Chromatin Regulator SRG3 Overexpression Protects against LPS/D-GalN-Induced Sepsis by Increasing IL10-Producing Macrophages and Decreasing IFN?-Producing NK Cells in the Liver
Abstract
:1. Introduction
2. Results
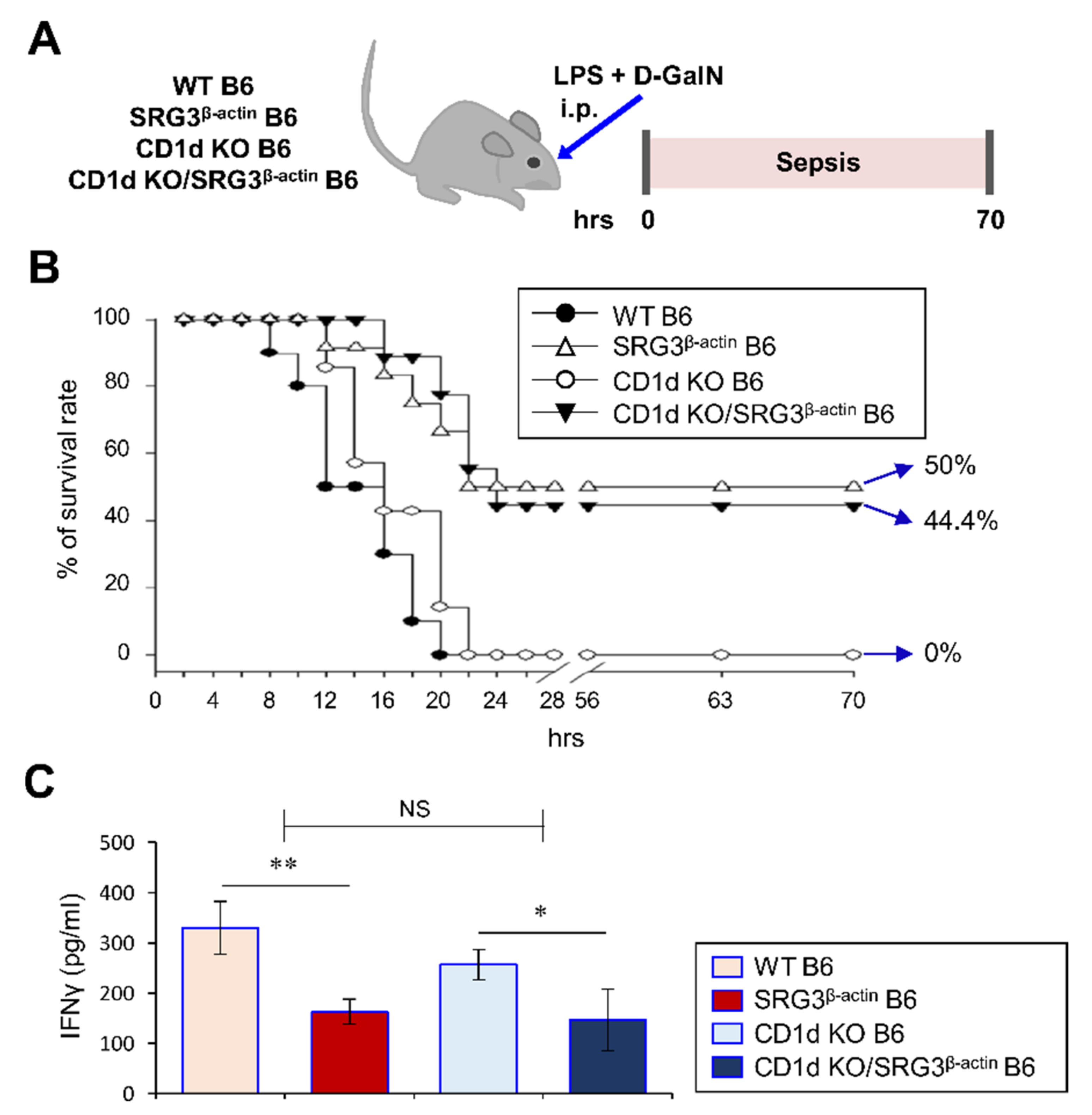
2.1. Ubiquitous Overexpression of the SRG3 Chromatin Remodeling Component Protects Mice against LPS/d-GalN-Induced Septic Shock
2.2. β-Actin Promoter-Driven Overexpression of SRG3 Suppresses LPS/d-GalN-Induced Pro-Inflammatory Cytokine Production in DCs and Macrophages
2.3. The Inhibitory Effects of SRG3 Overexpression on the Severity of LPS/D-GalN-Induced Sepsis Are Associated with Suppression of NK but Not NKT Cell Activation
2.4. The Protective Effect of SRG3 Overexpression on LPS/d-GalN-Induced Sepsis Is Independent of NKT Cells
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Mice and Reagents
4.3. Induction of Septic Shock
4.4. Genotyping of Tg and KO Mice
4.5. Flow Cytometry
4.6. Intracellular Cytokine Staining
4.7. Isolation of Liver Leukocytes
4.8. ELISA
4.9. Data Collection in the Human Protein Atlas
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ho, L.; Crabtree, G.R. Chromatin remodelling during development. Nat. Cell Biol. 2010, 463, 474–484. [Google Scholar] [CrossRef]
- Sohn, D.H.; Lee, K.Y.; Lee, C.; Oh, J.; Chung, H.; Jeon, S.H.; Seong, R.H. SRG3 Interacts Directly with the Major Components of the SWI/SNF Chromatin Remodeling Complex and Protects Them from Proteasomal Degradation. J. Biol. Chem. 2007, 282, 10614–10624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joliot, V.; Ait-Mohamed, O.; Battisti, V.; Pontis, J.; Philipot, O.; Robin, P.; Ito, H.; Ait-Si-Ali, S. The SWI/SNF Subunit/Tumor Suppressor BAF47/INI1 Is Essential in Cell Cycle Arrest upon Skeletal Muscle Terminal Differentiation. PLoS ONE 2014, 9, e108858. [Google Scholar] [CrossRef]
- Jeong, S.M.; Lee, C.; Lee, S.K.; Kim, J.; Seong, R.H. The SWI/SNF Chromatin-remodeling Complex Modulates Peripheral T Cell Activation and Proliferation by Controlling AP-1 Expression. J. Biol. Chem. 2010, 285, 2340–2350. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Jeon, S.; Choi, S.; Park, K.; Seong, R.H. The SWI/SNF chromatin remodeling complex regulates germinal center formation by repressing Blimp-1 expression. Proc. Natl. Acad. Sci. USA 2015, 112, E718–E727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.W.; Park, H.J.; Jeon, S.H.; Lee, C.; Seong, R.H.; Park, S.-H.; Hong, S. Ubiquitous Over-Expression of Chromatin Remodeling Factor SRG3 Ameliorates the T Cell-Mediated Exacerbation of EAE by Modulating the Phenotypes of both Dendritic Cells and Macrophages. PLoS ONE 2015, 10, e0132329. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, H.; Jeon, J.; Park, Y.; Kim, T.-C.; Jeon, S.; Seong, R.; Van Kaer, L.; Hong, S. Ubiquitous Overexpression of Chromatin Remodeling Factor SRG3 Exacerbates Atopic Dermatitis in NC/Nga Mice by Enhancing Th2 Immune Responses. Int. J. Mol. Sci. 2021, 22, 1553. