Abstract
As most recently demonstrated by the SARS-CoV-2 pandemic, congenital and perinatal infections are of significant concern to the pregnant population as compared to the general population. These outcomes can range from no apparent impact all the way to spontaneous abortion or fetal infection with long term developmental consequences. While some pathogens have developed mechanisms to cross the placenta and directly infect the fetus, other pathogens lead to an upregulation in maternal or placental inflammation that can indirectly cause harm. The placenta is a temporary, yet critical organ that serves multiple important functions during gestation including facilitation of fetal nutrition, oxygenation, and prevention of fetal infection in utero. Here, we review trophoblast cell immunology and the molecular mechanisms utilized to protect the fetus from infection. Lastly, we discuss consequences in the placenta when these protections fail and the histopathologic result following infection.
1. Introduction
Infections account for approximately 2 to 3% of all congenital anomalies [1]. The placenta is a dynamic organ which is necessary for creating a protective barrier that keeps pathogens out. The complex interplay of the physiologic pathways within the placenta, it’s multifaceted role in both prevention of fetal rejection by the maternal immune system and protection from transmission of infections to the fetus is worth investigating, especially as risks of pandemics increase. Maternal immune responses that occur at the maternal-fetal interface are critical for the acquisition or protection from congenital or perinatal infections.
In this review, the authors provide an overview of the cellular immunopathogenesis of the placental response towards invading infections. We discuss the molecular pathways involved and corresponding placental histopathologies with a specific focus on viral infections.
2. Role of the Placenta in Pregnancy Related Immune Responses
2.1. Mechanisms of Maternal-Fetal Tolerance
It was Peter Medawar in 1953 who first suggested that a pregnant woman’s immune system becomes inactive to tolerate a semi-allogenic fetus [2]. However, new research has uncovered that adaptation, not suppression, of the maternal immune system occurs during pregnancy. Maternal immune responses are dynamic, with the first and third trimester requiring a pro-inflammatory state for implantation and parturition to occur [3,4]. These responses are tightly regulated by signals originating from trophoblast cells of the placenta. There are three types of trophoblast cells which originate from the trophectoderm layer of the blastocyst: extravillous trophoblasts (EVTs), cytotrophoblasts (CTBs) and syncytiotrophoblasts (SYNs). EVTs invade into the maternal decidua and myometrium, promoting spiral artery remodeling which allows for nutrient transport to the fetus [5]. CTBs are mononuclear cells which make up the placental villi and proliferate in response to human chorionic gonadotrophin (hCG) [6]. Lastly, CTBs can fuse to from SYNs, the main barrier between the maternal immune system and the fetal placenta, which are critical for modulating maternal immunity and protecting the fetus from pathogens [7]. Specific mechanisms that trophoblast cells utilize to modulate maternal immunity will be further described in the next three sections.
2.2. Innate Immune Responses by Trophoblast Cells
The first line of control against a foreign cell or pathogen is the innate immune system. This response is non-specific and is driven by placental architecture and recognition of unique molecular patterns not typically seen in eukaryotes. The structure of the placenta makes up the strongest protective barrier through the SYNs, which form large, multi-nucleated cells that lack cell junctions commonly used by pathogens to get into tissues. Additionally, SYN cells have a dense cytoskeletal system which is highly effective at preventing transmission of cells and pathogens from the mother to the fetus, as demonstrated by Listeria monocytogenes infection [8]. Next, trophoblast cells can recognize foreign cells and pathogens through Toll-like receptors (TLR) and Retinoic acid-inducible gene I (RIG-I) receptors. TLRs1-10 are expressed on the placenta and recognize viruses (TLRs 3, 7 and 8) bacteria (TLRs 2, 9), and endogenous signals that indicate cell stress (TLR4) [9]. It has been demonstrated that TLR7/8 are increased in trophoblast cells exposed to hepatitis B virus (HBV), preventing maternal-fetal transmission; however, when downstream signaling through MyD88 is blocked, HBV can translocate across the placenta [10]. RIG-I, a cytoplasmic protein, is also expressed in trophoblasts and can recognize both RNA and DNA viruses leading to a conformational change, oligomerization with mitochondrial antiviral signaling proteins (MAVS) and activates transcription of NF-kB [11,12,13]. Others have demonstrated that RIG-I levels are increased upon Cytomegalovirus (CMV) and Herpes simplex virus 1 (HSV-1) infection [14,15]. Signaling though TLR and RIG-I leads to the production of type I interferons (IFN-α and IFN-β) which induce host anti-viral activity through the activation of both innate and adaptive immune responses.
2.3. Cell Mediated Immunologic Responses of Trophoblast Cells
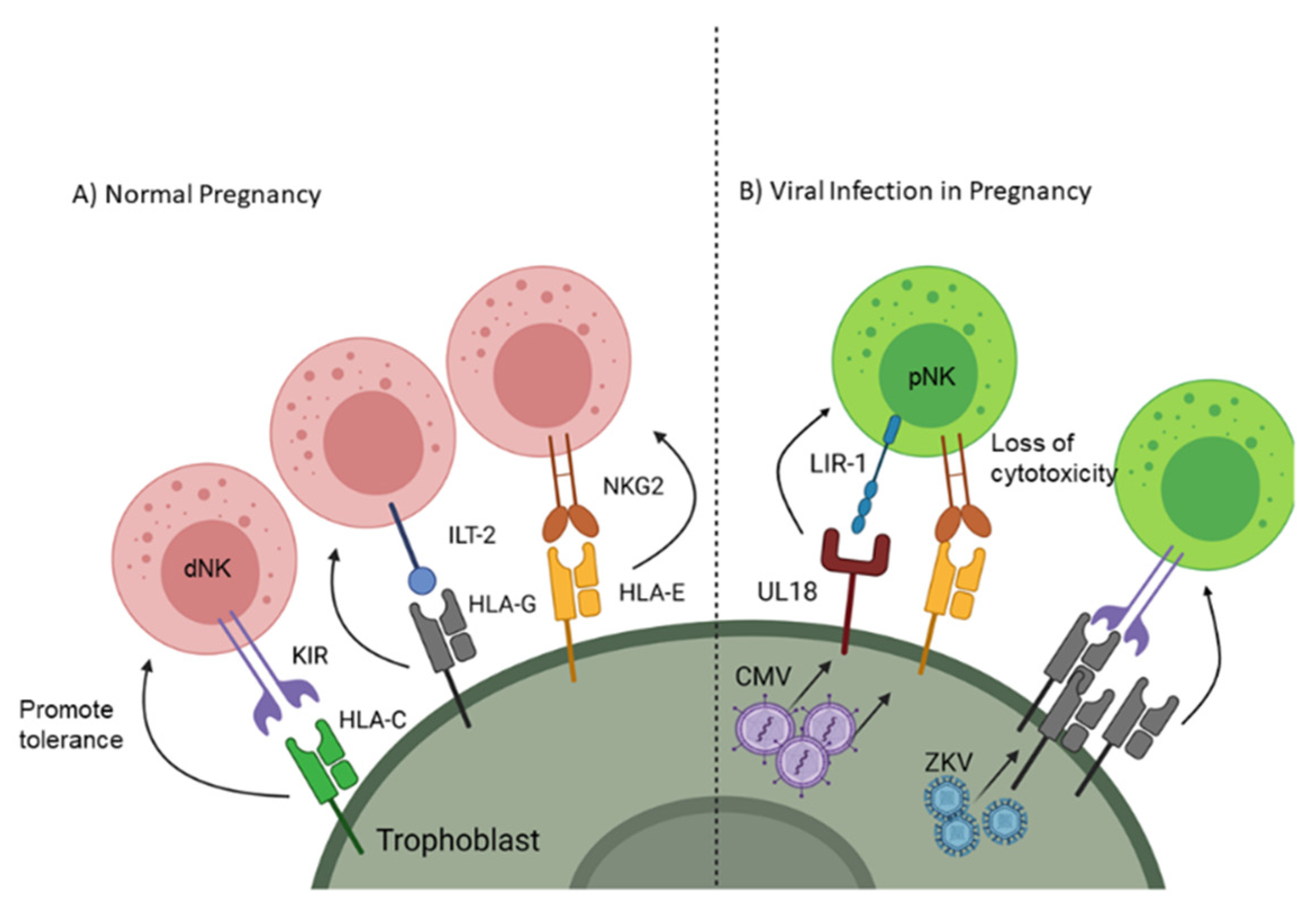
Adaptive immunity is a targeted response to a foreign cell or pathogen and takes a much longer time to generate an effective response compared to the innate response; however, the adaptive immune system generates an antigen-specific memory response so if that same antigen enters the body again it will quickly be destroyed. One component of adaptive immunity is cell mediated immunity which depends on phagocytes presenting foreign antigen to lymphocytes through major histocompatibility complex (MHC) class I and class II. As a fetus gets half of its genetic material from the father, presentation of paternal/fetal antigen would normally induce a specific response against the antigens of future offspring. Therefore, instead of expressing traditional MHC receptors, EVTs express non-classical MHC, including human leukocyte antigen (HLA)-G and -E [16,17], which are capable of processing and presenting foreign antigens through the TAP signaling complex [18] but instead maintain fetal-maternal tolerance [19]. Natural Killer (NK) cells make up a significant percentage of the leukocytes at the maternal-fetal interface; however, unlike peripheral NK cells (pNK) which have a cytotoxic phenotype, decidual NK cells (dNK) take on a tolerogenic phenotype (Figure 1A) [20]. dNK cells acquire this immunomodulatory function through signaling between natural killer group 2 receptor (NKG2) [21] and HLA-E as well as immunoglobulin-like transcript 2 receptor (ILT-2) with HLA-G [22]. EVTs further influence tolerance by blunting antigen-specific T cell cytotoxicity and promoting the differentiation of naive T cells towards a regulatory phenotype [19,23]. Additionally, EVTs do express class I HLA-C, which can influence NK cell phenotypes by restricting anti-fetal responses through killer cell Ig-like receptors (KIR) [24]. Trophoblast cells also express costimulatory receptors such as program death ligand I (PD-L1) and galectin-9, which interact with program death 1 (PD-1) and T cell immunoglobulin mucin 3 (Tim-3), respectively, to blunt T or NK cell activation [25,26,27]. Together, these cellular mechanisms ensure tolerance to the allogeneic fetus locally while systemically maintaining maternal defense against pathogens.
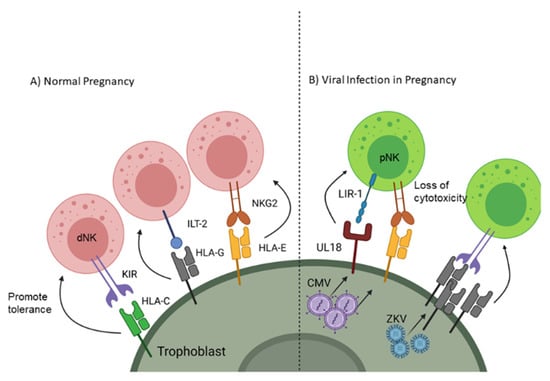
Figure 1.
Receptor signaling on trophoblast cells to promote natural killer (NK) cell tolerance to the fetus and viruses. (A) Non-classical MHC interactions with NK cells promotes decidual (d)NK phenotype and tolerance. (B) Overexpression of non-classical MHC and upregulation of decoy receptors are used by viruses (CMV and Zika virus (ZKV)) to blunt peripheral (p)NK cytotoxicity. Created with BioRender.com. Accessed on 6 February 2021
2.4. Humoral Mediated Immunologic Response in the Placenta
Another component of adaptive immunity is the humoral response, which is driven by the production of antibodies from activated B cells called plasma cells. These antibodies will recognize and bind to foreign pathogens, leading to their elimination through complement activation, neutralization, and enhancement of phagocytosis (opsonin). In the case of intracellular pathogens, antibodies binding to critical surface proteins, including virus spike proteins, may directly block cell entry. During pregnancy, passive immunity is acquired by the fetus from the mother through the transplacental passage of maternal IgG antibodies [28]. Transfer of these protective antibodies is mediated through Fc-receptors, specifically FcRn, on the SYN cells, where IgG within endosomes travel through the interstitial space be released into the fetal circulation [29,30,31]. Interestingly, maternal-fetal transfer of IgG is decreased during maternal infection with HIV and malaria [32,33]. IgG can also activate the complement system, leading to enhanced phagocytosis and protection from infection. Trophoblast cells express complement proteins, including C1q, C4d, CD46, CD55 and CD59 [34,35]. While complement activity is tightly regulated to prevent placental injury, in the case of infection the process of targeting the pathogen leads to inflammation, endothelial activation and coagulopathy which can harm the fetus [36]. Together, these regulatory processes in the placenta create a formidable blockade that protects the fetus from infection while facilitating maternal tolerance of the fetal hemiallograft; however, some pathogens have found unique mechanisms to exploit these placental safeguards which we cover in the next few sections.
3. Mechanisms of Viral Infection of the Placenta
Viral pathogens that reach the placenta do so via hematogenous spread, requiring that significant maternal viremia be present for placental infection to occur. The requirement for significant maternal viremia explains why some localized secondary infections, including shingles or recurrent HSV infections are unlikely to cause placental and fetal infections. As the SYN barrier directly contacts maternal blood as it bathes terminal villi, viral infection of the SYN itself is a usual precursor to transmission of infection to the fetal blood and downstream organs. While many viruses, including HIV, CMV, and SARS-CoV-2 directly infect the placenta by binding to viral receptors on the maternal aspect of SYNs, others may use antibody- dependent enhancement (ADE) to cross the SYN barrier. Viral replication in any cell is imperfect, resulting in newly synthesized viral proteins and nucleic acids being present throughout the infected cell and presented on the cell surface. Newly translated viral peptides are also processed for cell-surface antigen presentation in the context of MHC class I and II, along with the inhibitory HLA-E and HLA-G on trophoblasts. Members of the HSV family, and CMV, employ complex strategies for immune evasion. During infection, CMV engages mechanisms to evade the innate, cell-mediated, and humoral arms of the host immune response. In the setting of placental infection, evasive strategies by CMV, Zika virus, and other placental pathogens are synergistic with pre-existing adaptations of the maternal and fetal immune responses in the placenta, allowing for robust viral replication in this immunologically protected organ.
