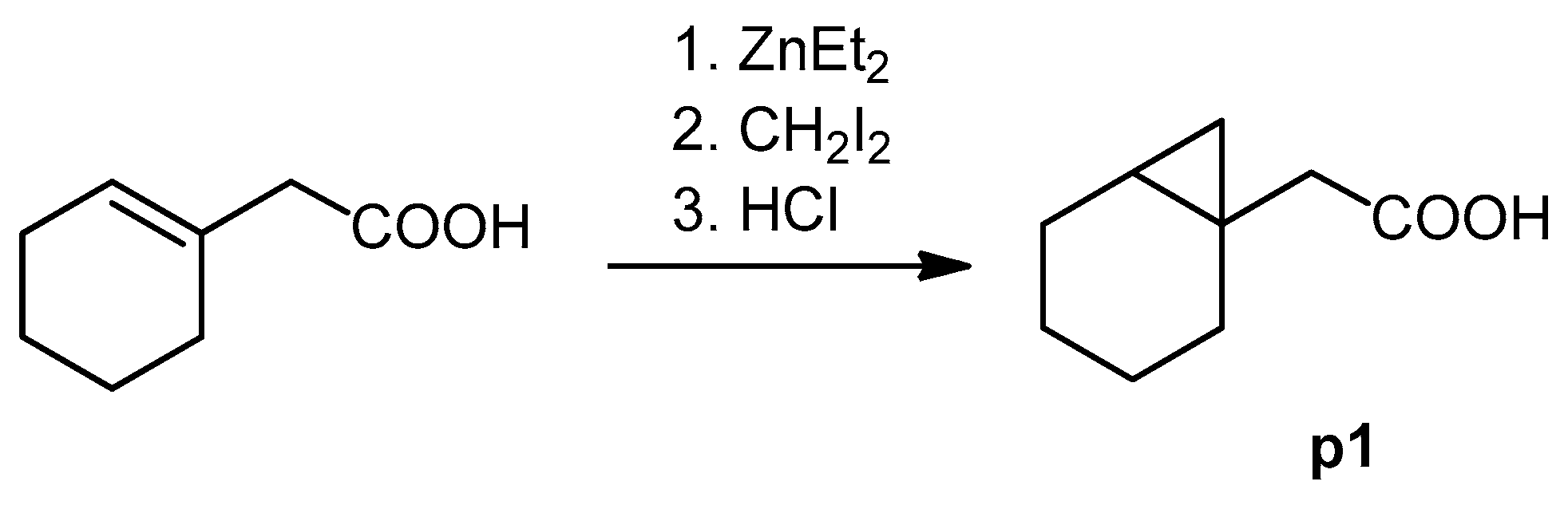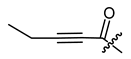Abstract
Melanoma is the deadliest form of skin cancer and accounts for about three quarters of all skin cancer deaths. Especially at an advanced stage, its treatment is challenging, and survival rates are very low. In previous studies, we showed that the constituents of the roots of Onosma paniculata as well as a synthetic derivative of the most active constituent showed promising results in metastatic melanoma cell lines. In the current study, we address the question whether we can generate further derivatives with optimized activity by synthesis. Therefore, we prepared 31, mainly novel shikonin derivatives and screened them in different melanoma cell lines (WM9, WM164, and MUG-Mel2 cells) using the XTT viability assay. We identified (R)-1-(1,4-dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 2-cyclopropyl-2-oxoacetate as a novel derivative with even higher activity. Furthermore, pharmacological investigations including the ApoToxGloTM Triplex assay, LDH assay, and cell cycle measurements revealed that this compound induced apoptosis and reduced cells in the G1 phase accompanied by an increase of cells in the G2/M phase. Moreover, it showed hardly any effects on the cell membrane integrity. However, it also exhibited cytotoxicity against non-tumorigenic cells. Nevertheless, in summary, we could show that shikonin derivatives might be promising drug leads in the treatment of melanoma.
1. Introduction
Malignant melanoma belongs to the most dangerous type of skin cancer and arises from melanocytes [1]. Only 2–3% of all diagnosed skin cancers are melanomas, however, they account for approx. 75% of all skin cancer deaths [2]. Melanomas are divided into four different stages: stages I and II display local primary tumors, stage III represents tumors with locoregional metastases, and stage IV tumors with distant metastases. In addition, tumors are typically categorized by thickness and ulceration [1,3]. When melanomas are detected at an early clinical stage and treated appropriately, the 10-year survival rate is around 98% [4]. However, when the tumor metastasizes, the survival rate drops dramatically and therapeutic success is limited due to tumor recurrence, severe side effects, and/or the development of resistances [2]. According to the Melanoma Research Alliance, the five-year survival rate for metastatic melanoma is currently only 22.5%. Therefore, the search for novel drug leads remains an important task.
In the discovery of novel therapeutics, natural products have always played a central role. In the case of anticancer drugs, the FDA approved in total 247 new chemical entities between 1981 and 2019, including 185 (75%) small molecules. Only 15.7% of the small molecules were totally synthetic compounds. All others were natural products or inspired by them [5]. Some of them, such as vinblastine, campthothecin, or taxol are most important in cancer chemotherapy today [6]. Shikonin (1) and its derivatives are naturally occurring, biologically active naphthoquinones and can be found in several members of the Boraginaceae family such as Arnebia euchroma (Royle ex Benth.) I.M. Johnst., Lithospermum erythrorhizon Siebold et Zucc., and Onosma paniculata Bureau & Franchet Their pharmacological spectrum comprises anti-oxidative, anti-inflammatory, anti-virus, and anti-cancer activities [7,8,9,10]. Plants containing these constituents are traditionally used for the treatment of eruptive exanthema, eczema, skin infections, burns, constipation, scalds, and cancer [11]. Concerning the anti-cancer activity, it has been reported that shikonin and derivatives led to induction of apoptosis, cell cycle arrest, and autophagy and inhibited cell growth and metastasis [7,8,9,10]. Shikonin was also shown to kill cancer cells synergistically in combination with established cancer therapeutics such as erlotinib [12].
In previous studies, we could show that β,β-dimethylacrylshikonin (2) was the most cytotoxic compound isolated from the roots of O. paniculata and exhibited the strongest cytotoxicity toward several melanoma cell lines [13,14]. Ongoing studies revealed that 2 favored catabolic processes and caused generation of reactive oxygen species, loss of mitochondrial membrane potential, and the upregulation of NOXA expression [15,16]. Finally, this led to apoptosis, autophagy, and cell cycle arrest. Using a mouse xenograft model, we could show that 2 exhibited also promising in vivo effects [16]. However, we were wondering whether the structure of 2 can be modified to further improve and optimize the pharmacological effects. In a first attempt, we ended up with cyclopropylacetylshikonin (3), which exhibited lower IC50 values than 2 in metastatic melanoma cell lines and induced apoptosis as well [17]. In the current study, we followed a two-fold strategy: (a) optimizing the cyclopropylacetyl group of our last hit 3 and (b) mounting a broad spectrum of structural features within the acyl residue of shikonin. To cover a broader spectrum of compounds and for comparison reasons, we also included some already known synthetic shikonin derivatives. However, most of the compounds are novel or not yet investigated in this context.
2. Results and Discussion
2.1. Syntheses of Shikonin Derivatives
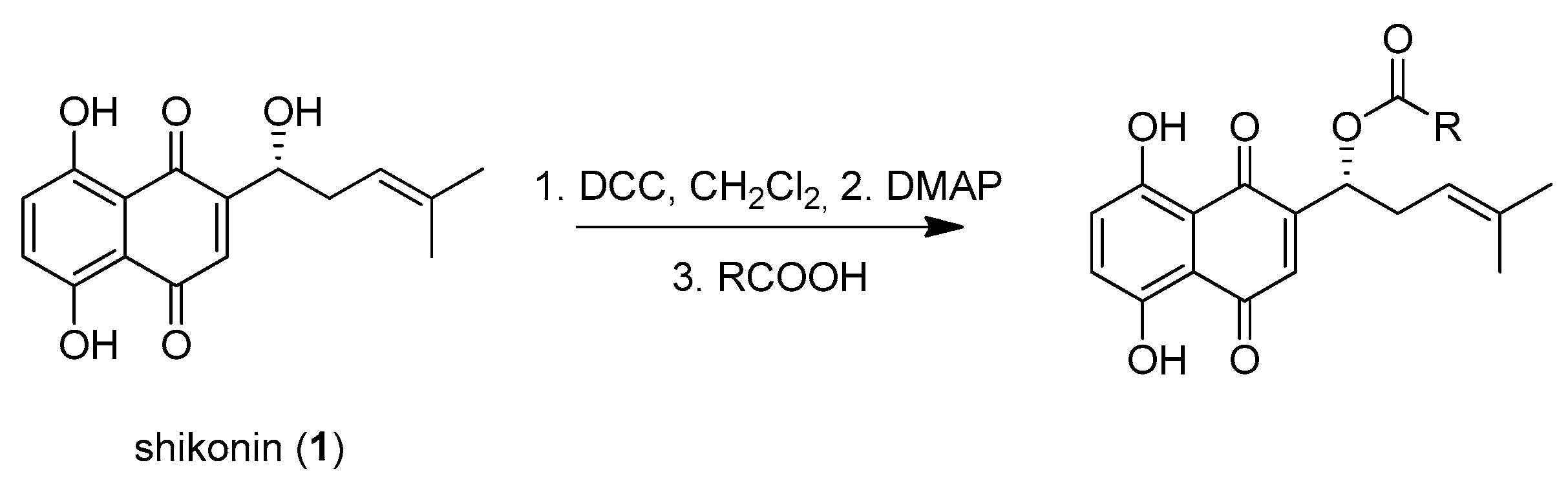
Shikonin derivatives have been shown to exhibit potent anti-cancer activities [7,8,9,10]. It was reported that the naphthoquinone scaffold with its hydroxyl groups is necessary for the pharmacological activity [18,19,20,21] and that the side chain modifies the activity. Similar features in the well-known anthracycline antitumor antibiotics and mitoxantrone seem to be important for DNA binding and bioavailability [22]. Therefore, we decided to focus on modifying the side chain. Shikonin (1, 100% R-isomer [17]) was chosen as starting material. Acylation of 1 was accomplished via Steglich esterification in dichloromethane with the corresponding carboxylic acid, as well as dicyclohexylcarbodiimide (DCC) as a coupling reagent and 4-dimethylaminopyridine (DMAP) as a catalyst (Figure 1) [17,20,23,24,25,26,27]. NMR spectra of all synthesized derivatives can be found in supplementary material (chapter 2).
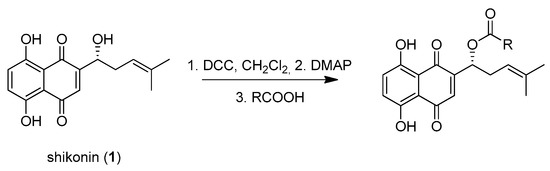
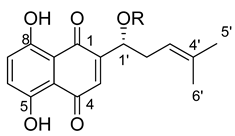
Figure 1.
Acylation of shikonin (1).
The first strategy was the optimization of the cyclopropylacetate in 3 [17]. In the bicyclus 4, 1′ and 2′ positions of cyclopropylacetate are connected as part of a cyclohexane. Replacement of the methylene spacer of 3 with a CO group, a C,C double bond, and an ethylene group resulted in 5, 6, and 7, respectively.
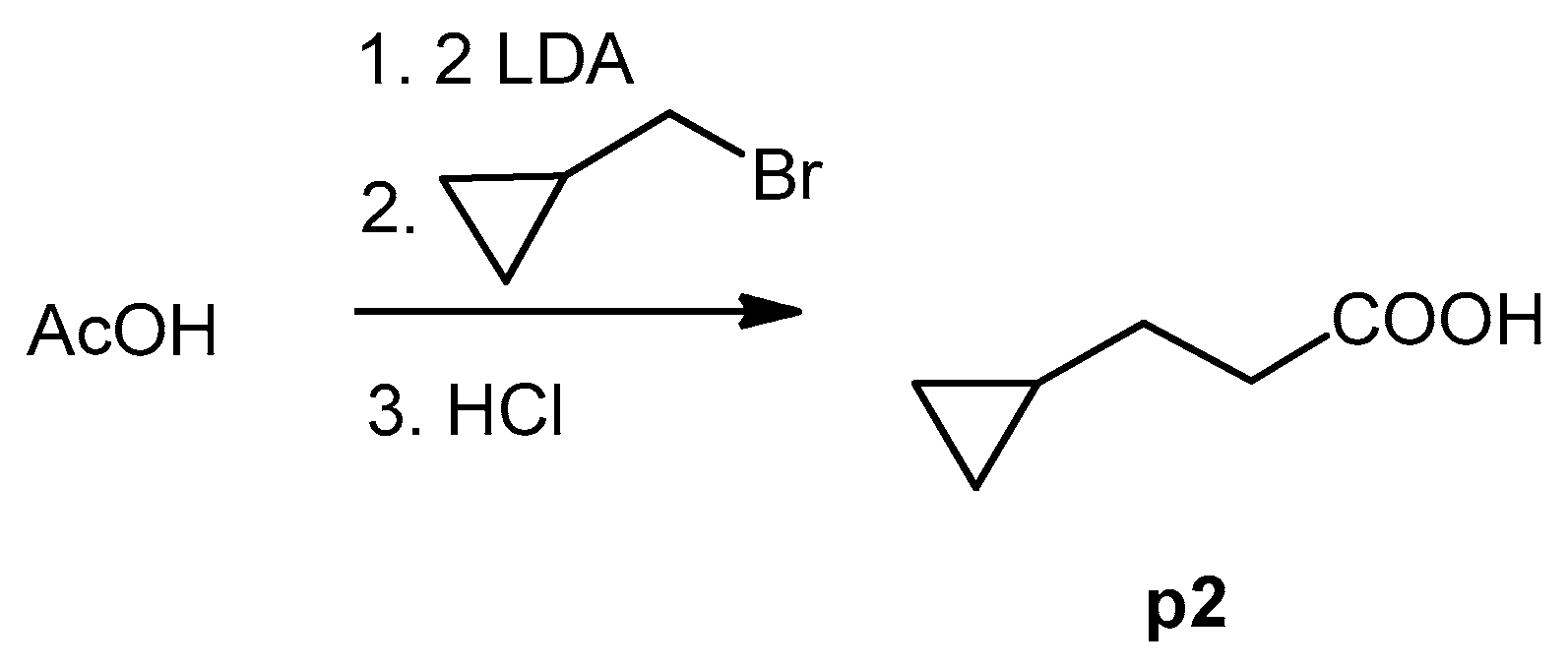
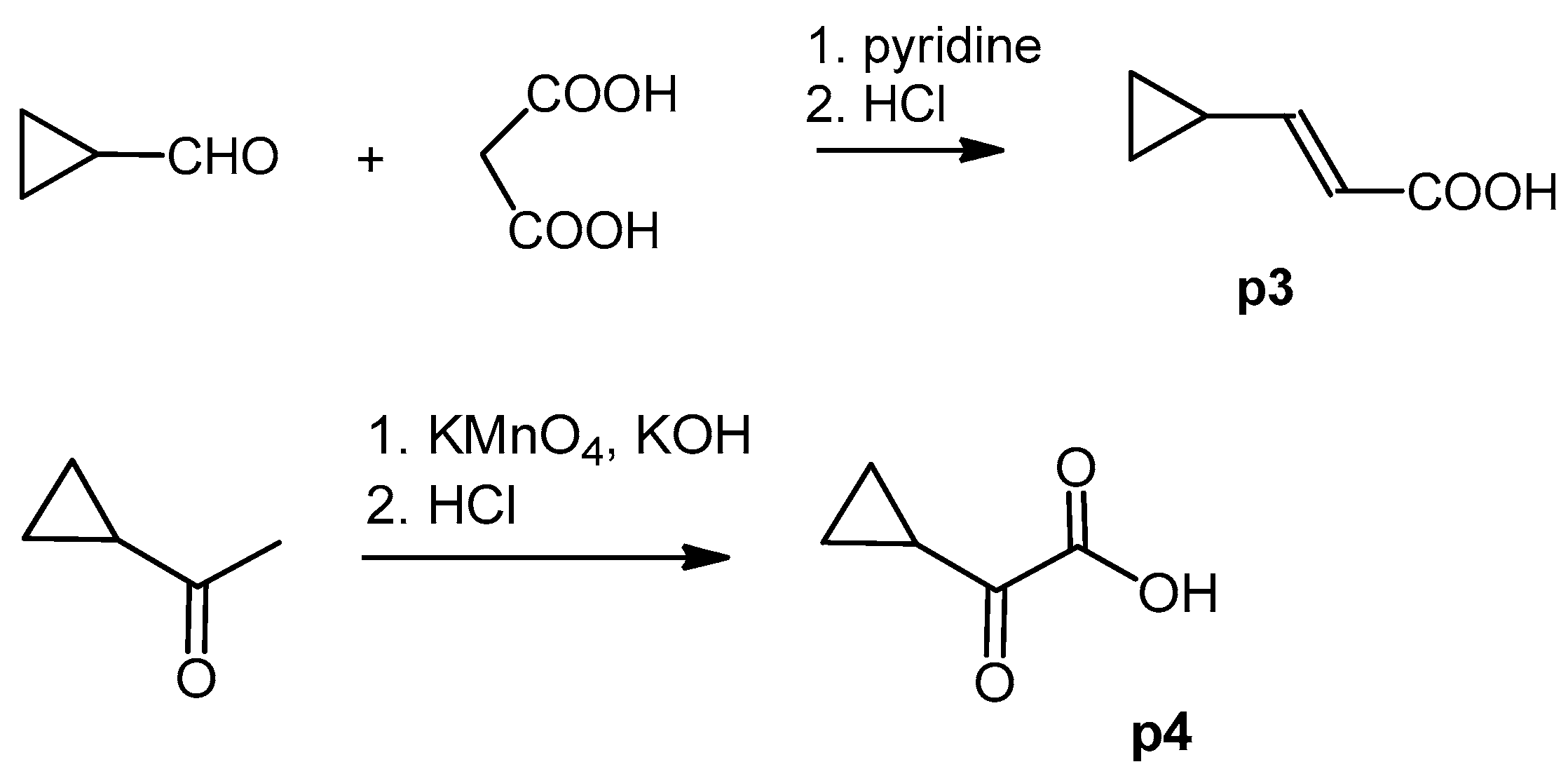
The cyclopropane precursor acids were synthesized in our laboratory. As outlined in Figure 2, the bicyclic acetic acid p1 was prepared from the corresponding (1-cyclohexenyl)-2-acetic via Furukawa modification of the Simmons–Smith reaction analogously to a procedure of Renaud and Fox [28]. 3-Cyclopropylpropanoic acid (p2) was prepared by α-alkylation of acetic acid in analogy to a procedure described by Barczak and Jarvo [29] (Figure 3). The Knoevenagel condensation of cyclopropanecarbaldehyde with malonic acid [30] resulted in β-cyclopropylacrylic acid p3. 2-Cyclopropyl-2-oxoacetic acid (p4) was obtained by oxidation of acetylcyclopropane with KMnO4 in a combination of the procedures given by Prokopenko et al. [31] and Xu et al. [32] (Figure 4). Details of the syntheses of the precursor acids are described in material and methods. Their NMR spectra can be found in the supplementary material (chapter 3). In all cases (precursor acids and shikonin derivatives), the structure and purity of each compound were analyzed using 1D and 2D NMR, LC-MS, and IR experiments.
