Chromatography-Independent Fractionation and Newly Identified Molecular Features of the Adzuki Bean (Vigna angularis Willd.) β-vignin Protein
Abstract
1. Introduction
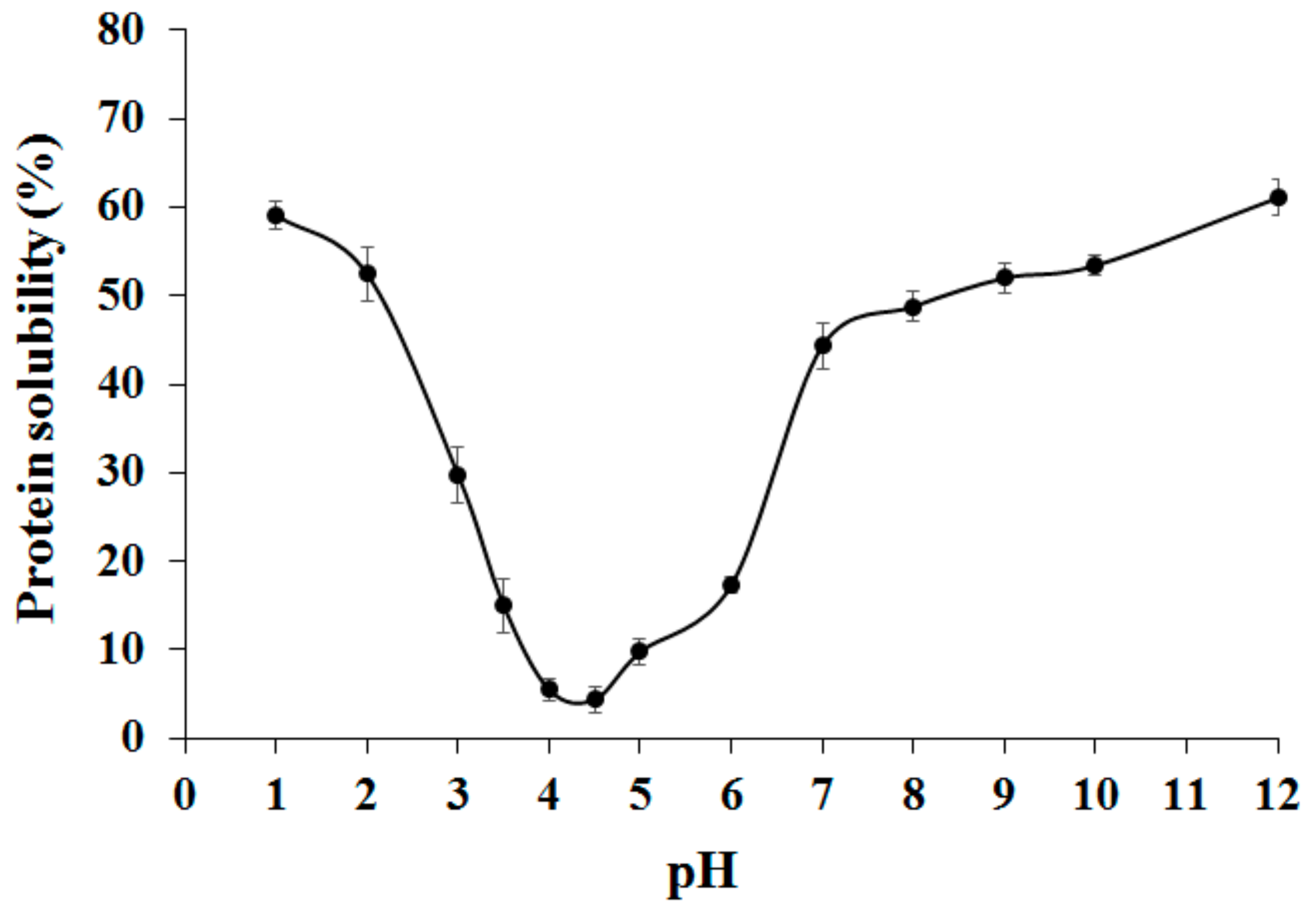
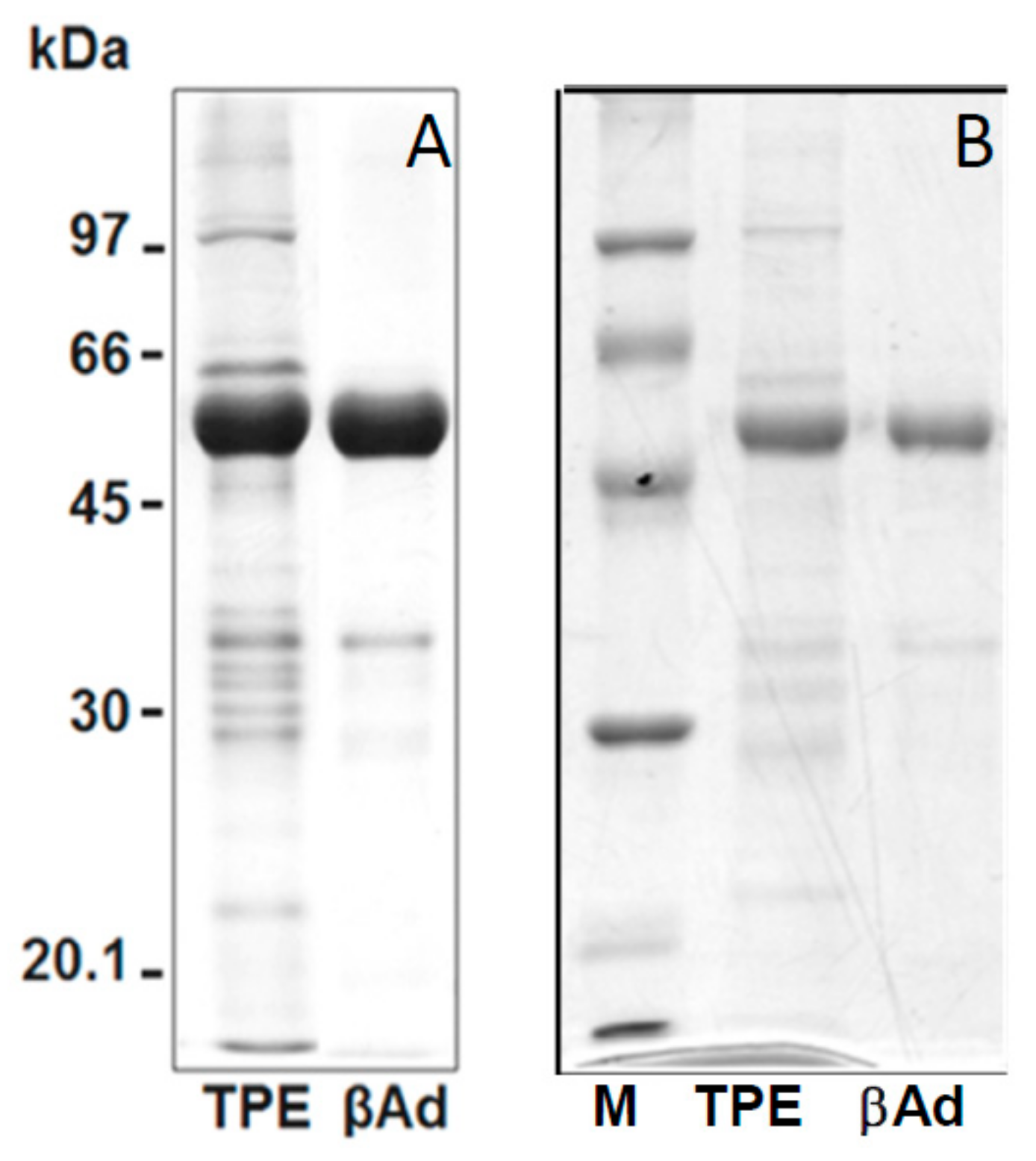
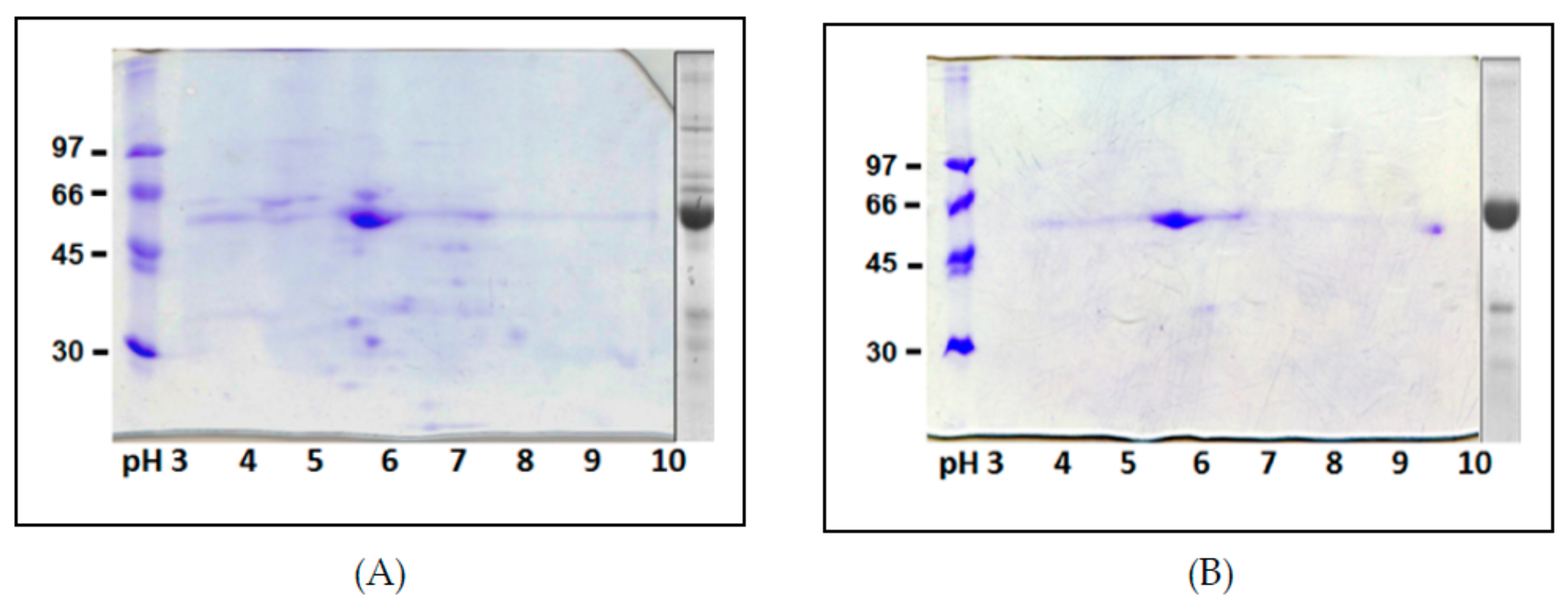
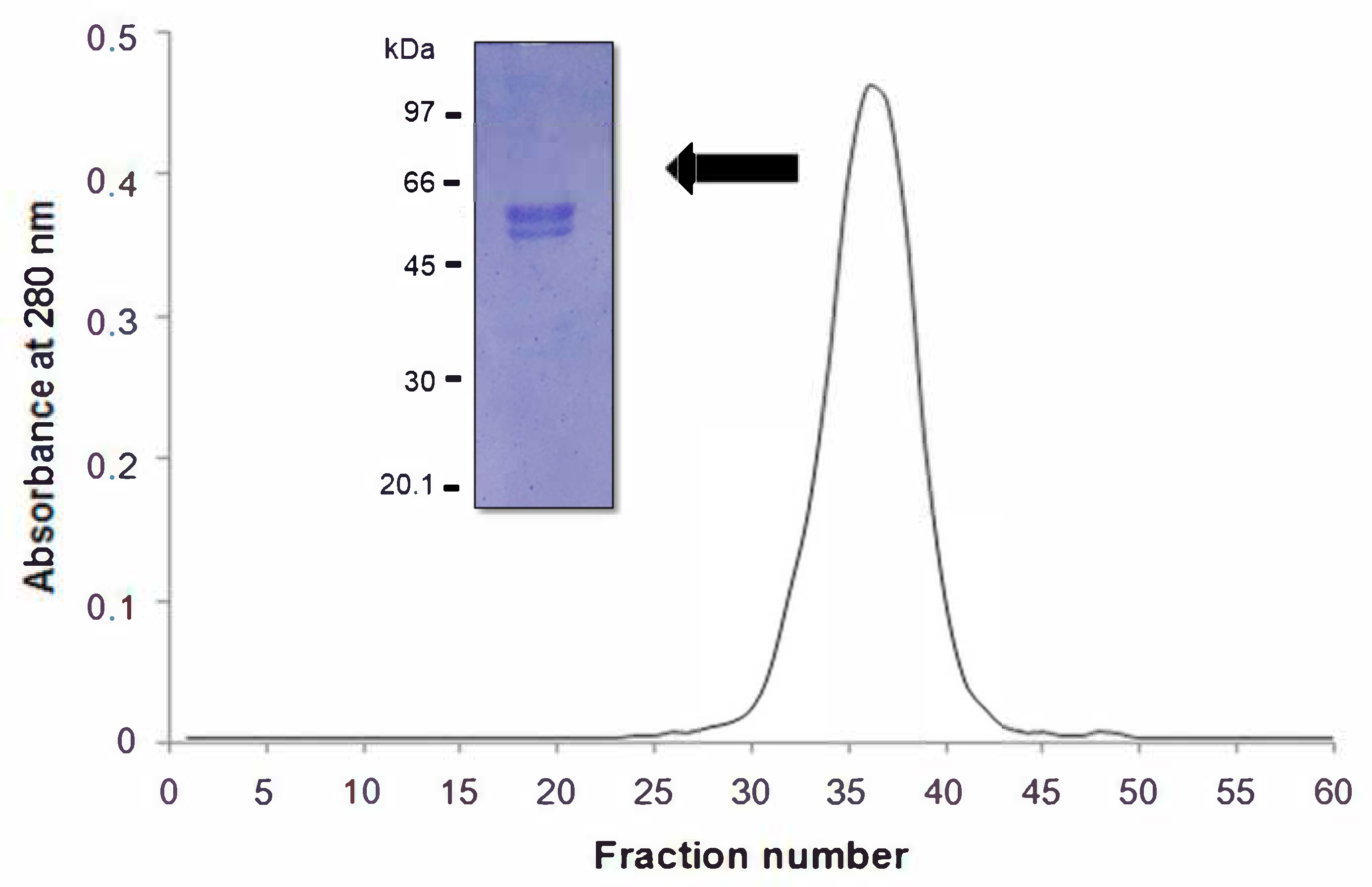
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material and Reagents
3.2. Chemical Composition
3.3. Protein Solubility
3.4. Protein Fractionation and Isolation of Raw β-vignin from Adzuki Seed
3.5. Molecular Exclusion Chromatography
3.6. Ion-Exchange Chromatography
3.7. Molecular Weight Determination
3.8. SDS-PAGE and IEF/SDS-PAGE
3.9. In Vitro Digestibility
3.10. Detection of N-glycosylated Polypeptides by Concanavalin A (ConA)
3.11. N-Terminal Amino Acid Sequencing
3.12. Inductively Coupled Plasma/Mass Spectrometry (ICP-MS) for Globulin Metal Content
3.13. Immobilised Metal Affinity Chromatography
3.14. IL-8 Expression in Caco-2 Cells
3.15. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TPE | Total protein extract |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel |
| IEF | Isoelectric focusing |
| DTT | Dithiothreitol |
| TNBS | 2,4,6-trinitrobenzenesulfonic acid |
| DH | Degree of hydrolysis |
| PVDF | Polyvinylidene difluoride |
| Ni-NTA | Nickel-charged affinity resin |
| Net-N-Glyc | N-Glycosylation sites |
| NCBI | National Center for Biotechnology Information |
| ConA | Concanavalin A |
| PDB | Protein Data Bank |
References
- Foyer, C.H.; Lam, H.M.; Nguyen, H.T.; Siddique, K.H.M.; Varshney, R.K.; Colmer, T.D.; Cowling, W.; Bramley, H.; Mori, T.A.; Hodgson, J.M.; et al. Neglecting legumes has compromised human health and sustainable food production. Nat. Plants 2016, 2, 1–10. [Google Scholar] [CrossRef]