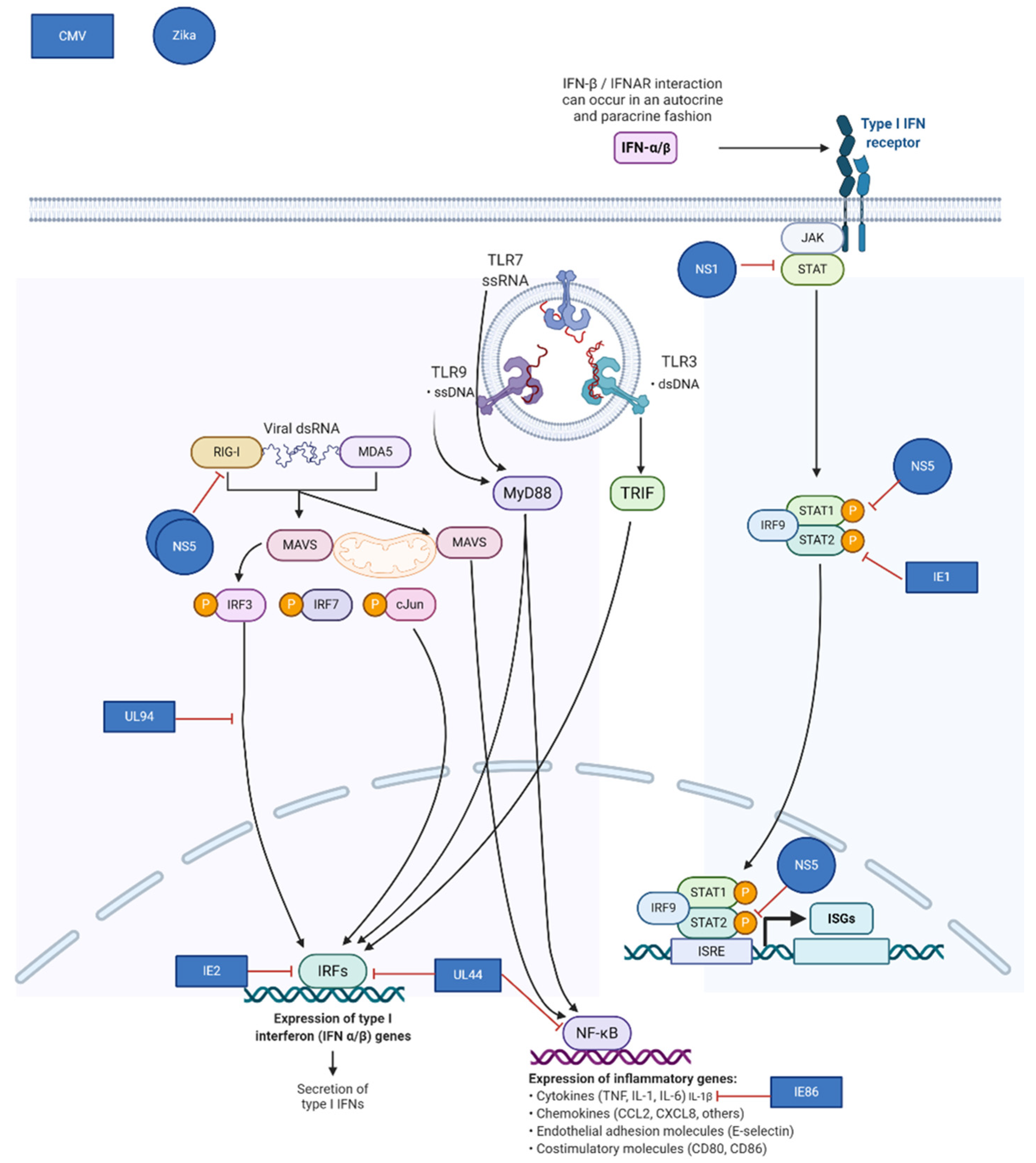
Virus-encoded mechanisms for evasion frequently target components of the type I interferon pathway. Congenital pathogens known to down-regulate this pathway include: Zika Virus, HIV, CMV, SARS-CoV-2, and others. Normally, infection with these viruses induces transcription of pro-inflammatory cytokines and type I interferons, resulting in the downstream establishment of an antiviral state in the infected cell by inducing transcription of hundreds of interferon-stimulated genes (ISGs). The ability of the placenta to mount an innate immune response is more robust at later times during gestation, and viruses may thus be more successful at productively infecting the placenta during early gestation [37,38]. CMV, Zika, and many other viruses employ multiple mechanisms to exploit the vulnerability of the placenta. Zika virus NS5 inhibits type I interferon receptor signaling and blocks induction of type I interferon via the RIG-I pathway [39,40,41]. (Figure 2) Like Zika and other flaviviruses, CMV uses multiple strategies to impair the innate host response to infection. CMV IE1, which is produced immediately upon infection, binds to and sequesters STAT2, preventing upregulation of ISGs [42].
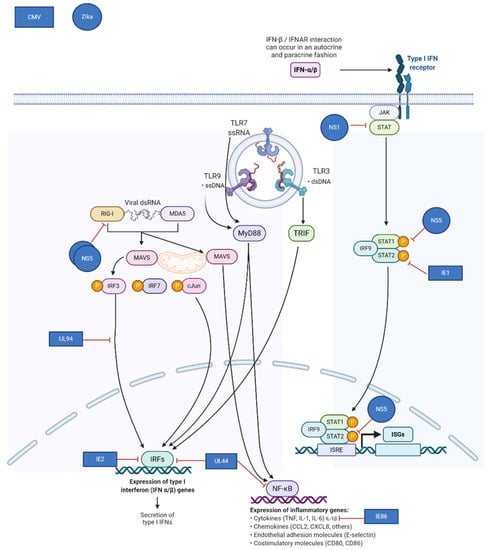
Figure 2.
Mechanisms of viral immune evasion in the placenta utilized by cytomegalovirus (CMV) and Zika virus. Adapted from “TLR Signaling Pathway”, by BioRender.com (Accessed on 6 February 2021). Retrieved from https://app.biorender.com/biorender-templates (Accessed on 6 February 2021).
Further downstream, CMV IE86 inhibits production of the pro-inflammatory cytokine IL-1β [43]. Other CMV gene products involved in inhibition of the innate response include UL44, IE2, and UL94 [42,44,45]. Both CMV and Zika virus, model congenital pathogens, target the host interferon response while causing robust infections during early gestation when innate immunity in the placenta is less effective.
Evasion of cell-mediated immunity is also known to occur during CMV and other herpesvirus infection, and viral inhibitory mechanisms are additive with changes already present in SYNs. In addition to the inherent SYN resistance to NK cell recognition conferred by cell-surface display of HLA-E, CMV encodes additional inhibitory molecules targeting antigen presentation and NK cell recognition. Mechanisms include downregulation of NK cell activation receptors and classical MHC I complexes, upregulation of HLA-E production, and expression of a virus-encoded inhibitory MHC decoy (UL18) [46]. Paradoxically, Zika virus upregulates expression of MHC class I in many cell types (Figure 1B). This is thought to decrease NK cell recognition and killing of infected cells but does not have the additional T cell inhibitory effects of HLA-E expression as seen with CMV infection [47].
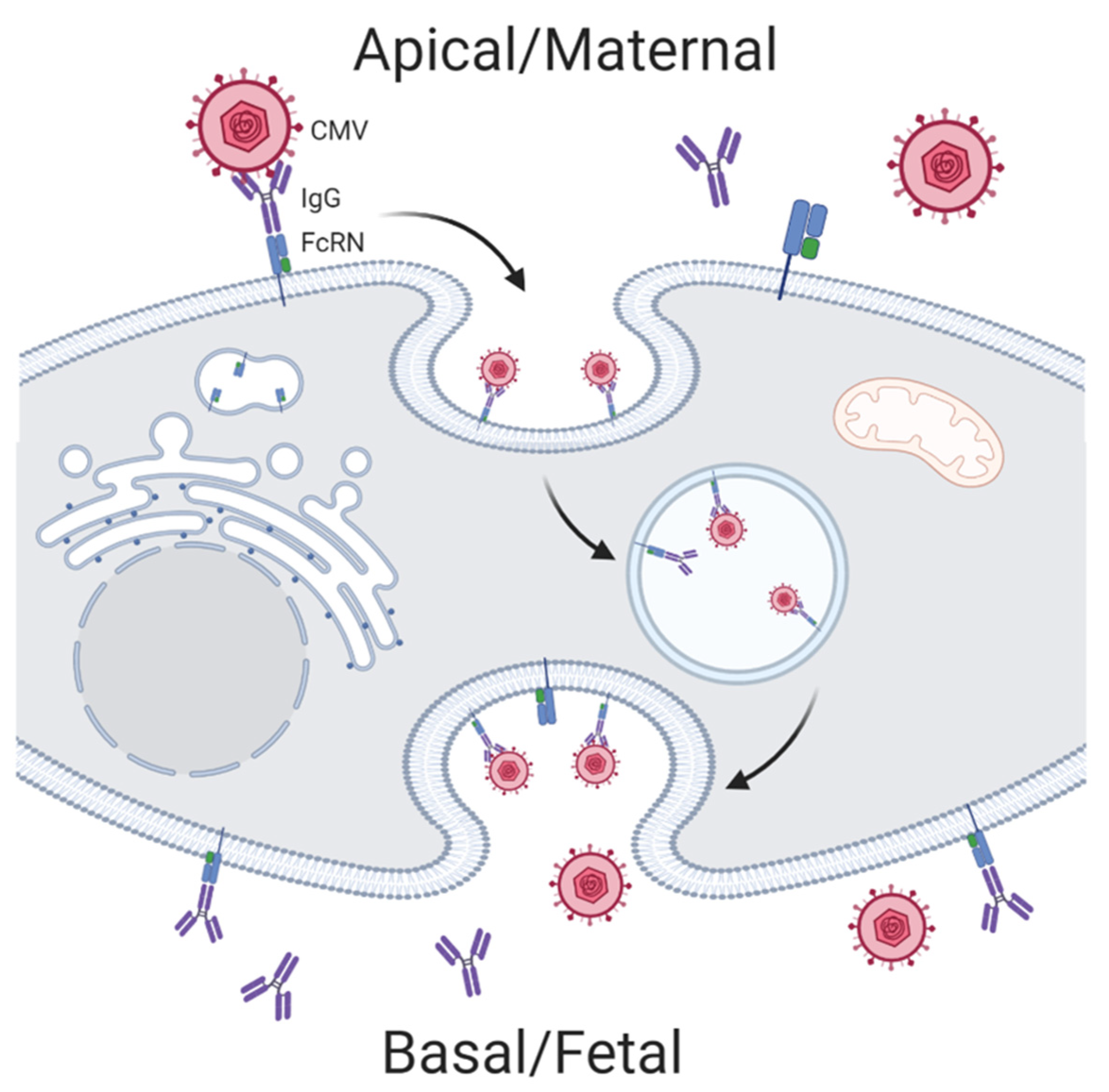
Viral pathogens have fewer known mechanisms of evading humoral immunity. Interestingly, the unique maternal-fetal IgG transport pathway of the trophoblast makes the placenta and fetus susceptible to some viral infections. Antibody-dependent enhancement (ADE) allows for low-affinity virus-specific IgG to facilitate transport of Zika virus across the cell membrane via FcR-mediated endocytosis. ADE is thought to be one mechanism by which Zika virus efficiently infects the placenta in women with previous Dengue virus infections (Figure 3) [39]. Both HIV and CMV have also been shown to use ADE to traverse the trophoblast during early infection when low-avidity IgG antibodies predominate [48,49,50].
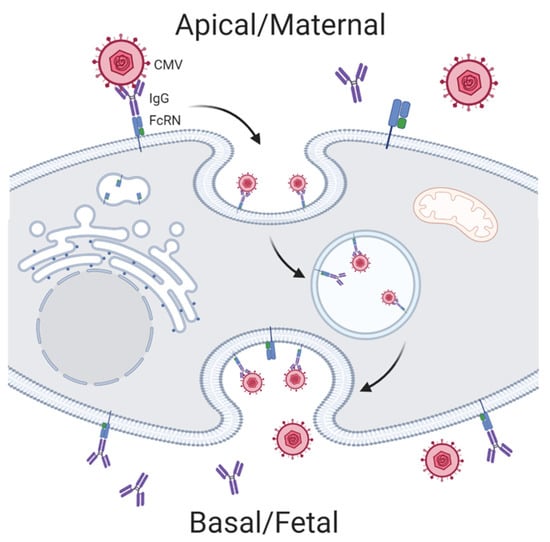
Figure 3.
Antibody-dependent enhancement mechanisms hijacked by cytomegalovirus (CMV) for mother to child transmission. Created with BioRender.com.
4. Clinical Pathophysiology of Specific Infections in Pregnancy
Several physiologic changes occur in pregnancy including significant adaptations in the maternal immune system to prevent rejection of an allogenic fetus. Consequently, this may increase the risk of certain maternal infections which may subsequently impact the fetus. It has been shown that EVT cells are more permissive to the transmission of infection compared to the SYN. Therefore, the window of transition from first trimester to second trimester is a particularly vulnerable time for infections due to decreased SYNs and increased EVT. This vulnerability starts decreasing from second trimester into third trimester due to the increased presence of SYN cells in the placenta.
Though there are numerous disease-causing infections, common viruses that result in significant congenital infections are reviewed in detail below. In addition to these infections, the SARS-CoV-2 virus which has resulted in the COVID-19 pandemic, is also of significant importance and will be discussed here.
