein |
| LDLR | Low-density lipoprotein receptor |
| LRP1 | LDLR-related protein 1 |
| MMP | Matrix metalloprotease |
| PA | Plasminogen activator |
| PAI-1 | Plasminogen activator inhibitor-1 |
| RCL | Reactive center loop |
| SASP | Senescence-associated secretory phenotype |
| SMB | Somatomedin B |
| TNF-α | Tumor necrosis factor-α [72] |
| tPA | Tissue-type plasminogen activator |
| uPA | Urokinase-type plasminogen activator |
| uPAR | uPA receptor |
References
- Gils, A.; Declerck, P.J. Three Decades of Research on Plasminogen Activator Inhibitor-1: A Multifaceted Serpin. Semin. Thromb. Hemost. 2013, 39, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Loskutoff, D.J.; Van Mourik, J.A.; Erickson, L.A.; Lawrence, D. Detection of an unusually stable fibrinolytic inhibitor produced by bovine endothelial cells. Proc. Natl. Acad. Sci. USA 1983, 80, 2956–2960. [Google Scholar] [CrossRef]
- Chmielewska, J.; Rånby, M.; Wiman, B. Evidence for a rapid inhibitor to tissue plasminogen activator in plasma. Thromb. Res. 1983, 31, 427–436. [Google Scholar] [CrossRef]
- Simpson, A.J.; Booth, N.A.; Moore, N.R.; Bennett, B. Distribution of plasminogen activator inhibitor (PAI-1) in tissues. J. Clin. Pathol. 1991, 44, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Crandall, D.L.; Quinet, E.M.; Morgan, G.A.; Busler, D.E.; McHendry-Rinde, B.; Kral, J.G. Synthesis and Secretion of Plasminogen Activator Inhibitor-1 by Human Preadipocytes. J. Clin. Endocrinol. Metab. 1999, 84, 3222–3227. [Google Scholar] [CrossRef]
- Rabieian, R.; Boshtam, M.; Zareei, M.; Kouhpayeh, S.; Masoudifar, A.; Mirzaei, H. Plasminogen Activator Inhibitor Type-1 as a Regulator of Fibrosis. J. Cell. Biochem. 2018, 119, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Takeshita, K.; Shimokawa, T.; Yi, H.; Isobe, K.-I.; Loskutoff, D.J.; Saito, H. Plasminogen activator inhibitor-1 is a major stress-regulated gene: Implications for stress-induced thrombosis in aged individuals. Proc. Natl. Acad. Sci. USA 2002, 99, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, P.; Riccio, A.; Welinder, K.; Douglas, R.; Sartorio, R.; Nielsen, L.; Oppenheimer, C.; Blasi, F.; Danø, K. Plasminogen activator inhibitor type-1: Reactive center and amino-terminal heterogeneity determined by protein and cDNA sequencing. FEBS Lett. 1986, 209, 213–218. [Google Scholar] [CrossRef]
- Gils, A.; Pedersen, K.E.; Skottrup, P.; Christensen, A.; Naessens, D.; Deinum, J.; Enghild, J.J.; Declerck, P.J.; Andreasen, P.A. Biochemical importance of glycosylation of plasminogen activator inhibitor-1. Thromb. Haemost. 2003, 90, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Booth, N.A.; Simpson, A.J.; Croll, A.; Bennett, B.; MacGregor, I.R. Plasminogen activator inhibitor (PAI-1) in plasma and platelets. Br. J. Haematol. 1988, 70, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Booth, N.; Cröll, A.; Bennett, B. The activity of plasminogen activator inhibitor-1 (PAI-1) of human platelet. Fibrinolysis 1990, 4, 138–140. [Google Scholar] [CrossRef]
- Declerck, P.J.; Alessi, M.C.; Verstreken, M.; Kruithof, E.K.; Juhan-Vague, I.; Collen, D. Measurement of plasminogen activator inhibitor 1 in biologic fluids with a murine monoclonal antibody-based enzyme-linked immunosorbent assay. Blood 1988, 71, 220–225. [Google Scholar] [CrossRef]
- Brogren, H.; Wallmark, K.; Deinum, J.; Karlsson, L.; Jern, S. Platelets Retain High Levels of Active Plasminogen Activator Inhibitor 1. PLoS ONE 2011, 6, e26762. [Google Scholar] [CrossRef]
- Morrow, G.B.; Whyte, C.S.; Mutch, N.J. Functional plasminogen activator inhibitor 1 is retained on the activated platelet membrane following platelet activation. Haematology 2019, 105, 2824–2833. [Google Scholar] [CrossRef]
- Torr-Brown, S.R.; Sobel, B.E. Attenuation of thrombolysis by release of plasminogen activator inhibitor type-1 from platelets. Thromb. Res. 1993, 72, 413–421. [Google Scholar] [CrossRef]
- Stringer, H.A.; Van Swieten, P.; Heijnen, H.F.; Sixma, J.J.; Pannekoek, H. Plasminogen activator inhibitor-1 released from activated platelets plays a key role in thrombolysis resistance. Studies with thrombi generated in the Chandler loop. Arterioscler. Thromb. J. Vasc. Biol. 1994, 14, 1452–1458. [Google Scholar] [CrossRef]
- Irving, J.A.; Pike, R.N.; Lesk, A.M.; Whisstock, J.C. Phylogeny of the Serpin Superfamily: Implications of Patterns of Amino Acid Conservation for Structure and Function. Genome Res. 2000, 10, 1845–1864. [Google Scholar] [CrossRef]
- Gettins, P.G.; Olson, S.T. Inhibitory serpins. New insights into their folding, polymerization, regulation and clearance. Biochem. J. 2016, 473, 2273–2293. [Google Scholar] [CrossRef][Green Version]
- Huntington, J.A.; Read, R.J.; Carrell, R.W. Structure of a serpin–protease complex shows inhibition by deformation. Nat. Cell Biol. 2000, 407, 923–926. [Google Scholar] [CrossRef]
- Perron, M.J.; Blouse, G.E.; Shore, J.D. Distortion of the Catalytic Domain of Tissue-type Plasminogen Activator by Plasminogen Activator Inhibitor-1 Coincides with the Formation of Stable Serpin-Proteinase Complexes. J. Biol. Chem. 2003, 278, 48197–48203. [Google Scholar] [CrossRef] [PubMed]
