Engineered Lactobacillus paracasei Producing Palmitoylethanolamide (PEA) Prevents Colitis in Mice
Abstract
1. Introduction
2. Results
2.1. Time-Dependent Production of PEA by pNAPE-LP and Exogenous Palmitate
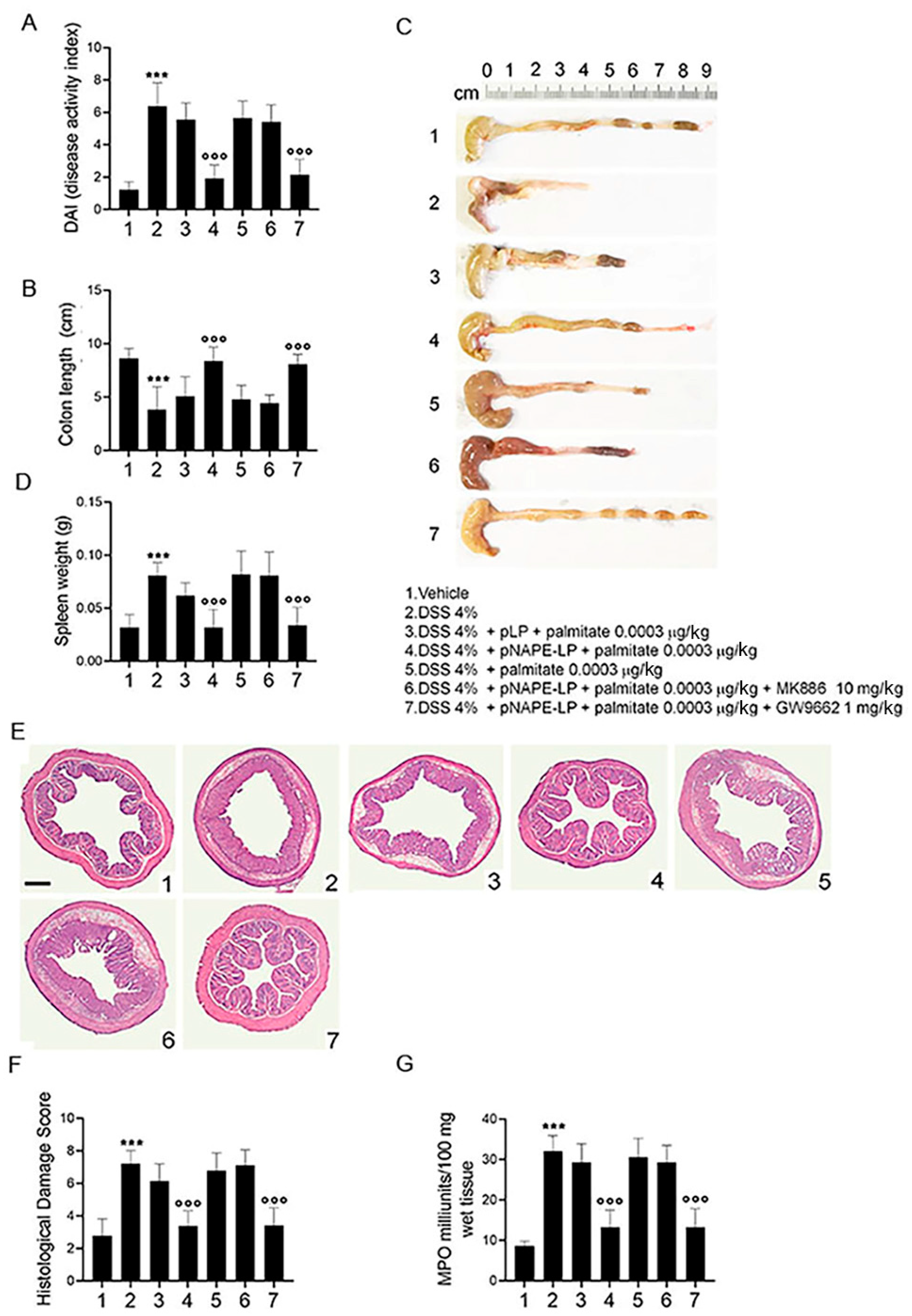
2.2. Co-Administration of pNAPE-LP and Palmitate Improves the Severity of DSS-Induced Colitis in Mice
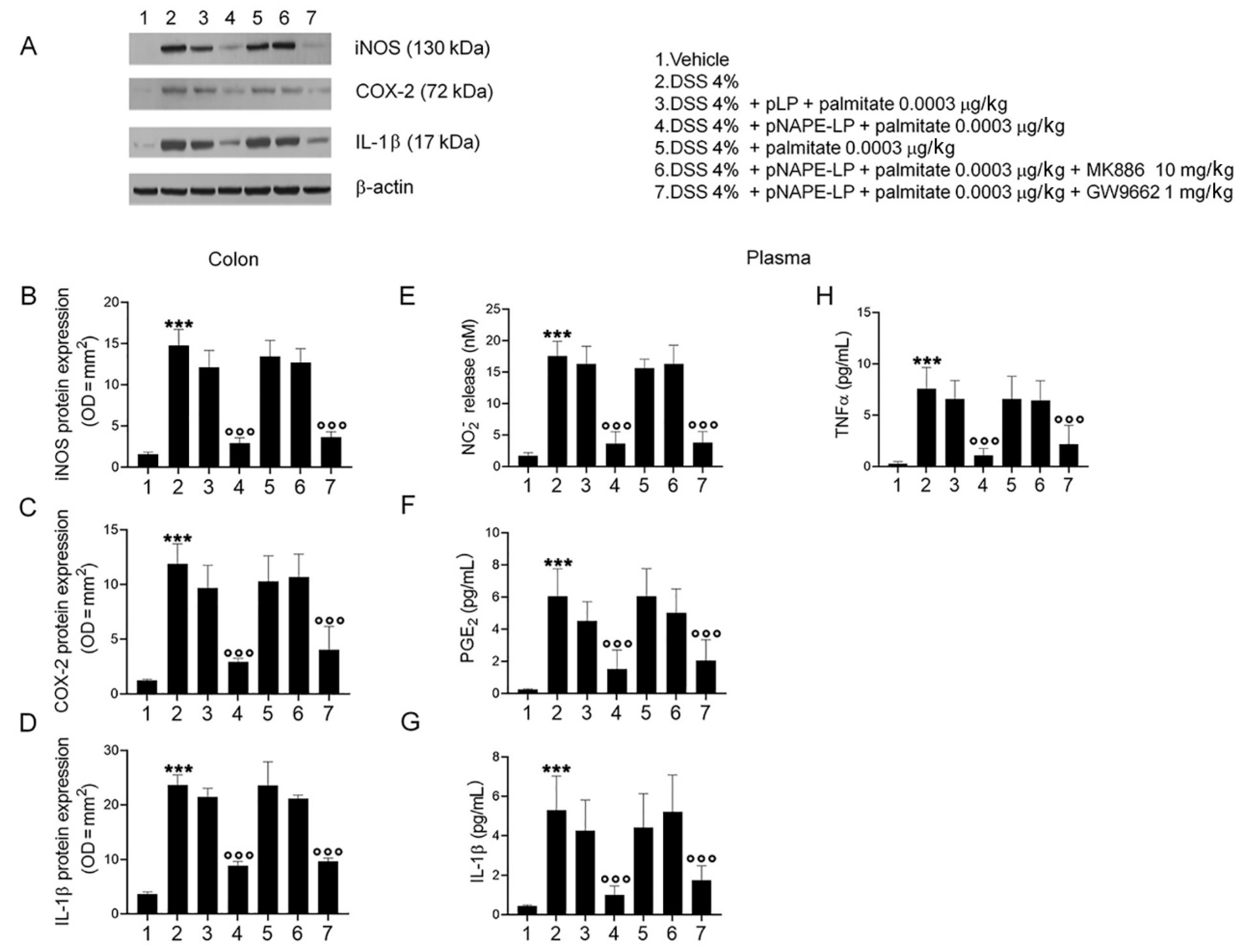
2.3. pNAPE-LP and Palmitate Co-Administration Improves Colon Histopathological Damage, Mucosal Neutrophils Infiltration and Decreases Inflammatory Markers Expression and Release in DSS-Treated Mice
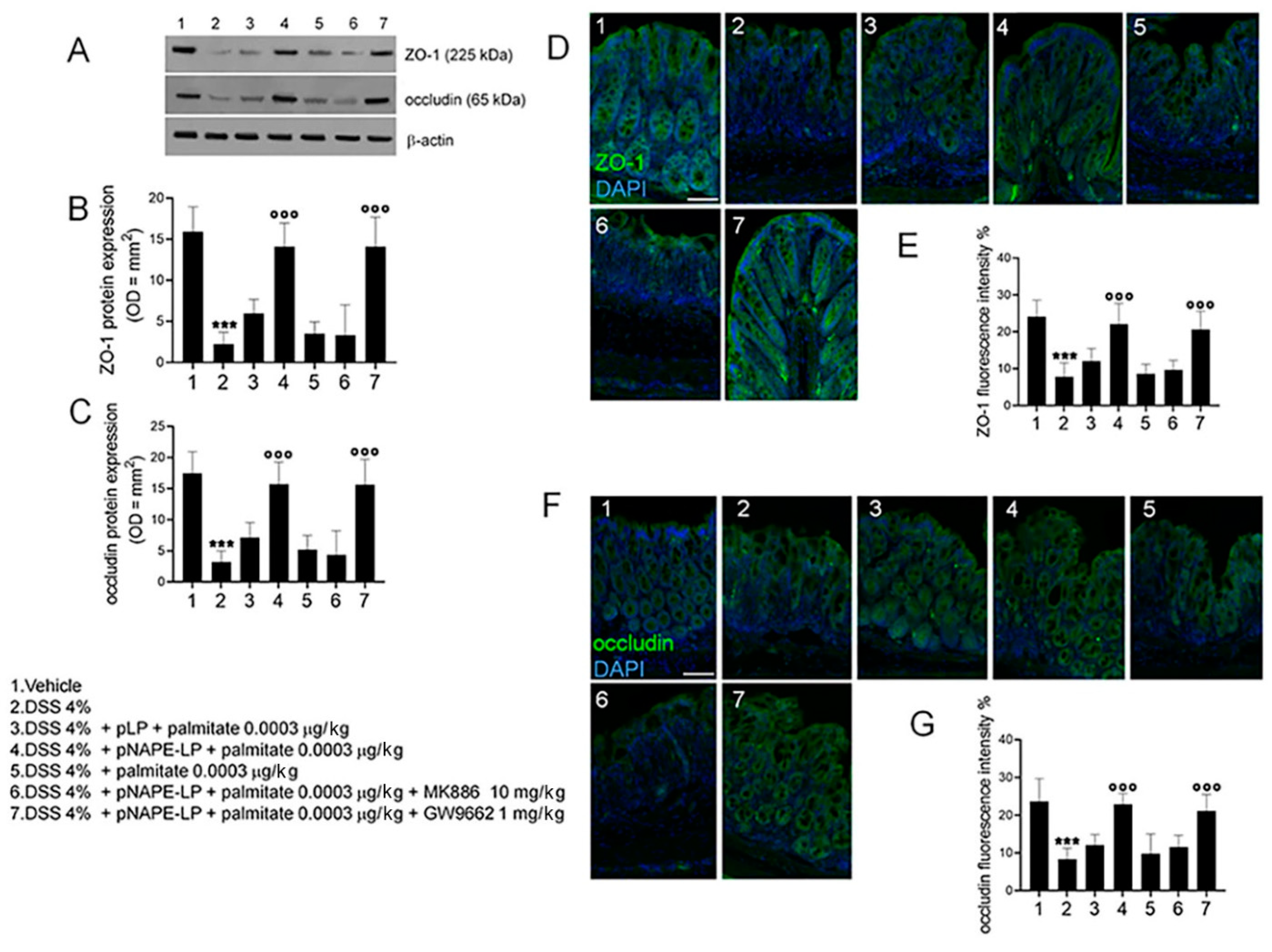
2.4. pNAPE-LP and Palmitate Co-Administration Restores DSS-Induced Mucosal Integrity
3. Discussion
4. Materials and Methods
4.1. Generation of Genetically Modified Strains of Lactobacillus paracasei subsp. paracasei F19
4.2. Animals and Experimental Design
4.3. In Vitro and In Vivo Quantification of Bacteria-Produced PEA by HPLC–MS Method
4.4. Disease Activity Index (DAI)
4.5. Histopathological Analysis
4.6. Protein Extraction and Western Blot Analysis
4.7. Preparation of Blood Samples
4.8. Enzyme-Linked Immunosorbent Assay for TNFα, PGE2 and IL-1β
4.9. NO Quantification
4.10. Myeloperoxidase Activity
4.11. Immunofluorescence Analysis
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hesselink, J.M.K.; Kopsky, D.J.; Witkamp, R.F. Palmitoylethanolamide (PEA)—‘Promiscuous’ anti-inflammatory and analgesic molecule at the interface between nutrition and pharma. PharmaNutrition 2014, 2, 19–25. [Google Scholar] [CrossRef]
- Al Houayek, M.; Bottemanne, P.; Subramanian, K.V.; Lambert, D.M.; Makriyannis, A.; Cani, P.; Muccioli, G.G. N -Acylethanolamine-hydrolyzing acid amidase inhibition increases colon N-palmitoylethanolamine levels and counteracts murine colitis. FASEB J. 2015, 29, 650–661. [Google Scholar] [CrossRef]