4.1. Cytomegalovirus
The cytomegalovirus (CMV) is a double stranded DNA virus belonging to the class of herpesviruses. The incidence varies based on geographic distribution and socioeconomic status; with rates of 0.7% in developed countries [51] and as high as 1–5% in developing countries [52]. It is transmitted by sexual contact or direct contact with infected body fluids including blood, urine and saliva and be classified as primary (infection in seronegative individual) or secondary (reactivation of infection) [53]. CMV is the leading cause of congenital viral infection, occurring in 0.2 to 2.2% of neonates and resulting in deafness and neurodevelopmental delay [51]. Congenital infection occurs through vertical transmission across the placenta, fetal exposure to contaminated genital tract secretions at the time of delivery or through breastmilk. As seen with other congenital infections, the risk of transmission increases with advancing gestational age, having reported rates of 5.2%, 16.4%, 36.5%, 40.1% and 65% in the preconception, periconceptional, first trimester, second trimester and third trimester, respectively [54]. However, the risk of disease severity is higher when infection occurs at earlier gestational ages and in the setting of primary infection [54]. Sonographic findings suggestive of congenital CMV include abdominal and liver calcifications, hepatosplenomegaly, echogenic bowel or kidneys, ascites, cerebral ventriculomegaly, microcephaly, hydrops and fetal growth restriction [55] If maternal serologic findings and sonographic findings are concerning for congenital CMV; amniocentesis for amniotic fluid CMV PCR can be performed for diagnostic testing. However, the detection of CMV in the amniotic fluid does not correlate with disease severity [56].
CMV has been identified in villous endothelial cells, epithelial cells of endometrial glands and endothelial cells of lymphatic vessels within decidua, EVTs, CTBs and Hofbauer cells [57,58]. In vitro studies of first trimester chorionic villi and isolated CTBs exposed to CMV identified transplacental transmission of the virus to occur via one of two pathways: 1) across SYNs via ADE with subsequent infection of CTBs, or 2) via direct infection of invasive CTBs in the uterine [53]. Inflammatory pathology from the anti-CMV placental immune response has been described in several studies. Following CMV infection of CTB and SYN, tumor necrosis factor (TNF)-α is secreted, causing apoptosis of uninfected cells in a paracrine fashion [59,60,61]. This inflammatory response at the maternal-fetal interface has detrimental effects on the placental structure and function that are separate from the effects of the infection itself.
Despite the varying risk of transmission throughout gestation, the data indicates the placenta also serves as a protective barrier during maternal infection [62]. As placental defense mechanisms are studied butremain largely enigmatic for many organisms, it is proposed that viral interactions between cells of the uterine and basal plate (vasculature, leukocytes, and CTBs) impact the severity and outcome of CMV infection [61]. Co-receptors-like integrin α5β3, α2β1 and α6β1, are necessary for internalization of CMV particles into fibroblasts and have been identified on CTBs. Studies that block these integrins prevent CMV entry into the placenta [63]. Lastly, the “double-hit-hypothesis” proposes that two concurrent infections weaken the defense, causing a more severe infection, as can be seen with CMV and with coinciding bacterial infections [64] and co-infection with HIV [65,66].
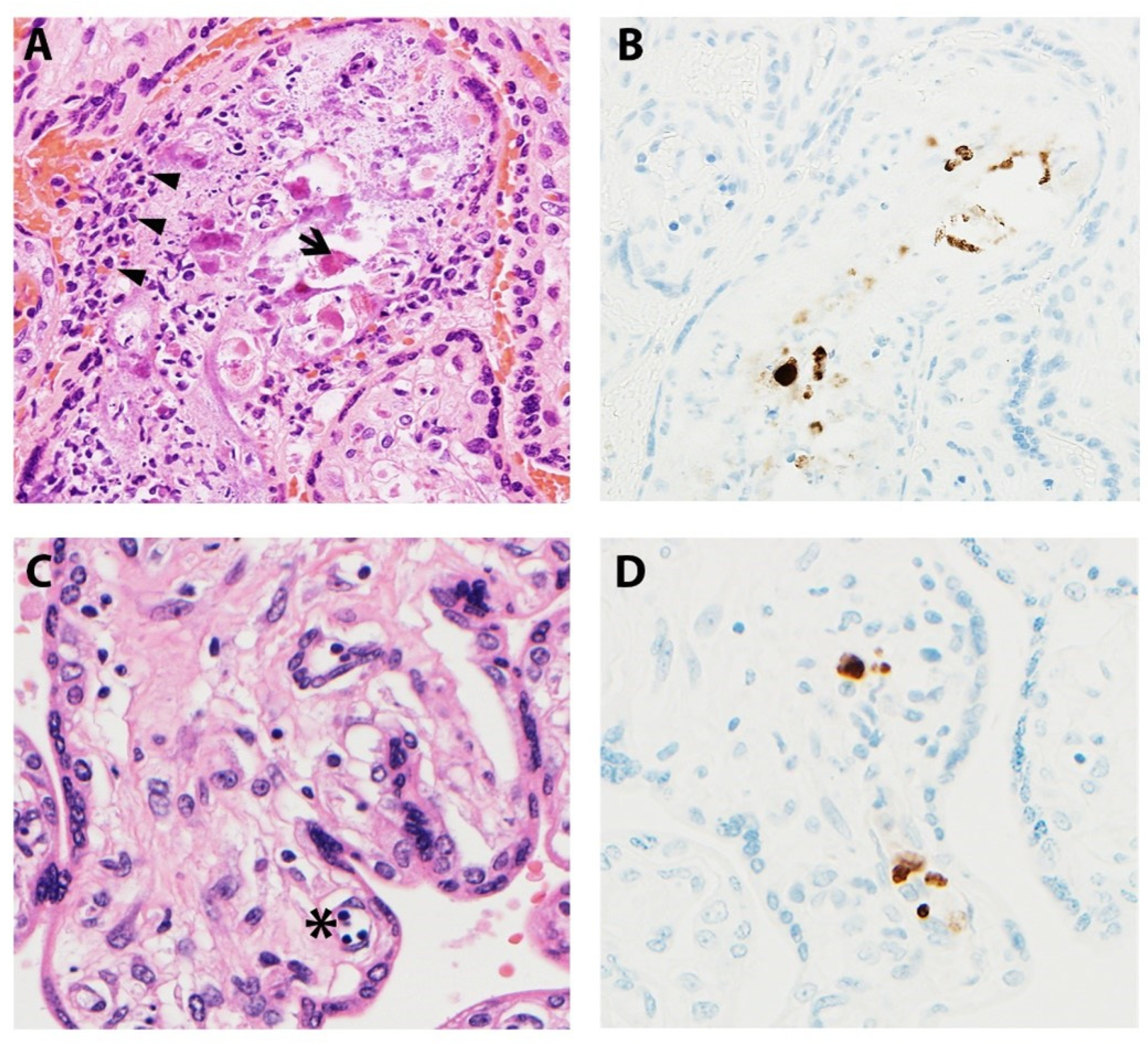
Microscopic manifestations of placental CMV infections vary with gestational age and infection progression (Figure 4). Studies have noted delayed villous maturation, villitis, cytomegalic cells, necrosis, and calcifications in term placentas when congenital CMV was diagnosed at mid-gestation [67]. Others have shown villous necrosis, but with infected endothelial cells identified in villous stroma not showing pathologic changes [62,68]. The viral cytopathic effect gives rise to the classic “owl-eye” inclusions, which are seen in only approximately 10% of cases [53,62,68]. As a result, IHC studies are an invaluable asset, highlighting insidious viral inclusions and/or viral proteins within the placenta. Infiltration of villous capillaries and villous stroma impacts villous development, characterized by delayed villous maturation. Longstanding infections lead to vascular sclerosis and obliteration, chorionic vessel thrombi, chronic lymphoplasmacytic villitis, and multifocal calcifications [68,69].
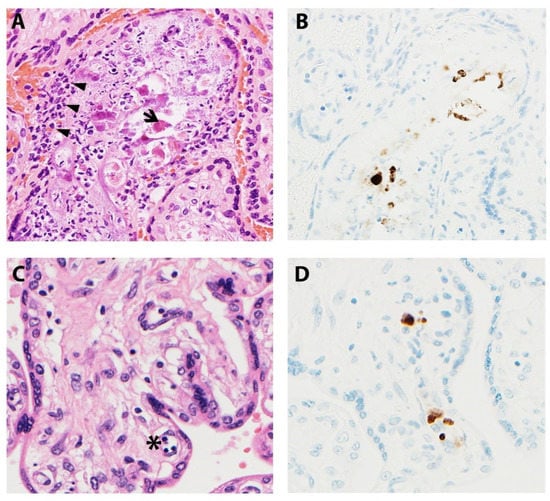
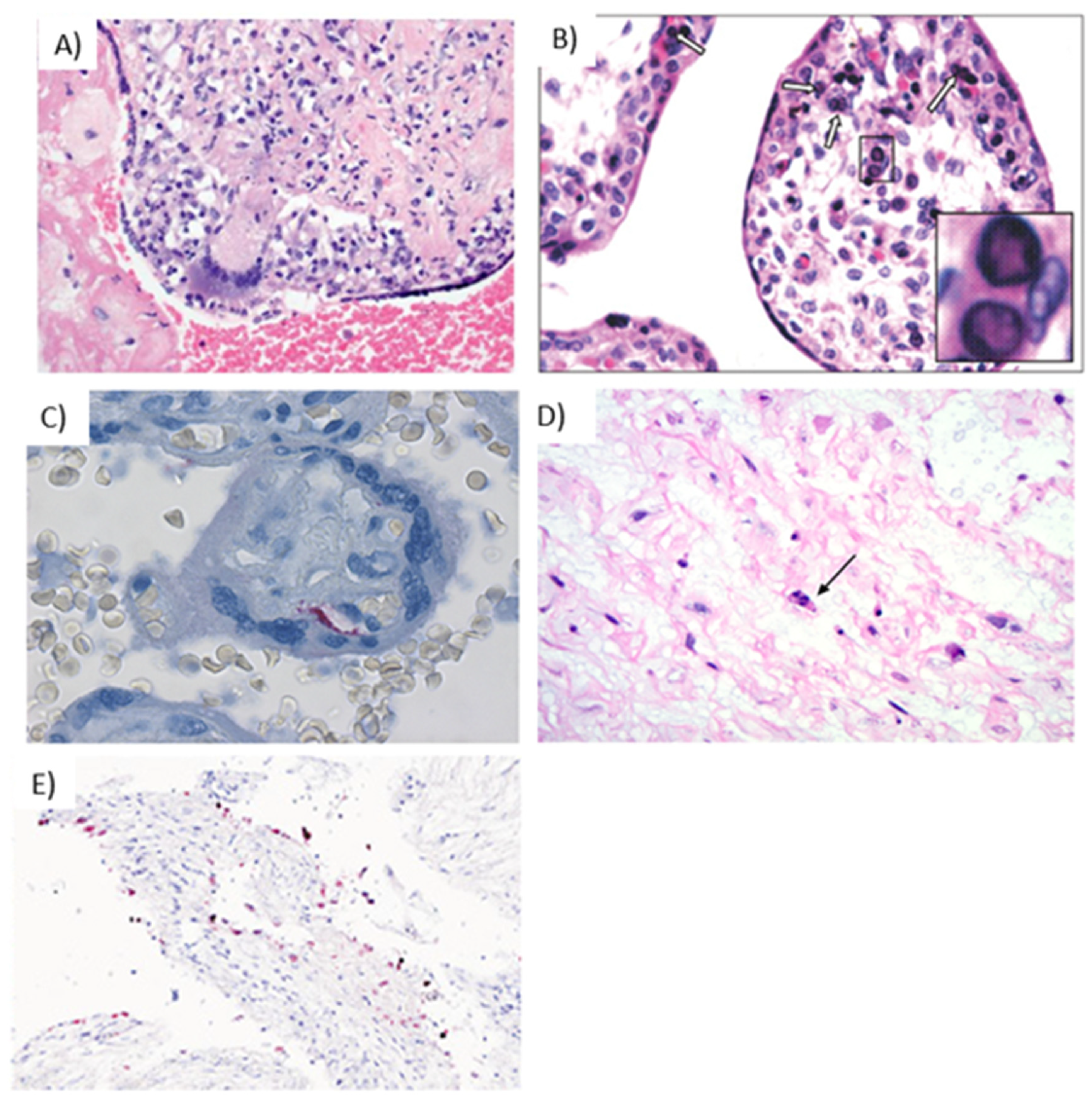
Figure 4.
Representative images of placentas infected by CMV. (A) Arrowheads illustrate chronic villitis and arrow indicates degraded CMV inclusions and cytopathic effect; (B) Immunostaining highlights viral inclusions; (C) Asterisk indicates erythroblastosis in early hydropic third trimester placenta; (D) Immunostaining highlights scattered positive CMV cells.
4.2. Hepatitis Viruses
Viral hepatitis is a common infection which can adversely affect pregnant women and their fetuses. Known viral hepatitis include Hepatitis A, B, C, D and E. Vaccinations are currently available for Hepatitis A and B; however, a large portion of women worldwide remain unvaccinated. Vertical transmission and clinical consequences are highest in patients with acute HBV infection. The HBV virus contains three principal antigens; namely Hepatitis B surface antigen (HBsAg) which is present on the viral surface, Hepatitis B core antigen (HbcAg) which is at the center of the viral particle, and Hepatitis Be antigen (HbeAg) which is coded by the same gene that codes for the core antigen.
The risk of HBV vertical transmission is approximately 10–20% in women with positive HBsAg and 90% in women with both HbsAg and HbeAg [70]. Perinatally acquired HBV infection confers 85–95% risk of chronic infection in the infant [71]. The frequency of vertical transmission also increases with advancing gestational age; transmission rates are 10% in the first trimester and up to 90% in the third trimester [70]. Transmission can occur prenatally or at birth. Due to this high risk of vertical transmission, routine screening of pregnant women for HbsAg at the first prenatal visit is recommended. Administration of Hepatitis B immunoglobulin (HBIG) can provide an immune barrier by limiting transcytosis of HBV and reduces the risk of vertical transmission of HBV in pregnancy. The HBIG antibodies can passively diffuse across the placenta at the materno-fetal interface; this process occurs at its maximum during the third trimester, which is the recommended window for administration of HBIG, with a dose of 100 IU to 200IU [72] In addition, HBIG should also be administered to neonates in the immediate postnatal period, within 12 h of birth [72]. Studies have reported varying mechanisms of transplacental viral transfer including a breach in the placental barrier during uterine contractions resulting in leakage of HbeAg [73], cell to cell transfer of virus from the maternal interface of an infected placenta [74,75]. In vitro studies also suggest that HBV results in intrauterine infection via infection of the placental trophoblasts; as seen by detection of HBV DNA in trophoblastic cells incubated with HBV positive serum [76].