- Aertgeerts, K.; De Bondt, H.L.; De Ranter, C.J.; Declerck, P.J. Mechanisms contributing to the conformational and functional flexibility of plasminogen activator inhibitor-1. Nat. Genet. 1995, 2, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Dewilde, M.; Strelkov, S.; Rabijns, A.; Declerck, P. High quality structure of cleaved PAI-1-stab. J. Struct. Biol. 2009, 165, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Declerck, P.; De Mol, M.; Vaughan, D.; Collen, D. Identification of a conformationally distinct form of plasminogen activator inhibitor-1, acting as a noninhibitory substrate for tissue-type plasminogen activator. J. Biol. Chem. 1992, 267, 11693–11696. [Google Scholar] [CrossRef]
- Urano, T.; Strandberg, L.; Johansson, L.B.-A.; Ny, T. A substrate-like form of plasminogen-activator-inhibitor type 1. Conversions between different forms by sodium dodecyl sulphate. JBIC J. Biol. Inorg. Chem. 1992, 209, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Audenaert, A.; Knockaert, I.; Collen, D.; Declerck, P. Conversion of plasminogen activator inhibitor-1 from inhibitor to substrate by point mutations in the reactive-site loop. J. Biol. Chem. 1994, 269, 19559–19564. [Google Scholar] [CrossRef]
- Van Meijer, M.; Smilde, A.; Tans, G.; Nesheim, M.E.; Pannekoek, H.; Horrevoets, A.J. The Suicide Substrate Reaction between Plasminogen Activator Inhibitor 1 and Thrombin Is Regulated by the Cofactors Vitronectin and Heparin. Blood 1997, 90, 1874–1882. [Google Scholar] [CrossRef] [PubMed]
- Mottonen, J.; Strand, A.; Symerský, J.; Sweet, R.M.; Danley, D.E.; Geoghegan, K.F.; Gerard, R.D.; Goldsmith, E.J. Structural basis of latency in plasminogen activator inhibitor-1. Nat. Cell Biol. 1992, 355, 270–273. [Google Scholar] [CrossRef]
- Declerck, P.J.; De Mol, M.; Alessi, M.C.; Baudner, S.; Pâques, E.P.; Preissner, K.T.; Müller-Berghaus, G.; Collen, D. Purification and characterization of a plasminogen activator inhibitor 1 binding protein from human plasma. Identification as a multimeric form of S protein (vitronectin). J. Biol. Chem. 1988, 263, 15454–15461. [Google Scholar] [CrossRef]
- Lindahl, T.L.; Sigurdardottir, O.; Wiman, B. Stability of Plasminogen Activator Inhibitor 1 (PAI-1). Thromb. Haemost. 1989, 62, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.K.; Thompson, L.C.; Bucci, J.C.; Nissen, P.; Gettins, P.G.W.; Peterson, C.B.; Andreasen, P.A.; Morth, J.P. Crystal Structure of Plasminogen Activator Inhibitor-1 in an Active Conformation with Normal Thermodynamic Stability. J. Biol. Chem. 2011, 286, 29709–29717. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.C.; Goswami, S.; Ginsberg, D.S.; Day, D.E.; Verhamme, I.M.; Peterson, C.B. Metals affect the structure and activity of human plasminogen activator inhibitor-1. I. Modulation of stability and protease inhibition. Protein Sci. 2010, 20, 353–365. [Google Scholar] [CrossRef]
- Keijer, J.; Linders, M.; Ehrlich, H.; Gebbink, R.K.; Pannekoek, H. Stabilisation of plasminogen activator inhibitor type 1 (PAI-1) activity by arginine: Possible implications for the interaction of PAI-1 with vitronectin. Fibrinolysis 1990, 4, 153–159. [Google Scholar] [CrossRef]
- Mimuro, J.; Schleef, R.R.; Loskutoff, D.J. Extracellular matrix of cultured bovine aortic endothelial cells contains functionally active type 1 plasminogen activator inhibitor. Blood 1987, 70, 721–728. [Google Scholar] [CrossRef]
- Smolarczyk, K.; Gils, A.; Boncela, J.; Declerck, P.J.; Cierniewski, C.S. Function-Stabilizing Mechanism of Plasminogen Activator Inhibitor Type 1 Induced upon Binding to α1-Acid Glycoprotein. Biochemestry 2005, 44, 12384–12390. [Google Scholar] [CrossRef] [PubMed]
- Sillen, M.; Weeks, S.D.; Strelkov, S.V.; Declerck, P.J. Structural Insights into the Mechanism of a Nanobody That Stabilizes PAI-1 and Modulates Its Activity. Int. J. Mol. Sci. 2020, 21, 5859. [Google Scholar] [CrossRef] [PubMed]
- De Taeye, B.; Gils, A.; Declerck, P.J. The story of the serpin plasminogen activator inhibitor 1: Is there any need for another mutant? Thromb. Haemost. 2004, 92, 898–924. [Google Scholar] [CrossRef] [PubMed]
- Sillen, M.; Declerck, P.J. Targeting PAI-1 in Cardiovascular Disease: Structural Insights Into PAI-1 Functionality and Inhibition. Front. Cardiovasc. Med. 2020, 7, 622473. [Google Scholar] [CrossRef]
- Bae, H.-B.; Zmijewski, J.W.; Deshane, J.S.; Zhi, D.; Thompson, L.C.; Peterson, C.B.; Chaplin, D.D.; Abraham, E. Vitronectin Inhibits Neutrophil Apoptosis through Activation of Integrin-Associated Signaling Pathways. Am. J. Respir. Cell Mol. Biol. 2012, 46, 790–796. [Google Scholar] [CrossRef]
- Wheaton, A.K.; Velikoff, M.; Agarwal, M.; Loo, T.T.; Horowitz, J.C.; Sisson, T.H.; Kim, K.K. The vitronectin RGD motif regulates TGF-β-induced alveolar epithelial cell apoptosis. Am. J. Physiol. Cell. Mol. Physiol. 2016, 310, L1206–L1217. [Google Scholar] [CrossRef] [PubMed]