- Hussain, Z.; Uyama, T.; Tsuboi, K.; Ueda, N. Mammalian enzymes responsible for the biosynthesis of N-acylethanolamines. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2017, 1862, 1546–1561. [Google Scholar] [CrossRef]
- Zhu, C.; De Solorzano, C.O.; Sahar, S.; Realini, N.; Fung, E.; Sassone-Corsi, P.; Piomelli, D. Proinflammatory Stimuli Control N-Acylphosphatidylethanolamine-Specific Phospholipase D Expression in Macrophages. Mol. Pharmacol. 2011, 79, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Romano, B.; Petrosino, S.; Pagano, E.; Capasso, R.; Coppola, D.; Battista, G.; Orlando, P.; Di Marzo, V.; Izzo, A.A. Palmitoylethanolamide, a naturally occurring lipid, is an orally effective intestinal anti-inflammatory agent. Br. J. Pharmacol. 2015, 172, 142–158. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E. Cannabinoids go nuclear: Evidence for activation of peroxisome proliferator-activated receptors. Br. J. Pharmacol. 2007, 152, 576–582. [Google Scholar] [CrossRef]
- Esposito, G.; Capoccia, E.; Turco, F.; Palumbo, I.; Lu, J.; Steardo, A.; Cuomo, R.; Sarnelli, G.; Steardo, L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut 2014, 63, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Couch, D.G.; Cook, H.; Ortori, C.; Barrett, D.; Lund, J.N.; O’Sullivan, S.E. Palmitoylethanolamide and Cannabidiol Prevent Inflammation-induced Hyperpermeability of the Human Gut In Vitro and In Vivo—A Randomized, Placebo-controlled, Double-blind Controlled Trial. Inflamm. Bowel Dis. 2019, 25, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Sarnelli, G.; Gigli, S.; Capoccia, E.; Iuvone, T.; Cirillo, C.; Seguella, L.; Nobile, N.; D’Alessandro, A.; Pesce, M.; Steardo, L.; et al. Palmitoylethanolamide Exerts Antiproliferative Effect and Downregulates VEGF Signaling in Caco-2 Human Colon Carcinoma Cell Line Through a Selective PPAR-α-Dependent Inhibition of Akt/mTOR Pathway. Phytotherapy Res. 2016, 30, 963–970. [Google Scholar] [CrossRef]