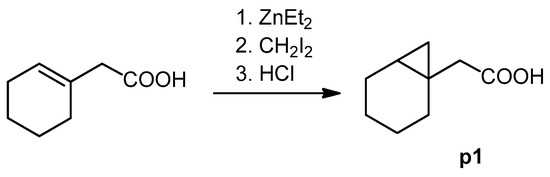
Figure 2.
Synthesis of the bicyclic acetic acid p1.
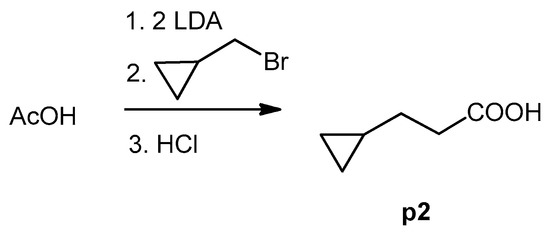
Figure 3.
Synthesis of 3-cyclopropylpropanoic acid (p2).
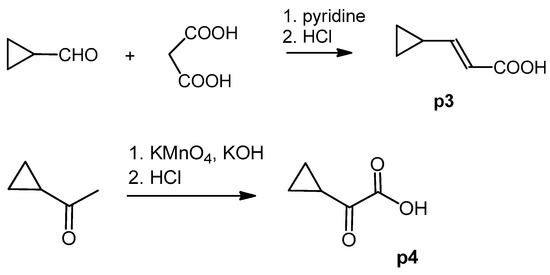
Figure 4.
Synthesis of precursor acids p3 and p4.
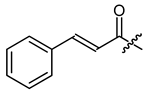
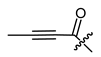
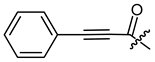
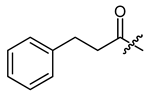
The second goal was to produce a broad spectrum of structural features within the acyl residue of shikonin to find potential novel drug leads. For better comparison and a more complete picture, we also included some already known derivatives. Phenylacetate 8 and cinnamate 9 showed, for example, cytotoxicity in previous studies and were synthesized for comparison reasons [26,33]. As there have been no reports about any shikonin alkynylacyl esters yet, we synthesized tetrolate 10, 2-butynoate 11, and 3-phenylpropiolate 12 to investigate the influence of a triple bond.
Except for three recently published studies about benzoylacrylates [34], succinamides, and maleinamides [35,36], all carbonyl groups in the acyl chain of shikonin or alkannin esters were part of acetoxy groups mainly derived from β-hydroxy acylates. However, the substances of Sun et al. [34] are of unknown chirality, among them the p-methylbenzoylacrylate 13. We also had a look on derivatives with the carbonyl carbon as a part of the carbon chain—either as a ketone or as part of a diester. Pyruvate 14 and 2-oxo-2-phenylacetate 15 represent α-ketoacylates. γ-Carbonyls are found in the keto esters 13 and 16 as well as in the diesters derived from monomethyl succinate 17 and monoethyl fumarate 18.
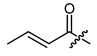
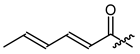
The known short chain alkyl and alkenyl esters 19 (isobutyrate) [37], 20 (isovalerate) [38], 21 (crotonate) [39], and 22 (sorbate) [38] were used to examine the influence of the saturation and branching on the activity. Compounds 19, 20, and 22 are already known to inhibit the growth of other types of cancer cells [23,37]. However, there have been no reports about their effects in melanoma cells. Derivative 21 was one of the very first non-natural 1′-O-acyl shikonines and shialkines [39], but no data about the biological activity have been reported so far.
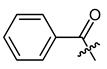
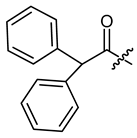
Other features, which we investigated, were carbocyclic and heterocyclic aryl groups. The most basic structures, i.e., phenylacetyl, benzoyl, and diphenylacetyl (8, 23, 24), were among the early synthetic 1′-O-acyl shikonines and shialkines. They were reported to bind to tubuline [38] and were cytotoxic against several cancer cell lines [23,28,40], however, no data were published about their effects in melanoma cells. Shikonin cinnamate 9 was intensively explored for its anticancer properties, too [26,41,42]. We used these derivatives to investigate the influence of the α,β double bond with the help of 3-phenylpropionate 25 [36].
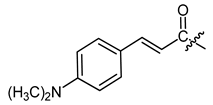
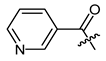
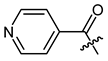
Additionally, as a p-dimethylamino group improved the activity of phenylacetate 8 [40], we prepared the p-dimethylaminocinnamate 26. Nicotinate 27 was reported as quite inactive [27,43], but more active than benzoate 23 [44]. Therefore, we tested nicotinate 27 and, additionally, isonicotinate 28. According to literature data, 3-(3-indolyl)propionate 29 showed up as the most active compound in another study [21]. As shikonin 2-furylcarboxylate was reported to be active [45] and we found that cinnamate was more active than benzoate, we prepared 3-(2-furyl)acrylate 30. The cytotoxicity of bromoacetate 31 was reported among other halogenated acetates [46]. Therefore, we prepared 31 together with novel ω-bromoalkylacylates 32, 33, and 34 to study the influence of chain length.
2.2. Results of the XTT Screening
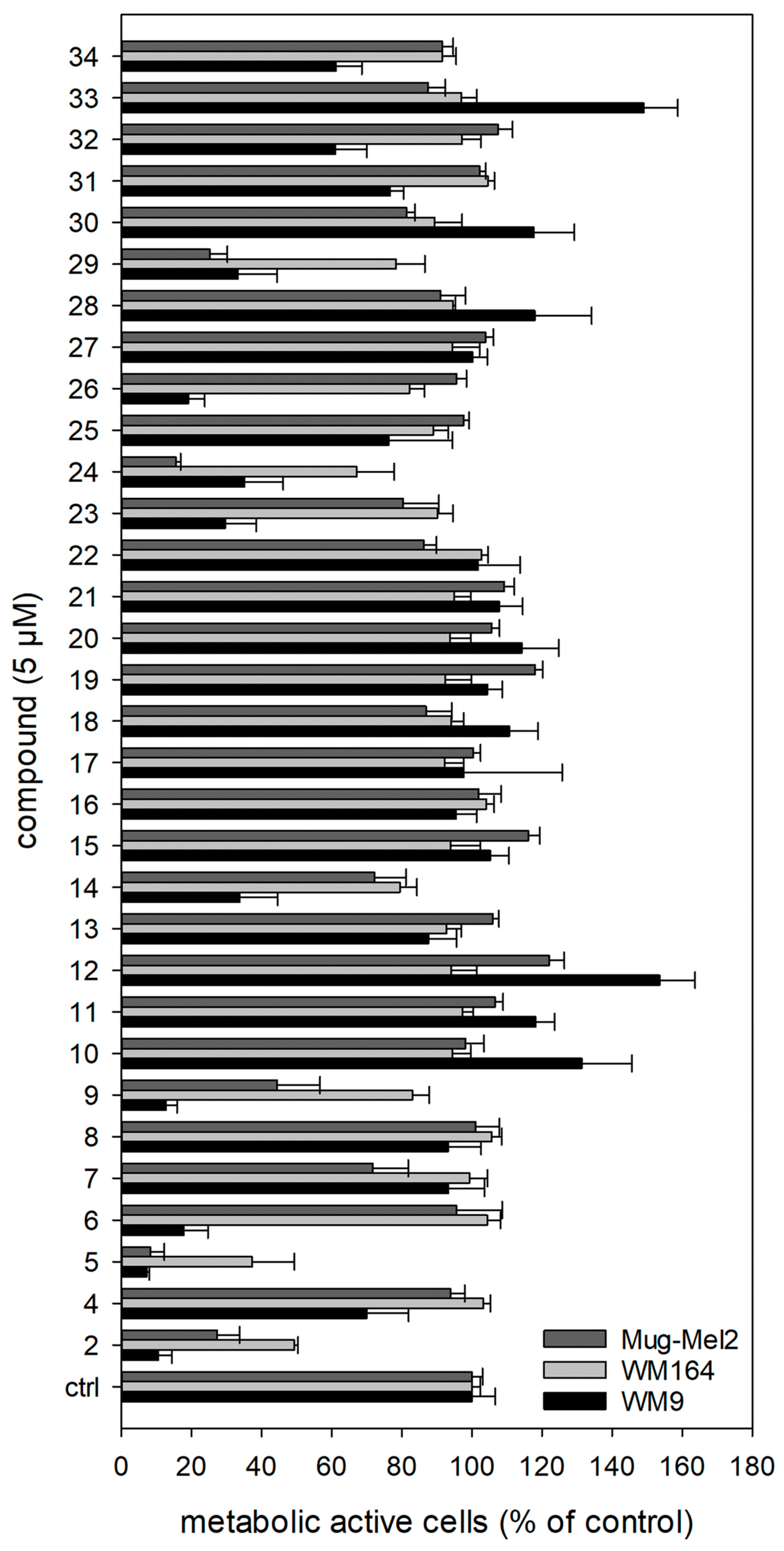
All prepared shikonin derivatives (see Table 1, compounds 4 to 34) were subjected to a cytotoxicity screening using the XTT viability assay. This assay is based on the activity of mitochondrial dehydrogenases. These enzymes cleave the yellow tetrazolium salt XTT leading to an orange formazan. This conversion only occurs in viable cells and can be directly quantified by measuring the absorbance [47]. Melanoma cells were treated with 1.0 µM, 5.0 µM, and 10.0 µM of each derivative for 72 h (Figure 5; Figures S1 and S2 in the Supplementary Material). In brief, the most active derivatives were 5, 6, 9, 14, 24, and 29, with 5 being the most cytotoxic. Its IC50 values are listed in Table 2. Moreover, the derivatives 11 and 28 showed no cytotoxicity at 10 µM, the derivatives 4, 16, 20, and 27 exhibited only a very weak cytotoxicity at 10 µM (Supplementary Figures S1 and S2).

Table 1.
Structures of shikonin (1) and derivatives thereof.
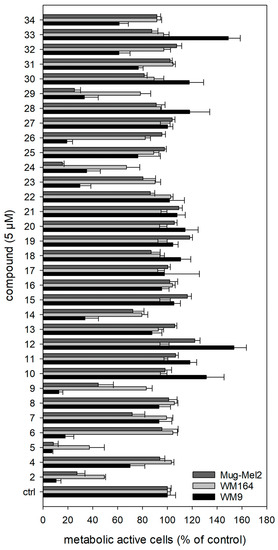
Figure 5.
Results of the XTT assay. For clarity reasons, only the results of the treatment with 5.0 µM for 72 h are shown. The complete results can be found in the Supplementary Material (Figures S1 and S2). 2 was tested as a reference at 5.0 µM. The strongest cytotoxicity was found for 5 (n = 6, mean ± sem). Vinblastine was used as positive control. At a concentration of 0.01 nM, it reduced the cell viability compared to control cells to: WM9 cells: 23.8 ± 1.5%, WM164 cells: 59.4 ± 4.4 %, and MUG-Mel2 cells: 65.0 ± 6.9 % (n = 6, mean ± sem, 72 h of treatment).

Table 2.
IC50 values (µM) of 2, 3, and 5 after 72 h of treatment and as determined using SigmaPlot 14.0 and the four-parameter logistic curve (n = 6, mean ± sem). n.d. = not determined.
Discussed in more detail, the results showed that the sensitivity of the three cell lines used was different. In general, WM164 cells reacted least to the treatment. Only a few compounds showed moderate activity, for example, the known derivatives 9, 24, and 29, as well as the novel derivative 14. WM9 cells were most affected by the derivatives. Next to 5 (novel), compounds 6 (novel), 9 (known), and 14 (novel) were the most cytotoxic derivatives in this cell line. MUG-Mel2 cells reacted in the case of some compounds (18, 19, 21, 22, 30, 33, and 34) more sensitive to the treatment than the WM9 and WM164 cells. This is of special interest, because MUG-Mel2 cells are NRAS mutated, while WM9 cells and WM164 cells are BRAF mutated. The mutational status is another category in melanoma diagnosis because some mutations lead to a poorer prognosis than others do. Around 50% of all melanomas exhibit a mutation in the BRAF gene and another 25% a mutation in the NRAS gene [48]. Tumors carrying a BRAF mutation are currently typically treated with a combination of MEK and BRAF inhibitors, however, tumor resistances often develop [49]. In the case of NRAS mutated melanoma, the therapeutic success is even lower because they are more difficult to treat [50]. This means that the most active derivatives of our study, can also display lead compounds for the development of further novel shikonin derivatives with a special focus on NRAS mutated melanoma cells.
Returning to the present results, we firstly modified the structure of our previous hit 3. Further substituents at the cyclopropane reduced the activity against all cell lines significantly. The modification of the spacer showed unequal effects: replacement of the methylene group with carbonyl (5) or ethylene (7) showed a similar activity on the cell lines with 5 being more cytotoxic towards WM9 cells. An α,β unsaturation resulted in less activity towards MUG-Mel2 cells but in an increased effect in WM9 cells.
When analyzing the other investigated structural features, it became obvious that hydrogenation of the acyl side chain of 2 reduced the cytotoxicity to a very low level in all cell lines. Shortening the side chain and removal of the branching β-methyl are among the few modifications, which had a bigger influence on the activity in WM9 cells than in MUG-Mel2 cells. The additional conjugated double bond in the sorbate 22 had no significant effect. However, the attachment of a phenyl group restored the properties to the activity level of 2. The p-dimethylamino group had no effect on the activity and, again, hydrogenation of the exocyclic double bond of the cinnamic residue lowered activity significantly. The removal or shortening of the spacer slightly improved the activity (compare 8 and 23). The replacement of the phenyl ring of 9 with a furan moiety (30) abolished the activity in WM9 cells but kept some effects in MUG-Mel2 cells. The diphenylacetate 24 and indolylpropionate 29 appeared as active as the cinnamate 9 and as 2. The replacement of the phenyl ring in benzoate 23 by a 3-pyridine or 4-pyridine abolished the cytotoxicity.
Moreover, the activity of the γ oxo esters 13, 16, 17, and 18 was generally low. While WM9 cells were less affected by saturated and unsaturated compounds, MUG-Mel2 cells showed a slight sensitivity towards unsaturated compounds. The introduction of an α carbonyl in 1′-O-acetylshikonin resulted in pyruvate 14 which showed an improved activity in WM9 cells, but a reduced one in MUG-Mel2 cells. Also, α-carbonylation of the benzoate 23, resulting in 2-oxo-2-phenylacetate 15, affected the cell lines differently. All the alkynylacyl esters (10, 11, and 12) showed no or only marginal effects on all cell lines. Even though ω-bromo compounds 31, 32, 33, and 34 appear more active, their potency is inferior to 2 and display no regularity concerning chain length and activity.
In summary, 5 appeared as the most cytotoxic compound in this series and, therefore, was investigated pharmacologically in more detail. First, 5 was also tested in non-tumorigenic HEK293 cells. As shown in Table 2, the IC50 value after 72 h was 3.4 µM, which is 2.3-fold higher than in WM9 cells, but also 1.3-fold lower than in WM164 cells. The cytotoxicity of chemotherapeutics against non-tumorigenic cells is a known problem in cancer therapy and one reason of undesirable side effects. For example, vinblastine and doxorubicin are two well-known and often clinically administered chemotherapeutics. In in vitro experiments, both also show cytotoxicity towards non-tumor cells to a similar or even greater extent [14,51,52]. However, it is difficult to extrapolate from in vitro to in vivo effects. Nevertheless, there is a certain risk that 5 will also cause side effects as they are already known for clinically used chemotherapeutics. However, it has already been shown for shikonin that there are ways to overcome this problem. Fayez et al. [53] reported recently that a combination of shikonin and silver nanoparticles synergistically inhibited the growth of lung cancer cells. Wang et al. [54] have shown that shikonin and JQ1, a bromodomain and extra-terminal motif (BET) inhibitor, encapsulated in lactoferrin nanoparticles changed the tumor immune microenvironment, activated immunogenic cell death, repolarized protumor phenotype, tumor-associated macrophages, and repressed glucose metabolism. They concluded that their system can be developed as a novel cancer immunotherapy due to several synergistic advantages. Another strategy could be a further development of our compounds to oxime derivatives. Huang et al. [55] demonstrated that such derivatives exhibited strong cytotoxicity towards cancer cells, but only a low cytotoxicity towards human skin fibroblasts.