- Gettins, P.G.W.; Dolmer, K. The High Affinity Binding Site on Plasminogen Activator Inhibitor-1 (PAI-1) for the Low Density Lipoprotein Receptor-related Protein (LRP1) Is Composed of Four Basic Residues. J. Biol. Chem. 2016, 291, 800–812. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Erickson, L.A.; Fici, G.J.; Lund, J.E.; Boyle, T.P.; Polites, H.G.; Marotti, K.R. Development of venous occlusions in mice transgenic for the plasminogen activator inhibitor-1 gene. Nat. Cell Biol. 1990, 346, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Eren, M.; Painter, C.A.; Atkinson, J.B.; Declerck, P.J.; Vaughan, D.E. Age-Dependent Spontaneous Coronary Arterial Thrombosis in Transgenic Mice That Express a Stable Form of Human Plasminogen Activator Inhibitor-1. Circulation 2002, 106, 491–496. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Pathophysiology of Myocardial Infarction. Compr. Physiol. 2015, 5, 1841–1875. [Google Scholar] [CrossRef] [PubMed]
- Schneiderman, J.; Sawdey, M.S.; Keeton, M.R.; Bordin, G.M.; Bernstein, E.F.; Dilley, R.B.; Loskutoff, D.J. Increased type 1 plasminogen activator inhibitor gene expression in atherosclerotic human arteries. Proc. Natl. Acad. Sci. USA 1992, 89, 6998–7002. [Google Scholar] [CrossRef]
- Lupu, F.; Bergonzelli, G.E.; Heim, D.A.; Cousin, E.; Genton, C.Y.; Bachmann, F.; Kruithof, E.K. Localization and production of plasminogen activator inhibitor-1 in human healthy and atherosclerotic arteries. Arterioscler. Thromb. J. Vasc. Biol. 1993, 13, 1090–1100. [Google Scholar] [CrossRef]
- Padró, T.; Steins, M.; Li, C.-X.; Mesters, R.M.; Hammel, D.; Scheld, H.H.; Kienast, J. Comparative analysis of plasminogen activator inhibitor-1 expression in different types of atherosclerotic lesions in coronary arteries from human heart explants. Cardiovasc. Res. 1997, 36, 28–36. [Google Scholar] [CrossRef][Green Version]
- Rylander, A.-C.J.; Lindgren, A.; Deinum, J.; Bergström, G.M.L.; Böttcher, G.; Kalies, I.; Wåhlander, K. Fibrinolysis inhibitors in plaque stability: A morphological association of PAI-1 and TAFI in advanced carotid plaque. J. Thromb. Haemost. 2017, 15, 758–769. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Khoukaz, H.B.; Ji, Y.; Braet, D.J.; Vadali, M.; Abdelhamid, A.A.; Emal, C.D.; Lawrence, D.A.; Fay, W.P. Drug Targeting of Plasminogen Activator Inhibitor-1 Inhibits Metabolic Dysfunction and Atherosclerosis in a Murine Model of Metabolic Syndrome. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Hamsten, A.; Wiman, B.; De Faire, U.; Blombäck, M. Increased Plasma Levels of a Rapid Inhibitor of Tissue Plasminogen Activator in Young Survivors of Myocardial Infarction. N. Engl. J. Med. 1985, 313, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Tofler, G.; Massaro, J.; O’Donnell, C.; Wilson, P.; Vasan, R.; Sutherland, P.; Meigs, J.; Levy, D.; D’Agostino, R. Plasminogen activator inhibitor and the risk of cardiovascular disease: The Framingham Heart Study. Thromb. Res. 2016, 140, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.G.; Motazedian, P.; Ramirez, F.D.; Simard, T.; Di Santo, P.; Visintini, S.; Faraz, M.A.; Labinaz, A.; Jung, Y.; Hibbert, B. Association between plasminogen activator inhibitor-1 and cardiovascular events: A systematic review and meta-analysis. Thromb. J. 2018, 16, 1–12. [Google Scholar] [CrossRef]
- Song, C.; Burgess, S.; Eicher, J.D.; O’Donnell, C.J.; Johnson, A.D.; Huang, J.; Sabater-Lleal, M.; Asselbergs, F.W.; Tregouet, D.; Shin, S.; et al. Causal Effect of Plasminogen Activator Inhibitor Type 1 on Coronary Heart Disease. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, M.E.; Lisman, T.; De Groot, P.G.; Meijers, J.C.M.; Le Cessie, S.; Doggen, C.J.M.; Rosendaal, F.R. Venous thrombosis risk associated with plasma hypofibrinolysis is explained by elevated plasma levels of TAFI and PAI-1. Blood 2010, 116, 113–121. [Google Scholar] [CrossRef]
- Chomiki, N.; Henry, M.; Alessi, M.C.; Anfosso, F.; Juhan-Vague, I. Plasminogen Activator Inhibitor-1 Expression in Human Liver and Healthy or Atherosclerotic Vessel Walls. Thromb. Haemost. 1994, 72, 44–53. [Google Scholar] [CrossRef]
- Juhan-Vague, I.; Alessi, M.C.; Vague, P. Increased plasma plasminogen activator inhibitor 1 levels. A possible link between insulin resistance and atherothrombosis. Diabetology 1991, 34, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Carratala, A.; Martinez-Hervas, S.; Rodriguez-Borja, E.; Benito, E.; Real, J.T.; Saez, G.T.; Carmena, R.; Ascaso, J.F. PAI-1 levels are related to insulin resistance and carotid atherosclerosis in subjects with familial combined hyperlipidemia. J. Investig. Med. 2017, 66, 17–21. [Google Scholar] [CrossRef]
- Ploplis, V.A. Effects of altered plasminogen activator inhibitor-1 expression on cardiovascular disease. Curr. Drug Targets 2011, 12, 1782–1789. [Google Scholar] [CrossRef]
- Festa, A.; D’Agostino, R.; Tracy, R.P.; Haffner, S.M. Elevated Levels of Acute-Phase Proteins and Plasminogen Activator Inhibitor-1 Predict the Development of Type 2 Diabetes: The Insulin Resistance Atherosclerosis Study. Diabetes 2002, 51, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinsky, J.; Barbieri, N.B.; Weinmann, T.; Ziegelmann, P.K.; Duncan, B.B.; Schmidt, M.I. Plasminogen activator inhibitor-1 and type 2 diabetes: A systematic review and meta-analysis of observational studies. Sci. Rep. 2016, 6, 17714. [Google Scholar] [CrossRef] [PubMed]