- Löwenberg, M.; D’Haens, G. Novel Targets for Inflammatory Bowel Disease Therapeutics. Curr. Gastroenterol. Rep. 2013, 15, 311. [Google Scholar] [CrossRef] [PubMed]
- Sarnelli, G.; D’Alessandro, A.; Iuvone, T.; Capoccia, E.; Gigli, S.; Pesce, M.; Seguella, L.; Nobile, N.; Aprea, G.; Maione, F.; et al. Palmitoylethanolamide Modulates Inflammation-Associated Vascular Endothelial Growth Factor (VEGF) Signaling via the Akt/mTOR Pathway in a Selective Peroxisome Proliferator-Activated Receptor Alpha (PPAR-α)-Dependent Manner. PLoS ONE 2016, 11, e0156198. [Google Scholar] [CrossRef]
- Russo, R.; Cristiano, C.; Avagliano, C.; De Caro, C.; La Rana, G.; Raso, G.M.; Canani, R.B.; Meli, R.; Calignano, A. Gut-brain Axis: Role of Lipids in the Regulation of Inflammation, Pain and CNS Diseases. Curr. Med. Chem. 2018, 25, 3930–3952. [Google Scholar] [CrossRef]
- Gabrielsson, L.; Mattsson, S.; Fowler, C.J. Palmitoylethanolamide for the treatment of pain: Pharmacokinetics, safety and efficacy. Br. J. Clin. Pharmacol. 2016, 82, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Djordjević, G. Positive Selection, Cloning Vectors for Gram-Positive Bacteria Based on a Restriction Endonuclease Cassette. Plasmid 1996, 35, 37–45. [Google Scholar] [CrossRef]
- Djordjevic, G.M.; Klaenhammer, T.R. Inducible gene expression systems in Lactococcus lactis. Mol. Biotechnol. 1998, 9, 127–139. [Google Scholar] [CrossRef]
- Mättö, J.; Fondén, R.; Tolvanen, T.; Von Wright, A.; Vilpponen-Salmela, T.; Satokari, R.; Saarela, M. Intestinal survival and persistence of probiotic Lactobacillus and Bifidobacterium strains administered in triple-strain yoghurt. Int. Dairy J. 2006, 16, 1174–1180. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Curr. Protoc. Immunol. 2014, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef]