2.3. Pharmacological Effects of Cyclopropyloxoacetate 5
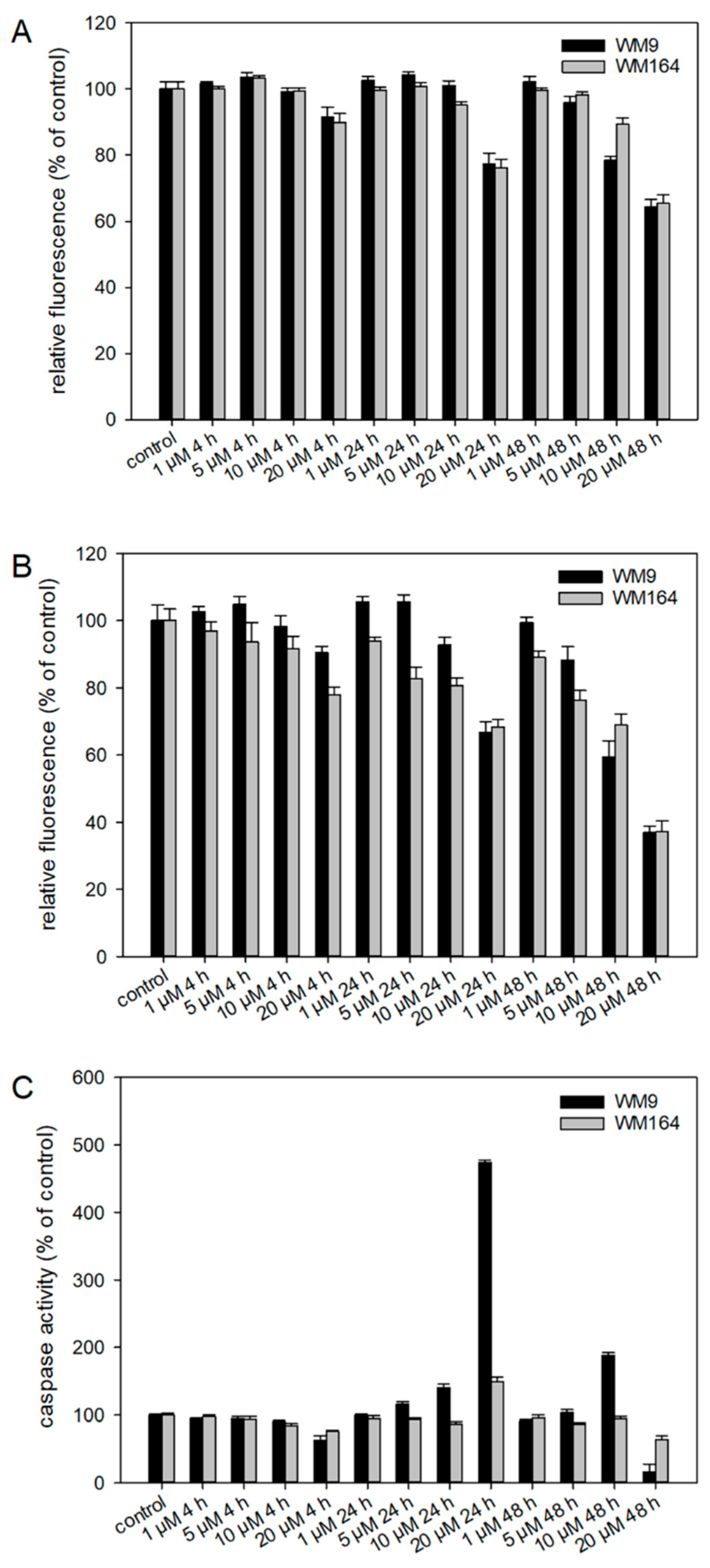
To investigate the effects of 5 in more detail, several pharmacological assays were performed. Since 5 was most active in the metastatic cell line WM9 (BRAF mutated), we decided to use this cell line and, in addition, another BRAF mutated cell line (WM164) for comparison. Using the ApoToxGlo™ Triplex Assay, we investigated the effects regarding cell viability, cytotoxicity, and apoptosis induction in more detail (Figure 6). The advantage of this assay is the combination of three assays in one single assay well. It has already been shown by our [14,16] and other groups [8,9] that several shikonin derivatives induced apoptosis in a variety of cancer types. We treated the cells with up to 20 µM of 5 for up to 48 h. Regarding cell viability, we found no statistically significant changes up 10 µM after 4 h and 24 h and up to 5 µM after 48 h. In the case of cytotoxicity, the fluorescence intensity decreased time- and dose-dependently, which can be an indicator for primary necrosis in combination with a reduced viability. Concerning apoptosis, we found a clear increase in caspase 3/7 activity after 24 h and 48 h in WM9 cells and a slight increase after 24 h in WM164. This agrees with the effects of our previous hit 3 [17] even if the activation of caspase 3/7 was weaker in case of 5. In summary, our results indicate that caspases are activated during the treatment with 5 further indicating that the cells undergo apoptotic cell death.
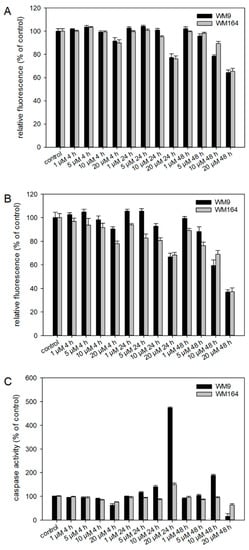
Figure 6.
Results of the ApoToxGlo™ Triplex Assay. WM9 and WM164 cells were treated with 1 µM, 5 µM, 10 µM, and 20 µM of 5 for 4 h, 24 h, or 48 h (n = 6, mean ± sem). (A) Viability of the cells measured as relative fluorescence of control cells. (B) Cytotoxicity of 5 towards the cells measured as relative fluorescence of control cells. (C) Activity of caspases 3 and 7 indicative for apoptosis induction. Staurosporine (25 µM) served as positive control (apoptosis increase in WM9 cells after 24 h: 1193.9 % and after 48 h: 297.4%; in WM164 cells after 24 h: 989.1% and after 48 h: 362.6%).
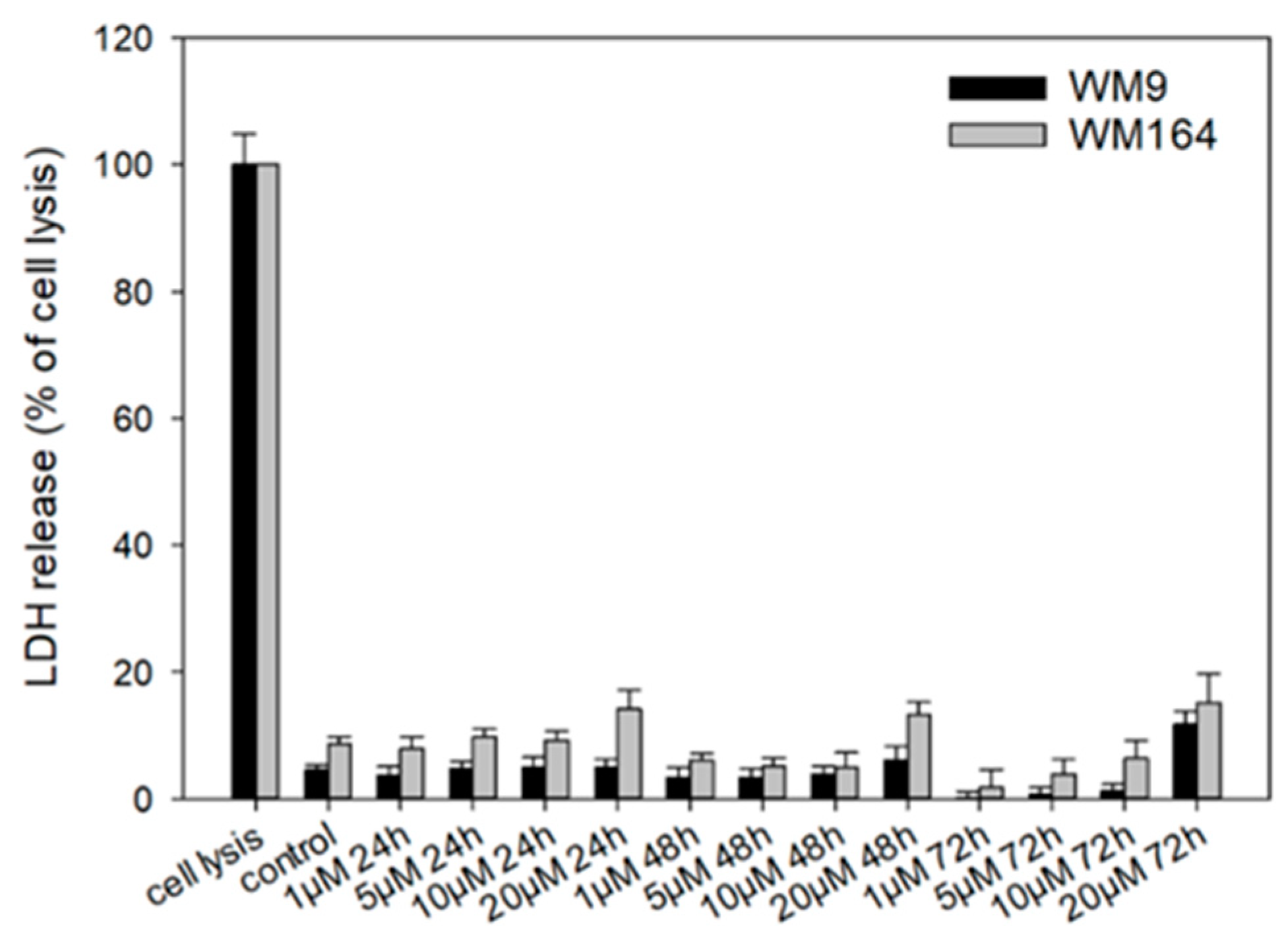
To investigate necrosis induction, we performed the CytoTox 96® Non-Radioactive Cytotoxicity Assay (LDH assay) (Figure 7). Lactate dehydrogenase (LDH) is released into the cell culture medium when the cell membrane is damaged. Therefore, this enzyme can be used as a marker for measuring necrotic cell death [56]. Cells were treated with 5 with up to 20 µM for up to 72 h. We found no LDH release up to 10 µM, which is 6.7-fold higher than the IC50 in WM9 cells and 2.2-fold higher than the IC50 in WM164 cells after 72 h. When the cells were treated with 20 µM 5, a slight increase of LDH release was found. However, compared to the maximal possible LDH release during complete cell lysis, the measured amount of LDH released by treated cells was generally quite low. In addition, these changes at 20 µM were not statistically significant in WM164 cells: 24 h: p = 0.182; 48 h: p = 0.115; 72 h: p = 0.269. In WM9 cells, the changes were statistically significant only at the highest tested concentration of 20 µM (p = 0.0197). In summary, necrosis seems to play only a minor role during the observed cells death at the concentrations tested. Nevertheless, it has been reported for shikonin that it induced necrosis in cancer cells such as lung and gastric cancer cells [57,58]. Cellular lysis appears also during the process of necroptosis [59] which has also been reported for shikonin [60]. Therefore, the increase of LDH release could also point to the induction of necroptosis by 5, which should be investigated in future studies.
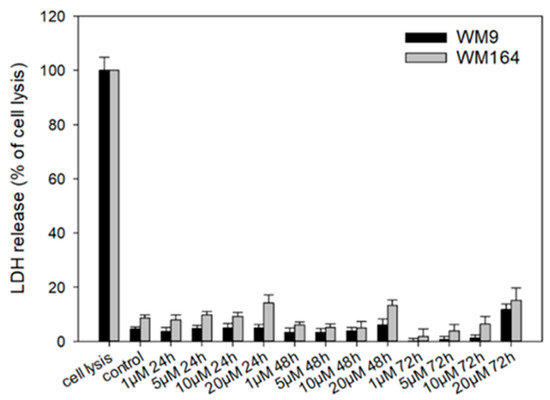
Figure 7.
Results of the LDH assay. WM9 and WM164 cells were treated with 1 µM, 5 µM, 10 µM, and 20 µM of 5 for 24 h, 48 h, or 72 h (n = 9, mean ± sem). Results are displayed as percentage of cell lysis. Control = vehicle treated cells (0.5% EtOH). Only at 20 µM, slight increases in LDH release were found.
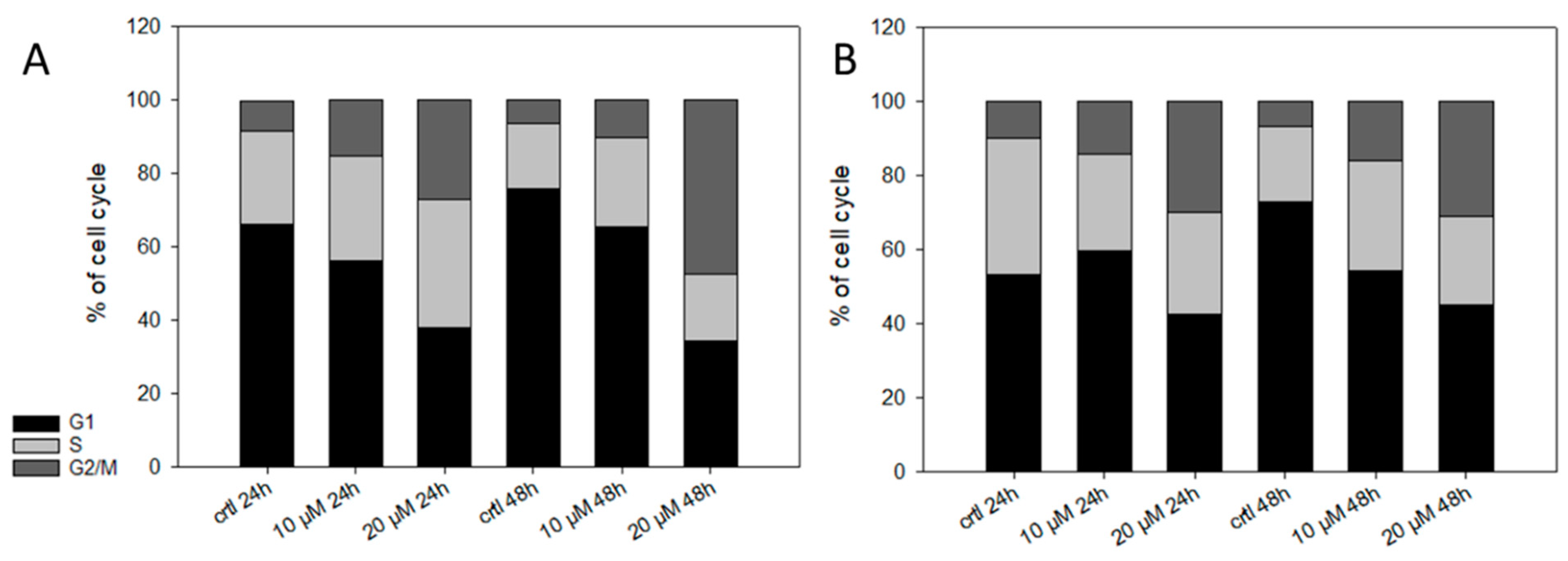
Finally, we investigated the effect of 5 on the cell cycle. It has been reported that shikonin derivatives are able to bind to tubulin and, therefore, lead to cell cycle arrest [43,61]. Also, in the case of 2, we found a cell cycle arrest in different types of melanoma cell lines [14]. Therefore, we treated the cells with up to 20 µM of 5 for up to 48 h (Figure 8). Only at higher concentrations, 5 changed the cell cycle distribution statistically significant (Table 3). We also tested the effect of 5 µM 5 on the cell cycle. However, 5 had no effect on the cell cycle at this concentration (data not shown). Our results agree with other studies since Baloch et al. [43] reported a quite high IC50 of 25.28 µM for shikonin concerning its inhibitory effect on tubulin polymerization.
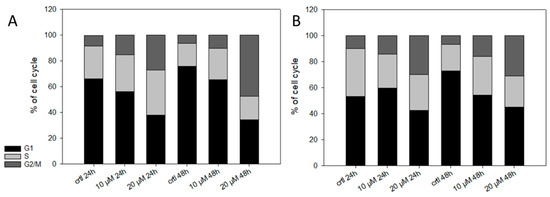
Figure 8.
Effects of 5 on the cell cycle. (A) WM9 and (B) WM164 cells were treated with 10 µM and 20 µM of 5 for 24 h or 48 h (n = 6, mean). Results are displayed as percentage of cell cycle distribution. Control = vehicle treated cells (0.5% EtOH). 5 influence the distribution of the cell cycle only at high concentrations.

Table 3.
Calculated p-values when comparing the amount of control and treated cells in the G2/M phase (student’s t-test, n = 6). The number of cells in the S-phase was not statistically significantly changed.
3. Materials and Methods
3.1. Chemicals
Shikonin was purchased from Chengdu Biopurify Phytochemicals Ltd. (Chengdu, China). β,β-Dimethylacrylshikonin (2) was isolated from dried roots of Onosma paniculata Bureau & Franchet (Boraginaceae) and identified as reported previously [14].
3.2. Synthesis of Shikonin Derivatives
The synthesis of shikonin derivatives is described below. Their NMR spectra can be found in the supplementary material, chapter 2. The purity of all compounds was analyzed using NMR experiments and always exceeded 95%. LC-ESI-MS measurements were performed on a Dionex Ultimate 3000 UHPLC (Thermo, San José, CA, USA). It was coupled with a Thermo LTQ XL linear ion trap mass spectrometer equipped with an H-ESI II probe (negative mode). The acquisition wavelength was 500 nm, source heater temperature: 250 °C, capillary temperature: 200 °C, source voltage: 3.5 kV, sheath gas flow: 50 arbitrary units, capillary voltage: −14 V, and auxiliary gas flow: 10 arbitrary units. A Kinetex C18 column (2.6 µm, 100 × 2.10 mm, Phenomenex, Torrance, CA, USA) was used as stationary phase. Water (A) and acetonitrile (B) were used as mobile phases (gradient program: 0–45 min: 55–100% B, flow rate: 0.2 mL/min, column temperature: 30 °C).
