Differential Methylation in the GSTT1 Regulatory Region in Sudden Unexplained Death and Sudden Unexpected Death in Epilepsy
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Study Population
2.2. Allele Frequencies in Sudden Unexplained Death and Sudden Unexpected Death in Epilepsy
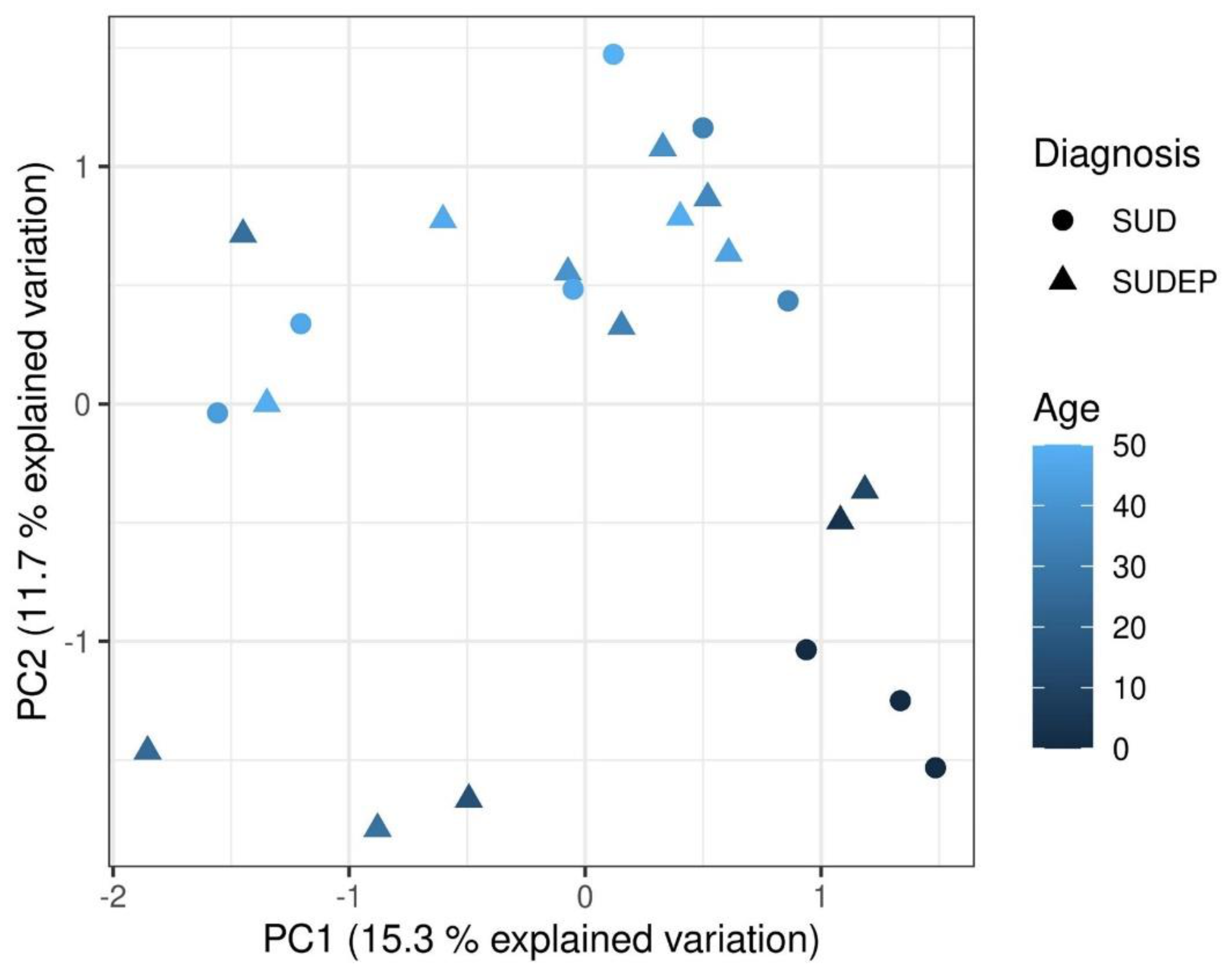
2.3. Principal Component Analysis
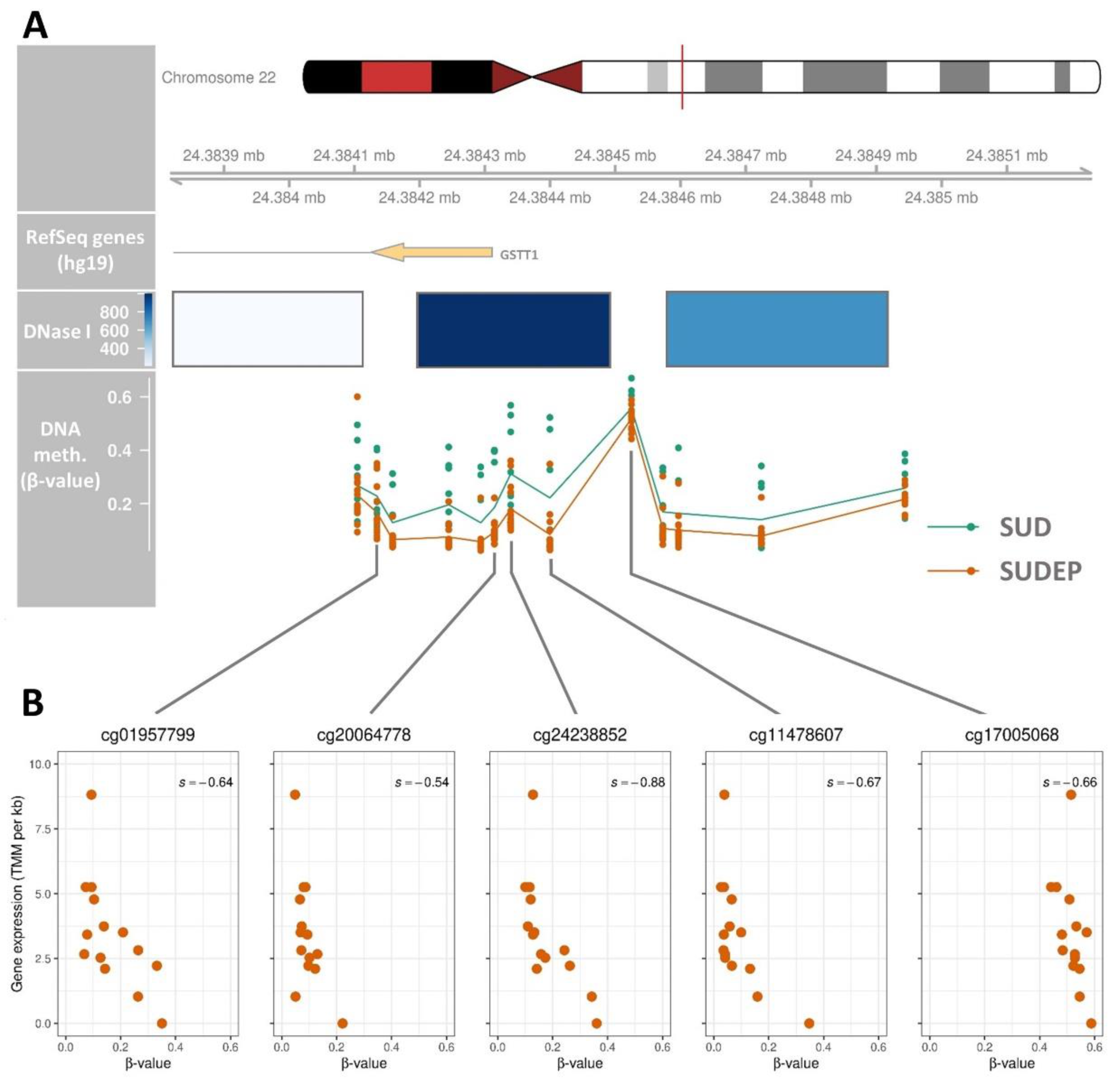
2.4. Regulatory Association of the GSTT1 DMR
2.5. Differentially Methylated Regions between Sudden Unexplained Death and Sudden Unexpected Death in Epilepsy
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Whole Genome Sequencing
4.3. Whole Transcriptome Sequencing
4.3.1. Preprocessing and Alignment of RNA Data
4.3.2. Normalisation of Read Counts
4.4. Quantification of DNA Methylation
4.5. Differentially Methylated Regions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skjelbred, T.; Lynge, T.H.; Nielsen, J.; Winkel, B.G.; Tfelt-Hansen, J. Symptoms and healthcare contact preceding sudden cardiac death in persons aged 1–49 years. Trends Cardiovasc. Med. 2020, 31, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Mellor, G.; Raju, H.; De Noronha, S.V.; Papadakis, M.; Sharma, S.; Behr, E.R.; Sheppard, M.N. Clinical characteristics and circumstances of death in the sudden arrhythmic death syndrome. Circ. Arrhythmia Electrophysiol. 2014, 7, 1078–1083. [Google Scholar] [CrossRef] [Green Version]
- Winkel, B.G.; Holst, A.G.; Theilade, J.; Kristensen, I.B.; Thomsen, J.L.; Ottesen, G.L.; Bundgaard, H.; Svendsen, J.H.; Haunsø, S.; Tfelt-Hansen, J. Nationwide study of sudden cardiac death in persons aged 1–35 years. Eur. Heart J. 2011, 32, 983–990. [Google Scholar] [CrossRef] [Green Version]
- Bagnall, R.D.; Weintraub, R.G.; Ingles, J.; Duflou, J.; Yeates, L.; Lam, L.; Davis, A.M.; Thompson, T.; Connell, V.; Wallace, J.; et al. A Prospective Study of Sudden Cardiac Death among Children and Young Adults. N. Engl. J. Med. 2016, 374, 2441–2452. [Google Scholar] [CrossRef]
- Risgaard, B.; Winkel, B.G.; Jabbari, R.; Behr, E.R.; Ingemann-Hansen, O.; Thomsen, J.L.; Ottesen, G.L.; Gislason, G.H.; Bundgaard, H.; Haunsø, S.; et al. Burden of Sudden Cardiac Death in Persons Aged 1 to 49 Years. Circ. Arrhythmia Electrophysiol. 2014, 7, 205–211. [Google Scholar] [CrossRef] [Green Version]
- Semsarian, C.; Ingles, J.; Wilde, A.A.M. Sudden cardiac death in the young: The molecular autopsy and a practical approach to surviving relatives. Eur. Heart J. 2015, 36, 1290–1296. [Google Scholar] [CrossRef]