- Sarnelli, G.; Pesce, M.; Seguella, L.; Lu, J.; Efficie, E.; Tack, J.; De Palma, F.D.E.; D’Alessandro, A.; Esposito, G. Impaired Duodenal Palmitoylethanolamide Release Underlies Acid-Induced Mast Cell Activation in Functional Dyspepsia. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Hammer, H.F. Gut Microbiota and Inflammatory Bowel Disease. Dig. Dis. 2011, 29, 550–553. [Google Scholar] [CrossRef]
- Lane, E.R.; Zisman, T.L.; Suskind, D.L. The microbiota in inflammatory bowel disease: Current and therapeutic insights. J. Inflamm. Res. 2017, 10, 63–73. [Google Scholar] [CrossRef]
- Gioacchini, G.; Rossi, G.; Carnevali, O. Host-probiotic interaction: New insight into the role of the endocannabinoid system by in vivo and ex vivo approaches. Sci. Rep. 2017, 7, 1261. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Abraham, B.P.; Quigley, E.M. Probiotics in Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2017, 46, 769–782. [Google Scholar] [CrossRef]
- Ganji-Arjenaki, M.; Rafieian-Kopaei, M. Probiotics are a good choice in remission of inflammatory bowel diseases: A meta analysis and systematic review. J. Cell. Physiol. 2018, 233, 2091–2103. [Google Scholar] [CrossRef]
- Steidler, L.; Hans, W.; Schotte, L.; Neirynck, S.; Obermeier, F.; Falk, W.; Fiers, W.; Remaut, E. Treatment of Murine Colitis by Lactococcus lactis Secreting Interleukin-10. Science 2000, 289, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
- Zurita-Turk, M.; Del Carmen, S.; Santos, A.C.; Pereira, V.B.; Cara, D.C.; Leclercq, S.Y.; dM de LeBlanc, A.; Azevedo, V.; Chatel, J.-M.; Leblanc, J.G.; et al. Lactococcus lactis carrying the pValac DNA expression vector coding for IL-10 reduces inflammation in a murine model of experimental colitis. BMC Biotechnol. 2014, 14, 73. [Google Scholar] [CrossRef]
- Braat, H.; Rottiers, P.; Hommes, D.W.; Huyghebaert, N.; Remaut, E.; Remon, J.; van Deventer, S.J.; Neirynck, S.; Peppelenbosch, M.P.; Steidler, L. A Phase I Trial with Transgenic Bacteria Expressing Interleukin-10 in Crohn’s Disease. Clin. Gastroenterol. Hepatol. 2006, 4, 754–759. [Google Scholar] [CrossRef]