3.3. General Procedure for the Acylation of Shikonin
A solution of shikonin in abs. CH2Cl2 (0.1 mmol/5 mL) was cooled to 0 °C under argon atmosphere and DCC was added. After 15 min of stirring, DMAP was added. After an additional 15 min stirring, the corresponding acid was added and stirred for another 5.5 h to 5 days with slowly warming up to room temperature. Afterwards, 1 mL cyclohexane/0.1 mmol shikonin was added and the mixture was concentrated at room temperature and under reduced pressure to ca. 0.5 mL/0.1 mmol shikonin. The mixture was then filtered over 3 mm silica and 2 mm celite® (eluent: petroleum ether/CH2Cl2 = 1:0 to 1:2). The resulting fractions were evaporated and subjected to flash CC and/or repeated PTLC (cyclohexane/CH2Cl2 mixtures).
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 2-(bicyclo[4 .1.0]heptan-1-yl)acetate (4), 50 µmol Shikonin, 0.18 mmol DCC, 15 µmol DMAP and 57 µmol 2-(1-bicyclo[4.1.0]heptyl)acetic acid (p1); reaction time 15 h; CC on silica (4 g; cyclohexane to cyclohexane/CH2Cl2 = 2:1; PTLC on silica with cyclohexane/CH2Cl2 = 2:1 (three times developed); 4, yield: 25%. 4: Rf = 0.49 (silica, CH2Cl2); IR (ATR): 2990 (w), ≈2950 (br) (OH), 2927 (w), 2856 (w), 1736 (m) (C=O), 1607 (s), 1568 (m), 1451 (m), 1263(m), 1230 (m), 1202 (m) (COC), 1141 (s), 782 (m) cm−1; 1 H-NMR (CDCl3): 0.33, 0.35 (2t, 2H J ≈ 5 Hz H-7′’), 0.50–0.58 (m, 2H, H-7′’), 0.84–0.92 (m, 2H, H-2′’), 1.09–1.37 (m, 8H, H-4′’, H-5′’), 1.55–1.64 (m, 2H, H-3′’), 1.58 (s, 6H, H-6′), 1.69 (s, 6H, H-5′), 1.68–1.80 (m, 4H, H-6′’), 1.90–2.00 (m, 2H, H-3′’), 2.20–2.34 (m, 4H, H-α), 2.42–2.52 (m, 2H, H-2′), 2.58–2.67 (m, 2H, H-2′), 5.14 (tquint, J = 7.3, 0.9 Hz, 2H, H-3′), 6.04 (J = 7.3, 4.4, 0.8 Hz, 2H, H-1′), 7.00 (d, J = 0.9 Hz, H-3), 7.01 (d, J = 1.0 Hz, 1H, H-3), 7.19 (s, 4H, H-6, H-7), 12.43 (s, 2H, C5-OH), 12.59 (s, 2H, C8-OH); 13 C-NMR (CDCl3): δ 16.7 (C-1′’), 2x 17.0 (C-7′’), 17.7, 17.8 (C-2′’), 18.0 (C-6′), 2x 21.0 (C-4′’), 2 × 21.4 (C-5′’), 2 × 23.7 (C-3′’), 25.8 (C-5′), 28.6, 28.7 (C-6′’), 32.9, 33.0 (C-2′), 2x 46.3 (C-α), 2 × 69.2 (C-1′), 111.6 (C-4a), 111.8 (C-8a), 118.0 (C-3′), 2 × 131.5 (C-3), 132.8 (C-7), 2 × 132.8 (C-6), 135.9 (C-4′), 148.5 (C-2), 2 × 166.9 (C-5), 2 × 167.4 (C-8), 171.5 (COO), 2 × 176.8 (C-1), 2 × 177.3, (C-4); MS (ESI−) m/z (%): 423.24 (100) [M-H]−, [M-H]− calculated for C25H28O6: 423.1808.
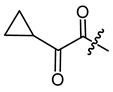
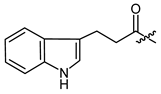
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 2-cyclopropyl-2-oxoacetate (5), 0.1 mmol Shikonin, 0.22 mmol DCC, 50 µmol DMAP and 0.15 mmol 2-cyclopropyl-2-oxoacetic acid (p4) reaction time 15 h; CC on silica (4 g; hexanes/CH2Cl2 = 1:0 to hexanes/CH2Cl2 = 0:1); PTLC on silica (developed four times with cyclohexane/CH2Cl2 = 2:1 and twice with cyclohexane/CH2Cl2 = 1:1); 5, yield: 45%. 5: Rf = 0.34 (silica, CH2Cl2); IR (ATR): 2971 (w), ≈2950 (br) (OH), 2915 (w), 2857 (w), 1735 (m) (C=O), 1715 (m) (C=O), 1608 (s), 1568 (m), 1451 (m), 1261 (s), 1230 (s), 1203 (s) (COC), 1057 (s), 778 (m) cm−1; 1H-NMR (CDCl3): 1.16–1.22 (m, 2H, H-2′’, H-3′’), 1.25–1.31 (m, 2H, H-2′’, H-3′’), 1.59 (s, 3H, H-6′), 1.68 (s, 3H, H-5′), 2.61 (dtm, J = 15.0, 7.5 Hz, 1H, H-2′), 2.67–2.76 (m, 2H, H-2′, H-1′’), 5.14 (tm, J = 7.3 Hz, 1H, H-3′), 6.17 (ddd, J = 7.1, 4.8, 0.5 Hz, 1H, H-1′), 7.11 (d, J = 0.7 Hz, 1H, H-3), 7.18 (s, 2H, H-6, H-7), 12.40 (s, 1H, C5-OH), 12.59 (s, 1H, C8-OH); 13C-NMR (CDCl3): δ 2 × 14.3 (C-2′’, C-3′’), 18.0 (C-6′), 18.2 (C-1′’), 25.8 (C-5′), 32.8 (C-2′), 71.4 (C-1′), 111.6 (C-4a), 111.8 (C-8a), 117.0 (C-3′), 131.4 (C-3), 133.3 (C-7), 133.6 (C-6), 136.9 (C-4′), 146.2 (C-2), 159.9 (COO), 168.8 (C-5), 169.3 (C-8), 174.6 (C-1), 176.2 (C-4), 193.3 (α-CO); MS (ESI−) m/z (%): 383.06 (100) [M-H]−, [M-H]− calculated for C21H20O7: 383.1131.
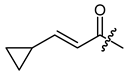
(R,E)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 3-cyclopropyl-2-propenoate (6), 0.1 mmol Shikonin, 0.20 mmol DCC, 30 µmol DMAP and 0.10 mmol β-cyclopropylacrylic acid (p3); reaction time 17 h; two consecutive PTLC on silica (developed twice with cyclohexane/CH2Cl2 = 2:1 each). 6, yield: 8 %. 6: Rf = 0.29 (silica, CH2Cl2). IR (ATR): 2917 (m), ≈2950 (br) (OH), 2851 (w), 1717 (s) (C=O), 1643 (m), 1609 (vs), 1570 (m), 1453 (m), 1263 (s), 1203 (s) (COC), 1140 (s), 779 (w) cm−1; 1H-NMR (CDCl3): 0.63–0.73 (m, 2H, H-2′’, H-3′’), 0.95–1.04 (m, 2H, H-2′’, H-3′’), 1.58 (s, 3H, H-6′), 1.58–1.68 (m, 1H, H-1′’), 1.69 (s, 3H, H-5′), 2.50 (dtm, J = 15.0, 7.3 Hz, 1H, H-2′), 2.64 (dtm, J = 14.9, 5.9 Hz, 1H, H-2′), 5.14 (tm, J = 7.3 Hz, 1H, H-3′), 5.97 (d, J = 15.4 Hz, 1H, H-α), 6.04 (ddd, J = 7.1, 4.4, 0.8 Hz, 1H, H-1′), 6.48 (dd, J = 15.4, 10.1 Hz, 1H, H-β), 6.98 (d, J = 0.9 Hz, 1H, H-3), 7.18 (s, 2H, H-6, H-7), 12.43 (s, 1H, C5-OH), 12.59 (s, 1H, C8-OH); 13C-NMR (CDCl3): δ 8.9 (C-2′’, C-3′’), 14.6 (C-1′’), 18.0 (C-6′), 25.8 (C-5′), 32.9 (C-2′), 69.2 (C-1′), 111.6 (C-4a), 111.7 (C-8a), 117.3 (C-α), 117.9 (C-3′), 131.6 (C-3), 132.5 (C-7), 132.6 (C-6), 135.9 (C-4′), 148.8 (C-2), 155.8 (C-β), 165.3 (COO), 166.3 (C-5), 166.8 (C-8), 177.4 (C-1), 178.8 (C-4); MS (ESI−) m/z (%): 785.31 (9) [2(M-H)+Na]−, 382.14 (54) [M]−, 381.25 (100) [M-H]−; [M]− calculated for C22H22O6: 382.1416.
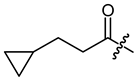
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 3-cyclopropylpropanoate (7), 0.1 mmol Shikonin, 0.26 mmol DCC, 50 µmol DMAP and 0.15 mmol 3-cyclopropylpropanoic acid (p2) reaction time 15 h; CC on silica (8 g; hexanes/CH2Cl2 = 1:0 to hexanes/CH2Cl2 = 1:2); 7, yield: 40%. 7: Rf = 0.49 (silica, CH2Cl2); IR (ATR): 3080 (w), 2972 (w), ≈2950 (br) (OH), 2916 (w), 2857 (w), 1742 (m) (C=O), 1610 (s), 1569 (m), 1454 (m), 1228 (s), 1204 (s) (COC), 784 (m) cm−1; 1H-NMR (CDCl3): 0.04–0.09 (m, 2H, H-2′’, H-3′’), 0.40–0.49 (m, 2H, H-2′’, H-3′’), 0.66–0.77 (m, 1H, H-1′’), 1.54 (q, J = 7.3 Hz, 2H, H-β), 1.57 (s, 3H, H-6′), 1.68 (s, 3H, H-5′), 2.42–2.51 (m, 1H, 1H, H-2′), 2.48 (td, J = 7.5, 2.2 Hz, 2H, H-α), 2.61 (dtm, J = 15.2, 5.6 Hz, 1H, H-2′), 5.12 (tm, J = 7.4 Hz, 1H, H-3′), 6.02 (ddd, J = 7.2, 4.4, 0.8 Hz, 1H, H-1′), 6.98 (d, J = 1.0 Hz, 1H, H-3), 7.18 (s, 2H, H-6, H-7), 12.42 (s, 1H, C5-OH), 12.58 (s, 1H, C8-OH); 13C-NMR (CDCl3): δ 4.4, 4.5 (C-2′’, C-3′’), 10.4 (C-1′’), 17.9 (C-6′), 25.7 (C-5′), 30.0 (C-β), 32.9 (C-2′), 34.4 (C-α), 69.3 (C-1′), 111.6 (C-4a), 111.8 (C-8a), 117.8 (C-3′), 131.5 (C-3), 132.7 (C-7), 132.8 (C-6), 136.0 (C-4′), 148.4 (C-2), 166.8 (C-5), 167.4 (C-8), 172.4 (COO), 176.8 (C-1), 178.3 (C-4); MS (ESI−) m/z (%): 383.30 (100) [M-H]−, [M-H]− calculated for C22H24O6: 383.4144.
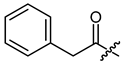
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl phenylacetate (8), 0.1 mmol Shikonin, 0.15 mmol DCC, 25 µmol DMAP and two portions of 0.1 mmol phenylacetic acid each; reaction time 17 h; CC on silica (8 g; CH2Cl2); 8, yield: 25%. 8: Rf = 0.25 (silica, cyclohexane/CH2Cl2 = 1:4); 1H- and 13C-NMR data fit with literature values [23,38].
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl cinnamate (9), 0.1 mmol Shikonin, 0.15 mmol DCC, 25 µmol DMAP and 0.1 mmol cinnamic acid; reaction time 17 h; CC on silica (8 g; CH2Cl2); 9, yield: 17%. 9: Rf = 0.31 (silica, CH2Cl2); 1H-NMR data fit with literature values [26] and the 13C-NMR data fit with those of the corresponding alkannin derivative [25].
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 2-butynoate (10), 0.1 mmol Shikonin, 0.20 mmol DCC, 25 µmol DMAP and 0.1 mmol 2-butynoic acid; reaction time 14 h; CC on silica (4 g; CH2Cl2); 10, yield: 42%. 10: Rf = 0.29 (silica, cyclohexane/CH2Cl2 = 1:4); IR (ATR): ≈ 3000 (vbr) (OH), 3052 (w), 2973 (w), 2928 (w), 2858 (w), 2238 (m) (C≡C), 1708 (s) (C=O), 1605 (s), 1568 (m), 1451 (m), 1238 (s) 1198 (s) (COC), 1066 (s), 768 (m), 738 (m) cm−1; 1H-NMR (CDCl3): 1.58 (s, 3H, H-6′), 1.70 (s, 3H, H-5′), 2.03 (s, 3H, H-γ), 2.51 (dtm, J = 15.0, 7.4 Hz, 1H, H-2′), 2.65 (dtm, J = 15.0, 6.0 Hz, 1H, H-2′), 5.12 (tm, J = 7.3 Hz, 1H, H-3′), 6.08 (ddd, J = 7.2, 4.6, 1.0 Hz, 1H, H-1′), 7.07 (d, J = 1.0 Hz, 1H, H-3), 7.18 (s, 2H, H-6, H-7), 12.42 (s, 1H, C5-OH), 12.57 (s, 1H, C8-OH); 13C-NMR (CDCl3): δ 3.9 (C-γ), 18.0 (C-6′), 25.7 (C-5′), 32.7 (C-2′), 70.3 (C-1′), 72.4 (C-α), 87.0 (C-β), 111.6 (C-4a), 111.8 (C-8a), 117.8 (C-3′), 131.5 (C-3), 133.0 (C-7), 133.2 (C-6), 136.5 (C-4′), 147.1 (C-2), 152.4 (COO), 167.7 (C-5), 168.2 (C-8), 175.8 (C-1), 177.4 (C-4); MS (ESI−) m/z (%): 354.07 (36) [M]−, 353.15 (100) [M-H]−; [M]− calculated for C20H18O6: 354.1103.
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 2-pentynoate (11), 0.1 mmol Shikonin, 0.15 mmol DCC, 25 µmol DMAP and 0.1 mmol 2-pentynoic acid; reaction time 16 h; CC on silica (4 g; cyclohexane / CH2Cl2 = 1:4); 11, yield: 35%. 11: Rf = 0.38 (silica, cyclohexane/CH2Cl2 = 1:4); IR (ATR): ≈3052 (w), 2980 (w), 2970 (vbr) (OH), 2937 (w), 2858 (w), 2236 (m) (C≡C), 1710 (s) (C=O), 1609 (s), 1569 (m), 1453 (m), 1232 (s) 1200 (s) (COC), 1080 (s), 1052 (s), 777 (m), 750 (m) cm−1; 1H-NMR (CDCl3): 1.24 (t, J = 7.5 Hz, 3H, H-δ), 1.58 (s, 3H, H-6′), 1.70 (s, 3H, H-5′), 2.39 (q, J = 7.5 Hz, 2H, H-γ), 2.51 (dtm, J = 14.6, 7.5 Hz, 1H, H-2′), 2.65 (dtm, J = 15.0, 5.9 Hz, 1H, H-2′), 5.14 (tm, J = 7.3 Hz, 1H, H-3′), 6.08 (ddd, J = 7.1, 4.6, 0.9 Hz, 1H, H-1′), 7.07 (d, J = 0.9 Hz, 1H, H-3), 7.18 (s, 2H, H-6, H-7), 12.43 (s, 1H, C5-OH), 12.57 (s, 1H, C8-OH); 13C-NMR (CDCl3): δ 12.4 (C-δ), 12.5 (C-γ), 18.0 (C-6′), 25.8 (C-5′), 32.7 (C-2′), 70.8 (C-1′), 72.0 (C-α), 92.1 (C-β), 111.6 (C-4a), 111.8 (C-8a), 117.3 (C-3′), 131.6 (C-3), 132.9 (C-7), 133.1 (C-6), 136.5 (C-4′), 147.2 (C-2), 152.6 (COO), 167.6 (C-5), 168.1 (C-8), 175.9 (C-1), 177.5 (C-4); MS (ESI−) m/z (%): 368.18 (24) [M]−, 367.21 (100), [M]− calculated for C21H20O6: 368.1260.