- Basso, C.; Carturan, E.; Pilichou, K.; Rizzo, S.; Corrado, D.; Thiene, G. Sudden cardiac death with normal heart: Molecular autopsy. Cardiovasc. Pathol. 2010, 19, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Ackerman, M.J.; Antzelevitch, C.; Bezzina, C.R.; Borggrefe, M.; Cuneo, B.F.; Wilde, A.A.M. Inherited cardiac arrhythmias. Nat. Rev. Dis. Prim. 2020, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Lahrouchi, N.; Raju, H.; Lodder, E.M.; Papatheodorou, E.; Ware, J.S.; Papadakis, M.; Tadros, R.; Cole, D.; Skinner, J.R.; Crawford, J.; et al. Utility of Post-Mortem Genetic Testing in Cases of Sudden Arrhythmic Death Syndrome. J. Am. Coll. Cardiol. 2017, 69, 2134–2145. [Google Scholar] [CrossRef]
- Christiansen, S.L.; Hertz, C.L.; Ferrero-Miliani, L.; Dahl, M.; Weeke, P.E.; LuCamp; Ottesen, G.L.; Frank-Hansen, R.; Bundgaard, H.; Morling, N. Genetic investigation of 100 heart genes in sudden unexplained death victims in a forensic setting. Eur. J. Hum. Genet. 2016, 24, 1797–1802. [Google Scholar] [CrossRef]
- Neubauer, J.; Lecca, M.R.; Russo, G.; Bartsch, C.; Medeiros-Domingo, A.; Berger, W.; Haas, C. Exome analysis in 34 sudden unexplained death (SUD) victims mainly identified variants in channelopathy-associated genes. Int. J. Legal Med. 2018, 132, 1057–1065. [Google Scholar] [CrossRef]
- Meder, B.; Haas, J.; Sedaghat-Hamedani, F.; Kayvanpour, E.; Frese, K.; Lai, A.; Nietsch, R.; Scheiner, C.; Mester, S.; Bordalo, D.M.; et al. Epigenome-Wide Association Study Identifies Cardiac Gene Patterning and a Novel Class of Biomarkers for Heart Failure. Circulation 2017, 136, 1528–1544. [Google Scholar] [CrossRef] [PubMed]
- Grunert, M.; Dorn, C.; Cui, H.; Dunkel, I.; Schulz, K.; Schoenhals, S.; Sun, W.; Berger, F.; Chen, W.; Sperling, S.R. Comparative DNA methylation and gene expression analysis identifies novel genes for structural congenital heart diseases. Cardiovasc. Res. 2016, 112, 464–477. [Google Scholar] [CrossRef]
- Haas, J.; Frese, K.S.; Park, Y.J.; Keller, A.; Vogel, B.; Lindroth, A.M.; Weichenhan, D.; Franke, J.; Fischer, S.; Bauer, A.; et al. Alterations in cardiac DNA methylation in human dilated cardiomyopathy. EMBO Mol. Med. 2013, 5, 413–429. [Google Scholar] [CrossRef]
- Movassagh, M.; Choy, M.K.; Knowles, D.A.; Cordeddu, L.; Haider, S.; Down, T.; Siggens, L.; Vujic, A.; Simeoni, I.; Penkett, C.; et al. Distinct epigenomic features in end-stage failing human hearts. Circulation 2011, 124, 2411–2422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holst, A.G.; Winkel, B.G.; Risgaard, B.; Nielsen, J.B.; Rasmussen, P.V.; Haunsø, S.; Sabers, A.; Uldall, P.; Tfelt-Hansen, J. Epilepsy and risk of death and sudden unexpected death in the young: A nationwide study. Epilepsia 2013, 54, 1613–1620. [Google Scholar] [CrossRef]