- Miljkovic, M.; Thomas, M.; Serror, P.; Rigottier-Gois, L.; Kojic, M. Binding activity to intestinal cells and transient colonization in mice of two Lactobacillus paracasei subsp. paracasei strains with high aggregation potential. World J. Microbiol. Biotechnol. 2019, 35, 85. [Google Scholar] [CrossRef]
- Zampieri, N.; Pietrobelli, A.; Biban, P.; Soffiati, M.; Dall’agnola, A.; Camoglio, F.S. Lactobacillus paracasei subsp. paracasei F19 in Bell’s stage 2 of necrotizing enterocolitis. Minerva Pediatr. 2013, 65, 353–360. [Google Scholar]
- Lavilla-Lerma, L.; Pérez-Pulido, R.; Martínez-Bueno, M.; Maqueda, M.; Valdivia, E. Characterization of functional, safety, and gut survival related characteristics of Lactobacillus strains isolated from farmhouse goat’s milk cheeses. Int. J. Food Microbiol. 2013, 163, 136–145. [Google Scholar] [CrossRef]
- Rossi, G.; Gioacchini, G.; Pengo, G.; Suchodolski, J.S.; Jergens, A.E.; Allenspach, K.; Gavazza, A.; Scarpona, S.; Berardi, S.; Galosi, L.; et al. Enterocolic increase of cannabinoid receptor type 1 and type 2 and clinical improvement after probiotic administration in dogs with chronic signs of colonic dysmotility without mucosal inflammatory changes. Neurogastroenterol. Motil. 2020, 32, e13717. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, S.; Di Marzo, V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br. J. Pharmacol. 2017, 174, 1349–1365. [Google Scholar] [CrossRef]
- Nestmann, E.R. Safety of micronized palmitoylethanolamide (microPEA): Lack of toxicity and genotoxic potential. Food Sci. Nutr. 2017, 5, 292–309. [Google Scholar] [CrossRef]
- Petrosino, S.; Cordaro, M.; Verde, R.; Moriello, A.S.; Marcolongo, G.; Schievano, C.; Siracusa, R.; Piscitelli, F.; Peritore, A.F.; Crupi, R.; et al. Oral Ultramicronized Palmitoylethanolamide: Plasma and Tissue Levels and Spinal Anti-hyperalgesic Effect. Front. Pharmacol. 2018, 9, 249. [Google Scholar] [CrossRef]