- Alessi, M.-C.; Poggi, M.; Juhan-Vague, I. Plasminogen activator inhibitor-1, adipose tissue and insulin resistance. Curr. Opin. Lipidol. 2007, 18, 240–245. [Google Scholar] [CrossRef]
- Aguilar-Salinas, C.A.; Viveros-Ruiz, T. Recent advances in managing/understanding the metabolic syndrome. F1000Research 2019, 8, 370. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, T.; Kubo, M.; Doi, Y.; Yonemoto, K.; Tanizaki, Y.; Rahman, M.; Arima, H.; Tsuryuya, K.; Iida, M.; Kiyohara, Y. Impact of Metabolic Syndrome on the Development of Cardiovascular Disease in a General Japanese Population. Stroke 2007, 38, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H.; Okamoto, R.; Kato, S.; Konishi, K.; Mizutani, H.; Yamada, N.; Isaka, N.; Nakano, T.; Ito, M. High glucose induces plasminogen activator inhibitor-1 expression through Rho/Rho-kinase-mediated NF-κB activation in bovine aortic endothelial cells. Atherosclerosis 2008, 196, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Alessi, M.C.; Juhan-Vague, I.; Kooistra, T.; Declerck, P.J.; Collen, D. Insulin Stimulates the Synthesis of Plasminogen Activator Inhibitor 1 by the Human Hepatocellular Cell Line Hep G2. Thromb. Haemost. 1988, 60, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.J.; Sobel, B.E. Synergistic augmentation of expression of plasminogen activator inhibitor type-1 induced by insulin, very-low-density lipoproteins, and fatty acids. Coron. Artery Dis. 1996, 7, 813–818. [Google Scholar] [CrossRef]
- Nordt, T.K.; Schneider, D.J.; Sobel, B.E. Augmentation of the synthesis of plasminogen activator inhibitor type-1 by precursors of insulin. A potential risk factor for vascular disease. Circulation 1994, 89, 321–330. [Google Scholar] [CrossRef]
- Chen, Y.; Billadello, J.J.; Schneider, D.J. Identification and localization of a fatty acid response region in the human plasminogen activator inhibitor-1 gene. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2696–2701. [Google Scholar] [CrossRef] [PubMed]
- Fattal, P.; Schneider, D.; Sobel, B.; Billadello, J. Post-transcriptional regulation of expression of plasminogen activator inhibitor type 1 mRNA by insulin and insulin-like growth factor 1. J. Biol. Chem. 1992, 267, 12412–12415. [Google Scholar] [CrossRef]
- Sawdey, M.S.; Loskutoff, D.J. Regulation of murine type 1 plasminogen activator inhibitor gene expression in vivo. Tissue specificity and induction by lipopolysaccharide, tumor necrosis factor-alpha, and transforming growth factor-beta. J. Clin. Investig. 1991, 88, 1346–1353. [Google Scholar] [CrossRef]
- Alessi, M.C.; Peiretti, F.; Morange, P.; Henry, M.; Nalbone, G.; Juhan-Vague, I. Production of Plasminogen Activator Inhibitor 1 by Human Adipose Tissue: Possible Link Between Visceral Fat Accumulation and Vascular Disease. Diabetes 1997, 46, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’Ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 2017, 4, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Loskutoff, D.J.; Samad, F. Molecular mechanisms of tumor necrosis factor-α-mediated plasminogen activator inhibitor-1 expression in adipocytes. FASEB J. 2005, 19, 1317–1319. [Google Scholar] [CrossRef] [PubMed]
- Rega, G.; Kaun, C.; Weiss, T.; Demyanets, S.; Zorn, G.; Kastl, S.; Steiner, S.; Seidinger, D.; Kopp, C.; Frey, M.; et al. Inflammatory Cytokines Interleukin-6 and Oncostatin M Induce Plasminogen Activator Inhibitor-1 in Human Adipose Tissue. Circulation 2005, 111, 1938–1945. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, L.; Liu, Z.; Liu, Y.; Luo, M.; Chen, N.; Deng, X.; Luo, Y.; He, J.; Zhang, L.; et al. PAI-1 Exacerbates White Adipose Tissue Dysfunction and Metabolic Dysregulation in High Fat Diet-Induced Obesity. Front. Pharmacol. 2018, 9, 1087. [Google Scholar] [CrossRef] [PubMed]
- Somodi, S.; Seres, I.; Lőrincz, H.; Harangi, M.; Fülöp, P.; Paragh, G. Plasminogen Activator Inhibitor-1 Level Correlates with Lipoprotein Subfractions in Obese Nondiabetic Subjects. Int. J. Endocrinol. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Levine, J.A.; Oleaga, C.; Eren, M.; Amaral, A.P.; Shang, M.; Lux, E.; Khan, S.S.; Shah, S.J.; Omura, Y.; Pamir, N.; et al. Role of PAI-1 in hepatic steatosis and dyslipidemia. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Morange, P.E.; Lijnen, H.R.; Alessi, M.C.; Kopp, F.; Collen, D.; Juhan-Vague, I. Influence of PAI-1 on Adipose Tissue Growth and Metabolic Parameters in a Murine Model of Diet-Induced Obesity. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1150–1154. [Google Scholar] [CrossRef]
- Schäfer, K.; Fujisawa, K.; Konstantinides, S.; Loskutoff, D.J. Disruption of the plasminogen activator inhibitor-1 gene reduces the adiposity and improves the metabolic profile of genetically obese and diabetic ob/ob mice. FASEB J. 2001, 15, 1840–1842. [Google Scholar] [CrossRef]
- Ma, L.-J.; Mao, S.-L.; Taylor, K.L.; Kanjanabuch, T.; Guan, Y.; Zhang, Y.; Brown, N.J.; Swift, L.L.; McGuinness, O.P.; Wasserman, D.H.; et al. Prevention of Obesity and Insulin Resistance in Mice Lacking Plasminogen Activator Inhibitor 1. Diabetes 2004, 53, 336–346. [Google Scholar] [CrossRef]