- Risgaard, B.; Waagstein, K.; Winkel, B.G.; Jabbari, R.; Lynge, T.H.; Glinge, C.; Albert, C.; Correll, C.U.; Hauns, S.; Fink-Jensen, A.; et al. Sudden cardiac death in young adults with previous hospital-based psychiatric inpatient and outpatient treatment: A nationwide cohort study from Denmark. J. Clin. Psychiatry 2015, 76, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Lynge, T.H.; Svane, J.; Pedersen-Bjergaard, U.; Gislason, G.; Torp-Pedersen, C.; Banner, J.; Risgaard, B.; Winkel, B.G.; Tfelt-Hansen, J. Sudden cardiac death among persons with diabetes aged 1-49 years: A 10-year nationwide study of 14 294 deaths in Denmark. Eur. Heart J. 2020, 41, 2699–2706. [Google Scholar] [CrossRef]
- Thurman, D.J.; Logroscino, G.; Beghi, E.; Hauser, W.A.; Hesdorffer, D.C.; Newton, C.R.; Scorza, F.A.; Sander, J.W.; Tomson, T. The burden of premature mortality of epilepsy in high-income countries: A systematic review from the Mortality Task Force of the International League against Epilepsy. Epilepsia 2017, 58, 17–26. [Google Scholar] [CrossRef]
- Shorvon, S.; Tomson, T. Sudden unexpected death in epilepsy. Lancet 2011, 378, 2028–2038. [Google Scholar] [CrossRef]
- Ali, A.; Wu, S.; Issa, N.P.; Rose, S.; Towle, V.L.; Warnke, P.; Tao, J.X. Association of sleep with sudden unexpected death in epilepsy. Epilepsy Behav. 2017, 76, 1–6. [Google Scholar] [CrossRef]
- Chahal, C.A.A.; Salloum, M.N.; Alahdab, F.; Gottwald, J.A.; Tester, D.J.; Anwer, L.A.; So, E.L.; Murad, M.H.; St Louis, E.K.; Ackerman, M.J.; et al. Systematic Review of the Genetics of Sudden Unexpected Death in Epilepsy: Potential Overlap With Sudden Cardiac Death and Arrhythmia-Related Genes. J. Am. Heart Assoc. 2020, 9, e012264. [Google Scholar] [CrossRef] [PubMed]
- Bagnall, R.D.; Singer, E.S.; Tfelt-Hansen, J. Sudden Cardiac Death in the Young. Hear. Lung Circ. 2020, 29, 498–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeGiorgio, C.M.; Markovic, D.; Mazumder, R.; Moseley, B.D. Ranking the leading risk factors for sudden unexpected death in epilepsy. Front. Neurol. 2017, 8, 473. [Google Scholar] [CrossRef]
- Devinsky, O.; Hesdorffer, D.C.; Thurman, D.J.; Lhatoo, S.; Richerson, G. Sudden unexpected death in epilepsy: Epidemiology, mechanisms, and prevention. Lancet Neurol. 2016, 15, 1075–1088. [Google Scholar] [CrossRef]