- Barac, M.B.; Pesic, M.B.; Stanojevic, S.P.; Kostic, A.Z.; Bivolarevic, V. Comparative study of the functional properties of three legume seed isolates: Adzuki, pea and soy bean. J. Food Sci. Technol. 2015, 52, 2779–2787. [Google Scholar] [CrossRef]
- Chen, T.H.H.; Gusta, L.V.; Tjahjadi, C.; Breene, W.M. Electrophoretic characterization of adzuki bean (Vigna angularis) seed proteins. J. Agric. Food Chem. 1984, 32, 396–399. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, J.; Lei, L.; Wang, L.; Wang, X.; Ying Ma, K.; Yang, X.; Chen, Z.Y. 7S protein is more effective than total soybean protein isolate in reducing plasma cholesterol. J. Funct. Foods 2017, 36, 18–26. [Google Scholar] [CrossRef]
- Ferreira, E.S.; Amaral, A.L.S.; Demonte, A.; Zanelli, C.F.; Capraro, J.; Duranti, M.; Neves, V.A. Hypocholesterolaemic effect of rat-administered oral doses of the isolated 7S globulins from cowpeas and adzuki beans. J. Nutr. Sci. 2015, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.T.; Ma, C.Y. Thermal properties of Phaseolus angularis (red bean) globulin. Food Chem. 2001, 73, 453–460. [Google Scholar] [CrossRef]
- Scarafoni, A.; Di Cataldo, A.; Vassilevskaia, T.D.; Bekman, E.P.; Rodrigues-Pousada, C.; Ceciliani, F.; Duranti, M. Cloning, sequencing and expression in the seeds and radicles of two Lupinus albus conglutin γ genes. Biochim. Biophys. Acta Gene Struct. Expr. 2001, 1519, 147–151. [Google Scholar] [CrossRef]
- Duranti, M.; Gius, C. Legume seeds: Protein content and nutritional value. F. Crop. Res. 1997, 53, 31–45. [Google Scholar] [CrossRef]
- Sakakibara, M.; Aoki, T.; Noguchi, H. Isolation and characterization of 7S protein-I of Phaseolus angularis (Adzuki bean). Agric. Biol. Chem. 1979, 43, 1951–1957. [Google Scholar] [CrossRef]