- Henkel, A.S.; Khan, S.S.; Olivares, S.; Miyata, T.; Vaughan, D.E. Inhibition of Plasminogen Activator Inhibitor 1 Attenuates Hepatic Steatosis but Does Not Prevent Progressive Nonalcoholic Steatohepatitis in Mice. Hepatol. Commun. 2018, 2, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.; Sloth, B.; Krog-Mikkelsen, I.; Flint, A.; Raben, A.; Tholstrup, T.; Brünner, N.; Astrup, A. A low-glycemic-index diet reduces plasma plasminogen activator inhibitor-1 activity, but not tissue inhibitor of proteinases-1 or plasminogen activator inhibitor-1 protein, in overweight women. Am. J. Clin. Nutr. 2008, 87, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Woo, C.-H.; Li, J.-D. Critical role of type 1 plasminogen activator inhibitor (PAI-1) in early host defense against nontypeable Haemophilus influenzae (NTHi) infection. Biochem. Biophys. Res. Commun. 2011, 414, 67–72. [Google Scholar] [CrossRef][Green Version]
- Goolaerts, A.; LaFargue, M.; Song, Y.; Miyazawa, B.; Arjomandi, M.; Carlès, M.; Roux, J.; Howard, M.; Parks, D.A.; Iles, K.E.; et al. PAI-1 is an essential component of the pulmonary host response during Pseudomonas aeruginosa pneumonia in mice. Thorax 2011, 66, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Kager, L.M.; Van Der Windt, G.J.; Wieland, C.W.; Florquin, S.; van’t Veer, C.; Van Der Poll, T. Plasminogen activator inhibitor type I may contribute to transient, non-specific changes in immunity in the subacute phase of murine tuberculosis. Microbes Infect. 2012, 14, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012, 122, 2731–2740. [Google Scholar] [CrossRef]
- Ozolina, A.; Sarkele, M.; Sabelnikovs, O.; Skesters, A.; Jaunalksne, I.; Serova, J.; Ievins, T.; Bjertnaes, L.J.; Vanags, I. Activation of Coagulation and Fibrinolysis in Acute Respiratory Distress Syndrome: A Prospective Pilot Study. Front. Med. 2016, 3, 64. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Inoue, H.; Ono, C.; Hashimoto, S.; Kioi, Y.; Matsumoto, H.; Matsuura, H.; Matsubara, T.; Shimizu, K.; et al. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc. Natl. Acad. Sci. USA 2020, 117, 22351–22356. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Tsantes, A.E.; Frantzeskaki, F.; Tsantes, A.G.; Rapti, E.; Rizos, M.; Kokoris, S.I.; Paramythiotou, E.; Katsadiotis, G.; Karali, V.; Flevari, A.; et al. The haemostatic profile in critically ill COVID-19 patients receiving therapeutic anticoagulant therapy. Medicine 2020, 99, e23365. [Google Scholar] [CrossRef]
- Kwaan, H.; Lindholm, P. The Central Role of Fibrinolytic Response in COVID-19—A Hematologist’s Perspective. Int. J. Mol. Sci. 2021, 22, 1283. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Mihalcioiu, C.; Rabbani, S.A. Multifaceted Role of the Urokinase-Type Plasminogen Activator (uPA) and Its Receptor (uPAR): Diagnostic, Prognostic, and Therapeutic Applications. Front. Oncol. 2018, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Devy, L.; Blacher, S.; Grignet-Debrus, C.; Bajou, K.; Masson, V.; Gerard, R.D.; Gils, A.; Carmeliet, G.; Carmeliet, P.; Declerck, P.J.; et al. The pro- or antiangiogenic effect of plasminogen activator inhibitor 1 is dose dependent. FASEB J. 2001, 16, 147–154. [Google Scholar] [CrossRef]
- Fang, H.; Placencio, V.R.; Declerck, Y.A. Protumorigenic Activity of Plasminogen Activator Inhibitor-1 Through an Antiapoptotic Function. J. Natl. Cancer Inst. 2012, 104, 1470–1484. [Google Scholar] [CrossRef] [PubMed]
- Balsara, R.D.; Ploplis, V.A. Plasminogen activator inhibitor-1: The double-edged sword in apoptosis. Thromb. Haemost. 2008, 100, 1029–1036. [Google Scholar] [CrossRef]
- Bajou, K.; Noel, A.; Gerard, R.D.; Masson, V.; Brunner, N.; Holsthansen, C.; Skobe, M.; Fusenig, N.E.; Carmeliet, P.; Collen, D.; et al. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat. Med. 1998, 4, 923–928. [Google Scholar] [CrossRef]
- Kubala, M.H.; Declerck, Y.A. The plasminogen activator inhibitor-1 paradox in cancer: A mechanistic understanding. Cancer Metastasis Rev. 2019, 38, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Bajou, K.; Maillard, C.; Jost, M.; Lijnen, R.H.; Gils, A.; Declerck, P.; Carmeliet, P.; Foidart, J.-M.; Noël, A. Host-derived plasminogen activator inhibitor-1 (PAI-1) concentration is critical for in vivo tumoral angiogenesis and growth. Oncogene 2004, 23, 6986–6990. [Google Scholar] [CrossRef] [PubMed]
- Isogai, C.; Laug, W.E.; Shimada, H.; Declerck, P.J.; Stins, M.F.; Durden, D.L.; Erdreich-Epstein, A.; Declerck, Y.A. Plasminogen activator inhibitor-1 promotes angiogenesis by stimulating endothelial cell migration toward fibronectin. Cancer Res. 2001, 61, 5587–5594. [Google Scholar]