- Manolis, T.A.; Manolis, A.A.; Melita, H.; Manolis, A.S. Sudden unexpected death in epilepsy: The neuro-cardio-respiratory connection. Seizure 2019, 64, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.N.; Hofman, N.; Haglund, C.M.; Cascino, G.D.; Wilde, A.A.M.; Ackerman, M.J. Identification of a possible pathogenic link between congenital long QT syndrome and epilepsy. Neurology 2009, 72, 224–231. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.H.; Bos, J.M.; Cascino, G.D.; Ackerman, M.J. Prevalence and spectrum of electroencephalogram-identified epileptiform activity among patients with long QT syndrome. Hear. Rhythm 2014, 11, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.M.; Glasscock, E.; Yoo, J.; Chen, T.T.; Klassen, T.L.; Noebels, J.L. Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained. Sci. Transl. Med. 2009, 2, 2ra6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagnall, R.D.; Crompton, D.E.; Petrovski, S.; Lam, L.; Cutmore, C.; Garry, S.I.; Sadleir, L.G.; Dibbens, L.M.; Cairns, A.; Kivity, S.; et al. Exome-based analysis of cardiac arrhythmia, respiratory control, and epilepsy genes in sudden unexpected death in epilepsy. Ann. Neurol. 2016, 79, 522–534. [Google Scholar] [CrossRef]
- Bagnall, R.D.; Ingles, J.; Yeates, L.; Berkovic, S.F.; Semsarian, C. Exome sequencing–based molecular autopsy of formalin-fixed paraffin-embedded tissue after sudden death. Genet. Med. 2017, 19, 1127–1133. [Google Scholar] [CrossRef] [Green Version]
- Tu, E.; Bagnall, R.D.; Duflou, J.; Semsarian, C. Post-mortem review and genetic analysis of sudden unexpected death in epilepsy (SUDEP) cases. Brain Pathol. 2011, 21, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Coll, M.; Allegue, C.; Partemi, S.; Mates, J.; Del Olmo, B.; Campuzano, O.; Pascali, V.; Iglesias, A.; Striano, P.; Oliva, A.; et al. Genetic investigation of sudden unexpected death in epilepsy cohort by panel target resequencing. Int. J. Legal Med. 2016, 130, 331–339. [Google Scholar] [CrossRef]
- Aurlien, D.; Leren, T.P.; Taubøll, E.; Gjerstad, L. New SCN5A mutation in a SUDEP victim with idiopathic epilepsy. Seizure 2009, 18, 158–160. [Google Scholar] [CrossRef] [Green Version]
- Jones, W.; Greytak, S.; Odeh, H.; Guan, P.; Powers, J.; Bavarva, J.; Moore, H.M. Deleterious effects of formalin-fixation and delays to fixation on RNA and miRNA-Seq profiles. Sci. Rep. 2019, 9, 6980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, M.T.P.; Sanchez, J.J.; Haselkorn, T.; Jewell, L.D.; Lucas, S.B.; Van Marck, E.; Børsting, C.; Morling, N.; Worobey, M. Multiplex PCR with minisequencing as an effective high-throughput SNP typing method for formalin-fixed tissue. Electrophoresis 2007, 28, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Do, H.; Dobrovic, A. Sequence artifacts in DNA from formalin-fixed tissues: Causes and strategies for minimization. Clin. Chem. 2015, 61, 64–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, T.J.; Buckley, M.J.; Statham, A.L.; Pidsley, R.; Samaras, K.; V Lord, R.; Clark, S.J.; Molloy, P.L. De novo identification of differentially methylated regions in the human genome. Epigenetics Chromatin 2015, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Bagnall, R.D.; Semsarian, C. Role of the molecular autopsy in the investigation of sudden cardiac death. Prog. Pediatr. Cardiol. 