Like HBV, HCV can also be vertically transmitted to the fetus prenatally or during delivery. The risk of transplacental transmission of HCV during chronic maternal infection is approximately 2–8% [77,78]. Studies propose that transmission occurs by transcytosis, transcellular vesicular transport of the virus, receptor mediated entry and/or infection of trophoblasts [79]. Placental infection with HCV results in damage of the placental barrier through enhanced placental NK T cell and γδ T-cell cytotoxicity while reducing expression of NK cell activation markers CD69, NKp44 and TRAIL creating a pathway for HCV transmission [80]. There are currently no vaccinations for HCV and no preventive strategies to reduce the risk of vertical transmission.
Reported histopathologic changes that occur following HBV infection of the placenta include stromal fibrosis, syncytial knotting, fibrinoid deposition, fibrinoid necrosis, villous capillary congestion and proliferation [81]. Xu et al. studied a cohort of 101 full-term placentas from HBsAg-positive pregnant women and reported HBV infection rates decreased in cell layers from the maternal side to the fetal surface. Large deposits of bilirubin can be seen in Hofbauer cells (fetal macrophages), trophoblasts, and macrophages of the fetal and extraplacental membranes in active HBV infections [82]. As HBV nucleocapsid contains two serologically distinct antigens (core and envelope), with a surrounding outer envelope surface antigen, the IHC stains both cytoplasm and membranes [76].
4.3. Varicella Zoster Virus
Varicella Zoster Virus (VZV), a Herpesvirus, causes varicella zoster (chicken pox) at time of primary infection and herpes zoster (shingles) at time of recurrence or reactivation of the latent herpes virus. Since the introduction of childhood VZV vaccination in 1995, the incidence of maternal varicella has decreased significantly [83]. The risk of congenital varicella syndrome is thus exceedingly rare given the low incidence of maternal disease. A large prospective study found the incidence of congenital varicella syndrome to be 0.4% if infection occurred before 12 weeks gestation, and 2% if infection occurred between 13 and 20 weeks of gestation [84]. Only 9 cases have been reported beyond 20 weeks of gestation [85]. The virus is transmitted from person to person via respiratory droplets to the nasal, oral or conjunctival mucosa, and via direct contact with vesicular fluids that contain the virus. Therefore, during pregnancy, mother to fetus transmission is possible in utero, perinatally, or postnatally.
Once the mother has a primary VZV infection, the virus replicates in various organs and lymph nodes, resulting in maternal viremia that may infect the placenta. The VZV DNA can be detected in multiple fetal organs, most notably in neural tissue, and infection contributes to limb hypoplasia, neurologic deficiencies (damage to autonomic nervous system) [84] and intrauterine encephalitis observed in infants with congenital varicella syndrome [86].
The primary mechanism of VZV transfer across the placenta remains unclear; however, it is postulated that T-cells infected with varicella virus bathe the decidua basalis, where the virus replicates and spreads into the intervillous space [87]. Both CD4 and CD8 T-cells are reprogrammed following VZV infection, rendering them more capable of crossing into the intervillous space [88]. This builds on previous studies showing that activated CD4 memory T-cells are highly susceptible to varicella infection [89]. A more recent study utilizing human tonsil tissue reported that the virus has capabilities to remodel naïve and memory T-cells, producing cell surface markers that favor tissue trafficking to the skin [88]. To counteract the immune system, VZV blocks JAK kinases from phosphorylating STAT1, thereby preventing the production and release of antiviral IFN-α and increasing VZV titers [90].
Reports vary on the histological features of VZV placental infection. Some studies indicate that regardless of a maternal history of varicella during pregnancy and time elapsed between maternal infection and birth, no viral-associated microscopic features are appreciated, perhaps indicating that VZV is transmitted to the fetus via the placenta, without apparent viral replication within the placenta [91,92]. Qureshi et al. [91] also reported nonspecific features, such as diffuse basal chronic villitis, mixed inflammatory infiltrate (lymphocytes, histiocytes, and multinucleated giant cells [Figure 5A]) and occasional nuclear clearing with faint eosinophilic center. Others have reported acute inflammation and diffuse or necrotizing chronic villitis with foci of granulomatous inflammation [93,94]. Immunohistochemistry images of the placenta usually reveal membranous and cytoplasmic staining, this is secondary to the VZV immunohistochemical stain which utilizes a mix of antibodies that highlight several glycoproteins, the nucleocapsid protein and the immediate early protein of the virus.
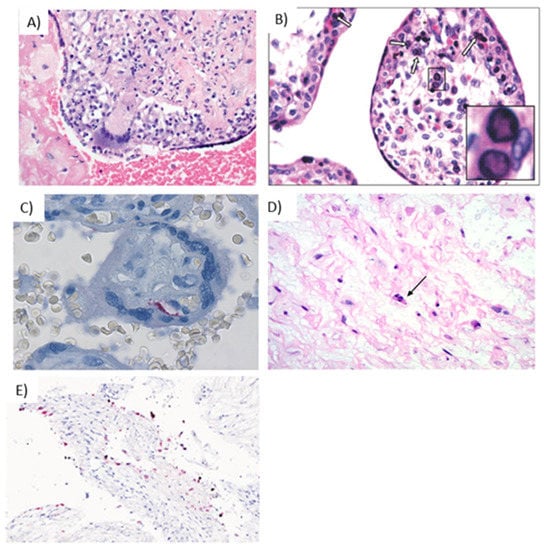
Figure 5.
Hallmarks of viral infections in placental tissue. (A) Giant cell inflammation caused by varicella infection [142] Reprinted from “Placental and Gestational Pathology” published by Cambridge University Press; reproduced with permission of the Licensor through PLSclear (ref. 47403) (B) Parvovirus B19 infection in the trophoblast cells, with intranuclear inclusions (white arrows) in the capillary cells of the chorionic villi, further shown at 40× in the inset [143]. Reprinted from Quemelo et al., 2007; used with permission from SciElo Brazil under the Creative Commons License (CC BY-NC 4.0). (C) Rubella infection in the placenta, demonstrated by immunostaining of endothelial cells [126]. Reprinted from Lazer et al., 2016; used with permission from Elsevier (ref. 5010950239116) (D) Ascending HSV infection shows infected cells in the cord stroma with nuclear irregularity, hyperchromasia, and individual cell necrosis (arrow) [69]. Reprinted from Heerema-McKenny, 2018; used with permission from John Wiley & Sons, Inc. (ref. 5010940997488) (E) Presence of SARS-COV-2 in decidual cells (red) by in situ hybridization (200×) [144]. Reprinted from Menter et al., 2021; used with permission from S. Karger AG.
4.4. Parvovirus B19 Virus
Parvovirus B19 is a single-stranded DNA virus that results in non-immune hydrops in fetuses, erythema infectiosum in children, and athropathies/myocarditis in adults [95]. The virus spreads mainly through respiratory droplets, infected blood products and by vertical transmission from mother to fetus [96]. Reported transmission rates include 50% with individuals residing with infected persons and approximately 20–30% for susceptible daycare workers and teachers exposed to infected students [97]. Within North America, approximately 65% of pregnant women show serologic evidence of prior infection, with the incidence of infection during pregnancy between 1–2%, increasing up to 10% during an epidemic [98]. Most women remain asymptomatic during an infection, although the transplacental transmission rate is 17–51% in acutely infected parvovirus B19 patients [99,100,101,102]. Maternal serologic screening is not routinely performed unless there are suspicious clinical findings or exposure. Management during pregnancy is dependent upon the severity of fetal anemia and gestational age [95]. For severe fetal anemia, intrauterine fetal transfusion of red cells is advised, with possible platelet administration to abate fetal thrombocytopenia. In mild cases of fetal anemia, invasive therapy is not recommended, however, the pregnancy will be followed by serial ultrasound examination and fetal middle cerebral artery (MCA)-peak systolic velocity (PVC) assessment [95]. In utero infection is suspected based on sonographic findings such as non-immune hydrops, placentomegaly, and stillbirth [103]. At 23 weeks’ gestation, the incidence of intrauterine fetal demise peaks, and is linked to the presence of sphingolipid globoside, abundant fetal erythropoiesis causing high viral loads and erythropoietin signaling from low fetal oxygen levels, all which support viral replication [57,100]. The main cellular receptor for Parvovirus B19 is the P blood group antigen globoside, that is strongly expressed on SYN and CTB cells and erythroid lineage cells. It is thought the presence of this receptor along trophoblast is involved in the virus’s ability to transmit across the placenta [104]. P-antigen is abundant in the placenta during the first half of pregnancy [98], increasing the risk of placental infection and transmission to the fetus. Although the cell-mediated immune response to placental parvovirus infection is not well characterized, one study noted a significant increase in interleukin-2 (IL-2) production in placentas from women with parvovirus B19 infections compared to uninfected placentas [104]. They also found that nearly 90% of the parvovirus-infected placentas had either a CD4 T-helper or CD8 cytolytic infiltrate into the villi.
Aside from identifying the eosinophilic circular viral inclusions within erythroid precursors in villous blood vessels, placental histological features also include hydropic chorionic villi, villous immaturity, nucleated red blood cells, and occasional chronic villitis (Figure 5B) [105]. If the inflammatory response is robust enough, placental dysfunction can occur leading to adverse fetal outcomes in the absence of fetal infection [98]. One study reviewed placentas from six women infected with parvovirus B19 during pregnancy and confirmed a mononuclear infiltrate present within the intervillous space and the chorionic villous stroma [106]. IHC is also available to highlight viral inclusion bodies in the nuclei of erythroid precursors, with viral antigen being detected in a cytoplasmic and nuclear pattern [107]
4.5. Human Immunodeficiency Virus
Human immunodeficiency virus (HIV) is a globally prevalent infection that is transmitted via sexual contact, exposure to infected blood or through perinatal transmission. Maternal to fetal transmission can occur in utero, during delivery or through breastfeeding. The risk of vertical transmission in utero is approximately 1 to 2% and is proportional to maternal CD4 counts and viral load [57]. American College of Obstetrics and Gynecology (ACOG) currently recommends screening all pregnant women for HIV at entry into prenatal care and at 28 weeks gestation. All pregnant women diagnosed with HIV should be started on antiretroviral therapy, such as Zidovudine. This specific drug is metabolized into its active form in the placenta and inhibits HIV replication specifically in trophoblast cells [108].
In vitro studies have identified that HIV- infected lymphocytes target the trophoblastic cell layer through endocytosis or transcytosis of the virion particle by direct cell-to-cell contact [75,109]. Pathologic studies of the human placenta identified CD4 receptors on trophoblastic cells and terminal villi [110], making them susceptible to infection by HIV. Additionally, there is evidence of HIV genomic material within CTB, SYN and Hofbauer cells [111]. Thus, HIV replication and transmission in the placenta may occur through CD4 positive Hofbauer cells or endothelial tissues [112]. Additionally, HIV uses the CCR5 and CXCR4 chemokine receptors to enter host cells, and these receptors are also expressed on placental tissue, further facilitating transfer of the virus [112]. As pregnancy results in a shift from Th1 to Th2 maternal immune responses, antiviral responses lead to Th1 cytokine production resulting in upregulation of CCR5 and CXCR4 receptors [113]. Consequently, placentas from HIV- transmitting mothers have a predominance of Th1 immunity, whereas those of from non-transmitting mothers sustain a strong predominance of Th2 immunity throughout pregnancy [114].
Regarding placental immune defense, Hofbauer cells act as an important mediator at the feto-maternal interface during ongoing HIV-1 infection. These specialized placental macrophages can isolate antibodies and antibody-virion immune complexes through expression of the following HIV-1 receptors along their surface: CD4, CCR5, CXC45, Fcγ and DC-SIGN [115,116,117]. Johnson et al. report in vitro models by which Hofbauer cells, although a target for HIV-1 infection, reduce mother-to-child transmission of HIV-1 by inducing immunoregulatory cytokines IL-10 and TGF-β1 [117]. Interestingly, these elevated levels of cytokines may indicate a mechanism by which the placenta provides an antiviral response at the maternal-fetal interface [115].
Microscopically, placental changes from an HIV infection have been described to impact villous maturity, characterized by distal villous immaturity, increased villous cellularity, and decreased vascularity [82]. In contrast, others have noted no associated placental lesions specific to HIV, despite in cases in which the virus was detected via cultures or electron microscopy [118]. Through IHC and in situ hybridization, the virus has been detected in villous trophoblasts, endothelial, and stromal cells as early as 8 weeks gestation [82,111,119]. Electron microscopy is an alternative method for identifying the presence of HIV. Using this technique, the virus can be appreciated within the SYN layer, cells of the decidua, umbilical vessels, and less commonly in Hofbauer cells [82,120].
4.6. Rubella Virus
Rubella virus is a member of the Togavirus family and is transmitted via direct droplet contact from nasopharyngeal secretions. The virus spreads from the respiratory tract, into the blood via infected lymphocytes and alveolar macrophages to local lymph nodes, causing lymphadenopathy [57]. This is followed by dissemination through the body to involve joints, skin, and the placenta, resulting in congenital rubella infection. Since the introduction of childhood Rubella vaccine, the incidence of congenital rubella has significantly decreased to less than 0.1 per 100,000 [121], and is usually seen in parts of the world where access to vaccination is challenging. Though rare, transplacental transfer of rubella can result in congenital rubella infection associated with spontaneous abortion, stillbirth and fetal growth restriction, or congenital rubella syndrome associated with birth defects including cataracts, congenital heart disease, hearing loss and fetal death [122]. These consequences occur secondary to virus- induced inhibition of cell division and cytopathic damage of affected fetal organs and blood vessels [123,124]. The risk of fetal infection with rubella is highest in the first trimester, especially prior to 10 weeks of gestation [125]. ACOG recommends routine screening for rubella immune status in all pregnant women. Those who are non-immune should receive MMR vaccine postpartum for protection against future infections with measles, mumps and rubella [121].