- Li, H.; Shinohara, E.; Cai, Q.; Chen, H.; Courtney, R.; Cao, C.; Wang, Z.; Teng, M.; Zheng, W.; Lu, B. Plasminogen Activator Inhibitor-1 Promoter Polymorphism is Not Associated with the Aggressiveness of Disease in Prostate Cancer. Clin. Oncol. 2006, 18, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Błasiak, J.; Smolarz, B. Plasminogen activator inhibitor-1 (PAI-1) gene 4G/5G promoter polymorphism is not associated with breast cancer. Acta Biochim. Pol. 2000, 47, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Dunning, A.M.; Moore, D.H.; Pharoah, P.D.; Ginzinger, D.G.; Chin, K.; Gray, J.W.; Waldman, F.M.; Ponder, B.A.; Werb, Z. Prognostic Value of PAI1 in Invasive Breast Cancer: Evidence That Tumor-Specific Factors Are More Important Than Genetic Variation in Regulating PAI1 Expression. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Jevrić, M.; Matić, I.Z.; Krivokuća, A.; Đorđić Crnogorac, M.; Besu, I.; Damjanović, A.; Branković-Magić, M.; Milovanović, Z.; Gavrilović, D.; Susnjar, S.; et al. Association of uPA and PAI-1 tumor levels and 4G/5G variants of PAI-1 gene with disease outcome in luminal HER2-negative node-negative breast cancer patients treated with adjuvant endocrine therapy. BMC Cancer 2019, 19, 71. [Google Scholar] [CrossRef]
- Duffy, M.J.; McGowan, P.M.; Harbeck, N.; Thomssen, C.; Schmitt, M. uPA and PAI-1 as biomarkers in breast cancer: Validated for clinical use in level-of-evidence-1 studies. Breast Cancer Res. 2014, 16, 1–10. [Google Scholar] [CrossRef]
- Duffy, M.J.; O’Donovan, N.; McDermott, E.; Crown, J. Validated biomarkers: The key to precision treatment in patients with breast cancer. Breast 2016, 29, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Kates, R.E.; Gauger, K.; Willems, A.; Kiechle, M.; Magdolen, V.; Schmitt, M.; Harbeck, N. Urokinase-type plasminogen activator (uPA) and its inhibitor PAI-1: Novel tumor-derived factors with a high prognostic and predictive impact in breast cancer. Thromb. Haemost. 2004, 91, 450–456. [Google Scholar] [CrossRef]
- Nakatsuka, E.; Sawada, K.; Nakamura, K.; Yoshimura, A.; Kinose, Y.; Kodama, M.; Hashimoto, K.; Mabuchi, S.; Makino, H.; Morii, E.; et al. Plasminogen activator inhibitor-1 is an independent prognostic factor of ovarian cancer and IMD-4482, a novel plasminogen activator inhibitor-1 inhibitor, inhibits ovarian cancer peritoneal dissemination. Oncotarget 2017, 8, 89887–89902. [Google Scholar] [CrossRef]
- Chan, O.T.; Furuya, H.; Pagano, I.; Shimizu, Y.; Hokutan, K.; Dyrskjøt, L.; Jensen, J.B.; Malmstrom, P.-U.; Segersten, U.; Janku, F.; et al. Association of MMP-2, RB and PAI-1 with decreased recurrence-free survival and overall survival in bladder cancer patients. Oncotarget 2017, 8, 99707–99721. [Google Scholar] [CrossRef]
- Becker, M.; Szarvas, T.; Wittschier, M.; Dorp, F.V.; Tötsch, M.; Schmid, K.W.; Rübben, H.; Ergün, S. Prognostic impact of plasminogen activator inhibitor type 1 expression in bladder cancer. Cancer 2010, 116, 4502–4512. [Google Scholar] [CrossRef]
- Sakakibara, T.; Hibi, K.; Koike, M.; Fujiwara, M.; Kodera, Y.; Ito, K.; Nakao, A. Plasminogen activator inhibitor-1 as a potential marker for the malignancy of colorectal cancer. Br. J. Cancer 2005, 93, 799–803. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zubac, D.P.; Wentzel-Larsen, T.; Seidal, T.; Bostad, L. Type 1 plasminogen activator inhibitor (PAI-1) in clear cell renal cell carcinoma (CCRCC) and its impact on angiogenesis, progression and patient survival after radical nephrectomy. BMC Urol. 2010, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulos, G.; Kotopouli, M.; Karampela, I.; Christodoulatos, G.S.; Antonakos, G.; Marinou, I.; Vogiatzakis, E.; Lekka, A.; Papavassiliou, A.; Dalamaga, M. Circulating plasminogen activator inhibitor-1 activity: A biomarker for resectable non-small cell lung cancer? J. BU ON Off. J. Balk. Union Oncol. 2019, 24, 943–954. [Google Scholar]
- Ghosh, A.K.; Vaughan, D.E. PAI-1 in tissue fibrosis. J. Cell. Physiol. 2011, 227, 493–507. [Google Scholar] [CrossRef]
- Lemaire, R.; Burwell, T.; Sun, H.; Delaney, T.; Bakken, J.; Cheng, L.; Rebelatto, M.C.; Czapiga, M.; De-Mendez, I.; Coyle, A.J.; et al. Resolution of Skin Fibrosis by Neutralization of the Antifibrinolytic Function of Plasminogen Activator Inhibitor 1. Arthritis Rheumatol. 2015, 68, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Y.; Long, J.; Wang, X. Association of the Plasminogen Activator Inhibitor-1 (PAI-1) Gene -675 4G/5G and -844 A/G Promoter Polymorphism with Risk of Keloid in a Chinese Han Population. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2014, 20, 2069–2073. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.-M. Oxidative Stress, Plasminogen Activator Inhibitor 1, and Lung Fibrosis. Antioxid. Redox Signal. 2008, 10, 303–320. [Google Scholar] [CrossRef]
- Courey, A.J.; Horowitz, J.C.; Kim, K.K.; Koh, T.J.; Novak, M.L.; Subbotina, N.; Warnock, M.; Xue, B.; Cunningham, A.K.; Lin, Y.; et al. The vitronectin-binding function of PAI-1 exacerbates lung fibrosis in mice. Blood 2011, 118, 2313–2321. [Google Scholar] [CrossRef]
- Małgorzewicz, S.; Skrzypczak-Jankun, E.; Jankun, J. Plasminogen activator inhibitor-1 in kidney pathology. Int. J. Mol. Med. 2013, 31, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Bergheim, I.; Guo, L.; Davis, M.A.; Duveau, I.; Arteel, G.E. Critical Role of Plasminogen Activator Inhibitor-1 in Cholestatic Liver Injury and Fibrosis. J. Pharmacol. Exp. Ther. 2005, 316, 592–600. [Google Scholar] [CrossRef]