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 3-phenylpropynoate (12), 50 µmol Shikonin, 0.12 mmol DCC, 15 µmol DMAP and 68 µmol phenylpropynoic acid; reaction time 19 h; PTLC on silica with cyclohexane/CH2Cl2 = 1:1 (twice developed); 12, yield: 67%. 12: Rf = 0.42 (silica, cyclohexane/CH2Cl2 = 1:4); IR (ATR): 3058 (w), 2971 (w), 2915 (w), 2857 (w), 2212 (m) (CΞC), 1711 (s) (C=O), 1608 (s), 1568 (m), 1452 (m), 1275 (s), 1238 (s) 1164 (s), 1164 (vs) (COC), 1111 (m), 755 (s), 687 (m) cm−1; 1H-NMR (CDCl3): 1.61 (s, 3H, H-6′), 1.72 (s, 3H, H-5′), 2.57 (dtm, J = 14.9, 7.4 Hz, 1H, H-2′), 2.70 (dtm, J = 14.8, 5.7 Hz, 1H, H-2′), 5.19 (tm, J = 7.3 Hz, 1H, H-3′), 6.17 (ddd, J = 7.3, 4.7, 1.0 Hz, 1H, H-1′), 7.14 (d, J = 0.9 Hz, 1H, H-3), 7.19 (s, 2H, H-6, H-7), 7.40 (tm, J = 7.4 Hz, 2H, H-3′’, H-5′’), 7.48 (tt, J = 7.5, 1.1 Hz, 1H, H-4′’), 7.63 (m, 2H, H-2′’, H-6′’), 12.43 (s, 1H, C5-OH), 12.59 (s, 1H, C8-OH); 13C-NMR (CDCl3): δ 18.0 (C-6′), 25.8 (C-5′), 32.8 (C-2′), 71.1 (C-1′), 80.2 (C-α), 87.6 (C-β), 111.6, (C-4a), 111.9 (C-8a), 117.2 (C-3′), 119.3 (C-1′’), 128.6 (C-3′’, C-5′’), 130.9 (C-4′’), 131.6 (C-3), 133.0 (C-7), 133.1 (C-2′’, C-3′’), 133.3 (C-6), 136.6 (C-4′), 147.0 (C-2), 152.8 (COO), 167.9 (C-5), 168.4 (C-8), 175.6 (C-1), 177.2 (C-4); MS (ESI−) m/z (%):416.18 (27) [M]−, 415.12 (100) [M-H]−, [M]− calculated for C25H20O6: 416.1260.
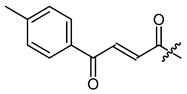
(R,E)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 4-oxo-4-(4-methylphenyl)-2-butenoate (13), 50 µmol Shikonin, 0.15 mmol DCC, 15 µmol DMAP and 95 µmol (E) 4-(4-methylphenyl)-3-oxo-2-butenoic acid; reaction time 16 h; PTLC on silica with cyclohexane/CH2Cl2 = 2:1 (four times developed); 13, yield: 4%. 13: Rf = 0.51 (silica, CH2Cl2); IR (ATR): 2967 (w), 2918 (m), ≈2950 (br) (OH), 2851 (w), 1728 (m) (C=O), 1669 (m), 1607 (vs), 1570 (m), 1453 (m), 1297 (s) (C-O-C), 1205 (m), 1161 (m), 756 (w) cm−1; 1H-NMR (CDCl3): 1.61 (s, 3H, H-6′), 1.70 (s, 3H, H-5′), 2.45 (s, 3H, Ph-CH3), 2.58 (dtm, J = 15.2, 7.6 Hz, 1H, H-2′), 2.70 (dtm, J = 15.2, 5.4 Hz, 1H, H-2′), 5.16 (tm, J = 7.2 Hz, 1H, H-3′), 6.17 (dd, J = 7.3, 4.7 Hz, 1H, H-1′), 6.95 (d, J = 15.5 Hz, 1H, H-α), 7.04 (d, J = 0.8 Hz, 1H, H-3), 7.19 (s, 2H, H-6, H-7), 7.32 (d, J = 8.2 Hz, 2H, H-3′’, H-5′’), 7.91 (d, J = 8.2 Hz, 2H, H-2′’, H-6′’), 7.96 (d, J = 15.5 Hz, 1H, H-β), 12.41 (s, 1H, C5-OH), 12.61 (s, 1H, C8-OH); 13C-NMR (CDCl3): δ 18.0 (C-6′), 21.8 (Ph-CH3), 25.8 (C-5′), 32.9 (C-2′), 70.5 (C-1′), 111.7 (C-4a), 111.8 (C-8a), 117.4 (C-3′), 129.0 (C-2′’, C-6′’), 129.7 (C-3′’, C-5′’), 131.2 (C-α), 131.3 (C-3), 133.1 (C-7), 133.3 (C-6), 134.0 (C-4′’), 136.5 (C-4′), 137.8 (C-β), 145.2 (C-1′’), 147.2 (C-2), 164.5 (COO), 168.0 (C-5), 168.6 (C-8), 175.5 (C-1), 177.0 (C-4), 188.6 (C-γ); MS (ESI−) m/z (%): 1417.45 (100) [3M-2H+K]−, 1401.56 (91) [3M-2H+Na]−, 1001.40 (55), 269.13 (42) [M-H-RCOOH]−; [3M-2H+K]− calculated for C27H24O7: 1417.4047.
Synthesis and spectroscopic data of this compound were published mislabeled as Z-isomer. 13C-NMR shift values fit with our data, but 1H-NMR data and assignment of 13C-NMR signals are deficient [34].
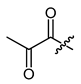
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 2-oxopropanoate (14), 0.25 mmol Shikonin, 0.75 mmol and 0.20 mmol DCC, 62 µmol DMAP and 0.25 mmol and 0.68 mmol pyruvic acid; reaction time 240 min plus 90 min; flash filtration on silica (10 g; cyclohexane/CH2Cl2 = 2:1 to 0:1); 14, from the acrylester derived from HBr elimination. 14, yield: 44%. 14: Rf = 0.31 (silica, CH2Cl2); IR (ATR): 2969 (w), ≈2950 (br) (OH), 2928 (w), 2856 (w), 1733 (s) (C=O), 1607 (s), 1569 (m), 1435 (m), 1411 (m), 1262 (m), 1231 (s), 1202 (s) (COC), 1113 (m), 779 (m) cm−1; 1H-NMR (CDCl3): 1.59 (s, 3H, H-6′), 1.69 (s, 3H, H-5′), 2.50 (s, 3H, H-β), 2.61 (dtm, J = 14.9, 7.4 Hz, 1H, H-2′), 2.70 (dtm, J = 15.0, 5.9 Hz, 1H, H-2′), 5.13 (tm, J = 7.1 Hz, 1H, H-3′), 6.15 (dd, J = 7.1, 5.1 Hz, 1H, H-1′), 7.09 (s, 1H, H-3), 7.17 (s, 2H, H-6 and H-7), 12.38 (s, 1H, C5-OH), 12.58 (s, 1H, C8-OH); 13C-NMR (CDCl3): δ 17.9 (C-6′), 25.7 (C-5′), 26.7 (C-2′), 32.8 (C-β), 70.3 (C-1′), 71.5 (C-2′’), 111.6, (C-4a), 111.8 (C-8a), 116.9 (C-3′), 131.5 (C-3), 133.5 (C-7), 133.7 (C-6), 136.9 (C-4′), 145.9 (C-2), 159.6 (COO), 169.2 (C-5), 169.7 (C-8), 174.1 (C-1), 175.7 (C-4), 190.9 (C-α); MS (ESI−) m/z (%): 1111.26 (100) [3M-2H+K]−, 269.25 (40) [M-H-RCOOH]−, [3M-2H+K]− calculated for C19H18O7: 1111.2638.
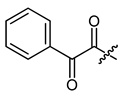
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 2-oxo-phenylacetate (15), 50 µmol Shikonin, 0.12 mmol DCC, 15 µmol DMAP and 67 µmol 2-oxo-phenylacetic acid; reaction time 19 h; PTLC on silica with cyclohexane/CH2Cl2 = 1:1 (twice developed); 15, yield: 57%. 15: Rf = 0.33 (silica, cyclohexane/CH2Cl2 = 1:4); IR (ATR): 3064 (w), 2972(w), 2930 (w) 2857 (w), 1742 (m) (C=O), 1688 (m) (C=O), 1609 (s), 1570 (m), 1451 (m), 1193 (s) 1173 (s) (COC), 982 (m), 682 (m) cm−1; 1H-NMR (CDCl3): 1.59 (s, 3H, H-6′), 1.72 (s, 3H, H-5′), 2.61 (dtm, J = 15.1, 7.7 Hz, 1H, H-2′), 2.76 (dtm, J = 15.1, 5.5 Hz, 1H, H-2′), 5.20 (tm, J = 7.3 Hz, 1H, H-3′), 6.37 (ddd, J = 7.1, 4.3, 0.8 Hz, 1H, H-1′), 7.18 (d, J = 0.8 Hz, 1H, H-3), 7.19 (s, 2H, H-6, H-7), 7.53 (tm, J = 7.8 Hz, 2H, H-3′’, H-5′’), 7.69 (tt, J = 7.7, 1.2 Hz, 1H, H-4′’), 8.00 (m, 2H, H-2′’, H-6′’), 12.41 (s, 1H, C5-OH), 12.62 (s, 1H, C8-OH); 13C-NMR (CDCl3): δ 18.0 (C-6′), 25.8 (C-5′), 33.0 (C-2′), 71.2 (C-1′), 111.6, (C-4a), 111.8 (C-8a), 117.2 (C-3′), 129.0 (C-3′’, C-5′’), 130.0 (C-2′’, C-3′’), 131.4 (C-3), 132.2 (C-1′’), 133.4 (C-7), 133.8 (C-6), 135.1 (C-4′’), 137.0 (C-4′), 146.0 (C-2), 162.6 (COO), 169.1 (C-5), 169.6 (C-8), 174.3 (C-1), 175.9 (C-4), 185.6 (C-α); MS (ESI−) m/z (%): 1297.25 (100) [3M-2H+K]−, 1281.41 (85) [3M-2H+Na]−, 269.18 (22) [M-H-RCOOH]−, [3M-2H+K]− calculated for C24H20O7: 1297.3108.
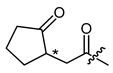
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 2-(2-oxocyclopentyl)acetate (16), 0.1 mmol Shikonin, 0.25 mmol DCC, 25 µmol DMAP and 0.18 mmol 2-(2-oxocyclopentyl)acetic acid; reaction time 15 h; CC on silica (4 g; cyclohexane/CH2Cl2 = 1:0 to cyclohexane/CH2Cl2 = 0:1); PTLC on silica (developed seven times with cyclohexane/CH2Cl2 = 2:1); 16, yield: 33%. 16: Rf = 0.11 (silica, CH2Cl2); IR (ATR): 2966 (w), ≈2950 (br) (OH), 2917 (w), 2878 (w), 1736 (m) (C=O), 1609 (s), 1569 (m), 1452 (m), 1261(m), 1231 (m), 1202 (m) (COC), 1160 (m), 782 (m) cm−1; 1H-NMR (CDCl3): 1.58 (s, 6H, H-6′), 1.58-1.68 (m, 2H, H-5′’), 1.69 (s, 6H, H-5′), 1.74-1.88 (m, 2H, H-4′’), 2.01–2.10 (m, 2H, H-4′’), 2.11–2.23 (m, 2H, H-3′’), 2.25–2.41 (m, 4H, H-5′’, H-4′’), 2.41–2.56 (m, 6H, H-2′, H-α, H-1′’), 2.57–2.67 (m, 2H, H-2′), 7.74–2.88 (m, 2H, H-α), 5.07–5.15 (m, 2H, H-3′), 6.00–6.07 (m, 2H, H-1′), 7.00 (d, J = 1.6 Hz, 1H, H-3), 7.03 (d, J = 0.8 Hz, 1H, H-3), 7.18 (s, 4H, H-6, H-7), 12.42 (s, 1H, C5-OH), 12.43 (s, 1H, C5-OH), 12.58 (s, 2H, C8-OH); 13C-NMR (CDCl3): δ 18.0 (C-6′), 20.6 (C-2′’), 25.7 (C-5′), 29.2, 29.3 (C-5′’), 32.8, 32.9 (C-2′), 33.9, 34.0 (C-α), 2 × 37.3 (C-3′’), 45.6 (C-1′’), 2 × 69.8 (C-1′), 111.6 (C-4a), 111.9 (C-8a), 117.7 (C-3′), 2x 131.5 (C-3), 2x 132.8 (C-7), 2 × 133.0 (C-6), 136.1, 136.2 (C-4′), 147.9 (C-2), 167.3, 167.5 (C-5), 167.7, 167.8 (C-8), 171.0 (COO), 176.3, 176.3 (C-1), 177.8, 177.9 (C-4), 218.8 (C-2′’); MS (ESI−) m/z (5): 411.27 (100) [M-H]−, [M-H]− calculated for C23H24O7: 411.1444.
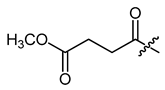
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl methyl butanedioate (17), 50 µmol Shikonin, 0.12 mmol DCC, 12.5 µmol DMAP and 76 µmol monomethyl succinate (4-methoxy-4-oxobutanoic acid); reaction time 18 h; CC on silica (8 g; cyclohexane/CH2Cl2 = 2:1 to 0:1); 17, yield: 15 %. 17: Rf = 0.36 (silica, CH2Cl2); IR (ATR): 2961 (m), 2918 (w), 2855 (w), 1735 (m) (C=O), 1607 (s), 1568 (m), 1452 (m), 1436 (m), 1410 (m), 1345 (m), 1261 (m), 1229 (m), 1200 (s) (COC), 1150 (s), 1111 (m), 1020 (m), 781 (m) cm−1; 1H-NMR (CDCl3): 1.58 (s, 3H, H-6′), 1.69 (s, 3H, H-5′), 2.49 (dtm, J = 14.9, 7.3 Hz, 1H, H-2′), 2.58-2.68 (m, 3H, H-2′, H-α*), 2.70–2.75 (m, 2H, H-β*), 3.70 (s, 3H, OCH3), 5.12 (tm, J = 7.3 Hz, 1H, H-3′), 6.04 (dd, J = 7.1, 4.6 Hz, 1H, H-1′), 7.01 (d, J = 0.8 Hz, 1H, H-3), 7.18 (s, 2H, H-6, H-7), 12.42 (s, 1H, C5-OH), 12.57 (s, 1H, C8-OH); 13C-NMR (CDCl3): δ 17.9 (C-6′), 25.7 (C-5′), 28.8, 29.2 (C-α, C-β), 32.8 (C-2′), 51.9 (OCH3), 69.9 (C-1′), 111.6, (C-4a), 111.8 (C-8a), 117.6 (C-3′), 131.5 (C-3), 132.8 (C-7), 132.9 (C-6), 136.2 (C-4′), 147.9 (C-2), 167.2 (C-5), 167.7 (C-8), 171.1 (C-1′’), 172.4 (COOCH3), 176.4 (C-1), 177.9 (C-4); MS (ESI−) m/z (%): 402.10 (28) [M]−, 401.15 (100) [M-H]−, [M]− calculated for C21H22O8: 402.1315.
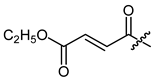
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl ethyl (2E)-but-2-enedioate (18), 0.1 mmol Shikonin, 0.15 mmol DCC, 20 µmol DMAP and 0.11 mmol monoethyl fumarate ((E)-4-methoxy-4-oxobut-2-enoic acid); reaction time 17 h; CC on silica (4 g; CH2Cl2); 18, yield: 7%. 18: Rf = 0.24 (silica, CH2Cl2); IR (ATR): 3074 (w), 2980 (m), ≈2950 (br) (OH), 2930 (w), 2857 (w), 1719 (s) (C=O), 1608 (s), 1569 (m), 1452 (m), 1294 (s), 1256 (s), 1230 (s), 1202 (s) (COC), 1149 (s), 1112 (m), 1026 (m), 771 (m) cm−1; 1H-NMR (CDCl3): 1.35 (t, J = 7.1 Hz, O-CH2-CH3), 1.57 (s, 3H, H-6′), 1.69 (s, 3H, H-5′), 2.54 (dtm, J = 15.0, 7.3 Hz, 1H, H-2′), 2.67 (dtm, J = 15.0, 5.0 Hz, 1H, H-2′), 4.29 (q, J = 7.1 Hz, O-CH2-CH3), 5.13 (tm, J = 7.3 Hz, 1H, H-1′), 6.17 (ddd, J = 7.3, 4.6, 0.8 Hz, 1H, H-1′), 6.90, 6.92 (2d, J = 15.9 Hz, 2H, H-α, H-β), 7.00 (d, J = 0.8 Hz, 1H, H-3), 7.19 (s, 2H, H-6, H-7), 12.41 (s, 1H, C5-OH), 12.59 (s, 1H, C8-OH); 13C-NMR (CDCl3): δ 14.1 (O-CH2-CH3), 18.0 (C-6′), 25.8 (C-5′), 32.8 (C-2′), 61.5 (O-CH2-CH3), 70.5 (C-1′), 111.6 (C-4a), 111.8 (C-8a), 117.3 (C-3′), 131.2 (C-3), 132.7 (C-α or C-β), 133.2 (C-7), 133.4 (C-6), 134.8 (C-α or C-β), 136.6 (C-4′), 147.1 (C-2), 163.7 (C-α-COO), 164.7 (COOEt), 168.2 (C-5), 168.7 (C-8), 175.3 (C-1), 176.8 (C-4); MS (ESI−) m/z (%):1279 (5) [3M-2H+K]−, 1265 (8) [3M-2H+Na]−, 1264 (14) [3M-2H+Na]−, 1263 (19) [3M-2H+Na]−, 414.14 (28) [M]−, 413.21 (100) [M-H]−; [M]− calculated for C22H22O8: 414.1315.
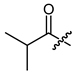
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 2-methylpropanoate (19), 0.1 mmol Shikonin, 0.20 mmol DCC, 25 µmol DMAP and 0.11 mmol isobutyric acid; reaction time 22 h; PTLC on silica (1 mm layer; developed once with cyclohexane/CH2Cl2 = 1:4); 19, yield: 14%. 19: Rf = 0.24 (silica, cyclohexane/CH2Cl2 = 1:4); 1H- and 13C-NMR data fit with literature values [62,63,64].
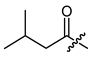
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 3-methyl-butanoate (20), 0.1 mmol Shikonin, 0.15 mmol DCC, 25 µmol DMAP and 0.1 mmol isovaleric acid; reaction time 17 h; PTLC on silica (developed three times with cyclohexane/CH2Cl2 = 2:5); 20, yield: 11 %. 20: Rf = 0.29 (silica, cyclohexane/CH2Cl2 = 1:4); 1H- and 13C-NMR data fit with literature values [62,64].