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Jeon, S.; Sohn, D.H.; Lee, C.; Ahn, S.; Kim, W.K.; Chung, H.; Seong, R.H. SRG3, a core component of mouse SWI/SNF complex, is essential for extra-embryonic vascular development. Dev. Biol. 2008, 315, 136–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.W.; Park, H.J.; Kim, N.; Hong, S. Natural Killer Dendritic Cells Enhance Immune Responses Elicited byα-Galactosylceramide-Stimulated Natural Killer T Cells. BioMed Res. Int. 2013, 2013, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.H.; Lee, S.W.; Park, H.J.; Lee, S.H.; Im, W.K.; Kim, Y.D.; Kim, K.H.; Park, S.J.; Hong, S.; Jeon, S.H. Anti-cancer activity of Angelica gigas by increasing immune response and stimulating natural killer and natural killer T cells. BMC Complement. Altern. Med. 2018, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Park, H.J.; Cheon, J.H.; Wu, L.; van Kaer, L.; Hong, S. iNKT Cells Suppress Pathogenic NK1.1(+)CD8(+) T Cells in DSS-Induced Colitis. Front. Immunol. 2018, 9, 2168. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, S.W.; Im, W.; Kim, M.; van Kaer, L.; Hong, S. iNKT Cell Activation Exacerbates the Development of Huntington’s Disease in R6/2 Transgenic Mice. Mediat. Inflamm. 2019, 2019, 3540974. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Park, H.J.; Pei, Y.; Yeo, Y.; Hong, S. Topical application of zwitterionic chitosan suppresses neutrophil-mediated acute skin inflammation. Int. J. Biol. Macromol. 2020, 158, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, S.W.; Park, S.H.; van Kaer, L.; Hong, S. Selective Expansion of Double Negative iNKT Cells Inhibits the Development of Atopic Dermatitis in Valpha14 TCR Transgenic NC/Nga Mice by Increasing Memory-Type CD8(+) T and Regulatory CD4(+) T Cells. J. Investig. Dermatol. 2020. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, S.W.; Van Kaer, L.; Hong, S. CD1d-Dependent iNKT Cells Control DSS-Induced Colitis in a Mouse Model of IFNγ-Mediated Hyperinflammation by Increasing IL22-Secreting ILC3 Cells. Int. J. Mol. Sci. 2021, 22, 1250. [Google Scholar] [CrossRef]
- Kinjo, Y.; Illarionov, P.; Vela, J.L.; Pei, B.; Girardi, E.; Li, X.; Li, Y.; Imamura, M.; Kaneko, Y.; Okawara, A.; et al. Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat. Immunol. 2011, 12, 966–974. [Google Scholar] [CrossRef]
- Lee, S.W.; Park, H.J.; van Kaer, L.; Hong, S.; Hong, S. Graphene oxide polarizes iNKT cells for production of TGFbeta and attenuates inflammation in an iNKT cell-mediated sepsis model. Sci. Rep. 2018, 8, 10081. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Holdbrooks, A.T.; Liu, Y.; Reynolds, S.L.; Yanagisawa, L.L.; Benveniste, E.N. SOCS3 Deficiency Promotes M1 Macrophage Polarization and Inflammation. J. Immunol. 2012, 189, 3439–3448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liangliang, Z.; Mu, G.; Song, C.; Zhou, L.; He, L.; Jin, Q.; Lu, Z. Role of M2 Macrophages in Sepsis-Induced Acute Kidney Injury. Shock 2018, 50, 233–239. [Google Scholar] [CrossRef]