- Chan, J.C.; Duszczyszyn, D.A.; Castellino, F.J.; Ploplis, V.A. Accelerated Skin Wound Healing in Plasminogen Activator Inhibitor-1-Deficient Mice. Am. J. Pathol. 2001, 159, 1681–1688. [Google Scholar] [CrossRef]
- Shioya, S.; Masuda, T.; Senoo, T.; Horimasu, Y.; Miyamoto, S.; Nakashima, T.; Iwamoto, H.; Fujitaka, K.; Hamada, H.; Hattori, N. Plasminogen activator inhibitor-1 serves an important role in radiation-induced pulmonary fibrosis. Exp. Ther. Med. 2018, 16, 3070–3076. [Google Scholar] [CrossRef]
- Huang, W.-T.; Vayalil, P.K.; Miyata, T.; Hagood, J.; Liu, R.-M. Therapeutic Value of Small Molecule Inhibitor to Plasminogen Activator Inhibitor–1 for Lung Fibrosis. Am. J. Respir. Cell Mol. Biol. 2012, 46, 87–95. [Google Scholar] [CrossRef]
- Senoo, T.; Hattori, N.; Tanimoto, T.; Furonaka, M.; Ishikawa, N.; Fujitaka, K.; Haruta, Y.; Murai, H.; Yokoyama, A.; Kohno, N. Suppression of plasminogen activator inhibitor-1 by RNA interference attenuates pulmonary fibrosis. Thorax 2010, 65, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Lassila, M.; Fukami, K.; Jandeleit-Dahm, K.; Semple, T.; Carmeliet, P.; Cooper, M.E.; Kitching, A.R. Plasminogen activator inhibitor-1 production is pathogenetic in experimental murine diabetic renal disease. Diabetologia 2007, 50, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, R.; Kaji, K.; Namisaki, T.; Moriya, K.; Kawaratani, H.; Kitade, M.; Takaya, H.; Aihara, Y.; Douhara, A.; Asada, K.; et al. Novel oral plasminogen activator inhibitor-1 inhibitor TM5275 attenuates hepatic fibrosis under metabolic syndrome via suppression of activated hepatic stellate cells in rats. Mol. Med. Rep. 2020, 22, 2948–2956. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Castellino, F.J.; Ploplis, V.A. Plasminogen activator inhibitor-1 (PAI-1) is cardioprotective in mice by maintaining microvascular integrity and cardiac architecture. Blood 2010, 115, 2038–2047. [Google Scholar] [CrossRef]
- Pedroja, B.S.; Kang, L.E.; Imas, A.O.; Carmeliet, P.; Bernstein, A.M. Plasminogen Activator Inhibitor-1 Regulates Integrin αvβ3 Expression and Autocrine Transforming Growth Factor β Signaling. J. Biol. Chem. 2009, 284, 20708–20717. [Google Scholar] [CrossRef]
- Soeda, S.; Koyanagi, S.; Kuramoto, Y.; Kimura, M.; Oda, M.; Kozako, T.; Hayashida, S.; Shimeno, H. Anti-apoptotic roles of plasminogen activator inhibitor-1 as a neurotrophic factor in the central nervous system. Thromb. Haemost. 2008, 100, 1014–1020. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, J.-H.; Kim, J.-H.; Lee, W.-H.; Lee, M.-S.; Suk, K. Plasminogen activator inhibitor type 1 regulates microglial motility and phagocytic activity. J. Neuroinflamm. 2012, 9, 149. [Google Scholar] [CrossRef]
- Rodríguez-Lorenzo, S.; Francisco, D.M.F.; Vos, R.; Hof, B.V.H.; Rijnsburger, M.; Schroten, H.; Ishikawa, H.; Beaino, W.; Bruggmann, R.; Kooij, G.; et al. Altered secretory and neuroprotective function of the choroid plexus in progressive multiple sclerosis. Acta Neuropathol. Commun. 2020, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gverić, D.; Herrera, B.; Petzold, A.; Lawrence, D.A.; Cuzner, M.L. Impaired fibrinolysis in multiple sclerosis: A role for tissue plasminogen activator inhibitors. Brain 2003, 126, 1590–1598. [Google Scholar] [CrossRef]
- Oh, J.; Lee, H.-J.; Song, J.-H.; Park, S.I.; Kim, H. Plasminogen activator inhibitor-1 as an early potential diagnostic marker for Alzheimer’s disease. Exp. Gerontol. 2014, 60, 87–91. [Google Scholar] [CrossRef]
- Jacobsen, J.S.; Comery, T.A.; Martone, R.L.; Elokdah, H.; Crandall, D.L.; Oganesian, A.; Aschmies, S.; Kirksey, Y.; Gonzales, C.; Xu, J.; et al. Enhanced clearance of A in brain by sustaining the plasmin proteolysis cascade. Proc. Natl. Acad. Sci. USA 2008, 105, 8754–8759. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhao, Y.; Zhai, Z.; Zheng, J.; Zhou, Y.; Zhai, Q.; Cao, X.; Tian, J.; Zhao, L. Role of plasminogen activator inhibitor-1 in the diagnosis and prognosis of patients with Parkinson’s disease. Exp. Ther. Med. 2018, 15, 5517–5522. [Google Scholar] [CrossRef]
- Reuland, C.J.; Church, F.C. Synergy between plasminogen activator inhibitor-1, α-synuclein, and neuroinflammation in Parkinson’s disease. Med. Hypotheses 2020, 138, 109602. [Google Scholar] [CrossRef]
- Yates, R.L.; Esiri, M.M.; Palace, J.; Jacobs, B.; Perera, R.; DeLuca, G.C. Fibrin(ogen) and neurodegeneration in the progressive multiple sclerosis cortex. Ann. Neurol. 2017, 82, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, F.; Čechová, K.; Průša, R.; Hort, J. Amyloid beta soluble forms and plasminogen activation system in Alzheimer’s disease: Consequences on extracellular maturation of brain-derived neurotrophic factor and therapeutic implications. CNS Neurosci. Ther. 2018, 25, 303–313. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Tchkonia, T.; Zhu, Y.; Van Deursen, J.; Campisi, J.; Kirkland, J.L. Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. J. Clin. Investig. 2013, 123, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Özcan, S.; Alessio, N.; Acar, M.B.; Mert, E.; Omerli, F.; Peluso, G.; Galderisi, U. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging 2016, 8, 1316–1329. [Google Scholar] [CrossRef]