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl (E)-2-butenoate (21), 0.1 mmol Shikonin, 0.15 mmol DCC, 25 µmol DMAP and two portions of 0.1 mmol crotonoic acid each; reaction time 16 plus 24 h; CC on silica (4 g; cyclohexane/CH2Cl2 = 1:1 to 0:1); 21, yield: 17%. 21: Rf = 0.27 (silica, cyclohexane/ CH2Cl2 = 1:4); 1H-NMR (CDCl3): 1.58 (s, 3H, H-6′), 1.69 (s, 3H, H-5′), 1.93 (dd, J = 6.8 Hz, 1.9, 3H, H-γ), 2.50 (dtm, J = 14.9, 7.2 Hz, 1H, H-2′), 2.64 (dtm, J = 15.1, 5.5 Hz, 1H, H-2′), 5.14 (tquint, J = 7.3, 1.3 Hz, 1H, H-3′), 5.91 (dq, J = 15.5, 1.5 Hz 2H, H-α), 6.07 (ddd, J = 6.2, 3.7, 0.8 Hz, 1H, H-1′), 6.98 (d, J = 1.0 Hz, 1H, H-3), 7.05 (dq, J = 15.5, 6.9 Hz, 1H, H-β), 7.18 (s, 2H, H-6, H-7), 12.43 (s, 1H, C5-OH), 12.59 (s, 1H, C8-OH); 13C-NMR (CDCl3): δ 17.9, 18.1 (C-γ, C-6′), 25.8 (C-5′), 29.7 (C-β), 32.9 (C-2′), 69.3 (C-1′), 111.6 (C-4a), 111.8 (C-8a), 117.8 (C-3′), 122.1 (C-α), 131.5 (C-3), 132.6, 132.7 (C-7, C-6), 136.0 (C-4′), 146.1 (C-ß), 148.5 (C-2), 165.1 (COO), 166.6 (C-5), 167.1 (C-8), 177.1 (C-1), 178.6 (C-4); 1H-NMR shift values fit with literature values [39].
(R,E,E)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 2,4-hexadienoate (22), 0.1 mmol Shikonin, 0.35 mmol DCC, 37.5 µmol DMAP and 0.1 mmol sorbic acid each; reaction time 17 h; PTLC on silica four times developed with cyclohexane/CH2Cl2 = 1:1 and once with CH2Cl2; 22, yield: 8%. 22: Rf = 0.40 (silica, CH2Cl2). 1H- and 13C-NMR data fit with literature values [23,38].
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl benzoate (23), 0.1 mmol Shikonin, 0.15 mmol DCC, 25 µmol DMAP and 0.1 mmol benzoic acid each; reaction time 17 h; PTLC on silica (twice developed with cyclohexane/CH2Cl2 = 1:1 and twice developed with cyclohexane/CH2Cl2 = 1:2); 23, yield: 5%. 23: Rf = 0.27 (silica, cyclohexane/CH2Cl2 = 1:4); 1H- and 13C-NMR data fit with literature values [23,38].
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl diphenylacetate (24), 50 µmol Shikonin, 0.12 mmol DCC, 15 µmol DMAP and 61 µmol diphenylacetic acid each; reaction time 13 h; CC on silica (8 g; cyclohexane/CH2Cl2 = 1:1 to 4:1); PTLC on silica with cyclohexane/CH2Cl2 = 1:1 (three times developed); PTLC on silica with cyclohexane/CH2Cl2 = 2:1 (three times developed); 24, yield: 29%. 24: Rf = 0.69 (silica, CH2Cl2);
1H- and 13C-NMR data fit with literature values [23,38].
(R,E)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 3-phenyl-propanoate (25), 50 µmol Shikonin, 0.12 mmol DCC, 20 µmol DMAP and 73 µmol 3-phenylpropanoic acid; reaction time 15 h; PTLC on silica with cyclohexane/CH2Cl2 = 1:1 (three times developed); 25, yield: 14%. 25: Rf = 0.45 (silica, cyclohexane/CH2Cl2 = 1:4); IR (ATR): 3028 (vw), 2965 (w), 2918 (w), ≈2950 (br) (OH), 2851 (w), 1738 (s) (C=O), 1608 (s), 1568 (m), 1453 (m), 1342 (m), 1264 (m), 1229 (m), 1201 (s) (C-O-C), 1146 (m), 1112 (m), 735 (m), 698 (m) cm−1; 1H- and 13C-NMR data fit with literature values [36].
(R,E)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 3-(4-dimethylaminophenyl)2-propenoate (26), 0.5 mmol Shikonin, 1.5 mmol DCC, 0.125 mmol DMAP and 0.675 mmol p-dimethylaminocinnamic acid; reaction time 24 h; flash CC on silica with cyclohexane/CH2Cl2 = 2:1 to 0:1; 26, yield: 6%. 26: Rf = 0.13 (silica, CH2Cl2). IR (ATR): 2920 (m, br), 2852 (w), 1707 (m), (C=O), 1597 (vs), 1526 (m), 1444 (m), 1232 (m), 1206 (m), 1182 (m), 1146 (s) (COC), 1113 (m), 815 (w) cm−1; 1H-NMR (CDCl3): 1.60 (d, J = 0.8 Hz, 3H, H-6′), 1.69 (d, J = 0.8 Hz, 3H, H-5′), 2.56 (dtm, J = 15.0, 7.1 Hz, 1H, H-2′), 2.68 (dtm, J = 15.0, 5.7 Hz, 1H, H-2′), 3.06 (s, 6H, NCH3), 5.19 (tm, J = 7.2 Hz, 1H, H-3′), 6.12 (ddd, J = 7.0, 4.4, 0.7 Hz, 1H, H-1′), 6.31 (d, J = 15.8 Hz, 1H, H-α), 6.83 (d, J = 8.0 Hz, 1H, H-3′’), 7.04 (d, J = 1.0 Hz, 1H, H-3), 7.19 (s, 2H, H-6 and H-7), 7.47 (d, J = 8.6 Hz, 1H, H-3′’), 7.67 (d, J = 15.8 Hz, 1H, H-β), 12.43 (s, 1H, C5-OH), 12.61 (s, 1H, C8-OH); 13C-NMR (CDCl3): δ 18.0 (C-6′), 25.8 (C-5′), 32.9 (C-2′), 40.9 (NCH3), 69.2 (C-1′), 111.6 (C-4a), 111.9 (C-8a), 112.4 (C-α), 112.9 (C-3′’, C-5′’), 117.9 (C-3′), 123.6 (C-3′’), 129.9 (C-2′’, C-6′’), 131.7 (C-3), 132.4 (C-7), 132.5 (C-6), 135.8 (C-4′), 146.2 (C-β), 148.8 (C-2), 166.2 (C-8), 166.3 (COO), 166.8 (C-5), 177.5 (C-4), 178.9 (C-1); MS no spectra available via (ESI−) or (ESI+), [M]− calculated for C27H27NO6: 461.1838.
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl pyridine-3-carboxylate (27), 50 µmol Shikonin, 0.12 mmol DCC, 55 µmol DMAP and 85 µmol nicotinic acid; reaction time 15 h; PTLC on silica with CH2Cl2 (three times developed) and PTLC on silica with CH2Cl2 (three times developed); 27, yield: 10%. 27: Rf = 0.50 (silica, CH2Cl2/MeOH = 40:1); 1H-NMR data fit with literature values [27].
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl pyridine-4-carboxylate (28), 50 µmol Shikonin, 100 µmol DCC, 7.5 µmol DMAP and 75 µmol isonicotinic acid; reaction time 22 h; flash CC on silica (8 g; CH2Cl2 to CH2Cl2/MeOH = 40:1), PTLC on silica with CH2Cl2 (three times developed) and PTLC on silica with cyclohexane/CH2Cl2 = 1:4 (four times developed); 28, yield: 1%. 28: Rf = 0.41 (silica, CH2Cl2 / MeOH = 40:1); IR (ATR): 2961 (w), 2918 (m), ≈2950 (br) (OH), 2851 (w), 1734 (s) (C=O), 1610 (s), 1570 (m), 1559 (m), 1456 (m), 1406 (m), 1269 (s) (C-O-C), 1205 (m), 1117 (m), 757 (w) cm−1; 1H-NMR (CDCl3): 1.62 (s, 3H, H-6′), 1.69 (s, 3H, H-5′), 2.68 (dtm, J = 15.2, 7.2 Hz, 1H, H-2′), 2.78 (dtm, J = 15.0, 5.6 Hz, 1H, H-2′), 5.18 (tm, J = 7.3 Hz, 1H, H-3′), 6.30 (ddd, J = 7.2, 4.8, 0.7 Hz, 1H, H-1′), 7.06 (d, J = 0.9 Hz, 1H, H-3), 7.19 (s, 2H, H-6 and H-7), 8.02 (s, 2H, H-3′’ and H-5′’), 8.87 (d, J = 7.3 Hz, 2H, H-2′’ and H-6′’), 12.38 (s, 1H, C5-OH), 12.63 (s, 1H, C8-OH); 13C-NMR (CDCl3 / MeOD = 2:1): δ 17.6 (C-6′), 25.8 (C-5′), 32.6 (C-2′), 70.9 (C-1′), 111.4, (C-4a), 111.8 (C-8a), 117.1 (C-3′), 123.0 (C-3′’, C-5′’), 130.6 (C-3), 133.2, 133.4 (C-6, C-7), 136.5 (C-4′), 137.2 (C-4′’), 146.4 (C-2), 150.0 (C-2′’, C-6′’), 163.6 (COO), 168.7 (C-8), 169.1 (C-5), 173.8 (C-1), 175.3 (C-4); MS (ESI−) m/z (%): 807 (14) [2(M-H)+Na], 393.09 (28) [M]−, 392.07 (100) [M-H]−; [M]− calculated for C22H19NO6: 393.1212.
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 3-(1H-indol-3-yl)-propanoate (29), 50 µmol Shikonin, 0.12 mmol DCC, 15 µmol DMAP and 63 µmol 3-(1H-indol-3-yl)-propanoic acid; reaction time 19 h; PTLC on silica CH2Cl2 (twice developed); 29, yield: 22%. 29: Rf = 0.22 (silica, CH2Cl2); 1H-NMR (CDCl3): 1.53 (s, 3H, H-6′), 1.64 (s, 3H, H-5′), 2.41 (dtm, J = 14.9, 7.5 Hz, 1H, H-2′), 2.54 (dtm, J = 14.7, 5.7 Hz, 1H, H-2′), 2.77-2.83 (m, 2H, H-α), 3.13 (tm, J = 7.4 Hz, 2H, H-β), 5.03 (tquint, J = 7.2, 1.1 Hz, 1H, H-3′), 5.99 (ddd, J = 6.9, 4.8, 0.7 Hz, 1H, H-1′), 6.70 (d, J = 0.7 Hz, 1H, H-3), 6.99 (d, J = 2.1 Hz, 1H, H-2′’), 7.11 (td, J = 7.4 Hz, 0.8 Hz, 1H, H-6′’), 7.16 (td, J = 7.8 Hz, 1.3 Hz, 1H, H-7′’), 7.18 (s, 2H, H-6, H-7), 7.34 (d, J = 7.3 Hz, 1H, H-8′’), 7.59 (d, J = 7.7 Hz, 1H, H-5′’), 8.00 (s br, 1H, NH), 12.43 (s, 1H, C5-OH), 12.56 (s, 1H, C8-OH); 1H-NMR data fit with literature values (Wang et al., 2014). 13C-NMR (CDCl3): δ 17.9 (C-6′), 20.7 (C-β), 25.7 (C-5′), 32.8 (C-2′), 35.0 (C-α), 69.4 (C-1′), 111.2 (C-8′’), 111.6 (C-4a), 111.8 (C-8a), 114.6 (C-3‘’), 117.6 (C-3′), 118.6 (C-5′’), 119.4 (C-6′’), 121.5 (C-2′’), 122.1 (C-7′’), 127.1 (C-4′’), 131.5 (C-3), 132.5 (C-7), 132.7 (C-6), 136.0 (C-4′), 136.3 (C-9‘‘), 148.3 (C-2), 166.5 (C-5), 167.0 (C-8), 172.3 (COO), 177.1 (C-1), 178.6 (C-4).
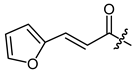
(R,E)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 3-(furan-2-yl)-2-propenoate (30), 50 µmol Shikonin, 0.12 mmol DCC, 15 µmol DMAP and 72 µmol 3-(furan-2-yl)-2-propenoic acid; reaction time 13 h; PTLC on silica with cyclohexane/CH2Cl2 = 1:1 (three times developed); 30, yield: 5%. 30: Rf = 0.43 (silica, cyclohexane/CH2Cl2 = 1:4); IR (ATR): 2969 (w), 2918 (m, br), 2853 (w), 1713 (m), (C=O), 1637 (m), 1609 (s), 1569 (m), 1454 (m), 1263 (m), 1232 (m), 1204 (s), 1155 (s), (COC), 1017 (m), 752 (m) cm−1; 1H-NMR (CDCl3): 1.60 (d, J = 0.8 Hz, 3H, H-6′), 1.69 (s, 3H, H-5′), 2.55 (dtm, J = 14.9, 7.4 Hz, 1H, H-2′), 2.68 (dtm, J = 14.8, 5.7 Hz, 1H, H-2′), 5.17 (tm, J = 7.2 Hz, 1H, H-3′), 6.12 (ddd, J = 7.2, 4.4, 0.5 Hz, 1H, H-1′), 6.38 (d, J = 15.7 Hz, 1H, H-α), 6.50 (dd, J = 3.4, 1.8 Hz, 1H, H-4′’), 6.66 (d, J = 3.4 Hz, 1H, H-3′’), 7.03 (d, J = 0.6 Hz, 1H, H-3), 7.19 (s, 2H, H-6 and H-7), 7.46 (d, J = 15.7 Hz, 1H, H-β), 7.52 (d, J = 1.5 Hz, 1H, H-5‘’), 12.42 (s, 1H, C5-OH), 12.60 (s, 1H, C8-OH); 13C-NMR (CDCl3): δ 18.0 (C-6′), 25.8 (C-5′), 32.9 (C-2′), 69.6 (C-1′), 111.6 (C-4a), 111.9 (C-8a), 112.4 (C-4′’), 114.9 (C-α), 115.5 (C-3′’), 117.8 (C-3′), 131.6 (C-3), 132.0 (C-β), 132.6 (C-7), 132.8 (C-6), 136.1 (C-4′), 145.1 (C-5′’), 148.4 (C-2), 150.7 (C-2′’), 165.7 (COO), 166.8 (C-8), 167.3 (C-5), 177.0 (C-4), 178.4 (C-1); MS (ESI−) m/z (%): 837 (51) [2(M-H)+Na]−, 408.07 (41) [M]−, 407.20 (67) [M-H]−, 137 (100), [M]− calculated for C23H20O7: 408.1209.
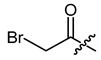
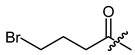
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl bromoacetate (31), 0.1 mmol Shikonin, 0.15 mmol DCC, 25 µmol DMAP and 0.1 mmol bromoacetic acid each; reaction time 17 h; CC on silica (8 g; CH2Cl2) and PTLC on silica with cyclohexane/CH2Cl2 = 2:1 (four times developed); 31, yield: 12%. 31: Rf = 0.33 (silica, CH2Cl2). 1H-NMR (CDCl3): 1.51 (s, 3H, H-6′), 1.70 (s, 3H, H-5′), 2.52 (dtm, J = 15.1, 7.5 Hz, 1H, H-2′), 2.66 (dtm, J = 15.1, 5.6 Hz, 1H, H-2′)), 3.87 (d, J = 12.1 Hz, 1H, CH2Br), 3.90 (d, J = 12.1 Hz, 1H, CH2Br), 5.13 (tquint, J = 7.3, 1.3 Hz, 1H, H-3′), 6.09 (ddd, J = 7.3, 4.5, 0.9 Hz, 1H, H-1′), 7.08 (d, J = 0.9 Hz, 1H, H-3), 7.18 (s, 2H, H-6, H-7), 12.41 (s, 1H, C5-OH), 12.57 (s, 1H, C8-OH); 13C-NMR (CDCl3): δ 18.0 (C-6′), 25.3 (CH2Br), 25.8 (C-5′), 32.8 (C-2′), 70.2 (C-1′), 111.6 (C-4a), 111.9 (C-8a), 117.2 (C-3′), 131.2 (C-3), 133.1 (C-7), 133.4 (C-6), 136.6 (C-4′), 146.8 (C-2), 166.1 (COO), 168.3 (C-5), 168.8 (C-8), 175.2 (C-1), 176.8 (C-4); disregarding the assignment of the signals the 1H-NMR spectrum fits with literature values [46].
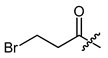
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 3-bromopropanoate (32), 0.3 mmol Shikonin, 16 mL CH2Cl2, two portions 0.45 mmol DCC, 75 µmol DMAP and two portions of 0.3 mmol 3-bromopropanoic acid; reaction time 17 h plus 4 days; CC on silica (10 g; CH2Cl2) and PTLC on silica with cyclohexane/CH2Cl2 = 2:1 (six times developed). It was impossible to separate 32, from the acryl ester derived from HBr elimination. 32, yield: 2%. 32: Rf = 0.38 (silica, CH2Cl2); IR (ATR): 2972 (w), ≈2950 (br) (OH), 2926 (w), 2857 (w), 1742 (m) (C=O), 1608 (s), 1568 (m), 1435 (m), 1408 (m), 1228 (s), 1201 (s) (COC), 1112 (m), 774 (m) cm−1; 1H-NMR (CDCl3): 1.59 (s, 3H, H-6′), 1.69 (s, 3H, H-5′), 2.51 (dtm, J = 14.8, 7.2 Hz, 1H, H-2′), 2.64 (dtm, J = 14.9, 5.5 Hz, 1H, H-2′), 3.01 (t, J = 6.7 Hz, 2H, H-α), 3.60 (2t, J = 6.8 Hz 2H, H-β), 5.13 (tm, J = 7.3 Hz, 1H, H-3′), 6.09 (ddd, J = 7.3, 4.6, 0.9 Hz, 1H, H-1′), 7.04 (d, J = 1.0 Hz, 1H, H-3), 7.18 (s, 2H, H-6, H-7), 12.41 (s, 1H, C5-OH), 12.58 (s, 1H, C8-OH); 13C-NMR (CDCl3): δ 18.0 (C-6′), 25.5 (C-5′), 29.7 (C-β), 32.9 (C-2′), 37.7 (C-α), 70.2 (C-1′), 111.6 (C-4a), 111.8 (C-8a), 117.5 (C-3′), 131.5 (C-3), 133.0 (C-7), 133.2 (C-6), 136.3 (C-4′), 147.5 (C-2), 167.7 (C-5), 168.2 (C-8), 169.4 (COO), 175.8 (C-1), 177.4 (C-4); MS (ESI−) m/z (%): 269.12 (100) [M-RCOOH-H]−, 241.08 (61) [269-CO]; [M]− calculated for C19H19BrO6: 422.0365 and 424.0345.