2017, 45, 17–23. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Di Ilio, C.; Federici, L. Glutathione transferases: Substrates, inihibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis 2018, 7, 8. [Google Scholar] [CrossRef]
- Haas, J.; Mester, S.; Lai, A.; Frese, K.S.; Sedaghat-Hamedani, F.; Kayvanpour, E.; Rausch, T.; Nietsch, R.; Boeckel, J.; Carstensen, A.; et al. Genomic structural variations lead to dysregulation of important coding and non-coding RNA species in dilated cardiomyopathy. EMBO Mol. Med. 2018, 10, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Shanks, G.W.; Tester, D.J.; Ackerman, J.P.; Simpson, M.A.; Behr, E.R.; White, S.M.; Ackerman, M.J. Importance of Variant Interpretation in Whole-Exome Molecular Autopsy. Circulation 2018, 137, 2705–2715. [Google Scholar] [CrossRef]
- Dulhunty, A.F.; Hewawasam, R.; Liu, D.; Casarotto, M.G.; Board, P.G. Regulation of the cardiac muscle ryanodine receptor by glutathione transferases. Drug Metab. Rev. 2011, 43, 236–252. [Google Scholar] [CrossRef]
- Glasscock, E. Genomic biomarkers of SUDEP in brain and heart. Epilepsy Behav. 2014, 38, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Lehnart, S.E.; Mongillo, M.; Bellinger, A.; Lindegger, N.; Chen, B.-X.; Hsueh, W.; Reiken, S.; Wronska, A.; Drew, L.J.; Ward, C.W.; et al. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J. Clin. Invest. 2008, 118, 2230–2245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabha, T.S.; Kumaraswami, K.; Kutala, V.K. Association of GSTT1 and GSTM1 polymorphisms in South Indian epilepsy patients. Indian J. Exp. Biol. 2016, 54, 783–787. [Google Scholar] [PubMed]
- Ercegovac, M.; Jovic, N.; Sokic, D.; Savic-Radojevic, A.; Coric, V.; Radic, T.; Nikolic, D.; Kecmanovic, M.; Matic, M.; Simic, T.; et al. GSTA1, GSTM1, GSTP1 and GSTT1 polymorphisms in progressive myoclonus epilepsy: A Serbian case-control study. Seizure 2015, 32, 30–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson-Smith, J.N.; Patel, M. Metabolic dysfunction and oxidative stress in epilepsy. Int. J. Mol. Sci. 2017, 18, 2365. [Google Scholar] [CrossRef] [Green Version]
- Coll, M.; Oliva, A.; Grassi, S.; Brugada, R.; Campuzano, O. Update on the genetic basis of sudden unexpected death in epilepsy. Int. J. Mol. Sci. 2019, 20, 1979. [Google Scholar] [CrossRef] [Green Version]