- Bai, L.; Liu, X.; Zheng, Q.; Kong, M.; Zhang, X.; Xiaohui, Z.; Lou, J.; Ren, F.; Chen, Y.; Zheng, S.; et al. M2-like macrophages in the fibrotic liver protect mice against lethal insults through conferring apoptosis resistance to hepatocytes. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Ma, T.; Lin, Y.; Lu, X.; Zhang, C.; Chen, S.; Jian, Z. IL-6/STAT3 pathway intermediates M1/M2 macrophage polarization during the development of hepatocellular carcinoma. J. Cell Biochem. 2018, 119, 9419–9432. [Google Scholar] [CrossRef]
- Stearns-Kurosawa, D.J.; Osuchowski, M.F.; Valentine, C.; Kurosawa, S.; Remick, D.G. The Pathogenesis of Sepsis. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 19–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, B.; Radaeva, S.; Park, O. Liver natural killer and natural killer T cells: Immunobiology and emerging roles in liver diseases. J. Leukoc. Biol. 2009, 86, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Park, H.J.; Lee, K.S.; Park, S.H.; Kim, S.; Jeon, S.H.; Hong, S. IL32gamma activates natural killer receptor-expressing innate immune cells to produce IFNgamma via dendritic cell-derived IL12. Biochem. Biophys. Res. Commun. 2015, 461, 86–94. [Google Scholar] [CrossRef]
- Michel, T.; Hentges, F.; Zimmer, J. Consequences of the crosstalk between monocytes/macrophages and natural killer cells. Front. Immunol. 2013, 3, 403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pervin, M.; Golbar, H.M.; Bondoc, A.; Izawa, T.; Kuwamura, M.; Yamate, J. Immunophenotypical characterization and influence on liver homeostasis of depleting and repopulating hepatic macrophages in rats injected with clodronate. Exp. Toxicol. Pathol. 2016, 68, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Bozorgzadeh, A.; Pierce, R.H.; Kurtis, J.; Crispe, I.N.; Orloff, M.S. TLR-dependent cross talk between human Kupffer cells and NK cells. J. Exp. Med. 2008, 205, 233–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emoto, M.; Miyamoto, M.; Yoshizawa, I.; Emoto, Y.; Schaible, U.E.; Kita, E.; Kaufmann, S.H. Critical role of NK cells rather than V alpha 14(+)NKT cells in lipopolysaccharide-induced lethal shock in mice. J. Immunol. 2002, 169, 1426–1432. [Google Scholar] [CrossRef] [Green Version]
- Etogo, A.O.; Nunez, J.; Lin, C.Y.; Toliver-Kinsky, T.E.; Sherwood, E.R. NK but Not CD1-Restricted NKT Cells Facilitate Systemic Inflammation during Polymicrobial Intra-Abdominal Sepsis1. J. Immunol. 2008, 180, 6334–6345. [Google Scholar] [CrossRef] [Green Version]
- Mühlen, K.A.; Schümann, J.; Wittke, F.; Stenger, S.; Van Rooijen, N.; Van Kaer, L.; Tiegs, G. NK Cells, but Not NKT Cells, Are Involved in Pseudomonas aeruginosaExotoxin A-Induced Hepatotoxicity in Mice. J. Immunol. 2004, 172, 3034–3041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuroda, E.; Ho, V.; Ruschmann, J.; Antignano, F.; Hamilton, M.; Rauh, M.J.; Antov, A.; Flavell, R.A.; Sly, L.M.; Krystal, G. SHIP Represses the Generation of IL-3-Induced M2 Macrophages by Inhibiting IL-4 Production from Basophils. J. Immunol. 2009, 183, 3652–3660. [Google Scholar] [CrossRef] [PubMed]