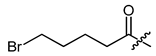
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 4-bromo-butanoate (33), 0.1 mmol Shikonin, 0.25 mmol DCC, 50 µmol DMAP and 0.5 mmol 4-bromobutanoic acid; reaction time 5 days; CC on silica (10 g; cyclohexane/CH2Cl2 = 2:5); 33, yield: 23%. 33: Rf = 0.34 (silica, cyclohexane/CH2Cl2 = 1:4); IR (ATR): 3055 (w), 2967(w), 2920 (m) (OH), 2855 (w), 1738 (s) (C=O), 1607 (s), 1568 (m), 1453 (m), 1198 (s) (COC), 1112 (s), 775 (m) cm−1; 1H-NMR (CDCl3): 1.59 (s, 3H, H-6′), 1.70 (s, 3H, H-5′), 2.20 (quint, J = 6.8 Hz, 2H, H-β), 2.49 (dtm, J = 14.9, 7.0 Hz, 1H, H-2′), 2.57-2.67 (m, 3H, H-2′, H-α), 3.47 (t, J = 6.4 Hz, 2H, H-γ), 5.12 (tm, J = 7.3 Hz, 1H, H-3′), 6.05 (ddd, J = 7.4, 4.6, 0.9 Hz, 1H, H-1′), 7.00 (d, J = 1.0 Hz, 1H, H-3), 7.19 (s, 2H, H-6, H-7), 12.42 (s, 1H, C5-OH), 12.59 (s, 1H, C8-OH); 13C-NMR (CDCl3): δ 18.0 (C-6′), 25.8 (C-5′), 27.5 (C-β), 32.38, 32.44 (C-α, C-γ), 32.9 (C-2′), 69.6 (C-1′), 111.6 (C-4a), 111.8 (C-8a), 117.6 (C-3′), 131.3 (C-3), 132.9 (C-7), 133.1 (C-6), 136.3 (C-4′), 147.9 (C-2), 167.5 (C-5), 168.0 (C-8), 171.4 (COO), 176.1 (C-1), 177.6 (C-4); MS (ESI−) m/z (%): 438.11 (27) [M]−, 437.17 (83) [M-H]−, 436.36 (35) [M]−, 435.30 (100) [M-H]−; [M]− calculated for C20H21BrO6: 436.0522 and 438.0501.
(R)-1-(1,4-Dihydro-5,8-dihydroxy-1,4-dioxonaphthalen-2-yl)-4-methylpent-3-enyl 5-bromopentanoate (34), 0.1 mmol Shikonin, 0.15 mmol DCC, 25 µmol DMAP and 0.1 mmol 5-brompentanoic acid each; reaction time 17 h; CC on silica (8 g; CH2Cl2) and PTLC on silica with cyclohexane/CH2Cl2 = 2:1 (four times developed); 34, yield: 20%. 34: Rf = 0.32 (silica, CH2Cl2); IR (ATR): 3055 (w), 2967(w), 2920 (m) (OH), 2855 (w), 1738 (s) (C=O), 1607 (s), 1568 (m), 1453 (m), 1198 (s) (COC), 1112 (s), 775 (m) cm−1; 1H-NMR (CDCl3): 1.59 (s, 3H, H-6′), 1.70 (s, 3H, H-5′), 1.77-1.86 (m, 2H, H-β), 1.87-1.96 (m, 2H, H-γ), 2.44 (td, J = 7.1, 1.7 Hz, 2H, H-α), 2.49 (dtm, J = 14.9, 7.4 Hz, 1H, H-2′), 2.61 (dtm, J = 14.7, 5.4 Hz, 1H, H-2′), 3.42 (t, J = 6.5 Hz, 2H, H-δ), 5.12 (tm, J = 7.3 Hz, 1H, H-3′), 6.05 (dd, J = 7.1, 6.1, Hz, 1H, H-1′), 6.99 (s, 1H, H-3), 7.18 (s, 2H, H-6 and H-7), 12.41 (s, 1H, C5-OH), 12.58 (s, 1H, C8-OH); 13C-NMR (CDCl3): δ 18.0 (C-6′), 23.4 (C-β), 25.7 (C-5′), 31.8 (C-γ), 32.8 (C-δ), 32.9 (C-2′), 33.3 (C-α), 69.5 (C-1′), 111.6 (C-4a), 111.8 (C-8a), 117.7 (C-3′), 131.3 (C-3), 132.9 (C-7), 133.0 (C-6), 136.1 (C-4′), 148.0 (C-2), 167.4 (C-5), 167.9 (C-8), 171.8 (COO), 176.2 (C-1), 177.7 (C-4); MS (ESI-) m/z (%): 452.04 (30) [M]−, 451.08 (100) [M-H]−, 450.16 (30) [M]−, 449.16 (98) [M-H]−; [M]− calculated for C21H23BrO6: 450.0678 and 452.0658.
3.4. Synthesis of Precursor Acids
3.4.1. 2-(1-Bicyclo[4.1.0]heptyl)acetic Acid (p1)
Under argon and at 0 °C, a solution of (1-cyclohexenyl)-2-acetic (226 mg, 1.61 mmol) in 1.3 mL toluene and and CH2I2 (1.06 g, 4.97 mmol) were added to a solution of ZnEt2 in toluene (3.2 mL, 1 M). After stirring overnight, the mixture was poured into HCl (2 M). The organic layer was separated, and the aqueous phase was extracted with Et2O. The combined organic layers were extracted with NaOH (1 M). The aqueous phase was acidified with 2 M HCl and extracted with CH2CI2. Evaporation of the solvent gave p1 [65], which was used without further purification. Yield: 60 %. 1H- and 13C NMR data fit with literature values [66].
3.4.2. 3-Cyclopropylpropanoic Acid (p2)
A solution of LDA (2. 5 mL, 2 M in THF/toluene/heptane) was added to THF (25 mL) under argon. The mixture was cooled to 0 °C and acetic acid (150 mg, 2.5 mmol) in THF (4 mL) were added dropwise. The mixture was then stirred for 30 min at 45 °C. After cooling to room temperature, bromomethylcyclopropane (405 mg, 3 mmol) was added within 15 min. The mixture was stirred at 45 °C for 5.5 h, cooled to room temperature, and poured into a mixture of Et2O and water (25 mL and 37 mL resp.). The organic layer was acidified with 2 N HCl and extracted AcOEt (3 × 25 mL). The extract was dried over Na2SO4 and most of the solvent was evaporated under reduced pressure resulting in a 74% solution of p2 in THF (67 mg, yield: 23%). This solution was used without further purification.
1H-NMR (CDCl3): 0.06 (q, J = 4.9 Hz, 2H, cyclpropyl-CH2), 0.39–49 (m, 2H, cyclpropyl-CH2), 0.67–0.79 (m, 1H), 1.53 (q, J = 7.3 Hz, 2H), 2.45 (t, J = 7.4 Hz, 2H, CH2-COOH), 10.72 (br.s, COOH); data fit with literature [67]; 13C NMR (CDCl3), δ: 4.45 (cyclpropyl-CH2), 10.4 (CH); 29.8 (CH2-CH2-COOH), 34.2 (CH2-CH2-COOH), 180.4 (COOH); data fit with literature, except δ(CO) [68,69].
3.4.3. (E) 3-Cyclopropylpropenoic Acid (p3)
A mixture of 500 mg (7,13 mmol) cyclopropanecarbaldehyde, 1.04 g (10 mmol) malonic acid, and 0.94 mL pyridine was stirred under argon for 6 h at 105 °C bath temperature, cooled to room temperature, acidified with ca. 7 mL 2 M HCl, and cooled in an ice bath. The precipitate was filtered with suction, washed with water, and dried over P2O5 in vacuo resulted in p3 (270 mg, yield 34%; drying caused loss due to sublimation) [70]. 1H-NMR (CDCl3) data fit with literature values [31]; 13C-NMR (CDCl3): δ 9.0 (CH2), 14.6 (cyclopropane CH), 117.4 (C-α), 157.1 (C-β), 172.2 (COOH).
3.4.4. 2-Cyclopropyl-2-Oxoacetic Acid (p4)
KMnO4 (4.42 g, 28 mmol) was solved in water (38 mL). Acetylcyclopropane (1.26 g, 15 mmol) was added under stirring, the mixture was heated to 30 °C, and 10% aq. KOH (6 mL) was added. The mixture was then gently heated with an oil bath and stirred for a further hour at 60 °C, cooled to room temperature, and filtered. The filter cake was washed with water (10 mL). The filtrate and washing solutions were combined, concentrated to about 15 mL, acidified with concentrated HCl to pH 2 and extracted with CH2CI2 (5 × 30 mL). Drying over Na2SO4 and evaporation of the solvent gave p4 (1.33 g; 78%) [71]. 1H and 13C; NMR data fit with literature [32].
3.5. Cell Culture
WM9, WM164, and MUG-Mel2 melanoma cell lines were cultivated in RPMI1640 medium (Gibco, Thermo Fisher Scientific, Waltham, MA, USA). The medium was supplemented with 2 mM L-glutamine, 10% fetal bovine serum (FBS, Gibco), and 1% penicillin/streptomycin solution (Pen/Strep, Gibco). HEK-293 (human epithelial cells) were grown in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12, Gibco®), supplemented with L-glutamine (2mM), 10 % FBS, and 1 % Pen/Strep. All cells were grown in a humidified 5% CO2 atmosphere and at 37 °C. They were passaged by trypsinization when reaching 90% confluence using a 0.25% trypsin-EDTA solution (Gibco).
3.6. XTT Viability Assay
To study cell viability, the cell proliferation kit II (XTT) (Roche Diagnostics, Mannheim, Germany; cat. no. 11 465 015 001) was used and performed according to the manufacturer’s instructions. Cell suspensions with 50,000 c/mL were prepared and 100 µL seeded in each well of a 96 well plate (clear plate, flat bottom). To allow the cells to adhere, cells were incubated for 24 h before the treatment was started. Control cells were then treated with 0.5% ethanol; all other cells were treated with different concentrations (1.0 µM, 5.0 µM, and 10.0 µM) of one of the shikonin derivatives. After 72 h of incubation, 50 µL of a freshly prepared XTT solution consisting of 5 mL XTT solution plus 100 µL electron coupling reagent were added to each well and incubated for another 2 h at 37 °C. Finally, the absorbance was measured with a Hidex Sense Microplate Reader (Hidex, Turku, Finland) at 490 nm and a reference wavelength of 650 nm. 2 was always tested as a reference at 5.0 µM. The assay was performed with three replicates and two different cell passages at least.
3.7. ApoToxGloTM Triplex Assay
The ApoToxGloTM Triplex Assay was purchased from Promega (Fitchburg, WI, USA, cat. no. G6320) and performed according to the manufacturer’s instructions. In brief, 10,000 cells/well (WM9 or MUG-Mel2 cells) in 100 µL of medium were pipetted in 96-well plates (white plates, flat bottom), incubated for 24 h and then treated with different concentrations of 5 for 4 h, 24 h and 48 h. Staurosporine (Abcam, Cambridge, UK) at a concentration of 25.0 µM is known to induce apoptosis and served as positive control. Subsequently, the viability/cytotoxicity reagent was prepared and 20 µL added to each well. After mixing for 30 s (orbital shaking, 300–500 rpm), the plates were incubated for another 30 min at 37 °C. Fluorescence was then measured at 400Ex/505Em (viability) and 485Ex/520Em (cytotoxicity) using a Hidex Sense Microplate Reader. Afterwards, the Caspase-Glo® 3/7 reagent was prepared and 100 µ were added to each well, followed by mixing (orbital shaking, 300–500 rpm, 30 s), and incubation at room temperature for another 30 min. Finally, luminescence was measured using a Hidex Sense Microplate Reader. The assay was performed at least two times, with three replicates each.
3.8. LDH Assay
To measure LDH release, the CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega) were used in accordance with the manufacturers protocol. Cell suspension of 100,000 c/mL were prepared and seeded into 96 well plates (clear, flat bottom, 100 µL/well). To allow the cells to adhere, cells were incubated for 24 h before the treatment was started. Afterwards, cells were treated with 1 µM, 5 µM, 10 µM, or 20 µM of 5 for 24 h, 48 h, or 72 h. To measure maximum LDH release, cells were lysed using the lyse reagent included in the assay kit. To quantify LDH release, the plates were centrifuged and 50 µL of each well were transferred into another 96 well plate. After addition of 50 µL CytoTox96 Reagent for 30 min, 50 µL of the stop solution were added and absorption were measured at 490 nm using a Hidex Sense Microplate Reader. Finally, using the following formula, the amount of LDH release was calculated: 100 × OD490 test well/OD490 maximum LDH release.
3.9. Cell Cycle Analysis
For cell cycle analysis, 2 mL of a 150,000 c/mL cell suspension were seeded into 6 well plates and incubated for 24 h. Afterwards, the cells were treated with 10 µM or 20 µM of 5 for 24 h and 48 h. The cells were collected including supernatants and by using trypsinization, centrifugation, and washing with RPMI cell culture medium. Finally, cells were resuspended in 500 µL PBS and fixed with 5 mL ice cold ethanol for 10 min. For FACS analyses, cells were centrifuged and resuspended with 200 µL PI lysis buffer. After incubation for 20 min in the dark, cells were analyzed using a LSRII™flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and ModFit software.
4. Conclusions
In previous studies, shikonin and derivatives have been demonstrated to be potent cytotoxic substances. In this study, we prepared 31 shikonin derivatives by synthesis. Most of them are novel derivatives, which have not yet been reported. One goal was to further optimize our previous hit 3. Another goal was to synthesize a broad spectrum of structural features to gain more insight into the structure-activity relationship. All derivatives were screened for their cytotoxicity in several melanoma cell lines. The results indicate that there is no strict structure-activity relationship and the different cell lines exhibited distinct sensitivities towards the derivatives. The most potent derivative was 5, which is a cyclopropyloxoacetate derivative of shikonin and, thus, structurally related to our previous hit 3. Compared to 3, 5 was more cytotoxic. Subsequent pharmacological investigations revealed that 5 leads to caspase 3/7 activation, no significant LDH release, and to a G2/M phase cell cycle arrest at higher concentrations. Nevertheless, it was also cytotoxic towards non-tumorigenic cells, which needs to be evaluated in more detail in future studies. In summary, our results indicate that shikonin derivatives might be potential drug leads for the development of novel melanoma treatment options.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/22/5/2774/s1.