- Nashef, L.; Walker, F.; Allen, P.; Sander, J.W.A.S.; Shorvon, S.D.; Fish, D.R. Apnoea and bradycardia during epileptic seizures: Relation to sudden death in epilepsy. J. Neurol. Neurosurg. Psychiatry 1996, 60, 297–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryvlin, P.; Nashef, L.; Lhatoo, S.D.; Bateman, L.M.; Bird, J.; Bleasel, A.; Boon, P.; Crespel, A.; Dworetzky, B.A.; Høgenhaven, H.; et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): A retrospective study. Lancet Neurol. 2013, 12, 966–977. [Google Scholar] [CrossRef]
- Stewart, M.; Silverman, J.B.; Sundaram, K.; Kollmar, R. Causes and Effects Contributing to Sudden Death in Epilepsy and the Rationale for Prevention and Intervention. Front. Neurol. 2020, 11, 765. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Mason-Suares, H.; Brockman, D.; Wang, M.; VanDenburgh, M.J.; Senol-Cosar, O.; Patterson, C.; Newton-Cheh, C.; Zekavat, S.M.; Pester, J.; et al. Rare Genetic Variants Associated With Sudden Cardiac Death in Adults. J. Am. Coll. Cardiol. 2019, 74, 2623–2634. [Google Scholar] [CrossRef]
- Robinson, M.D.; Kahraman, A.; Law, C.W.; Lindsay, H.; Nowicka, M.; Weber, L.M.; Zhou, X. Statistical methods for detecting differentially methylated loci and regions. Front. Genet. 2014, 5, 234. [Google Scholar] [CrossRef] [Green Version]
- Teschendorff, A.E.; Relton, C.L. Statistical and integrative system-level analysis of DNA methylation data. Nat. Rev. Genet. 2018, 19, 129–147. [Google Scholar] [CrossRef]
- Kling, T.; Wenger, A.; Beck, S.; Carén, H. Validation of the MethylationEPIC BeadChip for fresh-frozen and formalin-fixed paraffin-embedded tumours. Clin. Epigenetics 2017, 9, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, G.B.; Hager, H.; Hansen, L.L.; Tost, J. Improved reproducibility in genome-wide DNA methylation analysis for PAXgene-fixed samples compared with restored formalin-fixed and paraffin-embedded DNA. Anal. Biochem. 2015, 468, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Trudsø, L.C.; Andersen, J.D.; Jacobsen, S.B.; Christiansen, S.L.; Congost-Teixidor, C.; Kampmann, M.; Morling, N. A comparative study of single nucleotide variant detection performance using three massively parallel sequencing methods. PLoS ONE 2020, 15, e0239850. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. Available online: https://www.r-project.org/ (accessed on 5 March 2021).
- Andrews, S. FastQC—A quality control tool for high throughput sequence data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 5 March 2021).