- Fukuda, T.; Prak, K.; Fujioka, M.; Maruyama, N.; Utsumi, S. Physicochemical properties of native adzuki bean (Vigna angularis) 7S globulin and the molecular cloning of its cDNA isoforms. J. Agric. Food Chem. 2007, 55, 3667–3674. [Google Scholar] [CrossRef] [PubMed]
- Duranti, M.; Lovati, M.R.; Dani, V.; Barbiroli, A.; Scarafoni, A.; Castiglioni, S.; Ponzone, C.; Morazzoni, P. The α′ subunit from soybean 7S globulin lowers plasma lipids and upregulates liver β-VLDL receptors in rats fed a hypercholesterolemic diet. J. Nutr. 2004, 134, 1334–1339. [Google Scholar] [CrossRef]
- Fassini, P.G.; de Souza Ferreira, E.; da Silva, M.A.; Neves, V.A.; Demonte, A. Soybean glycinin (11S) increases HDL-cholesterol in hypercholesterolemic rats. Nutr. Food Sci. 2012, 42. [Google Scholar] [CrossRef]
- de Souza Ferreira, E.; Silva, M.A.; Demonte, A.; Neves, V.A. β-Conglycinin (7S) and glycinin (11S) exert a hypocholesterolemic effect comparable to that of fenofibrate in rats fed a high-cholesterol diet. J. Funct. Foods 2010, 2, 275–283. [Google Scholar] [CrossRef]
- Amaral, A.L.; Ferreira, E.S.; Neves, V.A.; Demonte, A. Legumin from chickpea: Hypolipidemic effect in the liver of hypercholesterolemic rats. Nutr. Food Sci. 2014, 44, 378–388. [Google Scholar] [CrossRef]
- Faridy, J.-C.M.; Stephanie, C.-G.M.; Gabriela, M.-M.O.; Cristian, J.-M. Biological activities of chickpea in human health (Cicer arietinum L.). A review. Plant Foods Hum. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Zanoni, C.; Arnoldi, A. Three peptides from soy glycinin modulate glucose metabolism in human hepatic HepG2 cells. Int. J. Mol. Sci. 2015, 16, 27362–27370. [Google Scholar] [CrossRef] [PubMed]