Author Contributions
Design and supervision of the study: N.K., A.H., B.R., R.B.; synthesis of derivatives, NMR recording and analyzing: A.H.; screening of derivatives: N.K., C.D., K.P., pharmacological investigations: N.K., C.D., K.P., B.L.; data processing and writing of the manuscript: N.K., A.H. and R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded in whole by the Austrian Science Fund (FWF) [P27505].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Eva-Maria Pferschy-Wenzig for her help in LC-MS measurements and Ing. Andrea Fleck for recording the IR spectra. Open Access Funding by the Austrian Science Fund (FWF) is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shain, A.H.; Bastian, B.C. From melanocytes to melanoma. Nat. Rev. Cancer 2016, 16, 345–358. [Google Scholar] [CrossRef]
- Corrie, P.; Hategan, M.; Fife, K.; Parkinson, C. Management of Melanoma. Br. Med. Bull. 2014, 111, 149–162. [Google Scholar] [CrossRef]
- Scolyer, R.A.; Rawson, R.V.; Gershenwald, J.E.; Ferguson, P.M.; Prieto, V.G. Melanoma pathology reporting and staging. Mod. Pathol. 2020, 33 (Suppl. 1), 15–24. [Google Scholar] [CrossRef]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Cragg, M.G.; Pezzuto, J.M. Natural products as vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Princ. Pract. 2016, 25 (Suppl. 2), 41–59. [Google Scholar] [CrossRef]
- Papageorgiou, V.P.; Assimopoulou, A.N.; Couladouros, E.A.; Hepworth, D.; Nicolaou, K.C. The chemistry and biology of alkannin, shikonin and related naphthazarin natural products. Angew. Chem. Int. Ed. Engl. 1999, 38, 270–301. [Google Scholar] [CrossRef]
- Chen, X.; Yang, L.; Oppenheim, J.J.; Howard, M.Z. Cellular pharmacology studies of shikonin derivatives. Phytother. Res. 2002, 16, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Andújar, I.; Rios, J.L.; Giner, R.M.; Recio, M.C. Pharmacological properties of shikonin—A review of literature since 2002. Planta Med. 2013, 79, 1685–1697. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Vong, C.T.; Chen, H.; Gao, Y.; Lyu, P.; Qiu, L.; Zhao, M.; Liu, Q.; Cheng, Z.; Zou, J.; et al. Naturally occurring anti-cancer compounds: Shining from Chinese herbal medicine. Chin. Med. 2019, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Duke, J.A.; Ayensu, S. Medicinal Plants of China; Reference Publications Inc.: Algonac, MI, USA, 1985; Volume 1. [Google Scholar]
- Zhao, Q.; Kretschmer, N.; Bauer, R.; Efferth, T. Shikonin and its derivatives inhibit the epidermal growth factor receptor signaling and synergistically kill glioblastoma cells in combination with erlotinib. Int. J. Cancer 2015, 137, 1446–1456. [Google Scholar] [CrossRef]
- Rinner, B.; Kretschmer, N.; Knausz, H.; Mayer, A.; Boechzelt, H.; Hao, X.J.; Heubl, G.; Efferth, T.; Schaider, H.; Bauer, R. A petrol ether extract of the roots of Onosma paniculatum induces cell death in a caspase dependent manner. J. Ethnopharmacol. 2010, 129, 182–188. [Google Scholar] [CrossRef]
- Kretschmer, N.; Rinner, B.; Deutsch, A.J.; Lohberger, B.; Knausz, H.; Kunert, O.; Blunder, M.; Boechzelt, H.; Schaider, H.; Bauer, R. Naphthoquinones from Onosma paniculata induce cell-cycle arrest and apoptosis in melanoma Cells. J. Nat. Prod. 2012, 75, 865–869. [Google Scholar] [CrossRef]
- Kretschmer, N.; Deutsch, A.; Durchschein, C.; Rinner, B.; Stallinger, A.; Higareda-Almaraz, J.C.; Scheideler, M.; Lohberger, B.; Bauer, R. Comparative Gene Expression Analysis in WM164 Melanoma Cells Revealed That β,β-Dimethylacrylshikonin Leads to ROS generation, Loss of Mitochondrial Membrane Potential, and Autophagy Induction. Molecules 2018, 23, 2823. [Google Scholar] [CrossRef] [PubMed]
- Stallinger, A.; Kretschmer, N.; Kleinegger, F.; Brvar, L.; Liegl-Atzwanger, B.; Prokesch, A.; Durchschein, C.; Bauer, R.; Deutsch, A.; Rinner, B. β,β-Dimethylacrylshikonin Induces Apoptosis in Melanoma Cell Lines by NOXA Upregulation. J. Nat. Prod. 2020, 83, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Durchschein, C.; Hufner, A.; Rinner, B.; Stallinger, A.; Deutsch, A.; Lohberger, B.; Bauer, R.; Kretschmer, N. Synthesis of novel shikonin derivatives and pharmacological effects of cyclopropylacetylshikonin on melanoma cells. Molecules 2018, 23, 2820. [Google Scholar] [CrossRef] [PubMed]
- Damianakos, H.; Kretschmer, N.; Syklowska-Baranek, K.; Pietrosiuk, A.; Bauer, R.; Chinou, I. Antimicrobial and cytotoxic isohexenylnapththazarins from Arnebia euchroma (Royle) Johnst. (Boraginaceae) callus and cell suspension culture. Molecules 2012, 17, 14310–14322. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, V.P. Naturally occurring isohexenylnaphthazarin pigments: A new class of drugs. Planta Med. 1980, 38, 193–203. [Google Scholar] [CrossRef]
- Rao, Z.; Liu, X.; Zhou, W.; Yi, J.; Li, S.S. Synthesis and antitumour activity of β-hydroxyisovalerylshikonin analogues. Eur. J. Med. Chem. 2001, 46, 3934–3941. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-M.; Lin, H.-Y.; Kong, W.-Y.; Guo, J.; Shi, J.; Huang, S.-C.; Qi, J.-L.; Yang, R.-W.; Gu, H.-W.; Yang, Y.-H. Synthesis and biological evaluation of heterocyclic carboxylic acyl shikonin derivatives. Chem. Biol. Drug Res. 2014, 83, 334–343. [Google Scholar] [CrossRef]
- Zhao, L.M.; Cao, F.X.; Jin, H.S.; Zhang, J.H.; Szwaya, J.; Wang, G. One-pot synthesis of 1,4-dihydroxy-2-(E-1-hydroxy-4-phenylbut-3-enyl)anthracene-9,10-diones as novel shikonin analogs and evaluation of their antiproliferative activities. Bioorg. Med. Chem. Lett. 2016, 26, 2691–2694. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.Z.; Baik, K.U. Process for Preparing 5,8-Dihydronaphthoquinone Derivatives, Novel 5,8-Dihydronaphthoquinone Derivatives and Their Use as Anticancer Agent. KR. Patent WO 95/02572, 26 January 1995. [Google Scholar]
- Baik, K.-U.; Song, G.Y.; Kim, Y.; Sok, D.-E.; Ahn, B.-Z. 2-Substituted naphthazarins; synthesis and antitumor activity. Arch. Pharm. Pharm. Med. Chem. 1997, 330, 377–382. [Google Scholar] [CrossRef]
- Shen, C.C.; Syu, W.J.; Li, S.Y.; Lin, C.H.; Lee, G.H.; Sun, C.M. Antimicrobial activities of naphthazarins from Arnebia euchroma. J. Nat. Prod. 2002, 65, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Chen, W.; Shi, J.; Kong, W.Y.; Qi, J.L.; Wang, X.M.; Yang, Y.H. Design, synthesis and biological evaluation of cinnamic acyl shikonin derivatives. Chem. Biol. Drug Des. 2013, 81, 275–283. [Google Scholar] [CrossRef]
- Baloch, S.K.; Ling, L.J.; Qiu, H.Y.; Ma, L.; Lin, H.Y.; Huang, S.C.; Qi, J.L.; Wang, X.M.; Lu, G.H.; Yang, Y.H. Synthesis and biological evaluation of novel shikonin ester derivatives as potential anti-cancer agents. RSC Adv. 2014, 4, 35588–35596. [Google Scholar] [CrossRef]
- Renaud, P.; Fox, M.A. Reaction of Dilithiated Carboxylic Acids with Iodine: Evidence for the Formation of a Radical Anion Intermediate. J. Org. Chem. 1988, 53, 3745–3752. [Google Scholar] [CrossRef]
- Barczak, N.T.; Jarvo, E.R. Regioselective Silver-Mediated Kondakov–Darzens Olefin Acylation. Chem. Eur. J. 2011, 17, 12912–12916. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, S.; Cooper, C.S.; Fung, A.K.L.; Lynch, J.K.; Plagge, F.; Chu, D.T.W. Asymmetric dipolar cycloaddition reaction: A practical, convergent synthesis of chiral pyrrolidines. Tetrahedron Asymmetry 1997, 8, 883–887. [Google Scholar] [CrossRef]
- Prokopenko, V.V.; Okonnishnikova, G.P.; Klimenko, I.P.; Shulishov, E.V.; Tomilov, Y.V. Synthesis and chemical transformations of 2-cyclopropyl-2-diazoacetates. Russ. Chem Bull. Int. Ed. 2007, 56, 1515–1521. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, W.; Shu, D.; Werness, J.B.; Tang, W. Synthesis of Cyclobutenes via Transition Metal Catalyzed Highly Selective Ring Expansion of Cyclopropanes. Angew. Chem. Int. Ed. 2008, 47, 8933–8936. [Google Scholar] [CrossRef]
- Kim, S.-H.; Song, G.-Y.; Sok, D.-E.; Ahn, B.-Z. Anti-cell adhesive effect of phenylacetylshikonin analogs related to their cytotoxicity in A549 cells. Arch. Pharm Res. 1997, 20, 155–157. [Google Scholar] [CrossRef]
- Sun, W.-X.; Han, H.-W.; Yang, M.-K.; Wen, Z.-L.; Wang, Y.-S.; Fu, J.-Y.; Lu, Y.-T.; Wang, M.-Y.; Bao, J.-X.; Lu, G.-H.; et al. Design, synthesis and biological evaluation of benzoylacrylic acid shikonin ester derivatives as irreversible dual inhibitors of tubulin and EGFR. Bioorg. Med. Chem. 2019, 27, 115153. [Google Scholar] [CrossRef]
- Yang, M.D.; Shen, X.B.; Hu, Y.S.; Chen, Y.Y.; Liu, X.H. Novel naphthalene-enoates: Design and anticancer activity through regulation cell autophagy. Biomed. Pharmacother. 2019, 113, 108747. [Google Scholar] [CrossRef]
- Shen, X.B.; Wang, Y.; Han, X.-Z.; Sheng, L.-Q.; Wu, F.; Liu, X. Design, synthesis, and anticancer activity of naphthoquinone derivatives. J. Enzyme Inhib. Med. Chem. 2020, 35, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.R.; Tsukada, M.; Suzuki, N.; Shimamura, T.; Gao, L.; Koyanagi, J.; Komada, F.; Saito, S. Comparison of the cytotoxic activities of naturally occurring hydroxyanthraquinones and hydroxynaphthoquinones. Eur. J. Med. Chem. 2008, 43, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.Z.; Baik, K.U.; Kweon, G.R.; Kyu, L.; Hwang, B.D. Acylshikonin analogues: Synthesis and inhibition of DNA topoisomerase-I. J. Med. Chem. 1995, 38, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Al-Arif, M.T. Cyclisation of Substituted 2-(4-Methyl-pent-3-enyl)-5,8-dihydroxy-1,4-naphthoquinones. J. Prakt Chem. 1982, 324, 865–869. [Google Scholar] [CrossRef]
- Kim, S.-H.; Song, G.-Y.; Jin, G.-Z.; Ahn, B.-Z. Antitumor activity of arylacetylshikonin analogs. Arch. Pharm. Res. 1996, 19, 416–422. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Singh, A.K.; Seth, P.; Misra, N.; Shafali, M.; Vijayavitthal, T.; Raj, K.; Maheshwari, R.K. Shikonin analogue (SA) 93/637 induces apoptosis by activation of caspase-3 in U937 cells. Front. Biosci. 2008, 13, 561–568. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, Y.; Wang, X.; Lin, H.; Shi, J.; Liu, H.; Kong, W.; Haori, Q.; Huang, S. Shikonin Octyl Methoxycinnamate Derivant as Well as Synthesis Method and Application Thereof. Patent CN102531893A, 4 July 2012. [Google Scholar]
- Baloch, S.K.; Ma, L.; Xu, G.H.; Bai, L.F.; Zhao, H.; Tang, C.Y.; Pang, Y.J.; Yang, R.W.; Wang, X.M.; Lu, G.H.; et al. A potent anticancer agent of shikonin derivative targeting tubulin. Chirality 2015, 27, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Liu, W.; Ding, J.; Cai, J.; Duan, W. Shikonin derivatives: Synthesis and inhibition of human telomerase. Bioorg. Med. Chem. Lett. 2002, 12, 1375–1378. [Google Scholar] [CrossRef]
- Yang, F.; Chen, Y.; Duan, W.; Zhang, C.; Zhu, H.; Ding, J. SH-7, a new synthesized shikonin derivative, exerting its potent antitumor activities as a topoisomerase inhibitor. Int. J. Cancer 2006, 119, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.-G.; Jin, G.-Z.; Song, G.-Y.; Hoon, C.; Ahn, B.-Z. Haloacetylshikonin Derivatives: Synthesis and Evaluation of Antitumor Activity. Yakhak Hoechi 1998, 42, 159–164. [Google Scholar]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar] [PubMed]
- Heppt, M.V.; Siepmann, T.; Engel, J.; Schubert-Fritschle, G.; Eckel, R.; Mirlach, L.; Kirchner, T.; Jung, A.; Gesierich, A.; Ruzicka, T.; et al. Prognostic Significance of BRAF and NRAS Mutations in Melanoma: A German Study from Routine Care. BMC Cancer 2017, 17, 536. [Google Scholar] [CrossRef]
- Mackiewicz, J.; Mackiewicz, A. BRAF and MEK Inhibitors in the Era of Immunotherapy in Melanoma Patients. Contemp. Oncol. (Poznan Poland) 2018, 22, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Couselo, E.; Adelantado, E.Z.; Ortiz, C.; García, J.S.; Perez-Garcia, J. NRAS-Mutant Melanoma: Current Challenges and Future Prospect. Oncol. Targets Ther. 2017, 10, 3941–3947. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.J.; Zhang, X.; Cheng, C.; Wang, F.; Wang, X.K.; Liang, Y.J.; To, K.K.W.; Zhou, W.; Huang, H.B.; Fu, L.W. Crizotinib (PF-02341066) reverses multidrug resistance in cancer cells by inhibiting the function of P-glycoprotein. Br. J. Pharmacol. 2012, 166, 1669–1683. [Google Scholar] [CrossRef]
- Akiyode, O.; George, D.; Getti, G.; Boateng, J. Systematic comparison of the functional physico-chemical characteristics and biocidal activity of microbial derived biosurfactants on blood-derived and breast cancer cells. J. Colloid Interface Sci. 2016, 479, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Fayez, H.; El-Motaleb, M.A.; Selim, A.A. Synergistic Cytotoxicity of Shikonin-Silver Nanoparticles as an Opportunity for Lung Cancer. J. Label. Comp. Radiopharm. 2020, 63, 25–32. [Google Scholar] [CrossRef]
- Wang, H.; Tang, Y.; Fang, Y.; Zhang, M.; Wang, H.; He, Z.; Wang, B.; Xu, Q.; Huang, Y. Reprogramming Tumor Immune Microenvironment (TIME) and Metabolism via Biomimetic Targeting Codelivery of Shikonin/JQ1. Nano Lett. 2019, 19, 2935–2944. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhao, H.R.; Meng, Q.Q.; Zhang, Q.J.; Dong, J.Y.; Zhu, B.Q.; Li, S.S. Synthesis and biological evaluation of sulfur-containing shikonin oxime derivatives as potential antineoplastic agents. Eur. J. Med. Chem. 2018, 143, 166–181. [Google Scholar] [CrossRef]
- Chan, F.K.; Moriwaki, K.; De Rosa, M.J. Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol. Biol. 2013, 979, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Liu, T.J.; Lai, H.C. Shikonin Induces Apoptosis, Necrosis, and Premature Senescence of Human A549 Lung Cancer Cells through Upregulation of p53 Expression. Evid. Based Complement. Alternat. Med. 2015, 2015, 620383. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kao, S.H.; Hunag, J.E.; Sheu, G.T.; Yeh, C.W.; Hseu, Y.C.; Wang, C.J.; Hsu, L.S. Shikonin time-dependently induced necrosis or apoptosis in gastric cancer cells via generation of reactive oxygen species. Chem. Biol. Interact. 2014, 211, 44–53. [Google Scholar] [CrossRef]
- Shan, B.; Pan, H.; Najafov, A.; Yuan, J. Necroptosis in development and diseases. Genes Dev. 2018, 32, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Sun, X.; Cao, Z. Shikonin-induced necroptosis in nasopharyngeal carcinoma cells via ROS overproduction and upregulation of RIPK1/RIPK3/MLKL expression. Oncol. Targets Ther. 2019, 12, 2605–2614. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Yin, Y.; Lian, B.P.; Leng, J.F.; Xia, Y.Z.; Kong, L.Y. Synthesis and biological evaluation of novel shikonin-benzo[b]furan derivatives as tubulin polymerization inhibitors targeting the colchicine binding site. Eur. J. Med. Chem. 2020, 190, 112105. [Google Scholar] [CrossRef] [PubMed]
- Albreht, A.; Vovk, I.; Simonovska, B.; Srbinoska, M. Identification of shikonin and its ester derivatives from the roots of Echium italicum L. J. Chromatogr. A 2009, 1216, 3156–3162. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jeong, H.J.; Park, J.-Y.; Kim, Y.M.; Park, S.-J.; Cho, J.K.; Park, K.H.; Ryu, Y.B.; Lee, W.S. Selective and slow-binding inhibition of shikonin derivatives isolated from Lithospermum erythrorhizon on glycosyl hydrolase 33 and 34 sialidases. Bioorg. Med. Chem. 2012, 20, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Chaouche, T.; Haddouchi, F.; Bekkara, F.A. Identification of Shikonin from the roots of Echium pycnanthum Pomel. Asian J. Pharm. Clin. Res. 2012, 5, 30–32. [Google Scholar]
- Sugahara, K.; Fujita, T.; Watanabe, S.; Sakamoto, M.; Sugimoto, K. A convenient lactonization of 2- and 3-cyclopropylalkanoic acids to γ- and δ-lactones. Synthesis 1990, 783–784. [Google Scholar] [CrossRef]
- Kantorowski, E.J.; Eisenberg, S.W.E.; Fink, W.H.; Kurth, M.J. Six- vs Seven-Membered Ring Formation from the 1-Bicyclo[4.1.0]heptanylmethyl Radical: Synthetic and ab Initio Studies. J. Org. Chem. 1999, 64, 570–580. [Google Scholar] [CrossRef]
- Maercker, A.; Theysohn, W. Versuche zur Umlagerung von 2-Cyclopropyl-äthyl-Anionen. Liebigs Ann. Chem. 1972, 759, 132–157. [Google Scholar] [CrossRef]
- Muehling, O.; Wessig, P. Photochemical synthesis of benzoyl spiro[2.2]pentanes; Photochem. Photobiol. Sci. 2006, 5, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, J.H.; Wallis, E.S. Kinetics of the addition of bromine to some cyclopropanes in aqueous acetic acid. J. Org. Chem. 1956, 21, 663–6966. [Google Scholar] [CrossRef]
- Henrick, C.A.; Willy, W.E.; Staal, G.B.; Ludvik, G.F. Ovicidal activity and its relation to chemical structure for the two-spotted spider mite (Tetranychus urticae Koch) in a new class of miticides containing the cyclopropyl group. J. Agric. Food Chem. 1976, 24, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Zbiral, E.; Werner, E. Reaktionen mit phosphororganischen Verbindungen, 10. Mitt.: [Oxydation mit Pb(OAc)4, (C6H5COO)2, C6H5J(OAc)2 und PbO2]. Zur Darstellung von α-Ketosäuremethylestern und α-Ketosäurethiophenylestern. Monatsh. Chem. 1966, 97, 1797–1820. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).