- Lindgreen, S. AdapterRemoval: Easy cleaning of next-generation sequencing reads. BMC Res. Notes 2012, 17, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Harrow, J.; Frankish, A.; Gonzalez, J.M.; Tapanari, E.; Diekhans, M.; Kokocinski, F.; Aken, B.L.; Barrell, D.; Zadissa, A.; Searle, S.; et al. GENCODE: The reference human genome annotation for the ENCODE project. Genome Res. 2012, 22, 1760–1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, J.D.; Jacobsen, S.B.; Trudsø, L.C.; Kampmann, M.-L.; Banner, J.; Morling, N. Whole genome and transcriptome sequencing of post-mortem cardiac tissues from sudden cardiac death victims identifies a gene regulatory variant in NEXN. Int. J. Legal Med. 2019, 133, 1699–1709. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2009, 26, 139–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortin, J.P.; Triche, T.J.; Hansen, K.D. Preprocessing, normalization and integration of the Illumina HumanMethylationEPIC array with minfi. Bioinformatics 2017, 33, 558–560. [Google Scholar] [CrossRef]
- Aryee, M.J.; Jaffe, A.E.; Corrada-Bravo, H.; Ladd-Acosta, C.; Feinberg, A.P.; Hansen, K.D.; Irizarry, R.A. Minfi: A flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 2014, 30, 1363–1369. [Google Scholar] [CrossRef] [Green Version]
- Hicks, S.C.; Irizarry, R.A. quantro: A data-driven approach to guide the choice of an appropriate normalization method. Genome Biol. 2015, 16, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touleimat, N.; Tost, J. Complete pipeline for Infinium® Human Methylation 450K BeadChip data processing using subset quantile normalization for accurate DNA methylation estimation. Epigenomics 2012, 4, 325–341. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Cook, D.; Lawrence, M. Plyranges: A grammar of genomic data transformation. Genome Biol. 2019, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.; Huber, W.; Pagès, H.; Aboyoun, P.; Carlson, M.; Gentleman, R.; Morgan, M.T.; Carey, V.J. Software for Computing and Annotating Genomic Ranges. PLoS Comput. Biol. 2013, 9, e1003118. [Google Scholar] [CrossRef] [PubMed]
- Hahne, F.; Ivanek, R. Visualizing genomic data using Gviz and bioconductor. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2016; Volume 1418, pp. 335–351. ISBN 978-1-4939-3578-9. [Google Scholar]
- ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57–74. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | SUD (n = 9) | SUDEP (n = 14) | p-Value |
|---|---|---|---|
| Males, n (%) | 6 (67) | 10 (71) | 1.000 |
| Median age at time of death, years (range) | 35 (0–50) | 35 (3–49) | 0.850 |
| Witnessed death, n (%) | 2 (22) | 1 (7) | 0.538 |
| Performed toxicology screen, n (%) | 6 (67) | 11 (79) | 0.643 |