- Awika, J.M.; Duodu, K.G. Bioactive polyphenols and peptides in cowpea (Vigna unguiculata) and their health promoting properties: A review. J. Funct. Foods 2017, 38, 686–697. [Google Scholar] [CrossRef]
- Amaral, A.L.; Ferreira, E.S.; Silva, M.A.; Neves, V.A.; Demonte, A. The Vicilin protein (Vigna radiata L.) of mung bean as a functional food: Evidence of “in vitro” hypocholesterolemic activity. Nutr. Food Sci. 2017, 47, 907–916. [Google Scholar] [CrossRef]
- Malaguti, M.; Dinelli, G.; Leoncini, E.; Bregola, V.; Bosi, S.; Cicero, A.F.G.; Hrelia, S. Bioactive peptides in cereals and legumes: Agronomical, biochemical and clinical aspects. Int. J. Mol. Sci. 2014, 15, 21120–21135. [Google Scholar] [CrossRef]
- Moreno-Valdespino, C.A.; Luna-Vital, D.; Camacho-Ruiz, R.M.; Mojica, L. Bioactive proteins and phytochemicals from legumes: Mechanisms of action preventing obesity and type-2 diabetes. Food Res. Int. 2020, 130, 108905. [Google Scholar] [CrossRef]
- Orrapin, S.; Intorasoot, A.; Roytrakul, S.; Dechsupa, N.; Kantapan, J.; Onphat, Y.; Srimek, C.; Sitthidet Tharinjaroen, C.; Anukool, U.; Butr-Indr, B.; et al. A novel recombinant javanicin with dual antifungal and anti-proliferative activities. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- E Silva, M.B.C.; da Cruz Souza, C.A.; Philadelpho, B.O.; da Cunha, M.M.N.; Batista, F.P.R.; da Silva, J.R.; Druzian, J.I.; Castilho, M.S.; Cilli, E.M.; Ferreira, E.S. In vitro and in silico studies of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase inhibitory activity of the cowpea Gln-Asp-Phe peptide. Food Chem. 2018, 259, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, M.; Maselli, P.; Nucara, A. Structural aspects of legume proteins and nutraceutical properties. Food Res. Int. 2015, 76, 19–30. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Liu, W.; Chen, H. Physicochemical characterization, antioxidant and anticancer activities of proteins from four legume species. J. Food Sci. Technol. 2017, 54, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Carbonaro, M.; Cappelloni, M.; Nicoli, S.; Lucarini, M.; Carnovale, E. Solubility-digestibility relationship of legume proteins. J. Agric. Food Chem. 1997, 45, 3387–3394. [Google Scholar] [CrossRef]
- Neves, V.A.; Pereira, D.D.; Shoshima, A.H.R.; Tavano, O.L. Isolamento da globulina principal de caupí (Vigna unguiculata (L.) Walp.) cultivar BR 14-Mulato. Alim. Nutr. 2003, 14, 47–55. [Google Scholar]
- Chau, C.F.; Cheung, P.C.K. Functional properties of flours prepared from three Chinese indigenous legume seeds. Food Chem. 1998, 61, 429–433. [Google Scholar] [CrossRef]
- Ferreira, E.S.; Silva, M.A.; Demonte, A.; Neves, V.A. β-conglycinin combined with fenofibrate or rosuvastatin have exerted distinct hypocholesterolemic effects in rats. Lipids Health Dis. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, M.; Jacquin, F.; Cassecuelle, F.; Savois, V.; Belghazi, M.; Aubert, G.; Quillien, L.; Huart, M.; Marget, P.; Burstin, J. A PQL (protein quantity loci) analysis of mature pea seed proteins identifies loci determining seed protein composition. Proteomics 2011, 11, 1581–1594. [Google Scholar] [CrossRef] [PubMed]
- Hajduch, M.; Ganapathy, A.; Stein, J.W.; Thelen, J.J. A Systematic Proteomic Study of Seed Filling in Soybean. Establishment of High-Resolution Two-Dimensional Reference Maps, Expression Profiles, and an Interactive Proteome Database. Plant Physiol. 2005, 137, 1397–1419. [Google Scholar] [CrossRef]