The exact mechanism by which Rubella traffics to the maternal-fetal interface has not been well studied. One hypothesis suggests that chronic infection causes monocytes to disseminate into the intervillous space and/or the lymphatic vessels within the basal plate. Placentas from congenital rubella syndrome were noted to have detectable antigens in CTBs, endothelial cells of villous capillaries, amniotic epithelium and various cells of the basal plate [126,127]. Adamo et al. [127] reported Rubella induces apoptosis in chorionic villous explants from human term placentas but not primary human embryo fibroblast cultures, suggesting the etiology for persistent congenital infection. In their models, they also showed elevated pathogenicity in human cell models compared to Vero monkey cell cultures, as humans are the natural host for Rubella. They postulate the persistence of the congenital infection is due to the intracellular location of the virus and its ability to avoid particular antibodies, the inability to activate apoptosis in parts of the infected cells during embryogenesis, and the slower growth rate limiting the duplication seen in clones of infected cells [127]. Others [128] have proposed the pathogenesis to include necrosis of chorion and endothelial cells, resulting in circulation of these infected cells to fetal organs such as eyes, heart, brain, and ears. Furthermore, Adamo et al. [127] discuss that the immune system may play a role in the pathogenesis, causing interferon and cytokines CCL5 or RANTES to be upregulated in fetal rubella-infected cells, contributing to growth and proliferation disruption of various differentiating cells, leading to congenital defects.
The histopathological findings in the rubella infected placenta include acute and chronic villitis and intervillositis, with necrosis of trophoblast cells and villi, vasculitis, and obliteration of stem villi [69,82]. In addition, there is delayed villous maturation and edema of stem and terminal villi [67]. Involvement of the umbilical cord demonstrates a generalized vasculitis with endothelial necrosis, thought due to the endothelial fixation of the virus [82]. Other reports describe an absence of destructive villitis and that the membranes, umbilical cord and decidua have chronic mononuclear inflammatory infiltrate, with mononuclear cell involvement of the decidua being one of the more common findings in rubella placentas [106]. Rubella viral inclusions appear eosinophilic round, intranuclear or cytoplasmic, and can be appreciated within the cells of decidua, EVTs, endothelial cells and amnion (Figure 5C) [69]
4.7. Herpes Simplex Virus
Herpes simplex virus (HSV) is a double-stranded DNA virus classified into HSV-1 (common cause of herpes labialis) and HSV-2 (common cause of herpes genitalis) based on its glycoprotein lipid bilayer envelope G1 and G2, respectively. It is transmitted through direct contact with sores, saliva, or genital secretions. Infection by HSV can be classified into three groups: primary, non- primary first episode, and recurrent infection. Primary infection is diagnosed when HSV-1 or HSV-2 is detected from lesions, without antibodies to any of the viral strains. Non- primary first infection refers to confirmation of either HSV-1 or -2 with antibodies to the other viral strain. Recurrent infection refers to detection of either HSV-1 or 2 and antibodies to the disease-causing viral strain. Approximately 1.6 million new cases of HSV-2 are diagnosed annually in the United States [129], and the incidence of new HSV-1 and HSV-2 infection diagnosed during pregnancy is 2%. Highlighting the insidious and ubiquitous nature of HSV, 60–80% of women that deliver an HSV-infected infant have an asymptomatic genital infection [130,131]. Interestingly, there is a lower risk of transmission to an exposed infant in recurrent HSV-2 lesions compared to new infection (2% vs. 57%) [132,133]. Neonatal HSV infection mainly presents with three clinical manifestations: (a) skin, eyes, and mouth herpes (SEM) disease, usually presenting as a vesicular rash occurring at 10–12 days of life; (b) CNS disease, with (60–70%) or without skin involvement, with clinical manifestations of encephalitis, potentially starting at any time within the first month of life and (c) disseminated disease, involving multiple organs including CNS, lungs, liver, adrenal, skin, eye, and/or mouth, with about 40% of infants never developing a vesicular rash [133].
The transmission of HSV to the neonate usually occurs through direct neonatal contact with infected maternal lesions at the time of delivery. Once infected, most women will develop viral specific antibodies after 12 weeks and this confers some protective effects to peripartum neonatal infection. This is supported by the findings that there is a higher risk of neonatal infection in newborns of mothers with primary or nonprimary first episode HSV-2 occurring near the time of labor and delivery when viral specific antibodies are more likely to be absent [134].
Robb et al. [135] proposed that transplacental HSV-1 infections may arise during transneural migration from perpetually infected dorsal root ganglia to the endometrium, or via direct placental infection through latently infected endometrium that is reactivated during pregnancy. EVTs are susceptible to infection by HSV-1 via entry mediators HveA, HveB, and HveC, which impact placental implantation resulting in dysfunction during early pregnancy and may lead to miscarriage [7]. Additionally, McDonagh et al. evaluated the relationship between pathogenic bacteria and concurrent infections with CMV and HSV from placental and decidual samples. They noted that HSV-1 and -2 infections were more focal within the decidua and that viral DNA was not found in the adjacent SYN cells of the placenta [136]. Attachment of the virus is facilitated by heparan sulfate, with entry being regulated by mediators HveA, HveB, and HveC [7]. The binding to heparan sulfate arises from interaction with virion glycoprotein C and then entry by the entry mediators [137,138]. Interestingly, enzymatic removal of heparan sulfate from cells reduces HSV’s ability to attach and cause infection by approximately 85% [7]. Koi et al. [7] report the placenta provides a defense mechanism against HSV through the SYN layer, limiting vertical transmission by not expressing the viral entry mediators HveA, HveB, and HveC. Koi et al. [7] further demonstrate that antibodies against HveB most effectively prevented HSV-1 entry into EVT cells [7].
Identification of HSV within the placenta is relatively uncommon, with a large study identifying a total of 64 cases between 1963 and 2009, supporting the rarity of intrauterine infection [139]. Histological manifestations of a placental HSV infection can include subacute necrotizing inflammation with stromal cell necrosis involving the umbilical cord and fetal surface/chorionic plate (Figure 5D) [140]. Additional investigation has described chronic chorioamnionitis with degradation of the amniotic lining, chorionic villi that are appropriate for gestational age, delayed villous maturation, and a HSV Cowdry type B intranuclear inclusion within subamniotic tissue [141]. Another study evaluating 64 cases of latent neonatal infection endorsed that although no HSV cytologic changes were found, the HSV-specific antigen was identified in individual cells of the subamnionic chorion, and/or individual cells of the subamniotic tissue, and/or perivascular tissue [135]. Through IHC, staining for HSV types 1 and 2 produce nuclear and granular cytoplasmic staining of the infected cells.
4.8. SARS-CoV-2 Virus
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an enveloped single-strand positive-sense RNA virus identified in December 2019 and is the etiologic organism of COVID-19 disease, a pandemic declared by the WHO in March of 2020 [145]. Evidence of the impact of SARS-CoV-2 in pregnancy is rapidly evolving and data on vertical transmission is limited. The effects on the fetus discussed in literature have included increased incidence of preterm delivery, higher rates of miscarriage, perinatal death, and intrauterine fetal distress [146].
SARS-CoV-2 enters cells by through binding its spike (S) protein to angiotensin-converting enzyme II (ACE2) receptor, with subsequent endocytosis, genomic replication, and mature virion release via exocytosis [147,148]. Reported main sites of ACE2 expression in the placenta have included the villi (SYN, CTB, endothelium of the villous vasculature, and smooth muscle cells of the primary villi), EVTs and decidual cells, and ACE2 is highly abundant in the early gestational placenta [149]. Additionally, SARS-CoV-2 utilizes S protein for receptor recognition to fuse with cell membranes [148,150]. However, in order to be completely functional, S protein requires proteolytic cleavage by human transmembrane protease serine 2 (TMPRSS2), cathepsin L (CTSL), furin, elastase, factor X or trypsin [148,151,152]. Single cell data of placental tissue from each trimester demonstrated that TMPRSS2 expression is low throughout the placenta, which likely explains why in utero infection has rarely been reported [153]. As SARS-CoV-2 research is ongoing, other proposed receptors for entry have surfaced, including dipeptidyl peptidase 4 (DPP4) and CD147/Basigin (BSG) [154]. Placental histopathology of third trimester placentas from 150 SARS-CoV-2 patients amongst 20 studies found that 2% of neonates and 21% of placental samples tested positive for SARS-CoV-2 [145,149]. SARS-CoV-2 is predominantly observed in the SYN layer with low levels in the villous CTB, indicating that the SYN cells provide a protective barrier against fetal transmission. A study by Kreis et al. evaluated the immune response of the placenta against SARS-CoV-2 and concluded that vertical transmission may occur, but this event is rare, owing to the effective placental innate immune response which induces type III IFN signaling, secretion of chromosome 19 miRNA cluster (C19MC) to restrict viral infections in autocrine and paracrine manners and regulation of the NF-κB pathway [148].
In addition to pathology, transcriptomic profiling using nucleic acid sequencing approaches have also demonstrated utility in understanding SARS-CoV-2 pathogenesis. A retrospective case-based analysis of liveborn neonates infected with SARS-CoV-2 via transplacental transmission, determined using RNA in situ hybridization against SARS-CoV-2 virus, identified SYN necrosis and chronic histiocytic intervillositis in the placenta [155]. Quantitative RT-PCR targeted to SARS-CoV-2 nucleocapside proteins showed that in one case of maternal-fetal transmission, viral titers were highest in the placenta (~1010 copies/100mg) followed by the cord blood (>105 copies/1mL); however, the virus was not identified in the fetal heart or lung tissue [156]. Lastly, single cell RNAseq from placental cells of COVID-19 hospitalized mothers demonstrated that although no viral transmission occurred during pregnancy (as defined by a negative SARS-CoV-2 RT-PCR test), there was altered gene expression observed which included increased expression of inflammatory cytokines, innate immune pathways and proteins critical for cytotoxicity as compared to uninfected controls [157]. Combining results from molecular pathways and pathological observations is a necessary step to further our knowledge and prenatal clinical care strategies for maternal SARS-CoV-2 infection and any future outbreaks.
Histopathological findings of SARS-CoV-2 infection in the placenta are met with conflicting opinions (Figure 5E). An insightful literature review by Sharps et al. summarizes 50 studies and discusses features of fetal vascular malperfusion (35.3%), maternal vascular malperfusion (46%), villitis (8.7%), chorioamnionitis (6%) and intervillositis (5.3%) being a trend [145]. Others have reported an increase in perivillous fibrin deposition in varying quantities [158,159,160,161]. In contrast, Hecht et al. reported on a series of 19 placentas, in which similar pathologies were identified; however, they concluded that given identical lesions were present in their control groups, SARS-CoV-2 is not associated with specific placental histopathology [162]. Interestingly, they also add that they suspected to see increased incidences of chronic villitis and chronic intervillositis, as these chronic inflammatory pathologies are more commonly reported in RNA viral placenta infections [162].
5. Conclusions
The understanding of placental molecular, immunologic and histopathic pathways and their role in transmission of, or protection from, infection is paramount to the care provided during pregnancy as well as the outcome of the infant. Continued research and focus in this area are critical to defining risks of vertical transmission of emerging infections as well as addressing prevention and treatment of existing infections.