- Pesce, M.; D’Alessandro, A.; Borrelli, O.; Gigli, S.; Seguella, L.; Cuomo, R.; Esposito, G.; Sarnelli, G. Endocannabinoid-related compounds in gastrointestinal diseases. J. Cell. Mol. Med. 2017, 22, 706–715. [Google Scholar] [CrossRef]
- Pesce, M.; Esposito, G.; Sarnelli, G. Endocannabinoids in the treatment of gastrointestinal inflammation and symptoms. Curr. Opin. Pharmacol. 2018, 43, 81–86. [Google Scholar] [CrossRef]
- Cani, P.; Plovier, H.; Van Hul, M.; Geurts, L.; Delzenne, N.M.; Druart, C.; Everard, A. Endocannabinoids—At the crossroads between the gut microbiota and host metabolism. Nat. Rev. Endocrinol. 2016, 12, 133–143. [Google Scholar] [CrossRef]
- Kim, J.J.; Shajib, S.; Manocha, M.M.; Khan, W.I. Investigating Intestinal Inflammation in DSS-induced Model of IBD. J. Vis. Exp. 2012, e3678. [Google Scholar] [CrossRef]
- De Filippis, D.; d’Amico, A.; Cipriano, M.; Petrosino, S.; Orlando, P.; Di Marzo, V.; Iuvone, T.; Endocannabinoid Research, Group. Levels of endocannabinoids and palmitoylethanolamide and their pharmacological manipulation in chronic granulomatous inflammation in rats. Pharmacol. Res. 2010, 61, 321–328. [Google Scholar] [CrossRef]
- Gachet, M.S.; Rhyn, P.; Bosch, O.G.; Quednow, B.B.; Gertsch, J. A quantitiative LC-MS/MS method for the measurement of arachidonic acid, prostanoids, endocannabinoids, N-acylethanolamines and steroids in human plasma. J. Chromatogr. B 2015, 976–977, 6–18. [Google Scholar] [CrossRef]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 1993, 69, 238–249. [Google Scholar] [PubMed]
- Li, R.; Kim, M.-H.; Sandhu, A.K.; Gao, C.; Gu, L. Muscadine Grape (Vitis rotundifolia) or Wine Phytochemicals Reduce Intestinal Inflammation in Mice with Dextran Sulfate Sodium-Induced Colitis. J. Agric. Food Chem. 2017, 65, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.; Radomski, M.; Carnuccio, R.; Moncada, S. Glucocorticoids inhibit the induction of nitric oxide synthase in macrophages. Biochem. Biophys. Res. Commun. 1990, 172, 1246–1252. [Google Scholar] [CrossRef]