- Lapaque, N.; Walzer, T.; Méresse, S.; Vivier, E.; Trowsdale, J. Interactions between Human NK Cells and Macrophages in Response toSalmonellaInfection. J. Immunol. 2009, 182, 4339–4348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.L.J.H.; Park, J.Y.; Choi, I. IFN-γ Regulates Expression of BRG1 Associated Factor 155/170 and Sensitivity to Steroid in Astrocytes. Immune Netw. 2004, 4, 224–228. [Google Scholar] [CrossRef] [Green Version]
- Sim, G.C.; Wu, S.; Jin, L.; Hwu, P.; Radvanyi, L.G. Defective STAT1 activation associated with impaired IFN-gamma production in NK and T lymphocytes from metastatic melanoma patients treated with IL-2. Oncotarget 2016, 7, 36074–36091. [Google Scholar] [CrossRef]
- Papillon, J.P.N.; Nakajima, K.; Adair, C.D.; Hempel, J.; Jouk, A.O.; Karki, R.G.; Mathieu, S.; Mobitz, H.; Ntaganda, R.; Smith, T.; et al. Discovery of Orally Active Inhibitors of Brahma Homolog (BRM)/SMARCA2 ATPase Activity for the Treatment of Brahma Related Gene 1 (BRG1)/SMARCA4-Mutant Cancers. J. Med. Chem. 2018, 61, 10155–10172. [Google Scholar] [CrossRef] [PubMed]
- Ju, A.; Lee, S.W.; Lee, Y.E.; Han, K.-C.; Kim, J.-C.; Shin, S.C.; Park, H.J.; Kim, E.E.; Hong, S.; Jang, M. A carrier-free multiplexed gene editing system applicable for suspension cells. Biomaterials 2019, 217, 119298. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Oksvold, P.; Fagerberg, L.; Lundberg, E.; Jonasson, K.; Forsberg, M.; Zwahlen, M.; Kampf, C.; Wester, K.; Hober, S.; et al. Towards a knowledge-based Human Protein Atlas. Nat. Biotechnol. 2010, 28, 1248–1250. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.W.; Park, H.J.; Jeon, J.; Park, Y.H.; Kim, T.-C.; Jeon, S.H.; Seong, R.H.; Van Kaer, L.; Hong, S. Chromatin Regulator SRG3 Overexpression Protects against LPS/D-GalN-Induced Sepsis by Increasing IL10-Producing Macrophages and Decreasing IFN?-Producing NK Cells in the Liver. Int. J. Mol. Sci. 2021, 22, 3043. https://doi.org/10.3390/ijms22063043
Lee SW, Park HJ, Jeon J, Park YH, Kim T-C, Jeon SH, Seong RH, Van Kaer L, Hong S. Chromatin Regulator SRG3 Overexpression Protects against LPS/D-GalN-Induced Sepsis by Increasing IL10-Producing Macrophages and Decreasing IFN?-Producing NK Cells in the Liver. International Journal of Molecular Sciences. 2021; 22(6):3043. https://doi.org/10.3390/ijms22063043
Chicago/Turabian StyleLee, Sung Won, Hyun Jung Park, Jungmin Jeon, Yun Hoo Park, Tae-Cheol Kim, Sung Ho Jeon, Rho Hyun Seong, Luc Van Kaer, and Seokmann Hong. 2021. "Chromatin Regulator SRG3 Overexpression Protects against LPS/D-GalN-Induced Sepsis by Increasing IL10-Producing Macrophages and Decreasing IFN?-Producing NK Cells in the Liver" International Journal of Molecular Sciences 22, no. 6: 3043. https://doi.org/10.3390/ijms22063043
APA StyleLee, S. W., Park, H. J., Jeon, J., Park, Y. H., Kim, T.-C., Jeon, S. H., Seong, R. H., Van Kaer, L., & Hong, S. (2021). Chromatin Regulator SRG3 Overexpression Protects against LPS/D-GalN-Induced Sepsis by Increasing IL10-Producing Macrophages and Decreasing IFN?-Producing NK Cells in the Liver. International Journal of Molecular Sciences, 22(6), 3043. https://doi.org/10.3390/ijms22063043