Funding
The authors received the following financial support for authorship and/or publication of this article: NIH K12 HD65987 (EALE).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
Images were created with BioRender.com, Accessed on 6 February 2021.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stegmann, B.J.; Carey, J.C. TORCH Infections. Toxoplasmosis, Other (syphilis, varicella-zoster, parvovirus B19), Rubella, Cytomegalovirus (CMV), and Herpes infections. Curr. Women’s Health Rep. 2002, 2, 253–258. [Google Scholar]
- Medawar, P.B. Some immunological and endocrinological problems raised by the evolution of viviparity in verte-brates. Symp. Soc. Exp. Biol. 1953, 7, 320–337. [Google Scholar]
- Gnainsky, Y.; Granot, I.; Aldo, P.; Barash, A.; Or, Y.; Mor, G.; Dekel, N. Biopsy-induced inflammatory conditions improve endometrial receptivity: The mecha-nism of action. Reproduction 2015, 149, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.J.; Telfer, J.F.; Young, A.; Campbell, S.; Stewart, C.J.; Cameron, I.T.; Greer, I.A.; Norman, J.E. Leukocytes infiltrate the myometrium during human parturition: Further evidence that labour is an inflammatory process. Hum. Reprod. 1999, 14, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Pollheimer, J.; Vondra, S.; Baltayeva, J.; Beristain, A.G.; Knöfler, M. Regulation of Placental Extravillous Trophoblasts by the Maternal Uterine Envi-ronment. Front. Immunol. 2018, 9, 2597. [Google Scholar] [CrossRef]
- Shi, Q.J.; Lei, Z.M.; Rao, C.V.; Lin, J. Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology 1993, 132, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Koi, H.; Zhang, J.; Makrigiannakis, A.; Getsios, S.; MacCalman, C.D.; Strauss, J.F.; Parry, S. Syncytiotrophoblast Is a Barrier to Maternal-Fetal Transmission of Herpes Simplex Virus1. Biol. Reprod. 2002, 67, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Zeldovich, V.B.; Clausen, C.H.; Bradford, E.; Fletcher, D.A.; Maltepe, E.; Robbins, J.R.; Bakardjiev, A.I. Placental Syncytium Forms a Biophysical Barrier against Pathogen Invasion. PLoS Pathog. 2013, 9, e1003821. [Google Scholar] [CrossRef]
- Koga, K.; Mor, G. Toll-Like Receptors at the Maternal-Fetal Interface in Normal Pregnancy and Pregnancy Disorders. Am. J. Reprod. Immunol. 2010, 63, 587–600. [Google Scholar] [CrossRef]
- Tian, T.; Sun, D.; Wang, P.; Wang, H.; Bai, X.; Yang, X.; Wang, Z.; Dong, M. Roles of Toll-like Receptor 7 and 8 in Prevention of Intrauterine Transmission of Hepatitis B Virus. Cell. Physiol. Biochem. 2015, 37, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Kikuchi, M.; Natsukawa, T.; Shinobu, N.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Akira, S.; Fujita, T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004, 5, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Peisley, A.; Wu, B.; Xu, H.; Chen, Z.J.; Hur, S. Structural basis for ubiquitin-mediated antiviral signal activation by RIG-I. Nature 2014, 509, 110–114. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Macmillan, J.B.; Chen, Z.J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 2009, 138, 576–591. [Google Scholar] [CrossRef]
- Jabłońska, A.; Świerzko, A.S.; Studzińska, M.; Suski, P.; Kalinka, J.; Leśnikowski, Z.J.; Cedzyński, M.; Paradowska, E. Insight into the expression of RIG-I-like receptors in human third trimester placentas following ex vivo cytomegalovirus or vesicular stomatitis virus infection. Mol. Immunol. 2020, 126, 143–152. [Google Scholar] [CrossRef]
- Jabłońska, A.; Studzińska, M.; Suski, P.; Kalinka, J.; Paradowska, E. Enhanced expression of IFI16 and RIG-I in human third-trimester placentas fol-lowing HSV-1 infection. Clin. Exp. Immunol. 2018, 193, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Kovats, S.; Main, E.K.; Librach, C.; Stubblebine, M.; Fisher, S.J.; Demars, R. A class I antigen, HLA-G, expressed in human trophoblasts. Science 1990, 248, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Ishitani, A.; Sageshima, N.; Lee, N.; Dorofeeva, N.; Hatake, K.; Marquardt, H.; Geraghty, D.E. Protein Expression and Peptide Binding Suggest Unique and Interacting Functional Roles for HLA-E, F, and G in Maternal-Placental Immune Recognition. J. Immunol. 2003, 171, 1376–1384. [Google Scholar] [CrossRef]
- Gobin, S.J.; Wilson, L.; Keijsers, V.; Elsen, P.J.V.D. Antigen processing and presentation by human trophoblast-derived cell lines. J. Immunol. 1997, 158, 3587–3592. [Google Scholar]
- Tilburgs, T.; Crespo, Â.C.; Van Der Zwan, A.; Rybalov, B.; Raj, T.; Stranger, B.; Gardner, L.; Moffett, A.; Strominger, J.L. Human HLA-G+ extravillous trophoblasts: Immune-activating cells that in-teract with decidual leukocytes. Proc. Natl. Acad. Sci. USA 2015, 112, 7219–7224. [Google Scholar] [CrossRef]
- Jabrane-Ferrat, N. Features of human decidual NK cells in healthy pregnancy and during viral infection. Front. Immunol. 2019, 10, 1397. [Google Scholar] [CrossRef]
- Lee, N.; Llano, M.; Carretero, M.; Ishitani, A.; Navarro, F.; López-Botet, M.; Geraghty, D.E. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc. Natl. Acad. Sci. USA 1998, 95, 5199–5204. [Google Scholar] [CrossRef]
- Colonna, M.; Navarro, F.; Bellón, T.; Llano, M.; García, P.; Samaridis, J.; Angman, L.; Cella, M.; López-Botet, M. A common inhibitory receptor for major histocompatibility complex class I mole-cules on human lymphoid and myelomonocytic cells. J. Exp. Med. 1997, 186, 1809–1818. [Google Scholar] [CrossRef]
- Le Gal, F.A.; Riteau, B.; Sedlik, C.; Khalil-Daher, I.; Menier, C.; Dausset, J.; Guillet, J.G.; Carosella, E.D.; Rouas-Freiss, N. HLA-G-mediated inhibition of antigen-specific cytotoxic T lymphocytes. Int. Immunol. 1999, 11, 1351–1356. [Google Scholar] [CrossRef]
- Sharkey, A.M.; Gardner, L.; Hiby, S.; Farrell, L.; Apps, R.; Masters, L.; Goodridge, J.; Lathbury, L.; Stewart, C.A.; Verma, S.; et al. Killer Ig-Like Receptor Expression in Uterine NK Cells Is Biased toward Recognition of HLA-C and Alters with Gestational Age. J. Immunol. 2008, 181, 39–46. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhou, W.H.; Tao, Y.; Wang, S.C.; Jiang, Y.L.; Zhang, D.; Piao, H.L.; Fu, Q.; Li, D.J.; Du, M.R. The Galectin-9/Tim-3 pathway is involved in the regulation of NK cell function at the ma-ternal–fetal interface in early pregnancy. Cell. Mol. Immunol. 2016, 13, 73–81. [Google Scholar] [CrossRef]
- Okuyama, M.; Mezawa, H.; Kawai, T.; Urashima, M. Elevated Soluble PD-L1 in Pregnant Women’s Serum Suppresses the Immune Reac-tion. Front. Immunol. 2019, 10, 86. [Google Scholar] [CrossRef]
- Enninga, E.A.L.; Harrington, S.M.; Creedon, D.J.; Ruano, R.; Markovic, S.N.; Dong, H.; Dronca, R.S. Immune checkpoint molecules soluble program death ligand 1 and galec-tin-9 are increased in pregnancy. Am. J. Reprod. Immunol. 2018, 79, e12795. [Google Scholar] [CrossRef] [PubMed]
- Madani, G.; Heiner, D. Antibody transmission from mother to fetus. Curr. Opin. Immunol. 1989, 1, 1157–1164. [Google Scholar] [CrossRef]
- Simister, N.E.; Mostov, K.E. An Fc receptor structurally related to MHC class I antigens. Nature 1989, 337, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Ober, R.J.; Martinez, C.; Lai, X.; Zhou, J.; Ward, E.S. Exocytosis of IgG as mediated by the receptor, FcRn: An analysis at the single-molecule level. Proc. Natl. Acad. Sci. USA 2004, 101, 11076–11081. [Google Scholar] [CrossRef] [PubMed]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef]
- Isabel de Moraes-Pinto, M.; Almeida, A.C.; Kenj, G.; Filgueiras, T.E.; Tobias, W.; Santos, A.M.; Carneiro-Sampaio, M.M.; Farhat, C.K.; Milligan, P.J.; Johnson, P.M.; et al. Placental transfer and maternally acquired neonatal IgG immunity in hu-man immunodeficiency virus infection. J. Infect. Dis. 1996, 173, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Brair, M.E.; Brabin, B.J.; Milligan, P.; Maxwell, S.; Hart, C.A. Reduced transfer of tetanus antibodies with placental malaria. Lancet 1994, 343, 208–209. [Google Scholar] [CrossRef]
- Buurma, A.; Cohen, D.; Veraar, K.; Schonkeren, D.; Claas, F.H.; Bruijn, J.A.; Bloemenkamp, K.W.; Baelde, H.J. Preeclampsia Is Characterized by Placental Complement Dysregulation. Hypertension 2012, 60, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, F.; Narchi, G.; Radillo, O.; Meri, S.; Ferrone, S.; Betterle, C. Susceptibility of human trophoblast to killing by human complement and the role of the complement regulatory proteins. J. Immunol. 1993, 151, 1562–1570. [Google Scholar]
- Conroy, A.; Serghides, L.; Finney, C.; Owino, S.O.; Kumar, S.; Gowda, D.C.; Liles, W.C.; Moore, J.M.; Kain, K.C. C5a Enhances Dysregulated Inflammatory and Angiogenic Responses to Malaria In Vitro: Potential Implications for Placental Malaria. PLoS ONE 2009, 4, e4953. [Google Scholar] [CrossRef]
- Enninga, E.A.L.; Theiler, R.N. Lymphocytic choriomeningitis virus infection demonstrates higher replicative capacity and de-creased antiviral response in the first-trimester placenta. J. Immunol. Res. 2019, 2019, 7375217. [Google Scholar] [CrossRef] [PubMed]
- Weisblum, Y.; Oiknine-Djian, E.; Vorontsov, O.M.; Haimov-Kochman, R.; Zakay-Rones, Z.; Meir, K.; Shveiky, D.; Elgavish, S.; Nevo, Y.; Roseman, M.; et al. Zika Virus Infects Early- and Midgestation Human Maternal Decidual Tissues, Inducing Distinct Innate Tissue Responses in the Maternal-Fetal Interface. J. Virol. 2016, 91. [Google Scholar] [CrossRef] [PubMed]
- Hertzog, J.; Junior, A.G.D.; Rigby, R.E.; Donald, C.L.; Mayer, A.; Sezgin, E.; Song, C.; Jin, B.; Hublitz, P.; Eggeling, C.; et al. Infection with a Brazilian isolate of Zika virus generates RIG-I stimulatory RNA and the viral NS5 protein blocks type I IFN induction and signaling. Eur. J. Immunol. 2018, 48, 1120–1136. [Google Scholar] [CrossRef]
- Schilling, M.; Bridgeman, A.; Gray, N.; Hertzog, J.; Hublitz, P.; Kohl, A.; Rehwinkel, J. RIG-I Plays a Dominant Role in the Induction of Transcriptional Changes in Zika Virus-Infected Cells, which Protect from Virus-Induced Cell Death. Cells 2020, 9, 1476. [Google Scholar] [CrossRef]
- Li, A.; Wang, W.; Wang, Y.; Chen, K.; Xiao, F.; Hu, D.; Hui, L.; Liu, W.; Feng, Y.; Li, G.; et al. NS5 Conservative Site Is Required for Zika Virus to Restrict the RIG-I Signaling. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Amsler, L.; Verweij, M.; DeFilippis, V.R. The tiers and dimensions of evasion of the type I interferon response by human cyto-megalovirus. J. Mol. Biol. 2013, 425, 4857–4871. [Google Scholar] [CrossRef]
- Botto, S.; Abraham, J.; Mizuno, N.; Pryke, K.; Gall, B.; Landais, I.; Streblow, D.N.; Fruh, K.J.; DeFilippis, V.R. Human cytomegalovirus immediate early 86-kda protein blocks transcription and in-duces degradation of the immature interleukin-1β protein during virion-mediated activation of the AIM2 inflammasome. mBio 2019, 10. [Google Scholar] [CrossRef]
- Zou, H.-M.; Huang, Z.-F.; Yang, Y.; Luo, W.-W.; Wang, S.-Y.; Luo, M.-H.; Fu, Y.-Z.; Wang, Y.-Y. Human Cytomegalovirus Protein UL94 Targets MITA to Evade the Antiviral Immune Response. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Fu, Y.Z.; Su, S.; Zou, H.M.; Guo, Y.; Wang, S.Y.; Li, S.; Luo, M.H.; Wang, Y.Y. Human Cytomegalovirus DNA Polymerase Subunit UL44 Antagonizes Antiviral Immune Re-sponses by Suppressing IRF3- and NF-κB-Mediated Transcription. J. Virol. 2019, 93. [Google Scholar] [CrossRef]
- De Pelsmaeker, S.; Romero, N.; Vitale, M.; Favoreel, H.W. Herpesvirus Evasion of Natural Killer Cells. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Glasner, A.; Oiknine-Djian, E.; Weisblum, Y.; Diab, M.; Panet, A.; Wolf, D.G.; Mandelboim, O. Zika Virus Escapes NK Cell Detection by Upregulating Major Histocompat-ibility Complex Class I Molecules. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Maidji, E.; McDonagh, S.; Genbacev, O.; Tabata, T.; Pereira, L. Maternal Antibodies Enhance or Prevent Cytomegalovirus Infection in the Placenta by Neonatal Fc Receptor-Mediated Transcytosis. Am. J. Pathol. 2006, 168, 1210–1226. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.E.; Kawamura, T.; Gorny, M.K.; Lake, D.; Xu, J.Y.; Matsumoto, Y.; Sugano, T.; Masuho, Y.; Mitchell, W.M.; Hersh, E. Human monoclonal antibodies to the human immunodeficiency virus type 1 (HIV-1) transmembrane glycoprotein gp4l enhance HIV-1 infection in vitro (antibody-dependent enhance-ment/complement/AIDS/vaccine development). Proc. Natl. Acad. Sci. USA 1990, 87, 3185–3189. [Google Scholar] [CrossRef] [PubMed]
- Willey, S.; Aasa-Chapman, M.M.I.; O’Farrell, S.; Pellegrino, P.; Williams, I.; Weiss, R.A.; Neil, S.J.D. Extensive complement-dependent enhancement of HIV-1 by autologous non-neutralising antibodies at early stages of infection. Retrovirology 2011, 8, 16. [Google Scholar] [CrossRef]
- Kenneson, A.; Cannon, M.J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 2007, 17, 253–276. [Google Scholar] [CrossRef]