- Mendoza, E.M.T.; Adachi, M.; Bernardo, A.E.N.; Utsumi, S. Mungbean [Vigna radiata (L.) Wilczek] globulins: Purification and characterization. J. Agric. Food Chem. 2001, 49, 1552–1558. [Google Scholar] [CrossRef]
- Sathe, S.K.; Venkatachalam, M. Fractionation and biochemical characterization of moth bean (Vigna aconitifolia L.) proteins. LWT Food Sci. Technol. 2007, 40, 600–610. [Google Scholar] [CrossRef]
- Klampfer, L. Cytokines, inflammation and colon cancer. Curr. Cancer Drug Targets. 2011, 11, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.S.; Capraro, J.; Sessa, F.; Magni, C.; Demonte, A.; Consonni, A.; Neves, V.A.; Cilli, E.M.; Duranti, M.; Scarafoni, A. New molecular features of cowpea bean (Vigna unguiculata L. Walp) β-vignin. Biosci. Biotechnol. Biochem. 2018, 82. [Google Scholar] [CrossRef]
- Han, I.H.; Swanson, B.G.; Baik, B.K. Protein digestibility of selected legumes treated with ultrasound and high hydrostatic pressure during soaking. Cereal Chem. 2007, 84, 518–521. [Google Scholar] [CrossRef]
- Tanoue, T.; Nishitani, Y.; Kanazawa, K.; Hashimoto, T.; Mizuno, M. In vitro model to estimate gut inflammation using co-cultured Caco-2 and RAW264.7 cells. Biochem. Biophys. Res. Commun. 2008, 374, 565–569. [Google Scholar] [CrossRef]
- Leonard, F.; Collnot, E.M.; Lehr, C.M. A three-dimensional coculture of enterocytes, monocytes and dendritic cells to model inflamed intestinal mucosa in vitro. Mol. Pharm. 2010, 7, 2103–2119. [Google Scholar] [CrossRef] [PubMed]