- Khan, S.S.; Shah, S.J.; Klyachko, E.; Baldridge, A.S.; Eren, M.; Place, A.T.; Aviv, A.; Puterman, E.; Lloyd-Jones, D.M.; Heiman, M.; et al. A null mutation in SERPINE1 protects against biological aging in humans. Sci. Adv. 2017, 3, eaao1617. [Google Scholar] [CrossRef]
- Vaughan, D.E.; Rai, R.; Khan, S.S.; Eren, M.; Ghosh, A.K. Plasminogen Activator Inhibitor-1 Is a Marker and a Mediator of Senescence. Arteroscler. Thromb. Vasc. Biol. 2017, 37, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, C.; Kiyici, S.; Budak, F.; Oral, B.; Guclu, M.; Duran, C.; Selimoglu, H.; Erturk, E.; Tuncel, E.; Imamoglu, S. The effect of metformin treatment on VEGF and PAI-1 levels in obese type 2 diabetic patients. Diabetes Res. Clin. Pract. 2008, 81, 56–60. [Google Scholar] [CrossRef]
- Brown, N.J.; Kumar, S.; Painter, C.A.; Vaughan, D.E. ACE Inhibition Versus Angiotensin Type 1 Receptor Antagonism. Hypertension 2002, 40, 859–865. [Google Scholar] [CrossRef][Green Version]
- Baluta, M.M.; Vintila, M.M. PAI-1 Inhibition—Another Therapeutic Option for Cardiovascular Protection. Maedica 2015, 10, 147–152. [Google Scholar]
- Fortenberry, Y.M. Plasminogen activator inhibitor-1 inhibitors: A patent review (2006–present). Expert Opin. Ther. Pat. 2013, 23, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Rouch, A.; Vanucci-Bacqué, C.; Bedos-Belval, F.; Baltas, M. Small molecules inhibitors of plasminogen activator inhibitor-1—An overview. Eur. J. Med. Chem. 2015, 92, 619–636. [Google Scholar] [CrossRef]
- D’Amico, S.; Martial, J.A.; Struman, I. A peptide mimicking the C-terminal part of the reactive center loop induces the transition to the latent form of plasminogen activator inhibitor type-1. FEBS Lett. 2012, 586, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Mathiasen, L.; Dupont, D.M.; Christensen, A.; Blouse, G.E.; Jensen, J.K.; Gils, A.; Declerck, P.J.; Wind, T.; Andreasen, P.A. A Peptide Accelerating the Conversion of Plasminogen Activator Inhibitor-1 to an Inactive Latent State. Mol. Pharmacol. 2008, 74, 641–653. [Google Scholar] [CrossRef]
- Jensen, J.K.; Malmendal, A.; Schiøtt, B.; Skeldal, S.; Pedersen, K.E.; Celik, L.; Nielsen, N.C.; Andreasen, P.A.; Wind, T. Inhibition of plasminogen activator inhibitor-1 binding to endocytosis receptors of the low-density-lipoprotein receptor family by a peptide isolated from a phage display library. Biochem. J. 2006, 399, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Fjellström, O.; Deinum, J.; Sjögren, T.; Johansson, C.; Geschwindner, S.; Nerme, V.; Legnehed, A.; McPheat, J.; Olsson, K.; Bodin, C.; et al. Characterization of a Small Molecule Inhibitor of Plasminogen Activator Inhibitor Type 1 That Accelerates the Transition into the Latent Conformation. J. Biol. Chem. 2013, 288, 873–885. [Google Scholar] [CrossRef]
- Lin, Z.; Jensen, J.K.; Hong, Z.; Shi, X.; Hu, L.; Andreasen, P.A.; Huang, M. Structural Insight into Inactivation of Plasminogen Activator Inhibitor-1 by a Small-Molecule Antagonist. Chem. Biol. 2013, 20, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Egelund, R.; Einholm, A.P.; Pedersen, K.E.; Nielsen, R.W.; Christensen, A.; Deinum, J.; Andreasen, P.A. A Regulatory Hydrophobic Area in the Flexible Joint Region of Plasminogen Activator Inhibitor-1, Defined with Fluorescent Activity-neutralizing Ligands. J. Biol. Chem. 2001, 276, 13077–13086. [Google Scholar] [CrossRef] [PubMed]
- Sillen, M.; Miyata, T.; Vaughan, D.; Strelkov, S.; Declerck, P. Structural Insight into the Two-Step Mechanism of PAI-1 Inhibition by Small Molecule TM5484. Int. J. Mol. Sci. 2021, 22, 1482. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-H.; Reinke, A.A.; Sanders, K.L.; Emal, C.D.; Whisstock, J.C.; Stuckey, J.A.; Lawrence, D.A. Mechanistic characterization and crystal structure of a small molecule inactivator bound to plasminogen activator inhibitor-1. Proc. Natl. Acad. Sci. USA 2013, 110, E4941–E4949. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sillen, M.; Declerck, P.J. A Narrative Review on Plasminogen Activator Inhibitor-1 and Its (Patho)Physiological Role: To Target or Not to Target? Int. J. Mol. Sci. 2021, 22, 2721. https://doi.org/10.3390/ijms22052721
Sillen M, Declerck PJ. A Narrative Review on Plasminogen Activator Inhibitor-1 and Its (Patho)Physiological Role: To Target or Not to Target? International Journal of Molecular Sciences. 2021; 22(5):2721. https://doi.org/10.3390/ijms22052721
Chicago/Turabian StyleSillen, Machteld, and Paul J. Declerck. 2021. "A Narrative Review on Plasminogen Activator Inhibitor-1 and Its (Patho)Physiological Role: To Target or Not to Target?" International Journal of Molecular Sciences 22, no. 5: 2721. https://doi.org/10.3390/ijms22052721
APA StyleSillen, M., & Declerck, P. J. (2021). A Narrative Review on Plasminogen Activator Inhibitor-1 and Its (Patho)Physiological Role: To Target or Not to Target? International Journal of Molecular Sciences, 22(5), 2721. https://doi.org/10.3390/ijms22052721