- Hyde, T.B.; Schmid, D.S.; Cannon, M.J. Cytomegalovirus seroconversion rates and risk factors: Implications for congenital CMV. Rev. Med. Virol. 2010, 20, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.; Genbacev, O.; Maidji, E.; Pereira, L. Human Cytomegalovirus Infection of Placental Cytotrophoblasts In Vitro and In Utero: Implications for Transmission and Pathogenesis. J. Virol. 2000, 74, 6808–6820. [Google Scholar] [CrossRef]
- Picone, O.; Vauloup-Fellous, C.; Cordier, A.G.; Guitton, S.; Senat, M.V.; Fuchs, F.; Ayoubi, J.M.; Grangeot Keros, L.; Benachi, A. A series of 238 cytomegalovirus primary infections during pregnancy: De-scription and outcome. Prenat. Diagn. 2013, 33, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Guerra, B.; Simonazzi, G.; Puccetti, C.; Lanari, M.; Farina, A.; Lazzarotto, T.; Rizzo, N. Ultrasound prediction of symptomatic congenital cytomegalovirus infection. Am. J. Obstet. Gynecol. 2008, 198, 380.e1–380.e7. [Google Scholar] [CrossRef] [PubMed]
- Liesnard, C.; Donner, C.; Brancart, F.; Gosselin, F.; Delforge, M.L.; Rodesch, F. Prenatal diagnosis of congenital cytomegalovirus infection: Prospective study of 237 pregnancies at risk. Obstet. Gynecol. 2000, 95, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Congenital Viral Infection: Traversing the Uterine-Placental Interface. Annu. Rev. Virol. 2018, 5, 273–299. [Google Scholar] [CrossRef]
- Pereira, L.; Petitt, M.; Fong, A.; Tsuge, M.; Tabata, T.; Fang-Hoover, J.; Maidji, E.; Zydek, M.; Zhou, Y.; Inoue, N.; et al. Intrauterine growth restriction caused by underlying congenital cytomegalovirus infec-tion. J. Infect. Dis. 2014, 209, 1573–1584. [Google Scholar] [CrossRef]
- León-Juárez, M.; Martínez–Castillo, M.; González-García, L.D.; Helguera-Repetto, A.C.; Zaga-Clavellina, V.; García-Cordero, J.; Flores-Pliego, A.; Herrera-Salazar, A.; Vázquez-Martínez, E.R.; Reyes-Muñoz, E. Cellular and molecular mechanisms of viral infection in the human placenta. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef]
- Muller, W.J.; Jones, C.A.; Koelle, D.M. Immunobiology of herpes simplex virus and cytomegalovirus infections of the fetus and newborn. Curr. Immunol. Rev. 2010, 6, 38–55. [Google Scholar] [CrossRef][Green Version]
- Weisblum, Y.; Panet, A.; Haimov-Kochman, R.; Wolf, D.G. Models of vertical cytomegalovirus (CMV) transmission and pathogene-sis. Semin. Immunopathol. 2014, 36, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Mühlemann, K.; Miller, R.K.; Metlay, L.; Menegus, M.A. Cytomegalovirus infection of the human placenta: An immunocytochemical study. Hum. Pathol. 1992, 23, 1234–1237. [Google Scholar] [CrossRef]
- Feire, A.L.; Koss, H.; Compton, T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly con-served disintegrin-like domain. Proc. Natl. Acad. Sci. USA. 2004, 101, 15470–15475. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Maidji, E.; McDonagh, S.; Genbacev, O.; Fisher, S. Human Cytomegalovirus Transmission from the Uterus tothe Placenta Correlates with the Presence of Pathogenic Bacteria andMaternalImmunity. J. Virol. 2003, 77, 13301–13314. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.L.; Mudd, J.C.; Shive, C.L.; Younes, S.-A.; Panigrahi, S.; Sieg, S.F.; Lee, S.A.; Hunt, P.W.; Calabrese, L.H.; Gianella, S.; et al. CD8 T-Cell Expansion and Inflammation Linked to CMV Coinfection in ART-treated HIV Infection. Clin. Infect. Dis. 2016, 62, 392–396. [Google Scholar] [CrossRef]
- Adachi, K.; Xu, J.; Ank, B.; Watts, D.H.; Camarca, M.; Mofenson, L.M.; Pilotto, J.H.; Joao, E.; Gray, G.; Theron, G.; et al. Congenital Cytomegalovirus and HIV Perinatal Transmission. Pediatr. Infect. Dis. J. 2018, 37, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.G.P.; Marques, R.L.S.; Lobato, Y.Y.; Fonseca, M.E.F.; dutra Wigg, M. Placental pathology in congenital rubella. Placenta 1985, 6, 281–295. [Google Scholar] [CrossRef]
- Costa, M.L.; Nobrega, G.D.M.; Antolini-Tavares, A. Key Infections in the Placenta. Obstet. Gynecol. Clin. N. Am. 2020, 47, 133–146. [Google Scholar] [CrossRef]
- Heerema-McKenney, A. Defense and infection of the human placenta. APMIS 2018, 126, 570–588. [Google Scholar] [CrossRef] [PubMed]
- Mast, E.E.; Margolis, H.S.; Fiore, A.E.; Brink, E.W.; Goldstein, S.T.; Wang, S.A.; Moyer, L.A.; Bell, B.P.; Alter, M.J. Advisory Committee on Immunization Practices (ACIP) A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: Immunization of infants, children, and adolescents. MMWR. Recomm. Rep. 2005, 54, 1–31. [Google Scholar]
- Lin, C.-L.; Kao, J.-H. Prevention of mother-to-child transmission: The key of hepatitis B virus elimination. Hepatol. Int. 2018, 12, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Eke, A.C.; Eleje, G.U.; Eke, U.A.; Xia, Y.; Liu, J. Hepatitis B immunoglobulin during pregnancy for prevention of mother-to-child trans-mission of hepatitis B virus. Cochrane Database Syst. Rev. 2017, 2, CD008545. [Google Scholar]
- Lin, H.H.; Lee, T.Y.; Chen, D.S.; Sung, J.L.; Ohto, H.; Etoh, T.; Kawana, T.; Mizuno, M. Transplacental leakage of HBeAg-positive maternal blood as the most likely route in caus-ing intrauterine infection with hepatitis B virus. J. Pediatr. 1987, 111 Pt 1, 877–881. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Yue, Y.-F.; Bai, G.-Q.; Shi, L.; Jiang, H. Mechanism of intrauterine infection of hepatitis B virus. World J. Gastroenterol. 2004, 10, 437–438. [Google Scholar] [CrossRef]
- Bhat, P.; Anderson, D.A. Hepatitis B Virus Translocates across a Trophoblastic Barrier. J. Virol. 2007, 81, 7200–7207. [Google Scholar] [CrossRef]
- Bai, H.; Zhang, L.; Ma, L.; Dou, X.G.; Feng, G.H.; Zhao, G.Z. Relationship of hepatitis B virus infection of placental barrier and hepatitis B virus intra-uterine transmission mechanism. World J. Gastroenterol. 2007, 13, 3625–3630. [Google Scholar] [CrossRef]
- Shebl, F.M.; El-Kamary, S.S.; Saleh, D.A.; Abdel-Hamid, M.; Mikhail, N.; Allam, A.; El-Arabi, H.; Elhenawy, I.; El-Kafrawy, S.; El-Daly, M.; et al. Prospective cohort study of mother-to-infant infection and clearance of hepatitis C in rural Egyptian villages. J. Med. Virol. 2009, 81, 1024–1031. [Google Scholar] [CrossRef]
- Tosone, G.; Maraolo, A.E.; Mascolo, S.; Palmiero, G.; Tambaro, O.; Orlando, R. Vertical hepatitis C virus transmission: Main questions and answers. World J. Hepatol. 2014, 6, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Le Campion, A.; Larouche, A.; Fauteux-Daniel, S.; Soudeyns, H. Pathogenesis of hepatitis C during pregnancy and childhood. Viruses 2012, 4, 3531–3550. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, C.W.; Golden-Mason, L.; Brocato, M.; Krull, M.; Narkewicz, M.R.; Rosen, H.R. Innate Immune Function in Placenta and Cord Blood of Hepatitis C—Seropositive Mother-Infant Dyads. PLoS ONE 2010, 5, e12232. [Google Scholar] [CrossRef]
- Liu, J.; Feng, Y.; Wang, J.; Li, X.; Lei, C.; Jin, D.; Feng, W.; Yang, Y.; He, Y.; Li, Y.; et al. An “immune barrier” is formed in the placenta by hepatitis B immunoglobulin to protect the fetus from hepatitis B virus infection from the mother. Hum. Vaccines Immunother. 2015, 11, 2068–2076. [Google Scholar] [CrossRef]
- Bittencourt, A.L.; Garcia, A.G.P. The Placenta in Hematogenous Infections. Pediatr. Pathol. Mol. Med. 2002, 21, 401–432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Patenaude, V.; Abenhaim, H.A. Maternal outcomes in pregnancies affected by varicella zoster virus infections: Population-based study on 7.7 million pregnancy admissions. J. Obstet. Gynaecol. Res. 2014, 41, 62–68. [Google Scholar] [CrossRef]
- Enders, G.; Bolley, I.; Miller, E.; Cradock-Watson, J.; Ridehalgh, M. Consequences of varicella and herpes zoster in pregnancy: Prospective study of 1739 cases. Lancet 1994, 343, 1548–1551. [Google Scholar] [CrossRef]
- Marin, M.; Güris, D.; Chaves, S.S.; Schmid, S.; Seward, J.F. Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recomm. Rep. 2007, 56, 1–40. [Google Scholar] [PubMed]
- Higa, K.; Dan, K.; Manabe, H. Varicella-zoster virus infections during pregnancy: Hypothesis concerning the mechanisms of congenital malformations. Obstet. Gynecol. 1987, 69, 214–222. [Google Scholar] [CrossRef]
- Hoo, R.; Nakimuli, A.; Vento-Tormo, R. Innate Immune Mechanisms to Protect against Infection at the Human Decidu-al-Placental Interface. Front. Immunol. 2020, 11, 2070. [Google Scholar] [CrossRef] [PubMed]
- Sen, N.; Mukherjee, G.; Sen, A.; Bendall, S.C.; Sung, P.; Nolan, G.P.; Arvin, A.M. Single-Cell Mass Cytometry Analysis of Human Tonsil T Cell Remodeling by Varicella Zoster Virus. Cell Rep. 2014, 8, 633–645. [Google Scholar] [CrossRef]
- Arvin, A.M.; Moffat, J.F.; Sommer, M.; Oliver, S.; Che, X.; Vleck, S.; Zerboni, L.; Ku, C.-C. Varicella-Zoster Virus T Cell Tropism and the Pathogenesis of Skin Infection. In Current Topics in Microbiology and Immunology; Abendroth, A., Arvin, A.M., Moffat, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 189–209. [Google Scholar]
- Ku, C.C.; Zerboni, L.; Ito, H.; Graham, B.S.; Wallace, M.; Arvin, A.M. Varicella-zoster virus transfer to skin by T Cells and modulation of viral replication by epi-dermal cell interferon-alpha. J. Exp. Med. 2004, 200, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, F.; Jacques, S.M. Maternal varicella during pregnancy: Correlation of maternal history and fetal outcome with pla-cental histopathology. Hum. Pathol. 1996, 27, 191–195. [Google Scholar] [CrossRef]
- Kawana, K.; Yoshikawa, H.; Sata, T. Post-partum detection of varicella-zoster virus DNA in the placenta. Int. J. Gynecol. Obstet. 1996, 55, 165–166. [Google Scholar] [CrossRef]
- Nikkels, A.F.; Delbecque, K.; Piérard, G.E.; Wienkötter, B.; Schalasta, G.; Enders, M. Distribution of Varicella-Zoster Virus DNA and Gene Products in Tissues of a First-Trimester Varicella-Infected Fetus. J. Infect. Dis. 2005, 191, 540–545. [Google Scholar] [CrossRef][Green Version]
- Altshuler, G.; Russell, P. The human placental villitides: A review of chronic intrauterine infection. Curr. Top. Pathol. 1975, 60, 64–112. [Google Scholar] [PubMed]
- Bonvicini, F.; Bua, G.; Gallinella, G. Parvovirus B19 infection in pregnancy—Awareness and opportunities. Curr. Opin. Virol. 2017, 27, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Servey, J.T.; Reamy, B.V.; Hodge, J. Clinical presentations of parvovirus B19 infection. Am. Fam. Physician 2007, 75, 373–376. [Google Scholar]
- American Academy of Pediatrics. Parvovirus B19. In Red Book: 2018 Report of the Committee on Infectious Diseases; Kimberlin, D.W., Brady, M.T., Jackson, M.A., Long, S.S., Eds.; American Academy of Pediatrics: Itasca, IL, USA, 2018; pp. 602–606. [Google Scholar]
- Lamont, R.F.; Sobel, J.; Vaisbuch, E.; Kusanovic, J.P.; Mazaki-Tovi, S.; Kim, S.K.; Uldbjerg, N.; Romero, R. Parvovirus B19 infection in human pregnancy. BJOG Int. J. Obstet. Gynaecol. 2010, 118, 175–186. [Google Scholar] [CrossRef]
- Harger, J.H.; Adler, S.P.; Koch, W.C.; Harger, G.F. Prospective evaluation of 618 pregnant women exposed to parvovirus B19: Risks and symptoms. Obstet. Gynecol. 1998, 91, 413–420. [Google Scholar] [CrossRef]
- Norbeck, O.; Papadogiannakis, N.; Petersson, K.; Hirbod, T.; Broliden, K.; Tolfvenstam, T. Revised Clinical Presentation of Parvovirus B19–Associated Intrauterine Fetal Death. Clin. Infect. Dis. 2002, 35, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.M. Prospective study of human parvovirus (B19) infection in pregnancy. Public Health Laboratory Service Working Party on Fifth Disease. BMJ 1990, 300, 1166–1170. [Google Scholar]
- Yaegashi, N. Pathogenesis of Nonimmune Hydrops Fetalis Caused by Intrauterine B19 Infection. Tohoku J. Exp. Med. 2000, 190, 65–82. [Google Scholar] [CrossRef]
- Levy, R.; Weissman, A.; Blomberg, G.; Hagay, Z.J. Infection by Parvovirus B 19 during Pregnancy: A Review. Obstet. Gynecol. Surv. 1999, 54, 69–74. [Google Scholar] [CrossRef]
- Jordan, J.A.; Huff, D.; Deloia, J.A. Placental Cellular Immune Response in Women Infected with Human Parvovirus B19 during Pregnancy. Clin. Diagn. Lab. Immunol. 2001, 8, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Spruill, L.S.; Batalis, N. Parvovirus B19 Infection: Characteristic Placental and Autopsy Findings in a Case of Intrauterine Fetal Demise. AJSP Rev. Rep. 2010, 15, 50–54. [Google Scholar] [CrossRef]
- Garcia, A.G.P.; Pegado, C.S.; Cubel, R.D.C.N.; Fonseca, M.E.F.; Sloboda, I.; Nascimento, J.P. feto-placentary pathology in human parvovirus b19 in-fection. Revista do Instituto de Medicina Tropical de São Paulo 1998, 40, 145–150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morey, A.L.; O’Neill, H.J.; Coyle, P.V.; Fleming, K.A. Immunohistological detection of human parvovirus B19 in formalinfixed, paraffin-embedded tissues. J. Pathol. 1992, 166, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Bui, T.; Ho, R.J.; Unadkat, J.D. Metabolism of 3′-azido-3′-deoxythymidine (AZT) in human placentae trophoblasts and Hof-bauer cells. Biochem. Pharmacol. 1994, 48, 383–389. [Google Scholar] [CrossRef]