- Muto, E.; Dell’Agli, M.; Sangiovanni, E.; Mitro, N.; Fumagalli, M.; Crestani, M.; De Fabiani, E.; Caruso, D. Olive oil phenolic extract regulates interleukin-8 expression by transcriptional and posttranscriptional mechanisms in Caco-2 cells. Mol. Nutr. Food Res. 2015, 59, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Akeson, W.R.; Stahmann, M.A. A Pepsin pancreatin digest index of protein quality evaluation. J. Nutr. 1964, 83, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, A.C.C.; Draghetta, W.; Del Lama, S.N.; Camargo, A.C.M.; Greene, L.J. A convenient manual trinitrobenzenesulfonic acid method for monitoring amino acids and peptides in chromatographic column effluents. Anal. Biochem. 1979, 96, 317–321. [Google Scholar] [CrossRef]
- Faye, L.; Chrispeels, M.J. Transport and processing of the glycosylated precursor of Concanavalin A in jack-bean. Planta 1987, 170, 217–224. [Google Scholar] [CrossRef]
- Mermet, J.-M.; Poussel, E. ICP Emission Spectrometers: 1995 Analytical Figures of Merit. Appl. Spectrosc. 1995, 49, 12A–18A. [Google Scholar] [CrossRef]
- Capraro, J.; De Benedetti, S.; Di Dio, M.; Bona, E.; Abate, A.; Corsetto, P.A.; Scarafoni, A. Characterization of Chenopodin Isoforms from Quinoa Seeds and Assessment of Their Potential Anti-Inflammatory Activity in Caco-2 Cells. Biomolecules 2020, 10, 795. [Google Scholar] [CrossRef] [PubMed]




| Fraction 1 | Protein | |
|---|---|---|
| mg/g of Seed | wt% 2 | |
| Wholemeal flour | 203.90 ± 2.55 | 100 |
| TPE | 164.50 ± 1.87 | 80.68 ± 0.43 |
| Albumin | 20.22 ± 0.72 | 9.92 ± 0.26 |
| Globulin | 104.56 ± 1.34 | 51.28 ± 0.38 |
| Prolamin | 5.18 ± 0.12 | 2.54 ± 0.05 |
| Glutelin | 13.13 ± 0.32 | 6.44 ± 0.14 |
| Insoluble protein | 7.93 ± 0.42 | 3.89 ± 0.23 |
| Sample * | Hydrolysis (%) | Digestibility (%) ‡ |
|---|---|---|
| β-vignin | 89.25 ± 2.09b | 92.18 ± 1.73b |
| Total protein extract (TPE) | 81.94 ± 1.66c | 84.63 ± 0.92c |