| No compounds detected, n (%) | 2 (33) | 2 (18) | 0.584 |
| One compound detected, n (%) | 2 (33) | 5 (46) | 1.000 |
| More than one compound detected, n (%) | 2 (33) | 4 (36) | 1.000 |
| Antiepileptic drug(s) detected, n (%) | 0 (0) | 8 (73) | 0.009 |
| Activity at time of death | |||
| Sleep, n (%) | 2 (22) | 5 (36) | 0.657 |
| At rest, n (%) | 3 (33) | 3 (21) | 0.643 |
| Passenger in car, n (%) | 1 (11) | 0 (0) | 0.391 |
| Unknown, n (%) | 3 (33) | 6 (43) | 1.000 |
| Chr | Start | End | Size | CpG | Min. FDR | Δβ | Nearest Gene | Distance |
|---|---|---|---|---|---|---|---|---|
| chr22 | 24,384,105 | 24,384,944 | 840 | 13 | 3.81×10–23 | 0.15 | GSTT1 | 0 |
| chr1 | 221,053,841 | 221,054,825 | 985 | 10 | 6.04×10–06 | −0.12 | HLX | 0 |
| chr1 | 110,230,252 | 110,230,633 | 382 | 7 | 1.12×10–05 | −0.14 | GSTM1 | 0 |
| chr22 | 24,373,322 | 24,373,618 | 297 | 3 | 1.17×10–04 | 0.19 | LOC391322 | 0 |
| chr11 | 5,959,658 | 5,959,945 | 288 | 3 | 4.61×10–04 | 0.11 | TRIM5 | 0 |
| chr22 | 24,348,549 | 24,348,715 | 167 | 3 | 6.15×10–04 | −0.16 | GSTT4 | 1290 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christiansen, S.N.; Jacobsen, S.B.; Andersen, J.D.; Kampmann, M.-L.; Trudsø, L.C.; Olsen, K.B.; Tfelt-Hansen, J.; Banner, J.; Morling, N. Differential Methylation in the GSTT1 Regulatory Region in Sudden Unexplained Death and Sudden Unexpected Death in Epilepsy. Int. J. Mol. Sci. 2021, 22, 2790. https://doi.org/10.3390/ijms22062790
Christiansen SN, Jacobsen SB, Andersen JD, Kampmann M-L, Trudsø LC, Olsen KB, Tfelt-Hansen J, Banner J, Morling N. Differential Methylation in the GSTT1 Regulatory Region in Sudden Unexplained Death and Sudden Unexpected Death in Epilepsy. International Journal of Molecular Sciences. 2021; 22(6):2790. https://doi.org/10.3390/ijms22062790
Chicago/Turabian StyleChristiansen, Steffan Noe, Stine Bøttcher Jacobsen, Jeppe Dyrberg Andersen, Marie-Louise Kampmann, Linea Christine Trudsø, Kristine Boisen Olsen, Jacob Tfelt-Hansen, Jytte Banner, and Niels Morling. 2021. "Differential Methylation in the GSTT1 Regulatory Region in Sudden Unexplained Death and Sudden Unexpected Death in Epilepsy" International Journal of Molecular Sciences 22, no. 6: 2790. https://doi.org/10.3390/ijms22062790
APA StyleChristiansen, S. N., Jacobsen, S. B., Andersen, J. D., Kampmann, M. -L., Trudsø, L. C., Olsen, K. B., Tfelt-Hansen, J., Banner, J., & Morling, N. (2021). Differential Methylation in the GSTT1 Regulatory Region in Sudden Unexplained Death and Sudden Unexpected Death in Epilepsy. International Journal of Molecular Sciences, 22(6), 2790. https://doi.org/10.3390/ijms22062790