- Cassini-Vieira, P.; Moreira, C.F.; da Silva, M.F.; da Silva Barcelos, L. Estimation of Wound Tissue Neutrophil and Macrophage Accumulation by Measuring Myeloperoxidase (MPO) and N-Acetyl-β-D-glucosaminidase (NAG) Activities. Bio-Protoc. 2015, 5, e1662. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, G.; Pesce, M.; Seguella, L.; Lu, J.; Corpetti, C.; Del Re, A.; De Palma, F.D.E.; Esposito, G.; Sanseverino, W.; Sarnelli, G. Engineered Lactobacillus paracasei Producing Palmitoylethanolamide (PEA) Prevents Colitis in Mice. Int. J. Mol. Sci. 2021, 22, 2945. https://doi.org/10.3390/ijms22062945
Esposito G, Pesce M, Seguella L, Lu J, Corpetti C, Del Re A, De Palma FDE, Esposito G, Sanseverino W, Sarnelli G. Engineered Lactobacillus paracasei Producing Palmitoylethanolamide (PEA) Prevents Colitis in Mice. International Journal of Molecular Sciences. 2021; 22(6):2945. https://doi.org/10.3390/ijms22062945
Chicago/Turabian StyleEsposito, Giuseppe, Marcella Pesce, Luisa Seguella, Jie Lu, Chiara Corpetti, Alessandro Del Re, Fatima Domenica Elisa De Palma, Giovanni Esposito, Walter Sanseverino, and Giovanni Sarnelli. 2021. "Engineered Lactobacillus paracasei Producing Palmitoylethanolamide (PEA) Prevents Colitis in Mice" International Journal of Molecular Sciences 22, no. 6: 2945. https://doi.org/10.3390/ijms22062945
APA StyleEsposito, G., Pesce, M., Seguella, L., Lu, J., Corpetti, C., Del Re, A., De Palma, F. D. E., Esposito, G., Sanseverino, W., & Sarnelli, G. (2021). Engineered Lactobacillus paracasei Producing Palmitoylethanolamide (PEA) Prevents Colitis in Mice. International Journal of Molecular Sciences, 22(6), 2945. https://doi.org/10.3390/ijms22062945