| Flour | 68.23 ± 2.02d | 70.47 ± 1,84d |
| Casein (standard) | 96.82 ± 0.82a | 100.00 ± 1.91a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Philadelpho, B.; Souza, V.; Souza, F.; Santos, J.; Batista, F.; Silva, M.; Capraro, J.; De Benedetti, S.; Heinzl, G.C.; Cilli, E.; et al. Chromatography-Independent Fractionation and Newly Identified Molecular Features of the Adzuki Bean (Vigna angularis Willd.) β-vignin Protein. Int. J. Mol. Sci. 2021, 22, 3018. https://doi.org/10.3390/ijms22063018
Philadelpho B, Souza V, Souza F, Santos J, Batista F, Silva M, Capraro J, De Benedetti S, Heinzl GC, Cilli E, et al. Chromatography-Independent Fractionation and Newly Identified Molecular Features of the Adzuki Bean (Vigna angularis Willd.) β-vignin Protein. International Journal of Molecular Sciences. 2021; 22(6):3018. https://doi.org/10.3390/ijms22063018
Chicago/Turabian StylePhiladelpho, Biane, Victória Souza, Fabiani Souza, Johnnie Santos, Fabiana Batista, Mariana Silva, Jessica Capraro, Stefano De Benedetti, Giuditta C. Heinzl, Eduardo Cilli, and et al. 2021. "Chromatography-Independent Fractionation and Newly Identified Molecular Features of the Adzuki Bean (Vigna angularis Willd.) β-vignin Protein" International Journal of Molecular Sciences 22, no. 6: 3018. https://doi.org/10.3390/ijms22063018
APA StylePhiladelpho, B., Souza, V., Souza, F., Santos, J., Batista, F., Silva, M., Capraro, J., De Benedetti, S., Heinzl, G. C., Cilli, E., Scarafoni, A., Magni, C., & Ferreira, E. (2021). Chromatography-Independent Fractionation and Newly Identified Molecular Features of the Adzuki Bean (Vigna angularis Willd.) β-vignin Protein. International Journal of Molecular Sciences, 22(6), 3018. https://doi.org/10.3390/ijms22063018