- Lagaye, S.; Derrien, M.; Menu, E.; Coıto, C.; Tresoldi, E.; Mauclere, P.; Scarlatti, G.; Chaouat, G.; Barre-Sinoussi, F.; Bomsel, M. Cell-to-cell contact results in a selective translocation of maternal human immunodefi-ciency virus type 1 quasispecies across a trophoblastic barrier by both transcytosis and infection. J. Virol. 2001, 75, 4780–4791. [Google Scholar] [CrossRef]
- Chandwani, S.; Greco, M.A.; Mittal, K.; Antoine, C.; Krasinski, K.; Borkowsky, W. Pathology and human immunodeficiency virus expression in placentas of sero-positive women. J. Infect. Dis. 1991, 163, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.H.; Fox, H.E.; Reynolds-Kohler, C.; Nelson, J.A. HIV-1 in trophoblastic and villous Hofbauer cells, and haematological pre-cursors in eight-week fetuses. Lancet 1990, 335, 565–568. [Google Scholar] [CrossRef]
- Al-Husaini, A.M. Role of placenta in the vertical transmission of human immunodeficiency virus. J. Perinatol. 2008, 29, 331–336. [Google Scholar] [CrossRef]
- Behbahani, H.; Popek, E.; Garcia, P.; Andersson, J.; Spetz, A.L.; Landay, A.; Flener, Z.; Patterson, B.K. Up-Regulation of CCR5 Expression in the Placenta Is Associated with Human Im-munodeficiency Virus-1 Vertical Transmission. Am. J. Pathol. 2000, 157, 1811–1818. [Google Scholar] [CrossRef]
- Patterson, B.K.; Behbahani, H.; Kabat, W.J.; Sullivan, Y.; O’Gorman, M.R.; Landay, A.; Flener, Z.; Khan, N.; Yogev, R.; Andersson, J. Leukemia inhibitory factor inhibits HIV-1 replication and is upregulated in placentae from nontransmitting women. J. Clin. Investig. 2001, 107, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.L.; Chakraborty, R. HIV-1 at the placenta: Immune correlates of protection and infection. Curr. Opin. Infect. Dis. 2016, 29, 248–255. [Google Scholar] [CrossRef]
- Simister, N.E. Human placental Fc receptors and the trapping of immune complexes. Vaccine 1998, 16, 1451–1455. [Google Scholar] [CrossRef]
- Johnson, E.L.; Chakraborty, R. Placental Hofbauer cells limit HIV-1 replication and potentially offset mother to child trans-mission (MTCT) by induction of immunoregulatory cytokines. Retrovirology 2012, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Nessmann, C.; Imbert, M.C.; Meuris, S.; Puissant, F.; Hustin, J. Morphological aspects of the placenta in HIV pregnancies. Placenta 1988, 9, 633–642. [Google Scholar] [CrossRef]
- Martin, A.W.; Brady, K.; Smith, S.I.; DeCoste, D.; Page, D.V.; Malpica, A.; Wolf, B.; Neiman, R.S. Immunohistochemical localization of human immunodeficiency virus p24 antigen in placental tissue. Hum. Pathol. 1992, 23, 411–414. [Google Scholar] [CrossRef]
- Villegas-Castrejon, H.; Paredes-Vivas, Y.; Flores-Rivera, E.; Gorbea-Robles, M.C.; Arredondo-Garcia, J.L. [Comparative study of the placenta from HIV+ mothers. Ultrastructural analysis]. Ginecol. Obstet. Mex. 1996, 64, 167–176. [Google Scholar]
- Reef, S.E.; Frey, T.K.; Theall, K.; Abernathy, E.; Burnett, C.L.; Icenogle, J.; McCauley, M.M.; Wharton, M. The changing epidemiology of rubella in the 1990s: On the verge of elimination and new challenges for control and prevention. JAMA 2002, 287, 464–472. [Google Scholar] [CrossRef]
- Reef, S.E.; Plotkin, S.; Cordero, J.F.; Katz, M.; Cooper, L.; Schwartz, B.; Zimmerman-Swain, L.; Danovaro-Holliday, M.C.; Wharton, M. Preparing for Elimination of Congenital Rubella Syndrome (CRS): Summary of a Workshop on CRS Elimination in the United States. Clin. Infect. Dis. 2000, 31, 85–95. [Google Scholar] [CrossRef]
- Naeye, R.L.; Blanc, W. Pathogenesis of congenital rubella. JAMA 1965, 194, 1277–1283. [Google Scholar] [CrossRef]
- Woods, W.A.; Johnson, R.T.; Hostetler, D.D.; Lepow, M.L.; Robbins, F.C. Immunofluorescent studies on rubella-infected tissue cultures and human tis-sues. J. Immunol. 1966, 96, 253–260. [Google Scholar] [PubMed]
- Miller, E.; Cradock-Watson, J.E.; Pollock, T.M. Consequences of confirmed maternal rubella at successive stages of pregnancy. Lancet 1982, 2, 781–784. [Google Scholar] [CrossRef]
- Lazar, M.; Perelygina, L.; Martines, R.; Greer, P.; Paddock, C.D.; Peltecu, G.; Lupulescu, E.; Icenogle, J.; Zaki, S.R. Immunolocalization and Distribution of Rubella Antigen in Fatal Congenital Ru-bella Syndrome. EBioMedicine 2016, 3, 86–92. [Google Scholar] [CrossRef]
- Adamo, P.; Asís, L.; Silveyra, P.; Cuffini, C.; Pedranti, M.; Zapata, M. Rubella Virus Does Not Induce Apoptosis in Primary Human Embryo Fibroblast Cultures: A Possible Way of Viral Persistence in Congenital Infection. Viral Immunol. 2004, 17, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Maraqa, N.F. Congenital Rubella. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507879/ (accessed on 10 August 2020).
- Armstrong, G.L.; Schillinger, J.; Markowitz, L.; Nahmias, A.J.; Johnson, R.E.; McQuillan, G.M.; Louis, M.E.S. Incidence of herpes simplex virus type 2 infection in the United States. Am. J. Epidemiol. 2001, 153, 912–920. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Whitley, R.J.; Corey, L.; Arvin, A.; Mintz, E.; Lakeman, F.D.; Sumaya, C.V.; Wright, P.F.; Dunkle, L.M.; Steele, R.W.; Soong, S.-J.; et al. Changing Presentation of Herpes Simplex Virus Infection in Neonates. J. Infect. Dis. 1988, 158, 109–116. [Google Scholar] [CrossRef]
- Whitley, R.J.; Nahmias, A.J.; Visintine, A.M.; Fleming, C.L.; Alford, C.A.; Yeager, A.; Arvin, A.; Haynes, R.; Hilty, M.; Luby, J.; et al. The natural history of herpes simplex virus infection of mother and newborn. Pediatrics 1980, 66, 489–494. [Google Scholar] [CrossRef]
- Yeager, A.S.; Arvin, A.M. Reasons for the absence of a history of recurrent genital infections in mothers of neonates infected with herpes simplex virus. Pediatrics 1984, 73, 188–193. [Google Scholar]
- James, S.H.; Kimberlin, D.W. Neonatal Herpes Simplex Virus Infection. Infect. Dis. Clin. N. Am. 2015, 29, 391–400. [Google Scholar] [CrossRef]
- Brown, Z.A.; Wald, A.; Morrow, R.A.; Selke, S.; Zeh, J.; Corey, L. Effect of serologic status and cesarean delivery on transmission rates of herpes sim-plex virus from mother to infant. JAMA 2003, 289, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Robb, J.A.; Benirschke, K.; Barmeyer, R. Intrauterine Latent Herpes Simplex Virus Infection: Spontaneous Abortion. Obstet. Gynecol. Surv. 1987, 42, 510–511. [Google Scholar] [CrossRef]
- McDonagh, S.; Maidji, E.; Ma, W.; Chang, H.T.; Fisher, S.; Pereira, L. Viral and bacterial pathogens at the maternal-fetal interface. J. Infect. Dis. 2004, 190, 826–834. [Google Scholar] [CrossRef]
- Krummenacher, C.; Nicola, A.V.; Whitbeck, J.C.; Lou, H.; Hou, W.; Lambris, J.D.; Geraghty, R.J.; Spear, P.G.; Cohen, G.H.; Eisenberg, R.J. Herpes simplex virus glycoprotein D can bind to poliovirus recep-tor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J. Virol. 1998, 72, 7064–7074. [Google Scholar] [CrossRef]
- Whitbeck, J.C.; Peng, C.; Lou, H.; Xu, R.; Willis, S.H.; De Leon, M.P.; Peng, T.; Nicola, A.V.; Montgomery, R.I.; Warner, M.S.; et al. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J. Virol. 1997, 71, 6083–6093. [Google Scholar] [CrossRef] [PubMed]
- Marquez, L.; Levy, M.L.; Munoz, F.M.; Palazzi, D.L. A Report of Three Cases and Review of Intrauterine Herpes Simplex Virus Infection. Pediatr. Infect. Dis. J. 2011, 30, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.E.; McKenney, A.; Rabinowitz, L.; Das, A. Diagnosis of Neonatal Herpes Simplex Infection from the Placenta. Case Rep. Pediatr. 2020, 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.S.; Popek, E.J.; Wise, B.; Hatzenbuehler, L.; Arunachalam, A.R.; Hair, A.B. Ascending in Utero Herpes Simplex Virus Infection in an Initially Healthy-Appearing Premature Infant. Pediatr. Dev. Pathol. 2015, 18, 155–158. [Google Scholar] [CrossRef]
- Roberts, D.J. Placental Infections. In Placental and Gestational Pathology. Diag-Nostic Pediatric Pathology; Roberts, D.J., Redline, R.W., Boyd, T.K., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 115–136. [Google Scholar]
- Quemelo, P.R.V.; Lima, D.M.; Fonseca, B.A.L.D.; Peres, L.C. Detection of parvovirus B19 infection in formalin-fixed and paraf-fin-embedded placenta and fetal tissues. Rev. Inst. Med. Trop. São Paulo 2007, 49, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Menter, T.; Mertz, K.D.; Jiang, S.; Chen, H.; Monod, C.; Tzankov, A.; Waldvogel, S.; Schulzke, S.M.; Hösli, I.; Bruder, E. Placental Pathology Findings during and after SARS-CoV-2 Infection: Features of Villitis and Malperfusion. Pathobiology 2021, 88, 69–77. [Google Scholar] [CrossRef]
- Sharps, M.C.; Hayes, D.J.; Lee, S.; Zou, Z.; Brady, C.A.; Almoghrabi, Y.; Kerby, A.; Tamber, K.K.; Jones, C.J.; Waldorf, K.M.A.; et al. A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection. Placenta 2020, 101, 13–29. [Google Scholar] [CrossRef]
- Di Mascio, D.; Khalil, A.; Saccone, G.; Rizzo, G.; Buca, D.; Liberati, M.; Vecchiet, J.; Nappi, L.; Scambia, G.; Berghella, V.; et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM. 2020, 2, 100107. [Google Scholar] [CrossRef]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar]
- Kreis, N.-N.; Ritter, A.; Louwen, F.; Yuan, J. A Message from the Human Placenta: Structural and Immunomodulatory Defense against SARS-CoV-2. Cells 2020, 9, 1777. [Google Scholar] [CrossRef]
- Valdes, G.; Neves, L.A.A.; Anton, L.; Corthorn, J.; Chacon, C.; Germain, A.M.; Merrill, D.C.; Ferrario, C.M.; Sarao, R.; Penninger, J.; et al. Distribution of angiotensin-(1-7) and ACE2 in human placentas of normal and patho-logical pregnancies. Placenta 2006, 27, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Millet, J.K.; Whittaker, G.R. Host cell proteases: Critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015, 202, 120–134. [Google Scholar] [CrossRef]
- Millet, J.K.; Whittaker, G.R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc. Natl. Acad. Sci. USA 2014, 111, 15214–15219. [Google Scholar] [CrossRef] [PubMed]
- Pique-Regi, R.; Romero, R.; Tarca, A.L.; Luca, F.; Xu, Y.; Alazizi, A.; Leng, Y.; Hsu, C.D.; Gomez-Lopez, N. Does the human placenta express the canonical cell entry mediators for SARS-CoV-2? eLife 2020, 9, e58716. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, W.; Zhou, Y.S.; Lian, J.Q.; Zhang, Z.; Du, P.; Gong, L.; Zhang, Y.; Cui, H.Y.; Geng, J.J.; et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv 2020. [Google Scholar] [CrossRef]
- Schwartz, D.A.; Baldewijns, M.; Benachi, A.; Bugatti, M.; Collins, R.R.; De Luca, D.; Facchetti, F.; Linn, R.L.; Marcelis, L.; Morotti, D.; et al. Chronic Histiocytic Intervillositis with Trophoblast Necrosis are Risk Factors Associated with Placental Infection from Coronavirus Disease 2019 (COVID-19) and Intrauterine Maternal-Fetal Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Transmission in Liveborn and Stillborn Infants. Arch. Pathol. Lab. Med. 2020, 31. [Google Scholar] [CrossRef]
- Hosier, H.; Farhadian, S.F.; Morotti, R.A.; Deshmukh, U.; Lu-Culligan, A.; Campbell, K.H.; Yasumoto, Y.; Vogels, C.B.; Casanovas-Massana, A.; Vijayakumar, P.; et al. SARS–CoV-2 infection of the placenta. J. Clin. Invest. 2020, 130, 4947–4953. [Google Scholar] [CrossRef]
- Lu-Culligan, A.; Chavan, A.R.; Vijayakumar, P.; Irshaid, L.; Courchaine, E.M.; Milano, K.M.; Tang, Z.; Pope, S.D.; Song, E.; Vogels, C.B.; et al. SARS-CoV-2 infection in pregnancy is associated with robust inflamma-tory response at the maternal-fetal interface. medRxiv. 2021. [Google Scholar] [CrossRef]
- Mulvey, J.J.; Magro, C.M.; Ma, L.X.; Nuovo, G.J.; Baergen, R.N. Analysis of complement deposition and viral RNA in placentas of COVID-19 patients. Ann. Diagn. Pathol. 2020, 46, 151530. [Google Scholar] [CrossRef] [PubMed]
- Shanes, E.D.; Mithal, L.B.; Otero, S.; Azad, H.A.; Miller, E.S.; Goldstein, J.A. Placental Pathology in COVID-19. Am. J. Clin. Pathol. 2020, 154, 23–32. [Google Scholar] [CrossRef]
- Richtmann, R.; Torloni, M.R.; Otani, A.R.O.; Levi, J.E.; Tobara, M.C.; de Almeida Silva, C.; Dias, L.; Miglioli-Galvão, L.; Silva, P.M.; Kondo, M.M.; et al. Fetal deaths in pregnancies with SARS-CoV-2 infection in Brazil: A case series. Case Rep. Women’s Health 2020, 27, e00243. [Google Scholar] [CrossRef]
- Baergen, R.N.; Heller, D.S. Placental Pathology in Covid-19 Positive Mothers: Preliminary Findings. Pediatr. Dev. Pathol. 2020, 23, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.L.; Quade, B.; Deshpande, V.; Mino-Kenudson, M.; Ting, D.T.; Desai, N.; Dygulska, B.; Heyman, T.; Salafia, C.; Shen, D.; et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental his-topathology: A series of 19 placentas from COVID-19-positive mothers. Mod. Pathol. 2020, 33, 2092–2103. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).